Abstract
Summary
Research has not examined changes in bone mineral density (BMD) between men and women following hip fracture. The aim was to evaluate sex differences in BMD following hip fracture. Men experienced significant declines in BMD, while not statistically greater than women, underscoring the necessity for better osteoporosis care in men.
Introduction
Each year in the USA, approximately 260,000 older adults experience a hip fracture. Women experiencing hip fracture have excess decline in BMD in the year following fracture compared to expected decrements due to aging, but few studies have assessed sex differences in the sequelae of hip fracture. Thus, our objective was to examine sex differences in BMD change in the year after hip fracture.
Methods
The sample (n = 286) included persons enrolled in the Baltimore Hip Studies 7th cohort, a study that matched (1:1) men and women experiencing hip fracture. Weighted estimating equations that accounted for missing data and selective survival were used to estimate sex differences in 12-month total hip (TH) and femoral neck (FN) BMD changes.
Results
Men had larger average adjusted percent decline in TH and FN BMD. Adjusted 12-month decreases at the FN showed a statistically significant decline of −4.60 % (95 % confidence interval [CI] −7.76 %, −0.20 %) in men and an insignificant change of-1.62 % (95 % CI −4.57 %, 1.32 %) in women. Yet, the difference in change between men and women was not statistically significant (P = 0.17). The estimated sex differences for TH BMD loss were smaller in magnitude.
Conclusions
There is evidence of significant BMD loss among men at the FN in the year after hip fracture. Although not statistically greater than women, these clinically significant findings highlight the need for improved osteoporosis care among men prior to and after hip fracture.
Keywords: Aging, DXA, Osteoporosis, Hip fracture, Sex
Introduction
Low bone mineral density (BMD) is an important determinant of hip fracture risk among the approximately 260,000 men and women who experience this acute medical event in the USA each year [1–3]. Declines in BMD associated with aging result in greater bone fragility, which substantially increases the risk of hip and other fractures in older men and women [2, 4]. However, decrements in BMD among men may have a different role in the experience of osteoporotic hip fractures compared to women [5]. Comparative findings from the Osteoporotic Fractures in Men Study (MrOS) and the Study of Osteoporotic Fractures (SOF) demonstrated that men had a higher adjusted rate of sustaining a non-vertebral fracture for every standard deviation decrease in total hip BMD compared to women: 2.31 (95 % confidence interval [CI] 1.90, 2.82) versus 1.74 (95 % CI 1.55, 1.95) [6]. Possible underlying sex differences in the pathogenesis of bone fragility or clinical care of osteoporosis may lead to differential changes in the consequences of decline in BMD between men and women over time.
Longitudinal studies of community-dwelling older adults generally show significant sex differences in total hip and femoral neck BMD decline between men and women. Men have higher BMD throughout the life course, while women experience greater BMD decrements that increase in magnitude over time [7–11]. Rates of BMD decline in post-menopausal women increase linearly with age and are predicted by prior BMD levels [12–16]. In contrast, BMD decrements accumulate in a non-linear manner as men age and are inversely associated with prior bone mineral content (BMC) [17]. These sex differences are a likely consequence of both menopause in women, which results in a loss of BMC and the development of osteoporosis, and an exponential age-related increase in bone turnover among older men [12, 17]. It has become more widely recognized that osteoporosis is also a significant problem among men, although it is frequently under-recognized and does not receive the same level of clinical attention, as evidenced by disproportionately lower treatment rates [18–20].
Of additional clinical importance are decrements in BMD that occur after hip fracture because they are a significant risk factor for new fractures [21]. Significant declines in BMD occur following hip fracture in older women, which are greatest in magnitude at the femoral neck; decrements have been estimated to be approximately 5 % by 12 months post-fracture [21, 22]. These changes following hip fracture are 12 times greater when compared to decrements due to normal aging occurring in community-dwelling older women (4.9 vs. 0.4 %, respectively) [23]. BMD changes following hip fracture are independent of body composition and functional status; BMD at the time of hip fracture explains 70 to 90 % of the variation in BMD decrements after hip fracture [21, 24]. Also, hip fracture patients with a higher BMD at the time of fracture have significantly faster declines compared to those with lower BMD [21]. These data are limited to women, and no studies we are aware of have examined sex differences in BMD change following hip fracture [5]. Thus, the objective of this study was to compare the change in total hip and femoral neck BMD between men and women in the year after hip fracture.
Materials and methods
Study data and sample
The Baltimore Hip Studies (BHS) 7th cohort is a prospective observational study designed to examine sex differences in the sequelae of hip fracture. Patients hospitalized for hip fracture were recruited from eight participating BHS network hospitals in the Baltimore metropolitan area. Men were continuously enrolled into the study, while recruitment of women was frequency matched with men on fracture timing within each hospital. Thus, the recruitment strategy ensured that an equal number of men and women were enrolled throughout the study and minimized the effect of secular changes in care and hospital practice differences. Participants were adults aged 65 years or older at the time of hospital admission for hip fracture (ICD-9 codes 820.00–820.9) who consented to enroll or had a proxy and provided informed consent within 15 days of being admitted. Exclusion criteria included pathologic fracture, not community-dwelling at the time of fracture, non-English speaker, being bedbound for 6 months before fracture, residence >70 mi from the hospital, weight >300 lb, no surgery, and hardware in the contralateral (i.e., non-fractured) hip. Protocols for this study were reviewed and approved by the University of Maryland Institutional Review Board (IRB) and the review boards of participating hospitals.
