Abstract
Mutations in the amyloid precursor protein (APP) gene inducing abnormal processing and deposition of β-amyloid protein in the brain have been implicated in the pathogenesis of Alzheimer's disease (AD). Although Tg2576 mice with the Swedish mutation (hAPPswe) exhibit age-related Aβ-plaque formation in brain regions like the hippocampus, the amygdala, and the cortex, these mice show a rather specific deficit in hippocampal-dependent learning and memory tasks. In view of recent findings showing that neural systems subserving different forms of learning are not simply independent but that depressing or enhancing one system affects learning in another system, we decided to investigate fronto-striatal synaptic plasticity and related procedural learning in these mutants. Fronto-striatal long-term depression (LTD) induced by tetanic stimulation of the cortico-striatal input was similar in Tg2576 and wild-type control mice. Behavioral data, however, pointed to an enhancement of procedural learning in the mutants that showed robust motor-based learning in the cross maze and higher active avoidance scores. Thus, in this mouse model of AD, an intact striatal function associated with an impaired hippocampal function seems to provide neural conditions favorable to procedural learning. Our results suggest that focusing on preserved or enhanced forms of learning in AD patients might be of interest to describe the functional reorganization of the brain when one memory system is selectively compromised by neurological disease.
Transgenic mice overexpressing the human amyloid precursor protein with the Swedish mutation (TgHuAPP695swe [Tg2576]; Hsiao et al. 1996) show age-dependent neural and cognitive alterations resembling those described in Alzheimer's disease (AD) patients (Chapman et al. 1999; King and Arendash 2002; Uryu et al. 2002). A main feature of Tg2576 mice is the presence of amyloid plaques that develop around 16-18 mo in selective brain regions like the cortex, the hippocampus, and the amygdala (Corcoran et al. 2002; Lehman et al. 2003). Although the β-amyloid peptides (Aβ) that accumulate in the brains of Tg2576 mice seem to be distinct from the chemically modified and insoluble peptides deposited in senile plaques of AD patients (Kalback et al. 2002), these mutants show several hallmarks of the human pathology, among which are an enhancement of oxidative stress (Smith et al. 1998), microglia activation (Frautschy et al. 1998), and glia-mediated inflammation (Benzing et al. 1999).
Electrophysiological experiments aimed at investigating the properties of the hippocampal synaptic transmission in this mouse strain have, however, yielded discrepant results. In particular, the question as to whether hippocampal basal synaptic transmission is normal while long-term potentiation (LTP) is altered (Chapman et al. 1999) or, conversely, if there is a deficit in basal synaptic transmission while LTP is induced and expressed normally (Fitzjohn et al. 2001), is still a matter of controversy. Nevertheless, these studies all point to a reduction in hippocampal function and the finding that Tg2576 mice perform poorly in hippocampus-depending learning tasks (Hsiao et al. 1996; Ashe 2001; Corcoran et al. 2002; King and Arendash 2002) is consistent with a deficit selectively affecting the hippocampal-based memory system.
It is worth remembering that the concept of the brain memory system arises from the observation that a dysfunction in an anatomical system abolishes specific memory operations but leaves other forms of acquisition intact. That is, treatments altering the hippocampal function disrupt declarative, spatial, or context-based memory in animals or humans but spare their capability to form stimulus-response or motor habits (Scoville and Milner 1957; O'Keefe and Nadel 1978; Cohen and Squire 1980; McDonald and White 1993). More recent evidence shows, however, that memory systems are not simply independent operating units but can also interact, because suppression of one system affects learning mediated by another system. For example, depressing the hippocampal function has been shown to enhance striatal-dependent procedural responding in rats tested in the water (Schroeder et al. 2002) or the standard version of the cross maze (Chang and Gold 2003). Thus, assuming that animal models of AD mainly show cognitive alterations reminiscent of a hippocampal dysfunction, investigations of their fronto-striatal plasticity and related procedural learning abilities (Calabresi et al. 1996; Spencer and Murphy 2000; Partridge et al. 2002; Geracitano et al. 2003) could be of interest. In the present experiments, we report superior motor-based learning and active avoidance performance together with normal development of fronto-striatal long-term depression in Tg2576 mice, suggesting that procedural learning was not only spared but enhanced under certain testing conditions in this mouse model of AD.
RESULTS
Experiment 1: Exploratory Activity and Spontaneous Alternation
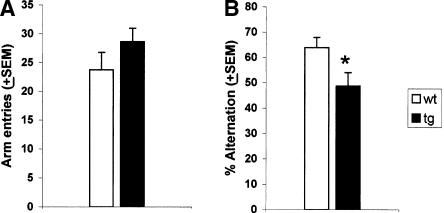
The data are shown in Figure 1. Statistical analyses performed by means of Student's t-tests for independent samples indicated that even though Tg2576 mice tended to visit more arms than did wild-type mice, the data failed to reach significance (t = 1.28, p > 0.05). Conversely, the percentage of spontaneous alternation was significantly lower in Tg2576 than in wild-type mice (t = -2.3, p < 0.05).
Figure 1.
