Abstract
The Hexosamine Biosynthetic Pathway (HBP) is highly dependent on multiple metabolic nutrients including glucose, glutamine and acetyl-CoA. Increased flux through HBP leads to elevated post-translational addition of β-D-N-acetylglucosamine sugars to nuclear and cytoplasmic proteins. Increased total O-GlcNAcylation is emerging as a general characteristic of cancer cells and recent studies suggest that O-GlcNAcylation is a central communicator of nutritional status to control key signaling and metabolic pathways that regulate multiple cancer cell phenotypes. This review summarizes our current understanding of changes of O-GlcNAc cycling enzymes in cancer, the role of O-GlcNAcylation in tumorigenesis and the current challenges in targeting this pathway therapeutically.
Keywords: glycosylation, signaling, hexosamine biosynthetic pathway, O-GlcNAcylation, OGT, cancer, metabolism, invasion, metastasis, angiogenesis, epithelial, ER stress, HIF-1α
Graphical abstract
Introduction
Linking Cellular Signaling Pathways to Metabolic Rewiring in Cancer
Cancer cells universally promote glycolysis in the presence of normal oxygen conditions 1,2. This altered metabolic state, known as the Warburg effect, allows cancer cells to take up a large share of nutrients from their environment (i.e., glucose and glutamine); a phenomenon that is widely used in vivo to monitor tumor growth using 2FDG-PET imaging 1,2. As a byproduct of the inefficient use of these nutrients, low amounts of ATP are produced, and sizeable amounts of carbon and nitrogen in the form of lactate and ammonia are secreted into the extracellular environment. These can then be absorbed and used by neighboring tumor cells as fuel. Although this may seem as an inefficient means of producing energy, aerobic glycolysis maintains a steady quantity of carbon backbones for macromolecular synthesis in the form of acetyl-CoA, NADPH and amino acids 1,3. This carbon skeleton then fuels the increase in biomass that is required for tumor cell growth and proliferation, while maintaining a redox balance through production of high amounts of NADPH 1,3. However, the understanding of how growth and proliferation factors work in concert with metabolic pathways is only now being elucidated.
Otto Warburg was the first to report that cancer cells engage in the production of lactate even in the presence of normal oxygen conditions 4, generated through an altered metabolic state that is now believed to be controlled largely by oncogenic activation of signal transduction pathways and transcription factors and/or loss of tumor suppressors 5,6,7,8. The phosphatidylinositol-3-kinase (PI3K)/Protein kinase B (or AKT)/mechanistic target of rapamycin (mTOR) is one such pathway (Figure 1) that lies downstream of receptor tyrosine kinase (RTK) activation and is known to be deregulated in a wide majority of cancer types 5,8,9. In normal cells, this ubiquitous system activates signaling in response to growth factor ligands. Ligand binding to receptor tyrosine kinases activates PI3K resulting in phosphorylation of phosphoinositol lipids at the plasma membrane which recruits and activates AKT. PI3K activity is tightly controlled by the phosphatase phosphatase and tensin homolog (PTEN), however many transformed cells contain inactivating mutations in PTEN 8. In response to changes in energy status, regulation of cell growth by AKT is accomplished through phosphorylation of mTOR complex 1 (mTORC1) inhibitors, tuberos sclerosis complex 1/2 (TSC1/2). For example, amino acid availability and upstream signaling activates mTORC1-dependent translation factors 4E-binding protein 1 (4E-BP1) and S6 kinase 1 (S6K1) to enhance protein synthesis of major metabolic regulators such as c-MYC, sterol regulatory element binding protein 1 (SREBP-1), and hypoxia inducible factor 1-alpha (HIF-1α) 5.
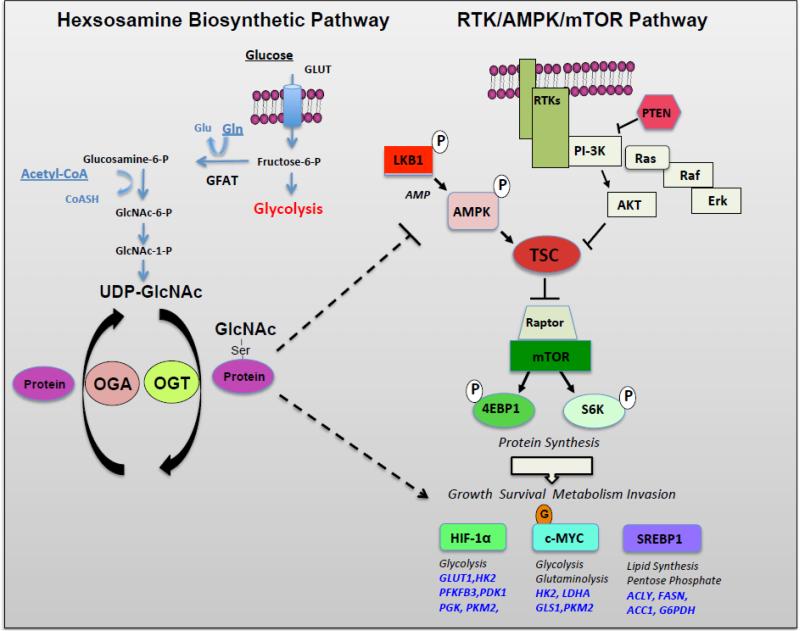
Figure 1. The Hexosamine Pathway Intersects with the Receptor Tyrosine Kinase/AMPK/mTOR Pathway.
In left panel, the hexosamine pathway controls O-GlcNAcylation of nuclear and cytoplasmic proteins. A small fraction (2-5%) of glucose enters the HBP and fructose-6-phosphate is converted to glucosamine-6-phosphate by the rate-limiting enzyme of this pathway glutamine:fructose-6- amidotransferase (GFAT). UDP-N-acetylglucosamine (UDP-GlcNAc) is generated in subsequent steps and used as substrate by O-GlcNAc transferase (OGT) to add GlcNAc to serine or threonine residues of nuclear or cytoplasmic target proteins. O-GlcNAc modifications can be removed from proteins by the glycoside hydrolase O-GlcNAcase (OGA). In right panel, the receptor tyrosine kinase (RTK)-mediated signaling pathways converge on mTOR to regulate key transcription factors involved in cancer metabolism including HIF-1α, c-MYC, and SREBP-1. LKB1/AMPK negatively regulate mTOR pathway and metabolism. OGT and O-GlcNAcylation can intersect with this pathway at level of AMPK and regulation of key metabolic transcription factors HIF-1α and c-Myc. Abbreviations: HK2, hexokinase, PFKFB3, 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase, isoform 3; PDK1, pyruvate dehydrogenase kinase, isozyme 1; PGK, phosphoglycerate kinase; PKM2, pyruvate kinase M2 isoform; LDHA, lactate dehydrogenase A; GLS1, glutaminae 1; ACLY, ATP citrate lyase; FASN, fatty acid synthase; ACC1, acetyl-CoA carboxylase 1.
The oncogenic transcription factor c-MYC is overexpressed in many cancer types, and supports glutamine uptake and metabolism, yielding the nitrogen required to produce nucleotides and amino acids. In addition, c-MYC dependent glutamate production feeds TCA cycle intermediates via an anaplerotic reaction to increase NADPH production, thus maintaining the redox balance in cancer cells 1,10. SREBP-1 also functions as a transcription factor downstream of mTORC1, to induce expression of genes involved in de novo fatty acid biosynthesis driving the production of membrane components required during cell proliferation 5. High SREBP-1 expression is associated with poor prognosis in a number of cancer types, and has been shown to sustain cancer cell growth and survival through lipogenic functions 11,12.
Many solid tumors outgrow blood supply reverting to a state of low oxygen tension, or hypoxia 13,14. Under low oxygen tension, hypoxia inducible factor, or HIF-1α, is stabilized and activates transcription of over 200 genes to promote glycolytic flux and survival – including glucose transporters, glycolytic enzymes and angiogenic factors 14. HIF-1 is a heterodimer consisting of the hypoxic transcription factor HIF-1α and the constitutively expressed HIF-1β also known as aryl hydrocarbon receptor nuclear translocator or ARNT 14. Independent of mTOR activity enhancing translation of HIF-1α, regulation at the protein level is a highly complex process 14. Under oxygen rich conditions, prolyl-hydroxylases use oxygen and α-ketoglutarate to hydroxylate HIF-1α at two proline residues (Pro402 and Pro564). This modification allows for HIF-1α to interact with the tumor suppressor Von Hippel-Lindau (pVHL) protein. This process is essential for HIF-1α ubiquitination and subsequent proteasomal degradation. Germline loss-of-function mutations in VHL are associated with the accumulation of HIF-1α in specific types of renal cancer 14. Under hypoxic conditions, however, prolyl hydroxylase activity is limited, thereby stabilizing HIF-1α, promoting nuclear translocation, and upregulating target gene expression and consequently promoting flux through glycolysis and enhancing glucose uptake (Figure 1) 13,14,15. Interestingly, low oxygen tension in tumors is associated with increased metastasis and decreased survival in breast and ovarian cancer patients, due to acquired resistance to radiation and chemotherapy 14,16. Cancer cells can retain HIF-1α activity even in environments where oxygen is abundant and undergo adaptive genetic changes to survive and proliferate in a hypoxic environment through several known mechanisms 14.