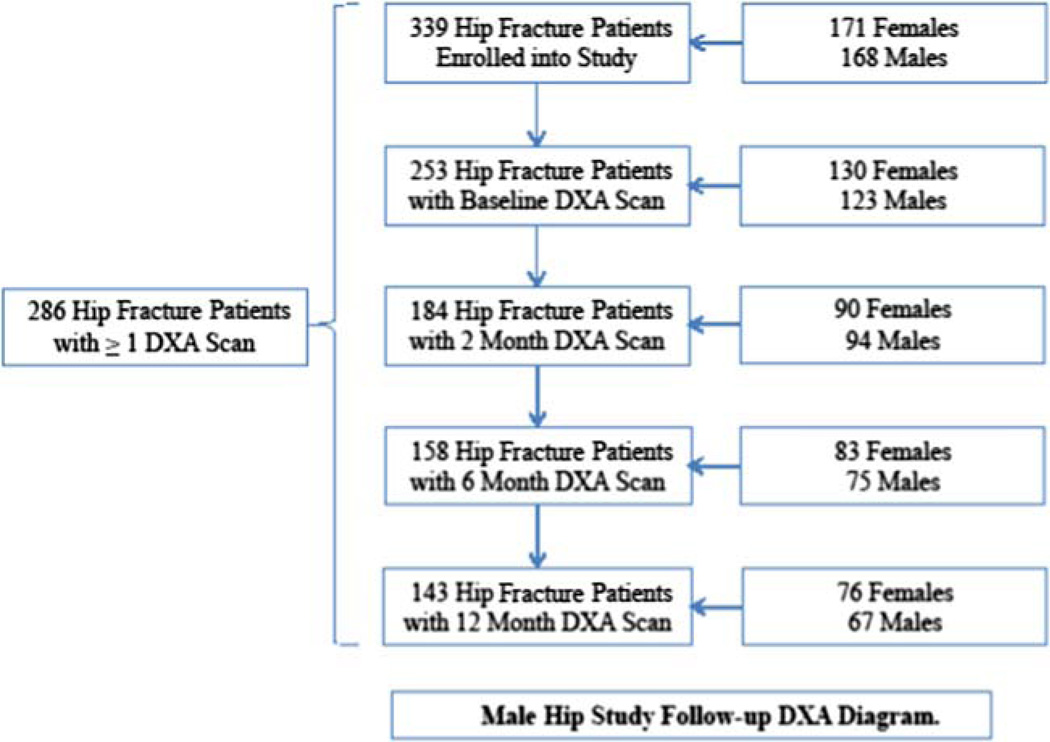
A total of 362 hip fracture patients were enrolled (180 males and 182 females). Five participants did not provide data at the baseline or 2-month follow-up visit, and another 18 participants were removed as a result of an IRB-requested post-procedure audit (6 participants were subsequently found to be ineligible because they did not meet study inclusion criteria and 12 participants were determined to be ineligible secondary to failures of the informed consent process), leaving a sample of 339 participants. A sample of 200 men and 200 women were planned for this study. Based on the maximum 1-year attrition (due to mortality and other loss to follow-up) of 50 %, effective sample sizes would be 100 participants per group. Assuming a 0.7 within-subject correlation for BMD, effect sizes as small as 0.28 standard deviations could be detected with 80 % power and 5 % type 1 error with two-sided tests. Study visits were conducted at baseline (within 22 days of admission) and at 2, 6, and 12 months after admission, which included questionnaires and measures of body composition and functional performance. Medical charts were abstracted and monthly telephone calls were made during the 1-year study period. The analytic sample for this study included 286 participants with at least one BMD measurement (Fig. 1).
Fig. 1.
Male hip study follow-up DXA diagram
BMD measurement
BMD was measured using dual-energy X-ray absorptiometry (DXA) at the total hip and femoral neck (grams per centimeter squared) of the contralateral hip. Participants had their BMD scans performed at one of seven study DXA facilities; four sites used Lunar Prodigy machines (Madison, WI, USA) and three sites used Hologic machines (Waltham, MA, USA). Men and women were matched within clinical sites, and thus also type of DXA machine. While the DXA site may have changed for a participant at later study visits, subsequent scans were on a machine of the same manufacturer, and men and women were scanned at an approximate equal proportion (1:1) on machines made by the same manufacturer over time (Supplementary Table SI). Standardized methods were used for quality control, certification of DXA operators, and scanning procedures to guarantee the reproducibility of results. Reproducibility measurements of every DXA machine were conducted separately at each clinical site and not provided by manufacturer. To account for any inter-site and machine differences, statistical models included a time-varying indicator to capture the different DXA sites and machines, an approach that has been used previously in the study of BMD changes in patients with osteoporosis [17]. Thus, the estimated sex differences were an average that represented a marginal effect across men and women who used the same type of scanner within the same clinical site at each time point.
Predictor variables
Demographic, anthropometric, behavioral, and clinical predictor variables measured at study baseline were selected a priori based on variables that were associated with BMD change among women in SOF and men in MrOS [25, 26]. Demographic, anthropometric, and behavioral measures included sex, age (years), race (white or non-white), height (meters), weight (kilograms), smoking (never, past, or current), and alcohol consumption (none, minimal, or moderate). Clinical characteristics were comorbidity assessed via the Charlson Comorbidity Index, depressive symptoms measured with the Center for Epidemiological Studies Depression Scale, and functional disability using Instrumental Activities of Daily Living (IADL) data obtained from a modified version of the Older Americans Resources and Services Instrument [27–29]. Concomitant medications that differed between men and women that could influence BMD change were bone-active drugs, glucocorticoids, hormone therapy, and calcium supplements. Medication use was assessed via patient-reported survey questions evaluating various types of treatments and coded as never, past, and current. Bone-active drugs included etidronate, alendronate, risedronate, ibandronate, teriparatide, calcitonin, zoledronic acid, and pamidronate. Glucocorticoid medications were prednisone, cortisone, hydrocortisone, dexamethasone, or other steroids. Hormone therapy was assessed using a single item asking about the use of estrogen (pill, vaginal cream, suppository, or patch) in women and testosterone (injection, patch, and gel) among men and was subsequently dichotomized into binary categories of Bever” and Bnever” due to small cell sizes. Calcium supplements were measured as the daily (“everyday” or “almost everyday”) consumption of Caltrate, Citracal, Os-Cal, or Tums.
Statistical analysis
To compare baseline characteristics of patients with BMD data between men and women, chi-square tests were used for categorical covariates and t tests or Wilcoxon rank-sum tests for continuous variables, and Wilcoxon rank tests for continuous measures with skewed distributions. These comparisons were also conducted separately among all cohort participants to determine if there were similar distributional trends in covariate measures by sex in the full study cohort. To account for missing baseline covariate data, missing outcome data, and truncation due to death in statistical models, an inverse probability of observation weighting approach was utilized in the primary analyses [30–32]. Further information about the construction of the missing data weights is provided in Appendix.
