Abstract
Background and Purpose
Current diagnosis of fetal posterior fossa anomalies by sonography and conventional MRI is limited by fetal position, motion, and by 2D, rather than 3D, representation. In this study, we aimed to validate the use of a novel MRI technique, 3D Super-Resolution Motion-Corrected MRI, to image the fetal posterior fossa.
Methods
From a database of pregnant women who received fetal MRIs at our institution, images of 49 normal fetal brains were reconstructed. Six measurements of the cerebellum, vermis, and pons were obtained for all cases on 2D conventional and 3D reconstructed MRI, and the agreement between the two methods was determined using concordance correlation coefficients. Concordance of axial and coronal measurements of the transcerebellar diameter was also assessed within each method.
Results
Between the two methods, the concordance of measurements was high for all six structures, (p<0.001), and was highest for larger structures such as the transcerebellar diameter. Within each method, agreement of axial and coronal measurements of the transcerebellar diameter was superior in 3D reconstructed MRI compared to 2D conventional MRI (p<0.001).
Conclusions
This comparison study validates the use of 3D Super-Resolution Motion-Corrected MRI for imaging the fetal posterior fossa, as this technique results in linear measurements that have high concordance with 2D conventional MRI measurements. Lengths of the transcerebellar diameter measured within a 3D reconstruction are more concordant between imaging planes, as they correct for fetal motion and orthogonal slice acquisition. This technique will facilitate further study of fetal abnormalities of the posterior fossa.
Keywords: fetal MRI, posterior fossa, cerebellum, vermis, 3D reconstruction, super resolution
Introduction
Despite remarkable gains in prenatal care, congenital neurologic syndromes remain a major cause of mortality and lifelong morbidity for thousands of children in the United States.1 The striking plasticity of the developing central nervous system makes early detection of brain malformations challenging but essential in order to advance our understanding and management of these conditions. The tools used at present, ultrasonography and conventional fetal MRI, have constraints in imaging the fetus in certain positions, in correcting for motion, and in generating a 3D representation of the fetal brain. As such, prenatal diagnoses of brain anomalies are primarily qualitative and subject to inter-expert variations in opinion.
As fetal MRI technology emerged during the late 1990s and early 2000s, there was hope that it could replace early postnatal MRI for diagnosing fetal anomalies.2 However, given inherent limitations in imaging very small structures, some posterior fossa anomalies, such as inferior vermian hypoplasia, can be misdiagnosed.3, 4 Furthermore, long-term neurodevelopmental outcomes for small or isolated posterior fossa lesions have not been rigorously studied, and are therefore less clearly characterized.5-8 As a result, parents and families often endure a significant burden of anxiety and prolonged waiting time when a prenatal diagnosis is uncertain or when the neurodevelopmental outcome is unclear.
In cases where a prenatal diagnosis is either very serious or unclear, a second MRI is nearly always performed after birth, as postnatal MRI has higher resolution and better motion control compared to prenatal MRI. Thus, postnatal MRI remains the “gold standard” technique for diagnosing many congenital brain anomalies, even those seen during early fetal development. Establishing more reliable and accurate fetal imaging biometrics in early to mid-gestation, paired with more rigorous long-term neurodevelopmental outcome studies, could significantly improve both counseling and management of these conditions.
Quantitative MRI methods, such as linear and volumetric measurements, are gaining clinical utility as imaging technology advances. Measuring the diameter of the lateral ventricle at the level of the atria has long been the preferred method to diagnose fetal ventriculomegaly.9 However, 3D biometrics, such as volume of the fetal ventricles,10 orbits,11 and posterior fossa12 are emerging as more sophisticated techniques. Standardizing the lengths and volumes of fetal structures for clinical use has the capacity to change subjective interpretation of fetal MRI into a more rigorous quantitative analysis, leading to more accurate diagnoses and, hopefully, to improved management of congenital anomalies.
