Abstract
Deficits in reward and motivation are common symptoms characterizing several psychiatric and neurological disorders. Such deficits may include anhedonia, defined as loss of pleasure, as well as impairments in anticipatory pleasure, reward valuation, motivation/effort, and reward learning. This chapter describes recent advances in the development of behavioral tasks used to assess different aspects of reward processing in both humans and non-human animals. While earlier tasks were generally developed independently with limited cross-species correspondence, a newer generation of translational tasks has emerged that are theoretically and procedurally analogous across species and allow parallel testing, data analyses, and interpretation between human and rodent behaviors. Such enhanced conformity between cross-species tasks will facilitate investigation of the neurobiological mechanisms underlying discrete reward and motivated behaviors and is expected to improve our understanding and treatment of neuropsychiatric disorders characterized by reward and motivation deficits.
1. Introduction
Feeling joy and satisfaction when engaging in social activities or accomplishing a task is an important process that promotes positive reinforcement, ensuring that events that are vital for survival and reproductive success are repeated. Conversely, inability to feel pleasure for normally pleasurable experiences can have severely debilitating effects on many aspects of life, including interpersonal relationships, work, and health. Without pleasure, experiences and activities that promote healthy lifestyles may not be appreciated, engaged in, and repeated.
The term anhedonia was coined by the French psychologist Ribot in the late 19th century to describe his patients, for which “it was impossible to find the least pleasure” (Ribot, 1896). Although the term anhedonia is still widely used more than a century later, the behaviors and neurobiological mechanisms that govern impaired reward processing have since evolved to expand beyond anhedonia. Indeed, pleasure is just one aspect of reward processing that contributes to positive reinforcement. As a result, the term anhedonia does not adequately capture the multi-faceted reward processes that, when disrupted, may each have debilitating effects on daily functioning and health even when other reward constructs remain intact.
Besides anhedonia, or loss of pleasure, deficits in other reward processes could result in behaviors that may be interpreted as loss of pleasure. For example, several reward-related processes precede the point at which an experience or activity could be perceived as pleasurable. Individuals must first: 1) anticipate or predict expected rewards that may occur in the future; 2) determine relative values of different rewards; 3) determine the cost or effort required to obtain different rewards; 4) become motivated to perform the necessary goal-directed actions to obtain worthwhile rewards; and 5) learn from previous experiences in order to repeat pleasurable goal-directed behaviors in the future. Deficits in any of these processes may preclude an individual from engaging in goal-directed actions for rewards, regardless of whether or not the reward is perceived as pleasant once obtained. Furthermore, unless carefully assessed, deficits in any of these processes may be incorrectly interpreted as anhedonia. Because each of these distinct reward processes are subserved by distinct neurobiological mechanisms (Der-Avakian and Markou, 2012), understanding which processes are affected in psychiatric and neurological disorders becomes important for elucidating pathophysiology and potential treatment options. This approach is aligned with the United States National Institute of Mental Health (NIMH) Research Domain Criteria (RDoC) initiative, which aims to classify mental disorders based on specific behavioral dimensions, such as reward-related subdomains, that can be linked to specific neurophysiological processes (Insel et al, 2010).
2. Deficits of Reward and Motivation in Psychiatric and Neurological Disorders
Several psychiatric and neurological disorders are marked by debilitating symptoms relating to reward and motivational deficits (American Psychiatric Association, 2013; World Health Organization, 1992). Anhedonia has been described in major depressive disorder (MDD; core symptom) (Klein, 1974), bipolar disorder (Leibenluft et al, 2003), schizophrenia (Haslam, 1809; Meehl, 1962), substance use disorder (particularly during withdrawal) (reviewed in (Markou et al, 1998)), eating disorders (Davis and Woodside, 2002), autism spectrum disorder (Chevallier et al, 2012), post-traumatic stress disorder (PTSD) (Nawijn et al, 2015), Alzheimer’s disease (Starkstein et al, 2005), and Parkinson’s disease (Isella et al, 2003) (see below for some caveats to these examples). Recent interest among clinical researchers has focused on investigating which specific reward processes, beyond anhedonia, are affected in the disorders described above (reviewed in Hyman and Fenton, 2003; Insel et al, 2010; Leboyer et al, 1998; Meyer-Lindenberg and Weinberger, 2006; Whitton et al, 2015). This approach is consistent with research in experimental animals, where broadly defined psychiatric disorders characterized by multiple symptoms cannot be modeled in non-human animals, but rather discrete behavioral processes that are often linked to circumscribed neurobiological mechanisms can be assessed (Geyer and Markou, 2002; Markou et al, 2009). Detailed investigation of the precise reward processes affected in each disorder requires novel clinical assessments that reliably distinguish one reward process from another. To take advantage of basic research in animals that can facilitate treatment development, new clinical behavioral assessments must be carefully designed.
3. Important Considerations for Cross-species Behavioral Assessments
Of the several human and animal behavioral assessments designed to measure different components of reward processing (see below and Barnes et al, 2014; Markou et al, 2013), most were developed independently between species. Thus, attempts to translate behavior across different species have traditionally been limited by several factors, including limited correspondence between human and animal tasks designed to assess the same construct. To improve correspondence between human and animal procedures and the predictive validity of data derived from animal behavioral procedures for human behaviors, the factors described below should be considered when new tasks are developed for any species.
With regard to clinical assessments, behavior is often measured using self-report questionnaires that are subjective in nature and require verbal communication between the experimenter and participant. For obvious reasons, these types of assessments cannot be implemented in animals. Animals may be observed for changes in non-verbal behavior (e.g., locomotor activity, orofacial responses) or may be trained to perform operant responses. Importantly, behavioral output in animals is generally quantifiable and data collection is (or at least should be) objective. Thus, for any clinical assessment to be successfully back-translated to animals, testing should require no verbal communication. In the examples described in the sections below (also, see Table 1), new clinical assessments that have either been translated from existing animal tasks or back-translated into novel animal tasks utilize a computer-based stimulus-response “game” (Anderson et al, 2012; Pizzagalli et al, 2005; Treadway et al, 2009).
Table 1.
Correspondence between human and non-human animal assessments of reward processes and associated neurobiological mechanisms
| Reward Processes |
Human Assessments |
Non-human Animal Assessments |
Correspondence | Neurobiological Mechanisms |
|---|---|---|---|---|
| Consummatory Pleasure |
Self-report (e.g., SHAPS, CPAS) |
Poor To Mid |
Nucleus accumbens Ventral pallidum Orbitofrontal cortex Opioids Endocannabinoids |
|
| Sucrose preference | Sucrose consumption and preference |
|||
| Anticipatory Pleasure |
Self-report (e.g., TEPS, ACIPS) |
Poor | Anterior cingulate cortex Orbitofrontal cortex Medial prefrontal cortex Basal ganglia Dopamine |
|
| Arousal, anticipatory locomotion, approach behaviors |
||||
| Ultrasonic vocalizations |
||||
| Successive contrast effects |
||||
| Reward Valuation |
Outcome devaluation task |
Outcome devaluation task |
High | Medial prefrontal cortex Dorsal striatum Nucleus accumbens Basolateral amygdala Orbitofrontal cortex Dopamine, Glutamate |
| Motivation | Self-report (e.g., BAS, MAP-SR) |
Mid to High |
Ventral tegmental area Nucleus accumbens Medial prefrontal cortex Anterior cingulate cortex Lateral hypothalamus Dopamine Glutamate |
|
| Progressive ratio task |
Progressive ratio task | |||
| EEfRT | Effort-related choice tasks |
|||
| Reward Learning |
RBPRT | RBPRT | High | Anterior cingulate cortex Orbitofrontal cortex Striatum Dorsal striatum Dopamine |
| PSST | PSST |
With regard to animal procedures, even though assessments generally meet the criteria above with regard to objectivity and non-verbal communication, behavior is sometimes measured in a manner that cannot be replicated in humans. For example, the forced swim and tail suspension tests commonly used with rodents, which are argued to reflect behavioral despair relating to depression, cannot be readily implemented in humans. Because often the experimenter is forced to interpret the behavior observed in animals in terms relating to human behavior, such an anthropomorphic interpretation can be very misleading and, even worse, has failed to predict treatment efficacy in humans for novel medications (Hyman, 2012; Insel et al, 2013; Markou et al, 2009; Nestler and Hyman, 2010).
Lastly, with regard to both human and animal tasks, behavioral output should ideally be accompanied by some structural or physiological measure that can be compared between species. While human and animal methods for visualizing neural structure and function each have their respective advantages and limitations, comparison of hypothesized neurobiological mechanisms across species will increase confidence that the similarities in observed behavior are manifested by similar biological mechanisms. Such neurobiological concordance across species will improve the probability that putative treatments for reward deficits tested in animals will translate to the clinic.
4. Translational Assessments of Reward and Motivation
4.1. Pleasure
To disentangle the subtle differences between pleasure and other reward processes, behavioral procedures are required that can measure aspects of pleasure that are not influenced by factors like motivation and learning. The sections below summarize the procedures commonly used to assess pleasure in humans and animals.
4.1.1. Human Assessments of Pleasure
Commonly used self-report scales that exclusively probe for pleasure deficits include the Snaith-Hamilton Pleasure Scale (SHAPS) (Snaith et al, 1995), the Fawcett-Clark Pleasure Scale (Fawcett et al, 1983), the Positive and Negative Affect Schedule (PANAS) (Watson et al, 1988), and the Revised Chapman Physical Anhedonia Scale (CPAS) (Chapman et al, 1976), among others. In addition, a subset of scores from questions relating to anhedonia from the Beck Depression Inventory (BDI) and Hamilton Rating Scale for Depression (HAM-D) may be extracted to distinguish deficits in reward processing from other symptoms of depression, although these subscores have generally not been validated. It should be noted that there are several other self-report measures of pleasure that are not listed here, but are widely used for diagnostic and research purposes.
There are important limitations related to relying on subjective observation or self-report of anhedonia that were described above. Recent adaptations of subjective anhedonia scales have begun to address some of these concerns by parsing reward deficits into discrete reward-related constructs, like consummatory and anticipatory anhedonia (see below). Nonetheless, these new scales remain subjective and require verbal communication, limiting their usefulness as translational measures.
4.1.2. Non-human Animal Assessments of Pleasure
In animals, common procedures used to assess anhedonia include the sucrose consumption and preference tests (Willner, 2005; Willner et al, 1987). The sucrose consumption test involves measuring the consumption of a palatable sucrose solution during or after exposure to an anhedonia-producing event. Similarly, the sucrose preference test is used to measure the preference for a sucrose solution when given a choice between the palatable drink and water. Decreased or no preference for the sucrose solution over water is argued to reflect anhedonia. While either test may be conducted without prior food or water restriction that is often used to increase motivation to respond during such tasks, the influence of motivation on these tasks cannot be ruled out. Exposure to various forms of stress, a precipitating factor for several psychiatric disorders, decreased sucrose preference in rodents (Willner et al, 1992) and non-human primates (Paul et al, 2000). However, the reliability of the sucrose preference test as a measure of anhedonia in animals has been questioned by several researchers who have been unable to replicate decreases in sucrose consumption or preference after chronic mild stress exposure (Forbes et al, 1996; Harris et al, 1997; Matthews et al, 1995; Reid et al, 1997).
4.1.3. Convergence of Human and Non-human Animal Assessments of Pleasure
While the human anhedonia scales cannot be translated into analogous animal tasks, the sucrose preference test has been adapted for use in humans. Interestingly, attempts to translate the animal findings of stress-induced anhedonia to humans have largely resulted in negative results. For example, patients with MDD, schizophrenia, or autism do not show deficits in the hedonic response to sucrose compared to healthy controls (Berlin et al, 1998; Damiano et al, 2014; Dichter et al, 2010). These results from human and animal studies suggest that either: a) the sucrose preference test is not a valid assessment of pleasure; or b) psychiatric disorders such as MDD, schizophrenia, and autism are not associated with deficits in pleasure. Notably, several lines of evidence have begun to confirm the latter point, particularly with regard to schizophrenia (Barch et al, 2015; Gard et al, 2007; Heerey and Gold, 2007).
4.2. Anticipation
Whereas hedonic, or consummatory, pleasure is defined as pleasure experienced while engaged in a rewarding activity, anticipatory pleasure is pleasure that is experienced at the thought of an event that is expected to occur in the future. Thus, deficits in anticipatory pleasure require the formulation of mental representations of future events. It has been well established that consummatory and anticipatory pleasure are mediated by distinct neural processes (Berridge and Robinson, 2003; Der-Avakian et al, 2012; Schultz, 2002). Consummatory pleasure is subserved by opioid and serotonergic mechanisms, while anticipatory pleasure is mediated primarily by dopaminergic mechanisms (Barbano and Cador, 2006, 2007). Thus, it is not surprising that anticipatory pleasure has been linked to motivation, another reward construct mediated by mesolimbic dopamine transmission (see below). For example, in MDD, anticipation for a rewarding event predicted the degree of an individual’s motivation to produce goal-directed actions to obtain the rewarding event (Sherdell et al, 2012). Importantly, clinical studies have highlighted a dissociation between consummatory and anticipatory pleasure in psychiatric disorders. For example, schizophrenia has been associated with disrupted anticipatory pleasure, but intact consummatory pleasure (Gard et al, 2007; Mote et al, 2014). Conversely, patients with Parkinson’s disease showed deficits in consummatory, but not anticipatory, pleasure (Loas et al, 2014). Thus, understanding the precise construct that is affected in different disorders has important implications for treatment development.