Weighted generalized estimating equations (WEEs) with an independence working correlation matrix were fit, where the weight was the inverse probability of observation given predictors of missing data. These models were used to estimate the association between sex and changes in total hip and femoral neck BMD, while accounting for potential selection bias from missing data. Time was modeled as categorical indicators, and the primary parameter of interest was the sex by time interaction testing whether the rate of BMD change from baseline differed between men and women at each time point. WEEs were used to calculate sex-specific baseline BMD values and the corresponding absolute and percent BMD changes during follow-up with 95 % confidence intervals (CIs). In adjusted models, covariates included every confounder selected a priori and measured at baseline and DXA site that was modeled as a time-varying covariate. Adjusted sex-specific baseline BMD values were calculated holding all covariate values at their sample mean.
Two sensitivity analyses were also conducted to test the robustness of the findings. First, standardized BMD (sBMD) values were calculated using measurement site-specific (e.g., femoral neck) conversion formulas as an additional method of accounting for any residual bias introduced by the different DXA machines [33]. The primary analyses were replicated using total hip and femoral neck sBMD values. Second, individual-level percent BMD change was used in unadjusted generalized estimating equations as the dependent variable, such that each patient served as his/her own control, and thus controlling for patient’s baseline BMD.
Results
Sample characteristics
The analytic sample was predominantly white (Table 1). Men were taller, heavier, and more likely to have more comorbidities and functional limitations than women. In addition, men had a higher frequency of smoking and alcohol consumption compared to women. Women had significantly higher rates of medication use at study enrollment, including bone-active drugs, glucocorticoids, hormone therapy, and calcium supplements. The most common bone-active drugs that men and women reported ever to have taken were bisphosphonates, particularly alendronate (Supplementary Table S2). Men generally had significantly higher total hip and femoral neck BMD at baseline, and measured BMD values were higher for patients scanned with a Lunar machine (Supplementary Table S1). However, the differences in BMD between men and women were similar regardless of machine type. Baseline total hip and femoral neck sBMD were higher for patients scanned with a Hologic machine, and the between-machine difference was lower; however, the observed sex differences were identical to the primary results and independent of machine manufacturer. Men were also significantly more likely to be lost to follow-up than women after 1 year due to death (20.57 vs. 6.21 %, respectively).
Table 1.
Baseline characteristics among men and women with DXA data and the full study cohort
| Variable | Analytic sample | Full cohort | ||||
|---|---|---|---|---|---|---|
| Men (n = 141) |
Women (n = 145) |
P | Men (n = 168) |
Women (n = 171) |
P | |
| White (n, %) | 126 (90.1 %) | 134 (93.7 %) | 0.26 | 146 (89.6 %) | 150 (91.5 %) | 0.56 |
| Age (m, SD) | 80.4 ± 7.6 | 81.0 ± 7.7 | 0.55 | 80.4 ± 7.8 | 81.4 ± 7.9 | 0.21 |
| Weight (m, SD) | 79.2 ± 14.3 | 63.4 ± 14.3 | <0.0001 | 78.2 ± 15.0 | 63.4 ± 14.4 | <0.0001 |
| Height (m, SD) | 1.8 ± 0.08 | 1.6 ± 0.08 | <0.0001 | 1.8 ± 0.08 | 1.6 ± 0.08 | <0.0001 |
| Smoking (n, %) | ||||||
| Never | 32 (22.9 %) | 67 (46.5 %) | <0.0001 | 37 (23.3 %) | 80 (48.8 %) | <0.0001 |
| Past | 97 (69.3 %) | 68 (47.2 %) | 110 (69.2 %) | 73 (44.5 %) | ||
| Present | 11 (7.9 %) | 9 (6.3 %) | 12 (7.6 %) | 11 (6.7 %) | ||
| Alcohol use (n, %) | ||||||
| None | 56 (40.0 %) | 72 (50.0 %) | 0.12 | 66 (41.5 %) | 84 (51.2 %) | 0.15 |
| Minimal | 80 (57.1 %) | 65 (45.1 %) | 88 (55.4 %) | 73 (44.5 %) | ||
| Moderate | 4 (2.9 %) | 7 (4.9 %) | 5 (3.1 %) | 7 (4.3 %) | ||
| Bone-active drugs (n, %) | ||||||
| Never | 129 (91.5 %) | 85 (58.6 %) | <0.0001 | 155 (92.3 %) | 107 (62.6 %) | <0.0001 |
| Past | 1 (0.7 %) | 21 (14.5 %) | 1 (0.6 %) | 23 (13.5 %) | ||
| Current | 11 (7.8 %) | 39 (26.9 %) | 12 (7.1 %) | 41 (24.0 %) | ||
| Glucocorticoids | ||||||
| Never | 110 (80.9 %) | 91 (64.1 %) | 0.006 | 123 (80.4 %) | 106 (65.8 %) | 0.009 |
| Past | 17 (12.5 %) | 29 (20.4 %) | 21 (13.7 %) | 32 (19.9 %) | ||
| Current | 9 (6.6 %) | 22 (15.5 %) | 9 (5.9 %) | 23 (14.3 %) | ||
