Abstract
Sarcopenia and physical inactivity synergistically progress in patients with chronic kidney disease (CKD) and are strong predictors of mortality in this population. Exercise training and essential amino acids and vitamin D supplements may contribute to improving sarcopenia and physical inactivity in CKD patients.
Keywords: Sarcopenia, Physical Inactivity, Chronic Kidney Disease
1. Context
Sarcopenia is characterized by the loss of skeletal muscle, which is defined by decreased muscle mass and strength (1). Sarcopenia often is observed in patients with chronic kidney disease (CKD) (2). The prevalence of sarcopenia increases in line with the progression of the stages of CKD (2).
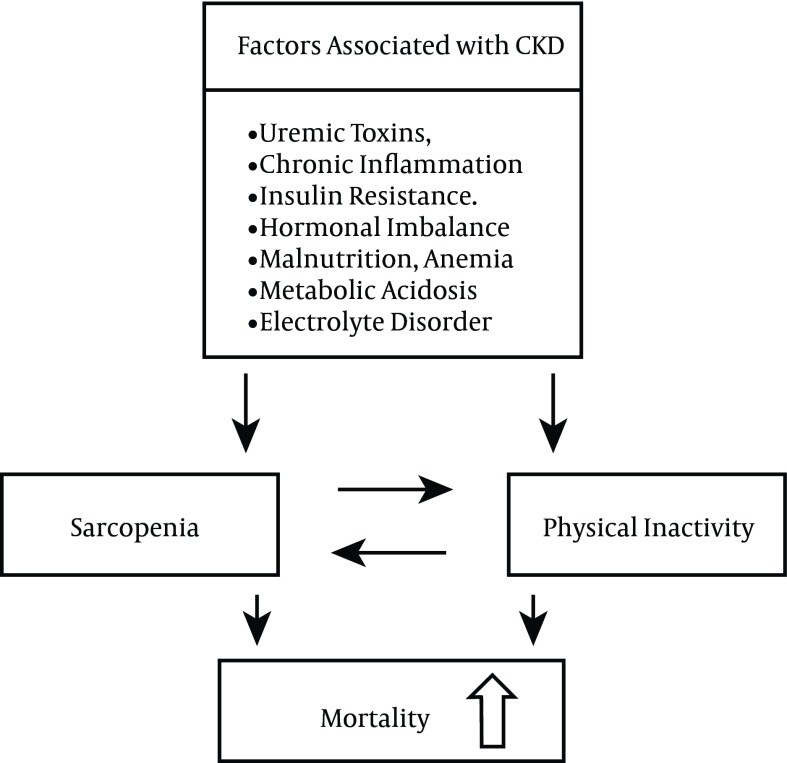
Various pathological conditions are associated with CKD, including accumulation of uremic toxins, chronic inflammation, insulin resistance, hormonal imbalance, malnutrition, vitamin D deficiency, inadequate oxygen transport as a consequence of anemia, metabolic acidosis, electrolyte disorder, and a protein-restricted diet. These pathological conditions all can contribute to the progression of sarcopenia and increased physical inactivity in CKD patients (2-7).
Sarcopenia induces physical inactivity through the loss of skeletal muscle, which in turn induces the loss of physical functions, and increased physical inactivity accelerates the progression of sarcopenia (8-10) (Figure 1). Consequently, sarcopenia and physical inactivity synergistically progress. Recent clinical studies show that both sarcopenia and physical inactivity have an association with increased mortality in CKD patients. Prevention and mitigation of the progression of sarcopenia and physical inactivity are important to improve the prognosis of CKD patients.
Figure 1. The Flow Diagram of This Systemic Review.
This review examines those clinical studies that have investigated the associations between sarcopenia, physical inactivity, and mortality and looks at strategies to prevent and improve sarcopenia and physical inactivity in CKD patients.
2. Evidence Acquisition
We systematically searched for clinical studies published in the English language in the MEDLINE (from 2000 to 2015). For this search, we used the following terms in various combinations: physical activity, exercise, muscle, sarcopenia, chronic kidney disease, dialysis, mortality, prognosis, and survival. We included cohort studies and randomized control trials that investigated the associations between sarcopenia, physical activity, and mortality in patients with chronic kidney disease.
3. Results
Computer and manual searches resulted in 1690 publications, of which 46 eligible articles were identified (Figure 1). Twenty-four articles were excluded because they did not meet the outcome assessment. Subsequently, five of these articles were excluded because two assessed muscle volume by midarm muscle circumference, which is not included in the definition of sarcopenia, and three articles included specific populations, such as patients with obesity, diabetes mellitus, and a low-protein diet. Thus, 17 articles were included in reference to the association between sarcopenia and physical inactivity, and mortality in CKD patients in this review. Furthermore, we discuss the therapeutic strategies to improve sarcopenia and physical inactivity in CKD patients.
