Abstract
Rabbits were given reinforced training of the nictitating membrane (NM) response using separate conditioned stimuli (CSs), which were a tone, light, and/or tactile vibration. Then, two CSs were compounded and given further pairings with the unconditioned stimulus (US). Evidence of both overexpectation and summation effects appeared. That is, responding to the individual CSs declined despite their continued pairing with the US on compound trials (overexpectation), and responding on the compound trials was greater than responding to the individual CSs (summation). The response loss appeared regardless of the testing regime, that is, whether the test presentations of the individual CSs were themselves reinforced (Experiment 2), not reinforced (Experiment 1), or deferred until the end of compound training (Experiment 2). The results are discussed with respect to the roles of excitatory versus inhibitory processes, elemental versus configural processes, and the possible roles of cerebellar and hippocampal pathways.
The present experiments were aimed at determining whether evidence of an overexpectation effect can be obtained in the rabbit nictitating membrane (NM) preparation. An overexpectation effect is said to occur when there is a decline in responding to a pair of well established conditioned stimuli (CSs) that have been given further reinforced training in compound with each other. This result has been observed repeatedly with rats, primarily in fear conditioning (Rescorla 1970, 1999; Kamin and Gaioni 1974; Kremer 1978; Lattal and Nakajima 1998; Blaisdell et al. 2001; McNally et al. 2004). However, other than a small demonstration in appetitive conditioning of pigeons (Khallad and Moore 1996), it is uncertain how widespread the overexpectation effect is. Yet, if widespread, the overexpectation effect provides a distinctive avenue for the study of behavioral and neural processes of response loss that are ordinarily studied using the extinction procedure.
Pavlov (1927) set the pattern for the study and explanation of response loss. At an operational level, extinction appeared to be the complement of acquisition. That is, acquisition of the CR required presentations of the CS paired with the unconditioned stimulus (US), and, in a complementary fashion, extinction of the conditioned response (CR) required presentations of the CS without the US. At a theoretical level, Pavlov (1927) postulated that CR acquisition was an expression of neural excitation whereas CR extinction reflected an accumulation of neural inhibition that counteracted excitation. Thus, extinction was seen as an active neural process rather than as a decline in underlying excitation. By doing so, Pavlov was able to explain why CRs could be quickly recovered even after extensive extinction training. For example, Pavlov (1927) was the first to report spontaneous recovery of CRs after a period of rest, rapid reacquisition of CRs when CS-US pairings are resumed after extinction training, reinstatement of CRs when the extinguished CS is presented following US-alone presentations, and external disinhibition, in which an extinguished CR reappears when the CS is presented in compound with a novel CS. For Pavlov, all these phenomena represented a lifting of the inhibition that allowed the still intact excitation to express itself.
Since Pavlov, extinction has often been explained as a form of new learning that inhibits the CR while leaving the underlying excitatory association intact (Hull 1943; Konorski 1948; Pearce and Hall 1980; Wagner and Brandon 1989; Bouton 1993). As did Pavlov, these theories assume that inhibition depends on presentation of the CS in the absence of the US. As may be apparent, the overexpectation effect presents a fundamental challenge to this traditional theoretical approach. Operationally, response loss occurs while each CS is still paired with the US. Moreover, the overexpectation effect was first predicted from a theory that assumes extinction entails “unlearning,” that is, a loss of underlying excitation rather than a growth of inhibition.
Specifically, the overexpectation effect was first predicted from Rescorla and Wagner's (1972) model of the effects of training with compounds of two or more CSs. Their model is based on a variant of an `error-correction' formula for explaining acquisition and extinction (Bush and Mosteller 1951). In the basic error-correction formula, changes in associative strength (ΔV) are proportional to the difference between the maximum level of association supportable by the US (λ) and the current strength of an association (V): ΔV ∝ (λ - V). Conventional CS-alone extinction reduces λ to zero, making ΔV negative and thus forcing a decline in associative strength (V) until it reaches a zero level.
Rescorla and Wagner (1972) amended the basic formula by assuming that ΔV for each element depends on the aggregate value of all concurrent CSs (∑V). Hence, the formula becomes ΔV ∝ (λ - ∑V). The addition of the summation term (∑V) expands the conditions under which excitatory associative strength can be reduced. Among other things, the Rescorla-Wagner formula predicts that loss of associative strength can occur when two CSs have each been trained separately to the asymptotic level (V ≈ λ) and then are compounded. At the start of reinforced compound training, the summated value of the two CSs (∑V ≈ 2λ) will greatly exceed the level supportable by the US (λ). Hence, there will be a negative ΔV for each element, and their associative strengths will be driven downward until their summated value can be supported by the US (∑V ≤ λ). Hence, one would observe a partial loss of responding to the individual CSs despite uninterrupted pairing with the US.
Given the theoretical challenge posed by the overexpectation effect, the present experiments were undertaken to determine whether it could be observed using bimodal compounds (e.g., tone + light) in a different species and different response system than that used in previous demonstrations. The rabbit NM preparation is particularly suitable for determining the generality of the overexpectation effect for two reasons.
First, the pathways for conditioning of the rabbit's NM response diverge from those that mediate fear conditioning in rats, in which the bulk of previous demonstrations of the overexpectation effect have occurred (Fendt and Fanselow 1999; Maren 2001; Medina et al. 2002). When the CS fills the entire CS-US interval (“delay” conditioning), the essential pathways for rabbit NM acquisition run through the brainstem and cerebellum (e.g., Thompson 1986; Steinmetz 2002). There is also evidence that the cerebellar circuits contain feedback loops that could correspond to the computation of the error term (λ - ∑V; Sears and Steinmetz 1991). When the CS does not fill the entire CS-US interval (trace conditioning), however, there is evidence of the involvement of higher centers, including some of those also involved in fear conditioning, most notably the hippocampus (Solomon et al. 1986; McEchron and Disterhoft 1999; Weible et al. 2000).
Second, the rabbit NM preparation has shown itself to be sensitive to compound stimulus manipulations (Kehoe 1998), but in a complex way that does not clearly predict whether an overexpectation effect will occur. When two CSs from different modalities have been given separate reinforced training, occasional test trials with their compound have yielded a higher level of responding to the compound than to the individual CSs. This effect suggests that the underlying associative strengths of the CSs summate, which is a necessary condition for the overexpectation effect according to the Rescorla-Wagner model (Kehoe 1986; Kehoe and Graham 1988; Kehoe et al. 1994). However, when a bimodal compound is presented on a sustained basis, there is good evidence of configural encoding. Among other things, rabbits that receive training from the start with a reinforced compound often show evidence of “spontaneous configuration,” in which responding to the elements is very weak despite the acquisition of high levels of responding to the compound (Kehoe 1982, 1986; Kehoe et al. 1994; Kehoe and Schreurs 1986a,b). This outcome suggests that a bimodal compound may be encoded as an event distinct from the individual CSs. On the basis of these findings, a shift from reinforced training with the individual CSs to sustained reinforced training of their compound could engage a configural encoding that would leave the associative strengths of the individual CSs intact and thereby preclude an overexpectation effect (Pearce 1987, 1994, 2002).