| Hormone therapy | 4 (2.9 %) | 54 (37.5 %) | <0.0001 | 5 (3.1 %) | 58 (35.4 %) | <0.0001 |
| Calcium supplements (n, %) | ||||||
| Never | 99 (71.2 %) | 45 (31.5 %) | <0.0001 | 114 (72.6 %) | 51 (31.3 %) | <0.0001 |
| Past | 15 (10.8 %) | 17 (11.9 %) | 15 (9.6 %) | 19 (11.7 %) | ||
| Current | 25 (18.0 %) | 81 (56.6 %) | 28 (17.8 %) | 93 (57.1 %) | ||
| Comorbidity (M, IQR) |
4 [2–6] | 4 [2–5] | 0.06 | 4 [2–6] | 2 [2–5] | 0.03 |
| IADL (M, IQR) | 2 [1–4] | 1 [0–3] | 0.01 | 2 [1–4] | 1 [0–3] | 0.01 |
| CES-D (m, SD) | 17.0 ± 9.6 | 17.7 ± 11.2 | 0.55 | 17.3 ± 10.4 | 17.6 ± 11.3 | 0.80 |
Total hip BMD
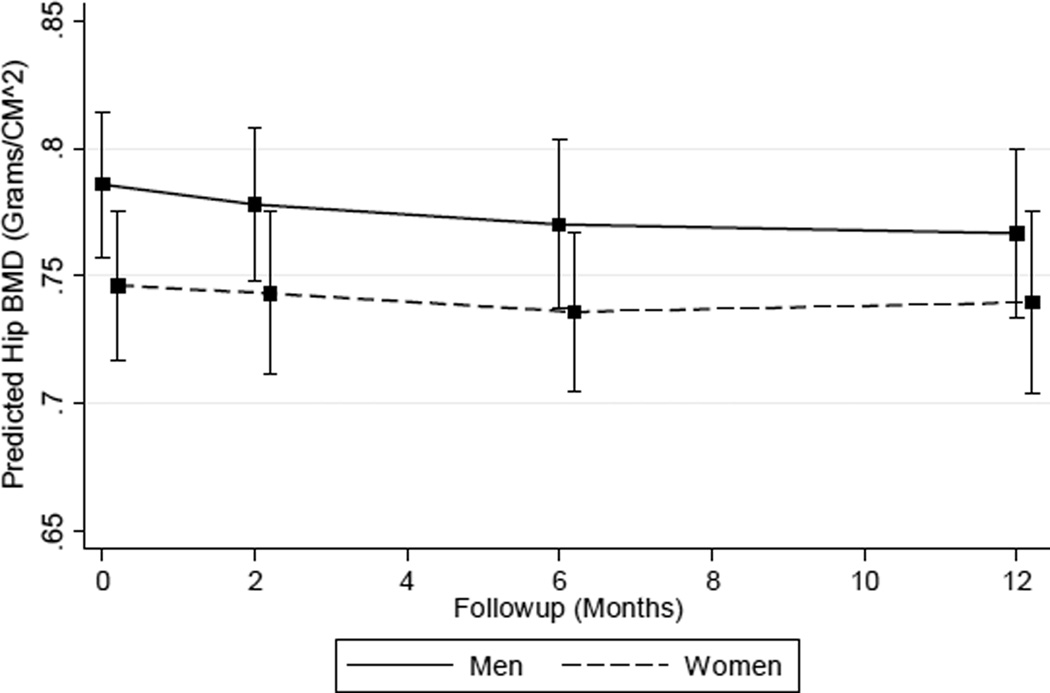
Unadjusted results showed a static decrement among women that changed little during follow-up (Table 2). Estimates ranged from −0.38 % (95 % CI −3.20, 2.45) at 2 months to −0.32 % (95 % CI 4.16, 3.53) at 12 months. Men had non-linear changes in total hip BMD that were smaller from baseline to 2 and 6 months, but larger by 12 months: −2.35 % (95 % CI −5.72, 1.03). The unadjusted model indicated that men had larger estimated long-term total hip BMD decreases, but this difference, when compared to the observed change in women, was not statistically significant (P = 0.39). Sensitivity analyses using unadjusted percent change as the outcome variable to control for baseline differences in BMD between men and women yielded similar results (data not shown). After adjustment for covariates (Table 2), estimates of percent total hip BMD change in men indicated a progressively greater cumulative decline from 2 to 12 months: −0.99 % (95 % CI −3.18, 1.18) to −2.44 % (95 % CI −5.61, 0.74). Adjusted results showed a non-linear pattern of decline among women that peaked at 6 months (−1.38; 95 % CI −3.71, 0.94) and was attenuated by 12 months (−0.88 %; 95 % CI −3.77, 2.01). There was a decreasing rate of decline in men and a non-linear pattern of change among women (Fig. 2), but the global test of the sex by time interaction was not statistically significant (P = 0.90). Sensitivity analysis results evaluating total hip sBMD were identical to the primary findings (Supplementary Table S3).
Table 2.
Unadjusted and adjusted absolute change (grams per centimeter squared) and percent change in total hip BMD between men and women following hip fracture patients at 2, 6, and 12 months
| Time point | Men | Women | |||||
|---|---|---|---|---|---|---|---|
| Unadjusted | BMD change | 95 % CI | *P | BMD change | 95 % CI | *P | **P |
| Baseline | 0.8116 | (0.7849, 0.8384) | REF | 0.7144 | (0.6911, 0.7378) | REF | *0.84 |
| Δ T2 | −0.0061 | (−0.0257, 0.0136) | 0.54 | −0.0027 | (−0.0228, 0.0175) | 0.79 | 0.81 |
| Δ T6 | −0.0018 | (−0.0293, 0.0257) | 0.89 | −0.0025 | (−0.0273, 0.0223) | 0.84 | 0.97 |
| Δ T12 | −0.0189 | (−0.0463, 0.0083) | 0.17 | −0.0022 | (−0.0297, 0.0252) | 0.87 | 0.39 |
| Unadjusted | % Change | 95 % CI | *P | % Change | 95 % CI | *P | **P |
| Baseline | REF | REF | REF | REF | REF | REF | *0.84 |
| Δ T2 | −0.75 | (−3.18, 1.68) | 0.54 | −0.38 | (−3.20, 2.45) | 0.79 | 0.81 |
| Δ T6 | −0.22 | (−3.62, 3.17) | 0.89 | −0.35 | (−3.82, 3.12) | 0.84 | 0.97 |
| Δ T12 | −2.35 | (−5.72, 1.03) | 0.17 | −0.32 | (−4.16, 3.53) | 0.87 | 0.39 |
| Adjusted | BMD change | 95 % CI | *P | BMD change | 95 % CI | *P | **P |
| Baseline | 0.7856 | (0.7574, 0.8139) | REF | 0.7461 | (0.7171, 0.7751) | REF | *0.90 |
| Δ T2 | −0.0078 | (−0.0250, 0.0093) | 0.37 | −0.003 | (−0.0188, 0.0128) | 0.70 | 0.67 |
| Δ T6 | −0.0157 | (−0.0371, 0.0056) | 0.14 | −0.0103 | (−0.0276, 0.0070) | 0.24 | 0.68 |
| Δ T12 | −0.0191 | (−0.0441, 0.0058) | 0.13 | −0.0066 | (0.0281, 0.0149) | 0.54 | 0.45 |
| Adjusted | % Change | 95 % CI | *P | % Change | 95 % CI | *P | **P |
| Baseline | REF | REF | REF | REF | REF | REF | *0.90 |
| Δ T2 | −0.99 | (−3.18, 1.18) | 0.37 | −0.41 | (−2.53, 1.72) | 0.7 | 0.67 |
| Δ T6 | −2.01 | (−4.73, 0.72) | 0.14 | −1.38 | (−3.71, 0.94) | 0.24 | 0.68 |
| Δ T12 | −2.44 | (−5.61, 0.74) | 0.13 | −0.88 | (−3.77, 2.01) | 0.54 | 0.45 |
P values for the sex- and time-specific changes;
P values for the *global test of the sex by time interaction and time-specific sex differences. Adjusted for potential confounders: race, age, weight, height, smoking, alcohol use, bisphosphonates, glucocorticoids, hormone therapy, calcium supplements, comorbidity count, instrumental activities of daily living, center for epidemiologic studies depression scale, and DXA measurement site
BMD bone mineral density, CI confidence interval
Fig. 2.