(Experiment 1) Tg2576 mice do not differ from wild-type mice in the number of arm entries but exhibit a lower rate of spontaneous alternation. (A) Mean number ± SEM of arm entries and (B) mean percentage ± SEM of spontaneous alternation rate, during a 5-min session of exploration in the Y maze. (tg) Tg2576 mice; (wt) wild-type mice; (*) P < 0.05.
Experiment 2: Cross Maze Learning
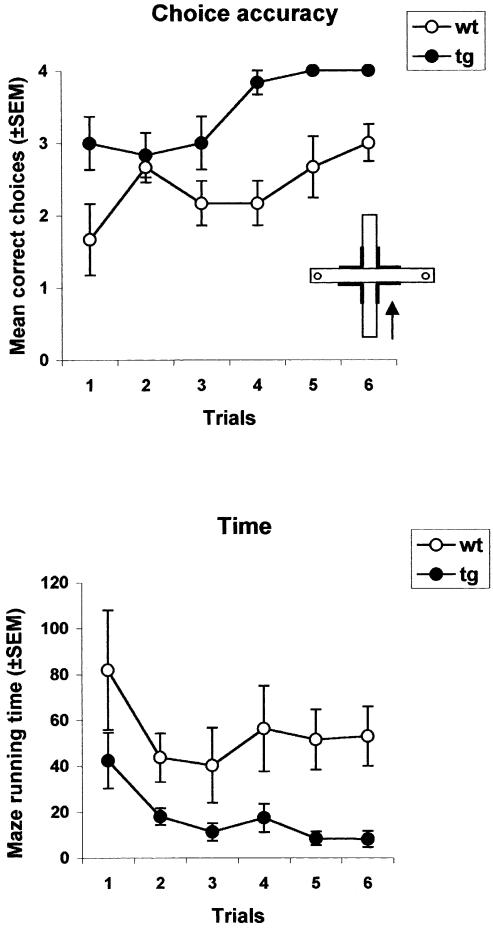
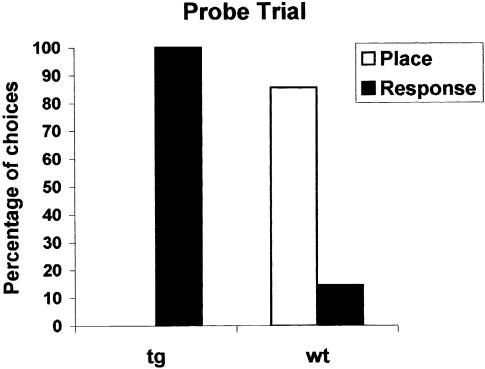
Figure 2 shows the number of correct choices and the time spent to complete each trial during cross maze training. An ANOVA with genotype as the between-group factor (two levels) and trials as the within-group factor (six levels) was performed on these two variables. The results showed that transgenic mice made more correct choices (F(1,14) = 24.9, p < 0.001) and run the maze faster (F(1,14) = 12.9, p < 0.01) than wild-type mice. The results of the probe trial are shown in Figure 3. The choices recorded in each group were tabulated, and differences in percentages between the two groups were evaluated using the two-tailed Fisher's Exact Test. Results show that the percentages of choices were differently distributed according to the genotype and the category (p < 0.005). Within-group comparisons revealed that all transgenic mice expressed a response strategy on the probe trial, thus maintaining the motor stratagem (turning right) learned during training (χ2 = 6, p < 0.05), whereas the majority of wild-type mice exhibited a place strategy (χ2 = 3.5, p < 0.05).
Figure 2.
(Experiment 2) Tg2576 mice show superior cross maze training performance. Mean number ± SEM of correct runs made during six daily four-run trials (top) and mean time ± SEM to complete each trial (bottom). (tg) Tg2576 mice; (wt) wild-type mice.
Figure 3.
(Experiment 2) All Tg2576 mice showed response learning on the cross maze probe trial, whereas wild-type mice were predominantly place learners. Number of choices corresponding to place (solid open bar) or response (solid filled bars) learning. (tg) Tg2576 mice; (wt) wild-type mice; (*) P < 0.05; (**) P < 0.01.
Experiment 3: Active Avoidance Learning
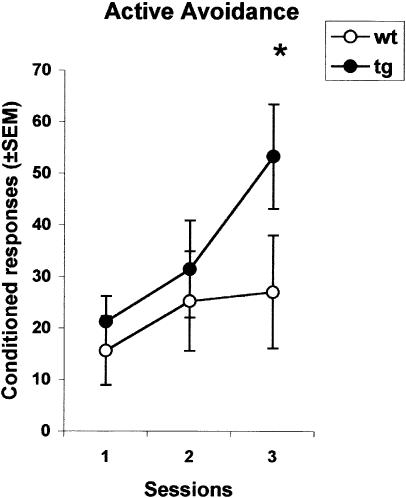
Figure 4 shows the conditioned responses recorded in each group during the three acquisition sessions. An ANOVA with genotype as the between-group factor (two levels) and sessions as the within-group factor (three levels) was performed on these data. The results showed no main effect of genotype (F(1,13) < 1), but a significant effect of sessions (F(2,26) = 10.66, p < 0.001), indicating that performance improved over time. Post hoc simple effects analysis revealed, however, an effect of genotype (p < 0.05) on the last training session. Pain thresholds recorded in naive and trained mice were compared by a t-test for unpaired samples. The results are shown in Table 1. No significant differences between mutant and wild-type mice were found in the minimal footshock intensity eliciting vocalization (t = -1.80, p > 0.05 for trained mice; t = -2.02, p > 0.05 for naive mice) or jumping (t = -1.50, p > 0.05 for trained mice; t = 0.14, p > 0.05 for naive mice).