3.1. Association Between Sarcopenia and Mortality in CKD Patients
Studies that have reported an association between sarcopenia and mortality in CKD patients at the pre-dialysis stage and those patients on hemodialysis are summarized in Table 1.
Table 1. Studies that Investigated the Association Between Sarcopenia and Mortality in CKD Patients.
| References | Year | Patients No. | Study Design | Duration of Study | Results |
|---|---|---|---|---|---|
| CKD Patients at Pre-Dialysis | |||||
| Chang et al. ( 11 ) | 2011 | 128 | Prospective observational study | 2.8 years | Reduced hand grip strength was an independent predictor of mortality and progression to ESRD (HR: 4.55, 95% CI: 1.49 - 13.87 in men; HR: 4.56, 95% CI: 1.27 - 16.41 in women). |
| Roshanravan et al. ( 12 ) | 2013 | 385 | Prospective observational study | 3 years | Walking speed < 0.8 m/s was associated with all-cause mortality (HR: 2.45, 95% CI: 1.09 - 5.54). |
| Pereira et al. ( 13 ) | 2015 | 287 | Prospective observational study | 3.3 years | Sarcopenia, defined as low skeletal muscle mass index and reduced hand grip strength, was an independent predictor of mortality (HR: 3.02, 95% CI: 1.30 - 7.05). |
| Patients on Hemodialysis | |||||
| Carrero et al. ( 14 ) | 2008 | 221 | Prospective observational study | 6 years | Moderate to severe muscle atrophy was associated with increased mortality (HR: 3.04, 95% CI: 1.61 - 5.71). |
| Kohl et al. ( 15 ) | 2012 | 52 | Prospective observational study | 12 years | Distance walked in the 6MWT was a survival predictor (HR: 0.53, 95% CI: 0.37 - 0.74 for each 100 meters walked with a 100-meter increment). |
| Matsuzawa et al. ( 16 ) | 2014 | 190 | Prospective observational study | 7 years | Knee extensor strength of < 40% was associated strongly with increased mortality risk (HR: 2.73, 95% CI: 1.14–6.52). |
| Isoyama et al. ( 17 ) | 2014 | 330 | Prospective observational study | 5 years | Sarcopenia, defined as low muscle mass and reduced hand grip strength, showed increased mortality risk (HR: 1.93, 95% CI: 1.01 - 3.71). |
| Kutner et al. ( 18 ) | 2015 | 752 | Prospective observational study | 3.3 years | Walk speed < 0.6 m/s was associated with increased mortality risk (HR: 2.17, 95% CI: 1.19 - 3.98). |
| Hemodialysis+ Peritoneal Dialysis | |||||
| Torino et al. ( 19 ) | 2014 | 296 | Secondary analysis of randomized controlled trial | 3.3 years | A 20-meter increase in walking during the 6MWT reduced the risk of all-cause death (HR: 0.89, 95% CI: 0.84 - 0.94). |
Abbreviations: ESRD, end stage renal disease; HR, hazard ratio; CI, confidence interval; CKD, chronic kidney disease; 6MWT, 6-minute walk test.
3.1.1. CKD Patients at the Pre-Dialysis Stage
The prevalence of sarcopenia was reported to increase in line with the progression of the CKD stages (2). The prevalence of sarcopenia for each CKD stage was reported to be 4.3% for normal renal function (estimated glomerular filtration rate [eGFR] ≥ 90 mL/minute/ 1.73 m2) and CKD Stage 1 (eGFR 60 - 89 mL/minute/ 1.73 m2), 6.3% for CKD Stage 2 (eGFR 60 - 89 mL/minute/ 1.73 m2) and 15.4% for CKD Stages 3 - 5 (< 60 mL/minute/ 1.73 m2). Sarcopenia was found to be a predictor of mortality in CKD patients (2). Several studies have reported that sarcopenia, as evaluated by loss of muscle mass and decreased gait speed and hand grip strength, increased mortality risk in CKD patients at the pre-dialysis stage (11-13). Renal outcome also was found to be associated negatively with sarcopenia in this population (11).
These findings suggest that sarcopenia should be evaluated carefully and that prevention and mitigation of the progression of sarcopenia and physical inactivity are important to improve the prognosis of CKD patients.
3.1.2. Hemodialysis Patients
The prevalence of sarcopenia in hemodialysis patients is higher than in CKD patients at the pre-dialysis stage. In elderly hemodialysis patients, the prevalence of sarcopenia increases up to 45% - 63% (20).
In addition to those factors observed in CKD patients at the pre-dialysis stage, loss of amino acids and albumin into the dialysate, systemic inflammation induced by contact with artificial substances, such as the dialysis membrane and circuits, and decreased physical activity due to bed rest during dialysis also contribute to the progression of sarcopenia in hemodialysis patients (2-6).
