Abstract
Background
Major depressive disorder (MDD) is increasingly recognized to involve functional deficits in both GABAergic and glutamatergic synaptic transmission. To elucidate the relationship between these phenotypes we made use of GABAA receptor γ2 subunit heterozygous (γ2+/−) mice, which we previously characterized as a model animal with construct, face and predictive validity for MDD.
Methods
To assess possible consequences of GABAergic deficits on glutamatergic transmission we quantitated the cell surface expression of NMDA- and AMPA-type glutamate receptors and the function of synapses in the hippocampus and medial prefrontal cortex of γ2+/− mice. In addition, we analyzed the effects of an acute dose of the experimental antidepressant ketamine on all these parameters in γ2+/− vs. wild-type mice.
Results
Modest defects in GABAergic synaptic transmission of γ2+/− mice resulted in a strikingly prominent homeostatic-like reduction in the cell surface expression of NMDA- and AMPA-type glutamate receptors, along with prominent functional impairment of glutamatergic synapses in the hippocampus and medial prefrontal cortex (mPFC). A single subanesthetic dose of ketamine lastingly normalized the glutamate receptor expression and synaptic function of γ2+/− mice to wild-type levels, along with antidepressant-like behavioral consequences selectively in γ2+/− mice. GABAergic synapses of γ2+/− mice were potentiated by ketamine in parallel but only in mPFC.
Conclusions
Depressive-like brain states that are caused by GABAergic deficits involve a homeostatic-like reduction of glutamatergic transmission that is reversible by an acute, subanesthetic dose of ketamine, along with regionally selective potentiation of GABAergic synapses. The data merge the GABAergic and glutamatergic deficit hypothesis of MDD.
Keywords: Major depressive disorder, homeostatic synaptic plasticity, antidepressant drug mechanisms, GABA, glutamate, neuroligin
Introduction
Major depressive disorder (MDD) is a leading cause of total disability with limited treatment options that are often ineffective (1, 2). The etiology of MDD is poorly understood but thought to involve combinations of endogenous vulnerability factors and precipitating life events such as uncontrollable stress (3). Candidate vulnerability factors include diverse defects in γ-aminobutyric acid (GABA)ergic inhibitory neurotransmission, such as reduced concentrations of GABA (4–9), reduced expression of glutamic acid decarboxylase (GAD) (10, 11), reduced expression of GABAA receptors (GABAARs) (12), and impaired function of GABAergic interneurons (10, 13, 14) (reviewed in 15, 16).
GABAergic deficit-induced changes in neural excitability (17) and reduced GAD-mediated conversion of glutamate to GABA may conceivably be causal for increased glutamate concentrations found in brain of MDD patients (18, 19). However, some studies of MDD point to reduced rather than increased brain content of glutamate (reviewed by 20), thereby suggesting a dynamic relationship between changes in GABAergic and glutamatergic transmission. Additional evidence suggestive of altered glutamatergic neurotransmission in MDD includes reduced expression and altered function of NMDARs (21, 22) and rapid therapeutic efficacy of subanesthetic doses of ketamine (23, 24). Ketamine exerts antidepressant activity as a non-competitive antagonist of NMDARs by ultimately enhancing glutamatergic synaptic transmission (25, 26). Importantly, ketamine is especially effective in otherwise treatment resistant forms of MDD associated with high anxiety (27). However, it is unclear how alterations in glutamatergic transmission and antidepressant efficacy of ketamine are functionally related to GABAergic deficits associated with MDD.
Stable functioning of neural networks in the face of rapid changes in neural excitability is critically dependent on homeostatic self-tuning mechanisms that, on a slower time course, preserve the balance of excitation and inhibition (E/I) and the average firing rates of neurons (28). Homeostatic mechanisms have most extensively been studied in cultured neurons. Of particular interest in the context of the present work is a slow form of homeostatic synaptic plasticity whereby pharmacologically induced hyperexcitability of cultured neurons is compensated by global scaling-down of glutamatergic synapses and scaling-up of inhibitory synapses (29–32).
Mice rendered hemizygous for the γ2 subunit gene (gabrg2) of GABAARs (γ2+/− mice) have been extensively characterized as a model with construct, face and predictive validity for anxious MDD (reviewed in 15, 33). They exhibit a modest impairment of GABAergic transmission characterized by loss of the γ2 subunit in approximately15% of GABAARs averaged across brain regions, with the most prominent reductions in neocortex and hippocampus (−25 to −35%, 34). The γ2-lacking GABAARs are functionally impaired as indicated by their reduced channel conductance (12 vs. 28 pS) and failure to accumulate at synapses (34–36). Behaviorally, γ2+/− mice exhibit signs of heightened anxiety, despair, anhedonia, and constitutive stress axis activation, and all these phenotypes are normalized by chronic treatment with the antidepressant desipramine (34, 37, 38). Cognitive alterations of γ2+/− mice in ambiguous cue conditioning tests mimic emotional pattern separation defects associated with MDD (34, 39, 40). Moreover, a phospho-site mutation that increases the cell surface expression of γ2-GABAARs has antidepressant-like behavioral consequences (41). Thus, the γ2+/− model lends support for a causative role of GABAergic deficits in the etiology of anxious MDD (15, 33).
Here we explored the consequences of GABAergic deficits on glutamatergic synapses. We found that γ2+/− mice exhibit reduced cell surface expression and function of NMDARs and AMPARs, along with reduced expression of the synaptic adhesion molecule neuroligin 1 (NL1) and defects in the density and function of glutamatergic synapses in the hippocampus and medial prefrontal cortex (mPFC). Similar defects were observed in γ2+/− cultured neurons. Moreover, treatment of γ2+/− mice (or cultures) with a subanesthetic dose of ketamine resulted in lasting (≥ 3 day) enhancement and normalization of glutamate receptor (GluR) expression and glutamatergic synapse function. Thus, depression-related brain states of γ2+/− mice involve a homeostatic-like reduction of glutamatergic transmission that can be normalized lastingly by the rapidly acting antidepressant ketamine. Notably, ketamine also potentiated the function of GABAergic synapses but only in anterior cingulate cortex (ACC). These data unite the GABAergic and glutamatergic deficit hypotheses of MDD, suggest that MDD may be caused by aberrant homeostatic plasticity, and provide novel insights into the synaptic mechanisms underlying antidepressant efficacy of ketamine.
Materials and Methods
For a more detailed and complete version of Material and Methods see Supplement 1.
Production and husbandry of mice
Two different GABAAR γ2+/− mouse lines were used as part of this study, with virtually identical germ line deletions of exon 8 of the gabrg2 locus. A first line of γ2+/− mice was maintained on a 129X1/SvJ background as previously described (34, 38, 42). A second line was generated on the C57BL/6J background by mating γ2f/f mice (43) with an oocyte-specific Cre line followed by outcrossing of the Cre transgene. Mice used for experimentation were littermates produced by crossing of γ2+/− mice and WT mice. The 129X1/SvJ line was used for preparation and analyses of cortical cultures, as well as biochemical and electrophysiological analyses of brain slices. The C57BL/6J line was used for biochemical and behavioral experimentation involving ketamine treatment.
Drug treatments
For treatment of cultures the drugs were diluted or dissolved in culture media to the following final concentrations: ketamine (10 μM, Ketaject®, Phoenix Pharmaceutical, Inc., St. Joseph, MO); 2-amino-5-phosphonovaleric acid (APV, 100 μM, Sigma-Aldrich, St. Louis, MO); bicuculline (20 μM, R&D Systems™, Minneapolis, MN); Ro25-6981 (10 μM, Sigma-Aldrich). For treatment of mice (8–9 weeks old), ketamine (Ketaject® diluted to 1 mg/ml in 0.9% saline) was administered at 10 mg/kg (biochemical and electrophysiological analyses) or 3 mg/kg (behavioral analyses) (i. p.) as previously described (26, 44).
Cell surface biotinylation
Cortical cultures from γ2+/− and WT mice were generated from embryonic day 14–15 embryos (129X1/SvJ line), subject to cell surface biotinylation at 21 days in vitro (DIV) and purification using NeutrAvidin agarose beads (Thermo, Rockford, IL) as described (45). For biotinylation of brain slices we adapted the protocol of Terunuma et al. (46). The biotinylated proteins were quantitated by SDS/PAGE/western blot using an Odyssey® CLx infrared imager (LI-COR, Lincoln, NE). Amounts of cell surface biotinylated proteins were normalized to amounts of β-tubulin in total extracts quantitated on parallel gels.
Immunofluorescent staining of cortical cultures
Immunofluorescent staining of neurons employed glia-free cortical cultures prepared from embryonic day 14–15 embryos as previously described (47). The cells were fixed, permeabilized and stained at 21 DIV as described (47) using rabbit anti MAP2 (1:1000, Ab5622), guinea pig anti VGluT1 (1:500, LV1439669), mouse anti PSD95 (1:1500, #28879, all from Millipore), mouse anti gephyrin (1:500, #147111), and rabbit anti VGAT (1:1000, #131002, both from Synaptic Systems). Synaptic immunoreactivities were developed and quantified as described (47).
