Abstract
Objective
Though sleep symptoms of insomnia can be quantified, none of the current diagnostic systems stipulate quantitative cut-offs for sleep-onset-latency (SOL) or wake-time-after-sleep-onset (WASO). Diagnoses are based instead on idiographic patient reports of ‘difficulty’ falling/staying asleep. Therefore, we examined whether remission of insomnia per diagnostic criteria results from a normalization of quantitative sleep disturbance, or if it is simply reflective of tolerance to sleep symptoms.
Methods
This study involved a year-long prospective investigation of 649 adults (48.1±11.6 y; 69.3% female) with DSM-5 based insomnia. Participants completed measures of sleep disturbance, perceived sleep-related distress, daytime sleepiness, functional impairment, and workplace productivity at baseline and follow-up one year later.
Results
271 participants no longer met DSM-5 based insomnia criteria at follow-up. However, 66% of these remitters reported ≥ 31 minutes of SOL and/or WASO. Importantly, daytime impairment in this subgroup of remitters was no different than among individuals who met diagnostic criteria at both baseline and follow-up (i.e., chronic insomniacs). By contrast, follow-up impairment was significantly lower (F = 12.3; p < .01) among remitters whose sleep disturbance returned below empirically-derived quantitative cut-offs (both SOL & WASO < 31 minutes) than in chronic insomniacs.
Conclusion
This is the first study on the long-term course of insomnia based on the newly established DSM-5 criteria. A troubling implication of findings is that a majority of insomniacs stop meeting diagnostic criteria despite continued sleep disturbance and impairment. ‘Remission’ in these cases is attributable instead to tolerance of sleep symptoms. Incorporating quantitative criteria into current diagnoses may offer a more sensitive assay of treatment needs.
Keywords: insomnia, remission, functional impairment, quantitative criteria, diagnostic considerations
1. Introduction
The primary obstacle to establishing clinically meaningful sleep-based cut-offs for insomnia disorder is that a diagnosis is based on not just sleep disturbance per se but the distress associated with sleep disturbance. Nearly all current diagnostic systems stipulate that nocturnal sleep disturbances cause clinically significant distress to warrant an insomnia diagnosis [1,2]. Importantly, data on the magnitude of nocturnal sleep symptoms, such as difficulty falling asleep (sleep onset latency: SOL) or staying asleep (wake-time after sleep onset: WASO), are judged to be clinically informative but unnecessary for a diagnosis. This diagnostic emphasis on sleep-related distress raises an important question: is there a subset of insomniacs who over time simply develop a tolerance for nocturnal insomnia symptoms despite no appreciable reduction in sleep disturbance? Further, can current diagnostic criteria capture this population?
In a classic study, Stepanski et al. [3] compared a group of research volunteers with insomnia to individuals who sought treatment for insomnia at a sleep clinic. While there were no differences between groups in sleep disturbance, the latter reported significantly higher distress, thus suggesting that distress is an important predictor of treatment seeking. Individuals who experience significant sleep disturbance but do not report sleep-related distress are hence a high-risk group; they face the significant morbidity associated with sleep disturbance [4,5], and yet may not seek treatment. This scenario is particularly germane to individuals with insomnia as this disorder takes a highly chronic course once it manifests [6,7]. However, it is presently unclear what proportion of insomniacs stop perceiving their sleep symptoms as distressing, and, more importantly, whether current diagnostic systems can capture this population. Further, nearly all prior studies [8–10] on insomnia-related impairment have been cross-sectional. As such, it is unknown whether remission of insomnia is accompanied by significant reductions in nocturnal sleep symptoms and a commensurate improvement in daytime functioning.
The primary aim of this study, therefore, was to examine whether remission of insomnia per diagnostic criteria is associated with a normalization of sleep disturbance according to empirically validated quantitative cut-offs [11] and, as such, with a reduction in functional impairment. In other words, we sought to determine whether insomnia remission per current diagnostic criteria is attributable to a reduction in sleep disturbance and daytime impairment, or simply to an increased tolerance of sleep symptoms. We analyzed longitudinal data from a large cohort of adults with insomnia per DSM-5 [1] based criteria. All participants provided extensive sleep disturbance data, including chronicity, frequency, and sleep-related distress at both baseline as well as at a follow-up assessment one year later. Additionally, participants completed measures of daytime sleepiness, functional impairment, and workplace productivity.
2. Methods
All study procedures were approved by the Institutional Review Board at Henry Ford Hospital.