4.2.1. Human Assessments of Anticipation
Clinical assessments that distinguish between consummatory and anticipatory anhedonia have been developed in recent years. The Temporal Experience of Pleasure Scale (TEPS) (Gard et al, 2006) is an 18-item self-report measure of trait anticipatory and consummatory pleasure that requires participants to respond using a Likert scale to indicate their agreement with statements that reflect either enjoyment of a reward or enjoyment relating to anticipation of a reward. An example of a statement probing anticipatory pleasure is: “When something exciting is coming up in my life, I really look forward to it.” Scores on the anticipatory pleasure-related items of the TEPS positively correlated with scores on the Behavioral Activation Scale (BAS), a measure of motivated behavior (Carver and White, 1994), further confirming the relationship between anticipatory pleasure and motivation (Gard et al, 2007).
Similar to the TEPS, the Anticipatory and Consummatory Interpersonal Pleasure Scale (ACIPS) is a 17-item self-report measure specifically designed to assess anticipatory pleasure relating to social-interpersonal interactions as well as consummatory pleasure relating to the experience of pleasure for social-interpersonal interactions when they occurred (Gooding and Pflum, 2014a). The ACIPS also uses a Likert scale that measures level of agreement with statements like: “I look forward to seeing people when I'm on my way to a party or get-together.” Scores on the ACIPS positively correlated with scores on the TEPS, as well as with scores on the BAS, again suggesting that anticipatory pleasure is strongly related to motivated behavior (Gooding et al, 2014a; Gooding and Pflum, 2014b).
The TEPS and ACIPS are important tools that have provided new and detailed insights into the disrupted behaviors associated with psychiatric and neurological disorders. The advent of these scales that assess anticipatory pleasure may prompt the development of additional clinical measures that can accurately distinguish consummatory and anticipatory pleasures from motivation, valuation, learning, and so on. Nonetheless, as discussed above, the required verbal communication required to respond to these scales precludes the possibility of developing animal versions of these measures.
4.2.2. Non-human Animal Assessments of Anticipation
Animal behavioral tasks developed to assess anticipatory pleasure generally measure arousal, anticipatory locomotor activity, and approach behavior prior to presentation of an expected reward. The reward is typically presented for several days at the same time of day. After training, anticipatory behavior is assessed on a test day immediately prior to the time of day when the reward is typically presented. Increased locomotor activity has been observed in rodents in anticipation of food (known as food-anticipatory activity) (Mistlberger, 1994), a sweet palatable reward (Hsu et al, 2010; Mendoza et al, 2005), a sexually receptive female (Mendelson and Pfaus, 1989; Pfaus and Phillips, 1991), or a drug reward (i.e., ethanol) (Buck et al, 2014a). While many of the studies on anticipatory pleasure involved rodents, food-anticipatory activity is conserved across species, including honeybees (Moore et al, 1989), fish (Weber and Spieler, 1987), birds (Wenger et al, 1991), rabbits (Jilge, 1992), and monkeys (Sulzman et al, 1977). In rodents, anticipatory activity was disrupted by manipulations known to produce depression-related behaviors in humans, including social stress (Kamal et al, 2010; van der Harst et al, 2005) and withdrawal from chronic amphetamine treatment (Barr et al, 1999a). Moreover, blocking dopamine, but not opioid, signaling disrupted anticipatory behavior in rats (Barbano et al, 2006), which is consistent with current knowledge of neurotransmitter systems mediating consummatory (opioid) and anticipatory (dopamine) pleasure (Barbano et al, 2007).
Anticipatory pleasure may also be assessed by measuring short, high ultrasonic vocalizations (~50 kHz) in rats, with higher frequency of ultrasonic vocalizations reflecting greater anticipation of an expected reward (Knutson et al, 2002). Indeed, increased anticipatory motor behavior was positively correlated with frequency of high ultrasonic vocalizations (Brenes and Schwarting, 2015). High ultrasonic vocalizations were emitted in anticipation of several rewarding stimuli, including food (Buck et al, 2014b; Opiol et al, 2015), a cocaine or ethanol reward (Buck et al, 2014a; Ma et al, 2010), and being reunited with a cage mate after a period of social isolation (Willey and Spear, 2012). As with anticipatory activity, blocking dopamine neurotransmission attenuated high ultrasonic vocalizations in response to food (Buck et al, 2014b).
Successive contrast effects reflect adaptations of behavior in response to unexpected changes of an anticipated reward. Positive contrast effects are observed when a greater reward (e.g., 4 food pellets) is unexpectedly obtained compared to an anticipated smaller reward (e.g., 1 food pellet), eliciting a greater behavioral response (e.g., lever pressing) than if the higher value reward was only ever experienced. Conversely, negative contrast effects are observed when a smaller reward is unexpectedly obtained compared to an anticipated greater reward, which typically elicits a greater depression of behavioral responding than if the smaller value reward was only ever experienced. The latter shift (i.e., negative contrast effect) is argued to reflect a state of disappointment that arises from the anticipated reward not being delivered. Successive contrast effects are highly conserved across species, including honeybees (Couvillon and Bitterman, 1984), rats (Barr and Phillips, 2002), dogs (Bentosela et al, 2009), and humans (Specht and Twining, 1999). As with anticipatory activity, depression-producing events, like amphetamine withdrawal, exacerbated negative contrast effects in rats (Barr et al, 2002), whereas acute amphetamine administration attenuated negative contrast effects (Phelps et al, 2015). Moreover, administration of a dopamine D1 receptor antagonist worsened negative contrast effects (Phelps et al, 2015), supporting the argument that reward anticipation is mediated by dopaminergic mechanisms. The role of dopamine in successive contrast effects is perhaps not surprising, given that the behavioral procedure may also be argued to reflect positive and negative reward prediction error processing, whereby greater- or lesser-than-expected rewards, respectively, are experienced. Positive and negative reward prediction errors are thought to be mediated by increased and decreased striatal dopamine signaling, respectively (Schultz et al, 1997).
4.2.3. Convergence of Human and Non-human Animal Assessments of Anticipation
While both human and animal assessments of anticipatory pleasure have played an important part in our understanding of reward processing in psychiatric and neurological disorders and their underlying neurobiological mechanisms, assessments are limited to the species in which they were developed. As with the assessments of consummatory pleasure reviewed above, the parallel development of objective, non-verbal assessments of anticipatory pleasure across species would benefit translational research efforts aimed at this reward construct. Nonetheless, the convergence of evidence surrounding the role of dopamine in anticipatory pleasure using different human and animal assessments suggests that the different species-specific tasks are tapping into similar behavioral and neurobiological mechanisms.
4.3. Reward Valuation
Reward valuation involves assessment of the relative value of rewards that guide approach and motivated behaviors. For example, rewards of higher value are expected to produce greater anticipation of and motivation to obtain the reward compared to rewards of lower value. Prior experiences allow individuals to create representations of reward value for future stimuli. Thus, reward valuation involves some aspects of pleasure, learning, memory, and decision making. Interestingly, pleasure and valuation have been dissociated in schizophrenia, with patients showing intact capacity to experience pleasure, but deficits in properly representing the value of future rewards (Gard et al, 2007; Gold et al, 2008). After reward valuation, effort calculations, based on the work required to obtain the reward, are integrated with value calculations to construct a cost-benefit analysis to determine whether the value of the reward justifies the effort required to obtain it. Accordingly, motivated behavior may be affected by disruptions in reward valuation. Reward valuation is highly conserved across species and has been observed in mice (Crombag et al, 2010; Hilario et al, 2007), rats (Balleine and Dickinson, 1992), sheep (Catanese et al, 2011), monkeys (Burke et al, 2014; West et al, 2011), and humans (Klossek et al, 2008).
4.3.1. Human Assessments of Reward Valuation
Outcome devaluation tasks have been developed for use in humans based on existing non-human animal tasks (see below). An outcome devaluation task is similar to a successive negative contrast task in that lowering the value of an expected outcome results in a decrease in behavior for that outcome. In a typical outcome devaluation task, participants are presented with two different stimuli (e.g., food or money) and perform one of two operant responses to receive either reward. One of the stimuli is then devalued, typically by overexposing the participant to that stimulus. As a result, participants tend to respond more for the stimulus that was not devalued compared to the devalued stimulus. By contrast, impaired outcome devaluation is reflected by a lack of decreased responding for the devalued stimulus.
Several manipulations have been shown to affect outcome devaluation in humans. For example, acute alcohol exposure in healthy individuals disrupted sensitivity to outcome devaluation (i.e., participants did not reduce their responding for the devalued stimulus) (Hogarth et al, 2012). fMRI studies revealed that sensitivity to outcome devaluation is associated with activity of the ventromedial prefrontal cortex (de Wit et al, 2009; de Wit et al, 2012) and orbitofrontal cortex (Valentin et al, 2007). Furthermore, consistent with neuroanatomical studies in rodents (see below), patients with Parkinson’s disease, which is characterized by dopamine depletion in the dorsal striatum, showed impaired outcome devaluation (de Wit et al, 2011), suggesting a corticostriatal mechanism involved in reward valuation.
4.3.2. Non-human Animal Assessments of Reward Valuation
Outcome devaluation in animals is often assessed in an instrumental conditioning task similar to that described above for humans (Adams and Dickinson, 1981). Subjects are presented with two different rewards of different values (e.g., food and sucrose pellets), each requiring a separate behavioral response to obtain the reward (e.g., in rodents, pressing a left vs. right lever for different valued rewards). As in humans, outcome devaluation can be achieved by satiating a subject that responds for a food reward or pairing the reward with a noxious stimulus. After diminishing the value of one of the rewards, the subject is given a choice to respond on either lever and typically chooses to respond more on the lever associated with the non-devalued reward compared to the lever associated with the devalued reward.
Several animal studies using an outcome devaluation task suggest that corticolimbic structures are involved in reward valuation. Lesions of the dorsomedial striatum or administration of a N-methyl-D-aspartate (NMDA) receptor antagonist into this region blocked the post-conditioning decrease in behavioral responding for a devalued reinforcer in rats, reflecting insensitivity to outcome devaluation (Yin et al, 2005a; Yin et al, 2005b). This role of the striatum in outcome devaluation is believed to involve the core, but not the shell, of the nucleus accumbens (Corbit et al, 2001). In agreement with studies in humans described above, lesions of the medial prefrontal cortex or basolateral amygdala of rats disrupted sensitivity to outcome devaluation (Balleine and Dickinson, 1998; Corbit and Balleine, 2003, 2005; Killcross and Coutureau, 2003). Moreover, smaller orbitofrontal cortex volume was associated with impaired outcome devaluation in monkeys (Burke et al, 2014). Overall, these results suggest an involvement of corticolimbic circuits in reward valuation. Interestingly, repeated daily administration of amphetamine, which increases cortical and striatal dopamine signaling, also disrupted sensitivity to outcome devaluation (Nelson and Killcross, 2006).
4.3.3. Convergence of Human and Rodent Assessments of Reward Valuation
While few examples exist of cross-species parallel comparisons of human and animal reward valuation tasks, results from outcome devaluation tasks are strikingly consistent across species. Perhaps the best example of cross-species correspondence in reward valuation is demonstrated by studies investigating the effects of stress on sensitivity to outcome devaluation. Humans exposed to a socially evaluated cold pressor test (Schwabe and Wolf, 2010) and rats exposed to chronic unpredictable stress, which includes social defeat, forced swim, and restraint (Dias-Ferreira et al, 2009), both showed decreased sensitivity to outcome devaluation. In humans, these effect of stress were associated with decreased volume of the medial prefrontal cortex and caudate (Soares et al, 2012). Indeed, several lines of evidence from both humans and animals suggest critical involvement of medial prefrontal and orbitofrontal cortices, as well as the striatum, in outcome devaluation (for review, see Balleine and O'Doherty, 2010). Importantly, because the outcome devaluation task was initially developed as an operant task in animals, translation to an analogous human task was possible and produced a framework in which to investigate reward valuation across species.
4.4. Motivation/Effort
Motivation is the incentive or desire to act or accomplish goals. Deficits in motivation may result from deficits in other reward constructs, such as pleasure or anticipation. For example, if an individual is unable to derive pleasure from a normally rewarding activity or from anticipation of that activity, then it is unlikely that the individual will be motivated to pursue that activity (Salamone et al, 2009; Sherdell et al, 2012). Moreover, deficits in motivation may contribute to the development of other symptoms of psychiatric illness, such as social withdrawal and cognitive impairment (Brebion et al, 2009), and can be severely debilitating with regard to functional outcome and reduced quality of life in patients (Barch and Dowd, 2010; Simpson et al, 2012).