Experiment 1
Experiment 1 was aimed at delineating the pattern of responding during sustained stimulus compounding versus continued training with the individual CSs. In brief, four groups of rabbits were all given initial acquisition training (Stage 1) in which pairings of a tone CS with the US (T+) were intermixed with pairings of a light CS with the US (L+). After the CR was established to both CSs, Stage 2 was conducted. The key experimental group (Group E+) received reinforced compound training in which the tone and light were presented simultaneously and paired with the US (TL+). This group also received occasional nonreinforced tests of the individual CSs (T, L) to determine whether or not the previously established levels of responding changed during Stage 2. To provide a between-subjects baseline against which to assess any changes in responding in Group E+, the second group (Group I+) continued to receive reinforced training with the individual CSs (T+, L+) as it had in Stage 1. Specifically, Group I+ provided a baseline for detecting any postasymptotic decrements or increments in responding to the CSs during their continued individual training (Kehoe and White 2002). The third group (Group E-) received compound training but without US (TL-), and the fourth group (Group I-) received extinction training of the individual CSs (T-, L-). These latter two groups were included to determine whether evidence for overexpectation and summation effects could be detected during extinction (Rescorla 2000).
RESULTS
Statistical analyses were conducted using planned contrasts for repeated measure designs (O'Brien and Kaiser 1985; Harris 1994). The Type I error rate was set to 0.05. In the text, means are accompanied by a figure in parentheses that represents the standard error of the mean (±SEM).
Stage 1
Rabbits from all four groups acquired conditioned responding to both the tone and the light during Stage 1. Differences among the four groups were small and not significant. Averaged across all days and all four groups, responding to the tone (M = 60% CRs ± 4%) was significantly greater than to the light (M = 48% CRs ± 4%), F(1,28) = 49.65, P < 0.01 (MS error = 1143.79). At the end of Stage 1 (Days 10-12), the four groups had collectively reached appreciable asymptotic levels of responding to the tone and light, specifically mean levels of 74% CRs and 66% CRs (±10%), respectively, F(1,28) = 16.00, P < 0.01 (MS error = 378.67).
Stage 2
Figure 1 shows the mean CR likelihood during the asymptotic portion of Stage 1 (Days 10-12) and each of the six days of Stage 2 (Days 13-18). There is a separate panel for each group. For Groups E+ and E-, the panels show curves for compound training trials (TL), tone test trials (T-), and light test trials (L-). In addition, a dotted line labeled as “Max” indicates the average CR likelihood calculated on the basis of whichever CS elicited the higher level of responding in each animal in each day's training. For Groups I+ and I-, the panels show curves for tone training trials (T), light training trials (L), tone test trials (T-), light test trials (L-), and the maximum likelihood for the two CSs (Max) on their test trials.
Figure 1.
Mean percent CRs in Stage 2 in Experiment 1 as a function of days. The panel for each group shows percentage CRs on their training trials (TL or T, L), test trials (T-, L-), and “Max,” which was calculated on the basis of whichever test stimulus elicited the higher level of responding in each animal in each session.
The Max measure was included to guard against two related, averaging artifacts. First, if some animals responded preferentially to the tone whereas other animals responded to the light, then the average responding to the tone and light CSs could appear lower than responding to the compound, when in fact each animal responded to one CS as much as to the compound (Meltzer and Hamm 1976; Aydin and Pearce 1997; Rescorla 1997). Second, if the tone overshadowed the light, then all the animals might have responded strongly to the tone and weakly to the light. In this case too, the average responding to the separate CSs would have been lower than responding to their compound.
During Stage 2, Groups E+ and I+ retained a high level of responding on their respective reinforced trials. Specifically, Group E+ showed a mean level of 71% CRs (±13%) on TL+ trials, and, similarly, Group I+ showed a mean level of 72% CRs (±11%) on T+ trials and 69% CRs on L+ trials (±12%). Any apparent differences between the two groups on reinforced trials were not significant.
On test trials of the individual CSs, Group E+ showed a decline in responding across sessions. For Group E+, within-subject comparisons between the final portion of Stage 1 (Days 10-12) and Stage 2 (Days 13-18) confirmed that there was a reduction in responding to the individual CSs despite their continued pairing with the US on TL+ trials. Specifically, the level of responding to tone in Stage 2 (M = 44% CRs ±9%) was significantly less than at the end of Stage 1 (M = 60% CRs ± 10%), F(1,28) = 4.85, P < 0.05 (MS error = 230). Similarly, the responding to light in Stage 2 (M = 30% CRs ± 10%) was less than at the end of Stage 1 (M = 51% CRs ± 10%), F(1,28) = 8.86, P < 0.01 (MS error = 212). There was also between-subject evidence of a relative decline in responding to the elements in Group E+ versus I+. Group E+'s maximum CR likelihood on test trials across Stage 2 (M = 50% CRs ± 14%) was significantly lower than that of Group I+ on their test trials (M = 81% CRs ± 11%), F(1,28) = 8.58, P < 0.01 (MS error = 5285). Within Group I+, responding to the individual CSs tended to rise slightly from Stage 1 to Stage 2, although not significantly (P >0.10).
For Group E+, there was within-subject evidence of behavioral summation. Across Stage 2 (Days 13-18), responding on compound trials (M = 71% CRs ± 13%) was significantly greater than the maximum CR likelihood to the individual CSs (M = 50% CRs ± 14%), F(1,28) = 24.55, P < 0.01 (MS error = 880).
As shown in the bottom panels of Figure 1, Groups E- and I- showed a rapid decline in responding that largely obscured any differences. Any apparent differences between groups were not significant, even on the first day of Stage 2, Fs < 1. Within Group E-, however, there was significant summation. Across all of Stage 2, responding on compound trials (M = 20% CRs ± 6%) was significantly greater than the maximum CR likelihood to the individual CSs (M = 9% CRs ± 3%), F(1,28) = 6.19, P < 0.05 (MS error = 880). On the first day of Stage 2, the difference was even more pronounced; the CR likelihood on compound trials was 64% CRs (±8%) versus a maximum CR likelihood to the individual CSs of 38% CRs (±9%), F(1,28) = 9.77, P < 0.01 (MS error = 585).