2.1. Setting and participants
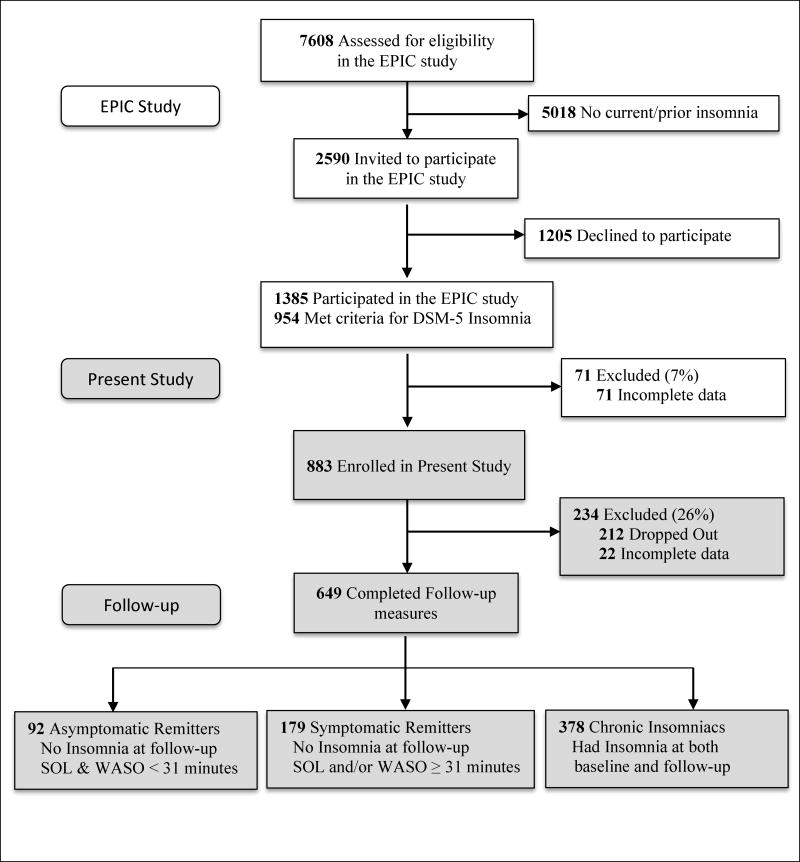
The present sample derives from the Evolution of Pathways to Insomnia Cohort (EPIC) study, a large NIMH-funded prospective investigation of individuals with current/past insomnia. A detailed description of recruitment strategies and sample characteristics appears in a previous report [12]. Briefly summarized, a total of 7608 individuals were randomly selected from a major statewide HMO database, and completed a web-delivered eligibility survey which assessed for insomnia history (Figure 1). Roughly 34% (n = 2590) of this initial sample met diagnostic criteria for current/prior insomnia and were invited to participate in the EPIC study, 46% (n = 1205) of whom declined. The remaining 1385 participated in the EPIC study. Here, we present data from EPIC study participants who met the following inclusion criteria: met DSM-5 based criteria for current insomnia (n = 954); completed baseline measures of sleep disturbance, daytime sleepiness, functional impairment, and workplace productivity (n = 883). A total of 649 participants from this initial sample completed follow-up measures one year later (retention rate: 74%).
Figure 1.
Flow of participants through the study.
2.2. Assessments
Insomnia
Classification of insomnia occurred per DSM-5 based criteria [1]. Accordingly, participants were classified as having insomnia if they reported experiencing one or more sleep complaints (e.g., ‘have you experienced difficulty falling asleep?’; ‘have you experienced difficulty staying asleep?’ etc.) for at least 3 nights a week for a period of 3 months or longer. Further, they had to endorse sleep-related daytime distress or impairment as measured by the following question: ‘to what extent do you consider your sleep problems to interfere with your daily functioning (e.g., daytime fatigue, ability to function at work/daily chores, concentration, memory, mood etc.)?’ Responses were coded on a 4-point Likert type scale: ‘0’ (‘not at all’); 1 (‘a little’); 2 (‘somewhat’), 3 (‘much’); ‘4’ (‘very much interfering’). Participants who reported a score of ‘2’ or higher were classified as insomniacs. This technique has been used to identify insomnia-related daytime impairment in recent epidemiological studies, and has been validated against clinician-administered diagnostic interviews [9,13].
Participants also reported the extent of sleep disturbance during the previous month: SOL i.e., ‘on average (including weekends and weekdays), how long did it take you to fall asleep’; and total WASO i.e., ‘on average (including weekdays and weekends), how long are you awake during the night’. Total sleep time (TST) was assessed over an average weekday (‘thinking about your average weekday, how long did you actually sleep each night’), given the tendency of insomniacs to engage in compensatory sleep (‘sleeping in’) on the weekends [14]. The chronicity of sleep problems was assessed via the following question: ‘how long have you had your sleep problem’? Any prescription/over-the-counter (OTC) medication use was assessed using the following questions: ‘in the past month, have you taken any prescription medications to help you sleep’; ‘in the past month, have you taken any OTC medications to help you sleep’. For each endorsed item above, participants also reported the frequency of use: ‘on average, how many times per month do you take this medication’.
Functional Impairment
The Epworth Sleepiness Scale (ESS) was used to assess levels of daytime sleepiness, with overall scores of 10 or greater indicating excessive or clinically significant sleepiness [15]. Daytime impairments per Research Diagnostic Criteria (RDC) for insomnia were also assessed [16]. Examples of RDC daytime impairment items are: ‘attention, concentration, or memory impairment’; ‘mood disturbance/irritability’; ‘daytime fatigue’. All such items were scored on a 4-point Likert type scale from 0 (‘None’) to 3 (‘Severe’), and were summed to produce an overall daytime impairment score. This scale exhibited high internal consistency (Cronbach’s α = .91).
Workplace productivity and role impairment (absenteeism/presenteeism) were assessed using the following questions from the World Health Organization’s Health and Work Performance Questionnaire (HPQ) – Short Form [17]: ‘how many days in the past 4 weeks did you miss an ENTIRE work day’; ‘how many days in the past 4 weeks did you miss PART of a work day’; ‘how many hours altogether did you work in the past 7 days?’ The above items achieved excellent internal consistency in the present sample (Cronbach’s α = .92).