Recent findings have begun to emerge highlighting the specific role of motivation in psychiatric disorders. For example, although deficits in motivation were recognized nearly a century ago in schizophrenia (Kraepelin, 1921), recent evidence suggests that such deficits are dissociable from consummatory pleasure, which is intact in individuals with schizophrenia (Barch et al, 2015; Gard et al, 2007; Heerey et al, 2007). Additionally, autism spectrum disorder is associated with anhedonia relating to social, but not other, stimuli (Chevallier et al, 2012; Damiano et al, 2014), but motivation to complete certain tasks can be greater in autism spectrum disorder compared to healthy controls (Damiano et al, 2012).
4.4.1. Human Assessments of Motivation/Effort
As with anticipatory pleasure assessments, clinical self-report questionnaires focused on motivated behavior and drive have emerged over the last two decades. The Behavioral Activation Scale (BAS) is a 13–item Likert-based self-report questionnaire probing aspects of reward relating to “reward responsiveness” (five items), “fun-seeking” (four items), and “drive” (four items) (Carver et al, 1994). Low BAS scores have been associated with increased MDD risk, severity of current MDD, and poor outcome with MDD (Kasch et al, 2002; McFarland et al, 2006; Meyer et al, 1999). Similarly, the Motivation and Pleasure Scale – Self Report (MAP-SR) is an 18-item Likert-based self-report questionnaire probing motivation related to social/interpersonal relationships and recreational/work activities (Llerena et al, 2013). In addition, experience-specific motivation scales are available to assess motivational impairment in areas such as academics (the Academic Motivation Scale) (Vallerand et al, 1992), athletics (the Sport Motivation Scale) (Pelletier et al, 1995), and fitness/health (the Exercise Motivation Scale) (Li, 1999). Again, while attempts to clinically distinguish motivational impairment from other aspects of reward processing are encouraging, these self-report measures offer minimal translational value.
Because several animal procedures exist that measure motivated behavior (see below), recent attempts have been made to adapt those procedures for use in humans in a clinical laboratory setting. For example, using a progressive ratio schedule of reinforcement, operant responding (e.g., lever pressing in rodents) for a food reward is measured as the response requirement to receive each subsequent reward is exponentially increased. Eventually, the subject terminates responding when the effort required to receive a single reward becomes too great, which is interpreted as the subject’s maximum level of motivation. The final ratio completed to earn a single reward is termed a breakpoint, with decreased breakpoints reflecting decreased motivation (Hodos, 1961). Human versions of the progressive ratio task have been developed to assess motivated behavior (Roane et al, 2001). Similar to rodents, human participants are instructed to perform an operant response (e.g., click a computer mouse or press a key on a keyboard) to earn a reward. Obtaining subsequent rewards then requires exponentially more clicks or key presses. Participants continue to perform the operant response to obtain a reward before ultimately giving up, presumably because the cumulative effort required to obtain the reward eventually outweighs the perceived value of the reward. Importantly, several studies have validated the human procedures, in which more preferred rewards elicited greater breakpoints than less preferred rewards (Glover et al, 2008; Penrod et al, 2008; Roane et al, 2001; Trosclair-Lasserre et al, 2008). Most studies utilizing progressive ratio reinforcement schedules in humans have done so using drugs of abuse as reinforcers. As expected, dependent individuals showed high levels of motivation (i.e., breakpoints) for drug administration (Stoops, 2008). Interestingly, using money as a reinforcer, patients with schizophrenia showed decreased breakpoints in a computerized progressive ratio task (Wolf et al, 2014).
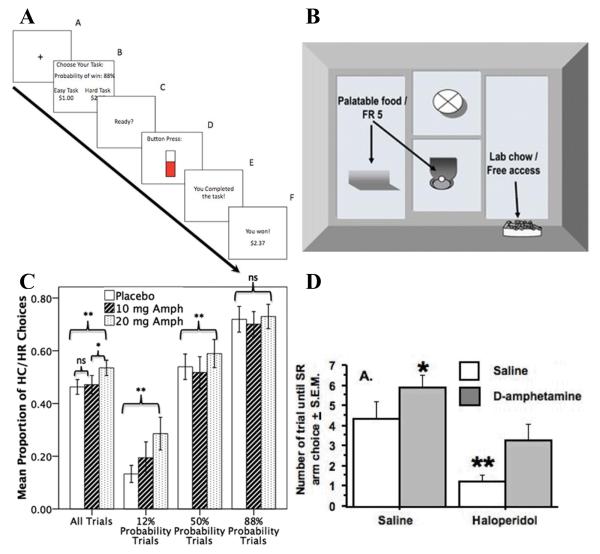
The effort-related choice tasks represent particularly intriguing examples of translation from rodent to human behavioral testing. In rodents, effort-related choice refers to the choice an animal makes to either exert effort for a high value reward or opt for a low value reward that is freely available (see below) (Salamone et al, 1991). The Effort-Expenditure for Rewards Task (EEfRT; Figure 1A) is a computer-based game that was recently developed as a human analogue of the effort-related choice task in rodents (Treadway et al, 2009). With EEfRT, participants may choose between performing a hard or easy task (i.e., completed number of key presses within a short or long period of time, respectively). Successful completion of the hard and easy tasks is subsequently rewarded with relatively high and low monetary rewards, respectively, and the probability of receiving a reward varies with each trial and is indicated prior to choosing task difficulty. Given a medium to high probability of receiving a reward, the proportion of choosing the hard task was inversely correlated with self-reported anhedonia (Treadway et al, 2009). With regard to psychiatric disorders, patients with MDD were less likely to choose the hard task compared to healthy controls (Treadway et al, 2012a), suggesting that willingness to exert effort to obtain rewards is diminished in MDD. Similarly, effort-related choice was disrupted in patients with schizophrenia using EEfRT (Barch et al, 2014; Fervaha et al, 2013; Treadway et al, 2015) as well as other similar computer-based effort-related choice tasks (Gold et al, 2013). Interestingly, using EEfRT, adults with autism spectrum disorder were more likely to choose the hard task compared to healthy controls, but were less influenced by reward probabilities, supporting the argument that people with autism spectrum disorder tend to be highly motivated, but only for very selective tasks (Damiano et al, 2012).
Figure 1.
Schematics of the human (A; EEfRT) and rat (B) effort-related choice tasks used to assess motivated behavior. The psychostimulant amphetamine (Amph) increased preference for the high cost/high reward (HC/HR) choice over the low cost/low reward option in humans (C) and rats (D). [FR5: fixed ratio 5 reinforcement schedule; SR: small reward] (Figures were reproduced with persmissions from Bardgett et al, 2009; Salamone et al, 2007; Treadway et al, 2009; Wardle et al, 2011)
4.4.2. Non-human Animal Assessments of Motivation/Effort
As described above, progressive ratio tests are often used to assess the motivational properties of natural and drug reinforcers in animals (Hodos, 1961; Hodos and Kalman, 1963). While decreased breakpoints are generally thought to reflect decreased motivation, alternate interpretations must be carefully considered. For example, motor impairment or satiety may impede sustained responding required to achieve high breakpoints. Decreased breakpoints may also reflect intolerance for the progressively increasing time delay between reward presentations. Conversely, increased breakpoints may stem from increased perseverative responding. Additionally, it is difficult to dissociate the hedonic from the motivational aspects of a reinforcer using a progressive ratio task alone. Addressing these caveats will help determine whether altered breakpoints reflect changes in motivation or another factor.
In rodents, several manipulations decrease breakpoints for a reward in a progressive ratio task. Withdrawal from chronic administration of several drugs of abuse, like cocaine (Carroll and Lac, 1987), amphetamine (Barr and Phillips, 1999b; Der-Avakian and Markou, 2010), nicotine (LeSage et al, 2006), and morphine (Zhang et al, 2007) decreased responding for a sucrose reward on a progressive ratio schedule of reinforcement. Similarly, rhesus monkeys chronically treated with escalating methylphenidate doses showed decreased breakpoints for a food reward (Rodriguez et al, 2010). Rats bred for congenital learned helplessness, a genetic animal model of behavioral despair, also showed decreased breakpoints for sucrose (Vollmayr et al, 2004). However, not all manipulations typically used to produce depression-like behaviors in non-human animals alter motivated behavior using the progressive ratio task. For example, chronic mild stress (Barr and Phillips, 1998) and neonatal maternal separation (Shalev and Kafkafi, 2002), two procedures commonly used to model depression-like behavior, failed to alter breakpoints for sucrose in rats.
The effort-related choice task was developed to address some of the limitations of the progressive ratio task described above (Figure 1B) (Salamone et al, 1991). This task is similar to the progressive ratio task in that increasing effort is required to obtain a reward. However, an added cost/benefit component is implemented in which the choice of a lesser reward is concurrently offered and can be obtained with little or no effort. With effort requirements being equal, individuals tend to prefer rewards of greater vs. lesser value. However, as the effort required to obtain the greater reward increases, preference eventually shifts towards the lesser reward requiring less effort (Salamone et al, 2007; Salamone et al, 1997). Thus, the effort-related choice between two different rewards addresses several of the caveats limiting the progressive ratio task described above. Notably, the effort-related choice task allows for a dissociation to be observed between motivation (i.e., exerting effort to obtain the greater reward) and consummatory pleasure (i.e., opting for the freely available lesser reward).
In rats, sucrose pellets or food pellets with high carbohydrate content may be used as a highly palatable reward, whereas standard lab chow is used as the non-preferred reward. The effort required to obtain the highly palatable reward may involve a greater number of lever presses or climbing a larger barrier as compared to obtaining the non-preferred reward (Salamone et al, 1994; Salamone et al, 1991). Regardless of the details of the procedure used, manipulating dopamine neurotransmission has been consistently shown to shift preference from high cost/high reward choices to low cost/low reward choices. For example, dopamine depletion in the nucleus accumbens or blocking the dopamine D1 or D2 receptor increases preference for freely available lab chow over lever pressing (Salamone et al, 1991) or climbing a barrier (Salamone et al, 1994; Yohn et al, 2015) for a more palatable reward. Similarly, dopamine D2 receptor over-expression in the striatum also increases preference for a low cost/low reward option in an effort-related choice task (Ward et al, 2012).
4.4.3. Convergence of Human and Non-human Animal Assessments of Motivation/Effort
Both human and animal versions of the progressive ratio task have been used to investigate the neurobiology of motivated behavior, particularly relating to drug and other addictions, although results have been mixed across species. For example, acute phenylalanine and tyrosine depletion in humans, which acts to decrease dopamine synthesis, decreased breakpoints for alcohol (Barrett et al, 2008), tobacco (Venugopalan et al, 2011), and money (Cawley et al, 2013), suggesting that the increased motivation for these rewards is related to elevated brain dopamine levels. In rats, dopamine depletion produced by 6-OHDA injected directly into the nucleus accumbens decreased breakpoints for a food reward (Aberman et al, 1998; Hamill et al, 1999), but increased breakpoints for apomorphine (Roberts, 1989), indicating that motivated behavior is mediated by dopamine D1-like and D2-like receptor signaling. Consistent with this view, administration of a dopamine D1-like or D2-like receptor antagonist in rhesus monkeys decreased breakpoints for a food pellet (Von Huben et al, 2006).
Not all manipulations have produced congruent results across species. Pretreatment with aripiprazole, a partial agonist at dopamine D2 and serotonin (5-HT) 1A receptors and an antagonist at 5-HT 2A receptors, decreased breakpoints for methamphetamine in recreational users (Stoops et al, 2013). Conversely, administration of a 5-HT 2A receptor antagonist failed to alter breakpoints for cocaine, nicotine, or food in rats (Fletcher et al, 2002; Fletcher et al, 2012). Thus, while the human and animal versions of the progressive ratio task are procedurally similar, parallel cross-species testing using similar manipulations (e.g., global vs. targeted dopamine depletion) and rewards (e.g., drug vs. non-drug reinforcers) will help elucidate the level of congruence between both tasks.
The effort-related choice task in humans (i.e., EEfRT) has only recently been developed, and thus data on congruence with the rodent version of the task is limited, but promising. The findings that effort-related choice is impaired in MDD and schizophrenia (Barch et al, 2014; Fervaha et al, 2013; Gold et al, 2013; Treadway et al, 2012a; Treadway et al, 2015), psychiatric disorders characterized by disrupted reward-related decision making that is argued to reflect deficits in striatal dopamine transmission, supports rodent studies indicating that effort-related choice is regulated by dopaminergic mechanisms (Salamone et al, 1994; Salamone et al, 1991). Perhaps even more convincing, acute amphetamine administration, which increases striatal dopamine levels (Kuczenski et al, 1991), increased preference for the high cost/high reward choice in both humans (Figure 1C) (Wardle et al, 2011) and rats (Figure 1D) (Bardgett et al, 2009) in an effort-related choice task. Moreover, willingness to expend effort for larger rewards in humans was positively correlated with increased dopamine in the striatum and ventromedial prefrontal cortex (PFC) in PET imaging studies (Treadway et al, 2012b), consistent with the argument for dopamine involvement in effort-related choice.
4.5. Reward Learning
Reward learning is a process by which individuals experience, learn, and repeat goal-directed actions that maximize the probability of receiving future rewards. Similarly, learning can occur to avoid actions that do not result in a reward. Conversely, impaired reward learning results in decisions that are made without regard for reward feedback. Thus, reward learning may involve several other reward processes, including pleasure, motivation, and decision making.