Predicted total hip BMD by sex
Femoral neck BMD
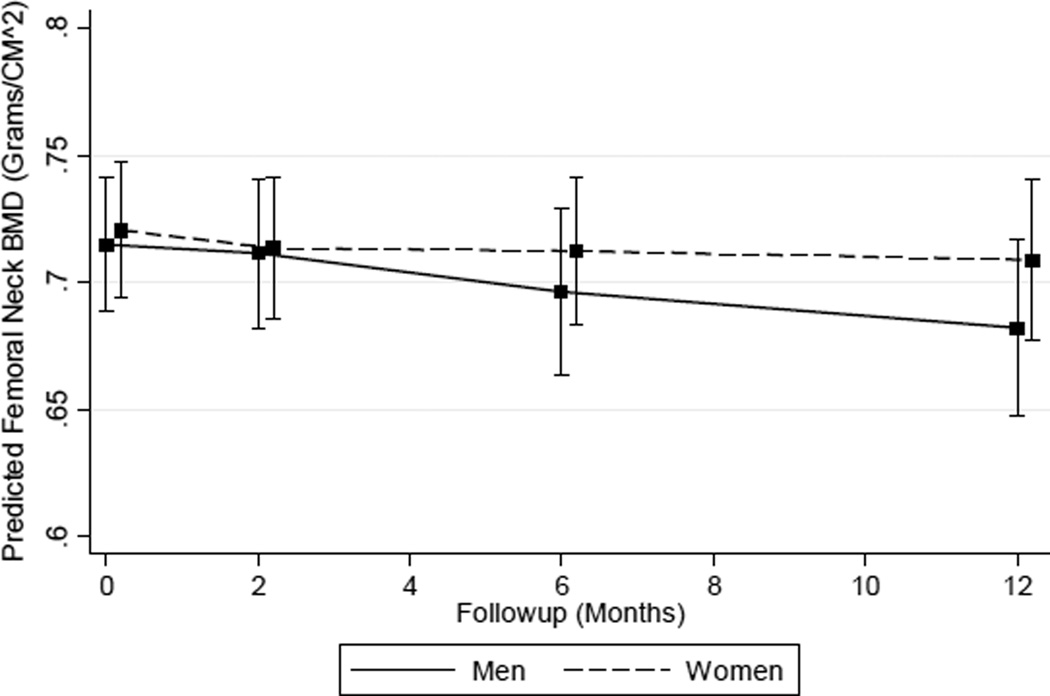
Similar to the total hip results, unadjusted estimations of percent femoral neck BMD decline among men displayed smaller changes at 2 and 6 months but a statistically significant decrease of −4.00 % (95 % CI −7.81, −0.20) at 12 months Table 3). However, this decrement was not significantly different (P = 0.12) than the estimated 12-month change among women of 0.31 % (95 % CI −3.88, 4.49). Results from sensitivity analyses using unadjusted percent change as the outcome variable did not differ compared to findings from the primary outcome model (data not shown). Adjustment resulted in estimated changes that showed a gradual increase in femoral neck BMD decrements over time, and the magnitude of these changes also was generally larger in both men and women (Table 3). Model-based estimates of femoral neck BMD percent decline at 2 and 12 months were −0.50 % (95 % CI −2.65, 1.66) to −4.60 % (95 % CI −7.76, −1.43) in men and −0.98 % (95 % CI −3.00, 1.04) to −1.61 % (95 % CI −4.57, 1.32) in women. Again, 12-month femoral neck estimates showed a significant decline among men (P = 0.003) and statistically insignificant change among women (P = 0.25). Also, the rate of femoral neck BMD decline among men continually increased, while in women, the prospective decrements decreased over time (Fig. 3). However, neither difference in change at 12 months (P = 0.17) or the global test of the sex by time interaction (P = 0.36) was statistically significant. Findings from sensitivity analyses using femoral neck sBMD did not differ from the primary results (Supplementary Table S4).
Table 3.