Electrophysiology
Coronal slices (350 μm) containing the dorsal hippocampus or anterior cingulate cortex were prepared using a vibratome (Leica VT1200S), from 7- to 13-week-old 129X1/SvJ mice (of either sex) in a solution containing (in mM): 210 sucrose, 7 D-glucose, 25 NaHCO3, 1.25 NaH2PO4, 2.5 KCl, 1.3 Na-ascorbate, 3 Na-pyruvate, 0.5 CaCl2, 7 MgCl2, saturated with 95% O2/5% CO2. During recordings slices were perfused with (in mM): 50 sucrose, 119 NaCl, 26.2 NaHCO3, 11 glucose, 2.5 KCl, 1 NaH2PO4, 2.5 CaCl2, and 1.3 MgCl2, saturated with 95% O2/5% CO2, and 0.5 μM TTX, 10 μM NBQX, 25 μM D-APV for mIPSCs, or 50 μM PTX for EPSCs, or 10 μM NBQX and 50 μM PTX were added to perfusate lacking MgCl2 for NMDAR-mediated EPSCs. Internal solutions consisted of (in mM): 127 CsCl, 8 NaCl, 1 CaCl2, 10 HEPES, 10 EGTA, 0.3 Na3-GTP, and 2 Mg-ATP, pH 7.2 for mIPSCs, or 127 CsMeSO4, 8 NaCl, 1 CaCl2, 10 HEPES, 10 EGTA, 0.3 Na3-GTP, and 2 Mg-ATP, 0.1 spermine, 5 QX-314, pH 7.2 for EPSCs. All recordings were obtained at Vh = 70 mV, unless otherwise indicated. EPSCs were evoked using borosilicate glass pipette stimulators positioned 50–200 μm away from the primary apical dendrite of the recorded cell. Recordings and analyses were performed using pCLAMP 10 software (Molecular Devices). Only cells with a stable access resistance throughout the recording period were included in the analysis.
Statistics
Statistical comparisons were performed using two-tailed student’s T-tests or ANOVAs followed by posthoc analyses as detailed in the text and Figure legends.
Results
GABAergic deficits of γ2-deficient neurons result in homeostatic downregulation of glutamate receptors
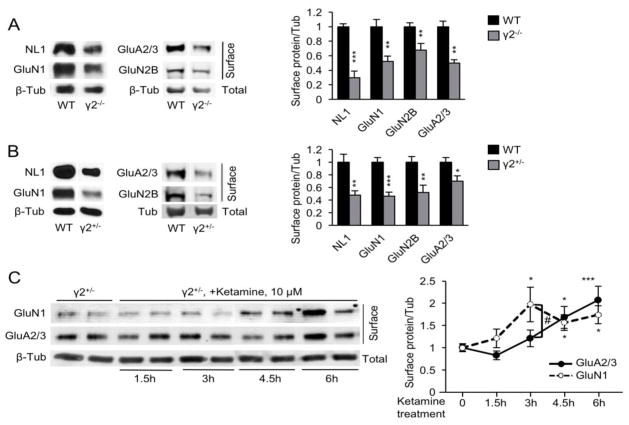
Chronic blockade of GABAARs in cultured neurons with bicuculline (BIC) results in homeostatic downregulation of NMDA and AMPAR function (29) (Fig. S1A in Supplement 1). Therefore, to begin to test whether similar changes in glutamate receptor (GluR) expression might occur in γ2+/− mice we began our analyses in γ2−/− and WT cultured cortical neurons (21 days in vitro). As a proxy for NMDARs we quantitated the cell surface expression of the obligatory subunit GluN1, as well as the GluN2B subunit, which is part of an NMDAR subtype that is implicated in mediating the detrimental effects of excess glutamate (48) and antidepressant effects of ketamine (49). In addition, we quantitated the cell surface expression of neuroligin 1 (NL1), a postsynaptic cell adhesion molecule that controls the accumulation of NMDARs at synapses (50). Lastly, we surveyed the expression of AMPARs using antibodies for GluA2/3 subunits. Using cell surface biotinylation assays we found that the plasma membrane accumulation of all four proteins was drastically reduced in γ2−/− vs. WT cultures (Fig. 1A). Remarkably, the cell surface expression of the same four proteins was reduced also in γ2+/− vs. WT cultures (Fig. 1B), with effect sizes comparable to those in γ2−/− vs. WT cultures (Fig. 1A) and BIC treated WT cultures (Fig. S1A in Supplement 1). Total expression of GluN1 and GluA2/3 was unaffected in γ2−/− vs. WT cultures and BIC-treated WT cultures, suggesting that the changes at the cell surface were due to impaired trafficking of receptors (Fig. S1A, B in Supplement 1). Therefore, a modest defect in GABAAR function in γ2+/− cultures (36, 51) leads to a profound downregulation of GluRs that is reminiscent of homeostatic scaling down of synapses induced by complete pharmacological blockade of GABAARs.
Figure 1. Analyses of cell surface expression of synaptic proteins in cortical cultures.
A γ2−/− cultures showed significantly reduced surface levels of NL1, GluN1, GluN2B and GluA2/3, compared to WT neurons (γ2−/− vs. WT: NL1, P < 0.001, n = 5–6; GluN1: P < 0.01, n = 5–7 cultures; GluN2B, P < 0.01, n = 12–13; GluA2/3, P < 0.01, n = 3–4, t-tests). B. The same markers were also reduced in γ2+/− cultures (γ2+/− vs. WT: NL1, P < 0.01, n = 4–5; GluN1, P < 0.001, n = 12; GluN2B, P < 0.01, n = 10–11; GluA2/3, P < 0.05, n = 14–15; t-tests). C. A time course of ketamine treatment of γ2+/− cultures revealed increased GluN1 at 3 h of ketamine treatment (γ2+/− vs. γ2+/− Ket, P < 0.01, n = 9) and remained elevated thereafter (P < 0.05, n = 6–13 for both the 4.5 and 6 h time points, ANOVA, Dunnett’s tests). GluA2/3 was significantly increased first at 4.5 h and then remained elevated (γ2+/− vs. γ2+/− Ket, 4h, P < 0.05, 6h, P < 0.001, n = 11–12, ANOVA, Dunnett’s tests). Cell surface GluN1 and GluA2/3 levels showed a greater 3h ketamine response for GluN1 than GluA2/3 [F(1,48) = 4.78, P < 0.05, ANOVA]. Data represent means ± SE. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Ketamine-induced reversal of glutamate receptor deficits
We hypothesized that down-regulation of GluRs was related to the depressive-like brain state of γ2+/− mice (37, 38) and hence that it should be reversible by antidepressant concentrations of ketamine. We treated γ2+/− cultures with ketamine (10 μM) for variable amounts of time and found that GluN1 cell surface expression was increased significantly within 3 h of treatment (Fig. 1C). Cell surface AMPARs were increased similarly but with about a 1.5 h delay. Ketamine also increased cell surface expression of NL1, with a time course similar to that of GluN1 (Fig. S2A in Supplement 1), which is consistent with a role of NL1 in cell surface trafficking of NMDARs (50). The effect of 10 μM ketamine on cell surface GluRs of γ2+/− cultures was reproduced by the GluN2B specific antagonist Ro25-6981 (10 μM) and the competitive NMDAR antagonist APV (100 μM) (Fig. S2B, C, in Supplement 1).
Glutamatergic synapse density is reduced by GABAAR deficits and normalized by ketamine treatment
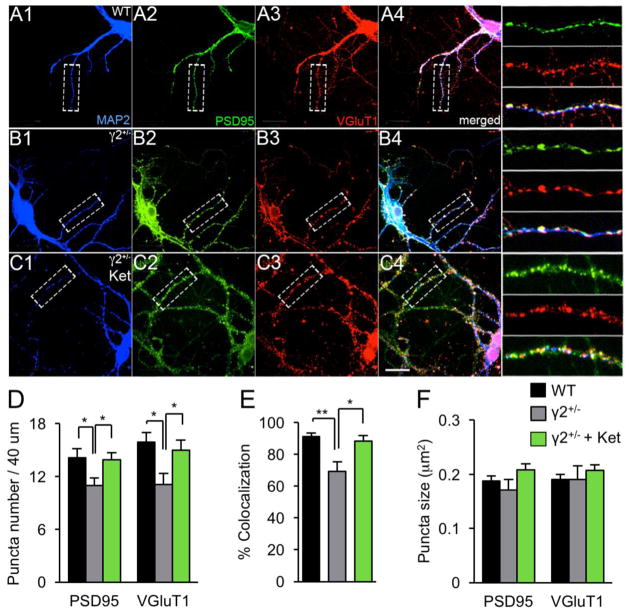
To address whether GABAAR deficits and ketamine impact the density of glutamatergic synapses we immunostained cultured cortical neurons for the pre- and postsynaptic markers vGluT1 and PSD95. The density of punctate immunoreactivity for both markers and their colocalization along dendrites was reduced in γ2+/− vs. WT neurons (Fig. 2A–E). Treatment of γ2+/− cultures with ketamine (10 μM, 6 h) normalized the expression and colocalization of vGluT1 and PSD95 to WT levels (Fig. 2A–E), while the size of immunoreactive puncta was unaffected by genotype and drug treatment (Fig. 2F). Thus, GABAAR deficit-induced reductions in GluR and NL1 cell surface expression and their normalization by ketamine correlate with changes in the number of synapses, rather than a change in protein accumulation at individual synapses.
Figure 2. Analyses of glutamatergic synapses of γ2+/− cortical cultures by immuno-staining.