Downstream of RTK activation, the RAS/RAF/MAPK pathway (Figure 1) is another key regulator of normal cell growth as well as malignant transformation 8. Mutations in oncogenic Ras play a number of roles in stimulating glucose uptake and utilization to feed anabolic pathways, promoting uptake of lysophospholipids to supply intracellular lipid pools. More recently, is has been demonstrated that RAS also promotes scavenging of proteins via micropinocytosis to fuel amino acid pools 8,17,18.
Another major regulator of metabolism and energy homeostasis is the liver kinase B1 (LKB1)/AMP-activated protein kinase (AMPK) signaling axis through its ability to sense changes in nutrient supplies 19,20. Downstream of LKB1, the heterotrimer AMPK senses changes in the cellular AMP to ATP ratio 19,20. In response to low energy conditions, LKB1 phosphorylates AMPK to turn on pathways limiting energy consumption and promoting ATP production in an attempt to restore metabolic homeostasis 19,21. The AMPK catalytic subunit directly phosphorylates the TSC1/2 complex and mTORC1 scaffold protein RAPTOR to inhibit mTOR activity 19,22. Upstream of AMPK, the tumor suppressor LKB1 is critical for the activation of AMPK is response to nutrient limiting conditions. LKB1 loss in somatic cells is associated with the progression of a number of cancers 19. Due to its role in the inhibition of anabolic processes vital to cancer growth, the activation of AMPK may provide assistance in combating cancer growth.
One metabolic pathway supported by the above mentioned signaling networks is the hexosamine biosynthetic pathway (HBP) 23,24. Due to its reliance on inputs from so many metabolites and anabolic signaling required to produce these metabolites, as well as the HBPs regulation of cellular responses to nutrients, HBP is at the nexus of cellular metabolism 24. As outlined below, the final product of this pathway the amino sugar uridine diphosphate N-acetylglucosamine (UDP-GlcNAc) derives its carbon skeleton from glucose, glutamine, nucleotide biosynthesis and lipid metabolites (Figure 1) 24. UDP-GlcNAc is used for synthesis of secreted or membrane glycoproteins it also serves as a donor substrate for O-GlcNAc transferase (OGT)-mediated O-GlcNAcylation of a number of nuclear and cytoplasmic proteins. Although O-GlcNAcylation regulates many functions of cellular physiology 25, this review will focus on how this post-translational modification functions as a critical node between cellular metabolism and signaling pathways to regulate cancer biology.
Hexosamine Biosynthetic Pathway regulates metabolism, signaling and cancer
The capacity to sense nutrients in the cellular environment is a crucial evolutionary mechanism required for adaptation and survival 5. In proliferating cells, glucose and glutamine consumption are regulated via growth factor signaling to support cell growth and survival. However, cancer cells, independent of growth factor stimulation, can utilize glucose and glutamine-derived carbon sources to fuel ATP synthesis, and support cell growth 5. While the majority of glucose molecules that enter the cell are metabolized through glycolysis, 3-5% percent of the glucose is diverted from glycolysis into the HBP 24,26. The HBP diverges from glycolysis where the rate-limiting enzyme glutamine fructose-6-phosphate amidotransferase (GFAT) converts fructose-6-phosphate to produce glucosamine-6-phosphate. This nutrient sensing pathway incorporates glucose, glutamine, nucleotides and Acetyl-CoA to produce the terminal amino sugar UDP-GlcNAc 24,27,28. Moreover, salvage pathways can introduce GlcNAc into the HBP directly by bypassing GFAT 29. Glucosamine can also enter the cell via glucose transporters, and once phosphorylated by GlcNAc kinase, can eventually be converted into UDP-GlcNAc 21,24.
UDP-GlcNAc can be used as a substrate for GlcNAc-1-phosphotransferase to cotranslationally modify proteins in the ER via N-linked glycosylation 27,28. Further glycan branching modifications in the Golgi are required for proper folding, stability and transport of surface receptor proteins 23,24. Alternatively, UDP-GlcNAc can be covalently added onto serine and threonine residues of a wide range of cellular proteins solely through the action of the enzyme OGT 23,24. This modification can be removed by the glycoside hydrolase O-GlcNAcase (OGA) (also referred to as MGEA5) that catalyzes cleavage of O-GlcNAc from proteins 30. This dynamic O-GlcNAc modification or O-GlcNAcylation modifies a myriad of intracellular proteins including signaling molecules, transcription factors and metabolic enzymes 24; 25.
The HBP has an essential role in sustaining vital cellular processes. For example, Thompson and colleagues 21 demonstrated that glucose metabolism through HBP is required to sustain sufficient growth factor signaling and glutamine uptake to support cell growth & survival. Additionally, the HBP has been shown to regulate ER stress pathways to maintain protein homeostasis, enhance degradation of misfolded proteins and reduce pro-apoptotic signaling 31. In response to oncogene activation, cancer cells experience elevated flux through the HBP. In a transgenic model, oncogenic RAS increases flux through glycolysis and the HBP, resulting in increased O-GlcNAcylation. Interestingly, these RAS-driven tumors require GFAT expression for growth in vitro and in vivo 18. Additionally, hypoxic cancer cells have been shown to contain elevated levels of the HBP genes including Gfpt1 and Gfpt2 as well as overall O-GlcNAcylation 15, and inhibition of the HBP rate-limiting enzyme blocks pancreatic cancer cell survival under low oxygen conditions. These studies suggest that flux through HBP is critical for cancer cell growth and survival. However, they do not distinguish the role of O-GlcNAcylation from other glycosylation mediated by HBP. A number of studies have now established alterations in O-GlcNAcylation and key enzymes OGT and OGA in multiple cancers (Table I) providing a link between O-GlcNAcylation and cancer phenotypes.
Table 1.
Deregulation of O-GlcNAcylation and O-GlcNac cycling enzymes in cancer
| Cancer: | Expression: | References: |
|---|---|---|
| Breast | Elevated OGT/O-GlcNAc in cancer cells and OGT RNA elevated in invasive breast cancer; OGT increase, OGA decrease associated with grade, lymph node metastasis; OGA levels decrease associated with poor overall survival; O-GlcNAcylation/OGT increased in primary malignant tumors compared to benign tumors |
Oncogene 2010 (34)
Cancer Research 2010 (35) Clin Exp Med. 2012 (37) Proteomics 2013 (38) Molecular Cell 2014 (39) |
| Prostate | Elevated OGT/O-GlcNAc in cancer cells; OGT increase associated with progression and poor survival; increased OGT associated with high Gleason Score and correlates with Myc expression |
J.Biol Chem 2012 (40)
Cancer Research 2013 (41) Prostate Can Prost Dis 2014 (42) Proteomics 2013 (43) |
| Colon | O-GlcNAcylation, OGT elevated in colon cancer tissue compared to adjacent tissue Metastatic CRC cell clones contains increased O-GlcNAcylation compared to primary clones; human colonic adenoma contain increased O-GlcNAcylation OGT & OGA levels; OGA+/− mice have higher incidence of colon tumors |
BBA 2011 (44)
J. Biol Chem 2012 (45) Oncol Rep 2013 (48) Oncogenesis 2014 (46) Oncotarget 2015 (47) |
| Lung | O-GlcNAcylation and OGT levels are elevated in lung squamous cell carcinoma tissue compared to adjacent lung tissue; PFK-1 is hyper-O-GlcNAcylated in |
BBA 2011 (44)
Science 2012 (53) |
| Liver | O-GlcNAcylation elevated in hepatocellular carcinoma compared to healthy liver; O-GlcNAcylation elevated in recurrent HCC patients; Low OGA, expression predicts metastatic recurrence in HCC patients. | Med Oncol 2012 (50) |
| Bladder | OGT transcript levels are significantly higher in grade II and III in comparison degrade I bladder cancer; significant increase in OGT expression between early bladder cancers and invasive or advanced bladder cancers | Clin Lab 2012 (49) |
| Pancreatic | Elevated O-GlcNAcylation, OGT and decreased OGA found in human pancreatic cancer cell lines compared to non-tumorigenic pancreatic epithelial cells | J. Biol Chem 2013 (54) |
| Leukemia | Chronic lymphocytic leukemia patients contain elevated O-GlcNAcylation compared to normal circulating B cells. Mouse model of T-cell acute lymphoblastic leukemia requires Ogt. |
Leukemia 2010 (51)
Nature Immunology 2016 (11) |
O-GlcNAc enzymes and O-GlcNAcylation in cancer
Many cancer types display elevated O-GlcNAcylation and aberrant expression of OGT and OGA (Table I) 32; 33. For example, multiple studies demonstrate that breast cancer cell lines and patient samples contain elevated levels of OGT and O-GlcNAc compared to normal counterparts 34; 35; 36; 37; 38. More recently, our lab demonstrated that within breast cancer subtypes, aggressive basal-like tumors contain high levels of OGT and O-GlcNAc compared to luminal type breast cancers 39. Various groups have shown increases in OGT and O-GlcNAc levels during breast cancer progression and this is related to the histological grade of the tumor 37; 38.
Similar trends have been observed in a number of cancer types including prostate 40; 41; 42; 43, colon 44; 45; 46; 47; 48, bladder 49, hepatocellular carcinoma 50, leukemia 51; 52, lung 44; 53 and pancreatic cancer 54 (Table I). Utilizing the Oncomine database, Lynch et al. demonstrated that OGT mRNA expression is elevated in prostate cancer tissue compared to normal adjacent tissue in over 200 patients and increased mRNA was associated with a decreased patient five year survival rate 40. Kamigaito and colleagues confirmed these results, implicating elevated O-GlcNAcylation as a prognostic factor in prostate cancer patient survival 42. In regards to colon cancer, metastatic clones were shown to contain significantly higher levels of O-GlcNAcylated proteins compared to clones derived from primary colon cancers 45. Together these results underscore the clinical importance of OGT mRNA and/or protein expression and O-GlcNAc levels in primary as well as metastatic tumors.