Because of the cognitive aspects associated with reward learning, it is perhaps expected that areas of the prefrontal cortex underlie reward learning. Indeed, increased activity in the dorsal anterior cingulate and orbitofrontal cortices, areas involved in integrating and utilizing reinforcement history to guide behavior, is correlated with elevated reward learning (Frank and Claus, 2006; Santesso et al, 2008). Additionally, dopaminergic mechanisms in the striatum are also involved in reward learning (Holroyd and Coles, 2002; Jocham et al, 2011, 2014; O'Doherty et al, 2004; Santesso et al, 2009; Vrieze et al, 2013a), suggesting some overlap with mechanisms responsible for anticipatory pleasure and motivation.
4.5.1. Human Assessments of Reward Learning
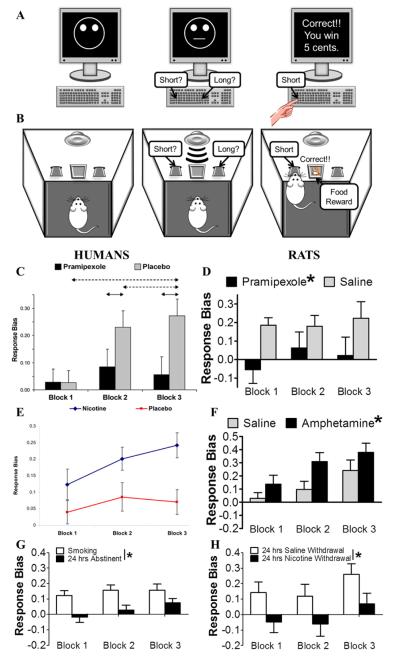
The Response Bias Probabilistic Reward task (RBPRT) is a laboratory-based task developed initially in humans to objectively assess normal and disrupted reward responsiveness, defined as the propensity to modulate future behavioral choices based on prior reward experiences (Figure 2A) (Pizzagalli et al, 2005; modified after Tripp and Alsop, 1999). The RBPRT combines aspects of a signal-detection task and a probabilistic reward task. In the signal detection component, participants must identify which of two ambiguous stimuli (e.g., a short or long mouth on a schematic face) is presented on a computer screen in order to receive monetary feedback. Unbeknownst to participants, a probabilistic reward component is implemented in the reinforcement schedule, whereby correct identification of one stimulus is reinforced three times more frequently (i.e., rich stimulus) than correct identification of the other stimulus (i.e., lean stimulus). Healthy participants (i.e., without a psychiatric diagnosis) develop a response bias for the rich stimulus, reflecting a shift from accurate responding when either stimulus is presented to increased responding on the key associated with the rich stimulus, regardless of which stimulus was presented. This pattern of change in behavior suggests that reward feedback from the differential reinforcement schedule was effective in modulating subsequent choices, and that healthy participants will tend to bias their responding to try to maximize the rewards received.
Figure 2.
Schematics of human (A) and rat (B) versions of the Response Bias Probabilistic Reward Task used to assess reward learning. The dopamine D2/D3 receptor agonist pramipexole blunted response bias in humans (C) and rats (D). The psychostimulants nicotine and amphetamine increased response bias in humans (E) and rats (F), respectively. Withdrawal from chronic nicotine exposure blunted response bias in humans (G) and rats (H). Altogether, these data suggest a high level of concordance between the human and rat versions of this reward learning task. (Figures were reproduced with permissions from Barr et al, 2008; Der-Avakian et al, 2013; Pergadia et al, 2014; Pizzagalli et al, 2008a)
Individuals with MDD and bipolar disorder did not develop a response bias for the rich stimulus in the RBPRT (Pizzagalli et al, 2008b; Pizzagalli et al, 2008c). These individuals instead tended to respond accurately when either rich or lean stimulus was presented, suggesting that the differential reinforcement of the two stimuli was ineffective in promoting a bias for the rich stimulus. Euthymic individuals with remitted depression (Pechtel et al, 2013a), individuals without any history of psychiatric illness, but with high trait levels of anhedonia based on a self-report questionnaire (Pizzagalli et al, 2005), and healthy individuals exposed to stress, a precipitating factor for several psychiatric disorders (Bogdan and Pizzagalli, 2006; Pizzagalli et al, 2007), also showed a blunted response bias compared to controls. Moreover, lower response biases predicted treatment outcome in MDD (Vrieze et al, 2013b). Conversely, response bias was not impaired in patients with schizophrenia (Ahnallen et al, 2012; Heerey et al, 2008).
Another reward learning task, the Probabilistic Stimulus Selection Task (PSST), was developed in humans to assess relative learning associated with positive versus negative reinforcement (Frank et al, 2004). In the task, participants are presented with one of three pairs of discrete symbols and instructed to select one of the two stimuli presented in each pair. Correct identification of one stimulus in the pair is rewarded at either an 80%, 70%, or 60% rate (i.e., depending on the pair that is presented). Incorrect identification is rewarded 20%, 30%, and 40% of the time, respectively. Participants train on this procedure until the relative reinforcement probabilities of each pair of stimuli are learned. Participants are then tested by being presented with the stimulus that was reinforced 80% of the time paired with one of the other four stimuli (i.e., stimuli that were reinforced 70%, 60%, 40%, and 30% of the time). Participants are also presented with the stimulus that was reinforced 20% of the time paired with the same four stimuli as above. Greater performance on the pairs that include the 80% reinforced stimulus reflect better learning from positive feedback, whereas greater performance on the pairs that include the 20% reinforced stimulus reflect better learning from negative feedback.
Healthy participants performed equally well when learning from positive and negative feedback using the PSST (Frank et al, 2007; Frank et al, 2004). However, when exposed to stress (e.g., threat of shock), healthy participants with high physiological and subjective responses to stress showed impairments in learning from positive, but not negative, feedback compared to non-stressed controls (Berghorst et al, 2013). Similarly, women with a history of childhood sexual assault were impaired in learning from positive, but not negative, feedback (Pechtel and Pizzagalli, 2013b). Patients with schizophrenia (Waltz et al, 2007) or Parkinson’s disease (Frank et al, 2004) were also impaired in learning from positive, but not negative, feedback. Interestingly, impairment in Parkinson’s disease was reversed with dopamine-enhancing medication (Frank et al, 2004). Collectively, these findings indicate that pathological or experimental conditions hypothesized to affect dopaminergic neurotransmission have negative effects on the ability to learn from positive feedback.
4.5.2. Non-human Animal Assessments of Reward Learning
The human RBPRT is an example of a clinical behavioral assessment of reward processing that does not rely on subjective self-report of behavior. Accordingly, a rat version of the RBPRT was developed based on the instructions and testing parameters used in the human version to assess reward learning in rats (Figure 2B) (Der-Avakian et al, 2013). The rat version of the task is conducted in operant boxes equipped with two levers, a speaker and tone generator, and a food dispenser. Rats are trained to distinguish between two stimuli (e.g., long and short tones) by pressing a lever associated with either stimulus, which results in delivery of a food pellet for some, but not all, correct responses. As in the human task, correct identification of one tone is reinforced three times more frequently (rich) than correct identification of the other tone (lean). Data collected from the RBPRT (i.e., response bias, discriminability, accuracy, and reaction time) are also analyzed identically between humans and rats. Because most of the studies using the rat version of the RBPRT were conducted either in parallel with the human RBPRT or in order replicate previous findings from the human RBPRT, details concerning the results of these studies are described in the next section.
A similar reward bias task was recently developed using ambiguous odor cues (Wang et al, 2013) based on a similar task previously developed for use with monkeys (Rorie et al, 2010; Samejima et al, 2005). In this task, rats respond on either a left or right nose-poke hole in response to one of two odor cues. Each odor is either presented separately or in different mixture combinations (i.e., ambiguous odors). When presented with an ambiguous odor cue, rats tend to be biased toward the nose-poke hole that dispensed a relatively greater reward. This procedure allows for assessment of response vigor as well, whereby time to initiation of subsequent trials is decreased as the net value of rewards is increased. Response vigor (i.e., time to trial initiation) was shown to depend on the dorsomedial striatum (Wang et al, 2013).
Like the RBPRT, a rat version of the PSST has been recently developed (Trecker et al, 2012). Using touch screen monitors, rats are presented with three pairs of discrete stimuli and trained to select one of the two stimuli in each pair. Correct identification results in delivery of a food pellet on 90%, 80%, and 70% of trials for each pair, whereas incorrect identification results in reward delivery on 10%, 20%, and 30% of trials, respectively. During test trials, rats are presented with either: 1) the stimulus associated with 90% reward paired with one of the other four novel stimuli; or 2) the stimulus associated with 10% reward paired with one of the other four novel stimuli, in order to assess learning from positive and negative feedback, respectively. Preliminary findings indicate that rats were able to learn the probabilistic contingencies for different stimulus pairs and learned from both positive and negative feedback during test trials. Future studies may explore the roles of stress and dopaminergic transmission in the striatum and prefrontal cortex to determine whether positive feedback learning is impaired, as was observed in humans (see above).
4.5.3. Convergence of Human and Non-human Animal Assessments of Reward Learning
Perhaps because of their novelty, the human reward learning tasks described above have only been modified for use in rats, and parallel data from humans and rats are only available for the RBPRT. As in humans, healthy rats developed a response bias for the rich stimulus, reflected by increasing accuracy for the rich stimulus and decreasing accuracy for the lean stimulus throughout the test session (Der-Avakian et al, 2013; Pizzagalli et al, 2005). Modulation of reward learning was similar between rats and humans in response to several manipulations. First, an acute, low-dose administration of the dopamine D2/D3 receptor agonist pramipexole blunted response bias in healthy humans (Figure 2C) (Pizzagalli et al, 2008a) and rats (Figure 2D) (Der-Avakian et al, 2013). The low doses of pramipexole used in both studies, which putatively decrease striatal dopamine levels by means of presynaptic autoreceptor activation, suggest that reward learning can be modulated by altering dopamine neurotransmission in the striatum. Second, and in support of the claim above, acute administration of the psychostimulant nicotine, which acts to increase striatal dopamine levels, increased response bias in humans (Figure 2E) (Barr et al, 2008). Similarly, administration of the psychostimulant amphetamine, which also increases striatal dopamine levels, increased response bias in rats (Figure 2F) (Der-Avakian et al, 2013). Third, withdrawal from chronic nicotine exposure blunted response bias in humans (Figure 2G) and rats (Figure 2H) (Pergadia et al, 2014). In rats, re-exposure to acute nicotine after withdrawal dose-dependently increased response bias, suggesting a mechanism by which nicotine-induced enhancement of reward learning in abstinent smokers may contribute to relapse to smoking.
5. Conclusions and Future Considerations
Identifying and treating reward deficits in psychiatric and neurological disorders has become increasingly important given not only the pervasiveness of the deficits across several disorders, but also our increasing understanding of the precise reward processes that are involved, like pleasure, anticipation, valuation, motivation, and reward learning. In order to understand the neurobiological mechanisms underlying these distinctive reward processes that will ultimately facilitate discovery of treatment targets, animal procedures are necessary that may be readily translated into analogous human tasks. Similarly, novel human tasks that do not already have a non-human analogue should be designed to assess behavior objectively using non-verbal communication. Ideally, moving forward, animal and human behavioral assessments that are developed in parallel will ensure that task parameters and psychometric properties are as analogous as possible.
Furthermore, many of the investigations probing the neurobiological mechanisms underlying different reward processes involved procedures that are not directly comparable across species. For example, human studies often relied on functional magnetic resonance imaging (fMRI) and electroencephalography (EEG), whereas non-human animal studies typically used brain lesion, intracranial microinjection, and gene knock-out techniques. While inferences may be made about similarities in brain function tied to behavior between humans and animals using different techniques, ideally, similar approaches to measuring brain function in different species would help strengthen and validate observed similarities in behavior. For example, recent advances in human electrophysiological techniques and animal brain imaging techniques now allow for such parallel cross-species comparisons. Such an approach would not only facilitate discovery of novel neurobiological mechanisms subserving different reward processes, but, importantly, would help determine whether the reward behavior, or disruptions in reward behavior, being assessed are mediated by similar neurobiological mechanisms across different species.
A limitation of the procedures described above is that as reward processes become more narrowly defined, tasks designed to assess aspects of those reward constructs tend to become more complicated. The level of task complication may not be problematic for assessments in humans, where detailed verbal instructions may be given to study participants. However, verbal instructions must be translated to training protocols for animal tasks that may require several weeks or months to train for the most complicated tasks. Such high levels of sophistication are necessary if tasks are required to probe increasingly discrete aspects of the reward spectrum that are not confounded by other behavioral factors. Moreover, enhancing the specificity of the reward construct being assessed improves the likelihood of discovering more focused, discrete neurobiological mechanisms that underlie a given reward process.
Ultimately, the value of this approach is in being able to use animal procedures to make specific testable hypotheses regarding novel treatment strategies that have a high degree of successfully translating to the clinic. It is anticipated that this new approach in cross-species translational research will lead to the development of safe and effective medications for the treatment of reward deficits in psychiatric and neurological disorders that have thus far eluded researchers.