Figure 4.
(Experiment 3) Tg2576 mice show superior active avoidance scores on the last training trial. Mean number of conditioned responses ± SEM during three consecutive daily sessions involving 90 US-CS pairings each. (tg) Tg2576 mice; (wt) wild-type mice; (*) P < 0.05.
Table 1.
(Experiment 3) Tg2576 and Wild-Type Mice Do Not Show Differences in Pain Threshold
| Vocalization
|
Jumping
|
|||
|---|---|---|---|---|
| Mean (mA) | SEM | Mean (mA) | SEM | |
| wt naive (n = 7) | 0.11 | ±0.01 | 0.22 | ±0.01 |
| tg naive (n = 8) | 0.17 | ±0.01 | 0.22 | ±0.01 |
| wt trained (n = 7) | 0.18 | ±0.04 | 0.24 | ±0.03 |
| tg trained (n = 7) | 0.25 | ±0.02 | 0.30 | ±0.03 |
Mean shock intensity (in milliamperes) ± SEM inducing vocalization (left), or jumping (right) in naive or trained Tg2576 and wild-type mice. (tg) Tg2576 mice; (wt) wild-type mice.
Experiment 4: Fronto-Striatal Long-Term Depression
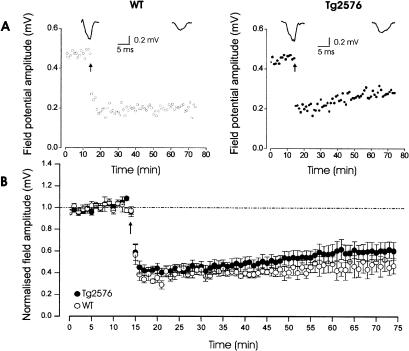
The data are shown in Figure 5. Stimulation of the cortico-striatal pathway elicited typical field potentials (fEPSPs) recorded with extracellular electrodes placed in striatal spiny neurons of brain slices obtained from Tg2576 (nine slices from five mice) and control mice (five slices from four mice).
Figure 5.
(Experiment 4) Field potentials induced in the striatum following stimulation of the fronto-striatal pathway are similar in Tg2576 and wild-type mice (p > 0.10). (A) Tetanic stimulation (arrow) of fronto-cortical fibers induced LTD in both wild-type (left) and in Tg2576 mice (right). The top traces are averages of six successive responses obtained immediately before and 50 min after the tetanic train. (B) Pooled data showing the normalized changes in field potential amplitude ± SEM induced by the tetanic stimulation (arrow) in Tg2576 (n = 9 slices from 5 mice) and wild-type (n = 5 slices from 4 mice). The degree of depression measured 10-60 min after the tetanic stimulation in both groups did not differ significantly (F(1,48) = 0.50, p > 0.10).
There is evidence (Jiang and North 1991; Calabresi et al. 1992) that AMPA glutamate receptors mediate the cortically evoked fEPSPs at striatal synapses. In agreement with these data, we found that fEPSPs recorded in both groups were unaffected by addition of the NMDA glutamate receptor antagonist AP5 (50 μM) while they were completely abolished by addition of the AMPA/Kainate glutamate receptor antagonist CNQX (10 μM; data not shown). The mean half-maximal amplitude of the field potentials (control mice: 0.45 ± 0.031 mV; transgenic mice: 0.48 ± 0.036 mV) and the stimulus intensity necessary to elicit these responses (control mice: 50.2 ± 4.1 V; transgenic mice: 46.12 ± 5.3 V) were similar in both groups (Student's t-test, p > 0.10 for each comparison). Tetanic stimulation induced a similar LTD in wild-type and transgenic mice (-52.5 ± 5% and -55.1 ± 9% of the pretetanic value, 1 h after tetanization, Student's t-test, p > 0.10). Development of LTD was evaluated using a two-way analysis of variance (ANOVA) with genotype as the between-group factor and time as the within-group factor. No effect of the genotype (F(1,48) < 1) and of the genotype × time window interaction (F(12,48) < 1) were found when the degree of depression was compared 10 to 60 min after tetanic stimulation. In both groups, however, field potentials tended to recover as time elapsed (significant effect of repeated measures, F(48,528) = 3.71, p < 0.01).