Mechanisms of OGT elevation in cancer
Although the levels of UDP-GlcNAc within the various cellular compartments exquisitely regulate the affinity of OGT for peptide substrates, little is known about the mechanisms of OGT regulation in cancer. Recent reports have demonstrated that oncogenic pathway activation mediates increases in OGT as well as overall O-GlcNAcylation in various cancer cells 47; 55; 56.
Sodi et al. recently demonstrated that the PI3K-mTOR-MYC signaling pathway is required for elevation of OGT and O-GlcNAcylation in human breast cancer cells 55. Interestingly, c-MYC regulation of OGT mechanistically requires the expression of chaperone HSP90A. Additionally, MMTV-MYC derived mammary tumor epithelial cells contain higher levels of OGT and O-GlcNAc and tissue from this transgenic oncogene driven model of breast cancer also revealed that progression in this model from normal tissue to late carcinoma corresponded with increased O-GlcNAcylation and OGT protein expression 55. Since Myc is itself O-GlcNAcylated which increases it stability (see below), Sodi et al. suggested a feed forward model in which Myc amplification in some cancers may lead to increase levels of O-GlcNAcylation, OGT and maintains Myc levels elevated to fuel cancer growth 55. Consistent with this idea, new studies by show that malignant transformation using a murine model of T-cell acute lymphoblastic leukemia required OGT and showed that c-Myc is a key controller of T cell O-GlcNAcylation 52.
In lung and prostate cancer cells, the MEK/ERK signaling pathway was shown to induce hyper-O-GlcNAcylation and enhance a malignant phenotype 56. The MEK/ERK pathway did not regulate OGT or O-GlcNAcylation in breast cancer cells 55 suggesting that pathways that regulate OGT and O-GlcNAcylation may be cancer-type specific. Nevertheless, since PI3K-mTOR-Myc and MEK/ERK pathways are elevated in nearly all cancers, this may explain why O-GlcNAcylation and OGT levels are elevated in many cancers (Table I). More recently, the histone demethylase LSD2 has been shown to regulate OGT protein degradation, in manner dependent on its E3 ligase activity in lung cancer cells 57. Since OGT itself contains post-translational tyrosine phosphorylation and is controlled by various enzymes involved in metabolic pathways (ie, GSK3B and AMPK), it would be interesting to explore these alternative regulatory mechanisms in cancer 25.
The role of O-GlcNAc cycling enzymes in the regulation of cancer hallmarks
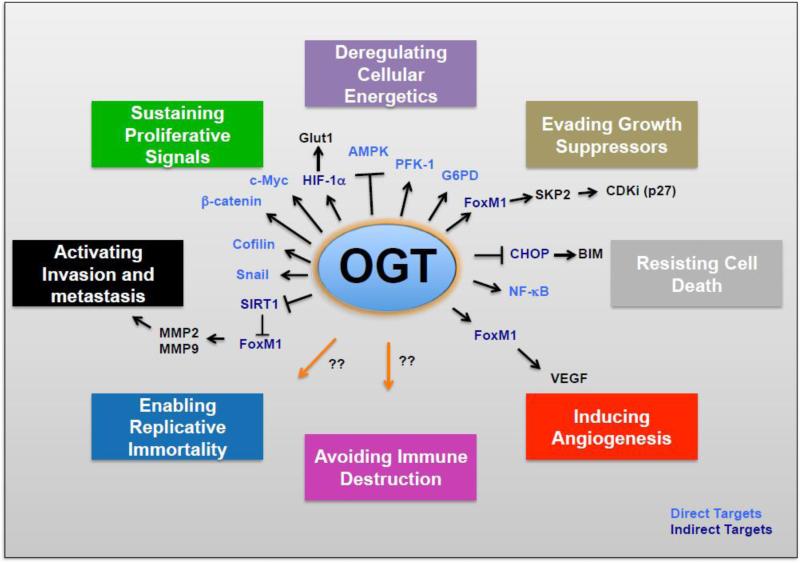
Studies in the past five years have implicated OGT and O-GlcNAcylation as regulators of many cancer phenotypes including multiple “Hallmarks of Cancer” 58 (Figure 2). Below we summarize recent studies that implicate OGT and O-GlcNAcylation directly and/or indirectly in the regulation of various cancer phenotypes.
Figure 2. OGT and O-GlcNAcylation Regulate Multiple “Hallmarks of Cancer”.
OGT via O-GlcNAcylation directly or indirectly regulate proteins that play major role in regulating multiple cancer phenotypes.
The role of OGT and O-GlcNAcylation in the regulation of cancer cell growth and survival
As mentioned previously, the nutrient-sensitive O-GlcNAc modification modulates protein dynamics including transcriptional and cellular signaling pathways in mammalian cells 24 and adjusts protein function according to nutritional status of the cell 59. Importantly, modulation of cellular O-GlcNAc levels has been linked to altered cellular development, mitotic progression, growth, and survival patterns 24,33. Indeed, complete knockout of OGT in mouse embryonic fibroblasts (MEFs) lead to defects in cell growth 60 and other studies have shown that O-GlcNAcylation is important in cell cycle progression 24. More recently, O-GlcNAcylation has been shown to play an important role in regulating embryonic stem cell self-renewal and differentiation 61. This demonstrates that a proper balance of O-GlcNAcylation is required for cell growth and maintenance.
Caldwell et al. was the first study to demonstrate that O-GlcNAc and OGT levels were highly elevated in breast cancer cells and normalizing OGT and O-GlcNAc levels via RNAi or pharmacological inhibition blocked anchorage-independent growth in vitro as well as inhibited in vivo primary tumor growth of breast cancer cells 34. A number of labs have seen similar reduction in tumor cell growth when OGT or O-GlcNAcylation is reduced. This includes cancer cells derived from prostate, colon, lung and pancreas (Table I). Mechanistically, reducing O-GlcNAc levels in breast cancer cells led to G1 cell cycle arrest and increased proteasomal degradation of the oncogenic transcription factor FOXM1 causing a decrease in multiple FOXM1-specific targets, including S-phase kinase-associated protein 2 (SKP2) and a concomitant increase in cyclin-dependent kinase (CDK) inhibitor p27 (Kip1) 34. Similar cell cycle effects were observed in prostate cancer cells where OGT and O-GlcNAc levels were reduced 40. Consistent with these results, Mina Bissell's group has shown that blocking the HBP or targeting OGT with RNAi can cause a reversion of cancer phenotypes in breast cancer cells including growth arrest, reestablishment of tissue polarity, suppression of oncogenic signaling and reduction of glycolytic activity 62.
More recently, Itkonen et al. 41 demonstrated that expression of c-MYC and OGT were tightly correlated in human prostate cancer cells. Inhibition of OGT in prostate cancer cells leads to decreased cell cycle progression and DNA replication in a MYC-dependent manner 41. It has been previously demonstrated that the oncoprotein c-MYC is O-GlcNAcylated at threonine 58 (Thr58) and this modification functions to stimulate growth 33. GSK3B phosphorylation at the nearby serine 62 site promotes c-MYC proteasomal degradation and is inhibited by the presence of O-GlcNAc at Thr58 33. Interestingly, Thr58 mutations are found in a variety of tumor types including lymphomas, resulting in reduced downstream expression of pro-apoptotic BIM 63. The contribution of O-GlcNAc to this phenotype could be a potential mechanism that remains to be explored.
Another major transcription factor involved in promoting cancer cell growth and survival is the nuclear factor kappa B (NF-κB). Various NF-κB subunits are modified by O-GlcNAc, including p65 and c-Rel 64,54. In a pancreatic adenocarcinoma (PDAC) cell line, the NF-κB p65 subunit and upstream kinases IKKα/IKKβ were found to be O-GlcNAcylated 54. Reducing O-GlcNAcylation in these cells decreased p65 phosphorylation, nuclear translocation, transcriptional activity, and tumor growth in vivo 54. Additionally, PDAC anchorage-independent growth and survival were dependent on p65 O-GlcNAcylation, as mutation of O-GlcNAc threonine residues resulted in PDAC cell death 54. In a separate study, elevated O-GlcNAcylation was found to promote colonic inflammation and tumorigenesis via NF-κB 47. Using a mouse model of induced colitis-associated colorectal cancer, the authors show that elevated O-GlcNAcylation, via heterozygous OGA deletion (+/−), directly leads to colorectal inflammation and tumorigenesis in these mice. Mechanistically, the authors demonstrated that OGA +/− mice contain elevated NF-κB signaling, and this activity requires an O-GlcNAc modification at two key sites (T322 and T352) on p65 47.