Unadjusted and adjusted absolute change (grams per centimeter squared) and percent change in femoral neck BMD between men and women following hip fracture patients at 2, 6, and 12 months
| Time point | Men | Women | |||||
|---|---|---|---|---|---|---|---|
| Unadjusted | BMD change | 95 % CI | *P | BMD change | 95 % CI | *P | **P |
| Baseline | 0.7431 | (0.7172, 0.7690) | REF | 0.6824 | (0.6590, 0.7058) | REF | *0.36 |
| Δ T2 | −0.0029 | (−0.0215, 0.0155) | 0.75 | −0.0059 | (−0.0260, 0.0141) | 0.55 | 0.83 |
| Δ T6 | −0.0020 | (−0.0257, 0.0217) | 0.86 | 0.0048 | (−0.0219, 0.0317) | 0.72 | 0.70 |
| Δ T12 | −0.0297 | (−0.0580, −0.0014) | 0.03 | 0.0021 | (−0.0264, 0.0306) | 0.88 | 0.12 |
| Unadjusted | % Change | 95 % CI | *P | % Change | 95 % CI | *P | **P |
| Baseline | REF | REF | REF | REF | REF | REF | *0.36 |
| Δ T2 | −0.40 | (−2.91, 2.10) | 0.75 | −0.87 | (−3.81, 2.06) | 0.55 | 0.83 |
| Δ T6 | −0.27 | (−3.46, 2.92) | 0.86 | 0.72 | (−3.21, 4.65) | 0.72 | 0.70 |
| Δ T12 | −4.00 | (−7.81, −0.20) | 0.03 | 0.31 | (−3.88, 4.49) | 0.88 | 0.12 |
| Adjusted | BMD change | 95 % CI | *P | BMD change | 95 % CI | *P | **P |
| Baseline | 0.7147 | (0.6882, 0.7413) | REF | 0.7204 | (0.6936, 0.7471) | REF | *0.36 |
| Δ T2 | −0.0035 | (−0.0189, 0.0118) | 0.65 | −0.0071 | (−0.0216, 0.0074) | 0.34 | 0.73 |
| Δ T6 | −0.0186 | (−0.0377, 0.0004) | 0.056 | −0.0081 | (−0.0251, 0.0087) | 0.34 | 0.40 |
| Δ T12 | −0.0328 | (−0.0554, −0.0102) | 0.005 | −0.0117 | (−0.0329, 0.0095) | 0.27 | 0.17 |
| Adjusted | % Change | 95 % CI | *P | % Change | 95 % CI | *P | **P |
| Baseline | REF | REF | REF | REF | REF | REF | *0.36 |
| Δ T2 | −0.50 | (−2.65, 1.66) | 0.65 | −0.98 | (−3.00, 1.04) | 0.34 | 0.73 |
| Δ T6 | −2.61 | (−5.28, 0.66) | 0.056 | −1.13 | (−3.48, 1.21) | 0.34 | 0.40 |
| Δ T12 | −4.60 | (−7.76, −1.43) | 0.005 | −1.62 | (−4.57, 1.32) | 0.27 | 0.17 |
P values for the sex- and time-specific changes;
P values for the *global test of the sex by time interaction and time-specific sex differences. Adjusted for potential confounders: race, age, weight, height, smoking, alcohol use, bisphosphonates, glucocorticoids, hormone therapy, calcium supplements, comorbidity count, instrumental activities of daily living, center for epidemiologic studies depression scale, and DXA measurement site
BMD bone mineral density, CI confidence interval
Fig. 3.
Predicted femoral neck BMD by sex
Discussion
This study examined differences in BMD change between men and women that occur following hip fracture. The results showed that women experienced statistically insignificant declines in total hip and femoral neck BMD, which are smaller in magnitude compared to estimates from previous BHS cohorts [21–24]. Although not statistically different from women, men had greater cumulative BMD decrements at both the total hip and femoral neck that were more than double the magnitude of the estimated declines among women. Studies of normal aging in community-dwelling older adults indicate that women experience significantly greater BMD decline than men; however, our results suggest a different pattern of sex differences in the loss of BMD after hip fracture [7–11].
The results demonstrated minimal, insignificant annual declines among women in total hip and femoral neck BMD (0.9 and 1.6 %, respectively). Previous BHS research has estimated BMD loss at the intertrochanteric region and femoral neck in women to be approximately 2 and 5 % in the year following hip fracture, respectively [21–24]. Clinical care for women with osteoporosis and osteoporotic fractures has improved, evidenced by decreasing US hip fracture rates that are expected to decline by 3.5 % by 2030, as well as physician practice guidelines that recommend aggressive pharmacological intervention to manage osteoporosis in Caucasian women [1, 34]. Further, higher BMC is associated with greater BMD decline and increases linearly with advancing age after menopause, but this loss is eventually attenuated in older age when total BMD has decreased considerably [12–16]. Smaller BMD decrements among women in BHS-7 may also be a consequence of general cohort differences compared to individuals who fractured when earlier BHS studies were conducted. More specifically, differences in early life exposure, osteoporosis care prior to and after hip fracture, or other factors such as greater life expectancy and increased age at the time of fracture, when women have lower BMC, could have resulted in slower rates of bone turnover and less BMD decline.
Men experienced greater decline at the total hip and a statistically significant decrease in femoral neck BMD at 12 months. Data from older Baltimore area men in the Men’s Osteoporosis Study suggest that men experience annual declines of 2.1 and 0.8 % at the femoral neck and total hip, respectively [35]. Our findings suggest that declines in BMD in the year after fracture among men are more than double the magnitude of normal decrements associated with aging. Men were significantly less likely to be using bone-active drugs, hormone therapy, and calcium supplements, and the lack of awareness about osteoporosis in men may explain, in part, the greater estimated BMD declines compared to women, although these differences in change were not statistically significant [18, 19]. However, research also indicates that among men, higher BMC is associated with smaller longitudinal BMD decrements, but the loss of total BMC during aging results in an age-related acceleration of BMD decline later in life [17]. Greater bone turnover in men is associated with greater hip bone loss, and thus, in addition to the under-screening and treatment of osteoporosis after hip fracture, larger BMD decrements may be related to accelerated declines that increase as men advance into older age [17, 36, 37].
Two potential mechanisms that may account for any sex differences in BMD decline after hip fracture are disparities in clinical care for osteoporosis and/or differential changes in bone turnover between men and women. Men are 10–20 times more likely to be under-treated with bisphosphonates and also have a lower likelihood of being referred for DXA scan and initiating osteoporosis treatment after hip fracture [18, 36]. Therefore, poorer clinical care in men could result in greater BMD decrements, while women receiving disproportionately better osteoporosis management may have more favorable outcomes. Bone loss in women starts earlier in life after menopause due to the acceleration of endocortical reabsorption and deceleration in periosteal apposition, while men have an age-related acceleration of bone loss later in life because of increased endocortical reabsorption that is not offset by stable periosteal apposition [38, 39]. Sex differences in BMD changes after hip fracture may also be related to this acceleration of bone loss that increases as men advance into older age combined with the attenuation in decline that occurs among women because of prior decrements in BMD that start after menopause. If men experience greater declines in BMD after hip fracture, there may be a higher risk for subsequent osteoporotic fractures that are potentially a consequence of modifiable factors.
There are several limitations of this study. First, the sample size may have precluded our ability to detect statistically significant differences in BMD trajectories between men and women. This issue may have been exacerbated by the variance inflation from inverse probability weighting to account for missing data and selective survival [40]. Second, the cohort was primarily comprised of white men and women recruited from the Baltimore metropolitan area, and therefore, the results from this study may lack generalizability to more ethnically and regionally diverse hip fracture samples. Medication use was also based on self-reported questionnaires, which does not capture information on compliance or adherence. Last, there is the potential for confounding by unmeasured factors not included in the analysis.