DISCUSSION
The major findings were as follows: For Group E+, there was converging evidence of an overexpectation effect. That is, responding to the elements declined, as seen both within Group E+ and in comparison of Group E+ to Group I+. Unfortunately, for the nonreinforced groups, the differences between Group E- and I- were too small to reach any conclusion as to whether or not the compounding procedure in Group E- caused responding to its elements to extinguish faster than they did when extinguished separately in Group I-. Second, a summation effect appeared in both Groups E+ and E-. That is, responding to the compound was significantly greater than responding to the elements. These demonstrations of behavioral summation in sustained compounding confirm the evidence of summation of tone and light stimuli obtained previously in the rabbit NM preparation when occasional test trials of the compound were interspersed among reinforced trials of the individual elements (e.g., Kehoe et al. 1994; Kehoe 1998).
MATERIALS AND METHODS
Subjects
The subjects were 32 naive, female, albino rabbits, (Oryctolagus cuniculus), 10-12-wks-old on arrival from the supplier. All were housed in individual cages and had unlimited access to food and water.
Apparatus
The apparatus and the recording procedure for the NM response were patterned after those described by Gormezano (1966). The rabbits were trained individually in one of eight sound-attenuating chambers. On the wall of each chamber in front of the subject was a stimulus panel. A speaker was mounted at the midpoint of the stimulus panel, 8 cm anterior to and 16 cm above the rabbit's head. The speaker provided an auditory CS, which was a 1000-Hz, 88-dB (SPL) tone of 250 msec duration superimposed on an ambient noise level of 81-dB, which was produced by an exhaust fan situated behind each subject. Mounted on the stimulus panel 4 cm above the speaker was an 8-W neon light that served as a houselight. The light CS consisted of a 20-Hz flashing of the houselight for 250 msec. The US was a 4-mA, 50-msec, 50-Hz AC current delivered via stainless steel Autoclip wound clips positioned 10 mm apart and 15 mm posterior to the dorsal canthus of the rabbit's right eye. On CS-US trials, the interstimulus interval between CS onset and US onset was 250 msec. The sequence and timing of stimulus events and the response recording were controlled by an Apple II computer equipped with interfaces and software developed by Scandrett and Gormezano (1980).
During training, each rabbit was restrained in a Perspex box (425 × 115 × 165 mm, internal dimensions) and held in place by inserting its head through an adjustable stock and securing its ears to the front of the stock with a polyurethane foam-covered metal clamp. A muzzlelike head set, fitted securely about the snout, supported a photoelectric transducer for detecting movements of the NM (Gormezano and Gibbs 1988). A small hook was attached to a silk loop sutured into the NM of the rabbit's right eye. The hook was connected to one end of an L-shaped crank that operated the photoelectric transducer. The signal from the transducer was amplified and transmitted to an analog-to-digital converter mounted in the computer.
Procedure
All rabbits received 1 d of preparation and 1 d of adaptation before training began. On the preparation day, hair surrounding each rabbit's right eye was clipped and, after being administered a local anesthetic (proxymetacaine hydrochloride), a small loop of surgical silk (000 Dynex) was sutured into, but not through, the NM of the right eye. The rabbits were then returned to their home cages. On the adaptation day, the rabbits were placed in the conditioning apparatus for 60 min, but neither the CSs nor the US were presented.
On the third day following adaptation, rabbits were randomly assigned to four groups (n = 8). Stage 1 training was the same for all groups. On each day, all animals received 40 training trials, which consisted of 20 pairings of the tone with the US (T+) intermixed with 20 pairings of the light (L+) with the US at a mean intertrial interval (ITI) of 60 sec (range 50-70 sec). Stage 1 lasted for 12 d.
Stage 2 lasted for 6 d. The four groups were split into two pairs. One experimental group in each pair received compounded tone + light presentations (E), and the other group received separate presentations of the individual tone and light stimuli (I). The two pairs differed as to whether the rabbits continued to receive reinforcement by the US (+) or not (-). Hence, in the first pair, the two groups were designated as Group E+ and Group I+, respectively, and in the second pair, the groups were Group E- and Group I-.
In Stage 2, Group I+ received the same number of tone and light presentations as in Stage 1. Specifically, each session contained 20 T+ trials intermixed with 20 L+ trials at a mean ITI of 60 sec. For Group E+, each session contained 20 reinforced trials of the compound (TL+) at a mean ITI of 120 sec (range 110-130 sec). Thus, Group E+ received the same number of reinforced exposures to the tone and light stimuli as Group I+.2 Similarly, for the nonreinforced groups, Group I- received 20 T- trials and 20 L- trials at a 60-sec mean ITI in each session, and Group E- received 20 TL- trials at a 120-sec mean ITI. For all groups, a nonreinforced test trial for one of the individual CSs was administered after every fifth trial; there were four tone test trials and four light test trials evenly distributed through the session.
Response Definition
A CR was defined as any extension of the NM exceeding 0.5 mm that occurred during a 250-msec period following the onset of a CS.
Experiment 2
Experiment 2 was aimed at providing converging evidence of the overexpectation and summation effects seen in Experiment 1 by removing any potential contamination from the nonreinforced tests of the elements. In previous studies using the rabbit NM preparation, a small number of elemental test trials, whether reinforced or nonreinforced, had not discernibly distorted the pattern of responding to a reinforced compound versus its elements (Kehoe 1986; Kehoe and Schreurs 1986a). Nevertheless, the mixture of reinforced compound trials (TL+) and nonreinforced CS presentations (T-, L-) constitutes a positive patterning schedule. Hence, the decline in responding to the individual CSs might, at least in part, reflect the acquisition of a configural discrimination between the compound and its elements. Accordingly, Experiment 2 examined reinforced compounding in which the test presentations of the individual CSs were either themselves reinforced or deferred until the end of compound training.
Experiment 2 contained two parts. Part 1 was a purely within-subject study aimed at replicating Group E+ from Experiment 1 but with a single change: the test trials of the individual CSs during the compound training in Stage 2 were still paired with the US. This group was labeled Group E++. In this group, any decline in responding to the individual CSs could not be attributed to a positive patterning effect or any extinction during the test trials. In fact, any incremental effect of the reinforced test trials on responding to the individual CSs would yield a conservative estimate of the overexpectation effect by counteracting the decremental effect of the reinforced compound training.
