Abstract
Most individuals with tuberous sclerosis complex (TSC) are born with a mutant allele of either TSC1 or TSC2 and a mosaic of psychological and cognitive defects. Tsc1 loss of heterozygosity contributes to severe dendritic abnormalities that are rescued by normalizing the levels of the actin-cross linking protein, Filamin A (FLNA). However, it is unclear whether dendrites and FLNA levels are abnormal in an heterozygote Tsc1 condition. Here, we examined dendritic morphology and FLNA levels in the olfactory bulb of Tsc1 wild type and heterozygote mice. Using in vivo neonatal electroporation to label newborn neurons followed by sholl analysis, we found that Tsc1 haploinsufficiency is associated with increased dendritic complexity and total dendritic length as well as increased FLNA levels. Since reducing FLNA levels has been shown to decrease Tsc1+/− dendritic complexity, these data suggest that increased FLNA levels in Tsc1+/− mice contribute to abnormal dendritic patterning in the Tsc1 heterozygote condition of individuals with TSC.
Keywords: dendrite, mTOR, tuberous sclerosis complex, Filamin A, circuit, network, neurogenesis
1. Introduction
TSC is an autosomal dominant monogenetic disorder observed in 1/6,000 individuals characterized by discrete lesions in diverse tissues, including the skin, heart, kidney, lung, and brain [1]). Most patients are born with at least one detectable mutation in TSC1 or TSC2 and are thus heterozygous for one of these genes [2;3]. Very often, there are subsequent inactivating mutations of the other functional allele during development leading to the formation of discrete lesions in several organs, including the brain [4–7].
The cortical lesions, called cortical tubers, are responsible for seizures occurring in >80% of TSC individuals [1]. However, TSC individuals display a mosaic of neuropsychological problems (e.g. autistic traits) and about half of them present with cognitive impairments (i.e. mental retardation). Although epilepsy can lead to both cognitive and psychiatric deficits, it is thought that heterozygosity for TSC1 and TSC2 is sufficient to account for some of these deficits. Indeed, Tsc1+/− and Tsc2+/− mice as well as mice with a heterozygote Tsc2 dominant negative allele (Tsc2ΔRG) display cognitive and social deficits despite the absence of brain lesions or seizures ([8] and for review see [9]). These deficits likely result from a collection of cellular and molecular alterations in heterozygote mice. At the molecular level, mTORC1 and ERK activities have been shown to be increased in Tsc1+/− and Tsc2ΔRG mice, respectively [10;11]. At the cellular level, retinogeniculate projections have been reported to show abnormalities in targeting in Tsc1+/− mice [12]. Alterations in dendritic patterning have not been observed in hippocampal neurons of Tsc1+/− mice in vivo [13]. This is somehow surprising considering that dendritic defects are often observed in disorders associated with cognitive or psychiatric deficits [14–17] and are a classical outcome of increased mTORC1 or ERK activity (see [9;18] for additional references). Because, the defects in axonal patterning were modest, it is possible that a modest defect in dendritic patterning may not be detectable by analyzing the number of first and second dendrites as performed in the published study using Tsc1+/− mice [13].
Here, we thus set out to examine the dendritic complexity of neurons in Tsc1+/− mice. To efficiently label dendrites, we used mice crossed with a tdTomato reporter line, in which expression of a Cre recombinase (Cre)-encoding plasmid leads to tdTomato expression. We electroporated a Cre plasmid in newborn neurons of the subventricular zone that migrate and become interneurons in the olfactory bulb (OB) [19]. We found that Tsc1+/− neurons display a modest but significant increase in dendritic complexity and total length compared to wild type Tsc1+/+ neurons. Intriguingly, a recent study reported that decreasing the level of an actin-cross-linking protein filamin A (FLNA) [20] in conditional Tsc1+/− neurons of the OB decreased their dendritic complexity [18]. The recent study also reported increased levels of FLNA in conditional Tsc1−/− (null) neurons compared to Tsc1+/− neurons and in TSC patients, but FLNA levels in Tsc1+/− versus wild type Tsc1 mice were not examined. In light of the data on dendrites in Tsc1+/− mice reported here, we examined FLNA levels in these mice and found that there were increased compared to those in Tsc1+/+ mice. In addition, correlation analysis between TSC1 and FLNA levels in Tsc1 mice suggest a gene-dosage relationship between Tsc1 and FLNA levels.