The potential sources of bias, however, are mitigated through the study strengths. Although the sample is small, it is one of the largest cohorts of both men and women hip fracture patients to examine post-hip fracture BMD changes. Furthermore, extensive clinical and patient-reported measures were available to control for a variety of potential confounders. The study also used novel methods to rigorously handle missing data and selective survival. This research is the first to assess sex differences in BMD following hip fracture, which is important because of the well-documented patterns in BMD change between men and women in the general population and its integral role in hip fracture risk and recovery.
Conclusion
Our findings indicate that men experience significant BMD decline during the year following hip fracture, particularly at the femoral neck. Although these decrements are not statistically significantly greater than those of women, these potentially clinically significant findings highlight the need for additional research assessing sex differences in the sequelae of hip fracture, as well as an increased awareness regarding the presence and treatment of osteoporosis among men before and after sustaining a hip fracture [5, 19]. Future research should examine differences in bone-active drug use and bone turnover between men and women after hip fracture to further elucidate how sex differentially impacts hip fracture recovery.
Supplementary Material
Acknowledgments
This research was supported by grants from the National Institute on Aging (R37 AG009901, R01 AG029315, P30 AG028747, T32 AG00262). We also acknowledge the cooperation of the hospitals, DXA facilities, residents, and families participating in this project.
Dr. Jay Magaziner has consulting agreements with Ammonett, Novartis, Sanofi, and Viking. Dr. Denise Orwig has consulting agreements with Kinexum and Viking. Dr. Thomas Beck is CEO of Beck Radiological Innovations Inc.
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s11657-016-0263-6) contains supplementary material, which is available to authorized users.
Compliance with ethical standards This observational research was conducted in accordance with the ethical standards of the Declaration of Helsinki, and the protocols for this study were reviewed and approved by the University of Maryland Institutional Review Board (IRB) and the review boards of participating hospitals.
Conflicts of interest Drs. Alan M. Rathbun, Michelle Shardell, J. Richard Hebel, Gregory E. Hicks, and Marc C. Hochberg have no disclosures to declare.
References
- 1.Stevens JA, Rudd RA. The impact of decreasing US hip fracture rates on future hip fracture estimates. Osteoporos Int. 2013;24:2725–2728. doi: 10.1007/s00198-013-2375-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Seeman E. Pathogenesis of bone fragility in women and men. Lancet. 2002;359:1841–1850. doi: 10.1016/S0140-6736(02)08706-8. [DOI] [PubMed] [Google Scholar]
- 3.Marshall D, Johnell O, Wedel H. Meta-analysis of how well measures of bone mineral density predict occurrence of osteoporotic fractures. BMJ (Clin Res Ed) 1996;312:1254–1259. doi: 10.1136/bmj.312.7041.1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johnell O, Kanis JA, Oden A, Johansson H, De Laet C, Delmas P, et al. Predictive value of BMD for hip and other fractures. J Bone Miner Res. 2005;20:1185–1194. doi: 10.1359/JBMR.050304. [DOI] [PubMed] [Google Scholar]
- 5.Orwig DL, Chan J, Magaziner J. Hip fracture and its consequences: differences between men and women. Orthop Clin North Am. 2006;37:611–622. doi: 10.1016/j.ocl.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 6.Cummings SR, Cawthon PM, Ensrud KE, Cauley JA, Fink HA, Orwoll ES. BMD and risk of hip and nonvertebral fractures in older men: a prospective study and comparison with older women. J Bone Miner Res. 2006;21:1550–1556. doi: 10.1359/jbmr.060708. [DOI] [PubMed] [Google Scholar]
- 7.Berger C, Langsetmo L, Joseph L, Hanley DA, Davison KS, Josse R, et al. Change in bone mineral density as a function of age in women and men and association with the use of antiresorptive agents. CMAJ: Canadian Medical Association journal = journal de 1'Association medicale canadienne. 2008;178:1660–1668. doi: 10.1503/cmaj.071416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Warming L, Hassager C, Christiansen C. Changes in bone mineral density with age in men and women: a longitudinal study. Osteoporos Int. 2002;13:105–112. doi: 10.1007/s001980200001. [DOI] [PubMed] [Google Scholar]
- 9.Hannan MT, Felson DT, Dawson-Hughes B, Tucker KL, Cupples LA, Wilson PWF. Risk factors for longitudinal bone loss in elderly men and women: the Framingham Osteoporosis Study. J Bone Miner Res. 2000;15:710–720. doi: 10.1359/jbmr.2000.15.4.710. [DOI] [PubMed] [Google Scholar]
- 10.Dennison E, Eastell R, Fall CHD, Kellingray S, Wood PJ, Cooper C. Determinants of bone loss in elderly men and women: a prospective population-based study. Osteoporos Int. 1999;10:384–391. doi: 10.1007/s001980050244. [DOI] [PubMed] [Google Scholar]
- 11.Burger H, de Laet CE, van Daele PL, Weel AE, Witteman JC, Hofman A, et al. Risk factors for increased bone loss in an elderly population: the Rotterdam Study. Am J Epidemiol. 1998;147:871–879. doi: 10.1093/oxfordjournals.aje.a009541. [DOI] [PubMed] [Google Scholar]
- 12.Hansen MA, Overgaard K, Christiansen C. Spontaneous postmenopausal bone loss in different skeletal areas—followed up for 15 years. J Bone Miner Res. 1995;10:205–210. doi: 10.1002/jbmr.5650100206. [DOI] [PubMed] [Google Scholar]
- 13.Ensrud KE, Palermo L, Black DM, Cauley J, Jergas M, Orwoll ES, et al. Hip and calcaneal bone loss increase with advancing age: longitudinal results from the study of osteoporotic fractures. J Bone Miner Res. 1995;10:1778–1787. doi: 10.1002/jbmr.5650101122. [DOI] [PubMed] [Google Scholar]
- 14.Greenspan SL, Maitland LA, Myers ER, Krasnow MB, Kido TH. Femoral bone loss progresses with age: a longitudinal study in women over age 65. J Bone Miner Res. 1994;9:1959–1965. doi: 10.1002/jbmr.5650091216. [DOI] [PubMed] [Google Scholar]