Ferrer et al. has shown that reducing OGT levels or pharmacologically inhibiting O-GlcNAcylation leads to selective apoptosis of breast cancer cells compared to non-transformed mammary epithelial cells 39. Reducing O-GlcNAcylation caused an increase in the ER stress response only in breast and lung cancer cells 39 and Myc-transformed mammary epithelial cells but not in non-transformed human mammary epithelial MCF-10A cells 55. The reduction of O-GlcNAcylation in cancer cells induced protein kinase RNA-like endoplasmic reticulum kinase (PERK) activation, eukaryotic initiation factor 2α (eIF-2α) phosphorylation and induction of the C/EBP homologous protein (CHOP) as well as pro-apoptotic Bcl-2 family members BIM, PUMA and NOXA 39. In MCF-10A cells, reducing O-GlcNAcylation, below levels seen in cancer cells, did not induce apoptosis, PERK activation or an ER stress response. The apoptosis in cancer cells was CHOP-dependent and also dependent on OGTs regulation of metabolism via HIF-1α and GLUT1 39. Thus, reducing O-GlcNAcylation in cancer cells induces an ER stress-mediated apoptosis that is very similar to the response of cancer cells to anti-glycolytics 65. Together these studies imply that O-GlcNAc contributes to tumor growth and survival through signaling mechanisms that are highly linked to cancer metabolism. Importantly, reducing O-GlcNAcylation had minimal effects on growth and survival of non-transformed mammary epithelial cells 34; 55, prostate epithelial cells 40 and pancreatic epithelial cells 54. These results suggest that cancer cells are highly dependent on O-GlcNAcylation for cell growth and survival compared to normal counterparts and thus may provide a therapeutic window to target OGT in cancer.
The role of OGT and O-GlcNAcylation in invasion and metastasis
Cancer cell invasion, metastatic dissemination and establishment at a secondary site are the result of multistep processes, which include epithelial-mesenchymal transition (EMT) 66. This phenomenon is often a result of aberrant signaling from the extracellular matrix mediated through β–catenin/Snail1 dependent transcription 66. This promotes EMT reprogramming by suppressing expression of cell junction protein E-cadherin while inducing expression of mesenchymal markers to promote cancer cell invasion via matrix metalloproteinases (MMPs) 66. Not surprisingly, metabolic alterations seen in cancer including hypoxia and loss of tumor suppressors have been linked to the promotion of an EMT phenotype thus contributing to carcinoma progression 15. Our group and others have demonstrated that inhibition of OGT reduces breast and prostate cancer cell epithelial-to-mesenchymal transition (EMT), invasion and metastasis in vivo 35; 40; 67. For example, Park et al. 67 revealed that O-GlcNAc modification of Snail1 at serine 112 (S112) stabilizes the transcription factor and thus increases its function to attenuate E-cadherin expression and promote EMT in MCF7 breast cancer cells. In a separate study, it was demonstrated that O-GlcNAc decreases cell surface E-cadherin through directly modifying β-catenin thereby contributing to metastasis in the 4T1 mouse breast cancer model 35. In addition, the actin binding protein cofilin is O-GlcNAcylated, which is required for its proper localization to invadopodia of breast cancer cells and contributes to breast cancer cell invasion 68. Recent studies have shown that EMT in lung cancer cells increases flux through the HBP and increases O-GlcnAcylation 69. These data indicate possible crosstalk between O-GlcNAcylation and EMT programs that may contribute to fine-tuning cancer cell invasion.
Lynch et al. demonstrated that OGT-mediated regulation of invasion was dependent upon regulation of the oncogenic transcription factor FOXM1 40 and its transcriptional targets MMP2 and MMP9. Additionally, upon OGT inhibition, we observed a decrease in the metastatic potential of prostate cancer cells in vivo 40. More recently, we have shown that in breast cancer cells, reduced O-GlcNAcylation leads to SIRT1-mediated proteasomal degradation of FOXM1, a mechanism that is dependent upon the MEK/ERK pathway. Reducing OGT levels, or O-GlcNAcylation, increases SIRT1 levels and activity. SIRT1 regulates the MEK/ERK pathway to increase FOXM1 degradation and reduces expression of its transcriptional targets MMP2 and MMP9. SIRT1 is critical for OGT-mediated regulation of FOXM1 ubiquitination as reducing SIRT1 activity reverses OGT-mediated regulation of FOXM1. Reducing OGT levels in aggressive triple negative breast cancer cells inhibits the ERK pathway that is dependent on OGT regulation of SIRT1 levels and activity. Moreover, we show that SIRT1 is required for the changes observed in invasion and metastasis in response to OGT modulation in breast cancer cells. O-GlcNAcylation regulation of SIRT1 levels in breast cancer cells and in mouse embryonic fibroblasts (MEFs) was dependent on AMPK. Thus, we implicate O-GlcNAcylation as a central component linking metabolism to invasion and metastasis via a AMPK/SIRT1/FOXM1 axis 70.
O-GlcNAcylation regulates cancer angiogenesis
Cancer cells require new blood vessel formation or angiogenesis for tumor expansion and metastatic spread 71. In prostate cancer cells reducing levels of OGT and O-GlcNAcylation blocked expression of vascular endothelial factor (VEGF) and importantly reduced angiogenic potential of prostate cancer cells in vitro 40. Conditioned media from prostate cancer cells containing OGT knockdown contained reduced HUVEC endothelial tube formation in vitro compared to control conditioned media. OGT regulation of VEGF expression and angiogenic potential of endothelial cells was found to require regulation of FOXM1 40. This data is consistent with studies linking FOXM1as a critical regulator of prostate cancer including angiogenesis 72. Future studies should examine whether OGT and O-GlcNAcylation directly regulate neovasculature that occurs during tumorigenesis in vivo.
O-GlcNAcylation regulates cancer glycolysis
It has been previously established that aberrant O-GlcNAcylation can contribute to metabolic disorders, such as insulin resistance33, implying it could also play a role in altered metabolism that occurs in cancer cells. Growing evidence indicates that O-GlcNAc is a crucial mechanism linking cancer metabolism and cellular signaling 39; 41; 53; 73,74. Many glycolytic enzymes have been shown to be direct substrates for OGT. One such enzyme is phosphofructokinase-1 (PFK-1), which catalyzes the committed step of glycolysis, the conversion of fructose-6-phosphate to fructose 1,6-bisphosphate. PFK-1 modification by O-GlcNAc was shown to enhance glycolytic activity and pentose phosphate pathway (PPP) flux, conferring a selective survival advantage to lung cancer cells 53. Similarly, O-GlcNAcylation of glucose-6-phosphate dehydrogenase (G6PDH) is induced under hypoxic conditions to elevate flux through the PPP, thereby increasing the nucleotide, lipid and antioxidant pools, contributing to the pathogenesis of lung cancer 73. The oncogenic isoform of pyruvate kinase, pyruvate kinase isozyme M2 (PKM2), is expressed in most human tumors. PKM2 has also been shown to be O-GlcNAc modified, resulting in reduced PKM2 activity in colon cancer cells 74. The exact functional importance of PKM2 O-GlcNAcylation in promoting cancer phenotypes remains to be explored.
Recently, we demonstrated that O-GlcNAcylation regulates glycolysis in cancer cells via hypoxia-inducible factor 1 (HIF-1α) (Figure 1) and its transcriptional target GLUT1 39. Reducing O-GlcNAcylation increases α-ketoglutarate, HIF-1 hydroxylation, and interaction with pVHL, resulting in HIF-1α degradation. HIF-1α and GLUT1 are critical for OGT-mediated regulation of metabolic stress, as overexpression of a stable form of HIF-1 or GLUT1 rescues metabolic defects caused by OGT depletion. Clinically, we demonstrated that human breast cancers with high levels of HIF-1α contain elevated OGT, and lower OGA levels correlate independently with poor patient outcome. This novel mechanism where O-GlcNAcylation regulates cancer cell metabolic reprograming via regulation of HIF-1α contributes further evidence supporting an important role for O-GlcNAcylation in the promotion of cancer glycolysis 39.
Cross-talk between O-GlcNAcylation and the AMPK pathway is emerging as important node in regulating cellular metabolism, growth and signaling in both normal physiology and diseased states such as cancer. Previous studies have shown that AMPK can phosphorylate OGT and regulate its substrate specificity and that AMPKα and γ subunits can be O-GlcNAcylated in skeletal muscle cells 75. In cancer cells, O-GlcNAcylation regulates AMPK indirectly (Figure 1) through regulation of metabolism. Reducing O-GlcNAcylation in cancer cells leads to a robust increase in LKB1 and AMPK activity that can be reversed by the addition of the permeable nutrient metabolite methyl-pyruvate 70 or through over-expression of the glucose transporter GLUT1 39, both of which support cellular bioenergetics. Regulation of bioenergetics by OGT also regulates mTOR as overexpression of stable HIFα-1 and GLUT1 also reverse OGT-inhibition of the mTOR pathway 39. Thus, OGT can directly or indirectly regulate the major players that control cancer metabolism including HIF-1α, c-MYC, AMPK, mTOR and GLUT1.