We previously reported that sarcopenia progressed in accordance with duration on hemodialysis even after adjustment for age and sex (21). Sarcopenia is a strong predictor of mortality in hemodialysis patients as well as in CKD patients at the pre-dialysis stage (14-19).
Several studies have reported an association between sarcopenia and mortality in hemodialysis patients (14-19). Sarcopenia, as estimated by low muscle mass, slow gait speed, and low hand grip strength, was found to increase mortality risk in hemodialysis patients (14-19). Muscle quality, calculated using hand grip strength divided by arm lean mass, also was reported to be an independent predictor of survival in hemodialysis patients (22).
These findings suggest that sarcopenia is an important condition that determines the prognosis of hemodialysis patients.
3.2. Association Between Physical Inactivity and Mortality in CKD Patients
Studies that have investigated the association between physical inactivity and mortality in CKD patients at the pre-dialysis stage and those patients on hemodialysis are summarized in Table 2.
Table 2. Studies that Investigated the Association Between Physical Activity and Mortality in CKD Patients.
| References | Year | Patients No. | Study Design | Duration of Study | Results |
|---|---|---|---|---|---|
| CKD Patients at Pre-Dialysis | |||||
| Beddhu et al. ( 23 ) | 2009 | 907 | Retrospective observational study | 7 years | Increased physical activity was associated with reduced mortality (HR: 0.58, 95% CI: 0.42 - 0.79 for insufficiently active group, and HR: 0.44, 95% CI: 0.33 - 0.58 for active group compared with physically inactive group). |
| Ricardo et al. ( 24 ) | 2013 | 2288 | Prospective observational study | 13 years | Regular physical activity was associated with decreased mortality (HR: 0.80, 95% CI: 0.65 - 0.99). |
| Navaneethan et al. ( 25 ) | 2014 | 2153 | Prospective observational study | 4.5 years | Low leisure time physical activity was associated with a higher risk of death (HR: 1.36, 95% CI: 1.003 - 1.85). |
| Chen et al. ( 26 ) | 2014 | 6363 | Prospective observational study | 2.5 years | Walking was associated with lower risk for overall mortality (HR: 0.67, 95% CI: 0.53 - 0.84). |
| Patients on Hemodialysis | |||||
| Tentori et al. ( 27 ) | 2010 | 20,920 | Prospective observational study/ | 1.8 years | Mortality risk was lower among regular exercisers (HR: 0.73, 95% CI: 0.69 - 0.78). |
| Matsuzawa et al. ( 28 ) | 2012 | 202 | Prospective observational study | 7 years | Engaging in habitual physical activity was associated with decreased mortality risk (HR: 0.78, 95% CI: 0.66 - 0.92) per 10 min/day increase in physical activity. |
| Lopes et al. ( 29 ) | 2014 | 5763 | Prospective observational study | 2.5 years | Aerobic activity was associated inversely with mortality (HR: 0.60, 95% CI:0.47 - 0.77) for very active group compared with never/rarely active group. |
| Hemodialysis + Peritoneal Dialysis | |||||
| O’Hare et al. ( 30 ) | 2003 | 2837 | Prospective observational study | 1 year | Sedentary behavior was associated with an increased mortality risk (HR: 1.62, 95% CI: 1.16 - 2.27). |
Abbreviations: CI, confidence interval; CKD, chronic kidney disease; HR, hazard ratio.
3.2.1. CKD Patients at the Pre-Dialysis Stage
CKD patients at the pre-dialysis stage often undertake less physical activity than the healthy population. A cross-sectional observational survey of 15,368 adults reported that physical inactivity rates, as determined by a questionnaire, were 28.0% for CKD patients and 13.5% for the non-CKD population (23). Another study reported that decreased renal function, estimated by eGFR, correlated with physical inactivity in CKD patients at the pre-dialysis stage (31).
Physical inactivity is a predictor of mortality in CKD patients at the pre-dialysis stage. Several studies have reported an association between physical inactivity and increased mortality risk in this population (23-25). Another study reported that walking habits were associated with mortality in patients in CKD stages 3 - 5 (26). In that study, increased walking was found to decrease overall mortality (26). A dose-dependent negative association was found between walking frequency (1 - 2, 3 - 4, 5 - 6 and ≥ 7 times per week) and hazard ratio of mortality (0.83, 0.72, 0.42, and 0.41) (26). That study also reported that increased walking inhibited decreased renal function and risk for induction of renal replacement therapy (26).
These findings suggest that increased physical activity may have beneficial effects on the prognosis of CKD patients at the pre-dialysis stage. CKD patients at the pre-dialysis stage should be encouraged to exercise to reduce mortality risk and reduce progression to end-stage renal diseases.
3.2.2. Hemodialysis Patients
A questionnaire survey examining the exercise habits of 20,920 hemodialysis patients found that 53.6% of these patients did not exercise at all or did so less than once per week (27). This sedentary behavior was observed more often in elder patients than in younger patients and more often in female patients than in male patients.