In this study, we aimed to refine the use of a novel reconstruction technique, 3D Super-Resolution Motion-Corrected MRI,13 to improve the diagnostic power of imaging the fetal posterior fossa. This method has the ability to both correct for fetal motion and improve the resolution of the images by reconstructing them into 3D space, allowing for re-alignment and re-slicing in any plane (Figure 1). It enables a shift from the limited qualitative assessment of 2D fetal snapshots to a fully 3D biometric assessment that is closer to what can be accomplished in postnatal imaging. Compared to the early works on volumetric reconstruction of fetal brain MRI,14, 15 which relied on scattered data interpolation for image reconstruction, 3D Super-Resolution Motion-Corrected MRI is based on a generative model of slice acquisition process which leads to an inverse problem that is solved for 3D image reconstruction. This technique allows robust estimation for systematic rejection of motion-corrupted slices and has shown superior performance compared to the conventional techniques based on scattered data interpolation.13
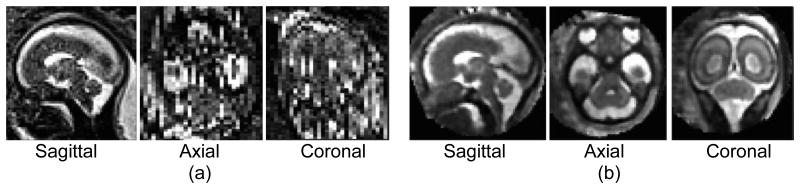
Figure 1. 2D Conventional MRI compared to 3D Super-Resolution Motion-Corrected MRI.
Three planar views of (a) an original 2D fetal MRI acquired in the sagittal plane - spatial resolution: 1.2×1.2×2 mm3, and (b) the reconstructed 3D super-resolution motion-corrected MRI with an isotropic spatial resolution of 1×1×1 mm3. The out-of-plane views in image (a) are disrupted by inter-slice fetal motion and limited resolution. The posterior fossa is much better visualized with coherent anatomic boundaries in all planes in the 3D reconstructed image (b).
While fetal brain MRI reconstruction has been used in several recent studies on fetal brain growth,16-25 there is concern in the clinical community that MRI reconstruction introduces error in the final image due to distortion or smoothing of the fetal structures, and thus reconstruction from scans with moderate to severe fetal motion may not be accurate representations of true fetal anatomy. If a 3D reconstruction preserves the original dimensions of the primary image, then linear measurements of the same structure should be nearly identical between original and reconstructed images.
Another issue is that in general, measurements of spheroidal structures, such as the cerebellum, pons, thalamus, and orbits, are unreliable when acquired in different planes via 2D fetal MRI. Due to the orientation of the cerebellum, its long diameter should measure the same if assessed in either axial or coronal planes. During 2D conventional fetal MRI acquisition, orthogonal planes are acquired in two sequential sequences that are hand-aligned, and are often slightly oblique to each other due to fetal motion between sequences. This has led to the inability to standardize certain fetal measurements.22, 26 As an example, in the case of suspected cerebellar hypoplasia, should the fetal transcerebellar diameter be measured in the axial plane, the coronal plane, or both? If the axial and coronal measurements do not agree, how does one choose the “correct” length? The final dimension often relies on the reader's clinical judgment as to which image is the “most” aligned to the proper axis of the fetus. A fully 3D image that can present image slices in perfectly orthogonal planes aligned to the correct axis of the fetus yields the ability to measure a structure in any plane (axial, coronal, sagittal) with high confidence that for spheroidal structures like the cerebellum, orthogonal measurements should be highly concordant. An extension of this hypothesis is that since the 3D reconstruction re-aligns the fetal brain into the correct orthogonal space, both linear and volumetric measurements should be highly accurate using this technology.
Standardized measurements of fetal posterior fossa structures throughout gestation could lead to improved diagnosis of abnormal growth patterns or structural anomalies. Furthermore, eventual development of more sophisticated volumetric measures of this region may lead to an improved ability to detect and diagnose anomalies earlier in gestation. To validate this technique for analyzing the fetal posterior fossa, we hypothesized that 3D Super-Resolution Motion-Corrected MRI preserves correct dimensions of the fetal posterior fossa, and that linear measurements of 2D conventional and 3D reconstructed MR images will be concordant. We further hypothesize that orthogonal (axial and coronal) measurements of the transcerebellar diameter are more concordant when measured in 3D reconstructed MRI, compared to 2D conventional MRI.