2. Materials and Methods
Animals
Research protocols were approved by the Yale University Institutional Animal Care and Use Committee. Experiments were performed on the following lines of transgenic mice of either gender: Tsc1fl/+ (fl, floxed, obtained from Jackson Labs), Tsc1+/+, and Tsc1+/− mice (obtained from the National Cancer Institute). All these lines were crossed with Rosa26R-STOP-tdTomato reporter mice (Jackson labs). Tail or toe samples were taken and were subjected to DNA isolation, PCR amplification using previously published primers [21] and amplicons separated by standard electrophoresis methods.
Neonatal electroporation and vectors
Postnatal electroporation was performed as previously described [19;22;23]. Plasmids (2–3 μg/μl) were diluted in PBS containing 0.1% fast green as a tracer. 0.5–1 μl of plasmid solution was injected into the lateral ventricles of neonatal pups using a pulled glass pipette (diameter <50 μm). 5 square-pulses of 50 ms-duration with 950 ms-intervals at 100 V were applied using the ECM 830 square wave pulse generator (BTX) and tweezer-type electrodes (model 520, BTX) placed on the heads of P0-P1 pups. The electrodes were positioned to direct current rostrally in the dorso-lateral SVZ. Vectors included pCAG-tdTomato [24] and pCAG-Cre (addgene, C. Cepko).
Morphometric analysis
Images of tdTomato+ basal dendrites were acquired in coronal sections using a Fluoview 1000 confocal microscope and traced with simple neurite tracer software (FIJI, GNU GPL v3, an ImageJ plugin, URL: http://fiji.sc/Simple_Neurite_Tracer). We analyzed the basal dendrites of granule cells in a specific layer for the following technical reason; the basal dendrite can be fully imaged and traced without confusion from a single labeled granule cell. Due to its length, the apical dendrite is often cut or partially cut, and this could lead to erroneous data. In addition, in the external plexiform layer where apical dendrites branch and terminate, it is difficult to distinguish whether the dendrite come from one cell or several cells. Sholl analyses were carried out using the number of intersections in 10 μm-increment concentric circles as a measure of morphological complexity. Z-stacks from 3 different square fields of view were taken from 3 different olfactory bulb (OB) sections. Analysis was performed blindly from at least 3 animals per condition.
Olfactory bulb lysate and western blot
Mice were anesthesized with pentobarbital (50 mg/kg) prior to olfactory bulb (OB) dissection. Western blotting protocol is detailed in [18]. Samples were homogenized in RIPA buffer, 1x Halt Protease and phosphatase inhibitor cocktail (Thermo Fisher Scientific), and 8 U/ml DNase. Samples were boiled in 2× Laemmli’s buffer. 10 or 20 μg protein/sample was loaded into a 4–15% polyacrylamide gel (Bio-Rad Mini Protean TGX gel). Proteins were transferred to PVDF and blocked in 5% milk or 5% BSA. Quantifications were performed using NIH Image J software. Primary antibodies included: FLNA (molecular weight: 281 kDa, Abcam, ab51217, 1:5,000), TSC1 (predicted band size: 130 kDa, observed at 150–160 kDa as reported for the company site, Abcam, ab32936, 1:5,000), and ERK1/2 (molecular weight of ERK1: 44 kDa, Santa Cruz, sc-94, 1:250,000). We used the Thermo Scientific BenchMark™ Pre-Stained Protein Ladder containing a mixture of 10 proteins spanning 6–180 kDa (Catalog number: 10748010, invitogen).
Statistics
Analysis was performed on N indicating the number of animals in each condition or n indicating the number of replicates. Data were presented in GraphPad Prism 6. Statistical significance was determined using unpaired Student t-test or one way ANOVA with p<0.05 or two way ANOVA with post hoc Bonferroni’s test for comparison of dendritic crossing. Data are presented as mean ± standard error of the mean (SEM).