O-GlcNAcylation and TET proteins: reprograming the cancer epigenome
The ten-eleven translocation (TET) family of dioxygenases (TET1/2/3) is responsible for converting 5-methylcytosine to 5-hydroxymethylcytosine thus providing a vital DNA demethylation mechanism 25; 76. Aberrant activity of the ten-eleven translocation (TET) family of 5-methylcytosine (5mC) hydroxylases directly results in epigenetic alterations that inhibit the expression of genes involved in cell differentiation 77. The nuclear isoform of OGT has been shown to stably interact with a number of TET family proteins in mouse embryonic stem cells (ESCs) 76. Functionally, the O-GlcNAc modification on TET1 promotes its association with CpG-rich promoters and Polycomb target genes in embryonic stem cells (ESCs), to promote cellular differentiation 76,78. Conversely, TET1-dependent recruitment of OGT to CpG islands overlaps at promoters with H3K4me3, directly coupling cellular metabolism to epigenetics and differentiation 25,77. Recent reports suggest that OGT catalyzes the O-GlcNAcylation of TET3, promotes TET3 nuclear export, and thus inhibits the formation of 5-hydroxymethylcytosine 76; 77. Thus, TET3 O-GlcNAcylation regulates subcellular localization and enzymatic activity, directly linking epigenetics and differentiation with glucose metabolism 76; 77. Since some cancers contain loss of function TETs, and TET can recruit OGT to chromatin 79, it will be of interest to determine if these mutations may alter OGT nuclear localization, alter its activity and/or epigenetic regulation in cancer cells. Whether changes in TET-OGT complexes at specific genomic regions results in chromatin alterations that promote cancer phenotypes remain to be explored.
O-GlcNAc Transferase as a therapeutic target in cancer
Various studies demonstrate a therapeutic window may exist to specifically target OGT and O-GlcNAcylation in cancer cells 39; 40; 80. A number of novel chemicals have been developed to target OGT and reduce O-GlcNAcylation in mammalian cells. Namely, small molecule OGT inhibitors 81 and UDP-GlcNAc salvage pathway analogs 29. In the past 10 years, Walker and colleagues 81; 82 have developed a number of OGT inhibitors aimed at providing insights to the biological and structural function of the enzyme in mammalian cells. Namely, the commercially available small molecule inhibitor ST045849, has been recently shown to stimulate cell death in prostate cancer cells when used in combination with alanine amidotransferase inhibitors 80.
Vocadlo and colleagues 29, have identified a biosynthetic thiosugar, Ac-5SGlcNAc, that can be salvaged in cells yielding a nucleotide sugar analog that acts to inhibit OGT. Interestingly, these OGT inhibitors have been shown to reduce breast and prostate cancer phenotypes including cell growth and invasion, and increase metabolic stress and apoptosis via reducing expression of potent oncogenes such as FOXM1, c-MYC and HIF-1α34,39,40; 41; 55. More recently, the PI3K inhibitor, GDC-0941 demonstrated synergy with OGT inhibition to sensitize ovarian cancer cells and induce apoptosis in vitro 83. The chemosensitizing effects of PI3K pathway inhibition with metabolic defects observed when targeting OGT may provide a promising combinatorial strategy. In addition, reducing O-GlcNAcylation in estrogen receptor positive breast cancer cells potentiated effects of tamoxifen on cell death 84. Thus, general inhibition of O-GlcNAcylation may be an effective way to increase sensitivity to existing therapy in treating cancer.
Little data exists to predict potential toxicity of targeting OGT using pharmacological inhibitors as a therapeutic strategy to combat cancer growth. Our lab and others have demonstrated through xenograft studies that RNAi inhibition results in decreased tumor growth in vivo, however systemic administration of an O-GlcNAcylation inhibitor and its potential toxicities to various tissues has yet to be explored. We can postulate that systemic inhibition of OGT with a specific inhibitor may result in adverse events in normal cells with high OGT expression such as the insulin secreting cells of the pancreas. Alejandro and colleagues 85 demonstrated that β-cell specific OGT genetic deletion at embryonic day 13.5 results in mice having β-cell failure and diabetes. OGT deficient β-cells displayed loss of mass and function, in part, due to elevated ER stress induced-apoptosis, much like what was seen in breast cancer cells upon OGT inhibition 39. However, OGT ablation in mature β-cells in 12 week old mice, had no effect on β-cell mass and mice did not develop diabetes 85. This study highlights that adult tissues may not be as sensitive to inhibition O-GlcNAcylation compared to developing tissues in mammals as well as the importance of understanding the various and complex roles of OGT in adult tissue.
Other potential toxicities may exist in tissues of the brain, as O-GlcNAc is found to be abundant within the brain and decreased O-GlcNAcylation correlates with Alzheimer's phenotypes, potentially through direct modification of Tau which is decreased in AD patient brain tissue 86. The review by Yuzwa et. al. highlights recent work in which pharmacological means to increase levels of O-GlcNAc in the brain results in protective effects in mouse models of AD 86. Inhibitory effects on neuronal excitability were seen when OGT was genetically deleted in AgRP neurons 87. The ablation of OGT in this cell type protected the animals from diet-induced obesity and diabetes showing the complex dynamics of O-GlcNAc in the brain. With this in mind, any negative impact of inhibition of OGT in this tissue could be strategically avoided through the development of pharmacological OGT inhibitors that are incapable of crossing the blood brain barrier.
O-GlcNAcylation may also have clinical use as a biomarker with diagnostic or prognostic value. For example, Rozanski et al. 49 were able to detect OGT mRNA in urine of patients with bladder cancer while OGT was not detected in urine of healthy patients. In addition, they found OGT levels increased in high-grade bladder cancers compared to lower grade tumors. Moreover, Tomic et al. showed that O-GlcNAc levels in blood and spleen reflected changes in tumor burden following treatment with resveratrol in mouse model of erythroleukemia 88. Thus, urine or blood content of OGT and O-GlcNAc may be useful in diagnosis or as markers for therapeutic responses in some cancers. In future studies, it will be interesting to determine whether OGT and O-GlcNAc can be detected in circulating tumor cells and whether its expression correlates with disease progression and patient outcome.
Emerging roles for O-GlcNAcylation
Cancer is a disease associated with aging 89 and a number of studies suggest a role for OGT in aging. Recent data shows that total levels of O-GlcNAcylation are elevated in aging tissue 90. Major areas of interest in the field of aging, including autophagy and the sirtuins, both appear to have links to OGT and O-GlcNAcylation.
Changes in autophagy have both a cause and effect role in the process of aging 91. Often, autophagy is enhanced by interventions shown to extend lifespan. These include caloric restriction (CR) which is the only recognized intervention known to extend both mean and maximal lifespan 92, and compounds such as resveratrol. Inhibition of autophagy serves to produce degenerations similar to aging phenotypes 91. There is evidence in C. elegans that OGT has a role in the regulation of autophagy. Perturbation of normal O-GlcNAc cycling by loss of either enzyme critical to this process (OGT and OGA) resulted in changes consistent with increased autophagy 93. In breast cancer cells, we have shown that reducing OGT and O-GlcNAcylation blocks the mTOR pathway, while increasing activation of nutrient sensors such as LKB1/AMPK, signaling changes that are consistent with an induction of autophagy 70. However, it has not been tested whether phenotypes caused by targeting OGT in cancer cells are associated with regulation of autophagy.
Intricate ties between the sirtuin family and aging put this class of deacetylases at the forefront of the aging field. Caloric restriction was shown to potently induce the expression of SIRT1 and SIRT3 in multiple tissues in vivo 94 and Sirt1−/− mice do not live longer on calorically restricted diet, demonstrating that sirtuins are important players in the life-extension induced by CR 95. Reducing OGT in cancer cells in vitro and in mammary tissue in vivo increases deacetylation of proteins by increasing expressing of the NAD-dependent deacetylases SIRT1 70, as mentioned previously in regards to OGTs role in invasion. The implications of a link between OGT and SIRT1, may extend beyond cancer and could provide insight into aging processes as well. Thus, since O-GlcNAcylation is elevated in aging tissue and regulates key pathways associated with autophagy and the sirtuins, it is possible the nutrient sensor O-GlcNAcylation may link altered glucose availability to key molecular pathways to regulate metabolic changes that occur during aging and also contribute to cancer.
Implications and Future Directions
Research into the evolving role of O-GlcNAcylation in normal biology and pathologies will continue to expand our understanding of the role of this modification in disease states such as cancer. Although there is ample evidence for a role of OGT in many “Hallmarks of Cancer” (Figure 2), it is still little evidence that OGT or O-GlcNAcylation may play a role in enabling replicative immortality or avoiding immune destruction. We must continue to expand our insight into the roles of OGT and O-GlcNAcylation in cancer and adult biology. We currently do not understand the role OGT plays in many adult systems. OGT knockout is embryonic lethal, owing to its role in development and therefore must play a strong role in developing cells 60. We can hypothesize that OGT and O-GlcNAc may play some role in adult stem cells and perhaps cancer stem cells, however these avenues of investigation have not yet been explored. New tools for genetic manipulation, such as the CRISPR-Cas9 system or novel mouse models, will accelerate our comprehension of the complex function of OGT in both normal biology and cancer. In addition, the discovery and improvement of new and existing OGT inhibitors will also help in understanding the role of OGT in normal biology and cancer. These new and more specific OGT inhibitors may also provide novel and effective therapeutic agents for cancers that exhibit deregulated O-GlcNAcylation.
Highlights.
O-GlcNAcylation levels are found elevated in nearly all cancers examined.
O-GlcNAc cycling enzymes O-GlcNAc transferase and O-GlcNAcase are altered in many cancers.
O-GlcNAcylation regulates many aspects of the “Hallmarks of Cancer”.
Reducing O-GlcNAcylation in cancer cells may be a potential treatment strategy.