Part 2 of Experiment 2 contained two groups. Both groups received initial reinforced training with three separate CSs, specifically, tone, light, and a vibrotactile stimulus. After CRs had been established to each of the three CSs, the rabbits in both groups received training in which two of the CSs were compounded and paired with the US. Across different rabbits in each group, all three possible compounds were used, that is, tone+light, tone+vibration, light+vibration. The remaining CS continued to receive individual pairings with the US. For example, the rabbits that received training with the tone+light compound (TL+) were also given training with the vibration (V+).
The two groups in Part 2 differed in one respect. One group (Group EI) received reinforced test trials of the two CSs that constituted the compound during compound training, and the other group (Group ED) did not receive any test trials of the individual CSs during compound training. At the completion of compound training, both groups were tested with all three CSs in extinction (Stage 3).
The expanded set of CSs and compounds further tested the generality of any overexpectation effect and limited the influence of any specific interaction among any pair of CSs, for example, any overshadowing effect of the tone on the light. Furthermore, reinforced training with the third CS in Stage 2 provided a within-subject baseline for detecting both summation of responding when the other two CSs were compounded and overexpectation, that is, any declines in responding to the other two CSs when they were tested during compound training. In this respect, the addition of the third CS provided a within-subject comparison that complemented the between-subject comparison of Groups E+ versus I+ in Experiment 1. As with the use of the reinforced test trials, any generalized incremental effects of the third CS on responding to the other two CSs from the compound could potentially counteract the decremental effect of compound training, thus providing a conservative estimate of the overexpectation effect.
RESULTS AND DISCUSSION
Part 1
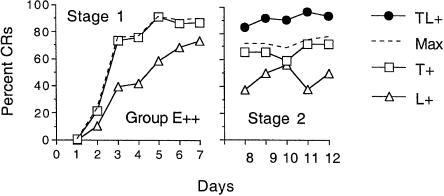
Figure 2 shows the mean CR likelihood during each day in both stages for Group E++. In Stage 1, responding to both CSs rose across days. As in Experiment 1, responding to the tone (M = 62% ± 8%) was consistently greater than responding to the light (M = 42% ± 9%) throughout Stage 1, F(1,7) = 8.24, P < 0.05, (MS error = 2866).
Figure 2.
Mean percent CRs in Group E++ in Stage 1 and Stage 2 as a function of days. The labeling conventions are the same as in Figure 1.
Inspection of the right panel of Figure 2 reveals a pattern of results similar to that of Group E+ in Experiment 1. Comparisons between the asymptotic portion of Stage 1 (Days 5-7) and Stage 2 (Days 8-12) revealed that there was a significant reduction in responding on both T+ trials and L+ trials. Specifically, the level of responding on T+ trials in Stage 2 (M = 67% CRs ± 11%) was less than in the final portion of Stage 1 (M = 88% CRs ± 6%), F(1,7) = 12.78, P < 0.01 (MS error = 143). Likewise, responding on L+ trials in Stage 2 (M = 46% CRs ± 10%) was less than in the final portion of Stage 1 (M = 67% CRs ± 6%), F(1,7) = 14.66, P < 0.01 (MS error = 115). In addition, behavioral summation appeared. Across Stage 2, CR likelihood on TL+ trials (M = 91% CRs ± 5%) was significantly greater than the maximum CR likelihood on the T+ and L+ trials (M = 73% CRs ± 14%), F(1,7) = 5.53, P < 0.05 (MS error = 2405).
Part 2: Stage 1
The left-hand panels of Figure 3 show the mean CR likelihood on A+, B+, and C+ trials during each day in Stage 1 for Groups EI and ED. The assignment of tone, light, and vibration was counterbalanced across A+, B+, and C+ trials. Responding to all three CSs rose steadily across days and reached asymptotic levels around 80% CRs. Responding on C+ trials in Group ED appeared lower than on A+ and B+ trials, but this and any other apparent differences among stimuli and groups were not significant, largest F(1,22) = 2.56, P > 0.10.
Figure 3.
Mean percent CRs in Groups EI and ED in Stages 1, 2, and 3. The labeling conventions are the same as in Figure 1.
Part 2: Stage 2
The middle panels of Figure 3 show the mean CR likelihood during the six days of Stage 2. Examination of the upper panel reveals that Group EI showed evidence of both overexpectation and summation effects. With regard to overexpectation, there were two pieces of converging evidence. First, responding on the test trials for the elements of the AB+ compound, namely, A+ trials (M = 62% CRs ± 8%) and B+ trials (M = 61% CRs ± 11%) showed a significant reduction in Stage 2 (Days 8-12) relative to responding during the final portion of Stage 1 (Days 6-8), (CSA, M = 79% CRs ± 4%; CSB, M = 76% CRs ±8%), smaller F(1,11) = 10.00, P < 0.01, (MS error = 161). In contrast, responding on C+ trials (M = 84% CRs ± 6%) rose significantly during Stage 2 compared to Stage 1 (M = 76% CRs ± 8%), F(1,11) = 9.20, P < 0.01 (MS error = 84). Second, within Stage 2, the level of responding on A+ and B+ trials was significantly less than on C+ trials, smaller F(1,11) = 4.64, P < 0.05, (MS error = 4270). With regard to summation, CR likelihood on AB+ trials (M = 95% CRs ± 2%) across all days of Stage 2 was significantly greater than the maximum CR likelihood on the A+ and B+ trials (M = 78% CRs ± 7%), F(1,11) = 14.59, P < 0.01 (MS error = 669).
Across all days of Stage 2, Groups EI and ED together showed further evidence of behavioral summation. A significantly higher level of responding appeared on AB+ trials (M = 91% CRs ± 1%) than on C+ trials (M = 81% CRs ± 6%), F(1,22) = 14.64, P < 0.01, (MS error = 1079). Any apparent differences between the two groups in this respect failed to reach significance.
Part 2: Stage 3
The right-hand panels of Figure 3 show the mean CR likelihood on the A-, B-, and C- trials. As may be apparent, both Groups EI and ED showed evidence of an overexpectation effect. Across the two groups, responding on A- trials (M = 24% CRs ± 9%) and B- trials (M = 25% CRs ± 8%) was significantly lower than on C- trials (M = 37% CRs ± 10%), smaller F(1,22) = 7.78, P < 0.01, (MS error = 2122). This difference appeared smaller in Group ED than in Group EI, but any apparent differences between the two groups failed to even approach significance, Fs < 1.