Several studies have reported that physical inactivity increases mortality risk in hemodialysis patients (27-30). Exercise, such as walking, was found to decrease mortality risk in hemodialysis patients (27). A dose-dependent negative association was found between walking frequency (1, 2 - 3, 4 - 5, and 6 - 7 times per week) and hazard ratio of mortality (0.82, 0.73, 0.72, and 0.69) (27).
These findings suggest that increased physical activity may have beneficial effects on the prognosis of hemodialysis patients.
3.3. Therapeutic Strategies to Improve Sarcopenia and Physical Inactivity
3.3.1. Exercise Training
Numerous studies have reported that both aerobic and resistance exercise training improve sarcopenia (muscle mass and strength) and physical inactivity in CKD patients at the pre-dialysis stage and in hemodialysis patients (32-40) (Table 3). Exercise training increases muscle mass and strength and improves physical functions. A recent meta-analysis reported that any type of regular exercise training is effective in improving exercise capacity, physical functions, and muscle mass and strength in all CKD patients (41). The American College of Sports Medicine guidelines recommend that CKD patients at the pre-dialysis stage and hemodialysis patients perform aerobic exercise training at mild-moderate levels for 20 - 60 minute/day, 3 - 5 days/week. The guidelines also recommend resistance exercise training for these patients, at 70% - 75% of one repetition maximum for a minimum one set of 10 - 15 repetitions, 2 - 3 days/week (42). kidney disease: improving global outcomes 2012’s clinical practice guidelines recommend regular exercise for at least 30 minutes, five times per week to prevent muscle loss and maintain physical activity in CKD patients (43). Cochrane collaboration guidelines also recommend that 30 minutes of exercise training three days/week can improve physical fitness, resulting in an improved health-related quality of life in hemodialysis patients (44).
Table 3. Studies that Investigated the Effects of Exercise Training on Sarcopenia (Muscle Mass and Strength) and Physical Function in CKD Patients.
| References | Year | Patients No. (Exercise) | Patients No. (Control) | Study | Duration of Study | Exercise Training | Results |
|---|---|---|---|---|---|---|---|
| CKD Patients at Pre-Dialysis | |||||||
| Rossi et al. ( 32 ) | 2014 | 59 | 48 | RCT | 12 weeks | Weight training and treadmill: 2 times/week. | Improved 6-min walk distance (P < 0.001). |
| Watson et al. ( 33 ) | 2015 | 21 | 14 | RCT | 8 weeks | Progressive resistance exercise: 3 times/week. | Increased rectus femoris anatomical cross-sectional area (P = 0.006), volume (P = 0.009), and knee extensor strength (P < 0.001). |
| Patients on Hemodialysis | |||||||
| DePaul et al. ( 34 ) | 2002 | 20 | 18 | RCT | 12 weeks | Resisted isotonic quadriceps and hamstring exercises and ergometer: 3 times/week. | Improved hamstring and quadriceps muscle strength (P = 0.02). |
| Castaneda et al. ( 35 ) | 2004 | 14 | 12 | RCT | 12 weeks | Resistance training: 45 min, 3 times/week. | Improved muscle strength (P = 0.001). |
| van Vilsteren et al. ( 36 ) | 2005 | 53 | 43 | RCT | 12 weeks | Cycling during dialysis, together with pre-dialysis strength training: 2 - 3 times/week. | Increased lower extremity muscle strength (P < 0.05). |
| Cheema et al. ( 37 ) | 2007 | 24 | 25 | RCT | 12 weeks | High-intensity, progressive resistance training during routine hemodialysis treatment. | Improved muscle strength (P = 0.002), midthigh circumference (P = 0.04) and midarm circumference (P = 0.004). |
| Kirkman et al. ( 38 ) | 2014 | 12 | 11 | RCT | 12 weeks | Resistance training: 3 times/week during hemodialysis sessions. | Increased thigh muscle volume (P = 0.007) and knee extensor strength (P = 0.012) |
| Matsufuji et al. ( 39 ) | 2015 | 12 | 15 | RCT | 12 weeks | Chair stand exercise 3 sessions/week. | Increased thigh circumference (p < 0.05). |
| Howden et al. ( 40 ) | 2015 | 36 | 36 | RCT | 48 weeks | Aerobic and resistance exercise 150 minutes/week. | Improved grip strength (P = 0.03) and 6-minutes’ walk distance (P < 0.001). |
Abbreviations: CI, confidence interval; CKD, chronic kidney disease; HR, hazard ratio; RCT randomized control study.
3.3.2. Essential Amino Acid Supplement
Essential amino acids are necessary for protein synthesis and maintenance and repair of muscle tissue. Inadequate amino acid intake often can be observed in CKD patients because of their protein-restricted diet (45). In addition, 6 - 8 g of amino acids can be lost into the dialysate during each hemodialysis session (46).