3. Results
To investigate whether Tsc1 haploinsufficiency would lead to alterations in dendritogenesis in vivo, we employed neonatal electroporation to fluorescently label developing OB neurons in Tsc1+/+, Tsc1+/−, and Tsc1fl/+ mice crossed with Rosa26R-STOP-tdTomato reporter mice (Fig. 1A). We electroporated pCAG-Cre to excise the floxed Tsc1 allele and the STOP cassette leading to tdTomato expression at P0 into neural progenitor cells of the subventricular zone. During division, electroporated neural progenitors pass their exogenous plasmids on to neuroblasts, which migrate and integrate into the OB [19]. By 2 weeks, these neuroblasts differentiate into GABAergic interneurons with both apical and basal dendrites. We assessed the complexity and length of the basal dendrites of tdTomato+ neurons in vivo at 14 days post-electroporation by sholl analysis in coronal sections from littermate mice (Fig. 1B). Sholl analysis revealed that the dendrites of Tsc1+/− neurons in both constitutive and conditional Tsc1+/− mice displayed a significant increase in their dendritic complexity (2-way ANOVA with post hoc Bonferroni’s test, n=27–30 neurons, N=3 mice each, Fig. 1C) and total dendritic length (One way ANOVA, P=0.0184, F=4.194, Fig. 1D).
Figure 1. Constitutive and conditional Tsc1+/− neurons display increased dendritic patterning compared to Tsc1+/+ neurons.
(A) Diagram illustrating electroporation in P0 neonates. Most transfected newborn neurons reach their final location in the olfactory bulb (OB) by P14. (B) Image and reconstruction of the basal dendrite of an OB granule cell expressing tdTomato with superimposed concentric circles for sholl analysis. (C) # crossings as a function of distance for the basal dendrites of tdTomato+ neurons at P14 in Tsc1+/+ (black), Tsc1+/− (violet) and Tsc1fl/+ (green) mice. Neurons were electroporated with pCAG-Cre leading to Tsc1 heterozygosity in Tsc1fl/+ mice and tdTomato expression due to crossing with R26R-Stop-tdTomato mice. *: P<0.05, **: P<0.01, purple for comparisons between Tsc1 wild type and heterozygote mice, and green between wild type and Tsc1fl/− mice using two way ANOVA followed by Bonferroni multiple comparisons. (D) Total dendritic length corresponding to the conditions in C.
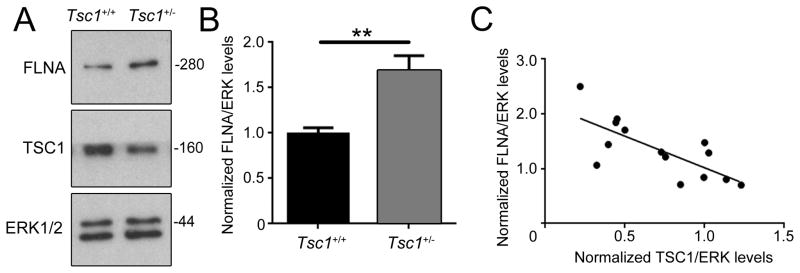
Considering that decreasing FLNA levels in conditional Tsc1+/− neurons decreased their dendritic complexity and total length [18], we examined whether FLNA levels were increased in Tsc1+/− versus Tsc1+/+ mice at P14. We performed immunoblotting for FLNA, TSC1 and the loading control ERK1/2, which is not altered in Tsc1 mice of different genotypes [10]. We found that FLNA levels were significantly increased in the OB as a result of Tsc1 haploinsufficiency (N=7 per condition, P=0.0045, unpaired t-test, Fig. 2A and B). In addition, correlation analysis between FLNA and TSC1 levels revealed that the levels of TSC1 and FLNA were inversely correlated (R2=0.53, P=0.003, N=14 from Tsc1+/+ and Tsc1+/− mice, Fig. 2C).
Figure 2. FLNA levels are increased in Tsc1+/− nice and correlate with TSC1 levels.
