Abstract
Background
The integrity of connections between the hippocampus and the anterior cingulate cortex (ACC) is critical for adaptive cognitive and emotional processing; these connections may be compromised in posttraumatic stress disorder (PTSD). However, there is a lack of PTSD research that combines structural and functional connectivity data, and no studies have examined whether abnormal ACC-hippocampal connectivity is associated with genetic variability, particularly for polymorphisms of a gene that has been previously associated with PTSD, FKBP5. This was the goal of the present study.
Methods
Fifty-four women with and without PTSD underwent diffusion tensor imaging and resting-state MRI. Probabilistic tractography was used to examine ACC-hippocampal structural connectivity; mean fractional anisotropy (FA) values were extracted from connectivity streamlines, which represent the cingulum bundle. Genotype data were collected for a single nucleotide polymorphism (SNP) of FKBP5, rs1360780.
Results
Participants with PTSD demonstrated poorer structural connectivity (lower cingulum FA) compared to traumatized controls (F1,50=6.77, p<.05). An interaction of FKBP5 genotype and diagnostic group was also observed (F1,37=4.52, p=.04), indicating lower cingulum FA in carriers of two risk alleles for this SNP, compared to other diagnostic and genotype groups. Carriers of two FKBP5 risk alleles also demonstrated poorer hippocampus-ACC connectivity at rest (p<.05). When cingulum FA was used a regressor in a brain-wide, seed-based regression analysis, significant associations were found between the hippocampus and dorsal regions of the ACC (p<.05).
Conclusions
Individuals with PTSD demonstrated compromised structural connectivity of the hippocampus-ACC pathway. Altered hippocampus-ACC connectivity may represent a highly salient intermediate neural phenotype for PTSD.
Keywords: PTSD, neuroimaging, diffusion tensor imaging, genetics, FKBP5, connectivity, hippocampus, anterior cingulate
Abnormalities in the microstructural integrity of specific white matter pathways appear to characterize individuals with pathological mood disruptions. Diffusion tensor imaging (DTI) studies have shown architectural deficits in paths that connect limbic regions to prefrontal brain regions (1) in individuals with affective disorders, such as posttraumatic stress disorder (PTSD). These prefrontal and limbic brain regions engage during learning, memory, executive functioning, and emotion regulation processes.
Poorer microstructural integrity of the cingulum bundle in particular has characterized psychiatric populations, including depressed individuals (2, 3) particularly those with mild cognitive impairment (4, 5) anorexics (6, 7), and schizophrenics (8). Abnormalities in this tract have also emerged in studies of traumatized populations, particularly those who have developed PTSD. Recently, a meta-analysis was conducted that included 7 studies of trauma-exposed adults (9); the findings indicated a region of reduced fractional anisotropy (FA; an index of white matter integrity) in the cingulum. Some studies indicate that these white matter abnormalities are most prominent in individuals who have developed post-traumatic psychopathology, as compared to similarly traumatized controls (10, 11).
Notably, abnormal cingulum integrity has been found in individuals with family histories of depression (12) and those with specific genetic polymorphisms, including polymorphisms of FKBP5, a gene that regulates glucocorticoid receptor activity and response to stress (13) and is thus thought to play a key role in the development of PTSD. Recent evidence indicates that cingulum integrity is largely heritable; results of a meta-analysis indicate nearly 70% heritability for the cingulum (14). The Val66Met polymorphism of the brain-derived neurotrophic factor gene, which has been consistently linked to depression (15), and more recently, to PTSD (16, 17), has also been associated with poorer integrity of the cingulum (18). Thus, abnormal connectivity of the cingulum may be a potential intermediate neural phenotype of disorders such as PTSD.
The integrity of this major limbic-cortical connection appears highly relevant to the development of post-traumatic sequelae, and may be heritable. Few studies have examined abnormalities in limbic-cortical white matter connections in traumatized populations, and the majority of previous investigations of PTSD populations (including our own, (13) employed Tract-Based Spatial Statistics (TBSS) (19)); a method with limited ability to measure connectivity within particular tracts of interest. This method customarily assumes a single-fiber orientation at each voxel and is dependent on accurate registration to an atlas or sample-based white matter skeleton. As such, TBSS is often dependent on the accuracy of registration; errors that occur using such methods can produce false positives in affected regions (20).
In comparison, probabilistic tractography methods can be restricted to specific data-driven connections between user-defined regions of interest, can be conducted directly in native diffusion space, and are able to detect complex, multi-fiber orientations at each voxel (21). Additionally, probabilistic tracking algorithms yield a large number of probable pathways between selected regions from a distribution of possible orientations (Jones, 2008). This multi-pathway approach addresses some of the expected uncertainty (e.g., error resulting from movement artifact and distortion) inherent to DTI analyses, and yields a quantitative connectivity metric that can be examined for each voxel along a given path (22, 23). Thus, tractography methods may provide a more sensitive and precise way to examine abnormalities in white matter microstructural connectivity.