A prospective observational study reported that dietary protein intake of less than 1.2 g/kg/day is an independent predictor of mortality in hemodialysis patients (47). The international society of renal nutrition and metabolism’s consensus statement recommends oral nutritional supplements, including essential amino acids, be taken by CKD patients at the pre-dialysis stage with a dietary protein intake of less than 0.7 g/kg/day and by hemodialysis patients with a dietary protein intake of less than 1.2 g/kg/day (48). Oral administration of 3.6 g of essential amino acids three times per day with meals was reported to improve hand grip strength significantly in hemodialysis patients with hypoalbuminemia, compared with a placebo group (49).
These findings suggest that essential amino acid supplementation may be considered for CKD patients with an inadequate dietary protein intake and with hypoalbuminemia to prevent sarcopenia, resulting in an improved prognosis.
3.3.3. Vitamin D Supplement
The vitamin D receptor is expressed in muscle tissue, and its activation promotes de novo protein synthesis in muscle (50).
The serum 25-hydroxy vitamin D (25-OHD) level was reported to be associated positively with muscle strength of the lower extremities, estimated using a micro manual muscle tester, in hemodialysis patients (51). Supplementation of 25-OHD was found to improve physical performance significantly, evaluated by the timed up and go test, gait velocity test, the timed chair stand test, and the stair climb test, in CKD patients at the pre-dialysis stage and hemodialysis patients who had vitamin D deficiency (a serum 25-OHD level less than 50 nmol/L) (52).
These findings suggest that 25-OHD supplementation is important to prevent sarcopenia and physical inactivity in CKD patients and that it may be considered for CKD patients who have a vitamin D deficiency.
4. Conclusions
Various pathological conditions associated with CKD can contribute to the progression of sarcopenia and increased physical inactivity in CKD patients (Figure 2). Sarcopenia and physical inactivity are associated strongly with an increased mortality risk in CKD patients. Therapeutic strategies, including exercise training and essential amino acid and vitamin D supplementation, may improve sarcopenia and physical inactivity. This may result in an improved prognosis for CKD patients.
Figure 2. The Various Factors Associated With CKD That Contribute to the Progression of Sarcopenia and Physical Inactivity in CKD Patients.
Sarcopenia and physical inactivity synergistically progress. Abbreviations: CKD, chronic kidney disease.
Acknowledgments
The authors thank the members of the division of nephrology, first department of integrated medicine, Saitama medical center, Jichi medical university, for helpful discussion.
Footnotes
Authors’ Contribution:Keiji Hirai developed the original idea and the protocol, abstracted and analyzed data, wrote the manuscript, and is the guarantor. Susumu Ookawara and Yoshiyuki Morishita contributed to the development of the protocol, abstracted data, and prepared the manuscript.
Conflict of Interest:The authors declare that they have no conflicts of interest.
Funding/Support:This study was supported by the division of nephrology, first department of integrated medicine, Saitama medical center, Jichi medical university.
References
- 1.Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39(4):412–23. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moon SJ, Kim TH, Yoon SY, Chung JH, Hwang HJ. Relationship between Stage of Chronic Kidney Disease and Sarcopenia in Korean Aged 40 Years and Older Using the Korea National Health and Nutrition Examination Surveys (KNHANES IV-2, 3, and V-1, 2), 2008-2011. PLoS One. 2015;10(6):e0130740. doi: 10.1371/journal.pone.0130740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carrero JJ, Stenvinkel P, Cuppari L, Ikizler TA, Kalantar-Zadeh K, Kaysen G, et al. Etiology of the protein-energy wasting syndrome in chronic kidney disease: a consensus statement from the International Society of Renal Nutrition and Metabolism (ISRNM). J Ren Nutr. 2013;23(2):77–90. doi: 10.1053/j.jrn.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 4.Bonanni A, Mannucci I, Verzola D, Sofia A, Saffioti S, Gianetta E, et al. Protein-energy wasting and mortality in chronic kidney disease. Int J Environ Res Public Health. 2011;8(5):1631–54. doi: 10.3390/ijerph8051631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Remuzzi A. Vitamin D, insulin resistance, and renal disease. Kidney Int. 2007;71(2):96–8. doi: 10.1038/sj.ki.5002047. [DOI] [PubMed] [Google Scholar]