Acknowledgments
AM was partially supported by RO1MH087989 and R56DA011946. DAP was partially supported by R01 MH068376 and R01 MH101521.
Footnotes
Disclosures Over the past three years, Dr. Markou received contract research support from Astra-Zeneca and Forest Laboratories, and honorarium/consulting fee from AbbVie, Germany for activities unrelated to this chapter. Over the past three years, Dr. Pizzagalli has received honoraria/consulting fees Otsuka America Pharmaceutical and Pfizer for activities unrelated to this chapter. Drs. Der-Avakian and Barnes have no financial disclosures to report.
REFERENCES
- Aberman JE, Ward SJ, Salamone JD. Effects of dopamine antagonists and accumbens dopamine depletions on time-constrained progressive-ratio performance. Pharmacol Biochem Behav. 1998;61(4):341–348. doi: 10.1016/s0091-3057(98)00112-9. [DOI] [PubMed] [Google Scholar]
- Adams CD, Dickinson A. Instrumental Responding Following Reinforcer Devaluation. Q J Exp Psychol-B. 1981 May;33:109–121. [Google Scholar]
- Ahnallen CG, Liverant GI, Gregor KL, Kamholz BW, Levitt JJ, Gulliver SB, et al. The relationship between reward-based learning and nicotine dependence in smokers with schizophrenia. Psychiatry Res. 2012;196(1):9–14. doi: 10.1016/j.psychres.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association . Diagnostic and statistical manual of mental disorders : DSM-V. American Psychiatric Association; Washington, DC: 2013. [Google Scholar]
- Anderson MH, Hardcastle C, Munafo MR, Robinson ES. Evaluation of a novel translational task for assessing emotional biases in different species. Cogn Affect Behav Neurosci. 2012;12(2):373–381. doi: 10.3758/s13415-011-0076-4. [DOI] [PubMed] [Google Scholar]
- Balleine B, Dickinson A. Signalling and incentive processes in instrumental reinforcer devaluation. Q J Exp Psychol B. 1992;45(4):285–301. [PubMed] [Google Scholar]
- Balleine BW, Dickinson A. Goal-directed instrumental action: contingency and incentive learning and their cortical substrates. Neuropharmacology. 1998;37(4-5):407–419. doi: 10.1016/s0028-3908(98)00033-1. [DOI] [PubMed] [Google Scholar]
- Balleine BW, O'Doherty JP. Human and rodent homologies in action control: corticostriatal determinants of goal-directed and habitual action. Neuropsychopharmacology. 2010;35(1):48–69. doi: 10.1038/npp.2009.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbano MF, Cador M. Differential regulation of the consummatory, motivational and anticipatory aspects of feeding behavior by dopaminergic and opioidergic drugs. Neuropsychopharmacology. 2006;31(7):1371–1381. doi: 10.1038/sj.npp.1300908. [DOI] [PubMed] [Google Scholar]
- Barbano MF, Cador M. Opioids for hedonic experience and dopamine to get ready for it. Psychopharmacology (Berl) 2007;191(3):497–506. doi: 10.1007/s00213-006-0521-1. [DOI] [PubMed] [Google Scholar]
- Barch DM, Dowd EC. Goal representations and motivational drive in schizophrenia: the role of prefrontal-striatal interactions. Schizophr Bull. 2010;36(5):919–934. doi: 10.1093/schbul/sbq068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barch DM, Pagliaccio D, Luking K. Mechanisms Underlying Motivational Deficits in Psychopathology: Similarities and Differences in Depression and Schizophrenia. Curr Top Behav Neurosci. 2015 doi: 10.1007/7854_2015_376. [DOI] [PubMed] [Google Scholar]
- Barch DM, Treadway MT, Schoen N. Effort, anhedonia, and function in schizophrenia: reduced effort allocation predicts amotivation and functional impairment. J Abnorm Psychol. 2014;123(2):387–397. doi: 10.1037/a0036299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardgett ME, Depenbrock M, Downs N, Points M, Green L. Dopamine modulates effort-based decision making in rats. Behav Neurosci. 2009;123(2):242–251. doi: 10.1037/a0014625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes SA, Der-Avakian A, Markou A. Anhedonia, avolition, and anticipatory deficits: assessments in animals with relevance to the negative symptoms of schizophrenia. Eur Neuropsychopharmacol. 2014;24(5):744–758. doi: 10.1016/j.euroneuro.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr AM, Fiorino DF, Phillips AG. Effects of withdrawal from an escalating dose schedule of d-amphetamine on sexual behavior in the male rat. Pharmacol Biochem Behav. 1999a;64(3):597–604. doi: 10.1016/s0091-3057(99)00156-2. [DOI] [PubMed] [Google Scholar]
- Barr AM, Phillips AG. Chronic mild stress has no effect on responding by rats for sucrose under a progressive ratio schedule. Physiol Behav. 1998;64(5):591–597. doi: 10.1016/s0031-9384(98)00060-2. [DOI] [PubMed] [Google Scholar]
- Barr AM, Phillips AG. Withdrawal following repeated exposure to d-amphetamine decreases responding for a sucrose solution as measured by a progressive ratio schedule of reinforcement. Psychopharmacology (Berl) 1999b;141(1):99–106. doi: 10.1007/s002130050812. [DOI] [PubMed] [Google Scholar]
- Barr AM, Phillips AG. Increased successive negative contrast in rats withdrawn from an escalating-dose schedule of D-amphetamine. Pharmacol Biochem Behav. 2002;71(1-2):293–299. doi: 10.1016/s0091-3057(01)00664-5. [DOI] [PubMed] [Google Scholar]
- Barr RS, Pizzagalli DA, Culhane MA, Goff DC, Evins AE. A single dose of nicotine enhances reward responsiveness in nonsmokers: implications for development of dependence. Biol Psychiatry. 2008;63(11):1061–1065. doi: 10.1016/j.biopsych.2007.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett SP, Pihl RO, Benkelfat C, Brunelle C, Young SN, Leyton M. The role of dopamine in alcohol self-administration in humans: individual differences. Eur Neuropsychopharmacol. 2008;18(6):439–447. doi: 10.1016/j.euroneuro.2008.01.008. [DOI] [PubMed] [Google Scholar]
- Bentosela M, Jakovcevic A, Elgier AM, Mustaca AE, Papini MR. Incentive contrast in domestic dogs (Canis familiaris) J Comp Psychol. 2009;123(2):125–130. doi: 10.1037/a0013340. [DOI] [PubMed] [Google Scholar]
- Berghorst LH, Bogdan R, Frank MJ, Pizzagalli DA. Acute stress selectively reduces reward sensitivity. Front Hum Neurosci. 2013;7:133. doi: 10.3389/fnhum.2013.00133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlin I, Givry-Steiner L, Lecrubier Y, Puech AJ. Measures of anhedonia and hedonic responses to sucrose in depressive and schizophrenic patients in comparison with healthy subjects. Eur Psychiatry. 1998;13(6):303–309. doi: 10.1016/S0924-9338(98)80048-5. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE. Parsing reward. Trends Neurosci. 2003;26(9):507–513. doi: 10.1016/S0166-2236(03)00233-9. [DOI] [PubMed] [Google Scholar]
- Bogdan R, Pizzagalli DA. Acute stress reduces reward responsiveness: implications for depression. Biol Psychiatry. 2006;60(10):1147–1154. doi: 10.1016/j.biopsych.2006.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brebion G, Bressan RA, Pilowsky LS, David AS. Depression, avolition, and attention disorders in patients with schizophrenia: associations with verbal memory efficiency. J Neuropsychiatry Clin Neurosci. 2009;21(2):206–215. doi: 10.1176/jnp.2009.21.2.206. [DOI] [PubMed] [Google Scholar]
- Brenes JC, Schwarting RK. Individual differences in anticipatory activity to food rewards predict cue-induced appetitive 50-kHz calls in rats. Physiol Behav. 2015 doi: 10.1016/j.physbeh.2015.05.012. [DOI] [PubMed] [Google Scholar]
- Buck CL, Malavar JC, George O, Koob GF, Vendruscolo LF. Anticipatory 50 kHz ultrasonic vocalizations are associated with escalated alcohol intake in dependent rats. Behav Brain Res. 2014a;271:171–176. doi: 10.1016/j.bbr.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck CL, Vendruscolo LF, Koob GF, George O. Dopamine D1 and mu-opioid receptor antagonism blocks anticipatory 50 kHz ultrasonic vocalizations induced by palatable food cues in Wistar rats. Psychopharmacology (Berl) 2014b;231(5):929–937. doi: 10.1007/s00213-013-3307-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke SN, Thome A, Plange K, Engle JR, Trouard TP, Gothard KM, et al. Orbitofrontal cortex volume in area 11/13 predicts reward devaluation, but not reversal learning performance, in young and aged monkeys. J Neurosci. 2014;34(30):9905–9916. doi: 10.1523/JNEUROSCI.3918-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll ME, Lac ST. Cocaine withdrawal produces behavioral disruptions in rats. Life Sci. 1987;40(22):2183–2190. doi: 10.1016/0024-3205(87)90009-9. [DOI] [PubMed] [Google Scholar]
- Carver CS, White TL. Behavioral-Inhibition, Behavioral Activation, and Affective Responses to Impending Reward and Punishment - the Bis Bas Scales. Journal of Personality and Social Psychology. 1994;67(2):319–333. [Google Scholar]
- Catanese F, Freidin E, Cuello MI, Distel RA. Devaluation of low-quality food during early experience by sheep. Animal. 2011;5(6):938–942. doi: 10.1017/S1751731110002661. [DOI] [PubMed] [Google Scholar]
- Cawley EI, Park S, aan het Rot M, Sancton K, Benkelfat C, Young SN, et al. Dopamine and light: dissecting effects on mood and motivational states in women with subsyndromal seasonal affective disorder. J Psychiatry Neurosci. 2013;38(6):388–397. doi: 10.1503/jpn.120181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman LJ, Chapman JP, Raulin ML. Scales for Physical and Social Anhedonia. Journal of Abnormal Psychology. 1976;85(4):374–382. doi: 10.1037//0021-843x.85.4.374. [DOI] [PubMed] [Google Scholar]
- Chevallier C, Grezes J, Molesworth C, Berthoz S, Happe F. Brief Report: Selective Social Anhedonia in High Functioning Autism. Journal of Autism and Developmental Disorders. 2012;42(7):1504–1509. doi: 10.1007/s10803-011-1364-0. [DOI] [PubMed] [Google Scholar]
- Corbit LH, Balleine BW. The role of prelimbic cortex in instrumental conditioning. Behav Brain Res. 2003;146(1-2):145–157. doi: 10.1016/j.bbr.2003.09.023. [DOI] [PubMed] [Google Scholar]
- Corbit LH, Balleine BW. Double dissociation of basolateral and central amygdala lesions on the general and outcome-specific forms of pavlovian-instrumental transfer. J Neurosci. 2005;25(4):962–970. doi: 10.1523/JNEUROSCI.4507-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbit LH, Muir JL, Balleine BW. The role of the nucleus accumbens in instrumental conditioning: Evidence of a functional dissociation between accumbens core and shell. J Neurosci. 2001;21(9):3251–3260. doi: 10.1523/JNEUROSCI.21-09-03251.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couvillon PA, Bitterman ME. The Overlearning-Extinction Effect and Successive Negative Contrast in Honeybees (Apis-Mellifera) J Comp Psychol. 1984;98(1):100–109. [PubMed] [Google Scholar]
- Crombag HS, Johnson AW, Zimmer AM, Zimmer A, Holland PC. Deficits in sensory-specific devaluation task performance following genetic deletions of cannabinoid (CB1) receptor. Learn Mem. 2010;17(1):18–22. doi: 10.1101/lm.1610510. [DOI] [PubMed] [Google Scholar]
- Damiano CR, Aloi J, Burrus C, Garbutt JC, Kampov-Polevoy AB, Dichter GS. Intact Hedonic Responses to Sweet Tastes in Autism Spectrum Disorder. Res Autism Spectr Disord. 2014;8(3):230–236. doi: 10.1016/j.rasd.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damiano CR, Aloi J, Treadway M, Bodfish JW, Dichter GS. Adults with autism spectrum disorders exhibit decreased sensitivity to reward parameters when making effort-based decisions. Journal of Neurodevelopmental Disorders. 2012;4 doi: 10.1186/1866-1955-4-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis C, Woodside DB. Sensitivity to the rewarding effects of food and exercise in the eating disorders. Compr Psychiatry. 2002;43(3):189–194. doi: 10.1053/comp.2002.32356. [DOI] [PubMed] [Google Scholar]
- de Wit S, Barker RA, Dickinson AD, Cools R. Habitual versus Goal-directed Action Control in Parkinson Disease. J Cognitive Neurosci. 2011;23(5):1218–1229. doi: 10.1162/jocn.2010.21514. [DOI] [PubMed] [Google Scholar]