Methods
Subject Enrollment
Pregnant women were enrolled as participants as part of a research MRI study protocol at our institution, approved by the institutional review board (IRB) committee. Participants were either healthy volunteers recruited from within our institution, or were referred for a concerning finding on ultrasound but ultimately found to have normal fetal anatomy on MRI. All participants were consented, entered into a secure RedCap database, and underwent a research fetal MRI, which was read by a clinical neuroradiologist. We searched the database for all normal fetal MRI studies performed from March 2013 to September 2014. All fetal MRIs with abnormal reads were excluded. We excluded a small number of early scans done at 1.5T to minimize variations in measurements that might be due to image resolution and quality. A total of 62 fetuses ranging from 19 to 38 weeks gestation met criteria for inclusion.
Image Collection and Reconstruction
Fetal MRI was performed without sedation or breath hold on a Siemens Skyra 3T scanner,22, 27 and involved repeated acquisitions of T2-weighted single shot fast spin echo (SSFSE) and TruFISP (True fast imaging with steady state precession) sequences in multiple planes. The imaging data was de-identified, uploaded to a secure server, and converted into 3D Super-Resolution Motion-Corrected reconstructed images using previously validated tools.13 For successful reconstruction, it was necessary to acquire at least 2 sequences of the same type encompassing the entire fetal head, ideally in orthogonal planes. Successful 3D reconstructions were then transformed into Talaraich space using the free software program MIPAV.28 Copies of the de-identified original 2D MR images were also saved on the secure server for direct comparison of measurements.
Measurements
The measurements were performed by the same researcher (DBP), who was trained by an experienced fetal radiologist (JAE) to properly measure 6 linear structures of the fetal posterior fossa. These structures included the transcerebellar diameter in the coronal (cTCD) and axial (aTCD) planes, the vermis height (VH), the vermis anterior-posterior diameter (VAP), the pons height (PH), and the anterior-posterior diameter of the pons (APDP). These six structures were measured on both 2D and 3D images according to standard techniques described in the literature29-32 and illustrated in Figure 2. Measurements were taken in both 2D conventional images and 3D reconstructed images using the free open-source software program OsiriX (Mac version 6.5.2, 32-bit).33 To reduce inter-measurement variability, all measurements were performed in triplicate on the same image using the OsiriX linear measurement tool, and were then averaged for a final assigned length. Measurements of the same fetus for the 2D conventional and 3D reconstructed images were performed on different days with at least 24 hours between them to minimize measurement and recall bias. Gestational ages were recorded in the RedCap database but were not taken into account during the measurement process.
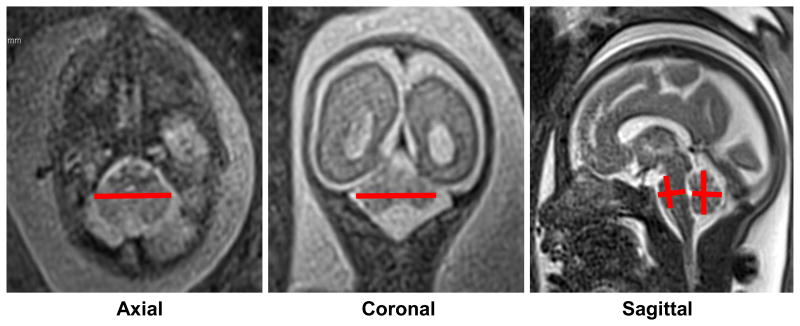
Figure 2. Linear MRI measurements of the fetal posterior fossa.
Examples of measurements taken of the six posterior fossa structures from 2D MR images. Axial and coronal transcerebellar diameter measurements were taken at the point of maximal length (left and middle panel). As is evident in the middle panel, it is common to obtain a slightly out of plane view of the cerebellum in one or more planes, which can introduce error into linear measurements. Vermis and pons heights and anterior-posterior diameters were achieved at the mid-sagittal plane at the level of the corpus callosum. Note that 3D measurements were achieved in an identical fashion.