(A) Immunoblots for FLNA, TSC1, and ERK1/2 from P14 OB of Tsc1+/+ (black) and Tsc1+/− (grey). (B) Normalized % changes for FLNA/ERK. (C) Plot of FLNA versus TSC1 levels.
4. Discussion
Our data suggest that Tsc1 haploinsufficiency as reported in TSC individuals is associated with increased dendritic patterning and increased levels of FLNA. We have previously reported that decreasing FLNA levels in heterozygote neurons was sufficient to reduce dendritic complexity [18], but it was unclear whether dendrites were altered in the heterozygote condition or whether FLNA was just required for dendritic maintenance. Together with the published findings, our data suggest that increased FLNA levels in TSC1 heterozygote condition contributes to altered dendritic complexity.
FLNA is an actin-cross linking protein with multiple binding partners and a spectrum of functions inside cells, including neurons [20;25;26]. As such the mechanism of FLNA action on dendritic development remains unclear and is outside the scope of this study. Nevertheless, our finding has several important implications. First, it was also recently reported that increased FLNA levels can alter spine morphology [27] in addition to dendrites as shown here. A change in dendritic morphology, including spine morphology is a hallmark of several disorders associated with cognitive and deficits [15;28]. In addition, Tsc1+/− mice have both cognitive and social deficits [8;13]. Our findings thus beg the question as to whether increased FLNA levels could contribute to cognitive and psychiatric deficits in TSC. Second, our findings are not limited to TSC. Indeed, we recently reported that increased FLNA levels are increased as a result of increased MEK/ERK1/2 signaling in Tsc1 null condition [18]. Several neurodevelopmental disorders are considered to be part of a RAS/MAPK syndrome associated with increased MEK/ERK activity. It would thus be intriguing to examine whether FLNA levels are increased in these disorders associated with neurocognitive deficits. As part of this non-neurocognitive group is cancer which is associated with altered FLNA levels [29]. Another condition leading to increased FLNA levels is hypoxia, in which increased FLNA contributed to altered spine morphology.
Finding a correlation between FLNA and TSC1 levels suggests that TSC individuals will have differences in their FLNA levels due to allele-specific variations in Tsc1 mRNA expression [30] that could contribute to a spectrum of dendritic dysmorphogenesis and cognitive or psychiatric disparities. In addition, a copy number variation of FLNA is found in the genome of patients suffering from autism disorder [31]. Whether this could contribute to patterning abnormalities remain to be examined.
All together these findings emphasize the need to further examine FLNA contribution to neuronal morphology and patterning as well as network activity and behavior in several disorders associated with alterations in RAS/MAPK and AKT/TSC/mTOR signaling.
Highlights.
Tsc1 heterozygote neurons display increased dendritic arborization.
Tsc1 heterozygosity leads to increased filamin A levels.
Filamin A and TSC1 levels are correlated.