Further, it is likely that abnormal white matter connectivity between limbic and prefrontal regions influences the function of these regions, even during a state of rest. Lesser hippocampal-prefrontal functional connectivity has been previously observed in PTSD patients compared to controls. Chen and Etkin (24) found that PTSD participants demonstrated decreased connectivity of the posterior hippocampus with dorsal and perigenual ACC regions, as well as posterior cingulate cortex (PCC) and precuneus regions, both at rest and in response to a task during fMRI. The posterior hippocampus and anterior cingulate have been recognized as components of the default mode network, which is thought to be compromised in PTSD; to this end, lesser functional connectivity has been observed between the PCC and medial and superior prefrontal regions in PTSD (25). Although increasing data supports the notion that impaired hippocampal-frontal functional connectivity characterizes PTSD, there is little data on how this perturbation may be influenced by genetic factors. FKBP5 is a gene worthy of investigation, in this regard; polymorphisms of this gene are likely to impact hippocampal-frontal connectivity via their effects on glucocorticoid activity. Glucocorticoid fluctuations influence structural connections between regions highly susceptible to stress (particularly, the hippocampus and prefrontal cortex) via dendritic remodeling and effects on postsynaptic dendritic spine plasticity (26). Given the well-established links between FKBP5 polymorphisms and PTSD susceptibility(27), as well as what is known about the effects of glucocorticoid oscillations on neural architecture, it is possible that this gene enhances susceptibility for PTSD via its effects on hippocampal-prefrontal connectivity.
Thus, in the present study, we used probabilistic tractography to examine associations between PTSD symptoms, FKBP5 genotype, and cingulum connectivity. We hypothesized that, even after accounting for trauma exposure, age and depressive symptomatology, individuals with PTSD would demonstrate poorer connectivity in this pathway compared to controls. Further, we examined whether these microstructural differences would affect functional connectivity of the brain at rest. Regarding FKBP5, and in light of our earlier findings (13), we predicted that those who carried two copies of the T “risk” allele for a putatively functional polymorphism (rs1360780) would demonstrate poorer microstructural and functional connectivity between the hippocampus and ACC, compared to those with no or one copy of this allele.
Method
Participants
The Institutional Review Board of Emory University approved all study procedures. A total of 54 African-American women aged 20–62 years were recruited through an ongoing study of risk factors for PTSD. Individuals were approached in general medical clinics of a publicly-funded hospital that serves low income individuals in inner-city Atlanta. Eligibility criteria for participation included ability to understand English (assessed by a study researcher) and willingness to provide informed consent. Participants were initially screened for the following exclusion criteria: current psychotropic medication use, current alcohol or substance dependence, medical or physical conditions that preclude MRI scanning (e.g., metal implants), a history of bipolar disorder, schizophrenia or other psychotic disorder, medical conditions that contribute significantly to psychiatric symptoms (e.g., dementia), history of head injury with loss of consciousness for longer than 5 minutes, or a history of neurological illness. Clinical assessments were administered during a separate appointment. Participants were given a pregnancy test to confirm that they were not pregnant and a urine drug screen to rule out substance use on the day prior to scanning. Clinical, genetic and DTI data from 22 participants were published previously (13).
Sample demographics and clinical characteristics are provided in Table 1.
Table 1.
Demographic and Clinical Characteristics
| Trauma Control | PTSD | ||
|---|---|---|---|
| (n=41) | (n=13) | ||
| Mean (SD) | Mean (SD) | F | |
| Age | 39.7 (11.3) | 36.6 (14.3) | .63 |
| CAPS re-experiencing | 2 (3.3) | 10.3 (6.8) | 35.1** |
| CAPS avoidance and numbing | 4.2 (5.5) | 21.4 (8.5) | 70.4** |
| CAPS hyperarousal | 9.3 (8.5) | 17.8 (8.7) | 9.6** |
| BDI total | 10.7 (10.1) | 19.5 (10.7) | 7.3* |
| TEI total | 4.5 (2.4) | 6.4 (3.8) | 4.7* |
| TEI adult trauma | 4 (2.1) | 4.9 (3.2) | 1.5 |
| CTQ total score | 38.8 (15.1) | 51.6 (23) | 5.4* |
| % | % | Kruskal-Wallis p | |
| Education | .52 | ||
| < 12th grade | 9.8 | 7.7 | |
| 12th grade/high school graduate | 34.1 | 7.7 | |
| GED | 2.4 | 7.7 | |
| Some college/technical school | 31.7 | 69.2 | |
| College/tech school graduate | 17.1 | 7.7 | |
| Graduate school | 4.9 | 0 | |
| Monthly Income | .17 | ||
| $0 – 249 | 12.8 | 30.8 | |
| $250 – 499 | 15.4 | 7.7 | |
| $500 – 999 | 30.8 | 38.5 | |
| $1000–1999 | 33.3 | 23.1 | |
| $2000+ | 7.7 | 0 | |
p < .05
p< .01
CAPS = Clinician Administered PTSD Scale (frequency and intensity score combined)
TEI = Traumatic Events Inventory
BDI = Beck Depression Inventory
CTQ = Childhood Trauma Questionnaire
Clinical measures
Given that individuals in our population have demonstrated frequent exposure to trauma, and the possibility that trauma exposure itself would impact connectivity, we assessed for trauma exposure in both childhood and adulthood. The Traumatic Events Inventory (TEI) was administered to detail frequency and type of trauma(s) experienced throughout the lifetime; total level of trauma exposure was measured by a sum score reflecting the total number of different types of traumas to which a participant had been exposed over the course of their life (TEI total) or adulthood (TEI adult trauma). The Clinician Administered PTSD Scale for DSM-IV (28) was administered to determine presence and severity of PTSD symptoms. Based on the CAPS, 13 participants met diagnostic criteria for PTSD (PTSD+), whereas 41 did not meet criteria for the disorder (Controls); subscale scores are provided in Table 1. We administered the Childhood Trauma Questionnaire (CTQ) to measure potential associations between connectivity and childhood maltreatment (29).