Acknowledgements
This work is supported by NIH-NCI grants CA183574 (to C.M.F.), CA192868 (to V.L.S.) and CA155413 (to M.J.R.)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Boroughs LK, DeBerardinis RJ. Metabolic pathways promoting cancer cell survival and growth. Nat Cell Biol. 2015;17:351–9. doi: 10.1038/ncb3124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–33. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaelin WG., Jr. Cancer and altered metabolism: potential importance of hypoxia-inducible factor and 2-oxoglutarate-dependent dioxygenases. Cold Spring Harb Symp Quant Biol. 2011;76:335–45. doi: 10.1101/sqb.2011.76.010975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Warburg O. On the origin of cancer cells. Science. 1956;123:309–14. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 5.DeBerardinis RJ, Lum JJ, Hatzivassiliou G, Thompson CB. The biology of cancer: metabolic reprogramming fuels cell growth and proliferation. Cell Metab. 2008;7:11–20. doi: 10.1016/j.cmet.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 6.Shaw RJ, Cantley LC. Ras, PI(3)K and mTOR signalling controls tumour cell growth. Nature. 2006;441:424–30. doi: 10.1038/nature04869. [DOI] [PubMed] [Google Scholar]
- 7.Jones RG, Thompson CB. Tumor suppressors and cell metabolism: a recipe for cancer growth. Genes Dev. 2009;23:537–48. doi: 10.1101/gad.1756509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dang CV, Semenza GL. Oncogenic alterations of metabolism. Trends Biochem Sci. 1999;24:68–72. doi: 10.1016/s0968-0004(98)01344-9. [DOI] [PubMed] [Google Scholar]
- 9.Zoncu R, Efeyan A, Sabatini DM. mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol. 2011;12:21–35. doi: 10.1038/nrm3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DeBerardinis RJ, Mancuso A, Daikhin E, Nissim I, Yudkoff M, Wehrli S, Thompson CB. Beyond aerobic glycolysis: transformed cells can engage in glutamine metabolism that exceeds the requirement for protein and nucleotide synthesis. Proc Natl Acad Sci U S A. 2007;104:19345–50. doi: 10.1073/pnas.0709747104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Flavin R, Peluso S, Nguyen PL, Loda M. Fatty acid synthase as a potential therapeutic target in cancer. Future Oncol. 2010;6:551–62. doi: 10.2217/fon.10.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Menendez JA, Lupu R. Fatty acid synthase and the lipogenic phenotype in cancer pathogenesis. Nat Rev Cancer. 2007;7:763–77. doi: 10.1038/nrc2222. [DOI] [PubMed] [Google Scholar]
- 13.Harris AL. Hypoxia--a key regulatory factor in tumour growth. Nat Rev Cancer. 2002;2:38–47. doi: 10.1038/nrc704. [DOI] [PubMed] [Google Scholar]
- 14.Semenza GL. Defining the role of hypoxia-inducible factor 1 in cancer biology and therapeutics. Oncogene. 2010;29:625–34. doi: 10.1038/onc.2009.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guillaumond F, Leca J, Olivares O, Lavaut MN, Vidal N, Berthezene P, Dusetti NJ, Loncle C, Calvo E, Turrini O, Iovanna JL, Tomasini R, Vasseur S. Strengthened glycolysis under hypoxia supports tumor symbiosis and hexosamine biosynthesis in pancreatic adenocarcinoma. Proc Natl Acad Sci U S A. 2013;110:3919–24. doi: 10.1073/pnas.1219555110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Generali D, Berruti A, Brizzi MP, Campo L, Bonardi S, Wigfield S, Bersiga A, Allevi G, Milani M, Aguggini S, Gandolfi V, Dogliotti L, Bottini A, Harris AL, Fox SB. Hypoxia-inducible factor-1alpha expression predicts a poor response to primary chemoendocrine therapy and disease-free survival in primary human breast cancer. Clin Cancer Res. 2006;12:4562–8. doi: 10.1158/1078-0432.CCR-05-2690. [DOI] [PubMed] [Google Scholar]
- 17.Palm W, Park Y, Wright K, Pavlova NN, Tuveson DA, Thompson CB. The Utilization of Extracellular Proteins as Nutrients Is Suppressed by mTORC1. Cell. 2015;162:259–70. doi: 10.1016/j.cell.2015.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ying H, Kimmelman AC, Lyssiotis CA, Hua S, Chu GC, Fletcher-Sananikone E, Locasale JW, Son J, Zhang H, Coloff JL, Yan H, Wang W, Chen S, Viale A, Zheng H, Paik JH, Lim C, Guimaraes AR, Martin ES, Chang J, Hezel AF, Perry SR, Hu J, Gan B, Xiao Y, Asara JM, Weissleder R, Wang YA, Chin L, Cantley LC, DePinho RA. Oncogenic Kras maintains pancreatic tumors through regulation of anabolic glucose metabolism. Cell. 2012;149:656–70. doi: 10.1016/j.cell.2012.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shackelford DB, Shaw RJ. The LKB1-AMPK pathway: metabolism and growth control in tumour suppression. Nat Rev Cancer. 2009;9:563–75. doi: 10.1038/nrc2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sengupta S, Peterson TR, Sabatini DM. Regulation of the mTOR complex 1 pathway by nutrients, growth factors, and stress. Mol Cell. 2010;40:310–22. doi: 10.1016/j.molcel.2010.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wellen KE, Lu C, Mancuso A, Lemons JM, Ryczko M, Dennis JW, Rabinowitz JD, Coller HA, Thompson CB. The hexosamine biosynthetic pathway couples growth factor-induced glutamine uptake to glucose metabolism. Genes Dev. 2010;24:2784–99. doi: 10.1101/gad.1985910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gwinn DM, Shackelford DB, Egan DF, Mihaylova MM, Mery A, Vasquez DS, Turk BE, Shaw RJ. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell. 2008;30:214–26. doi: 10.1016/j.molcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hanover JA, Krause MW, Love DC. The hexosamine signaling pathway: O-GlcNAc cycling in feast or famine. Biochim Biophys Acta. 2010;1800:80–95. doi: 10.1016/j.bbagen.2009.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bond MR, Hanover JA. A little sugar goes a long way: the cell biology of O-GlcNAc. J Cell Biol. 2015;208:869–80. doi: 10.1083/jcb.201501101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hardiville S, Hart GW. Nutrient regulation of signaling, transcription, and cell physiology by O-GlcNAcylation. Cell Metab. 2014;20:208–13. doi: 10.1016/j.cmet.2014.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marshall S, Bacote V, Traxinger RR. Discovery of a metabolic pathway mediating glucose-induced desensitization of the glucose transport system. Role of hexosamine biosynthesis in the induction of insulin resistance. J Biol Chem. 1991;266:4706–12. [PubMed] [Google Scholar]
- 27.Dennis JW, Lau KS, Demetriou M, Nabi IR. Adaptive regulation at the cell surface by N-glycosylation. Traffic. 2009;10:1569–78. doi: 10.1111/j.1600-0854.2009.00981.x. [DOI] [PubMed] [Google Scholar]
- 28.Love DC, Hanover JA. The hexosamine signaling pathway: deciphering the “O-GlcNAc code”. Sci STKE. 2005;2005:re13. doi: 10.1126/stke.3122005re13. [DOI] [PubMed] [Google Scholar]
- 29.Gloster TM, Zandberg WF, Heinonen JE, Shen DL, Deng L, Vocadlo DJ. Hijacking a biosynthetic pathway yields a glycosyltransferase inhibitor within cells. Nat Chem Biol. 2011;7:174–81. doi: 10.1038/nchembio.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gao Y, Wells L, Comer FI, Parker GJ, Hart GW. Dynamic O- glycosylation of nuclear and cytosolic proteins: cloning and characterization of a neutral, cytosolic beta-N-acetylglucosaminidase from human brain. J Biol Chem. 2001;276:9838–45. doi: 10.1074/jbc.M010420200. [DOI] [PubMed] [Google Scholar]
- 31.Vincenz L, Hartl FU. Sugarcoating ER Stress. Cell. 2014;156:1125–7. doi: 10.1016/j.cell.2014.02.035. [DOI] [PubMed] [Google Scholar]
- 32.Lynch TP, Reginato MJ. O-GlcNAc transferase: a sweet new cancer target. Cell Cycle. 2011;10:1712–3. doi: 10.4161/cc.10.11.15561. [DOI] [PubMed] [Google Scholar]
- 33.Slawson C, Hart GW. O-GlcNAc signalling: implications for cancer cell biology. Nat Rev Cancer. 2011;11:678–84. doi: 10.1038/nrc3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Caldwell SA, Jackson SR, Shahriari KS, Lynch TP, Sethi G, Walker S, Vosseller K, Reginato MJ. Nutrient sensor O-GlcNAc transferase regulates breast cancer tumorigenesis through targeting of the oncogenic transcription factor FoxM1. Oncogene. 2010;29:2831–42. doi: 10.1038/onc.2010.41. [DOI] [PubMed] [Google Scholar]