Acknowledgments
This work was supported by grants from NIH-NINDS R01NS093704 (AB), training grant from the China Scholarship Council (LZ and TH), and Young Investigator Grant of Xiangya Hospital, CSU (2015Q02, LZ).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Crino PB, Nathanson KL, Henske EP. The tuberous sclerosis complex. N Engl J Med. 2006;355:1345–1356. doi: 10.1056/NEJMra055323. [DOI] [PubMed] [Google Scholar]
- 2.van Slegtenhorst M, de Hoogt R, Hermans C, Nellist M, Janssen B, Verhoef S, Lindhout D, van den OA, Halley D, Young J, Burley M, Jeremiah S, Woodward K, Nahmias J, Fox M, Ekong R, Osborne J, Wolfe J, Povey S, Snell RG, Cheadle JP, Jones AC, Tachataki M, Ravine D, Sampson JR, Reeve MP, Richardson P, Wilmer F, Munro C, Hawkins TL, Sepp T, Ali JB, Ward S, Green AJ, Yates JR, Kwiatkowska J, Henske EP, Short MP, Haines JH, Jozwiak S, Kwiatkowski DJ. Identification of the tuberous sclerosis gene TSC1 on chromosome 9q34. Science. 1997;277:805–808. doi: 10.1126/science.277.5327.805. [DOI] [PubMed] [Google Scholar]
- 3.Kwiatkowski DJ, Short MP. Tuberous sclerosis. Arch Dermatol. 1994;130:348–354. [PubMed] [Google Scholar]
- 4.Green AJ, Smith M, Yates JR. Loss of heterozygosity on chromosome 16p13.3 in hamartomas from tuberous sclerosis patients. Nat Genet. 1994;6:193–196. doi: 10.1038/ng0294-193. [DOI] [PubMed] [Google Scholar]
- 5.Sepp T, Yates JR, Green AJ. Loss of heterozygosity in tuberous sclerosis hamartomas. J Med Genet. 1996;33:962–964. doi: 10.1136/jmg.33.11.962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Henske EP, Scheithauer BW, Short MP, Wollmann R, Nahmias J, Hornigold N, van Slegtenhorst M, Welsh CT, Kwiatkowski DJ. Allelic loss is frequent in tuberous sclerosis kidney lesions but rare in brain lesions. Am J Hum Genet. 1996;59:400–406. [PMC free article] [PubMed] [Google Scholar]
- 7.Crino PB, Aronica E, Baltuch G, Nathanson KL. Biallelic TSC gene inactivation in tuberous sclerosis complex. Neurology. 2010;74:1716–1723. doi: 10.1212/WNL.0b013e3181e04325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sato A, Kasai S, Kobayashi T, Takamatsu Y, Hino O, Ikeda K, Mizuguchi M. Rapamycin reverses impaired social interaction in mouse models of tuberous sclerosis complex. Nat Commun. 2012;3:1292. doi: 10.1038/ncomms2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feliciano DM, Hartman NW, Lin TV, Bartley C, Kubera C, Hsieh L, Lafourcade C, O’Keefe RA, Bordey A. A circuitry and biochemical basis for tuberous sclerosis symptoms: from epilepsy to neurocognitive deficits. Int J Dev Neurosci. 2013:667–678. doi: 10.1016/j.ijdevneu.2013.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bartley CM, O’Keefe RA, Bordey A. FMRP S499 is phosphorylated independent of mTORC1-S6K1 activity. PLoS ONE. 2014;9:e96956. doi: 10.1371/journal.pone.0096956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chevere-Torres I, Maki JM, Santini E, Klann E. Impaired social interactions and motor learning skills in tuberous sclerosis complex model mice expressing a dominant/negative form of tuberin. Neurobiol Dis. 2012;45:156–164. doi: 10.1016/j.nbd.2011.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nie D, Di NA, Han JM, Baharanyi H, Kramvis I, Huynh T, Dabora S, Codeluppi S, Pandolfi PP, Pasquale EB, Sahin M. Tsc2-Rheb signaling regulates EphA-mediated axon guidance. Nat Neurosci. 2010;13:163–172. doi: 10.1038/nn.2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goorden SM, van Woerden GM, van der WL, Cheadle JP, Elgersma Y. Cognitive deficits in Tsc1+/− mice in the absence of cerebral lesions and seizures. Ann Neurol. 2007;62:648–655. doi: 10.1002/ana.21317. [DOI] [PubMed] [Google Scholar]
- 14.Jan YN, Jan LY. Branching out: mechanisms of dendritic arborization. Nat Rev Neurosci. 2010;11:316–328. doi: 10.1038/nrn2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaufmann WE, Moser HW. Dendritic anomalies in disorders associated with mental retardation. Cereb Cortex. 2000;10:981–991. doi: 10.1093/cercor/10.10.981. [DOI] [PubMed] [Google Scholar]