Statistical Analysis
R software34 was used for all statistical analyses. We evaluated the agreement between 2D conventional MRI and 3D reconstructed MRI using the concordance correlation coefficients (CCC) method. The CCC of each of the six structures was individually computed using the variance components procedure. The measurement model in this approach included a random effect for subject, a fixed effect for the method used (2D vs. 3D), and a random interaction term for the subject and method. The variance components for the model were estimated using restricted maximum likelihood.35 The agreement of axial and coronal measurements of the transcerebellar diameter within each method was then computed for all cases using the same technique as above.
Results
49 of the 62 (79%) qualifying normal fetal MRIs were successfully reconstructed using the 3D Super-Resolution Motion-Corrected technique. Of the 13 scans that were unable to be reconstructed, 9 cases had severe fetal motion such that no more than one single sequence encompassed the entire fetal head, and 4 cases had low signal-to-noise ratio and severe intensity non-uniformity artifacts due to radiofrequency penetration and standing waves as well as B1 field inhomogeneity artifacts,22 for which the reconstruction algorithm could not correct.
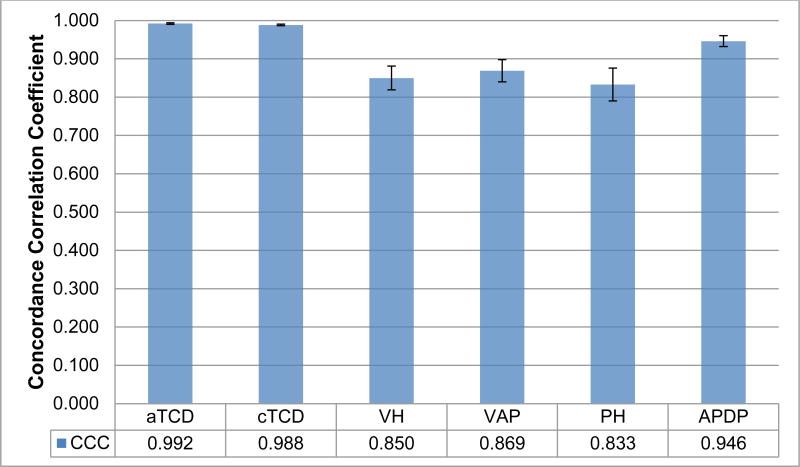
In the 49 successfully reconstructed cases, measurements within the same fetal structure between the two sets of images had high concordance correlation coefficients (p<0.001, Figure 3), indicating excellent agreement. As noted in Figure 3, the largest structures measured, (transcerebellar diameter), had extremely high concordance (CCC>0.9). Smaller structures, (pons and vermis), while slightly less concordant than larger structures, still had high concordance (CCC>0.8).
Figure 3. Concordance Correlation Coefficients (CCC) between 2D conventional and 3D reconstructed MRI measurements of 6 posterior fossa structures.
CCC = concordance correlation coefficient. aTCD = axial transcerebellar diameter. cTCD = coronal transcerebellar diameter. VH = vermis height. VAP = vermis AP diameter. PH = pons height. APDP = anterior-posterior diameter of pons. Measurements for all six structures had high concordance between the two imaging types (2D vs. 3D). Error bars reflect the standard error for each structure's CCC (ranging from 0.002 to 0.043). These estimates are highly significant (p<0.001).
Finally, the measurements of the transcerebellar diameter in axial and coronal planes had higher concordance in 3D reconstructed images as compared to 2D conventional images, (CCC=0.999 vs. 0.989, SE=0.0004 vs. 0.0029; p<0.001) (Table 1, Figure 4). Thus, measurements of the same fetal structure between orthogonal planes are more concordant within a 3D reconstructed image.
Table 1. Agreement between Axial and Coronal Transcerebellar Diameter in 2D conventional and 3D reconstructed MRI.
| MRI method | CCC | SE | 95% Confidence Interval |
|---|---|---|---|
| 2D conventional | 0.989 | 0.0029 | 0.981 - 0.993 |
| 3D reconstructed | 0.999 | 0.0004 | 0.998 - 0.999 |
CCC = concordance correlation coefficient. SE = standard error.