A–C. Representative micrographs of neurons cultured form WT (A) and γ2+/− (B, C) embryos (DIV 20–21) subjected to treatment with ketamine (Ket) (C) and immunostained for the dendritic marker MAP2 (A1, B1, C1, blue), PSD95 (A2, B2, C2, green) and vGluT1 (A3, B3, C3, red). Boxed dendritic segments including merged images are shown enlarged to the right of the panels (A4, B4, C4). Scale bar, 16.7 μm. D. The density of punctate dendritic immunoreactivity of tear-drop-shaped cells for PSD and VGluT1 was significantly reduced in γ2+/− vs. WT cultures [PSD95, F(2,33) = 4.11, VGluT1, F(2,33) = 5.17, P < 0.05 for both proteins, ANOVA; WT vs. γ2+/−, P < 0.05, n = 21–23 cells for both PSD95 and VGluT1, Tukey’s test]. Puncta densities γ2+/− cultures treated with ketamine were reversed to WT levels (γ2+/− vs. γ2+/− Ket, P < 0.05, n = 21–23, for PSD95 and VGluT1, Tukey’s test). E. The fraction of VGluT1 puncta colocalized with PSD95 was reduced in γ2+/− vs. WT cultures and restored by ketamine treatment of γ2+/− cultures [F(2,33) = 8.71, P < 0.01, ANOVA. γ2+/− vs. WT and γ2+/− vs. γ2+/− Ket, P < 0.01, n = 21–23 for both comparisons, Tukey’s test). F. Puncta sizes were unaltered across conditions [PSD95, F(2,33) = 5.05; VGluT1, F(2,33) = 1.392, ANOVA, P, n.s.]. Data represent means ± SE. *, P < 0.05; **, P < 0.01, Tukey’s tests.
The GABAAR γ2+/− model shows increased sensitivity to anxiolytic- and antidepressant-like behavioral effects of ketamine
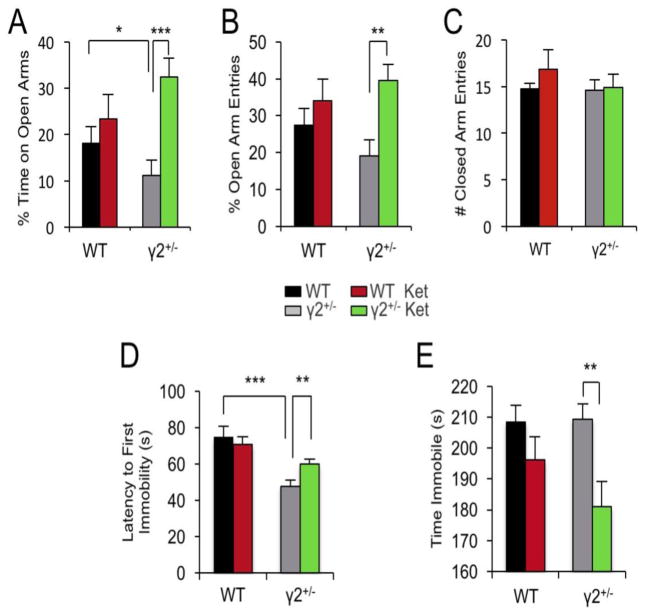
Assuming that ketamine induced surface expression of GluRs was related to its antidepressant activity we predicted that ketamine exerts increased antidepressant behavioral effects in γ2+/− compared to WT mice. However, preliminary experiments designed to address behavioral effects of ketamine revealed, inexplicably, that γ2+/− and WT mice maintained on a 129X1/SvJ strain background failed to show antidepressant-like behavioral responses to ketamine (not shown), reminiscent of other mouse strains that are insensitive to ketamine (52, 53). We therefore re-derived γ2+/− mice on a C57BL/6J genetic background (Materials and Methods), which has been widely used for studies of ketamine. Interestingly, eight hours after a single dose of ketamine (3 mg/kg, i.p.) the anxiety-like phenotype of γ2+/− mice in the Elevated Plus Maze was fully normalized to WT levels, without effects in WT mice (Fig. 3A). Moreover, ketamine had antidepressant-like consequences in the Forced Swim Test selectively in γ2+/− but not WT mice (Fig. 3B). Thus, γ2+/− mice are more sensitive than WT to the anxiolytic- and antidepressant-like behavioral effects of ketamine.
Figure 3. GABAAR γ2+/− mice exhibit increased behavioral sensitivity to anxiolytic- and antidepressant-like effects of ketamine.
Separate groups of GABAAR γ2+/− mice and WT littermates were injected with vehicle (saline) or ketamine (3 mg/kg, i.p.) and subjected to behavioral testing 8 h after treatment. A-C. In the Elevated Plus Maze, the % time spent on open arms (A) showed a significant overall treatment effect [F(1,43) = 8.17, P = 0.006, Two-way ANOVA] and a strong trend towards a genotype x treatment interaction [F(1,43) = 3.81, P = 0.057]. Posthoc analyses revealed an anxiety-like reduction in the % time spent on open arms in vehicle treated γ2+/− vs. WT mice (P < 0.05, n = 12-16) consistent with the phenotype previously reported for γ2+/− mice on the 129X1/SvJ background (34, 37). Ketamine treatment resulted in an anxiolytic-like increase in the % time on open arms in γ2+/− [γ2+/− Ket vs. Veh, P < 0.001, n = 12-14) but not WT mice (WT Ket vs. Veh, P > 0.05, n = 13-16, Fisher’s test). Similarly, analyses of % open arm entries (B) showed a significant overall treatment effect [F(1,51) = 7.71, P = 0.008 Two-way ANOVA] and a significant anxiolytic-like effect of ketamine selectively in γ2+/− (γ2+/− Ket vs. Veh, P < 0.01, n = 12-14) but not WT mice (WT Ket vs. Veh. P > 0.05, n = 14-16, Fisher tests). The four groups were indistinguishable with respect to closed arm entries (C) [genotype: F(1,51) = 0.52, P = 0.473, treatment: F(1,51) = 0.71, P = 0.404, Two-way ANOVA]. D, E. In the Forced Swim Test, the latency to first immobility (D) showed an overall genotype effect [F(1,90) = 19.78, P < 0.001, n = 18-27, Two-way ANOVA] and a genotype x treatment interaction [F(1,90) = 4.45, P = 0.038]. Posthoc group comparisons showed a reduced Latency to First Immobility specifically in vehicle-treated γ2+/− vs. WT mice (γ2+/− Veh vs. WT Veh. P < 0.001, n = 18-25; WT Ket vs. Veh, P > 0.05, n = 20-21, t-tests) and an increased Latency to First Immobility in ketamine-treated γ2+/− vs. vehicle-treated γ2+/− mice (γ2+/− Veh vs. γ2+/− Ket. P < 0.01, n = 18-23, t-test), consistent with the depressive-like phenotype previously reported for γ2+/− mice on the 129X1/SvJ background (37, 38) and with ketamine-induced changes in GluR expression and synapse function that were limited to or greater in γ2+/− vs. WT mice. Measurements of total time spent immobile (E) showed an overall treatment effect [F(1,90) = 9.32, P = 0.003, n = 18-27, Two-way ANOVA] and an antidepressant-like drug effect specifically in ketamine vs. vehicle treated γ2+/− but not WT mice (γ2+/− Ket vs. Veh, P < 0.01, n = 18-25; WT Ket vs. Veh, P > 0.05, n = 20-21, Fisher’s tests).
GABAAR deficits of γ2+/− mice result in downregulation of glutamate receptors in vivo
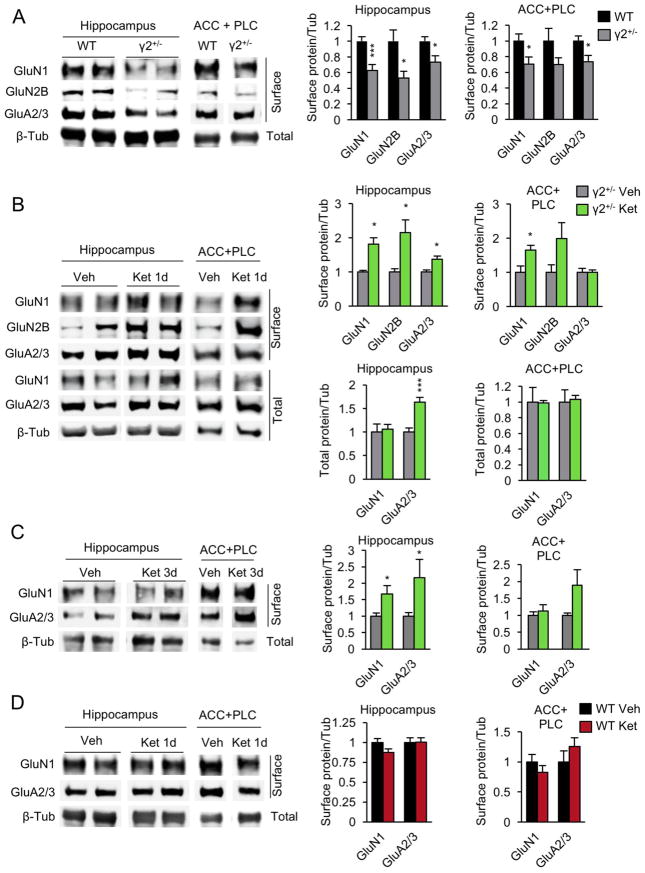
To begin to assess the fate of glutamatergic synapses in vivo we quantified the cell surface expression of GluRs in acute brain slices of the hippocampus and the anterior cingulate/prelimbic cortex (ACC/PLC), which are among the brain areas with the greatest GABAAR deficit in γ2+/− mice (34) and essential for antidepressant drug-induced behavioral effects in rodents (54, 55). The cell surface AMPARs and NMDARs were drastically reduced in γ2+/− vs. WT mice in both hippocampus and ACC/PLC (Fig. 4A).