Comorbid Sleep Disorders
The Berlin Apnea Questionnaire (BAQ) was used to assess risk for obstructive sleep apnea [18]. The BAQ identifies respondents at risk for Obstructive Sleep Apnea based on a combination of signs and symptoms: snoring; daytime sleepiness and fatigue; obesity and hypertension. Restless Legs Syndrome (RLS) was assessed using an empirically validated question set developed by the International RLS Study Group [19]. Participants were identified to be at risk for RLS if they met the following diagnostic criteria: (1) urge to move your legs/uncomfortable or unpleasant feelings in your legs; (2) these symptoms occur during periods of rest/inactivity, such as while sitting or lying down; (3) these symptoms improve if you get up and start walking; (4) these symptoms only occur during the evening/night.
2.3. Data analysis
All statistical analyses were conducted using IBM SPSS Statistics [20] for Windows – Version 19 (Armonk, NY). ANCOVA models were used for between-group comparisons involving multiple factors or covariates. Given the extensive array of variables, covariates were selected for inclusion in omnibus models per recommendations outlined by Mickey & Greenland [21]. Specifically, only those variables which were related to the dependent variable in univariate analyses at a significance level of p < .20 were retained in the final model. Between-group differences in change scores were accomplished via ANOVA models; though ANCOVA models with difference scores as the dependent variable (DV) and baseline scores as a covariate are widely used, recent simulation studies conclude that this approach is inappropriate for evaluating change scores across two time points in naturally occurring groups [22].
3. Results
A total of 271 participants from the initial sample of 649 insomniacs no longer met diagnostic criteria for insomnia at follow-up (remission rate: 41.8%), which is consistent with existing data on the natural course of insomnia [23]. However, mean levels of sleep disturbance among remitters remained high: SOL (mean = 43.52 ± 43.58 minutes); WASO (mean = 59.93 ± 63.69); TST (mean = 366.18 ± 74.90). Importantly, 66.1% reported ≥ 31 minutes of SOL and/or WASO at follow-up, a cut-off suggested in prior reports [11] as an empirically valid threshold for diagnosing insomnia disorder. Despite these continued sleep disturbances, almost 86% of remitters reported that their sleep problems interfered only ‘a little’ or ‘not at all’ in their daily functioning. To further explore this finding, participants were categorized into three groups: ‘chronic insomniacs’ who met DSM-5 based insomnia criteria at both baseline and follow-up assessment (n = 378); symptomatic remitters, who no longer met these criteria at follow-up, but continued to report ≥ 31 minutes of SOL and/or WASO (n = 179); asymptomatic remitters, who stopped meeting insomnia criteria at follow-up and also reported < 31 minutes of SOL and WASO (n = 92).
3.1. Demographic and clinical characteristics
Though some racial diversity was observed (African American: 24.8%), this sample was predominantly white (69%) and middle-aged (48.1 ± 11.6 years). Further, women were over-represented (69%), reflecting the gender disparity in the prevalence of insomnia disorder [24]. As for comorbid sleep disorders, the proportion (~29%) of participants at risk for OSA was comparable to the estimated prevalence of OSA (29% – 67%) among insomniacs in the general population [25]. The prevalence of RLS (~7%) in the present sample was similar to population-based (7% – 10%) estimates [26].
A chi-square test of independence between gender and follow-up group membership was statistically significant (χ2 = 13.61, p < .01). Examination of individual cells suggested that this effect was driven primarily by the male asymptomatic remitters group; there were significantly more male asymptomatic remitters (z = 2.8, p < .05) than expected per the null hypothesis (Table 1). The conditional distribution of race across groups revealed too few cases for ‘Asian’ and ‘Other’ in a number of cells for chi-square analyses. We therefore assessed the independence between group membership and race for the two most prevalent categories of the race variable: ‘White’ and ‘African-American’; chi-square analyses did not reveal a significant relationship (χ2 = 4.36, p = .11). There were no between-group differences at baseline in marital status, employment rates, risk for comorbid sleep disorders, or medication use (Table 1). Given the gender disparity across groups, between-group differences in other continuous measures were estimated via ANCOVA models with gender as a covariate. There were no baseline group differences in ESS scores or any indices of workplace productivity. Gender was not significantly associated with ESS scores, daytime impairment, or workplace productivity.
Table 1.