DISCUSSION
The pattern of responding in Groups E++, EI, and ED paralleled that seen during reinforced compounding in Experiment 1. By reinforcing the presentations of the elements as well as the compound in Groups E++ and EI, the decline in responding to the elements could not be attributed to an explicit discrimination between the compound and its elements. By deferring testing of the elements until the completion of compound training in Group ED, the decline in responding to the elements can be safely attributed to training with the compound and not exposure to the elements, whether reinforced or not. Moreover, in Group ED, all three CSs had been paired with the US equally often throughout Stages 1 and 2. Finally, Group EI in Stage 2 showed evidence of overexpectation and summation on a within-subjects basis. Specifically, responding on A+ and B+ trials was less than on C+ trials, whereas responding on AB+ trials was greater than on C+ trials.
MATERIALS AND METHODS
Part 1
There was a single group of rabbits labeled Group E++ (n = 8). This group received training identical to that of Group E+ in Experiment 1 with the single exception that the test presentations of the tone and light were paired with the US. Stage 1 training was conducted for 7 d, and Stage 2 training lasted for 5 d.
Part 2
There were two groups (n = 12) labeled Group EI and Group ED. In Stage 1, both groups received 8 d of training with three CSs, namely, tone, light, and a vibrotactile stimulus. The tone and light were the same as those used in the other experiments. The vibrotactile stimulation was delivered to each animal's back by a small DC motor mounted on a velcro strap wrapped around the animal about 10 cm behind the animal's head. A 27-Hz vibration was created by a small off-center brass weight mounted on the shaft of the motor. The motor was shielded on two sides with an aluminum casing attached to the motor with epoxy cement. The casing surrounding the motor was attached to a velcro strap with contact adhesive. The aluminum casing was in direct contact with the animal's skin. This vibrotactile stimulus produced a small auditory component that a pilot study indicated could serve as a CS, even with masking noise. Nevertheless, the tone and vibrotactile stimulus used in this experiment are highly distinctive to rabbits; no generalization between them has been detected (Weidemann and Kehoe 2004).
Each day of Stage 1 contained 20 intermixed pairings of each CS with the US. No one type of trial occurred more than three times in succession. All three CS durations and the CS-US intervals were 400 msec to allow time for recruitment of the vibrotactile stimulus, which required 120 msec. Thus, the effective CS-US interval for this stimulus was approximately 280 msec, which produces only a slightly higher rate and level of CR acquisition than the 400-msec CS-US intervals for the tone and light (Kehoe and Macrae 2002). In all other respects, the apparatus and procedure were identical to those used in Experiment 1.
Stage 2 lasted 6 d. In each session, both groups received 20 reinforced compound trials containing two of the CSs (AB+). The assignment of the tone, light, and vibration to the compound was counterbalanced so that four animals in each group received one of the three possible compounds. The remaining stimulus was also presented on 20 reinforced trials in each session (C+). The compound trials and single-element trials were intermixed such that no more than one type of trial occurred more than three times in succession. Group EI also received four A+ trials and four B+ trials. These trials occurred on Trials 8, 17, 25, 34, 42, 51, 59, and 68 of each session, alternating semi-randomly between A+ and B+ presentations. In contrast, Group ED received “blank” trials with no programmed stimulus presentations in the positions corresponding to the A+ and B+ trials in Group EI.
Stage 3 entailed a single day of extinction testing. Both groups received 24 presentations of each of the three CSs. The presentations of the CSs were intermixed such that no more than one type of trial occurred more than three times in succession.
DISCUSSION
The results of the present experiments provide converging evidence of an overexpectation effect in the rabbit NM preparation, specifically, a reduction in responding to the elements of the compound despite their continued pairing with the US. Irrespective of the differences in testing regimes across experiments, the magnitude of the reduction from Stage 1 to Stage 2 was similar. In Groups E+, E++, and EI, the reductions were 19%, 21%, and 16%, respectively. By the same token, behavioral summation consistently appeared; responding to the compound was significantly greater than the level of responding to either element or, in the case of Group EI, a stimulus that received individual training. Within Groups E+, E++, and EI, the differences between the level of responding to the compound and the maximum CR likelihood to the elements were 21%, 18%, and 17%, respectively.
The evidence of an overexpectation effect expands the generality of this phenomenon to a new species and new response, one which is known to have neural pathways distinct from those that mediate fear conditioning. The evidence of behavioral summation extends previous demonstrations of summation in the rabbit NM preparation for bimodal compounds (Kehoe 1982, 1986, 1998; Kehoe and Graham 1988; Kehoe et al. 1994). In all previous demonstrations of summation, the compound was only tested occasionally during reinforced training of the elements. Conversely, summation was seen in the present experiments when the compound was presented on a sustained basis and the elements were tested occasionally.
The combined evidence of overexpectation and summation effects are entirely consistent with Rescorla and Wagner's formulation of the error correction rule ΔV ∝ (λ - ∑V). In particular, these findings indicate that the excitatory associative strengths of the elements summated (∑V) to exceed the value of λ, when the US was present (λ = 1). Conversely, the present results indicate that the shift from reinforced training with the elements to reinforced training with the compound did not engage a strong configural process that effectively fused the elements into a single event distinct from those of the elements. Had a configural encoding largely displaced the encoding of the elements, the associative strengths of elements would have remained intact and would have been expressed in undiminished responding on test trials (Pearce 1987, 1994, 2002).
The evidence of response loss during continued pairings of the CS with the US challenges the traditional excitation-inhibition theories that (1) tie response loss to the acquisition of inhibition and (2) tie inhibition to the nonreinforced presentation of the CS. However, it is not necessary to abandon both these assumptions and adopt an unlearning model. Rather, only the relation between inhibition and nonreinforcement needs to be abandoned. Specifically, an error-correction model can be reconciled with excitation-inhibition models by partitioning the associative strength of each element into an excitatory and an inhibitory component (Pearce 1987, 1994; Klopf 1988; Macrae and Kehoe 1999). Whenever ΔV is positive (λ > V), the excitatory component would be increased, and, whenever ΔV is negative (λ < V), the inhibitory component would be increased. Moreover, declines in excitation and increases in inhibition may not be mutually exclusive; response loss may reflect the aggregate effect of both processes operating in parallel (Klopf 1988). In these ways, inhibition can be divorced from its historic tie to nonreinforcement.