- 6.Souza VA, Oliveira D, Mansur HN, Fernandes NM, Bastos MG. Sarcopenia in chronic kidney disease. J Bras Nefrol. 2015;37(1):98–105. doi: 10.5935/0101-2800.20150014. [DOI] [PubMed] [Google Scholar]
- 7.Clyne N, Jogestrand T, Lins LE, Pehrsson SK, Ekelund LG. Factors limiting physical working capacity in predialytic uraemic patients. Acta Med Scand. 1987;222(2):183–90. doi: 10.1111/j.0954-6820.1987.tb10657.x. [DOI] [PubMed] [Google Scholar]
- 8.Kortebein P, Ferrando A, Lombeida J, Wolfe R, Evans WJ. Effect of 10 days of bed rest on skeletal muscle in healthy older adults. JAMA. 2007;297(16):1772–4. doi: 10.1001/jama.297.16.1772-b. [DOI] [PubMed] [Google Scholar]
- 9.Lee JS, Auyeung TW, Kwok T, Lau EM, Leung PC, Woo J. Associated factors and health impact of sarcopenia in older chinese men and women: a cross-sectional study. Gerontology. 2007;53(6):404–10. doi: 10.1159/000107355. [DOI] [PubMed] [Google Scholar]
- 10.Pillard F, Laoudj-Chenivesse D, Carnac G, Mercier J, Rami J, Riviere D, et al. Physical activity and sarcopenia. Clin Geriatr Med. 2011;27(3):449–70. doi: 10.1016/j.cger.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 11.Chang YT, Wu HL, Guo HR, Cheng YY, Tseng CC, Wang MC, et al. Handgrip strength is an independent predictor of renal outcomes in patients with chronic kidney diseases. Nephrol Dial Transplant. 2011;26(11):3588–95. doi: 10.1093/ndt/gfr013. [DOI] [PubMed] [Google Scholar]
- 12.Roshanravan B, Robinson-Cohen C, Patel KV, Ayers E, Littman AJ, de Boer IH, et al. Association between physical performance and all-cause mortality in CKD. J Am Soc Nephrol. 2013;24(5):822–30. doi: 10.1681/ASN.2012070702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pereira RA, Cordeiro AC, Avesani CM, Carrero JJ, Lindholm B, Amparo FC, et al. Sarcopenia in chronic kidney disease on conservative therapy: prevalence and association with mortality. Nephrol Dial Transplant. 2015;30(10):1718–25. doi: 10.1093/ndt/gfv133. [DOI] [PubMed] [Google Scholar]
- 14.Carrero JJ, Chmielewski M, Axelsson J, Snaedal S, Heimburger O, Barany P, et al. Muscle atrophy, inflammation and clinical outcome in incident and prevalent dialysis patients. Clin Nutr. 2008;27(4):557–64. doi: 10.1016/j.clnu.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 15.Kohl LM, Signori LU, Ribeiro RA, Silva AMV, Moreira PR, Dipp T, et al. Prognostic value of the six-minute walk test in end-stage renal disease life expectancy: a prospective cohort study. Clinics. 2012;67(6):581–6. doi: 10.6061/clinics/2012(06)06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matsuzawa R, Matsunaga A, Wang G, Yamamoto S, Kutsuna T, Ishii A, et al. Relationship between lower extremity muscle strength and all-cause mortality in Japanese patients undergoing dialysis. Phys Ther. 2014;94(7):947–56. doi: 10.2522/ptj.20130270. [DOI] [PubMed] [Google Scholar]
- 17.Isoyama N, Qureshi AR, Avesani CM, Lindholm B, Barany P, Heimburger O, et al. Comparative associations of muscle mass and muscle strength with mortality in dialysis patients. Clin J Am Soc Nephrol. 2014;9(10):1720–8. doi: 10.2215/CJN.10261013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kutner NG, Zhang R, Huang Y, Painter P. Gait Speed and Mortality, Hospitalization, and Functional Status Change Among Hemodialysis Patients: A US Renal Data System Special Study. Am J Kidney Dis. 2015;66(2):297–304. doi: 10.1053/j.ajkd.2015.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Torino C, Manfredini F, Bolignano D, Aucella F, Baggetta R, Barilla A, et al. Physical performance and clinical outcomes in dialysis patients: a secondary analysis of the EXCITE trial. Kidney Blood Press Res. 2014;39(2-3):205–11. doi: 10.1159/000355798. [DOI] [PubMed] [Google Scholar]
- 20.Lamarca F, Carrero JJ, Rodrigues JC, Bigogno FG, Fetter RL, Avesani CM. Prevalence of sarcopenia in elderly maintenance hemodialysis patients: the impact of different diagnostic criteria. J Nutr Health Aging. 2014;18(7):710–7. doi: 10.1007/s12603-014-0455-y. [DOI] [PubMed] [Google Scholar]