- 16.Dierssen M, Ramakers GJ. Dendritic pathology in mental retardation: from molecular genetics to neurobiology. Genes Brain Behav. 2006;5(Suppl 2):48–60. doi: 10.1111/j.1601-183X.2006.00224.x. [DOI] [PubMed] [Google Scholar]
- 17.Nestor MW, Hoffman DA. Aberrant dendritic excitability: a common pathophysiology in CNS disorders affecting memory? Mol Neurobiol. 2012;45:478–487. doi: 10.1007/s12035-012-8265-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang L, Bartley CM, Gong X, Hsieh LS, Lin TV, Feliciano DM, Bordey A. MEK-ERK1/2-Dependent FLNA Overexpression Promotes Abnormal Dendritic Patterning in Tuberous Sclerosis Independent of mTOR. Neuron. 2014;84:78–91. doi: 10.1016/j.neuron.2014.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lacar B, Young SZ, Platel JC, Bordey A. Imaging and recording subventricular zone progenitor cells in live tissue of postnatal mice. Front Neurosci. 2010;4 doi: 10.3389/fnins.2010.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nakamura F, Stossel TP, Hartwig JH. The filamins: organizers of cell structure and function. Cell Adh Migr. 2011;5:160–169. doi: 10.4161/cam.5.2.14401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Feliciano DM, Su T, Lopez J, Platel JC, Bordey A. Single-cell Tsc1 knockout during corticogenesis generates tuber-like lesions and reduces seizure threshold in mice. J Clin Invest. 2011;121:1596–1607. doi: 10.1172/JCI44909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Feliciano DM, Lafourcade CA, Bordey A. Neonatal subventricular zone electroporation. J Vis Exp. 2013 doi: 10.3791/50197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Platel JC, Dave KA, Gordon V, Lacar B, Rubio ME, Bordey A. NMDA receptors activated by subventricular zone astrocytic glutamate are critical for neuroblast survival prior to entering a synaptic network. Neuron. 2010;65:859–872. doi: 10.1016/j.neuron.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pathania M, Torres-Reveron J, Yan L, Kimura T, Lin TV, Gordon V, Teng ZQ, Zhao X, Fulga TA, Van VD, Bordey A. miR-132 enhances dendritic morphogenesis, spine density, synaptic integration, and survival of newborn olfactory bulb neurons. PLoS ONE. 2012;7:e38174. doi: 10.1371/journal.pone.0038174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Feng Y, Walsh CA. The many faces of filamin: a versatile molecular scaffold for cell motility and signalling. Nat Cell Biol. 2004;6:1034–1038. doi: 10.1038/ncb1104-1034. [DOI] [PubMed] [Google Scholar]
- 26.Sarkisian MR, Bartley CM, Rakic P. Trouble making the first move: interpreting arrested neuronal migration in the cerebral cortex. Trends Neurosci. 2008;31:54–61. doi: 10.1016/j.tins.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 27.Segura I, Lange C, Knevels E, Moskalyuk A, Pulizzi R, Eelen G, Chaze T, Tudor C, Boulegue C, Holt M, Daelemans D, Matondo M, Ghesquiere B, Giugliano M, Ruiz de AC, Dewerchin M, Carmeliet P. The Oxygen Sensor PHD2 Controls Dendritic Spines and Synapses via Modification of Filamin A. Cell Rep. 2016;14:2653–2667. doi: 10.1016/j.celrep.2016.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Penzes P, Cahill ME, Jones KA, VanLeeuwen JE, Woolfrey KM. Dendritic spine pathology in neuropsychiatric disorders. Nat Neurosci. 2011;14:285–293. doi: 10.1038/nn.2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Savoy RM, Ghosh PM. The dual role of filamin A in cancer: can’t live with (too much of) it can’t live without it. Endocr Relat Cancer. 2013;20:R341–R356. doi: 10.1530/ERC-13-0364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jentarra GM, Rice SG, Olfers S, Saffen D, Narayanan V. Evidence for population variation in TSC1 and TSC2 gene expression. BMC Med Genet. 2011;12:29. doi: 10.1186/1471-2350-12-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gilman SR, Chang J, Xu B, Bawa TS, Gogos JA, Karayiorgou M, Vitkup D. Diverse types of genetic variation converge on functional gene networks involved in schizophrenia. Nat Neurosci. 2012;15:1723–1728. doi: 10.1038/nn.3261. [DOI] [PMC free article] [PubMed] [Google Scholar]