- de Wit S, Corlett PR, Aitken MR, Dickinson A, Fletcher PC. Differential Engagement of the Ventromedial Prefrontal Cortex by Goal-Directed and Habitual Behavior toward Food Pictures in Humans. Journal of Neuroscience. 2009;29(36):11330–11338. doi: 10.1523/JNEUROSCI.1639-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit S, Watson P, Harsay HA, Cohen MX, van de Vijver I, Ridderinkhof KR. Corticostriatal connectivity underlies individual differences in the balance between habitual and goal-directed action control. J Neurosci. 2012;32(35):12066–12075. doi: 10.1523/JNEUROSCI.1088-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Der-Avakian A, D'Souza MS, Pizzagalli DA, Markou A. Assessment of reward responsiveness in the response bias probabilistic reward task in rats: implications for cross-species translational research. Transl Psychiatry. 2013;3:e297. doi: 10.1038/tp.2013.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Der-Avakian A, Markou A. Withdrawal from chronic exposure to amphetamine, but not nicotine, leads to an immediate and enduring deficit in motivated behavior without affecting social interaction in rats. Behav Pharmacol. 2010;21(4):359–368. doi: 10.1097/FBP.0b013e32833c7cc8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Der-Avakian A, Markou A. The neurobiology of anhedonia and other reward-related deficits. Trends Neurosci. 2012;35(1):68–77. doi: 10.1016/j.tins.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias-Ferreira E, Sousa JC, Melo I, Morgado P, Mesquita AR, Cerqueira JJ, et al. Chronic stress causes frontostriatal reorganization and affects decision-making. Science. 2009;325(5940):621–625. doi: 10.1126/science.1171203. [DOI] [PubMed] [Google Scholar]
- Dichter GS, Smoski MJ, Kampov-Polevoy AB, Gallop R, Garbutt JC. Unipolar depression does not moderate responses to the Sweet Taste Test. Depress Anxiety. 2010;27(9):859–863. doi: 10.1002/da.20690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fawcett J, Clark DC, Scheftner WA, Gibbons RD. Assessing Anhedonia in Psychiatric-Patients - the Pleasure Scale. Arch Gen Psychiat. 1983;40(1):79–84. doi: 10.1001/archpsyc.1983.01790010081010. [DOI] [PubMed] [Google Scholar]
- Fervaha G, Graff-Guerrero A, Zakzanis KK, Foussias G, Agid O, Remington G. Incentive motivation deficits in schizophrenia reflect effort computation impairments during cost-benefit decision-making. J Psychiatr Res. 2013;47(11):1590–1596. doi: 10.1016/j.jpsychires.2013.08.003. [DOI] [PubMed] [Google Scholar]
- Fletcher PJ, Grottick AJ, Higgins GA. Differential effects of the 5-HT(2A) receptor antagonist M100907 and the 5-HT(2C) receptor antagonist SB242084 on cocaine-induced locomotor activity, cocaine self-administration and cocaine-induced reinstatement of responding. Neuropsychopharmacology. 2002;27(4):576–586. doi: 10.1016/S0893-133X(02)00342-1. [DOI] [PubMed] [Google Scholar]
- Fletcher PJ, Rizos Z, Noble K, Soko AD, Silenieks LB, Le AD, et al. Effects of the 5-HT2C receptor agonist Ro60-0175 and the 5-HT2A receptor antagonist M100907 on nicotine self-administration and reinstatement. Neuropharmacology. 2012;62(7):2288–2298. doi: 10.1016/j.neuropharm.2012.01.023. [DOI] [PubMed] [Google Scholar]
- Forbes NF, Stewart CA, Matthews K, Reid IC. Chronic mild stress and sucrose consumption: validity as a model of depression. Physiol Behav. 1996;60(6):1481–1484. doi: 10.1016/s0031-9384(96)00305-8. [DOI] [PubMed] [Google Scholar]
- Frank MJ, Claus ED. Anatomy of a decision: striato-orbitofrontal interactions in reinforcement learning, decision making, and reversal. Psychol Rev. 2006;113(2):300–326. doi: 10.1037/0033-295X.113.2.300. [DOI] [PubMed] [Google Scholar]
- Frank MJ, Samanta J, Moustafa AA, Sherman SJ. Hold your horses: impulsivity, deep brain stimulation, and medication in parkinsonism. Science. 2007;318(5854):1309–1312. doi: 10.1126/science.1146157. [DOI] [PubMed] [Google Scholar]
- Frank MJ, Seeberger LC, O'Reilly RC. By carrot or by stick: cognitive reinforcement learning in parkinsonism. Science. 2004;306(5703):1940–1943. doi: 10.1126/science.1102941. [DOI] [PubMed] [Google Scholar]
- Gard DE, Gard MG, Kring AM, John OP. Anticipatory and consummatory components of the experience of pleasure: A scale development study. J Res Pers. 2006;40(6):1086–1102. [Google Scholar]
- Gard DE, Kring AM, Gard MG, Horan WP, Green MF. Anhedonia in schizophrenia: distinctions between anticipatory and consummatory pleasure. Schizophr Res. 2007;93(1-3):253–260. doi: 10.1016/j.schres.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geyer MA, Markou A. The Role of Preclinical Models in the Development of Psychotropic Drugs. In: Davis KL, Charney D, Coyle JT, Nemeroff CB, editors. Neuropsychopharmacology: The Fifth Generation of Progress. Lippincott Williams & Wilkins; 2002. pp. 445–455. [Google Scholar]
- Glover AC, Roane HS, Kadey HJ, Grow LL. Preference for reinforcers under progressive- and fixed-ratio schedules: a comparison of single and concurrent arrangements. J Appl Behav Anal. 2008;41(2):163–176. doi: 10.1901/jaba.2008.41-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold JM, Strauss GP, Waltz JA, Robinson BM, Brown JK, Frank MJ. Negative symptoms of schizophrenia are associated with abnormal effort-cost computations. Biol Psychiatry. 2013;74(2):130–136. doi: 10.1016/j.biopsych.2012.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold JM, Waltz JA, Prentice KJ, Morris SE, Heerey EA. Reward processing in schizophrenia: a deficit in the representation of value. Schizophr Bull. 2008;34(5):835–847. doi: 10.1093/schbul/sbn068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gooding DC, Pflum MJ. The assessment of interpersonal pleasure: introduction of the Anticipatory and Consummatory Interpersonal Pleasure Scale (ACIPS) and preliminary findings. Psychiatry Res. 2014a;215(1):237–243. doi: 10.1016/j.psychres.2013.10.012. [DOI] [PubMed] [Google Scholar]
- Gooding DC, Pflum MJ. Further validation of the ACIPS as a measure of social hedonic response. Psychiatry Res. 2014b;215(3):771–777. doi: 10.1016/j.psychres.2013.11.009. [DOI] [PubMed] [Google Scholar]
- Hamill S, Trevitt JT, Nowend KL, Carlson BB, Salamone JD. Nucleus accumbens dopamine depletions and time-constrained progressive ratio performance: effects of different ratio requirements. Pharmacol Biochem Behav. 1999;64(1):21–27. doi: 10.1016/s0091-3057(99)00092-1. [DOI] [PubMed] [Google Scholar]
- Harris RB, Zhou J, Youngblood BD, Smagin GN, Ryan DH. Failure to change exploration or saccharin preference in rats exposed to chronic mild stress. Physiol Behav. 1997;63(1):91–100. doi: 10.1016/s0031-9384(97)00425-3. [DOI] [PubMed] [Google Scholar]
- Haslam J. Observations on madness and melancholy; including practical remarks on those diseases, together with cases, and an account of the morbid appearances on dissection. 2d edn Callow; London: 1809. p. vii.p. 345. [Google Scholar]
- Heerey EA, Bell-Warren KR, Gold JM. Decision-making impairments in the context of intact reward sensitivity in schizophrenia. Biol Psychiatry. 2008;64(1):62–69. doi: 10.1016/j.biopsych.2008.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heerey EA, Gold JM. Patients with schizophrenia demonstrate dissociation between affective experience and motivated behavior. J Abnorm Psychol. 2007;116(2):268–278. doi: 10.1037/0021-843X.116.2.268. [DOI] [PubMed] [Google Scholar]
- Hilario MR, Clouse E, Yin HH, Costa RM. Endocannabinoid signaling is critical for habit formation. Front Integr Neurosci. 2007;1:6. doi: 10.3389/neuro.07.006.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodos W. Progressive ratio as a measure of reward strength. Science. 1961;134(3483):943–944. doi: 10.1126/science.134.3483.943. [DOI] [PubMed] [Google Scholar]
- Hodos W, Kalman G. Effects of increment size and reinforcer volume on progressive ratio performance. J Exp Anal Behav. 1963;6:387–392. doi: 10.1901/jeab.1963.6-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogarth L, Attwood AS, Bate HA, Munafo MR. Acute alcohol impairs human goal-directed action. Biol Psychol. 2012;90(2):154–160. doi: 10.1016/j.biopsycho.2012.02.016. [DOI] [PubMed] [Google Scholar]
- Holroyd CB, Coles MG. The neural basis of human error processing: reinforcement learning, dopamine, and the error-related negativity. Psychol Rev. 2002;109(4):679–709. doi: 10.1037/0033-295X.109.4.679. [DOI] [PubMed] [Google Scholar]
- Hsu CT, Patton DF, Mistlberger RE, Steele AD. Palatable meal anticipation in mice. Plos One. 2010;5(9) doi: 10.1371/journal.pone.0012903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman SE. Revolution stalled. Sci Transl Med. 2012;4(155) doi: 10.1126/scitranslmed.3003142. 155cm111. [DOI] [PubMed] [Google Scholar]
- Hyman SE, Fenton WS. Medicine. What are the right targets for psychopharmacology? Science. 2003;299(5605):350–351. doi: 10.1126/science.1077141. [DOI] [PubMed] [Google Scholar]
- Insel T, Cuthbert B, Garvey M, Heinssen R, Pine DS, Quinn K, et al. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. Am J Psychiatry. 2010;167(7):748–751. doi: 10.1176/appi.ajp.2010.09091379. [DOI] [PubMed] [Google Scholar]
- Insel TR, Voon V, Nye JS, Brown VJ, Altevogt BM, Bullmore ET, et al. Innovative solutions to novel drug development in mental health. Neurosci Biobehav Rev. 2013;37(10 Pt 1):2438–2444. doi: 10.1016/j.neubiorev.2013.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isella V, Iurlaro S, Piolti R, Ferrarese C, Frattola L, Appollonio I, et al. Physical anhedonia in Parkinson's disease. J Neurol Neurosurg Psychiatry. 2003;74(9):1308–1311. doi: 10.1136/jnnp.74.9.1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jilge B. Restricted Feeding - a Nonphotic Zeitgeber in the Rabbit. Physiology & Behavior. 1992;51(1):157–166. doi: 10.1016/0031-9384(92)90218-q. [DOI] [PubMed] [Google Scholar]
- Jocham G, Klein TA, Ullsperger M. Dopamine-mediated reinforcement learning signals in the striatum and ventromedial prefrontal cortex underlie value-based choices. J Neurosci. 2011;31(5):1606–1613. doi: 10.1523/JNEUROSCI.3904-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jocham G, Klein TA, Ullsperger M. Differential modulation of reinforcement learning by D2 dopamine and NMDA glutamate receptor antagonism. J Neurosci. 2014;34(39):13151–13162. doi: 10.1523/JNEUROSCI.0757-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamal A, Van der Harst JE, Kapteijn CM, Baars AJ, Spruijt BM, Ramakers GM. Announced reward counteracts the effects of chronic social stress on anticipatory behavior and hippocampal synaptic plasticity in rats. Exp Brain Res. 2010;201(4):641–651. doi: 10.1007/s00221-009-2083-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasch KL, Rottenberg J, Arnow BA, Gotlib IH. Behavioral activation and inhibition systems and the severity and course of depression. Journal of Abnormal Psychology. 2002;111(4):589–597. doi: 10.1037//0021-843x.111.4.589. [DOI] [PubMed] [Google Scholar]
- Killcross S, Coutureau E. Coordination of actions and habits in the medial prefrontal cortex of rats. Cereb Cortex. 2003;13(4):400–408. doi: 10.1093/cercor/13.4.400. [DOI] [PubMed] [Google Scholar]
- Klein DF. Endogenomorphic Depression - Conceptual and Terminological Revision. Arch Gen Psychiat. 1974;31(4):447–454. doi: 10.1001/archpsyc.1974.01760160005001. [DOI] [PubMed] [Google Scholar]
- Klossek UMH, Russell J, Dickinson A. The control of instrumental action following outcome devaluation in young children aged between 1 and 4 years. Journal of Experimental Psychology-General. 2008;137(1):39–51. doi: 10.1037/0096-3445.137.1.39. [DOI] [PubMed] [Google Scholar]
- Knutson B, Burgdorf J, Panksepp J. Ultrasonic vocalizations as indices of affective states in rats. Psychol Bull. 2002;128(6):961–977. doi: 10.1037/0033-2909.128.6.961. [DOI] [PubMed] [Google Scholar]
- Kraepelin E. Dementia Praecox and Paraphrenia. The Journal of Nervous and Mental Disease. 1921;54(4):384. [Google Scholar]
- Kuczenski R, Segal DS, Aizenstein ML. Amphetamine, cocaine, and fencamfamine: relationship between locomotor and stereotypy response profiles and caudate and accumbens dopamine dynamics. J Neurosci. 1991;11(9):2703–2712. doi: 10.1523/JNEUROSCI.11-09-02703.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leboyer M, Bellivier F, Nosten-Bertrand M, Jouvent R, Pauls D, Mallet J. Psychiatric genetics: search for phenotypes. Trends Neurosci. 1998;21(3):102–105. doi: 10.1016/s0166-2236(97)01187-9. [DOI] [PubMed] [Google Scholar]