3D reconstructed MRI had higher CCC between axial and coronal transcerebellar diameters than 2D conventional MRI (Z score = 5.676, p<0.001). The p-value corresponds to the null hypothesis that CCC between axial and coronal measurements is the same in 2D conventional and 3D reconstructed MRI. Note that 3D reconstructed MRI had a smaller standard error than 2D conventional MRI, and the 95% confidence intervals do not overlap.
Figure 4. Comparison of Axial and Coronal TCD measurements in 2D conventional and 3D reconstructed MRI.
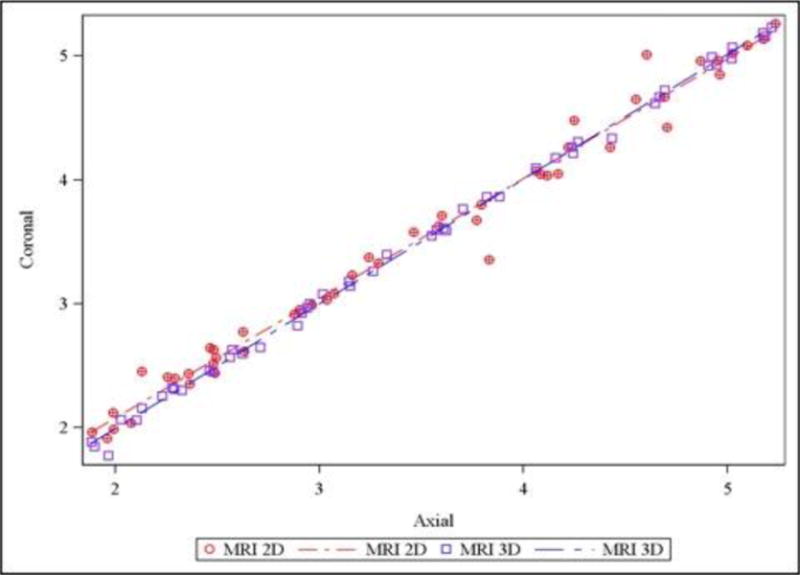
Agreement between axial and coronal transcerebellar diameter was superior in 3D reconstructed MRI (purple), compared to 2D conventional MRI (red; p<0.001). Note that the 3D MRI measurements fall closer to the agreement line as compared to the 2D MRI measurements.
Discussion
This study validates the use of 3D Super-Resolution Motion-Corrected MRI for imaging the fetal posterior fossa. Our results show that this technique yields high quality 3D images, with linear measurements of the posterior fossa that significantly agree with 2D conventional measurements. From this, we conclude that the 3D reconstruction process does not distort the proportions of the fetal posterior fossa, and the final 3D images are accurate representations of the original fetal dimensions.
Whereas measurements of the transcerebellar diameter within each image modality are overall highly concordant in this study, 3D reconstructed MRI is superior to 2D conventional MRI when comparing the transcerebellar diameter in axial and coronal planes (Table 1). Given the significant plasticity of the developing fetal brain, even the small differences observed in this study may have significant clinical implications for long term neurodevelopment. The scans chosen for this study were from normal volunteers with healthy fetuses, and the 2D conventional scans were close to the ideal scenario with respect to the ability to obtain orthogonal slices and accurate measurements. In clinical circumstances, during which a pregnant woman is likely to be anxious or have difficulty lying still, the posterior fossa often cannot be ideally imaged in all planes, which forces the radiologist to choose the best subjective sequence for measurements. Thus, the small improvement in agreement between planes shown in this study may produce a significant improvement in clinical practice.
The current study has several limitations. Reconstruction of 3D fetal MRI from 2D images was not successful in cases of excessive fetal motion, or when the pregnant subject could not tolerate a full study. The current rate of success for reconstruction for healthy volunteers in our cohort was 79%, but as this technology continues to be refined, we predict that the reconstruction success rate will increase.
Another limitation of this study arises due to the constraints of linear measurements on a 3D image. Ideally one would compare the volumes of structures, rather than just lengths, to ensure their overall dimensions were preserved after a reconstruction process. Whereas 3D images yield the ability to obtain automatic volumes, conventional 2D measurements in fetuses can only be done with laborious hand tracing. As hand tracing often introduces measurement errors, we limited this study to comparing 2D (linear) dimensions.