Figure 4. Downregulation of glutamate receptors in γ2+/− vs. WT mice is reversed by ketamine treatment.
A. The cell surface expression of GluN1 and GluA2/3 was reduced in γ2+/− vs. WT mice both in hippocampus (GluN1, P < 0.001; GluA2/3, P < 0.05, n = 12–13 mice for both comparisons) and ACC/PLC (P < 0.05, n = 12–13, for both comparisons). GluN2B cell surface expression was reduced in hippocampus of γ2+/− mice (γ2+/− vs. WT, P < 0.05, n = 6–7) and trended lower in ACC/PLC (P = 0.15, n = 6–7). B. In γ2+/− animals analyzed 24h after an acute dose of ketamine (10 mg/kg, i.p.) the cell surface expression of NMDARs and AMPARs in hippocampus was increased compared to vehicle-treated γ2+/− controls (P < 0.05, n = 7–11, for the GluN1, GluN2B and GluA2/3 comparisons) to levels comparable to those of WT mice in (A) (γ2+/−+Ket vs. WT, P, n.s., n = 9–12). In ACC/PLC, ketamine treatment of γ2+/− mice resulted in increased expression of NMDARs (γ2+/− Ket vs. Veh: GluN1, P < 0.05) but not AMPARs (GluA2/3, P, n.s., n = 8–9 for both comparisons). Cell surface GluN2B levels trended in the same direction as GluN1 but the effect was not significant (P = 0.12, n = 3). C. In hippocampus of γ2+/− animals analyzed three days after ketamine treatment the cell surface expression of NMDARs and AMPARs remained increased compared to controls (P < 0.05, n = 10–12, for both GluN1 and GluA2/3). By contrast, in ACC/PLC, the GluN1 cell surface level had returned to base line (P, n.s., n = 8–9) but the GluA2/3 cell surface expression showed a strong trend of an increase (P = 0.08, n = 6/group) that was not yet evident at one day after treatment (B). D. In WT mice analyzed 24 h after ketamine treatment the expression of NMDARs and AMPARs was unchanged compared to vehicle controls in both hippocampus and ACC/PLC (WT Veh vs. Ket: P, n.s., n = 5–6, for all four comparisons). Data are from mice maintained on a C57BL/6J background. The genotype differences were reproduced with 129X1/SvJ mice (Fig. S1C in Supplement 1). Data represent means ± SE. *, P < 0.05; ***, P < 0.001, t-tests.
A subanesthetic dose of ketamine given to γ2+/− mice normalizes NMDAR cell surface expression in vivo
To examine whether ketamine affected expression of GluR in vivo we treated γ2+/− mice with ketamine (C57BL/6J, strain, 10 mg/kg, i.p.) and 24 h later harvested hippocampal and ACC/PLC brain slices for quantification of cell surface proteins. Ketamine treatment of γ2+/− mice resulted in significant upregulation and normalization of cell surface NMDARs in both hippocampus and ACC/PLC (Fig. 4B). Expression of the GluN2B subunit appeared increased to similar levels as GluN1, although the effect was more variable and significant only in hippocampus. Total expression of NMDARs remained unaltered, indicating that ketamine acted posttranslationally to normalize impaired cell surface accumulation of NMDARs. Unlike NMDARs, cell surface AMPARs of γ2+/− mice were upregulated by ketamine only in hippocampus and unaffected in ACC/PLC. Moreover, this drug effect in hippocampus involved increased total expression of AMPARs, rather than merely a change in cell surface accumulation (Fig. 4B). Notably, comparing the hippocampal cell surface expression of GluRs of ketamine treated γ2+/− and drug naïve WT mice (Fig. 4A and B, data normalized to values of drug naïve γ2+/− mice) indicated that ketamine fully restored the expression of GluRs from γ2+/− to WT levels [γ2+/−+Ket vs. WT: P, n.s., n = 9–12, for both GluN1 and GluA2/3, ANOVA, Tukey test).
Given the lasting therapeutic effects of ketamine in patients (23) we further assessed whether the drug effects on GluR expression seen one day after treatment remained measurable three days after treatment. Interestingly, in hippocampus, both GluN1 and GluA2/3 remained significantly elevated compared to vehicle-treated γ2+/− mice three days after treatment (Fig. 4C). By contrast, in PFC, the effects of ketamine on GluN1 were no longer detectable. Instead, there was a strong trend for upregulation of GluA2/3 that was not observed one day after treatment (Fig. 4 B, C). Thus, a subanesthetic dose of ketamine can lastingly reverse homeostatic downregulation of glutamatergic synapses in the hippocampus, induced by impaired GABAergic transmission for at least three days post treatment. Moreover, ketamine induced augmentation of AMPAR expression in PFC may be delayed relative to that of NMDAR and slower than in hippocampus.
Importantly, ketamine had no effect on GluR cell surface expression in WT mice, independent of brain region analyzed (Fig. 4D, 1-day treatment), and consistent with the selective behavioral effects of ketamine in γ2+/− but not WT mice described in Fig 3. Notably, downregulation of GluRs was evident in γ2+/− mice of both genetic backgrounds [Fig. 4A (C57BL/6J) and Fig. S1C in Supplement 1 (129X1/SvJ)], suggesting that differential behavioral sensitivity of the two genetic backgrounds to the effects of ketamine was due to strain differences acting downstream of altered GluR expression.
GABAAR deficits reduce the number of functional glutamatergic synapses in vivo and this defect is reversed by a single dose of ketamine
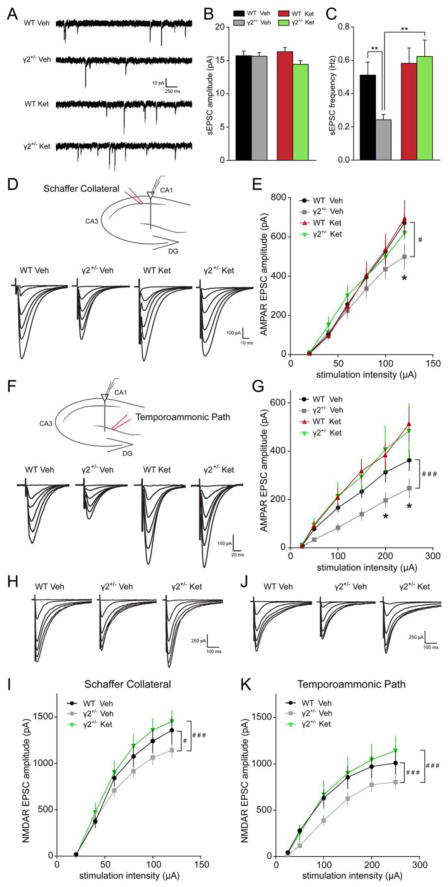
We next assessed functional defects in glutamatergic transmission using voltage clamp recordings of CA1 pyramidal cells in hippocampal slices. The frequency of spontaneous excitatory postsynaptic currents (sEPSCs) was drastically reduced in γ2+/− vs. WT cells, while the amplitude was unaffected (Fig. 5A–C). Ketamine administered to mice 24 h prior to recordings normalized the sEPSC frequency of γ2+/− mice to WT levels and had no effect in WT mice (Fig. 5C). Neither ketamine nor genotype affected the amplitude of sEPSCs (Fig. 5B). Consistent with results obtained by immunostaining of cultures (Fig. 2), these findings suggest that GABAAR deficits and ketamine affect glutamatergic transmission through a change in the number of functional synapses rather than through changes in the abundance of AMPARs at synapses.
Figure 5. Downregulation of glutamatergic synaptic inputs to γ2+/− CA1 pyramidal neurons is reversed by ketamine.