These conclusions imply that processes of neural inhibition may not be aligned closely with nonreinforcement. In the rabbit NM preparation, the cerebellar and brainstem pathways essential for CR acquisition could mediate response loss during reinforced compounding in a manner consistent with Rescorla and Wagner's error correction formula, ΔV ∝ (λ - ∑V; Sears and Steinmetz 1991). Specifically, the inferior olive may supply the error signal (λ - ∑V). The inferior olive receives excitatory inputs from US pathways (λ) in the brainstem and inhibitory inputs (-∑V) by routes originating in the anterior interpositus nucleus that drives the CR via the red nucleus and cranial facial nuclei (Andersson et al. 1988; Nelson et al. 1989; Weiss et al. 1991; Hesslow and Ivarsson 1996; Hesslow and Yeo 2002; Krupa and Thompson 2003). This signal is then sent, via climbing fibers, to sites of plasticity in the anterior interpositus nucleus and cerebellar cortex. If this scheme is approximately correct, then inactivating the anterior interpositus during reinforced stimulus compounding would block transmission of the inhibitory activity (-∑V) to the inferior olive and thereby prevent the overexpectation effect. If anything, such a blockade should enhance excitatory conditioning to the compound and its elements, because the US input (λ) through the inferior olive to the cerebellum would be transmitted unhindered by inhibitory outputs (-∑V).
As yet, knowledge of the neural pathways that underpin extinction of the rabbit NM response is modest. Nevertheless, the known pathways appear to be organized in a hierarchical fashion that could mediate a mixture of excitatory and inhibitory processes (Hesslow and Yeo 2002; Christian and Thompson 2003). Both cerebellar and hippocampal pathways appear to contribute to conventional extinction of the CR in the rabbit NM preparation. In the cerebellum, reversible inactivation of the anterior interpositus nucleus, which is essential to CR acquisition, prevents extinction of the CR (Hardiman et al. 1996; Ramnani and Yeo 1996) as can lesions of the anterior lobe of cerebellar cortex (Perrett and Mauk 1995; Garcia et al. 1999). By the same token, lesions of the hippocampus have impeded extinction of well established CRs during the conventional CS-alone procedure (Schmaltz and Theios 1972; Powell and Buchanan 1980; Akase et al. 1989; Moyer et al. 1990) and during the reversal of a discrimination (Berger and Orr 1983).
In addition to extinction, the hippocampus is thought to be a substrate for configural learning using compounds of two or more CSs (Sutherland and Rudy 1989; Rudy and Sutherland 1995). This function, if it exists in rabbits, is probably not engaged in producing an overexpectation effect. The present experiments consistently yielded evidence of summation, that is, greater responding to the compound than its elements. Had a configural function been strongly engaged, then responding to the compound would have been less than the level of responding to the elements, not greater (Kehoe and Gormezano 1980; Bellingham et al. 1985; Pearce 2002).
Caution, however, must be exercised in reaching any conclusions about the role of the hippocampus in the overexpectation effect or any other compound stimulus effect in the rabbit NM preparation. The available findings are sparse. On the one hand, hippocampal lesions have reduced the ability of prior training of one element (A+) to block CR acquisition to the other element of a compound (AB+; Solomon 1977). On the other hand, hippocampal lesions have failed to impair acquisition of discriminative NM responding in the conditioned inhibition procedure, in which one element is reinforced (A+) whereas the compound is not (AB-; Solomon 1977). What is needed is an experiment to determine whether lesions of the hippocampus in the rabbit would perhaps leave the overexpectation effect in tact while impairing learning in a patently configural task such as negative patterning (A+, B+, AB-) which has been repeatedly demonstrated in the rabbit NM preparation (Kehoe and Graham 1988; Weidemann et al. 1999).
In conclusion, the present findings add to the empirical generality of the overexpectation effect. From a theoretical perspective, the present results support the basic predictions from an error correction rule, in particular, that an extinction-like loss of responding can occur despite continued reinforcement. To the extent that inhibition plays a role in response loss, inhibition appears to depend not on the absence of the US but rather on a discrepancy between the predicted value of the reinforcer (∑V) and its actual value (λ). However, it remains an open question as to what is the precise mix of excitatory loss and inhibitory acquisition.
Acknowledgments
Preparation of this manuscript was supported by Australian Research Council Grants A79917018 and DP0344082. We thank Gabrielle Weidemann and Stephanie Dartnall for their assistance in data collection. These experiments were approved by the UNSW Animal Care and Ethics Committee in accordance with relevant legislation.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Article and publication are at http://www.learnmem.org/cgi/doi/10.1101/lm.77604.
Footnotes
Reducing the total number of trials and increasing the ITI for the compounding groups necessarily increased the spacing of the trials. However, on the basis of previous findings in the rabbit NM preparation (Kehoe and Macrae 2002), this increase, at worst, introduced a bias against seeing an overexpectation effect by perhaps slightly elevating the overall level of responding to the compound and elements. In contrast to this bias, the alternative tactic of using 40 TL trials per session at a 60-sec ITI could have artifactually produced an overexpectation effect. According to the Rescorla-Wagner model, doubling the number of tone and light presentations in the compounding groups would exaggerate the overexpectation effect by roughly doubling any decline in the associative strength of the elements relative to the control groups.
References
- Akase, E., Alkon, D.L., and Disterhoft, J.F. 1989. Hippocampal lesions impair memory of short delay conditioned eye blink in rabbits. Behav. Neurosci. 103: 935-943. [DOI] [PubMed] [Google Scholar]
- Andersson, G., Garwicz, M., and Hesslow, G. 1988. Evidence for a GABA-mediated cerebellar inhibition of the inferior olive in the cat. Exp. Brain Res. 72: 450-456. [DOI] [PubMed] [Google Scholar]
- Aydin, A. and Pearce, J.M. 1997. Some determinants of response summation. Anim. Learn. Behav. 25: 106-121. [Google Scholar]
- Bellingham, W.P., Gillette-Bellingham, K., and Kehoe, E.J. 1985. Summation and configuration in patterning schedules with the rat and rabbit. Anim. Learn. Behav. 13: 152-164. [Google Scholar]
- Berger, T.W. and Orr, W.B. 1983. Hippocampectomy selectively disrupts discrimination reversal conditioning of the rabbit nictitating membrane response. Behav. Brain Res. 8: 49-68. [DOI] [PubMed] [Google Scholar]
- Blaisdell, A.P., Denniston, J.C., and Miller, R.R. 2001. Recovery from the overexpectation effect: Contrasting performance-focused and acquisition models of retrospective revaluation. Anim. Learn. Behav. 29: 367-380. [Google Scholar]
- Bouton, M.E. 1993. Context, time, and memory retrieval in the interference paradigms of Pavlovian conditioning. Psychol. Bull. 114: 80-99. [DOI] [PubMed] [Google Scholar]
- Bush, R.R. and Mosteller, F.A. 1951. A mathematical model for simple learning. Psychol. Rev. 58: 313-323. [DOI] [PubMed] [Google Scholar]
- Christian, K.M., and Thompson, R.F. 2003. Neural substrates of eyeblink conditioning: Acquisition and retention. Learn. Mem. 11: 427-455. [DOI] [PubMed] [Google Scholar]
- Fendt, M. and Fanselow, M.S. 1999. The neuroanatomical and neurochemical basis of conditioned fear. Neurosci. Biobehav. Rev. 83: 803-834. [DOI] [PubMed] [Google Scholar]
- Garcia, K.S., Steele, P.M., and Mauk, M.D. 1999. Cerebellar cortex lesions prevent acquisition of conditioned eyelid responses. J. Neurosci. 19: 10940-10947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gormezano, I. 1966. Classical conditioning. In Experimental methods and instrumentation in psychology (ed. J.B. Sidowski), pp. 385-420. McGraw-Hill, New York.