Finally, it is important to note that a gold standard to measure fetal brain structures is exceedingly difficult, as postmortem brain specimens of normal fetuses are remarkably rare, and often damaged or destroyed in the procurement/fixation process. We thus acknowledge that we were not able to compare our data to any gold-standard measurement; however, we have demonstrated that this technique is not inferior to 2D conventional MRI with respect to linear measurements, and is superior for measurements of the same structure in orthogonal planes. These findings pave the way for further studies using non-linear measurements only possible in 3D space, which will have important future clinical applications.
The 3D Super-Resolution Motion-Corrected MRI has several advantages. This technique is only modestly expensive from a computational standpoint, and can be implemented on parallel computing hardware. With optimal implementation on appropriate hardware it would be possible to achieve super-resolution reconstruction in near real time. The adoption of super-resolution reconstruction in clinical practice would simply require a focus of resources on the logistics of applying the reconstruction techniques to increase the convenience for the potential consumers of such reports. This technology also requires minimal added cost or use of resources, and has the potential to improve both prenatal counseling and postnatal outcomes, as improved quantitative evaluation may lead to better prognostication and planning. It may also direct decisions regarding perinatal care, such as delivery at a hospital with Level 3 NICU as opposed to a community hospital. Finally, reducing or obviating the immediate need for confirmatory imaging after birth may lead to cost savings in the future, and reduce the burden of transferring critically ill newborns from their NICU bed to the imaging facility. This 3D technique has many exciting potential applications going forward, including the use of automated segmentation and volumetric assessment of fetal brain structures, which are likely to have direct clinical applications for this technology.
In showing that the reconstruction process does not alter the proportions of the final 3D images, we conclude that using data from 3D reconstructions will yield accurate representations of in vivo volumes. This technique may be particularly useful for future applications in analyzing abnormal fetal structures, particularly when a structure appears abnormal in only one plane on conventional fetal MRI. Our future research will involve using this technique on abnormal fetal posterior fossa structures to evaluate whether diagnostic accuracy is improved in fetal life when compared to confirmatory postnatal MRI. Ultimately, this volumetric data, paired with more rigorous long-term neurodevelopmental follow-up, could significantly improve our prenatal counseling and management of patients with prenatally diagnosed posterior fossa anomalies.
Acknowledgments
This research was supported in part by National Institutes of Health (NIH) grants R25 3R25NS070682, R01 EB013248, and R01 EB018988.
Footnotes
Disclosures: The authors have no financial conflicts of interests.
References
- 1.Parker SE, Mai CT, Canfield MA, et al. Updated National Birth Prevalence estimates for selected birth defects in the United States, 2004-2006. Birth Defects Res A Clin Mol Teratol. 2010;88:1008–16. doi: 10.1002/bdra.20735. [DOI] [PubMed] [Google Scholar]
- 2.Blaicher W, Bernaschek G, Deutinger J, Messerschmidt A, Schindler E, Prayer D. Fetal and early postnatal magnetic resonance imaging--is there a difference? J Perinat Med. 2004;32:53–7. doi: 10.1515/JPM.2004.009. [DOI] [PubMed] [Google Scholar]