A–C. sEPSC recordings from CA1 pyramidal neurons of γ2+/− and WT mice injected 24 h earlier with saline (Veh) or ketamine (Ket). Representative traces are shown in (A), with summary quantification of sEPSC amplitude in (B) and frequency in (C). Note the significant decrease in frequency of sEPSC recorded from vehicle-treated γ2+/− vs. WT mice that was normalized by ketamine treatment (genotype x treatment interaction for sEPSC frequency, F(1,86) = 4.473, P = 0.037, Two-way ANOVA; P < 0.01, n = 12–41 cells, for both group comparisons, Tukey’s test). D–G. AMPAR EPSCs recorded from CA1 pyramidal cells evoked by stimulation of the SC (D, E) or TA path (F, G). Schematics with sites of stimulation and recording and average traces at progressively larger stimulation intensities are shown in (D) and (F). AMPAR responses were reduced in γ2+/− vs. WT mice at SC (E) and TA (G) synapses. Moreover, ketamine treatment restored synaptic responses of γ2+/− mice to WT levels [(E) # WT Veh vs. γ2+/− Veh F(1,138) = 5.391, P = 0.022, Two-way ANOVA; P < 0.05, n = 9–17 cells, Tukey’s test; (G) ### WT Veh vs. γ2+/− Veh, F(1,102) = 23.78, P < 0.001, Two-way ANOVA, P < 0.05, Tukey’s test). H–K. NMDAR EPSCs recorded from CA1 pyramidal cells evoked by stimulation of the SC (H, I) or TA path (J, K). Schematics with sites of stimulation and recordings, along with average sample traces at progressively larger stimulation intensities are shown in (H) and (J), with summary quantification in (I) and (K). NMDAR responses in γ2+/− mice were reduced compared to WT at both SC (I) and TA (K) synapses. Ketamine treatment restored the synaptic NMDAR responses of γ2+/− mice to WT levels [(I) # WT Veh vs. γ2+/− Veh, F(1,89) = 4.952, P = 0.029, Two-way ANOVA; ### γ2+/− Veh vs. γ2+/− Ket, F(1,95) = 12.31, P < 0.001, Two-way ANOVA; (K) ### WT Veh vs. γ2+/− Veh F(1,90) = 15.55, P < 0.001, Two-way ANOVA; ### γ2+/− Veh vs. γ2+/− Ket F(1,96) = 12.55, P < 0.001, Two-way ANOVA; n = 8–9 cells for all groups in (I) and (K)]. Data represent means ± SE. *, P < 0.05; **, P < 0.01.
Chronic stress has been shown to preferentially affect temporoammonic (TA) synapses onto CA1 pyramidal neurons rather than Schaffer collateral (SC) synapses (56). To further characterize the synaptic deficits of γ2+/− CA1 pyramidal cells we recorded AMPAR- and NMDAR EPSCs evoked by selective stimulation of either the SC or TA pathway. AMPAR EPSC amplitudes were significantly reduced in γ2+/− vs. WT slices, with a more pronounced effect at TA synapses (Fig. 5D–G). Moreover, ketamine treatment of γ2+/− mice normalized SC-evoked AMPAR EPSCs to WT levels, without corresponding effects in WT mice (Fig. 5E). At TA synapses, ketamine potentiated the AMPAR responses, with greater effects in γ2+/− than WT mice (WT Ket as % WT Veh vs. γ2+/− Ket as % γ2+/− Veh, F(1,163) = 17.73, P < 0.0001, Two-way ANOVA) (Fig. 5F, G). Similar to AMPAR EPSCs, NMDAR currents of SC-CA1 and TA-CA1 synapses of γ2+/− mice were impaired at baseline, and restored to WT levels by ketamine pretreatment (Fig. 5H–K).
To further examine the idea that the reduced sEPSC frequency of γ2+/− neurons reflected a reduction in the density of synapses (Fig. 5A, B) we assessed the synaptic release probability by measuring paired pulse ratios (PPRs) from SC and TA path-stimulated pyramidal cells (Fig. S3 in Supplement 1). The PPRs were unaffected by genotype for both types of synapses and increased by ketamine selectively at TA-CA1 synapses. Thus, genotype- and treatment-dependent alterations of glutamatergic transmission primarily reflect changes in synapse number rather than release probability. Further, neither genotype nor ketamine treatment affected the rectification of AMPAR responses evoked by SC or TA pathway stimulation (Fig. S4 in Supplement 1), indicating that the calcium permeability of AMPARs does not play a significant role in the forms of plasticity examined here.
Ketamine enhances GABAergic synaptic inhibition in frontal cortex
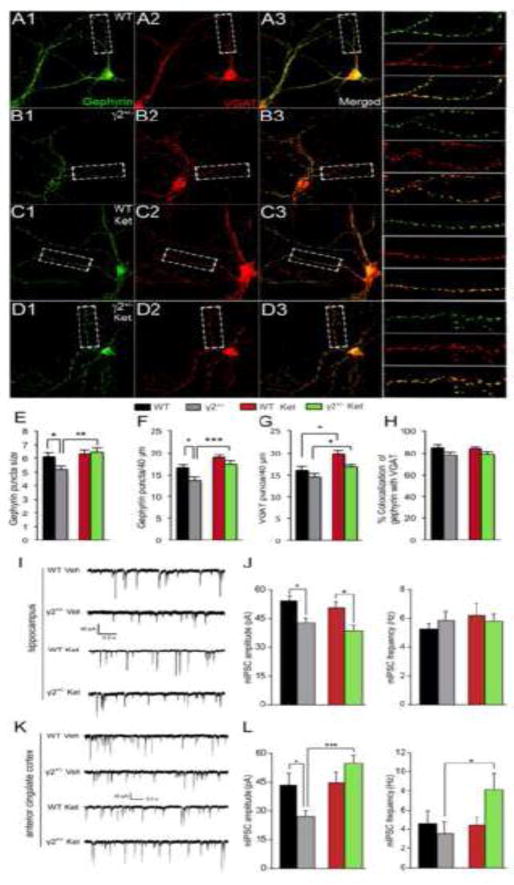
Ketamine has potent seizure-suppressing effects in animal models of epilepsy and in patients (57–59). We therefore wondered whether ketamine-induced potentiation of glutamatergic transmission was accompanied by an increase in GABAergic inhibition. Comparison of γ2+/− and WT cultured cortical neurons by immunofluorescent staining for the pre- and postsynaptic markers vesicular GABA transporter (VGAT) and gephyrin revealed a significant reduction in the size and density of punctate gephyrin immunoreactivity in γ2+/− vs. WT cultures, with both of these defects fully reversed by 6 h treatment of γ2+/− cultures with 10 μM ketamine (Fig. 6 A–G). A modest but significant increase in the density of gephyrin puncta was also observed in WT cultures (Fig. 6F). VGAT staining of γ2+/− cultures was increased by ketamine in parallel with gephyrin (Fig. 6G), suggesting that ketamine potentiated the postsynaptic apparatus while also increasing the number of GABAergic synapses in γ2+/− cultures. Consistent with this interpretation, the colocalization of VGAT and gephyrin was unaffected by genotype or ketamine treatment (Fig. 6H). As expected, the amplitude of miniature inhibitory postsynaptic currents (mIPSCs) recorded from CA1 and L2/3 ACC pyramidal cells of γ2+/− brain slices was reduced compared to WT, while the frequency was unaltered (Fig. 6 I–L). Therefore, γ2+/− neurons display a defect in GABAergic inhibition that is not compensated for by any process resembling homeostatic scaling up of inhibitory synaptic strength seen following prolonged treatment of cultured neurons with BIC. Curiously, we found that ketamine had no effect on mIPSCs recorded from CA1 neurons of γ2+/− mice (Fig. 6 I, J). However, ketamine fully restored the amplitude of mIPSCs recorded from γ2+/− L2/3 ACC pyramidal cells to WT levels, along with a prominent increase in the frequency of mIPSCs observed selectively in γ2+/− but not WT mice (Fig. 6 K, L). Ketamine did not affect the amplitude or frequency of mIPSCs of WT mice, consistent with our other observations that showed that ketamine effects on GluR expression, synapse density and emotional behavior were enhanced or specific for γ2+/− mice representing the pathological condition. Notably, glutamatergic synapses of L2/3 pyramidal cells were downregulated in γ2+/− mice, and restored by ketamine administration to WT levels (Fig. S5A, B in Supplement 1), similar to results obtained with CA1 pyramidal cells. Thus, GABAergic synapses in ACC of γ2+/− mice are potentiated by ketamine both pre- and post-synaptically, in concert with restoration of glutamatergic synapses and antidepressant behavioral effects.
Figure 6. Characterization of GABAergic synapse deficits and their reversal by ketamine in γ2+/− cultured cortical neurons, and CA1 and L2/3 ACC of γ2+/− mice.
A–H. Cortical cultures (DIV21) prepared from WT (A, C) and γ2+/− (B, D) embryos were either untreated (A, B) or treated with ketamine (C, D) (10 μM, 6 h) and subjected to immunofluorescent staining for gephyrin (green, A1–D1) and VGAT (red, A2–D2). Colocalization in merged images is shown in yellow (A3–D3) with enlarged dendritic segments depicted to the right. Scale bar, 16.7 μm. E. Quantitation of punctate immunoreactivity in dendritic segments of pyramidal neurons showed that the size of gephyrin clusters was reduced in γ2+/− vs. WT neurons and restored to WT levels by ketamine treatment [F(3, 73) = 5.3, P < 0.01, ANOVA, γ2+/− vs. WT, P = 0.05; γ2+/− vs. γ2+/− Ket, P < 0.01, WT vs. WT Ket, P, n.s., n = 18–21, Bonferroni]. F. The density of punctate gephyrin staining per 40 μm was significantly reduced in γ2+/− vs. WT cultures [F(3, 73) = 64.9, P < 0.001, ANOVA; γ2+/− vs. WT, P < 0.05, n = 18–21, Tukey’s test] and increased by ketamine selectively in γ2+/− cultures (γ2+/− vs. γ2+/− Ket, P < 0.001, WT vs. WT Ket, P, n.s., n = 18–21, both comparisons, Tukey’s test). G. The density of VGAT puncta along 40 μm segments of dendrite was increased by ketamine independent of genotype [F(1/73) = 16.4, P < 0.001, ANOVA; WT vs. WT Ket, P < 0.01, γ2+/− vs. γ2+/− Ket, P < 0.05, n = 18–21, t-tests). H. The fraction of gephyrin puncta colocalized with VGAT was unaltered across conditions [F(3,73) = 1.99, P = 0.12, ANOVA]. I–L. Representative traces of mIPSC recordings from CA1 (I) and L2/3 ACC pyramidal neurons (K) of WT and γ2+/− mice injected with saline (Veh) or Ket 24 h prior to recording. (J) Quantification of mIPSC amplitude and frequency for CA1 neurons showed a significant decrease in amplitude in γ2+/− vs. WT mice, regardless of treatment (mIPSC amplitude by genotype, F(1,45) = 18.02, P = 0.001, Two-way ANOVA, P < 0.05, n = 10–13 cells; Tukey’s test). (L) Quantification of mIPSC amplitude and frequency for ACC neurons showed a significant decrease in amplitude in γ2+/− vs. WT mice that was reversed by ketamine treatment of γ2+/− mice (mIPSC amplitude, genotype x treatment interaction, F(1,25) = 7.879, P < 0.01, n = 6–8 cells; Two-way ANOVA; treatment comparison F(1,25) = 9.388, P = 0.005, Bonferroni). Data represent means ± SE. * P < 0.05, ** P < 0.01; *** P < 0.001.