Demographic and clinical characteristics statistics stratified by follow-up group membershipa
| Groups based on follow-up insomnia and sleep disturbance
|
|||||
|---|---|---|---|---|---|
| Overall Sample (n = 649) | Asymptomatic Remitters (n = 92) | Symptomatic Remitters (n = 179) | Chronic Insomniacs (n = 378) | χ2 | |
| % | % | % | % | ||
| Gender (Women) | 69.3 | 53.3b | 69.8 | 73.0 | 13.6; p < .01 |
| Race | |||||
| White | 69.0 | 81.5 | 68.2 | 66.4 | 4.4; p = .11 |
| African American | 24.8 | 17.4 | 25.7 | 26.2 | |
| Asian | 1.1 | 1.1 | 1.7 | 0.8 | |
| Other | 5.1 | 0.0 | 4.4 | 6.6 | |
| Marital Status: | |||||
| Married | 61.6 | 63.0 | 63.1 | 60.6 | 0.4; p = .81 |
| Comorbid Sleep Disorders | |||||
| Risk for OSA | 29.1 | 30.4 | 24.6 | 33.3 | 4.4; p = .11 |
| Risk for RLS | 6.8 | 5.4 | 6.1 | 7.4 | 0.6; p = .74 |
| Medication Use (Yes/No) | |||||
| Prescription Medication | 19.1 | 14.1 | 19.0 | 20.4 | 1.9; p = .39 |
| OTC Medication | 19.3 | 16.3 | 15.6 | 21.7 | 3.5; p = .18 |
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | F-test | |
| Age | 48.1 (11.6) | 46.9 (12.3) | 47.5 (12.1) | 48.7 (11.2) | 1.3; p = .28 |
| Medication Use (days/month) | |||||
| Prescription Medication | 14.0 (12.0 | 15.1 (10.3) | 12.4 (11.5) | 14.4 (12.4) | 0.6; p = .57 |
| OTC Medication | 11.0 (10.0) | 12.8 (11.3) | 7.9 (7.6) | 11.1 (10.3) | 1.7; p = .19 |
| Epworth Sleepiness Scale | 8.6 (4.6) | 8.6 (4.3) | 8.1 (4.3) | 8.9 (4.7) | 2.0; p = .14 |
| Workplace Productivity | |||||
| Number of missed days | 0.4 (1.5) | 0.2 (0.8) | 0.4 (1.3) | 0.5 (1.7) | 1.9; p = .14 |
| Number of part-day misses | 0.4 (1.6) | 0.3 (1.2) | 0.3 (1.6) | 0.5 (1.6) | 1.1; p = .32 |
| Number of hours worked | 28.0 (23.2) | 30.8 (21.0) | 30.2 (26.4) | 26.8 (23.6) | 1.8; p = .17 |
Though baseline statistics are presented here, groups were created based on insomnia status and sleep disturbance at follow-up assessment;
The observed count in this cell was significantly less than expected per the null hypothesis;
SD, Standard deviation; OTC, Over the counter; OSA, Obstructive sleep apnea; RLS, restless leg syndrome.
3.2. Changes in nocturnal sleep symptoms
Next, we examined between-group differences in sleep disturbance changes from baseline to follow-up. Preliminary analyses did not reveal an independent relationship between changes in sleep disturbance and any potential covariates, including gender or ESS scores. Therefore, per guidelines for evaluating change scores across two time points in naturally occurring groups [26], ANOVA models were run with change scores in SOL, WASO, and TST as the dependent variables (DV) and group membership as the independent variable (Table 2). The overall ANOVA model for SOL changes scores did not achieve statistical significance (F = 2.38; p = .09). However, post-hoc, Fisher’s LSD tests indicated that the asymptomatic remitters (mean = −16.25 ± 26.85 minutes) reported a significantly larger decrease in SOL than both the symptomatic remitters (mean = −5.25 ± 50.45 minutes; p < .05) and the chronic insomniacs (mean = −5.82 ± 42.99 minutes; p < .05); chronic insomniacs and symptomatic remitters did not differ significantly. With respect to WASO, the ANOVA model was statistically significant (F = 3.19; p < .01; partial η2 = .01), such that asymptomatic remitters (mean = −28.48 ± 49.63 minutes) reported a significantly larger decrease in WASO from baseline to follow-up than did the chronic insomniacs (mean = −6.96 ± 79.30 minutes); there were no other significant group differences. Finally, there were significant differences between all three groups on changes in TST (F = 7.65; p < .01; partial η2 = .02); chronic insomniacs reported the least change (mean = +1.76 ± 74.20 minutes), whereas the asymptomatic remitters reported the largest increase (mean = +32.88 ± 61.93 minutes).
Table 2.
Changes in sleep disturbance and daytime impairment
| Baseline M (SD) | Follow-up M (SD) | Change M (SD) | ||
|---|---|---|---|---|
|
|
||||
| Sleep onset latency (minutes) | F = 2.4; p = .09; partial η2 = .01 | |||
| Asymptomatic Remitters | 33.8 (26.7) | 17.5 (8.9) | − 16.3 (26.9) | |
| Symptomatic Remitters | 62.2 (49.8) | 56.9 (48.2) | − 5.3 (50.5)a | |
| Chronic Insomniacs | 64.3 (49.9) | 58.5 (45.3) | − 5.8 (43.0)a | |
| Wake Time After Sleep Onset (minutes) | F = 3.2; p < .05; partial η2 = .01 | |||
| Asymptomatic Remitters | 44.2 (50.6) | 15.8 (9.9) | − 28.5 (49.6) | |
| Symptomatic Remitters | 93.7 (74.2) | 82.6 (67.7) | − 11.1 (70.2) | |
| Chronic Insomniacs | 93.5 (71.2) | 86.6 (74.0) | − 7.0 (79.3)a | |
| Total sleep time (minutes) | F = 7.7; p < .01; partial η2 = .02 | |||
| Asymptomatic Remitters | 351.0 (71.7) | 383.9 (71.7) | + 32.9 (61.9)b,c | |
| Symptomatic Remitters | 340.6 (86.3) | 357.2 (75.1) | + 16.5 (75.4)a,c | |
| Chronic Insomniacs | 328.2 (77.4) | 329.9 (78.5) | + 1.8 (74.2)a,b | |
| Daytime Impairment | F = 12.3; p < .01; partial η2 = .04 | |||
| Asymptomatic Remitters | 29.5 (7.2) | 25.7 (7.7) | − 3.8 (5.0) | |
| Symptomatic Remitters | 32.0 (7.0) | 28.9 (7.0) | − 3.1 (5.4) | |
| Chronic Insomniacs | 38.3 (8.4) | 37.4 (9.4) | − 0.9 (5.6)a | |
| Epworth Sleepiness Scale | F = 1.1; p = .34; partial η2 = .00 | |||
| Asymptomatic Remitters | 8.6 (4.3) | 7.9 (4.2) | − 0.7 (2.6) | |
| Symptomatic Remitters | 8.1 (4.3) | 7.6 (4.0) | − 0.5 (3.4) | |
| Chronic Insomniacs | 8.9 (4.7) | 8.7 (4.7) | − 0.2 (3.5) | |
| Number of hours worked: | F = 3.2; p < .05; partial η2 = .01 | |||
| Asymptomatic Remitters | 30.8 (21.0) | 35.9 (29.9) | + 5.2 (30.0) | |
| Symptomatic Remitters | 30.2 (26.4) | 27.4 (22.8) | − 2.8 (24.6)a | |
| Chronic Insomniacs | 26.8 (23.6) | 27.1 (22.6) | + 0.3 (23.2) | |
significantly different from the ‘Asymptomatic Remitters’ group;
significantly different from the ‘Symptomatic Remitters’ group,
significantly different from the ‘Chronic Insomniacs’ group; SD, Standard deviation.