- 21.Morishita Y, Kubo K, Haga Y, Miki A, Ishibashi K, Kusano E, et al. Skeletal muscle loss is negatively associated with single-pool Kt/V and dialysis duration in hemodialysis patients. Ther Apher Dial. 2014;18(6):612–7. doi: 10.1111/1744-9987.12174. [DOI] [PubMed] [Google Scholar]
- 22.Yoda M, Inaba M, Okuno S, Yoda K, Yamada S, Imanishi Y, et al. Poor muscle quality as a predictor of high mortality independent of diabetes in hemodialysis patients. Biomed Pharmacother. 2012;66(4):266–70. doi: 10.1016/j.biopha.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 23.Beddhu S, Baird BC, Zitterkoph J, Neilson J, Greene T. Physical activity and mortality in chronic kidney disease (NHANES III). Clin J Am Soc Nephrol. 2009;4(12):1901–6. doi: 10.2215/CJN.01970309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ricardo AC, Madero M, Yang W, Anderson C, Menezes M, Fischer MJ, et al. Adherence to a healthy lifestyle and all-cause mortality in CKD. Clin J Am Soc Nephrol. 2013;8(4):602–9. doi: 10.2215/CJN.00600112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Navaneethan SD, Kirwan JP, Arrigain S, Schold JD. Adiposity measures, lean body mass, physical activity and mortality: NHANES 1999-2004. BMC Nephrol. 2014;15:108. doi: 10.1186/1471-2369-15-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen IR, Wang SM, Liang CC, Kuo HL, Chang CT, Liu JH, et al. Association of walking with survival and RRT among patients with CKD stages 3-5. Clin J Am Soc Nephrol. 2014;9(7):1183–9. doi: 10.2215/CJN.09810913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tentori F, Elder SJ, Thumma J, Pisoni RL, Bommer J, Fissell RB, et al. Physical exercise among participants in the Dialysis Outcomes and Practice Patterns Study (DOPPS): correlates and associated outcomes. Nephrol Dial Transplant. 2010;25(9):3050–62. doi: 10.1093/ndt/gfq138. [DOI] [PubMed] [Google Scholar]
- 28.Matsuzawa R, Matsunaga A, Wang G, Kutsuna T, Ishii A, Abe Y, et al. Habitual physical activity measured by accelerometer and survival in maintenance hemodialysis patients. Clin J Am Soc Nephrol. 2012;7(12):2010–6. doi: 10.2215/CJN.03660412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lopes AA, Lantz B, Morgenstern H, Wang M, Bieber BA, Gillespie BW, et al. Associations of self-reported physical activity types and levels with quality of life, depression symptoms, and mortality in hemodialysis patients: the DOPPS. Clin J Am Soc Nephrol. 2014;9(10):1702–12. doi: 10.2215/CJN.12371213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O'Hare AM, Tawney K, Bacchetti P, Johansen KL. Decreased survival among sedentary patients undergoing dialysis: results from the dialysis morbidity and mortality study wave 2. Am J Kidney Dis. 2003;41(2):447–54. doi: 10.1053/ajkd.2003.50055. [DOI] [PubMed] [Google Scholar]
- 31.Finkelstein J, Joshi A, Hise MK. Association of physical activity and renal function in subjects with and without metabolic syndrome: a review of the Third National Health and Nutrition Examination Survey (NHANES III). Am J Kidney Dis. 2006;48(3):372–82. doi: 10.1053/j.ajkd.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 32.Rossi AP, Burris DD, Lucas FL, Crocker GA, Wasserman JC. Effects of a renal rehabilitation exercise program in patients with CKD: a randomized, controlled trial. Clin J Am Soc Nephrol. 2014;9(12):2052–8. doi: 10.2215/CJN.11791113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Watson EL, Greening NJ, Viana JL, Aulakh J, Bodicoat DH, Barratt J, et al. Progressive Resistance Exercise Training in CKD: A Feasibility Study. Am J Kidney Dis. 2015;66(2):249–57. doi: 10.1053/j.ajkd.2014.10.019. [DOI] [PubMed] [Google Scholar]
- 34.DePaul V, Moreland J, Eager T, Clase CM. The effectiveness of aerobic and muscle strength training in patients receiving hemodialysis and EPO: a randomized controlled trial. Am J Kidney Dis. 2002;40(6):1219–29. doi: 10.1053/ajkd.2002.36887. [DOI] [PubMed] [Google Scholar]
- 35.Castaneda C, Gordon PL, Parker RC, Uhlin KL, Roubenoff R, Levey AS. Resistance training to reduce the malnutrition-inflammation complex syndrome of chronic kidney disease. Am J Kidney Dis. 2004;43(4):607–16. doi: 10.1053/j.ajkd.2003.12.025. [DOI] [PubMed] [Google Scholar]