DISCUSSION
LTD induced in striatal spiny neurons by tetanic stimulation of cortico-striatal fibers developed normally in Tg2576 mice indicating that the hAPPSwe mutation spares some plasticity mechanisms in the striatum. In view of evidence showing that long-lasting changes in the synaptic efficacy of cortico-striatal synapses play an important role in the regulation of the excitatory input to basal ganglia mediating “stimulus-response” or “habit” learning (Spencer and Murphy 2000; Partridge et al. 2002), the intact development of striatal LTD in Tg2576 mice indicates that the neural bases for certain forms of learning and memory are preserved in this mouse model of AD. This observation is consistent with recent data suggesting that amyloidosis alters neural plasticity within only certain brain structures because extracellular field potentials in vitro and field potentials in vivo elicited by prefrontal cortico-cortical stimulation were unchanged in APP23 transgenic mice, another murine model of AD (Roder et al. 2003).
Interestingly, in spite of their similar cortico-striatal LTD, Tg2576 mice performed better than wild-type mice in two tasks largely depending on the striatum (Delacour et al. 1977; Kelly et al. 1977; Vecsei and Beal 1991; Packard and Mc Gaugh 1996). First, cross maze training performance was more accurate in the mutants. Clearly, the learning of this simple motor habit (turning right) was likely facilitated by the fact that Tg2576 mice show reduced spontaneous alternation, that is, a natural tendency of rodents to explore different arms of a maze on successive runs. Thus, the hippocampal dysfunction produced by the mutation (Chapman et al. 1999; Fitzjohn et al. 2001) might disrupt spontaneous behaviors that, in normal animals, interfere negatively with the learning of a simple motor habit. This assumption is in good agreement with data showing that lesioning (Matthews and Best 1995) or inactivating (Schroeder et al. 2002; Chang and Gold 2003) the hippocampus facilitates learning of nonspatial responses.
On the probe trial, however, Tg2576 mice did not simply behave as rodents with a dysfunctioning hippocampus. In fact, all the transgenic mice maintained the motor stratagem implemented during training (turning right), whereas there is evidence that, on probe trials administered following a similar short amount of training, mice (Passino et al. 2002; Middei et al. 2004) or rats (Packard and McGaugh 1996) with an altered hippocampal function show random responding. In fact, only a few experimental conditions were shown to favor response learning when the present cross maze protocol was used: (1) drastic reduction of extra-maze cues, which prevents spatial orientation (Restle 1957); (2) extensive training (16 daily trials), which makes normal animals shifting from cognitive spatial orientation to automatic motor-based responding (Packard and McGaugh 1996); (3) intrastriatal injections of glutamate aimed at enhancing the striatal function (Packard 1999). In that case, response learning became predominant on probe trials administered following short training, as in the present Tg2576 mice.
Indeed, that the hAPPswe mutation but not hippocampal inactivation enhances procedural learning is intriguing. A tentative explanation could be that the different behavior shown by Tg2576 mice and hippocampal inactivated animals depends on the chronic versus temporary disruption of their hippocampus. In particular, the long-lasting alteration of the hippocampal function induced by the mutation is more likely to promote a striatal-based compensatory mechanism than a temporarily disabled hippocampus. Interestingly, predominant response learning has also been reported in rats chronically exposed to intracerebroventricular infusion of β-amyloid 1-42 protein (Ammassari-Teule et al. 2002).
In addition, we found higher active avoidance scores in Tg2576 than in their wild-type littermates. This observation disagrees with previous findings showing similar scores in the two genotypes (King and Arendash 2002) even though differences in the experimental procedure and the genetic background can account for this discrepancy. In particular, training was notably distributed in the King and Arendash experiments (10 US-CS pairings on 12 consecutive days), whereas we used a more standard massed training procedure (90 US-CS pairings on three consecutive days). In addition, the Tg2576 mice used in their experiments were in a 75% C57BL/6 background, that is, in 75% of an inbred strain performing poorly in the active avoidance task (Bovet et al. 1969; Lipp et al. 1989), whereas we used the original Tg2576 mutant mice deriving from a C57BL/6 × SJL hybrid successively backcrossed to SJL (Hsiao et al. 1996). Therefore, the possibility that variations in the genetic background mediate a different effect of the targeted mutation on active avoidance performance cannot be excluded. Clearly, the former observation that hippocampal lesions enhanced active avoidance performance (for review, see O'Keefe and Nadel 1978) provides an additional evidence that suppression of hippocampal-dependent operations enhance striatal-dependent stimulus-response learning even if, importantly, a variety of factors such as the age of the subjects (Molino 1975), the site (Myhrer 1975), or the nature (Schmaltz 1971) of the lesions are crucial in promoting the performance enhancement.
Altogether, our data indicate enhanced procedural learning and normal fronto-striatal LTD in Tg2576 mice that otherwise show defect in hippocampal synaptic transmission (Chapman et al. 1999; Fitzjohn et al. 2001). Indeed, a preserved excitatory cortical input to the basal ganglia can only warrant normal striatal-dependent learning in animals having an intact hippocampus. However, in view of increasing evidence showing that processing of information by the hippocampus negatively interferes with performance in tasks depending on other neural systems (Chang and Gold 2003; Gold 2003; McIntyre et al. 2003; Savage et al. 2003), it could be that an intact striatal function associated with an impaired hippocampal function provides neural conditions favorable to procedural learning.