3.3. Changes in daytime impairment and workplace productivity
To examine between-group differences in changes from baseline to follow-up in daytime impairment, we ran an ANCOVA model with daytime impairment change scores as the DV, group membership as the independent variable, and changes in SOL, WASO, and TST as covariates (Table 2). The overall model was significant (Omnibus F = 10.68; p < .01; Adjusted R2 = .07). All three sleep parameter change scores were significantly associated with daytime impairment changes, such that larger improvements in sleep parameters were associated with larger improvements in daytime impairment: delta SOL (F = 5.10; p < .05; partial η2 = .01); delta WASO (F = 7.41; p < .01; partial η2 = .01); delta TST (F = 4.58; p < .05; partial η2 = .01). Group differences were also statistically significant (F = 12.26; p < .01; partial η2 = .04); asymptomatic remitters (mean = −3.82 ± 4.99) reported a significantly larger decrease in daytime impairment than the chronic insomniacs (mean = −0.92 ± 5.64).
There were no significant differences between groups in ESS change scores.
3.4. Changes in workplace productivity
Preliminary analyses did not reveal any independent relationships between changes in ‘number of hours worked’ and any potential covariates, including sleep disturbance changes. Hence, we ran an ANOVA model with changes in ‘number of hours worked’ from baseline to follow-up as the DV and group membership as the IV. Group differences were statistically significant (F = 3.18; p < .05; partial η2 = .01), such that asymptomatic remitters reported a significantly larger increase (mean = +5.18 ± 29.98 hours) in hours worked than the symptomatic remitters (mean = −2.76 ± 24.59 hours; p < .05). Changes in number of days/partial-days missed were not significantly different between groups.
4. Discussion
Though sleep disturbance in insomnia can be quantified, none of the current diagnostic systems enforce quantitative thresholds for nocturnal insomnia symptoms. Instead, diagnoses are determined based on whether or not patients report ‘difficulty’ falling/staying asleep. This more sensitive albeit less specific approach is largely defensible in light of the significant morbidity associated with insomnia disorder [27]. The emergence of safe and effective behavioral [28] and pharmacological [29] treatments further justifies casting a wide net to capture this debilitating disorder. However, in the present study, we found that a paradoxical effect of this diagnostic reliance on patient report is that a significant proportion of insomniacs stop meeting DSM-5 based insomnia criteria despite continued sleep disturbance. Indeed, the surprising majority (> 65%) of remitters i.e., individuals who no longer met diagnostic criteria for insomnia, reported sleep disturbances in excess of standardized [11] quantitative cut-offs (≥ 31 minutes of SOL and/or WASO). Only a small proportion reported a relative normalization of sleep disturbance (both SOL and WASO < 31 minutes) in conjunction with insomnia remission. Importantly, these groups differed significantly on various indices of functional impairment, a primary determinant of treatment seeking [3,30].
4.1. Symptomatic Remitters
Changes in sleep parameters, including SOL and WASO, from baseline to follow-up in this subset of remitters were not significantly different than corresponding changes among chronic insomniacs. Average follow-up sleep disturbance (SOL ~ 57 minutes; WASO ~ 83 minutes) in these symptomatic remitters was clinically significant by all current cut-offs [8,11,31]. Understandably, this group, not unlike the chronic insomniacs, reported no significant improvements in overall functioning. The most striking clinical feature of this group, therefore, was that over 85% of these individuals reported that their sleep disturbance interfered only ‘a little’ or ‘not at all’ in their daily functioning; by definition, 0% characterized their sleep disturbance as such at baseline. One potential explanation for this dramatic change in perception is that this group did on average experience a significantly larger improvement in average nightly TST than did chronic insomniacs. However, the effect size was small (mean increase ~ 17 minute from baseline), and, more importantly, the average nightly TST for symptomatic remitters was still below 6 hours (see Table 2). Recent studies show that insomnia combined with chronic insufficient sleep (< 6 hours) is associated with increased risk for morbidity, including diabetes and hypertension. [32,33]
This is the first study to identify such a group of symptomatic remitters i.e., former insomniacs who fail to meet criteria due to a lack of sleep-related distress. Notably, a series of studies by another research team [23] adopted a similar approach to categorize sleep disturbance: ‘good sleepers’, who did not report insomnia symptoms; an ‘insomnia symptoms’ group, composed of individuals who reported insomnia symptoms but did not meet diagnostic criteria; and an ‘insomnia syndrome’ group of individuals who met diagnostic criteria for insomnia. However, the ‘insomnia symptoms’ group in these prior studies included both individuals who were satisfied with their sleep despite significant sleep disturbance (> 30 minutes of SOL/WASO), as well as those who were dissatisfied with sleep in the absence of significant sleep disturbance. The present study is the first to parse out sleep disturbance from sleep satisfaction in the context of insomnia. That current diagnostic systems are ill-equipped to capture symptomatic remitters is concerning, given both the severity of sleep disturbance in this population and the significant morbidity associated with chronic sleep disturbance.