Discussion
We have shown here that the GABAergic deficit-induced depression-related brain state of γ2+/− mice involves a homeostatic-like reduction of glutamatergic transmission. A modest reduction in GABAergic transmission in γ2+/− mice, consisting of a ~20% reduction in the mIPSC amplitude of principal cells, leads to a robust ~30–50% reduction in cell surface NMDA and AMPARs and a ~50% reduction in sEPSC frequency. Hyperexcitability of cultured neurons induced by chronic BIC treatment is known to result in homeostatic scaling-down of glutamatergic synapses in concert with scaling-up of inhibitory synapses (29, 30) and increased expression of postsynaptic GABAARs (31). Compared to complete (but transient) blockade of GABAARs by BIC treatment of cultures, γ2+/− mice and cultures display only a modest reduction of GABAAR function. However, unlike in BIC-treated cultures, inhibitory synapses are not strengthened in γ2+/− mice, possibly due to the limiting amounts of γ2-GABAARs. It is conceivable then that homeostatic mechanisms of γ2+/− mice that serve to restore the balance of excitation and inhibition ended up exacerbating downregulation of glutamatergic transmission. Similar homeostatic mechanisms are likely to operate under conditions of chronic or repeated stress, which are used to model depression in rodents, involve downregulation of AMPARs and NMDARs (60), result in increased behavioral responsiveness to ketamine (61), and hence involve changes in glutamatergic transmission comparable to those observed in the γ2+/− model. Importantly, chronic stress also increases the chloride reversal potential, which renders GABAergic inhibition ineffective or excitatory (62–64). Thus, stress-induced downregulation of GluRs may be a consequence of excessive excitatory drive and impaired upregulation of inhibition analogous to mechanisms observed in the γ2+/− model. Our findings merge the GABAergic (15, 16) and glutamatergic (21, 60) deficit hypotheses of MDD and provide a novel mechanism for how these two neurotransmitter systems interact in the etiopathology of MDD.
Second, we showed that the GABAergic deficit-induced adaptations of glutamatergic synapses in γ2+/− mice are reversed by an acute subanesthetic dose of ketamine. Normalization of GluR expression and synapse density was also observed in γ2+/− cultures in the continuous presence of ketamine or APV or Ro25-6981, while WT cultures were unaffected by ketamine or Ro25-6981. These observations suggest that downregulation of NMDARs in γ2+/− cultures and mice involves excessive or untimely activation of NMDARs, most likely due to chronically increased release of glutamate. Recent evidence indicates that the initial effect of a subanesthetic dose of ketamine is to block a subpopulation of NMDARs that are active at rest (44), followed by increased expression of AMPARs (26). Our data extend these findings and show that initial antagonistic effects of ketamine on NNMDARs are followed by restoration of previously compromised expression and function of postsynaptic NMDARs, in addition to increased expression and function of AMPARs. Moreover, the effects of ketamine were greatly enhanced or specific for γ2+/− vs. WT mice, i.e. under conditions representing the pathological condition.
Third, we showed that in concert with enhanced glutamatergic transmission ketamine potentiates the pre- and postsynaptic function of GABAergic inhibitory synapses of L2/3 ACC pyramidal cells of γ2+/− mice. Notably, this drug effect occurred despite the impairment of inhibitory synapses that is evident in untreated γ2+/− mice. An enduring potentiation of synaptic inhibition is consistent with the known seizure suppressing effects of ketamine (57–59).
Fourth, downregulation of cell surface NMDARs in γ2+/− neurons and its reversal by ketamine were paralleled by corresponding changes in the cell surface expression of NL1, a synaptogenic cell adhesion protein that contributes to activity-dependent balancing of glutamatergic and GABAergic synaptic transmission (65) and controls the synaptic accumulation of NMDARs (50). Thus, aberrant trafficking of NL1 may play a key role in maladaptations observed in γ2+/− mice and their reversal by ketamine.
GABAAR-deficit-induced downregulation of cell surface GluRs is reminiscent of chronic stress-induced downregulation of AMPARs and NMDARs in mPFC (66–68) and downregulation of AMPARs selectively at TA-CA1 synapses (56, 69). Similar to selective stress-induced impairment of TA-CA1 synapses, the functional deficit in γ2+/− mice was greater at TA-CA1 than at SC-CA1 synapses (TA-CA1 γ2+/− as % WT vs. SC-CA1 γ2+/− as % WT, F(1,120) = 5.714, P = 0.0184, Two-way ANOVA) (Fig. 5E vs. G). TA-CA1 synapses map to distal apical dendrites of CA1 pyramidal cells that are targets of somatostatin (SST)-positive oriens-lacunosum moleculare (O-LM) cells (70). SST interneurons are highly sensitive to stress and functionally impaired in MDD patients (71). Thus, endogenous or stress-induced GABAergic deficits may be key for stress-induced downregulation of CA1 pyramidal neuron glutamatergic synapses. Elucidating the signaling pathways underlying homeostatic plasticity of GABAergic and glutamatergic synaptic transmission should lead to novel approaches for the treatment of MDD.
Supplementary Material
Acknowledgments
We thank Yao Guo for technical assistance and Dr. Qiuying Shen for expert advise on statistical analyses. This publication was made possible by grants MH089111 and MH099851 to B.L. from the National Institutes of Mental Health (NIMH) and grants from the Canadian Institutes of Health Research and Natural Sciences and Engineering Research Council to D.S.
Footnotes
Author contributions:
Z.R., H.P., D.S. and B.L. conceived and designed the experiments; Z.R., H.P, S.J.J., M.S. and T.F. performed the experiments; Z.R., H.P, S.J.J., M.S and T.F. analyzed the data; Z.R, H.P. and BL wrote the manuscript; S.J.J., T.F. and D.S. critically read the manuscript.
Additional information:
Supplementary Information is available at the Journal’s website
Financial Disclosures: All authors report no biomedical financial interests or potential conflicts of interest.