These observations allow the following points to be made. Cooperation and competition between separate memory systems are currently tested in normal animals by examining how enhancing or depressing one neural system experimentally affects performance in tasks governed by a different system. The present findings suggest that this approach should be extended to animal models of neurodegenerative diseases that frequently show a selective dysfunction in a brain region and/or neurotransmitter system mediating specific cognitive operations. In particular, in view of the strong convergence between animal and human studies in this field (Packard and Knowlton 2002; Poldrack and Packard 2003; Moody et al. 2004), focusing on preserved or paradoxically enhanced forms of cognition in AD patients may be helpful to describe the functional reorganization of the brain occurring when one memory system is selectively compromised by neurological disease.
METHODS
Animals
Mice overexpressing the APP695 fragment with the Swedish mutation (TgHuAPP695swe: Tg2576) and wild-type mice obtained from a hybrid genetic background (87% C57BL/6 × 12.5% SJL) subsequently backcrossed to C57BL/6 × SJL F1 mice were used. At the beginning of the experiment, they were 15 mo old and their weights ranged from 23 to 28 g. They were housed in a temperature-controlled room (22°C) with a light-dark 12:12 cycle (lights on 07:00-19:00 h). Food and water were given ad libitum. Eight mice per group were used in the training experiments. Unequal numbers of subjects in experiment 3 were caused by one Tg2576 mouse dying before the completion of testing. Posttraining footshock sensitivity thresholds were measured in seven Tg2576 and seven wild-type mice because one wild-type mouse died after active avoidance training. Footshock sensitivity thresholds were measured in eight Tg2576 and seven wild-type naive mice. All experiments were carried out according to the guidelines on the ethical use of animals from the European Communities Council Directive of 24 November 1986 (86/609/EEC).
Experiment 1: Spontaneous Alternation and Exploratory Activity
Spontaneous alternation and exploratory activity were measured in a wooden Y maze painted white with 13-cm-high walls. Each arm was 40 cm long and 6 cm large. Mice were placed in the center of the maze for a 5-min trial during which free exploration was allowed. Two dependent variables were recorded: the number of arms visited and the sequence of arm choices allowing the alternation rate to be determined. Spontaneous alternation values are expressed in percentage and correspond to the ratio of arm choices differing from the previous two choices divided by the number of total choices minus two (King and Arendash 2002).
Experiment 2: Cross Maze Learning
The apparatus was a wooden cross maze painted white that consisted of four identical arms (north, south, east, west) diverging from a squared platform (perimeter 6 × 6 cm). Each arm was 35 cm long and 6 cm wide. Transparent plexiglas 15-cm-high walls were fixed on both sides of each arm from their point of connection with the central platform until halfway. Four sliding doors made of the same transparent material separated each arm from the central platform. A food cup, 1 cm deep and 2 cm in diameter, was inserted in the floor at the distal end of the east and the west arms. The maze was elevated 40 cm above the floor and maintained in a constant orientation during the experiment. Behavioral testing was conducted in a diffusely illuminated 12-m2 room containing numerous items (posters, panels). The experimenter was seated behind the south arm. The day before pretraining began, the mice were food-deprived to bring down their weights to 85% of the initial level. Food deprivation was maintained during the whole training period. After pretraining and, subsequently, after each training trial, mice were given a daily ration of food varying between 3.5 and 4.5 g. Pretraining consisted of placing each mouse on the starting point of the south arm and allowing them to visit the maze where food pellets had been scattered in both choice arms. The door giving access to the north arm was closed. This procedure was repeated for two consecutive days. On the following days, mice were placed again on the starting point of the south arm. As during pretraining, the access to the north arm was blocked. On each trial, a single food pellet was located in the food cup at the end of the east arm. Mice were allowed to run the maze and after they entered the east arm (correct response) or the west arm (incorrect response), the door was closed and no correction procedure was used. After consuming the food pellet (east arm) or being held for 15 sec in the nonrewarded arm (west arm), mice were removed from the maze and placed in a cage located behind the south starting point for a 30-sec intertrial interval. Mice were given four daily training trials for six consecutive days. On the seventh day, mice were given a probe trial during which they were released from the north arm and allowed to choose either the east arm (place learning) or the west arm (response learning). The access to the south arm was closed. During probe trials, food was available in both the east and the west arms.
Experiment 3: Active Avoidance Learning and Pain Threshold Reactivity
Active avoidance learning was carried out in a battery of eight two-way shuttle-boxes (40 × 10 × 15 cm). Each shuttle-box was divided into two compartments by a partition with an opening at the floor level connecting the two compartments. The shuttle-boxes had a transparent cover with a lightbulb (10 W) attached above each compartment. The floor was a stainless grid. Mice were placed in the dark compartment and submitted to an active avoidance test (duration 45 min, 90 avoidance trials) during three consecutive days. Each trial consisted of a 30-sec light signal (10 W) presented in one compartment (conditioned stimulus, CS) 5 sec before the onset of an electric footshock (0.7 mA, 25 sec) in the other compartment (unconditioned stimulus, US). Three dependent variables were recorded: escape responses (crossings during US presentation), conditioned responses (crossings during CS presentation), and intertrial crossings (crossings independent from US or CS presentation).