- 3.Limperopoulos C, Robertson RL, Estroff JA, et al. Diagnosis of inferior vermian hypoplasia by fetal magnetic resonance imaging: potential pitfalls and neurodevelopmental outcome. Am J Obstet Gynecol. 2006;194:1070–6. doi: 10.1016/j.ajog.2005.10.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pinto J, Paladini D, Severino M, et al. Delayed rotation of the cerebellar vermis: an important pitfall in early second trimester fetal MR imaging studies. Ultrasound Obstet Gynecol. 2015 doi: 10.1002/uog.15782. [DOI] [PubMed] [Google Scholar]
- 5.Adamsbaum C, Moutard ML, Andre C, et al. MRI of the fetal posterior fossa. Pediatr Radiol. 2005;35:124–40. doi: 10.1007/s00247-004-1316-3. [DOI] [PubMed] [Google Scholar]
- 6.Bolduc ME, Limperopoulos C. Neurodevelopmental outcomes in children with cerebellar malformations: a systematic review. Dev Med Child Neurol. 2009;51:256–67. doi: 10.1111/j.1469-8749.2008.03224.x. [DOI] [PubMed] [Google Scholar]
- 7.D'Antonio F, Khalil A, Garel C, et al. Systematic Review and Meta-Analysis of Isolated Posterior Fossa Malformations on Prenatal Ultrasound: Nomenclature, Diagnostic Accuracy and Associated Anomalies. Ultrasound Obstet Gynecol. 2015 doi: 10.1002/uog.14900. [DOI] [PubMed] [Google Scholar]
- 8.D'Antonio F, Khalil A, Garel C, et al. Systematic review and meta-analysis of isolated posterior fossa malformations on prenatal imaging (part 2): neurodevelopmental outcome. Ultrasound Obstet Gynecol. 2015 doi: 10.1002/uog.15755. [DOI] [PubMed] [Google Scholar]
- 9.Pilu G, Reece EA, Goldstein I, Hobbins JC, Bovicelli L. Sonographic evaluation of the normal developmental anatomy of the fetal cerebral ventricles: II. The atria Obstet Gynecol. 1989;73:250–6. [PubMed] [Google Scholar]
- 10.Gholipour A, Akhondi-Asl A, Estroff JA, Warfield SK. Multi-atlas multi-shape segmentation of fetal brain MRI for volumetric and morphometric analysis of ventriculomegaly. Neuroimage. 2012;60:1819–31. doi: 10.1016/j.neuroimage.2012.01.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Velasco-Annis C, Gholipour A, Afacan O, Prabhu SP, Estroff JA, Warfield SK. Normative biometrics for fetal ocular growth using volumetric MRI reconstruction. Prenat Diagn. 2015;35:400–8. doi: 10.1002/pd.4558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vatansever D, Kyriakopoulou V, Allsop JM, et al. Multidimensional analysis of fetal posterior fossa in health and disease. Cerebellum. 2013;12:632–44. doi: 10.1007/s12311-013-0470-2. [DOI] [PubMed] [Google Scholar]
- 13.Gholipour A, Estroff JA, Warfield SK. Robust super-resolution volume reconstruction from slice acquisitions: application to fetal brain MRI. IEEE Trans Med Imaging. 2010;29:1739–58. doi: 10.1109/TMI.2010.2051680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rousseau F, Glenn OA, Iordanova B, et al. Registration-based approach for reconstruction of high-resolution in utero fetal MR brain images. Acad Radiol. 2006;13:1072–81. doi: 10.1016/j.acra.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 15.Jiang S, Xue H, Glover A, Rutherford M, Rueckert D, Hajnal JV. MRI of moving subjects using multislice snapshot images with volume reconstruction (SVR): application to fetal, neonatal, and adult brain studies. IEEE Trans Med Imaging. 2007;26:967–80. doi: 10.1109/TMI.2007.895456. [DOI] [PubMed] [Google Scholar]
- 16.Kim K, Habas PA, Rousseau F, Glenn OA, Barkovich AJ, Studholme C. Intersection based motion correction of multislice MRI for 3-D in utero fetal brain image formation. IEEE Trans Med Imaging. 2010;29:146–58. doi: 10.1109/TMI.2009.2030679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuklisova-Murgasova M, Quaghebeur G, Rutherford MA, Hajnal JV, Schnabel JA. Reconstruction of fetal brain MRI with intensity matching and complete outlier removal. Med Image Anal. 2012;16:1550–64. doi: 10.1016/j.media.2012.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clouchoux C, Kudelski D, Gholipour A, et al. Quantitative in vivo MRI measurement of cortical development in the fetus. Brain Struct Funct. 2012;217:127–39. doi: 10.1007/s00429-011-0325-x. [DOI] [PubMed] [Google Scholar]