Its contents are solely the responsibility of the authors and do not necessarily represent the views of the funding agencies.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, et al. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R) JAMA. 2003;289:3095–3105. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- 2.McIntyre RS, Filteau MJ, Martin L, Patry S, Carvalho A, Cha DS, et al. Treatment-resistant depression: definitions, review of the evidence, and algorithmic approach. J Affect Disord. 2014;156:1–7. doi: 10.1016/j.jad.2013.10.043. [DOI] [PubMed] [Google Scholar]
- 3.Willner P, Scheel-Kruger J, Belzung C. The neurobiology of depression and antidepressant action. Neurosci Biobehav Rev. 2013;37:2331–2371. doi: 10.1016/j.neubiorev.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 4.Sanacora G, Mason GF, Rothman DL, Behar KL, Hyder F, Petroff OA, et al. Reduced cortical gamma-aminobutyric acid levels in depressed patients determined by proton magnetic resonance spectroscopy. Arch Gen Psychiatry. 1999;56:1043–1047. doi: 10.1001/archpsyc.56.11.1043. [DOI] [PubMed] [Google Scholar]
- 5.Hasler G, van der Veen JW, Tumonis T, Meyers N, Shen J, Drevets WC. Reduced prefrontal glutamate/glutamine and gamma-aminobutyric acid levels in major depression determined using proton magnetic resonance spectroscopy. Arch Gen Psychiatry. 2007;64:193–200. doi: 10.1001/archpsyc.64.2.193. [DOI] [PubMed] [Google Scholar]
- 6.Gabbay V, Mao X, Klein RG, Ely BA, Babb JS, Panzer AM, et al. Anterior cingulate cortex gamma-aminobutyric acid in depressed adolescents: relationship to anhedonia. Arch Gen Psychiatry. 2012;69:139–149. doi: 10.1001/archgenpsychiatry.2011.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bhagwagar Z, Wylezinska M, Jezzard P, Evans J, Ashworth F, Sule A, et al. Reduction in occipital cortex gamma-aminobutyric acid concentrations in medication-free recovered unipolar depressed and bipolar subjects. Biol Psychiatry. 2007;61:806–812. doi: 10.1016/j.biopsych.2006.08.048. [DOI] [PubMed] [Google Scholar]
- 8.Price RB, Shungu DC, Mao X, Nestadt P, Kelly C, Collins KA, et al. Amino acid neurotransmitters assessed by proton magnetic resonance spectroscopy: relationship to treatment resistance in major depressive disorder. Biol Psychiatry. 2009;65:792–800. doi: 10.1016/j.biopsych.2008.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Croarkin PE, Levinson AJ, Daskalakis ZJ. Evidence for GABAergic inhibitory deficits in major depressive disorder. Neurosci Biobehav Rev. 2011;35:818–825. doi: 10.1016/j.neubiorev.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 10.Feyissa AM, Woolverton WL, Miguel-Hidalgo JJ, Wang Z, Kyle PB, Hasler G, et al. Elevated level of metabotropic glutamate receptor 2/3 in the prefrontal cortex in major depression. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:279–283. doi: 10.1016/j.pnpbp.2009.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guilloux JP, Douillard-Guilloux G, Kota R, Wang X, Gardier AM, Martinowich K, et al. Molecular evidence for BDNF- and GABA-related dysfunctions in the amygdala of female subjects with major depression. Mol Psychiatry. 2012;17:1130–1142. doi: 10.1038/mp.2011.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klumpers UM, Veltman DJ, Drent ML, Boellaard R, Comans EF, Meynen G, et al. Reduced parahippocampal and lateral temporal GABAA-[11C]flumazenil binding in major depression: preliminary results. Eur J Nucl Med Mol Imaging. 2010;37:565–574. doi: 10.1007/s00259-009-1292-9. [DOI] [PubMed] [Google Scholar]
- 13.Sibille E, Morris HM, Kota RS, Lewis DA. GABA-related transcripts in the dorsolateral prefrontal cortex in mood disorders. Int J Neuropsychopharmacol. 2011;14:721–734. doi: 10.1017/S1461145710001616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rajkowska G, O’Dwyer G, Teleki Z, Stockmeier CA, Miguel-Hidalgo JJ. GABAergic neurons immunoreactive for calcium binding proteins are reduced in the prefrontal cortex in major depression. Neuropsychopharmacology. 2007;32:471–482. doi: 10.1038/sj.npp.1301234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luscher B, Shen Q, Sahir N. The GABAergic deficit hypothesis of major depressive disorder. Mol Psychiatry. 2011;16:383–406. doi: 10.1038/mp.2010.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pittenger C, Sanacora G, Krystal JH. The NMDA receptor as a therapeutic target in major depressive disorder. CNS Neurol Disord Drug Targets. 2007;6:101–115. doi: 10.2174/187152707780363267. [DOI] [PubMed] [Google Scholar]
- 17.Levinson AJ, Fitzgerald PB, Favalli G, Blumberger DM, Daigle M, Daskalakis ZJ. Evidence of cortical inhibitory deficits in major depressive disorder. Biol Psychiatry. 2010;67:458–464. doi: 10.1016/j.biopsych.2009.09.025. [DOI] [PubMed] [Google Scholar]
- 18.Sanacora G, Gueorguieva R, Epperson CN, Wu YT, Appel M, Rothman DL, et al. Subtype-specific alterations of gamma-aminobutyric acid and glutamate in patients with major depression. Arch Gen Psychiatry. 2004;61:705–713. doi: 10.1001/archpsyc.61.7.705. [DOI] [PubMed] [Google Scholar]
- 19.Hashimoto K, Sawa A, Iyo M. Increased levels of glutamate in brains from patients with mood disorders. Biol Psychiatry. 2007;62:1310–1316. doi: 10.1016/j.biopsych.2007.03.017. [DOI] [PubMed] [Google Scholar]
- 20.Niciu MJ, Ionescu DF, Richards EM, Zarate CA., Jr Glutamate and its receptors in the pathophysiology and treatment of major depressive disorder. J Neural Transm. 2014;121:907–924. doi: 10.1007/s00702-013-1130-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paul IA, Skolnick P. Glutamate and depression: clinical and preclinical studies. Ann N Y Acad Sci. 2003;1003:250–272. doi: 10.1196/annals.1300.016. [DOI] [PubMed] [Google Scholar]
- 22.Feyissa AM, Chandran A, Stockmeier CA, Karolewicz B. Reduced levels of NR2A and NR2B subunits of NMDA receptor and PSD-95 in the prefrontal cortex in major depression. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:70–75. doi: 10.1016/j.pnpbp.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zarate CA, Jr, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, et al. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. 2006;63:856–864. doi: 10.1001/archpsyc.63.8.856. [DOI] [PubMed] [Google Scholar]
- 24.Abdallah CG, Averill LA, Krystal JH. Ketamine as a promising prototype for a new generation of rapid-acting antidepressants. Ann N Y Acad Sci. 2015;1344:66–77. doi: 10.1111/nyas.12718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moghaddam B, Adams B, Verma A, Daly D. Activation of glutamatergic neurotransmission by ketamine: a novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. J Neurosci. 1997;17:2921–2927. doi: 10.1523/JNEUROSCI.17-08-02921.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M, et al. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 2010;329:959–964. doi: 10.1126/science.1190287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ionescu DF, Luckenbaugh DA, Niciu MJ, Richards EM, Slonena EE, Vande Voort JL, et al. Effect of baseline anxious depression on initial and sustained antidepressant response to ketamine. J Clin Psychiatry. 2014;75:e932–938. doi: 10.4088/JCP.14m09049. [DOI] [PubMed] [Google Scholar]
- 28.Turrigiano G. Homeostatic synaptic plasticity: local and global mechanisms for stabilizing neuronal function. Cold Spring Harb Perspect Biol. 2012;4:a005736. doi: 10.1101/cshperspect.a005736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Watt AJ, van Rossum MC, MacLeod KM, Nelson SB, Turrigiano GG. Activity coregulates quantal AMPA and NMDA currents at neocortical synapses. Neuron. 2000;26:659–670. doi: 10.1016/s0896-6273(00)81202-7. [DOI] [PubMed] [Google Scholar]
- 30.Peng YR, Zeng SY, Song HL, Li MY, Yamada MK, Yu X. Postsynaptic spiking homeostatically induces cell-autonomous regulation of inhibitory inputs via retrograde signaling. J Neurosci. 2010;30:16220–16231. doi: 10.1523/JNEUROSCI.3085-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rannals MD, Kapur J. Homeostatic strengthening of inhibitory synapses is mediated by the accumulation of GABA(A) receptors. J Neurosci. 2011;31:17701–17712. doi: 10.1523/JNEUROSCI.4476-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pribiag H, Peng H, Shah WA, Stellwagen D, Carbonetto S. Dystroglycan mediates homeostatic synaptic plasticity at GABAergic synapses. Proc Natl Acad Sci U S A. 2014;111:6810–6815. doi: 10.1073/pnas.1321774111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith KS, Rudolph U. Anxiety and depression: mouse genetics and pharmacological approaches to the role of GABA(A) receptor subtypes. Neuropharmacology. 2012;62:54–62. doi: 10.1016/j.neuropharm.2011.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Crestani F, Lorez M, Baer K, Essrich C, Benke D, Laurent JP, et al. Decreased GABAA-receptor clustering results in enhanced anxiety and a bias for threat cues. Nat Neurosci. 1999;2:833–839. doi: 10.1038/12207. [DOI] [PubMed] [Google Scholar]
- 35.Essrich C, Lorez M, Benson J, Fritschy J-M, Luscher B. Postsynaptic clustering of major GABA(A) receptor subtypes requires the gamma2 subunit and gephyrin. Nat Neurosci. 1998;1:563–571. doi: 10.1038/2798. [DOI] [PubMed] [Google Scholar]