In each genotype, footshock sensitivity was evaluated both posttraining and in groups of naive mice. The animals were placed in a plexiglas cage (28 cm long × 28 cm wide × 10 cm high) with a grid floor connected to a shock producer. For each mouse, the pain threshold was evaluated by increasing current intensity from 0 to a maximum of 0.3 mA. The minimal intensity eliciting vocalization and jumping was retained as the score.
Experiment 4: Fronto-Striatal Long-Term Depression
Preparation of Brain Slices
Mice were anaesthetized with halothane and decapitated. Preparation and maintenance of brain slices have been described previously (Calabresi et al. 1992). Briefly, the brain was rapidly removed and coronal cortico-striatal slices (300 μm) were cut from tissue blocks with a vibratome in an artificial cerebrospinal fluid solution (ACSF, 20°-25°C). Slices were incubated in a holding chamber (1 h, 34°C) and then transferred to a recording chamber, completely submerged in ACSF (33°-34°C, 2-3 mL/min; gassed with 95% O2-5% CO2). ACSF composition was 126 mM NaCl, 1.2 mM NaH2PO4, 1.3 mM MgCl2, 2.4 mM CaCl2, 2.5 mM KCl, 18 mM NaHCO3, and 10 mM glucose.
Induction of Fronto-Striatal Long-Term Depression
Synaptic field potentials within the dorsal striatum were recorded using glass microelectrodes filled with 2 M NaCl (5-10 MΩ). Signals were fed to an Iso-DAM8 amplifier (WPI Inc.), filtered at 1 kHz, and acquired and analyzed with the “LTP program” (Anderson and Collingridge 2001). A bipolar Ni/Cr insulated stimulating electrode was placed in the corpus callosum to activate cortico-striatal fibers. A test stimulus (50 msec duration) was delivered every 60 sec to evoke half-maximal responses. After 10 min of stable responses, a tetanus consisting of three trains (100 Hz, 1 sec, 6 sec interval) was delivered. During the tetanus, the intensity of the stimulus was set to produce maximal responses. The responses were recorded for 60 min following the tetanus. The field potential amplitude was defined as the mean amplitude of the peak negativity, measured from the peak of the early and of the late positivity (Calabresi et al. 1992).
Acknowledgments
This research was supported by a grant from the Italian Minister of Health, Progetto Finalizzato Strategico Alzheimer (ICS120.3/RA00.86).
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Article and publication are at http://www.learnmem.org/cgi/doi/10.1101/lm.80604.
References
- Ammassari-Teule, M., Middei, S., Passino, E., and Restivo, L. 2002. Enhanced procedural learning following β-amyloid protein (1-42) infusion in the rat. NeuroReport 13: 1679-1682. [DOI] [PubMed] [Google Scholar]
- Anderson, W.W. and Collingridge, G.L. 2001. The LTP Program: A data acquisition program for on-line analysis of long-term potentiation and other synaptic events. J. Neurosci. Methods 108: 71-83. [DOI] [PubMed] [Google Scholar]
- Ashe, K.H. 2001. Learning and memory in transgenic mice modeling Alzheimer's disease. Learn. Mem. 8: 301-308. [DOI] [PubMed] [Google Scholar]
- Benzing, W.C., Wujek, J.R., Ward, E.K., Shaffer, D., Ashe, K.H., Younkin, S.G., and Brunden, K.R. 1999. Evidence for glial mediated inflammation in aged APP(SW) transgenic mice. Neurobiol. Aging 20: 581-589. [DOI] [PubMed] [Google Scholar]
- Bovet, D., Bovet-Nitti, F., and Oliverio, A. 1969. Genetic aspects of learning and memory in mice. Science 163: 139-149. [DOI] [PubMed] [Google Scholar]
- Calabresi, P., Maj, R., Pisani, A., Mercuri, N.B., and Bernardi, G. 1992. Long-term synaptic depression in the striatum: Physiological and pharmacological characterization. J. Neurosci. 12: 4224-4233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabresi, P., Pisani, A., Mercuri, N.B., and Bernardi, G. 1996. The corticostriatal projection: From synaptic plasticity to dysfunctions of the basal ganglia. Trends Neurosci. 19: 19-24. [DOI] [PubMed] [Google Scholar]
- Chang, Q. and Gold, P.E. 2003. Intra-hippocampal lidocaine injections impair acquisition of a place task and facilitate acquisition of a response task in rats. Behav. Brain Res. 144: 19-24. [DOI] [PubMed] [Google Scholar]
- Chapman, P.F., White, G.l., Jones, M.W., Cooper-Blacketer, D., Marshall, V.J., Irizzary, M., Younkin, L., Good, M.A., Bliss, T.V., Hyman, B.T., et al. 1999. Impaired synaptic plasticity and learning in aged amyloid precursor protein transgenic mice. Nat. Neurosci. 2: 271-276. [DOI] [PubMed] [Google Scholar]
- Cohen, N.J. and Squire, L.R. 1980. Preserved learning and retention of pattern-analyzing skill in amnesia: Dissociation of knowing how and knowing that. Science 210: 207-210. [DOI] [PubMed] [Google Scholar]