- Leibenluft E, Charney DS, Pine DS. Researching the pathophysiology of pediatric bipolar disorder. Biol Psychiatry. 2003;53(11):1009–1020. doi: 10.1016/s0006-3223(03)00069-6. [DOI] [PubMed] [Google Scholar]
- LeSage MG, Burroughs D, Pentel PR. Effects of nicotine withdrawal on performance under a progressive-ratio schedule of sucrose pellet delivery in rats. Pharmacol Biochem Behav. 2006;83(4):585–591. doi: 10.1016/j.pbb.2006.03.021. [DOI] [PubMed] [Google Scholar]
- Li FZ. The exercise motivation scale: Its multifaceted structure and construct validity. J Appl Sport Psychol. 1999;11(1):97–115. [Google Scholar]
- Llerena K, Park SG, McCarthy JM, Couture SM, Bennett ME, Blanchard JJ. The Motivation and Pleasure Scale-Self-Report (MAP-SR): Reliability and validity of a self-report measure of negative symptoms. Compr Psychiat. 2013;54(5):568–574. doi: 10.1016/j.comppsych.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loas G, Duru C, Godefroy O, Krystkowiak P. Hedonic deficits in Parkinson's disease: is consummatory anhedonia specific? Front Neurol. 2014;5:24. doi: 10.3389/fneur.2014.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma ST, Maier EY, Ahrens AM, Schallert T, Duvauchelle CL. Repeated intravenous cocaine experience: development and escalation of pre-drug anticipatory 50-kHz ultrasonic vocalizations in rats. Behav Brain Res. 2010;212(1):109–114. doi: 10.1016/j.bbr.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markou A, Chiamulera C, Geyer MA, Tricklebank M, Steckler T. Removing obstacles in neuroscience drug discovery: the future path for animal models. Neuropsychopharmacology. 2009;34(1):74–89. doi: 10.1038/npp.2008.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markou A, Kosten TR, Koob GF. Neurobiological similarities in depression and drug dependence: a self-medication hypothesis. Neuropsychopharmacology. 1998;18(3):135–174. doi: 10.1016/S0893-133X(97)00113-9. [DOI] [PubMed] [Google Scholar]
- Markou A, Salamone JD, Bussey TJ, Mar AC, Brunner D, Gilmour G, et al. Measuring reinforcement learning and motivation constructs in experimental animals: relevance to the negative symptoms of schizophrenia. Neurosci Biobehav Rev. 2013;37(9 Pt B):2149–2165. doi: 10.1016/j.neubiorev.2013.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews K, Forbes N, Reid IC. Sucrose consumption as an hedonic measure following chronic unpredictable mild stress. Physiol Behav. 1995;57(2):241–248. doi: 10.1016/0031-9384(94)00286-e. [DOI] [PubMed] [Google Scholar]
- McFarland BR, Shankman SA, Tenke CE, Bruder GE, Klein DN. Behavioral activation system deficits predict the six-month course of depression. J Affect Disorders. 2006;91(2-3):229–234. doi: 10.1016/j.jad.2006.01.012. [DOI] [PubMed] [Google Scholar]
- Meehl PE. Schizotaxia, Schizotypy, Schizophrenia. American Psychologist. 1962;17(12):827–838. [Google Scholar]
- Mendelson SD, Pfaus JG. Level searching: a new assay of sexual motivation in the male rat. Physiol Behav. 1989;45(2):337–341. doi: 10.1016/0031-9384(89)90136-4. [DOI] [PubMed] [Google Scholar]
- Mendoza J, Angeles-Castellanos M, Escobar C. Entrainment by a palatable meal induces food-anticipatory activity and c-Fos expression in reward-related areas of the brain. Neuroscience. 2005;133(1):293–303. doi: 10.1016/j.neuroscience.2005.01.064. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Weinberger DR. Intermediate phenotypes and genetic mechanisms of psychiatric disorders. Nat Rev Neurosci. 2006;7(10):818–827. doi: 10.1038/nrn1993. [DOI] [PubMed] [Google Scholar]
- Meyer B, Johnson SL, Carver CS. Exploring behavioral activation and inhibition sensitivities among college students at risk for bipolar spectrum symptomatology. J Psychopathol Behav. 1999;21(4):275–292. doi: 10.1023/A:1022119414440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mistlberger RE. Circadian food-anticipatory activity: formal models and physiological mechanisms. Neurosci Biobehav Rev. 1994;18(2):171–195. doi: 10.1016/0149-7634(94)90023-x. [DOI] [PubMed] [Google Scholar]
- Moore D, Siegfried D, Wilson R, Rankin MA. The influence of time of day on the foraging behavior of the honeybee, Apis mellifera. J Biol Rhythms. 1989;4(3):305–325. doi: 10.1177/074873048900400301. [DOI] [PubMed] [Google Scholar]
- Mote J, Minzenberg MJ, Carter CS, Kring AM. Deficits in anticipatory but not consummatory pleasure in people with recent-onset schizophrenia spectrum disorders. Schizophr Res. 2014;159(1):76–79. doi: 10.1016/j.schres.2014.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawijn L, van Zuiden M, Frijling JL, Koch SB, Veltman DJ, Olff M. Reward functioning in PTSD: a systematic review exploring the mechanisms underlying anhedonia. Neurosci Biobehav Rev. 2015;51:189–204. doi: 10.1016/j.neubiorev.2015.01.019. [DOI] [PubMed] [Google Scholar]
- Nelson A, Killcross S. Amphetamine exposure enhances habit formation. J Neurosci. 2006;26(14):3805–3812. doi: 10.1523/JNEUROSCI.4305-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ, Hyman SE. Animal models of neuropsychiatric disorders. Nat Neurosci. 2010;13(10):1161–1169. doi: 10.1038/nn.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Doherty J, Dayan P, Schultz J, Deichmann R, Friston K, Dolan RJ. Dissociable roles of ventral and dorsal striatum in instrumental conditioning. Science. 2004;304(5669):452–454. doi: 10.1126/science.1094285. [DOI] [PubMed] [Google Scholar]
- Opiol H, Pavlovski I, Michalik M, Mistlberger RE. Ultrasonic vocalizations in rats anticipating circadian feeding schedules. Behav Brain Res. 2015;284:42–50. doi: 10.1016/j.bbr.2015.02.003. [DOI] [PubMed] [Google Scholar]
- Paul IA, English JA, Halaris A. Sucrose and quinine intake by maternally-deprived and control rhesus monkeys. Behav Brain Res. 2000;112(1-2):127–134. doi: 10.1016/s0166-4328(00)00173-x. [DOI] [PubMed] [Google Scholar]
- Pechtel P, Dutra SJ, Goetz EL, Pizzagalli DA. Blunted reward responsiveness in remitted depression. J Psychiatr Res. 2013a;47(12):1864–1869. doi: 10.1016/j.jpsychires.2013.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pechtel P, Pizzagalli DA. Disrupted reinforcement learning and maladaptive behavior in women with a history of childhood sexual abuse: a high-density event-related potential study. JAMA Psychiatry. 2013b;70(5):499–507. doi: 10.1001/jamapsychiatry.2013.728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelletier LG, Tuson KM, Fortier MS, Vallerand RJ, Briere NM, Blais MR. Toward a New Measure of Intrinsic Motivation, Extrinsic Motivation, and Amotivation in Sports - the Sport Motivation Scale (Sms) J Sport Exercise Psy. 1995;17(1):35–53. [Google Scholar]
- Penrod B, Wallace MD, Dyer EJ. Assessing potency of high- and low-preference reinforcers with respect to response rate and response patterns. J Appl Behav Anal. 2008;41(2):177–188. doi: 10.1901/jaba.2008.41-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pergadia ML, Der-Avakian A, D'Souza MS, Madden PA, Heath AC, Shiffman S, et al. Association between nicotine withdrawal and reward responsiveness in humans and rats. JAMA Psychiatry. 2014;71(11):1238–1245. doi: 10.1001/jamapsychiatry.2014.1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaus JG, Phillips AG. Role of dopamine in anticipatory and consummatory aspects of sexual behavior in the male rat. Behav Neurosci. 1991;105(5):727–743. doi: 10.1037//0735-7044.105.5.727. [DOI] [PubMed] [Google Scholar]
- Phelps CE, Mitchell EN, Nutt DJ, Marston HM, Robinson ES. Psychopharmacological characterisation of the successive negative contrast effect in rats. Psychopharmacology (Berl) 2015 doi: 10.1007/s00213-015-3905-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzagalli DA, Bogdan R, Ratner KG, Jahn AL. Increased perceived stress is associated with blunted hedonic capacity: potential implications for depression research. Behav Res Ther. 2007;45(11):2742–2753. doi: 10.1016/j.brat.2007.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzagalli DA, Evins AE, Schetter EC, Frank MJ, Pajtas PE, Santesso DL, et al. Single dose of a dopamine agonist impairs reinforcement learning in humans: behavioral evidence from a laboratory-based measure of reward responsiveness. Psychopharmacology (Berl) 2008a;196(2):221–232. doi: 10.1007/s00213-007-0957-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzagalli DA, Goetz E, Ostacher M, Iosifescu DV, Perlis RH. Euthymic patients with bipolar disorder show decreased reward learning in a probabilistic reward task. Biol Psychiatry. 2008b;64(2):162–168. doi: 10.1016/j.biopsych.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzagalli DA, Iosifescu D, Hallett LA, Ratner KG, Fava M. Reduced hedonic capacity in major depressive disorder: evidence from a probabilistic reward task. J Psychiatr Res. 2008c;43(1):76–87. doi: 10.1016/j.jpsychires.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzagalli DA, Jahn AL, O'Shea JP. Toward an objective characterization of an anhedonic phenotype: a signal-detection approach. Biol Psychiatry. 2005;57(4):319–327. doi: 10.1016/j.biopsych.2004.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid I, Forbes N, Stewart C, Matthews K. Chronic mild stress and depressive disorder: a useful new model? Psychopharmacology (Berl) 1997;134(4):365–367. doi: 10.1007/s002130050471. discussion 371-367. [DOI] [PubMed] [Google Scholar]
- Ribot T. La psychologie des sentiments. F. Alcan; Paris: 1896. p. xi.p. 443. [Google Scholar]
- Roane HS, Lerman DC, Vorndran CM. Assessing reinforcers under progressive schedule requirements. J Appl Behav Anal. 2001;34(2):145–166. doi: 10.1901/jaba.2001.34-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts DC. Breaking points on a progressive ratio schedule reinforced by intravenous apomorphine increase daily following 6-hydroxydopamine lesions of the nucleus accumbens. Pharmacol Biochem Behav. 1989;32(1):43–47. doi: 10.1016/0091-3057(89)90208-6. [DOI] [PubMed] [Google Scholar]
- Rodriguez JS, Morris SM, Hotchkiss CE, Doerge DR, Allen RR, Mattison DR, et al. The effects of chronic methylphenidate administration on operant test battery performance in juvenile rhesus monkeys. Neurotoxicol Teratol. 2010;32(2):142–151. doi: 10.1016/j.ntt.2009.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rorie AE, Gao J, McClelland JL, Newsome WT. Integration of sensory and reward information during perceptual decision-making in lateral intraparietal cortex (LIP) of the macaque monkey. Plos One. 2010;5(2):e9308. doi: 10.1371/journal.pone.0009308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamone JD, Correa M, Farrar A, Mingote SM. Effort-related functions of nucleus accumbens dopamine and associated forebrain circuits. Psychopharmacology (Berl) 2007;191(3):461–482. doi: 10.1007/s00213-006-0668-9. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Correa M, Farrar AM, Nunes EJ, Pardo M. Dopamine, behavioral economics, and effort. Front Behav Neurosci. 2009;3:13. doi: 10.3389/neuro.08.013.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamone JD, Cousins MS, Bucher S. Anhedonia or anergia? Effects of haloperidol and nucleus accumbens dopamine depletion on instrumental response selection in a T-maze cost/benefit procedure. Behav Brain Res. 1994;65(2):221–229. doi: 10.1016/0166-4328(94)90108-2. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Cousins MS, Snyder BJ. Behavioral functions of nucleus accumbens dopamine: empirical and conceptual problems with the anhedonia hypothesis. Neurosci Biobehav Rev. 1997;21(3):341–359. doi: 10.1016/s0149-7634(96)00017-6. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Steinpreis RE, McCullough LD, Smith P, Grebel D, Mahan K. Haloperidol and nucleus accumbens dopamine depletion suppress lever pressing for food but increase free food consumption in a novel food choice procedure. Psychopharmacology (Berl) 1991;104(4):515–521. doi: 10.1007/BF02245659. [DOI] [PubMed] [Google Scholar]
- Samejima K, Ueda Y, Doya K, Kimura M. Representation of action-specific reward values in the striatum. Science. 2005;310(5752):1337–1340. doi: 10.1126/science.1115270. [DOI] [PubMed] [Google Scholar]