- 15.Steiger P, Cummings SR, Black DM, Spencer NE, Genant HK. Age-related decrements in bone mineral density in women over 65. J Bone Miner Res. 1992;7:625–632. doi: 10.1002/jbmr.5650070606. [DOI] [PubMed] [Google Scholar]
- 16.Hansen MA, Overgaard K, Riis BJ, Christiansen C. Role of peak bone mass and bone loss in postmenopausal osteoporosis: 12 year study. BMJ (Clin Res Ed) 1991;303:961–964. doi: 10.1136/bmj.303.6808.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cawthon PM, Ewing SK, McCulloch CE, Ensrud KE, Cauley JA, Cummings SR, et al. Loss of hip BMD in older men: the osteoporotic fractures in men (MrOS) study. J Bone Miner Res. 2009;24:1728–1735. doi: 10.1359/JBMR.090419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bor A, Matuz M, Gyimesi N, Biczók Z, Soós G, Doró P. Gender inequalities in the treatment of osteoporosis. Maturitas. 2015;80:162–169. doi: 10.1016/j.maturitas.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 19.Lambert JK, Zaidi M, Mechanick JI. Male osteoporosis: epidemiology and the pathogenesis of aging bones. current Osteoporosis Reports. 2011;9:229–236. doi: 10.1007/s11914-011-0066-z. [DOI] [PubMed] [Google Scholar]
- 20.Crandall C. Gender differences in osteoporosis treatment: a review of clinical research. J Gender-Specific Med JGSM. 1999;3:42–46. [PubMed] [Google Scholar]
- 21.Wehren LE, Hawkes WG, Hebel JR, Orwig D, Zimmerman SI, Fox KM, et al. Predictors of bone loss after hip fracture. Osteoporos Int. 2004;15:125–131. doi: 10.1007/s00198-003-1498-9. [DOI] [PubMed] [Google Scholar]
- 22.Fox KM, Magaziner J, Hawkes WG, Yu-Yahiro J, Hebel JR, Zimmerman SI, et al. Loss of bone density and lean body mass after hip fracture. Osteoporos Int. 2000;11:31–35. doi: 10.1007/s001980050003. [DOI] [PubMed] [Google Scholar]
- 23.Magaziner J, Wehren L, Hawkes WG, Orwig D, Hebel JR, Fredman L, et al. Women with hip fracture have a greater rate of decline in bone mineral density than expected: another significant consequence of a common geriatric problem. Osteoporos Int. 2006:971–977. doi: 10.1007/s00198-006-0092-3. [DOI] [PubMed] [Google Scholar]
- 24.Wehren LE, Hawkes WG, Hebel JR, Orwig DL, Magaziner J. Bone mineral density, soft tissue body composition, strength, and functioning after hip fracture. J Gerontol Ser A Biol Sci Med Sci. 2005;60:80–84. doi: 10.1093/gerona/60.1.80. [DOI] [PubMed] [Google Scholar]
- 25.Orwoll E, Blank JB, Barrett-Connor E, Cauley J, Cummings S, Ensrud K, et al. Design and baseline characteristics of the osteoporotic fractures in men (MrOS) study—a large observational study of the determinants of fracture in older men. Contemp Clin Trials. 2005;26:569–585. doi: 10.1016/j.cct.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 26.Cummings SR, Black DM, Nevitt MC, Browner WS, Cauley JA, Genant HK, et al. Appendicular bone density and age predict hip fracture in women. JAMA. 1990;263:665–668. [PubMed] [Google Scholar]
- 27.Plotnikoff RC, Brez S, Hotz SB. Exercise behavior in a community sample with diabetes: understanding the determinants of exercise behavioral change. Diabetes Educ. 2000;26:450–459. doi: 10.1177/014572170002600312. [DOI] [PubMed] [Google Scholar]
- 28.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 29.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174. [PubMed] [Google Scholar]
- 30.Robins JM, Rotnitzky A, Zhao LP. Estimation of regression coefficients when some regressors are not always observed. J Am Stat Assoc. 1994;89:846–866. [Google Scholar]
- 31.Robins JM, Rotnitzky A, Zhao LP. Analysis of semiparametric regression models for repeated outcomes in the presence of missing data. J Am Stat Assoc. 1995;90:106–121. [Google Scholar]
- 32.Shardell M, Hicks GE, Miller RR, Magaziner J. Semiparametric regression models for repeated measures of mortal cohorts with non-monotone missing outcomes and time-dependent covariates. Stat Med. 2010;29:2282–2296. doi: 10.1002/sim.3985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lu Y, Fuerst T, Hui S, Genant H. Standardization of bone mineral density at femoral neck, trochanter and Ward’s triangle. Osteoporos Int. 2001;12:438–444. doi: 10.1007/s001980170087. [DOI] [PubMed] [Google Scholar]
- 34.Adler RA, Fuleihan GEH, Bauer DC, Camacho PM, Clarke BL, Clines GA, et al. Managing Osteoporosis in Patients on Long-Tenn Bisphosphonate Treatment: Report of a Task Force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2015 [Google Scholar]
- 35.Tracy JK, Meyer WA, Flores RH, Wilson PD, Hochberg MC. Racial differences in rate of decline in bone mass in older men: the Baltimore men’s osteoporosis study. J Bone Miner Res. 2005;20:1228–1234. doi: 10.1359/JBMR.050310. [DOI] [PubMed] [Google Scholar]
- 36.Antonelli M, Einstadter D, Magrey M. Screening and treatment of osteoporosis after hip fracture: comparison of sex and race. J Clin Densitom. 2014;17:479–483. doi: 10.1016/j.jocd.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 37.Bauer DC, Garnero P, Harrison SL, Cauley JA, Eastell R, Ensrud KE, et al. Biochemical markers of bone turnover, hip bone loss, and fracture in older men: the MrOS study. J Bone Miner Res. 2009;24:2032–2038. doi: 10.1359/JBMR.090526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Szulc P, Seeman E, Duboeuf F, Sornay-Rendu E, Delmas PD. Bone fragility: failure of periosteal apposition to compensate for increased endocortical resorption in postmenopausal women. J Bone Miner Res. 2006;21:1856–1863. doi: 10.1359/jbmr.060904. [DOI] [PubMed] [Google Scholar]
- 39.Szulc P, Delmas PD. Bone loss in elderly men: increased endosteal bone loss and stable periosteal apposition. The prospective MINOS study. Osteoporos Int. 2007;18:495–503. doi: 10.1007/s00198-006-0254-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cole SR, Hernan MA. Constructing inverse probability weights for marginal structural models. Am J Epidemiol. 2008;168:656–664. doi: 10.1093/aje/kwn164. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.