- 36.Lorez M, Benke D, Luscher B, Mohler H, Benson JA. Single-channel properties of neuronal GABAA receptors from mice lacking the 2 subunit. J Physiol. 2000;527(Pt 1):11–31. doi: 10.1111/j.1469-7793.2000.t01-1-00011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Earnheart JC, Schweizer C, Crestani F, Iwasato T, Itohara S, Mohler H, et al. GABAergic control of adult hippocampal neurogenesis in relation to behavior indicative of trait anxiety and depression states. J Neurosci. 2007;27:3845–3854. doi: 10.1523/JNEUROSCI.3609-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shen Q, Lal R, Luellen BA, Earnheart JC, Andrews AM, Luscher B. gamma-Aminobutyric acid-type A receptor deficits cause hypothalamic-pituitary-adrenal axis hyperactivity and antidepressant drug sensitivity reminiscent of melancholic forms of depression. Biol Psychiatry. 2010;68:512–520. doi: 10.1016/j.biopsych.2010.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Leal SL, Tighe SK, Jones CK, Yassa MA. Pattern separation of emotional information in hippocampal dentate and CA3. Hippocampus. 2014;24:1146–1155. doi: 10.1002/hipo.22298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fujii T, Saito DN, Yanaka HT, Kosaka H, Okazawa H. Depressive mood modulates the anterior lateral CA1 and DG/CA3 during a pattern separation task in cognitively intact individuals: a functional MRI study. Hippocampus. 2014;24:214–224. doi: 10.1002/hipo.22216. [DOI] [PubMed] [Google Scholar]
- 41.Vithlani M, Hines RM, Zhong P, Terunuma M, Hines DJ, Revilla-Sanchez R, et al. The ability of BDNF to modify neurogenesis and depressive-like behaviors is dependent upon phosphorylation of tyrosine residues 365/367 in the GABA(A)-receptor gamma2 subunit. J Neurosci. 2013;33:15567–15577. doi: 10.1523/JNEUROSCI.1845-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gunther U, Benson J, Benke D, Fritschy JM, Reyes G, Knoflach F, et al. Benzodiazepine-insensitive mice generated by targeted disruption of the gamma 2 subunit gene of gamma-aminobutyric acid type A receptors. Proc Natl Acad Sci U S A. 1995;92:7749–7753. doi: 10.1073/pnas.92.17.7749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schweizer C, Balsiger S, Bluethmann H, Mansuy M, Fritschy JM, Mohler H, et al. The gamma2 subunit of GABA(A) receptors is required for maintenance of receptors at mature synapses. Mol Cell Neurosci. 2003;24:442–450. doi: 10.1016/s1044-7431(03)00202-1. [DOI] [PubMed] [Google Scholar]
- 44.Autry AE, Adachi M, Nosyreva E, Na ES, Los MF, Cheng PF, et al. NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature. 2011;475:91–95. doi: 10.1038/nature10130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yuan X, Yao J, Norris D, Tran DD, Bram RJ, Chen G, et al. Calcium-modulating cyclophilin ligand regulates membrane trafficking of postsynaptic GABA(A) receptors. Mol Cell Neurosci. 2008;38:277–289. doi: 10.1016/j.mcn.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Terunuma M, Xu J, Vithlani M, Sieghart W, Kittler J, Pangalos M, et al. Deficits in phosphorylation of GABA(A) receptors by intimately associated protein kinase C activity underlie compromised synaptic inhibition during status epilepticus. J Neurosci. 2008;28:376–384. doi: 10.1523/JNEUROSCI.4346-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Alldred MJ, Mulder-Rosi J, Lingenfelter SE, Chen G, Luscher B. Distinct gamma2 subunit domains mediate clustering and synaptic function of postsynaptic GABAA receptors and gephyrin. J Neurosci. 2005;25:594–603. doi: 10.1523/JNEUROSCI.4011-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu Y, Wong TP, Aarts M, Rooyakkers A, Liu L, Lai TW, et al. NMDA receptor subunits have differential roles in mediating excitotoxic neuronal death both in vitro and in vivo. J Neurosci. 2007;27:2846–2857. doi: 10.1523/JNEUROSCI.0116-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Miller OH, Yang L, Wang CC, Hargroder EA, Zhang Y, Delpire E, et al. GluN2B-containing NMDA receptors regulate depression-like behavior and are critical for the rapid antidepressant actions of ketamine. eLife. 2014;3:e03581. doi: 10.7554/eLife.03581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Budreck EC, Kwon OB, Jung JH, Baudouin S, Thommen A, Kim HS, et al. Neuroligin-1 controls synaptic abundance of NMDA-type glutamate receptors through extracellular coupling. Proc Natl Acad Sci U S A. 2013;110:725–730. doi: 10.1073/pnas.1214718110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ren Z, Sahir N, Murakami S, Luellen BA, Earnheart JC, Lal R, et al. Defects in dendrite and spine maturation and synaptogenesis associated with an anxious-depressive-like phenotype of GABAA receptor-deficient mice. Neuropharmacology. 2015;88:171–179. doi: 10.1016/j.neuropharm.2014.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sato Y, Seo N, Kobayashi E. Genetic background differences between FVB and C57BL/6 mice affect hypnotic susceptibility to pentobarbital, ketamine and nitrous oxide, but not isoflurane. Acta Anaesthesiol Scand. 2006;50:553–556. doi: 10.1111/j.1399-6576.2006.001002.x. [DOI] [PubMed] [Google Scholar]
- 53.Bechtholt-Gompf AJ, Smith KL, John CS, Kang HH, Carlezon WA, Jr, Cohen BM, et al. CD-1 and Balb/cJ mice do not show enduring antidepressant-like effects of ketamine in tests of acute antidepressant efficacy. Psychopharmacology. 2011;215:689–695. doi: 10.1007/s00213-011-2169-8. [DOI] [PubMed] [Google Scholar]
- 54.Samuels BA, Hen R. Neurogenesis and affective disorders. Eur J Neurosci. 2011;33:1152–1159. doi: 10.1111/j.1460-9568.2011.07614.x. [DOI] [PubMed] [Google Scholar]
- 55.Chang CH, Chen MC, Lu J. Effect of antidepressant drugs on the vmPFC-limbic circuitry. Neuropharmacology. 2015;92:116–124. doi: 10.1016/j.neuropharm.2015.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kallarackal AJ, Kvarta MD, Cammarata E, Jaberi L, Cai X, Bailey AM, et al. Chronic stress induces a selective decrease in AMPA receptor-mediated synaptic excitation at hippocampal temporoammonic-CA1 synapses. J Neurosci. 2013;33:15669–15674. doi: 10.1523/JNEUROSCI.2588-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schneider PG, Rodriguez de Lores Arnaiz G. Ketamine prevents seizures and reverses changes in muscarinic receptor induced by bicuculline in rats. Neurochem Int. 2013;62:258–264. doi: 10.1016/j.neuint.2012.12.013. [DOI] [PubMed] [Google Scholar]
- 58.Chen JW, Wasterlain CG. Status epilepticus: pathophysiology and management in adults. Lancet Neurol. 2006;5:246–256. doi: 10.1016/S1474-4422(06)70374-X. [DOI] [PubMed] [Google Scholar]
- 59.Synowiec AS, Singh DS, Yenugadhati V, Valeriano JP, Schramke CJ, Kelly KM. Ketamine use in the treatment of refractory status epilepticus. Epilepsy Res. 2013;105:183–188. doi: 10.1016/j.eplepsyres.2013.01.007. [DOI] [PubMed] [Google Scholar]
- 60.Thompson SM, Kallarackal AJ, Kvarta MD, Van Dyke AM, LeGates TA, Cai X. An excitatory synapse hypothesis of depression. Trends Neurosci. 2015;38:279–294. doi: 10.1016/j.tins.2015.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Garcia LS, Comim CM, Valvassori SS, Reus GZ, Stertz L, Kapczinski F, et al. Ketamine treatment reverses behavioral and physiological alterations induced by chronic mild stress in rats. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:450–455. doi: 10.1016/j.pnpbp.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 62.Hewitt SA, Bains JS. Brain-derived neurotrophic factor silences GABA synapses onto hypothalamic neuroendocrine cells through a postsynaptic dynamin-mediated mechanism. J Neurophysiol. 2006;95:2193–2198. doi: 10.1152/jn.01135.2005. [DOI] [PubMed] [Google Scholar]
- 63.Wake H, Watanabe M, Moorhouse AJ, Kanematsu T, Horibe S, Matsukawa N, et al. Early changes in KCC2 phosphorylation in response to neuronal stress result in functional downregulation. J Neurosci. 2007;27:1642–1650. doi: 10.1523/JNEUROSCI.3104-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.MacKenzie G, Maguire J. Chronic stress shifts the GABA reversal potential in the hippocampus and increases seizure susceptibility. Epilepsy Res. 2015;109:13–27. doi: 10.1016/j.eplepsyres.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chubykin AA, Atasoy D, Etherton MR, Brose N, Kavalali ET, Gibson JR, et al. Activity-dependent validation of excitatory versus inhibitory synapses by neuroligin-1 versus neuroligin-2. Neuron. 2007;54:919–931. doi: 10.1016/j.neuron.2007.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gourley SL, Kedves AT, Olausson P, Taylor JR. A history of corticosterone exposure regulates fear extinction and cortical NR2B, GluR2/3, and BDNF. Neuropsychopharmacology. 2009;34:707–716. doi: 10.1038/npp.2008.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yuen EY, Wei J, Liu W, Zhong P, Li X, Yan Z. Repeated stress causes cognitive impairment by suppressing glutamate receptor expression and function in prefrontal cortex. Neuron. 2012;73:962–977. doi: 10.1016/j.neuron.2011.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li N, Liu RJ, Dwyer JM, Banasr M, Lee B, Son H, et al. Glutamate N-methyl-D-aspartate receptor antagonists rapidly reverse behavioral and synaptic deficits caused by chronic stress exposure. Biol Psychiatry. 2011;69:754–761. doi: 10.1016/j.biopsych.2010.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cai X, Kallarackal AJ, Kvarta MD, Goluskin S, Gaylor K, Bailey AM, et al. Local potentiation of excitatory synapses by serotonin and its alteration in rodent models of depression. Nat Neurosci. 2013;16:464–472. doi: 10.1038/nn.3355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Klausberger T. GABAergic interneurons targeting dendrites of pyramidal cells in the CA1 area of the hippocampus. Eur J Neurosci. 2009;30:947–957. doi: 10.1111/j.1460-9568.2009.06913.x. [DOI] [PubMed] [Google Scholar]
- 71.Lin LC, Sibille E. Somatostatin, neuronal vulnerability and behavioral emotionality. Mol Psychiatry. 2015;20:377–387. doi: 10.1038/mp.2014.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.