Finally, in addition to these diagnostic issues, the identification of such symptomatic remitters in the present study also has important treatment implications, especially for cognitive behavior therapy for insomnia (CBTI). The cognitive module of CBTI aims to address maladaptive and dysfunctional beliefs about sleep among insomniacs, given that this population often overestimates both sleep need as well as the daytime consequences of sleep loss [34,35]. A potential side-effect of treatment, therefore, is that a subset of patients (i.e., symptomatic remitters) may learn to tolerate clinically significant levels of sleep disturbance. Thus, clinicians are urged to strike a careful balance between re-structuring unreasonable expectations about sleep, and, in the same vein, emphasizing the minimal sleep requirement for healthy functioning. After all, the goal of treatment is not just to minimize insomnia symptoms, but to promote optimal sleep health.
4.2. Asymptomatic Remitters
Few studies [8,36] have examined the prospective relationship between nocturnal insomnia symptoms and daytime functioning. The present data are among the first to show that a decline over time in the severity of nocturnal insomnia symptoms, including TST, SOL, and WASO, is significantly associated with improvements in daytime functioning. Asymptomatic remitters, i.e., former insomniacs whose sleep disturbance remitted below quantitative clinical cut-offs (< 31 minutes of SOL and WASO), showed a significantly larger improvement in daytime functioning than chronic insomniacs. This group also reported a significantly greater increase in number of weekly work hours (~ +5 hours) than symptomatic remitters. Note that asymptomatic remitters reported significantly lower levels of baseline impairment, thus speaking to the strength of these effects; per the law of initial values, a significant decrease in scores is more difficult to detect when baseline scores are low. Notably, there were no remarkable changes in daytime sleepiness in any of the groups, with ESS scores remaining sub-threshold throughout the study. This finding is highly consistent with recent reports suggesting that hyper-arousal is a stable, trait-like feature of insomnia, and is largely independent of nocturnal sleep disturbance [37].
Certain baseline differences between this group and the other two groups were also quite intriguing. First, both the symptomatic remitters and chronic insomniacs had more severe sleep disturbance at baseline. Second, only 54% of the eventual asymptomatic remitters reported quantitatively significant SOL/WASO at baseline. By contrast, this proportion was 90% among symptomatic remitters and 87% in chronic insomniacs. Finally, while symptomatic remitters and chronic insomniacs evidenced the well-known gender disparity in insomnia, gender distribution was largely even (54% female) among asymptomatic remitters. A relatively small proportion (~ 14%) of the overall sample, asymptomatic remitters may therefore represent mild insomnia cases, and thus account for the sample variability in sleep disturbance at baseline.
4.3. Limitations and Future Directions
Current findings must be interpreted in light of certain methodological limitations. First, insomnia was classified based on self-report questionnaires and not gold-standard clinician interviews. Note that nearly identical techniques have been used to identify insomnia in other large population-based survey studies [9]. Reported sleep parameter estimates were not corroborated by more objective assessment techniques such as PSG or actigraphy. However, prior attempts to establish sensitive and specific quantitative cut-offs for both PSG- and actigraphy-defined sleep parameters have been unsuccessful [38,39], thus rendering these assessment techniques largely superfluous to the present study’s aims. We also lacked data on whether and what proportion of participants received treatment for insomnia between baseline and follow-up. Notably, medication use, a proxy for treatment, did not vary between groups. Finally, though we present prospective data, this study only included two time-points a whole year apart. More frequent assessments in future studies can offer a more nuanced and sensitive perspective on the trajectory of sleep disturbance and diagnostic changes.
Nevertheless, we believe this study carries significant clinical and diagnostic implications. To the best of our knowledge, it is the first to delineate the long-term clinical course of insomnia disorder based on the newly established DSM-5 diagnostic criteria [1]. This report is especially unique in its focus on the changes in sleep and daytime impairment associated with remission in a large and representative sample of former insomniacs. Findings point to an important problem with current diagnostic criteria for insomnia i.e., that it is possible to ‘remit’ by diagnostic criteria despite continued and significant sleep disturbance and daytime impairment. An important ramification is that a substantial portion of individuals with insomnia may prematurely drop off the clinician’s radar. Our findings are therefore largely supportive of recent efforts to derive quantitative thresholds for the severity of nocturnal sleep symptoms and to incorporate these into future diagnostic nosologies.
Supplementary Material
Highlights.
Many insomniacs stop meeting diagnostic criteria despite continued sleep disturbance (≥ 31 minutes of SOL/WASO).
‘Remission’ in these cases is attributable instead to tolerance of sleep symptoms.
Incorporating quantitative criteria for nocturnal insomnia symptoms may help solve this problem.