- Corcoran, K.A., Lu, Y., Turner, R.S., and Maren, S. 2002. Overexpression of hAPPswe impairs rewarded alternation and contextual fear conditioning in a transgenic mouse model of Alzheimer's disease. Learn. Mem. 9: 243-252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delacour, J., Echavarria, M.T., Senault, B., and Houcine, O. 1977. Specificity of avoidance deficits produced by 6-hydroxydopamine lesions of the nigrostriatal system of the rat. J. Comp. Physiol. Psychol. 91: 875-885. [DOI] [PubMed] [Google Scholar]
- Fitzjohn, S.M., Morton, R.A., Kuenzi, F., Roshal, T.W., Shearman, M., Lewis, H., Smith, D., Reynolds, D.S., Davies, C.H., Colingride, G.L., et al. 2001. Age-related impairment of synaptic transmission but normal long term potentiation in transgenic mice that overexpress the human APP695SWE mutant form of amyloid precursor protein. J. Neurosci. 21: 4691-4698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frautschy, S.A., Yang, F., Irrizzary, M., Hyman, B., Saido, T.C., Hsiao, K., and Cole G.M. 1998. Microglial response to amyloid plaques in APPsw transgenic mice. Am. J. Pathol. 152: 307-317. [PMC free article] [PubMed] [Google Scholar]
- Geracitano, R., Paolucci, E., Prisco, S., Guatteo, E., Zona, C., Longone, P., Ammassari-Teule, M., Bernardi, G., Berretta N., and Mercuri, N.B. 2003. Altered long-term corticostriatal synaptic plasticity in transgenic mice overexpressing human CU/ZN superoxide dismutase (GLY93 → ALA) mutation. Neuroscience 118: 399-408. [DOI] [PubMed] [Google Scholar]
- Gold, P.E. 2003. Acetylcholine modulation of neural systems involved in learning and memory. Neurobiol. Learn. Mem. 80: 194-210. [DOI] [PubMed] [Google Scholar]
- Hsiao, K., Chapman, P., Nilsen, S., Eckman, C., Harigaya, Y., Younkin, S., Yang F., and Cole, G. 1996. Correlative memory deficits, Aβ elevation, and amyloid plaques in transgenic mice. Science 274: 99-102. [DOI] [PubMed] [Google Scholar]
- Jiang, Z.G. and North, R.A. 1991. Membrane properties and synaptic responses of rat striatal neurones in vitro. J. Physiol. 443: 533-553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalback, W., Watson, M.D., Kokjohn, T.A., Kuo, Y.M., Weiss, N., Luehrs, D.C., Lopez, J., Brune, D., Sisodia, S.S., Staufenbeil, M., et al. 2002. APP Transgenic mice Tg2576 accumulate Aβ peptides that are distinct from the chemically modified and insoluble peptides deposited in Alzheimer's disease senile plaques. Biochemistry 41: 922-928. [DOI] [PubMed] [Google Scholar]
- Kelly, J., Alheid, G.F., McDermott, L., Halaris, A., and Grossman, S.P. 1977. Behavioral and biochemical effects of knife cuts that preferentially interrupt principal afferent and efferent connections of the striatum in the rat. Pharmacol. Biochem. Behav. 6: 31-45. [DOI] [PubMed] [Google Scholar]
- King, D.L. and Arendash, G.W. 2002. Behavioral characterization of the Tg2576 transgenic model of Alzheimer's disease through 19 months. Physiol. Behav. 75: 627-642. [DOI] [PubMed] [Google Scholar]
- Lehman, E.J., Kulnane, L.S., and Lamb, B.T. 2003. Alterations in β-amyloid production and deposition in brain regions of two transgenic models. Neurobiol. Aging 24: 645-653. [DOI] [PubMed] [Google Scholar]
- Lipp, H.-P., Schwegler, H., Crusio, W.E., Wolfer, D.P., Leisinger-Trigona, M.-C., Heimrich, B., and Driscoll, P. 1989. Using genetically-defined rodent strains for the identification of hippocampal traits relevant for two-way avoidance behavior: A non invasive approach. Experientia 45: 845-859. [DOI] [PubMed] [Google Scholar]
- Matthews, D.B. and Best, P. 1995. Fimbria/fornix lesions facilitate learning of a nonspatial response task. Psychon. Bull. Rev. 2: 113-116. [DOI] [PubMed] [Google Scholar]
- McDonald, R.J. and White, N.M. 1993. A triple dissociation of memory systems: Hippocampus, amygdala, and dorsal striatum. Behav. Neurosci. 107: 3-22. [DOI] [PubMed] [Google Scholar]
- McIntyre, C.K., Marriott, L.K., and Gold P.E. 2003. Cooperation between memory systems: Acetylcholine release in the amygdala correlates positively with performance on a hippocampal-dependent task. Behav. Neurosci. 117: 320-326. [DOI] [PubMed] [Google Scholar]
- Middei, S., Restivo, L., Sgobio, C., Passino, E., and Ammassari-Teule, M. 2004. Reversible inactivation of hippocampus and dorsolateral striatum in C57BL/6 and DBA/2 inbred mice failed to show interaction between memory systems in these genotypes. Behav. Brain Res. (in press). [DOI] [PubMed]
- Molino, A. 1975. Sparing of function after infant lesions of selected limbic structures in the rat. J. Comp. Physiol. Psychol. 89: 868-881. [DOI] [PubMed] [Google Scholar]
- Moody, T.D., Bookheimer, S.Y., Vanek, Z., and Knowlton, B.J. 2004. An implicit learning task activates medial temporal lobe in patients with Parkinson's disease. Behav. Neurosci. 118: 438-442. [DOI] [PubMed] [Google Scholar]
- Myhrer, T. 1975. Locomotor and avoidance behaviour in rats with partial or total lesions of the perforant path. Physiol. Behav. 15: 217-224. [DOI] [PubMed] [Google Scholar]
- O'Keefe, J. and Nadel, L. 1978. The hippocampus as a cognitive map, p. 465. Clarendon Press, Oxford, UK.