- Santesso DL, Dillon DG, Birk JL, Holmes AJ, Goetz E, Bogdan R, et al. Individual differences in reinforcement learning: behavioral, electrophysiological, and neuroimaging correlates. Neuroimage. 2008;42(2):807–816. doi: 10.1016/j.neuroimage.2008.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santesso DL, Evins AE, Frank MJ, Schetter EC, Bogdan R, Pizzagalli DA. Single dose of a dopamine agonist impairs reinforcement learning in humans: evidence from event-related potentials and computational modeling of striatal-cortical function. Hum Brain Mapp. 2009;30(7):1963–1976. doi: 10.1002/hbm.20642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W. Getting formal with dopamine and reward. Neuron. 2002;36(2):241–263. doi: 10.1016/s0896-6273(02)00967-4. [DOI] [PubMed] [Google Scholar]
- Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science. 1997;275(5306):1593–1599. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- Schwabe L, Wolf OT. Socially evaluated cold pressor stress after instrumental learning favors habits over goal-directed action. Psychoneuroendocrinology. 2010;35(7):977–986. doi: 10.1016/j.psyneuen.2009.12.010. [DOI] [PubMed] [Google Scholar]
- Shalev U, Kafkafi N. Repeated maternal separation does not alter sucrose-reinforced and open-field behaviors. Pharmacol Biochem Behav. 2002;73(1):115–122. doi: 10.1016/s0091-3057(02)00756-6. [DOI] [PubMed] [Google Scholar]
- Sherdell L, Waugh CE, Gotlib IH. Anticipatory pleasure predicts motivation for reward in major depression. J Abnorm Psychol. 2012;121(1):51–60. doi: 10.1037/a0024945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson EH, Waltz JA, Kellendonk C, Balsam PD. Schizophrenia in translation: dissecting motivation in schizophrenia and rodents. Schizophr Bull. 2012;38(6):1111–1117. doi: 10.1093/schbul/sbs114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snaith RP, Hamilton M, Morley S, Humayan A, Hargreaves D, Trigwell P. A Scale for the Assessment of Hedonic Tone - the Snaith-Hamilton Pleasure Scale. Brit J Psychiat. 1995;167:99–103. doi: 10.1192/bjp.167.1.99. [DOI] [PubMed] [Google Scholar]
- Soares JM, Sampaio A, Ferreira LM, Santos NC, Marques F, Palha JA, et al. Stress-induced changes in human decision-making are reversible. Transl Psychiatry. 2012;2:e131. doi: 10.1038/tp.2012.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Specht SM, Twining RC. Human taste contrast and self-reported measures of anxiety. Percept Mot Skills. 1999;88(2):384–386. doi: 10.2466/pms.1999.88.2.384. [DOI] [PubMed] [Google Scholar]
- Starkstein SE, Jorge R, Mizrahi R, Robinson RG. The construct of minor and major depression in Alzheimer's disease. Am J Psychiatry. 2005;162(11):2086–2093. doi: 10.1176/appi.ajp.162.11.2086. [DOI] [PubMed] [Google Scholar]
- Stoops WW. Reinforcing Effects of Stimulants in Humans: Sensitivity of Progressive-Ratio Schedules. Exp Clin Psychopharm. 2008;16(6):503–512. doi: 10.1037/a0013657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoops WW, Bennett JA, Lile JA, Sevak RJ, Rush CR. Influence of aripiprazole pretreatment on the reinforcing effects of methamphetamine in humans. Prog Neuropsychopharmacol Biol Psychiatry. 2013;47:111–117. doi: 10.1016/j.pnpbp.2013.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulzman FM, Fuller CA, Mooreede MC. Feeding Time Synchronizes Primate Circadian-Rhythms. Physiology & Behavior. 1977;18(5):775–779. doi: 10.1016/0031-9384(77)90182-2. [DOI] [PubMed] [Google Scholar]
- Treadway MT, Bossaller NA, Shelton RC, Zald DH. Effort-based decision-making in major depressive disorder: a translational model of motivational anhedonia. J Abnorm Psychol. 2012a;121(3):553–558. doi: 10.1037/a0028813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treadway MT, Buckholtz JW, Cowan RL, Woodward ND, Li R, Ansari MS, et al. Dopaminergic mechanisms of individual differences in human effort-based decision-making. J Neurosci. 2012b;32(18):6170–6176. doi: 10.1523/JNEUROSCI.6459-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treadway MT, Buckholtz JW, Schwartzman AN, Lambert WE, Zald DH. Worth the 'EEfRT'? The effort expenditure for rewards task as an objective measure of motivation and anhedonia. Plos One. 2009;4(8):e6598. doi: 10.1371/journal.pone.0006598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treadway MT, Peterman JS, Zald DH, Park S. Impaired effort allocation in patients with schizophrenia. Schizophr Res. 2015;161(2-3):382–385. doi: 10.1016/j.schres.2014.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trecker A, Robbins TW, Mar AC, editors. Development of a Visual-guided Probabilistic Selection Task for Rats. Proceedings of Measuring Behavior 2012; Utrecht, The Netherlands. August 28-31, 2012; 2012. Utrecht, The Netherlands. [Google Scholar]
- Tripp G, Alsop B. Sensitivity to reward frequency in boys with attention deficit hyperactivity disorder. J Clin Child Psychol. 1999;28(3):366–375. doi: 10.1207/S15374424jccp280309. [DOI] [PubMed] [Google Scholar]
- Trosclair-Lasserre NM, Lerman DC, Call NA, Addison LR, Kodak T. Reinforcement magnitude: an evaluation of preference and reinforcer efficacy. J Appl Behav Anal. 2008;41(2):203–220. doi: 10.1901/jaba.2008.41-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentin VV, Dickinson A, O'Doherty JP. Determining the neural substrates of goal-directed learning in the human brain. J Neurosci. 2007;27(15):4019–4026. doi: 10.1523/JNEUROSCI.0564-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallerand RJ, Pelletier LG, Blais MR, Briere NM, Senecal C, Vallieres EF. The Academic Motivation Scale - a Measure of Intrinsic, Extrinsic, and Amotivation in Education. Educ Psychol Meas. 1992;52(4):1003–1017. [Google Scholar]
- van der Harst JE, Baars AM, Spruijt BM. Announced rewards counteract the impairment of anticipatory behaviour in socially stressed rats. Behav Brain Res. 2005;161(2):183–189. doi: 10.1016/j.bbr.2005.02.029. [DOI] [PubMed] [Google Scholar]
- Venugopalan VV, Casey KF, O'Hara C, O'Loughlin J, Benkelfat C, Fellows LK, et al. Acute phenylalanine/tyrosine depletion reduces motivation to smoke cigarettes across stages of addiction. Neuropsychopharmacology. 2011;36(12):2469–2476. doi: 10.1038/npp.2011.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollmayr B, Bachteler D, Vengeliene V, Gass P, Spanagel R, Henn F. Rats with congenital learned helplessness respond less to sucrose but show no deficits in activity or learning. Behav Brain Res. 2004;150(1-2):217–221. doi: 10.1016/S0166-4328(03)00259-6. [DOI] [PubMed] [Google Scholar]
- Von Huben SN, Davis SA, Lay CC, Katner SN, Crean RD, Taffe MA. Differential contributions of dopaminergic D1- and D2-like receptors to cognitive function in rhesus monkeys. Psychopharmacology (Berl) 2006;188(4):586–596. doi: 10.1007/s00213-006-0347-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrieze E, Ceccarini J, Pizzagalli DA, Bormans G, Vandenbulcke M, Demyttenaere K, et al. Measuring extrastriatal dopamine release during a reward learning task. Hum Brain Mapp. 2013a;34(3):575–586. doi: 10.1002/hbm.21456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrieze E, Pizzagalli DA, Demyttenaere K, Hompes T, Sienaert P, de Boer P, et al. Reduced reward learning predicts outcome in major depressive disorder. Biol Psychiatry. 2013b;73(7):639–645. doi: 10.1016/j.biopsych.2012.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waltz JA, Frank MJ, Robinson BM, Gold JM. Selective reinforcement learning deficits in schizophrenia support predictions from computational models of striatal-cortical dysfunction. Biol Psychiatry. 2007;62(7):756–764. doi: 10.1016/j.biopsych.2006.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang AY, Miura K, Uchida N. The dorsomedial striatum encodes net expected return, critical for energizing performance vigor. Nat Neurosci. 2013;16(5):639–647. doi: 10.1038/nn.3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward RD, Simpson EH, Richards VL, Deo G, Taylor K, Glendinning JI, et al. Dissociation of hedonic reaction to reward and incentive motivation in an animal model of the negative symptoms of schizophrenia. Neuropsychopharmacology. 2012;37(7):1699–1707. doi: 10.1038/npp.2012.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardle MC, Treadway MT, Mayo LM, Zald DH, de Wit H. Amping up effort: effects of d-amphetamine on human effort-based decision-making. J Neurosci. 2011;31(46):16597–16602. doi: 10.1523/JNEUROSCI.4387-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and Validation of Brief Measures of Positive and Negative Affect - the Panas Scales. Journal of Personality and Social Psychology. 1988;54(6):1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Weber DN, Spieler RE. Effects of the Light-Dark Cycle and Scheduled Feeding on Behavioral and Reproductive Rhythms of the Cyprinodont Fish, Medaka, Oryzias-Latipes. Experientia. 1987;43(6):621–624. doi: 10.1007/BF02126355. [DOI] [PubMed] [Google Scholar]
- Wenger D, Biebach H, Krebs JR. Free-Running Circadian-Rhythm of a Learned Feeding Pattern in Starlings. Naturwissenschaften. 1991;78(2):87–89. [Google Scholar]
- West EA, DesJardin JT, Gale K, Malkova L. Transient inactivation of orbitofrontal cortex blocks reinforcer devaluation in macaques. J Neurosci. 2011;31(42):15128–15135. doi: 10.1523/JNEUROSCI.3295-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitton AE, Treadway MT, Pizzagalli DA. Reward processing dysfunction in major depression, bipolar disorder and schizophrenia. Curr Opin Psychiatry. 2015;28(1):7–12. doi: 10.1097/YCO.0000000000000122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willey AR, Spear LP. Development of anticipatory 50 kHz USV production to a social stimuli in adolescent and adult male Sprague-Dawley rats. Behav Brain Res. 2012;226(2):613–618. doi: 10.1016/j.bbr.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willner P. Chronic mild stress (CMS) revisited: consistency and behavioural-neurobiological concordance in the effects of CMS. Neuropsychobiology. 2005;52(2):90–110. doi: 10.1159/000087097. [DOI] [PubMed] [Google Scholar]
- Willner P, Muscat R, Papp M. Chronic mild stress-induced anhedonia: a realistic animal model of depression. Neurosci Biobehav Rev. 1992;16(4):525–534. doi: 10.1016/s0149-7634(05)80194-0. [DOI] [PubMed] [Google Scholar]
- Willner P, Towell A, Sampson D, Sophokleous S, Muscat R. Reduction of sucrose preference by chronic unpredictable mild stress, and its restoration by a tricyclic antidepressant. Psychopharmacology (Berl) 1987;93(3):358–364. doi: 10.1007/BF00187257. [DOI] [PubMed] [Google Scholar]
- Wolf DH, Satterthwaite TD, Kantrowitz JJ, Katchmar N, Vandekar L, Elliott MA, et al. Amotivation in schizophrenia: integrated assessment with behavioral, clinical, and imaging measures. Schizophr Bull. 2014;40(6):1328–1337. doi: 10.1093/schbul/sbu026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . The ICD-10 classification of mental and behavioural disorders : clinical descriptions and diagnostic guidelines. World Health Organization; Geneva: 1992. p. xii.p. 362. [Google Scholar]
- Yin HH, Knowlton BJ, Balleine BW. Blockade of NMDA receptors in the dorsomedial striatum prevents action-outcome learning in instrumental conditioning. Eur J Neurosci. 2005a;22(2):505–512. doi: 10.1111/j.1460-9568.2005.04219.x. [DOI] [PubMed] [Google Scholar]
- Yin HH, Ostlund SB, Knowlton BJ, Balleine BW. The role of the dorsomedial striatum in instrumental conditioning. Eur J Neurosci. 2005b;22(2):513–523. doi: 10.1111/j.1460-9568.2005.04218.x. [DOI] [PubMed] [Google Scholar]
- Yohn SE, Santerre JL, Nunes EJ, Kozak R, Podurgiel SJ, Correa M, et al. The Role of Dopamine D1 Receptor Transmission in Effort-Related Choice Behavior: Effects of D1 agonists. Pharmacol Biochem Behav. 2015 doi: 10.1016/j.pbb.2015.05.003. [DOI] [PubMed] [Google Scholar]
- Zhang D, Zhou X, Wang X, Xiang X, Chen H, Hao W. Morphine withdrawal decreases responding reinforced by sucrose self-administration in progressive ratio. Addict Biol. 2007;12(2):152–157. doi: 10.1111/j.1369-1600.2007.00068.x. [DOI] [PubMed] [Google Scholar]