Acknowledgments
This study was supported by an NIMH Grant (R01 MH082785) and an investigator initiated research award from Merck & Co, both to Dr. Drake. The NIMH and Merck had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Footnotes
Conflicts of Interest Disclosures: Dr. Roth has served as consultant for Abbott, Accadia, AstraZenca, Aventis, AVER, Bayer, BMS, Cypress, Ferrer, Glaxo Smith Kline, Impax, Intec, Jazz, Johnson and Johnson, Merck, Neurocrine, Novartis, Proctor and Gamble, Pfizer, Purdue, Shire, Somaxon, Transcept; has received research support from Cephalon, Merck, and Transcept; and has served on speakers bureau for Purdue. Dr. Drake has received research support from Merck, Interclinic, Aladdin Dreamer, and Teva; and has served on speakers bureau for Teva and Merck. Dr. Pillai indicated no financial conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-5™. 5. Arlington, VA US: American Psychiatric Publishing, Inc; 2013. [Google Scholar]
- 2.American Academy of Sleep Medicine. International Classification of Sleep Disorders: Diagnostic and Coding Manual. 3. Rochester, MN: Sleep Disorders Association; 2014. [Google Scholar]
- 3.Stepanski E, Koshorek G, Zorick F, Glinn M, Roehrs T, Roth T. Characteristics of individuals who do or do not seek treatment for chronic insomnia. Psychosomatics. 1989;30:421–7. doi: 10.1016/S0033-3182(89)72248-9. [DOI] [PubMed] [Google Scholar]
- 4.Luyster FS, Strollo PJ, Zee PC, Walsh JK. Sleep: a health imperative. Sleep. 2012;35:727–34. doi: 10.5665/sleep.1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dew MA, Hoch CC, Buysse DJ, Monk TH, Begley AE, Houck PR, et al. Healthy older adults’ sleep predicts all-cause mortality at 4 to 19 years of follow-up. Psychosom Med. 65:63–73. doi: 10.1097/01.psy.0000039756.23250.7c. [DOI] [PubMed] [Google Scholar]
- 6.Janson C, Lindberg E, Gislason T, Elmasry A, Boman G. Insomnia in men--a 10-year prospective population based study. Sleep J Sleep Sleep Disord Res. 2001;24:430. doi: 10.1093/sleep/24.4.425. [DOI] [PubMed] [Google Scholar]
- 7.Mendelson W. Long-term follow-up of chronic insomnia. Sleep J Sleep Res Sleep Med. 1995;18:701. doi: 10.1093/sleep/18.8.698. [DOI] [PubMed] [Google Scholar]
- 8.Drake CL, Vargas I, Roth T, Friedman NP. Quantitative measures of nocturnal insomnia symptoms predict greater deficits across multiple daytime impairment domains. Behav Sleep Med. 2015;13:73–87. doi: 10.1080/15402002.2014.880345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Espie CA, Kyle SD, Hames P, Cyhlarova E, Benzeval M. The daytime impact of DSM-5 insomnia disorder: comparative analysis of insomnia subtypes from the Great British Sleep Survey. J Clin Psychiatry. 2012;73:e1478–84. doi: 10.4088/JCP.12m07954. [DOI] [PubMed] [Google Scholar]
- 10.Shekleton JA, Rogers NL, Rajaratnam SMW. Searching for the daytime impairments of primary insomnia. Sleep Med Rev. 2010;14:47–60. doi: 10.1016/j.smrv.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 11.Lichstein KL, Durrence HH, Taylor DJ, Bush AJ, Riedel BW. Quantitative criteria for insomnia. Behav Res Ther. 2003;41:427–45. doi: 10.1016/s0005-7967(02)00023-2. [DOI] [PubMed] [Google Scholar]
- 12.Pillai V, Roth T, Drake CL. The nature of stable insomnia phenotypes. Sleep. 2015;38:127–38. doi: 10.5665/sleep.4338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kessler RC, Coulouvrat C, Hajak G, Lakoma MD, Roth T, Sampson N, et al. Reliability and validity of the brief insomnia questionnaire in the America insomnia survey. Sleep. 2010;33:1539–49. [PMC free article] [PubMed] [Google Scholar]
- 14.Jefferson CD, Drake CL, Scofield HM, Myers E, McClure T, Roehrs T, et al. Sleep hygiene practices in a population-based sample of insomniacs. Sleep. 2005;28:611–5. doi: 10.1093/sleep/28.5.611. [DOI] [PubMed] [Google Scholar]
- 15.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–5. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 16.Edinger JD, Bonnet MH, Bootzin RR, Doghramji K, Dorsey CM, Espie CA, et al. Derivation of research diagnostic criteria for insomnia: report of an American Academy of Sleep Medicine Work Group. Sleep. 2004;27:1567–96. doi: 10.1093/sleep/27.8.1567. [DOI] [PubMed] [Google Scholar]
- 17.Kessler RC, Barber C, Beck A, Berglund P, Cleary PD, McKenas D, et al. The World Health Organization Health and Work Performance Questionnaire (HPQ) J Occup Environ Med. 2003;45:156–74. doi: 10.1097/01.jom.0000052967.43131.51. [DOI] [PubMed] [Google Scholar]
- 18.Netzer NC, Stoohs RA, Netzer CM, Clark K, Strohl KP. Using the Berlin Questionnaire to identify patients at risk for the sleep apnea syndrome. Ann Intern Med. 1999;131:485–91. doi: 10.7326/0003-4819-131-7-199910050-00002. [DOI] [PubMed] [Google Scholar]