- Packard, M.G. 1999. Glutamate infused posttraining into the hippocampus or caudate-putamen differentially strengthens place and response learning. Proc. Natl. Acad. Sci. 96: 12881-12886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packard, M.G. and Knowlton, B.J. 2002. Learning and memory functions of the basal ganglia. Ann. Rev. Neurosci. 25: 5563-5593. [DOI] [PubMed] [Google Scholar]
- Packard, M.G. and McGaugh, J.L. 1996. Inactivation of the hippocampus or caudate nucleus with lidocaine differentially affects expression of place and response learning. Neurobiol. Learn. Mem. 65: 65-72. [DOI] [PubMed] [Google Scholar]
- Partridge, J.G., Apparsundaram, S., Gerhardt, G.A., Ronesi, J., and Lovinger, D.M. 2002. Nicotinic acetylcholine receptors interact with dopamine induction of striatal long term depression. J. Neurosci. 22: 2541-2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passino, E., Middei, S., Restivo, L., Bertaina Anglade, V., and Ammassari-Teule, M. 2002. Genetic approach to variability of memory systems: Analysis of place vs. response learning and Fos-related expression in hippocampal and striatal areas of C57BL/6 and DBA/2 mice. Hippocampus 12: 63-75. [DOI] [PubMed] [Google Scholar]
- Poldrack, R.A. and Packard, M.G. 2003. Competition among multiple memory systems: Converging evidence from animals and human brain studies. Neuropsychologia 41: 245-251. [DOI] [PubMed] [Google Scholar]
- Restle, F. 1957. Discrimination of cues in mazes: A resolution of the place vs. the response question. Psychol. Rev. 6: 217-228. [DOI] [PubMed] [Google Scholar]
- Roder, S., Danober, L., Pozza, M.F., Lingenhoehl, K., Wiederhold, K-H., and Olpe, H-R. 2003. Electrophysiological studies on the hippocampus and prefrontal cortex assessing the effects of amyloidosis in amyloid precursor protein 23 transgenic mice. Neuroscience 120: 705-720. [DOI] [PubMed] [Google Scholar]
- Savage, L.M., Chang, Q., and Gold, P.E. 2003. Diencephalic damage decreases hippocampal acetylcholine release during spontaneous alternation testing. Learn. Mem. 10: 242-246. [DOI] [PubMed] [Google Scholar]
- Schmaltz, L.W. 1971. Deficit in active avoidance learning in rats following penicillin injection into hippocampus. Physiol. Behav. 6: 667-674. [DOI] [PubMed] [Google Scholar]
- Schroeder, J.P., Wingard, J.C., and Packard, M.G. 2002. Post-training reversible inactivation of hippocampus reveals interference between memory systems. Hippocampus 12: 280-284. [DOI] [PubMed] [Google Scholar]
- Scoville, W.B. and Milner, B. 1957. Loss of recent memory after bilateral hippocampal lesions. J. Neuropsychiatry Clin. Neurosci. 12: 103-113. [DOI] [PubMed] [Google Scholar]
- Smith, M.A., Hirai, K., Hsiao, K., Pappolla, M.A., Harris, P.L., Siedlak, S.L., Tabaton, M., and Perry, G. 1998. Amyloid-β deposition in Alzheimer transgenic mice is associated with oxidative stress. J. Neurochem. 70: 2212-2215. [DOI] [PubMed] [Google Scholar]
- Spencer, J.P. and Murphy, K.P. 2000. Bi-directional changes in synaptic plasticity induced at corticostriatal synapses in vitro. Exp. Brain Res. 135: 497-503. [DOI] [PubMed] [Google Scholar]
- Uryu, K., Laurer, H., McIntosh, T., Pratico, T., Martinez, D., Leight, S., Lee, V.M., and Trojanowski, J.Q. 2002. Repetitive mild brain trauma accelerates Aβ deposition, lipid peroxidation, and cognitive impairment in a transgenic mouse model of Alzheimer amyloidosis. J. Neurosci. 22: 446-454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vecsei, L. and Beal, M.F. 1991. Comparative behavioral and neurochemical studies with striatal kainic acid- or quinolinic acid-lesioned rats. Pharmacol. Biochem. Behav. 39: 473-478. [DOI] [PubMed] [Google Scholar]