- Gormezano, I. and Gibbs, C.M. 1988. Transduction of the rabbit's nictitating membrane response. Behavior Research Methods, Instrumentation, and Computers 20: 18-21. [Google Scholar]
- Hardiman, M.J., Ramnani, N., and Yeo, C.H. 1996. Reversible inactivation of the cerebellum with muscimol prevents acquisition and extinction of conditioned nictitating membrane responses in the rabbit. Exp. Brain Res. 110: 235-247. [DOI] [PubMed] [Google Scholar]
- Harris, R.J. 1994. ANOVA: An analysis of variance primer. Peacock, Itasca, IL.
- Hesslow, G. and Ivarsson, M. 1996. Inhibition of the inferior olive during conditioned responses in the decerebrate ferret. Exp. Brain Res. 110: 36-46. [DOI] [PubMed] [Google Scholar]
- Hesslow, G. and Yeo, C.H. 2002. The functional anatomy of skeletal conditioning. In A neuroscientist's guide to classical conditioning (ed. J.W. Moore), pp. 86-146. Springer, New York.
- Hull, C.L. 1943. Principles of behavior. Appleton-Century-Crofts, New York.
- Kamin, L.J. and Gaioni, S.J. 1974. Compound conditioned emotional response conditioning with differentially salient elements in rats. J. Comp. Physiol. Psychol. 87: 591-597. [DOI] [PubMed] [Google Scholar]
- Kehoe, E.J. 1982. Overshadowing and summation in compound stimulus conditioning of the rabbit's nictitating membrane response. J. Exp. Psychol. Anim. Behav. Process. 8: 313-328. [PubMed] [Google Scholar]
- ____. 1986. Summation and configuration in conditioning of the rabbit's nictitating membrane response to compound stimuli. J. Exp. Psychol. Anim. Behav. Process. 12: 186-195. [Google Scholar]
- ____. 1998. Can the whole be something other than the sum of its parts? In Models of action: Mechanisms for adaptive behavior (eds. C.D.L. Wynne and J.E.R. Staddon), pp. 87-126. Erlbaum, Mahwah, NJ.
- Kehoe, E.J. and Gormezano, I. 1980. Configuration and combination laws in conditioning with compound stimuli. Psychol. Bull. 87: 351-378. [PubMed] [Google Scholar]
- Kehoe, E.J. and Graham, P. 1988. Summation and configuration in negative patterning of the rabbit's conditioned nictitating membrane response. J. Exp. Psychol. Anim. Behav. Process. 14: 320-333. [Google Scholar]
- Kehoe, E.J. and Macrae, M. 2002. Fundamental behavioral methods and findings in classical conditioning. In A neuroscientist's guide to classical conditioning (ed. J.W. Moore), pp. 171-231. Springer, New York.
- Kehoe, E.J. and Schreurs, B.G. 1986a. Compound conditioning of the rabbit's nictitating membrane response: Test trial manipulations. Bull. Psychon. Soc. 24: 79-81. [Google Scholar]
- ____. 1986b. Compound-component differentiation as a function of CS-US interval and CS duration in the rabbit's nictitating membrane response. Anim. Learn. Behav. 14: 144-154. [Google Scholar]
- Kehoe, E.J. and White, N.E. 2002. Extinction revisited: Similarities between extinction and reductions in US intensity in classical conditioning of the rabbit's nictitating membrane response. Anim. Learn. Behav. 30: 96-111. [DOI] [PubMed] [Google Scholar]
- Kehoe, E.J., Horne, A.J., Horne, P.S., and Macrae, M. 1994. Summation and configuration between and within sensory modalities in classical conditioning of the rabbit. Anim. Learn. Behav. 22: 19-26. [Google Scholar]
- Khallad, Y. and Moore, J. 1996. Blocking, unblocking, and overexpectation in autoshaping with pigeons. J. Exp. Anal. Behav. 65: 575-591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klopf, A.H. 1988. A neuronal model of classical conditioning. Psychobiology 16: 85-125. [Google Scholar]
- Konorski, J. 1948. Conditioned reflexes and neuron organization. Cambridge University Press, Cambridge, UK.