- 36.van Vilsteren MC, de Greef MH, Huisman RM. The effects of a low-to-moderate intensity pre-conditioning exercise programme linked with exercise counselling for sedentary haemodialysis patients in The Netherlands: results of a randomized clinical trial. Nephrol Dial Transplant. 2005;20(1):141–6. doi: 10.1093/ndt/gfh560. [DOI] [PubMed] [Google Scholar]
- 37.Cheema B, Abas H, Smith B, O'Sullivan A, Chan M, Patwardhan A, et al. Progressive exercise for anabolism in kidney disease (PEAK): a randomized, controlled trial of resistance training during hemodialysis. J Am Soc Nephrol. 2007;18(5):1594–601. doi: 10.1681/ASN.2006121329. [DOI] [PubMed] [Google Scholar]
- 38.Kirkman DL, Mullins P, Junglee NA, Kumwenda M, Jibani MM, Macdonald JH. Anabolic exercise in haemodialysis patients: a randomised controlled pilot study. J Cachexia Sarcopenia Muscle. 2014;5(3):199–207. doi: 10.1007/s13539-014-0140-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Matsufuji S, Shoji T, Yano Y, Tsujimoto Y, Kishimoto H, Tabata T, et al. Effect of chair stand exercise on activity of daily living: a randomized controlled trial in hemodialysis patients. J Ren Nutr. 2015;25(1):17–24. doi: 10.1053/j.jrn.2014.06.010. [DOI] [PubMed] [Google Scholar]
- 40.Howden EJ, Coombes JS, Strand H, Douglas B, Campbell KL, Isbel NM. Exercise training in CKD: efficacy, adherence, and safety. Am J Kidney Dis. 2015;65(4):583–91. doi: 10.1053/j.ajkd.2014.09.017. [DOI] [PubMed] [Google Scholar]
- 41.Heiwe S, Jacobson SH. Exercise training in adults with CKD: a systematic review and meta-analysis. Am J Kidney Dis. 2014;64(3):383–93. doi: 10.1053/j.ajkd.2014.03.020. [DOI] [PubMed] [Google Scholar]
- 42.Wolters Kluwer Health.. American College of Sports Medicine: ACMS's Guidelines for Exercise Testing and Prescription Medicine. Lippincott Williams and Wilkins; 2013. [Google Scholar]
- 43.Levin A, Stevens PE, Bilous RW, Coresh J, De Francisco ALM, de Jong PE, et al. Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group: KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3(1):e37443 [Google Scholar]
- 44.Heiwe S, Jacobson SH, Heiwe S. Exercise training for adults with chronic kidney disease. 2011 doi: 10.1002/14651858.CD003236.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Volpi E, Kobayashi H, Sheffield-Moore M, Mittendorfer B, Wolfe RR. Essential amino acids are primarily responsible for the amino acid stimulation of muscle protein anabolism in healthy elderly adults. Am J Clin Nutr. 2003;78(2):250–8. doi: 10.1093/ajcn/78.2.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Borrelli S, De Nicola L, Sagliocca A, Liberti ME, Santangelo S, Donnarumma G, et al. [Amino acid loss during dialysis treatment]. G Ital Nefrol. 2011;28(1):26–31. [PubMed] [Google Scholar]
- 47.Antunes AA, Delatim Vannini F, de Arruda Silveira LV, Martin LC, Barretti P, Caramori JC. Influence of protein intake and muscle mass on survival in chronic dialysis patients. Ren Fail. 2010;32(9):1055–9. doi: 10.3109/0886022X.2010.510233. [DOI] [PubMed] [Google Scholar]
- 48.Ikizler TA, Cano NJ, Franch H, Fouque D, Himmelfarb J, Kalantar-Zadeh K, et al. Prevention and treatment of protein energy wasting in chronic kidney disease patients: a consensus statement by the International Society of Renal Nutrition and Metabolism. Kidney Int. 2013;84(6):1096–107. doi: 10.1038/ki.2013.147. [DOI] [PubMed] [Google Scholar]
- 49.Eustace JA, Coresh J, Kutchey C, Te PL, Gimenez LF, Scheel PJ, et al. Randomized double-blind trial of oral essential amino acids for dialysis-associated hypoalbuminemia. Kidney Int. 2000;57(6):2527–38. doi: 10.1046/j.1523-1755.2000.00112.x. [DOI] [PubMed] [Google Scholar]
- 50.Bischoff-Ferrari HA. Relevance of vitamin D in muscle health. Rev Endocr Metab Disord. 2012;13(1):71–7. doi: 10.1007/s11154-011-9200-6. [DOI] [PubMed] [Google Scholar]
- 51.Zahed N, Chehrazi S, Falaknasi K. The evaluation of relationship between vitamin D and muscle power by micro manual muscle tester in end-stage renal disease patients. Saudi J Kidney Dis Transpl. 2014;25(5):998–1003. doi: 10.4103/1319-2442.139885. [DOI] [PubMed] [Google Scholar]
- 52.Taskapan H, Baysal O, Karahan D, Durmus B, Altay Z, Ulutas O. Vitamin D and muscle strength, functional ability and balance in peritoneal dialysis patients with vitamin D deficiency. Clin Nephrol. 2011;76(2):110–6. doi: 10.5414/cn107160. [DOI] [PubMed] [Google Scholar]