- 35.Gu Y, Mi W, Ge Y, Liu H, Fan Q, Han C, Yang J, Han F, Lu X, Yu W. GlcNAcylation plays an essential role in breast cancer metastasis. Cancer Res. 2010;70:6344–51. doi: 10.1158/0008-5472.CAN-09-1887. [DOI] [PubMed] [Google Scholar]
- 36.Gu Y, Ande SR, Mishra S. Altered O-GlcNAc modification and phosphorylation of mitochondrial proteins in myoblast cells exposed to high glucose. Arch Biochem Biophys. 2011;505:98–104. doi: 10.1016/j.abb.2010.09.024. [DOI] [PubMed] [Google Scholar]
- 37.Krzeslak A, Forma E, Bernaciak M, Romanowicz H, Brys M. Gene expression of O-GlcNAc cycling enzymes in human breast cancers. Clin Exp Med. 2012;12:61–5. doi: 10.1007/s10238-011-0138-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Champattanachai V, Netsirisawan P, Chaiyawat P, Phueaouan T, Charoenwattanasatien R, Chokchaichamnankit D, Punyarit P, Srisomsap C, Svasti J. Proteomic analysis and abrogated expression of O-GlcNAcylated proteins associated with primary breast cancer. Proteomics. 2013;13:2088–99. doi: 10.1002/pmic.201200126. [DOI] [PubMed] [Google Scholar]
- 39.Ferrer CM, Lynch TP, Sodi VL, Falcone JN, Schwab LP, Peacock DL, Vocadlo DJ, Seagroves TN, Reginato MJ. O-GlcNAcylation regulates cancer metabolism and survival stress signaling via regulation of the HIF-1 pathway. Mol Cell. 2014;54:820–31. doi: 10.1016/j.molcel.2014.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lynch TP, Ferrer CM, Jackson SR, Shahriari KS, Vosseller K, Reginato MJ. Critical role of O-Linked beta-N-acetylglucosamine transferase in prostate cancer invasion, angiogenesis, and metastasis. J Biol Chem. 2012;287:11070–81. doi: 10.1074/jbc.M111.302547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Itkonen HM, Minner S, Guldvik IJ, Sandmann MJ, Tsourlakis MC, Berge V, Svindland A, Schlomm T, Mills IG. O-GlcNAc transferase integrates metabolic pathways to regulate the stability of c-MYC in human prostate cancer cells. Cancer Res. 2013;73:5277–87. doi: 10.1158/0008-5472.CAN-13-0549. [DOI] [PubMed] [Google Scholar]
- 42.Kamigaito T, Okaneya T, Kawakubo M, Shimojo H, Nishizawa O, Nakayama J. Overexpression of O-GlcNAc by prostate cancer cells is significantly associated with poor prognosis of patients. Prostate Cancer Prostatic Dis. 2014;17:18–22. doi: 10.1038/pcan.2013.56. [DOI] [PubMed] [Google Scholar]
- 43.Gu Y, Gao J, Han C, Zhang X, Liu H, Ma L, Sun X, Yu W. O-GlcNAcylation is increased in prostate cancer tissues and enhances malignancy of prostate cancer cells. Mol Med Rep. 2014;10:897–904. doi: 10.3892/mmr.2014.2269. [DOI] [PubMed] [Google Scholar]
- 44.Mi W, Gu Y, Han C, Liu H, Fan Q, Zhang X, Cong Q, Yu W. O-GlcNAcylation is a novel regulator of lung and colon cancer malignancy. Biochim Biophys Acta. 2011;1812:514–9. doi: 10.1016/j.bbadis.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 45.Yehezkel G, Cohen L, Kliger A, Manor E, Khalaila I. O-linked beta-N- acetylglucosaminylation (O-GlcNAcylation) in primary and metastatic colorectal cancer clones and effect of N-acetyl-beta-D-glucosaminidase silencing on cell phenotype and transcriptome. J Biol Chem. 2012;287:28755–69. doi: 10.1074/jbc.M112.345546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang YR, Jang HJ, Yoon S, Lee YH, Nam D, Kim IS, Lee H, Kim H, Choi JH, Kang BH, Ryu SH, Suh PG. OGA heterozygosity suppresses intestinal tumorigenesis in Apc(min/+) mice. Oncogenesis. 2014;3:e109. doi: 10.1038/oncsis.2014.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang YR, Kim DH, Seo YK, Park D, Jang HJ, Choi SY, Lee YH, Lee GH, Nakajima K, Taniguchi N, Kim JM, Choi EJ, Moon HY, Kim IS, Choi JH, Lee H, Ryu SH, Cocco L, Suh PG. Elevated O-GlcNAcylation promotes colonic inflammation and tumorigenesis by modulating NF- kappaB signaling. Oncotarget. 2015;6:12529–42. doi: 10.18632/oncotarget.3725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Phueaouan T, Chaiyawat P, Netsirisawan P, Chokchaichamnankit D, Punyarit P, Srisomsap C, Svasti J, Champattanachai V. Aberrant O-GlcNAc-modified proteins expressed in primary colorectal cancer. Oncol Rep. 2013;30:2929–36. doi: 10.3892/or.2013.2794. [DOI] [PubMed] [Google Scholar]
- 49.Rozanski W, Krzeslak A, Forma E, Brys M, Blewniewski M, Wozniak P, Lipinski M. Prediction of bladder cancer based on urinary content of MGEA5 and OGT mRNA level. Clin Lab. 2012;58:579–83. [PubMed] [Google Scholar]
- 50.Zhu Q, Zhou L, Yang Z, Lai M, Xie H, Wu L, Xing C, Zhang F, Zheng S. O-GlcNAcylation plays a role in tumor recurrence of hepatocellular carcinoma following liver transplantation. Med Oncol. 2012;29:985–93. doi: 10.1007/s12032-011-9912-1. [DOI] [PubMed] [Google Scholar]
- 51.Shi Y, Tomic J, Wen F, Shaha S, Bahlo A, Harrison R, Dennis JW, Williams R, Gross BJ, Walker S, Zuccolo J, Deans JP, Hart GW, Spaner DE. Aberrant O-GlcNAcylation characterizes chronic lymphocytic leukemia. Leukemia. 2010;24:1588–98. doi: 10.1038/leu.2010.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Swamy M, Pathak S, Grzes KM, Damerow S, Sinclair LV, van Aalten DM, Cantrell DA. Glucose and glutamine fuel protein O-GlcNAcylation to control T cell self-renewal and malignancy. Nature immunology. 2016;17:712–20. doi: 10.1038/ni.3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yi W, Clark PM, Mason DE, Keenan MC, Hill C, Goddard WA, 3rd, Peters EC, Driggers EM, Hsieh-Wilson LC. Phosphofructokinase 1 glycosylation regulates cell growth and metabolism. Science. 2012;337:975–80. doi: 10.1126/science.1222278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ma Z, Vocadlo DJ, Vosseller K. Hyper-O-GlcNAcylation is anti- apoptotic and maintains constitutive NF-kappaB activity in pancreatic cancer cells. J Biol Chem. 2013;288:15121–30. doi: 10.1074/jbc.M113.470047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sodi VL, Khaku S, Krutilina R, Schwab LP, Vocadlo DJ, Seagroves TN, Reginato MJ. mTOR/MYC Axis Regulates O-GlcNAc Transferase Expression and O-GlcNAcylation in Breast Cancer. Mol Cancer Res. 2015;13:923–33. doi: 10.1158/1541-7786.MCR-14-0536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang X, Ma L, Qi J, Shan H, Yu W, Gu Y. MAPK/ERK signaling pathway-induced hyper-O-GlcNAcylation enhances cancer malignancy. Mol Cell Biochem. 2015;410:101–10. doi: 10.1007/s11010-015-2542-8. [DOI] [PubMed] [Google Scholar]
- 57.Yang Y, Yin X, Yang H, Xu Y. Histone demethylase LSD2 acts as an E3 ubiquitin ligase and inhibits cancer cell growth through promoting proteasomal degradation of OGT. Molecular cell. 2015;58:47–59. doi: 10.1016/j.molcel.2015.01.038. [DOI] [PubMed] [Google Scholar]
- 58.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 59.Zeidan Q, Hart GW. The intersections between O-GlcNAcylation and phosphorylation: implications for multiple signaling pathways. J Cell Sci. 2010;123:13–22. doi: 10.1242/jcs.053678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.O'Donnell N, Zachara NE, Hart GW, Marth JD. Ogt-dependent X- chromosome-linked protein glycosylation is a requisite modification in somatic cell function and embryo viability. Mol Cell Biol. 2004;24:1680–90. doi: 10.1128/MCB.24.4.1680-1690.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jang H, Kim TW, Yoon S, Choi SY, Kang TW, Kim SY, Kwon YW, Cho EJ, Youn HD. O-GlcNAc regulates pluripotency and reprogramming by directly acting on core components of the pluripotency network. Cell Stem Cell. 2012;11:62–74. doi: 10.1016/j.stem.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 62.Onodera Y, Nam JM, Bissell MJ. Increased sugar uptake promotes oncogenesis via EPAC/RAP1 and O-GlcNAc pathways. J Clin Invest. 2014;124:367–84. doi: 10.1172/JCI63146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dang CV, O'Donnell K A, Juopperi T. The great MYC escape in tumorigenesis. Cancer cell. 2005;8:177–8. doi: 10.1016/j.ccr.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 64.Ramakrishnan P, Clark PM, Mason DE, Peters EC, Hsieh-Wilson LC, Baltimore D. Activation of the transcriptional function of the NF-kappaB protein c-Rel by O-GlcNAc glycosylation. Sci Signal. 2013;6:ra75. doi: 10.1126/scisignal.2004097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.El Mjiyad N, Caro-Maldonado A, Ramirez-Peinado S, Munoz-Pinedo C. Sugar-free approaches to cancer cell killing. Oncogene. 2011;30:253–64. doi: 10.1038/onc.2010.466. [DOI] [PubMed] [Google Scholar]