- Kremer, E.F. 1978. The Rescorla-Wagner model: Losses in associative strength in compound conditioned stimuli. J. Exp. Psychol. Anim. Behav. Process. 4: 22-36. [DOI] [PubMed] [Google Scholar]
- Krupa, D.J. and Thompson, R.F. 2003. Inhibiting the expression of a classically conditioned behavior prevents its extinction. J. Neurosci. 23: 10577-10584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lattal, K.M. and Nakajima, S. 1998. Overexpectation in appetitive Pavlovian and instrumental conditioning. Anim. Learn. Behav. 26: 351-360. [Google Scholar]
- Macrae, M. and Kehoe, E.J. 1999. Savings after extinction in conditioning of the rabbit's nictitating membrane response. Psychobiology 27: 85-94. [Google Scholar]
- Maren, S. 2001. Neurobiology of Pavlovian fear conditioning. Annu. Rev. Neurosci. 24: 897-931. [DOI] [PubMed] [Google Scholar]
- McEchron, M.D. and Disterhoft, J.F. 1999. Hippocampal encoding of non-spatial trace conditioning. Hippocampus 9: 385-396. [DOI] [PubMed] [Google Scholar]
- McNally, G., Pigg, M., and Weidemann, G. 2004. Blocking, unblocking, and overexpectation of fear: A role for opioid receptors in the regulation of Pavlovian association formation. Behav. Neurosci. 118: 111-120. [DOI] [PubMed] [Google Scholar]
- Medina, J.F., Christopher, R.J., Mauk, M.D., and LeDoux, J.E. 2002. Parallels between cerebellum- and amygdala-dependent conditioning. Nat. Rev. Neurosci. 3: 122-131. [DOI] [PubMed] [Google Scholar]
- Meltzer, D. and Hamm, R.J. 1976. Response summation in the pigeon. Bull. Psychon. Soc. 7: 515-518. [Google Scholar]
- Moyer, J.R., Deyo, R.A., and Disterhoft, J.F. 1990. Hippocampectomy disrupts trace eye-blink conditioning in rabbits. Behav. Neurosci. 104: 243-252. [DOI] [PubMed] [Google Scholar]
- Nelson, B.J., Adams, J.C., Barmack, N.H., and Mugnaini, E. 1989. Comparative study of glutamate decarboxylase immunoreactive boutons in the mammalian inferior olive. J. Comp. Neurol. 286: 514-539. [DOI] [PubMed] [Google Scholar]
- O'Brien, R.G. and Kaiser, M.K. 1985. MANOVA method for analyzing repeated measures designs: An extensive primer. Psychol. Bull. 97: 316-333. [PubMed] [Google Scholar]
- Pavlov, I.P. 1927. Conditioned reflexes: An investigation of the physiological activity of the cerebral cortex, pp. 60-61. Oxford University Press, London. [DOI] [PMC free article] [PubMed]
- Pearce, J.M. 1987. A model for stimulus generalization in Pavlovian conditioning. Psychol. Rev. 94: 61-73. [PubMed] [Google Scholar]
- ____. 1994. Similarity and discrimination: A selective review and a connectionist model. Psychol. Rev. 101: 587-607. [DOI] [PubMed] [Google Scholar]
- ____. 2002. Evaluation and development of a connectionist theory of configural learning. Anim. Learn. Behav. 30: 73-95. [DOI] [PubMed] [Google Scholar]
- Pearce, J.M. and Hall, G. 1980. A model for Pavlovian learning: Variations in the effectiveness of conditioned but not of unconditioned stimuli. Psychol. Rev. 87: 532-552. [PubMed] [Google Scholar]
- Perrett, S.P. and Mauk, M.D. 1995. Extinction of conditioned eyelid responses requires the anterior lobe of the cerebellar cortex. J. Neurosci. 15: 2074-2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell, D.A. and Buchanan, S. 1980. Autonomic-somatic relationships in the rabbit (Oryctolagus cuniculus): Effects of hippocampal lesions. Physiol. Psychol. 8: 455-462. [Google Scholar]
- Ramnani, N. and Yeo, C.H. 1996. Reversible inactivations of the cerebellum prevent the extinction of conditioned nictitating membrane responses in rabbits. J. Physiol. 495: 159-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rescorla, R.A. 1970. Reduction in the effectiveness of reinforcement after prior excitatory conditioning. Learn. Motiv. 1: 372-381. [Google Scholar]
- ____. 1997. Summation assessment of a configural theory. Anim. Learn. Behav. 25: 200-209. [Google Scholar]
- ____. 1999. Summation and overexpectation with qualitatively different outcomes. Anim. Learn. Behav. 27: 50-62. [Google Scholar]
- ____. 2000. Extinction can be enhanced by a concurrent excitor. J. Exp. Psychol. Anim. Behav. Process. 26: 251-260. [DOI] [PubMed] [Google Scholar]
- Rescorla, R.A. and Wagner, A.R. 1972. A theory of Pavlovian conditioning: Variations in the effectiveness of reinforcement and nonreinforcement. In Classical conditioning II (eds. A.H. Black and W.F. Prokasy), pp. 64-99. Appleton-Century-Crofts, New York.
- Rudy, J.W. and Sutherland, R.J. 1995. Configural association theory and the hippocampal formation: An appraisal and reconfiguration. Hippocampus 5: 375-389. [DOI] [PubMed] [Google Scholar]
- Scandrett, J. and Gormezano, I. 1980. Microprocessor control and A/D data acquisition in classical conditioning. Behavior Research Methods and Instrumentation 12: 120-125. [Google Scholar]
- Schmaltz, L.W. and Theios, J. 1972. Acquisition and extinction of a classically conditioned response in hippocampectomized rabbits (Oryctolagus cuniculus). J. Comp. Physiol. Psychol. 79: 328-333. [DOI] [PubMed] [Google Scholar]
- Sears, L.L. and Steinmetz, J.E. 1991. Dorsal accessory inferior olive activity diminishes during acquisition of the rabbit classically conditioned eyelid response. Brain Res. 545: 114-122. [DOI] [PubMed] [Google Scholar]
- Solomon, P.R. 1977. Role of hippocampus in blocking and conditioned inhibition of the rabbit's nictitating membrane response. J. Comp. Physiol. Psychol. 91: 407-417. [DOI] [PubMed] [Google Scholar]
- Solomon, P.R., Vander Schaaf, E.R., Weisz, D.J., and Thompson, R.F. 1986. Hippocampus and trace conditioning of the rabbit's classically conditioned nictitating membrane response. Behav. Neurosci. 100: 729-744. [DOI] [PubMed] [Google Scholar]
- Steinmetz, J.E. 2002. The cerebellum and classical eyeblink conditioning: Much ado about something. In Model systems and the neurobiology of associative learning: A festschrift in honor of Richard F. Thompson (eds. J. E. Steinmetz et al.), pp. 217-244. Erlbaum, Mahwah, NJ.
- Sutherland, R.J. and Rudy, J.W. 1989. Configural association theory: The role of the hippocampal formation in learning, memory, and amnesia. Psychobiology 17: 129-144. [Google Scholar]
- Thompson, R.F. 1986. The neurobiology of learning and memory. Science 233: 941-947. [DOI] [PubMed] [Google Scholar]
- Wagner, A.R. and Brandon, S.E. 1989. Evolution of a structured connectionist model of Pavlovian conditioning (AESOP). In Contemporary learning theories: Pavlovian conditioning and the status of traditional learning theory (eds. S.B. Klein and R.R. Mowrer), pp. 149-190. Erlbaum, Hillsdale, NJ.
- Weible, A.P., McEchron, M.D., and Disterhoft, J.F. 2000. Cortical involvement in acquisition and extinction of trace eye-blink conditioning. Behav. Neurosci. 114: 1058-1067. [DOI] [PubMed] [Google Scholar]
- Weidemann, G. and Kehoe, E.J. 2004. Recovery of the rabbit's conditioned nictitating membrane response without direct reinforcement after extinction. Learn. Behav. (in press). [DOI] [PubMed]
- Weidemann, G., Georgilas, A., and Kehoe, E.J. 1999. Temporal specificity in patterning of the rabbit's conditioned nictitating membrane response. Anim. Learn. Behav. 27: 99-107. [Google Scholar]
- Weiss, C., Houk, J.C., and Gibson, A.R. 1991. Inhibition of sensory responses of cat inferior olive neurons produced by stimulation of red nucleus. J. Neurophysiol. 64: 1170-1185. [DOI] [PubMed] [Google Scholar]