- 19.Allen RP, Picchietti D, Hening WA, Trenkwalder C, Walters AS, Montplaisi J. Restless legs syndrome: diagnostic criteria, special considerations, and epidemiology. A report from the restless legs syndrome diagnosis and epidemiology workshop at the National Institutes of Health. Sleep Med. 2003;4:101–19. doi: 10.1016/s1389-9457(03)00010-8. [DOI] [PubMed] [Google Scholar]
- 20.IBM SPSS Statistics for Windows, Version 19.0. 2010. [Google Scholar]
- 21.Mickey RM, Greenland S. The impact of confounder selection criteria on effect estimation. Am J Epidemiol. 1989;129:125–37. doi: 10.1093/oxfordjournals.aje.a115101. [DOI] [PubMed] [Google Scholar]
- 22.Jamieson J. Analysis of covariance (ANCOVA) with difference scores. Int J Psychophysiol. 2004;52:277–83. doi: 10.1016/j.ijpsycho.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 23.Morin CM, Bélanger L, LeBlanc M, Ivers H, Savard J, Espie CA, et al. The natural history of insomnia: a population-based 3-year longitudinal study. Arch Intern Med. 2009;169:447–53. doi: 10.1001/archinternmed.2008.610. [DOI] [PubMed] [Google Scholar]
- 24.Roth T, Coulouvrat C, Hajak G, Lakoma MD, Sampson NA, Shahly V, et al. Prevalence and perceived health associated with insomnia based on DSM-IV-TR; International Statistical Classification of Diseases and Related Health Problems, Tenth Revision; and Research Diagnostic Criteria/International Classification of Sleep Disorders. Biol Psychiatry. 2011;69:592–600. doi: 10.1016/j.biopsych.2010.10.023. [DOI] [PubMed] [Google Scholar]
- 25.Ong JC, Crisostomo MI. The More the Merrier? Working Towards Multidisciplinary Management of Obstructive Sleep Apnea and Comorbid Insomnia. J Clin Psychol. 2013;69:1066–77. doi: 10.1002/jclp.21958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gamaldo CE, Earley CJ. Restless legs syndrome: a clinical update. Chest. 2006;130:1596–604. doi: 10.1378/chest.130.5.1596. [DOI] [PubMed] [Google Scholar]
- 27.Roth T, Drake C. Defining insomnia: the role of quantitative criteria. Sleep. 2006;29:424–5. doi: 10.1093/sleep/29.4.424. [DOI] [PubMed] [Google Scholar]
- 28.Harvey AG, Tang NK. Cognitive behaviour therapy for primary insomnia: can we rest yet? Sleep Med Rev. 2003;7:237–62. doi: 10.1053/smrv.2002.0266. [DOI] [PubMed] [Google Scholar]
- 29.Roehrs T, Roth T. Insomnia pharmacotherapy. Neurotherapeutics. 2012;9:728–38. doi: 10.1007/s13311-012-0148-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Davidson JR, Aime A, Ivers H, Morin CM. Characteristics of individuals with insomnia who seek treatment in a clinical setting versus those who volunteer for a randomized controlled trial. Behav Sleep Med. 2009;7:37–52. doi: 10.1080/15402000802577769. [DOI] [PubMed] [Google Scholar]
- 31.Lineberger MD, Carney CE, Edinger JD, Means MK. Defining insomnia: quantitative criteria for insomnia severity and frequency. Sleep. 2006;29:479–85. doi: 10.1093/sleep/29.4.479. [DOI] [PubMed] [Google Scholar]
- 32.Vgontzas AN, Liao D, Bixler EO, Chrousos GP, Vela-Bueno A. Insomnia with objective short sleep duration is associated with a high risk for hypertension. Sleep. 2009;32:491. doi: 10.1093/sleep/32.4.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vgontzas AN, Liao D, Pejovic S, Calhoun S, Karataraki M, Bixler EO. Insomnia with objective short sleep duration is associated with type 2 diabetes: A population-based study. Diabetes Care. 2009;32:1980–5. doi: 10.2337/dc09-0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morin CM, Vallières A, Ivers H. Dysfunctional beliefs and attitudes about sleep (DBAS): validation of a brief version (DBAS-16) Sleep. 2007;30:1547–54. doi: 10.1093/sleep/30.11.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Harvey AG. A cognitive model of insomnia. Behav Res Ther. 2002;40:869–93. doi: 10.1016/s0005-7967(01)00061-4. [DOI] [PubMed] [Google Scholar]
- 36.Fortier-Brochu E, Beaulieu-Bonneau S, Ivers H, Morin CM. Insomnia and daytime cognitive performance: a meta-analysis. Sleep Med Rev. 2012;16:83–94. doi: 10.1016/j.smrv.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 37.Roehrs TA, Randall S, Harris E, Maan R, Roth T. MSLT in primary insomnia: stability and relation to nocturnal sleep. Sleep. 2011;34:1647–52. doi: 10.5665/sleep.1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Edinger JD, Ulmer CS, Means MK. Sensitivity and specificity of polysomnographic criteria for defining insomnia. J Clin Sleep Med. 2013;9:481–91. doi: 10.5664/jcsm.2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Levenson JC, Troxel WM, Begley A, Hall M, Germain A, Monk TH, et al. A quantitative approach to distinguishing older adults with insomnia from good sleeper controls. J Clin Sleep Med. 2013;9:125–31. doi: 10.5664/jcsm.2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.