- 19.Scott JA, Habas PA, Rajagopalan V, et al. Volumetric and surface-based 3D MRI analyses of fetal isolated mild ventriculomegaly: brain morphometry in ventriculomegaly. Brain Struct Funct. 2013;218:645–55. doi: 10.1007/s00429-012-0418-1. [DOI] [PubMed] [Google Scholar]
- 20.Studholme C. Mapping the developing human brain in utero using quantitative MR imaging techniques. Semin Perinatol. 2015;39:105–12. doi: 10.1053/j.semperi.2015.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tourbier S, Bresson X, Hagmann P, Thiran JP, Meuli R, Cuadra MB. An efficient total variation algorithm for super-resolution in fetal brain MRI with adaptive regularization. Neuroimage. 2015;118:584–97. doi: 10.1016/j.neuroimage.2015.06.018. [DOI] [PubMed] [Google Scholar]
- 22.Gholipour A, Estroff JA, Barnewolt CE, et al. Fetal MRI: A Technical Update with Educational Aspirations. Concepts Magn Reson Part A Bridg Educ Res. 2014;43:237–66. doi: 10.1002/cmr.a.21321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Habas PA, Kim K, Corbett-Detig JM, et al. A spatiotemporal atlas of MR intensity, tissue probability and shape of the fetal brain with application to segmentation. Neuroimage. 2010;53:460–70. doi: 10.1016/j.neuroimage.2010.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Limperopoulos C, Clouchoux C. Advancing fetal brain MRI: targets for the future. Semin Perinatol. 2009;33:289–98. doi: 10.1053/j.semperi.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 25.Dittrich E, Kasprian G, Prayer D, Langs G. Atlas learning in fetal brain development. Top Magn Reson Imaging. 2011;22:107–11. doi: 10.1097/RMR.0b013e318267fe94. [DOI] [PubMed] [Google Scholar]
- 26.Senapati GM, Levine D, Smith C, et al. Frequency and cause of disagreements in imaging diagnosis in children with ventriculomegaly diagnosed prenatally. Ultrasound Obstet Gynecol. 2010;36:582–95. doi: 10.1002/uog.7680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Victoria T, Johnson AM, Edgar JC, Zarnow DM, Vossough A, Jaramillo D. Comparison Between 1.5-T and 3-T MRI for Fetal Imaging: Is There an Advantage to Imaging With a Higher Field Strength? AJR Am J Roentgenol. 2016;206:195–201. doi: 10.2214/AJR.14.14205. [DOI] [PubMed] [Google Scholar]
- 28.Bazin PL, Cuzzocreo JL, Yassa MA, et al. Volumetric neuroimage analysis extensions for the MIPAV software package. J Neurosci Methods. 2007;165:111–21. doi: 10.1016/j.jneumeth.2007.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Garel C. Fetal cerebral biometry: normal parenchymal findings and ventricular size. Eur Radiol. 2005;15:809–13. doi: 10.1007/s00330-004-2610-z. [DOI] [PubMed] [Google Scholar]
- 30.Triulzi F, Parazzini C, Righini A. MRI of fetal and neonatal cerebellar development. Semin Fetal Neonatal Med. 2005;10:411–20. doi: 10.1016/j.siny.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 31.Tilea B, Alberti C, Adamsbaum C, et al. Cerebral biometry in fetal magnetic resonance imaging: new reference data. Ultrasound Obstet Gynecol. 2009;33:173–81. doi: 10.1002/uog.6276. [DOI] [PubMed] [Google Scholar]
- 32.Sanz-Cortes M, Egana-Ugrinovic G, Zupan R, Figueras F, Gratacos E. Brainstem and cerebellar differences and their association with neurobehavior in term small-for-gestational-age fetuses assessed by fetal MRI. Am J Obstet Gynecol. 2014;210:452e1–8. doi: 10.1016/j.ajog.2013.12.008. [DOI] [PubMed] [Google Scholar]
- 33.Rosset A, Spadola L, Ratib O. OsiriX: an open-source software for navigating in multidimensional DICOM images. J Digit Imaging. 2004;17:205–16. doi: 10.1007/s10278-004-1014-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.R Development Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2008. http://www.R-project.org. [Google Scholar]
- 35.Carrasco JL, Jover L. Estimating the generalized concordance correlation coefficient through variance components. Biometrics. 2003;59:849–58. doi: 10.1111/j.0006-341x.2003.00099.x. [DOI] [PubMed] [Google Scholar]