- 66.Thiery JP, Sleeman JP. Complex networks orchestrate epithelial mesenchymal transitions. Nat Rev Mol Cell Biol. 2006;7:131–42. doi: 10.1038/nrm1835. [DOI] [PubMed] [Google Scholar]
- 67.Park SY, Kim HS, Kim NH, Ji S, Cha SY, Kang JG, Ota I, Shimada K, Konishi N, Nam HW, Hong SW, Yang WH, Roth J, Yook JI, Cho JW. Snail1 is stabilized by O-GlcNAc modification in hyperglycaemic condition. EMBO J. 2010;29:3787–96. doi: 10.1038/emboj.2010.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Huang X, Pan Q, Sun D, Chen W, Shen A, Huang M, Ding J, Geng M. O-GlcNAcylation of cofilin promotes breast cancer cell invasion. J Biol Chem. 2013;288:36418–25. doi: 10.1074/jbc.M113.495713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lucena MC, Carvalho-Cruz P, Donadio JL, Oliveira IA, de Queiroz RM, Marinho-Carvalho MM, de Paula IF, Gondim KC, McComb ME, Costello CE, Whelan SA, Todeschini AR, Dias WB. Epithelial mesenchymal transition induces aberrant glycosylation through hexosamine biosynthetic pathway activation. The Journal of biological chemistry. 2016 doi: 10.1074/jbc.M116.729236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ferrer CM, Lu T, Bacigalupa ZA, Katsetos CD, Sinclair DA, Reginato MJ. O-GlcNAcylation regulates breast cancer metastasis via SIRT1 modulation of FoxM1 pathway. Oncogene. 2016 doi: 10.1038/onc.2016.228. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hida K, Maishi N, Torii C, Hida Y. Tumor angiogenesis-characteristics of tumor endothelial cells. Int J Clin Oncol. 2016;21:206–12. doi: 10.1007/s10147-016-0957-1. [DOI] [PubMed] [Google Scholar]
- 72.Cai Y, Balli D, Ustiyan V, Fulford L, Hiller A, Misetic V, Zhang Y, Paluch AM, Waltz SE, Kasper S, Kalin TV. Foxm1 expression in prostate epithelial cells is essential for prostate carcinogenesis. J Biol Chem. 2013;288:22527–41. doi: 10.1074/jbc.M113.455089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rao X, Duan X, Mao W, Li X, Li Z, Li Q, Zheng Z, Xu H, Chen M, Wang PG, Wang Y, Shen B, Yi W. O-GlcNAcylation of G6PD promotes the pentose phosphate pathway and tumor growth. Nat Commun. 2015;6:8468. doi: 10.1038/ncomms9468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chaiyawat P, Chokchaichamnankit D, Lirdprapamongkol K, Srisomsap C, Svasti J, Champattanachai V. Alteration of O-GlcNAcylation affects serine phosphorylation and regulates gene expression and activity of pyruvate kinase M2 in colorectal cancer cells. Oncology reports. 2015;34:1933–42. doi: 10.3892/or.2015.4178. [DOI] [PubMed] [Google Scholar]
- 75.Bullen JW, Balsbaugh JL, Chanda D, Shabanowitz J, Hunt DF, Neumann D, Hart GW. Cross-talk between two essential nutrient-sensitive enzymes: O-GlcNAc transferase (OGT) and AMP-activated protein kinase (AMPK). J Biol Chem. 2014;289:10592–606. doi: 10.1074/jbc.M113.523068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Vella P, Scelfo A, Jammula S, Chiacchiera F, Williams K, Cuomo A, Roberto A, Christensen J, Bonaldi T, Helin K, Pasini D. Tet proteins connect the O-linked N-acetylglucosamine transferase Ogt to chromatin in embryonic stem cells. Mol Cell. 2013;49:645–56. doi: 10.1016/j.molcel.2012.12.019. [DOI] [PubMed] [Google Scholar]
- 77.Balasubramani A, Rao A. O-GlcNAcylation and 5-methylcytosine oxidation: an unexpected association between OGT and TETs. Mol Cell. 2013;49:618–9. doi: 10.1016/j.molcel.2013.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Myers SA, Panning B, Burlingame AL. Polycomb repressive complex 2 is necessary for the normal site-specific O-GlcNAc distribution in mouse embryonic stem cells. Proc Natl Acad Sci U S A. 2011;108:9490–5. doi: 10.1073/pnas.1019289108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Huang Y, Rao A. Connections between TET proteins and aberrant DNA modification in cancer. Trends Genet. 2014;30:464–74. doi: 10.1016/j.tig.2014.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Itkonen HM, Gorad SS, Duveau DY, Martin SE, Barkovskaya A, Bathen TF, Moestue SA, Mills IG. Inhibition of O-GlcNAc transferase activity reprograms prostate cancer cell metabolism. Oncotarget. 2016 doi: 10.18632/oncotarget.7039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ortiz-Meoz RF, Jiang J, Lazarus MB, Orman M, Janetzko J, Fan C, Duveau DY, Tan ZW, Thomas CJ, Walker S. A small molecule that inhibits OGT activity in cells. ACS Chem Biol. 2015;10:1392–7. doi: 10.1021/acschembio.5b00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gross BJ, Kraybill BC, Walker S. Discovery of O-GlcNAc transferase inhibitors. J Am Chem Soc. 2005;127:14588–9. doi: 10.1021/ja0555217. [DOI] [PubMed] [Google Scholar]
- 83.Kwei KA, Baker JB, Pelham RJ. Modulators of sensitivity and resistance to inhibition of PI3K identified in a pharmacogenomic screen of the NCI-60 human tumor cell line collection. PLoS One. 2012;7:e46518. doi: 10.1371/journal.pone.0046518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kanwal S, Fardini Y, Pagesy P, N'Tumba-Byn T, Pierre-Eugene C, Masson E, Hampe C, Issad T. O-GlcNAcylation-inducing treatments inhibit estrogen receptor alpha expression and confer resistance to 4-OH-tamoxifen in human breast cancer-derived MCF-7 cells. PloS one. 2013;8:e69150. doi: 10.1371/journal.pone.0069150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Alejandro EU, Bozadjieva N, Kumusoglu D, Abdulhamid S, Levine H, Haataja L, Vadrevu S, Satin LS, Arvan P, Bernal-Mizrachi E. Disruption of O-linked N-Acetylglucosamine Signaling Induces ER Stress and beta Cell Failure. Cell Rep. 2015;13:2527–38. doi: 10.1016/j.celrep.2015.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yuzwa SA, Vocadlo DJ. O-GlcNAc and neurodegeneration: biochemical mechanisms and potential roles in Alzheimer's disease and beyond. Chem Soc Rev. 2014;43:6839–58. doi: 10.1039/c4cs00038b. [DOI] [PubMed] [Google Scholar]
- 87.Ruan HB, Dietrich MO, Liu ZW, Zimmer MR, Li MD, Singh JP, Zhang K, Yin R, Wu J, Horvath TL, Yang X. O-GlcNAc transferase enables AgRP neurons to suppress browning of white fat. Cell. 2014;159:306–17. doi: 10.1016/j.cell.2014.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tomic J, McCaw L, Li Y, Hough MR, Ben-David Y, Moffat J, Spaner DE. Resveratrol has anti-leukemic activity associated with decreased O-GlcNAcylated proteins. Experimental hematology. 2013;41:675–86. doi: 10.1016/j.exphem.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 89.Wu LE, Gomes AP, Sinclair DA. Geroncogenesis: metabolic changes during aging as a driver of tumorigenesis. Cancer Cell. 2014;25:12–9. doi: 10.1016/j.ccr.2013.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yang YR, Song M, Lee H, Jeon Y, Choi EJ, Jang HJ, Moon HY, Byun HY, Kim EK, Kim DH, Lee MN, Koh A, Ghim J, Choi JH, Lee-Kwon W, Kim KT, Ryu SH, Suh PG. O-GlcNAcase is essential for embryonic development and maintenance of genomic stability. Aging Cell. 2012;11:439–48. doi: 10.1111/j.1474-9726.2012.00801.x. [DOI] [PubMed] [Google Scholar]
- 91.Rubinsztein DC, Marino G, Kroemer G. Autophagy and aging. Cell. 2011;146:682–95. doi: 10.1016/j.cell.2011.07.030. [DOI] [PubMed] [Google Scholar]
- 92.Hursting SD, Lavigne JA, Berrigan D, Perkins SN, Barrett JC. Calorie restriction, aging, and cancer prevention: mechanisms of action and applicability to humans. Annu Rev Med. 2003;54:131–52. doi: 10.1146/annurev.med.54.101601.152156. [DOI] [PubMed] [Google Scholar]
- 93.Wang P, Hanover JA. Nutrient-driven O-GlcNAc cycling influences autophagic flux and neurodegenerative proteotoxicity. Autophagy. 2013;9:604–6. doi: 10.4161/auto.23459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Haigis MC, Guarente LP. Mammalian sirtuins--emerging roles in physiology, aging, and calorie restriction. Genes Dev. 2006;20:2913–21. doi: 10.1101/gad.1467506. [DOI] [PubMed] [Google Scholar]
- 95.Mercken EM, Hu J, Krzysik-Walker S, Wei M, Li Y, McBurney MW, de Cabo R, Longo VD. SIRT1 but not its increased expression is essential for lifespan extension in caloric-restricted mice. Aging Cell. 2014;13:193–6. doi: 10.1111/acel.12151. [DOI] [PMC free article] [PubMed] [Google Scholar]