MRI acquisition, image processing and statistical analyses
Scanning was conducted on a research-dedicated Siemens 3-Tesla TIM-Trio scanner at Emory University Hospital. Diffusion-weighted images were acquired with maximum gradient strength of 40mTm−1 with the following parameters: 39×2.5 mm thick axial slices, matrix = 128×128, field of view (FOV) = 220×220 mm, voxel size = 1.72×1.72×2.5 mm. Diffusion weighting was isotropically distributed along 60 directions using a b-value of 1,000 s/mm2. Four normalization images, with no diffusion encoding (b=0), were acquired and averaged for each direction using linear rigid body registration (FLIRT; 30). All diffusion-weighted image processing and analysis was conducted using FMRIB Software Library (FSL version 4.1; www.fmrib.ax.ac.uk/fsl; 31). A high-resolution T1-weighted structural scan was also acquired for co-registration purposes using an MPRAGE sequence: 176 slices, FOV= 256 mm cubic voxels; 1mm isotropic slices; repetition time (TR)= 2600 msec; echo time (TE) = 3.02 msec; inversion time (TI)= 900msec; flip angle=8°. Resting state functional MRI were acquired using the Z-SAGA pulse sequence, which has been shown (32) to recover susceptibility signal losses including amygdala and prefrontal cortex. Volumes were acquired axially, parallel to the anterior-posterior commissure line; 150 volumes, 30 slices, 3.44×3.44×4 mm3, repetition time TR = 2950 ms, TE = 30/67ms, flip angle = 90°. Resting state data were available for a total of 30 participants, 7 of whom met criteria for PTSD.
DTI Data Processing and Probabilistic Tractography
Correction for head motion and eddy current distortion was performed for data from each participant using an automated affine registration algorithm. Both diffusion-weighted and T1 images were skull-stripped using the FSL brain extraction tool(33). FA maps were generated using the DTIfit in the FMRIB Diffusion Toolbox. Markov Chain Monte Carlo sampling was used to calculate within-voxel probability density functions of the principal diffusion direction using FSL’s BEDPOSTX tool, which also accounts for the possibility of crossing fibers within a voxel (21). Probabilistic fiber tracking was conducted with PROBTRACKX implemented in FSL; this method repeatedly samples the distribution at each voxel to produce ‘streamlines’ that connect voxels from selected seed regions (5000 streamline samples, .5 mm step length, curvature threshold = .2). A mask of the cingulum, created using the JHU White Matter Tractography Atlas (34), was used as an anatomical waypoint to constrain probabilistic tracts; a separate exclusion mask was created to further eliminate the likelihood of pathways in irrelevant white matter tracts, gray matter regions and CSF. Hippocampus and ACC masks were used as seed regions for the tracts, created using the Harvard-Oxford Subcortical Structural Atlas (http://www.cma.mgh.harvard.edu/fsl_atlas.html). Only streamlines that passed through seed regions and the waypoint were retained. The resulting streamlines were transformed to Montreal Neurological Institute (MNI) space. Given the large size and extent of cingulum streamlines, these paths were thresholded by 10% to reduce the likelihood of including extraneous tracts. An example thresholded probabilistic tract of the cingulum is provided in Figure 1. Fractional anisotropy was used as our measure of tract integrity, given that earlier studies have indicated it to be a reliable assessment of microstructural integrity of white matter fibers (35). Individual FA maps were linearly aligned to a standard MNI brain using FLIRT; mean FA for all streamlines was then extracted and entered into statistical analyses.
Figure 1.

Example probabilistic tracts of the cingulum.
All data were visually inspected for major artifacts before being included in analyses. Cingulum data was available for 54 participants. Using IBM SPSS version 20, ANCOVA and partial correlational analyses were conducted with mean FA values of all tracts, along with clinical and neuropsychological variables. Age and depressive symptoms were controlled for in these analyses, as these factors have been known to influence white matter integrity (10, 36–38). A threshold of p<.05, two-tailed, was used to define statistical significance.
Resting State Functional Connectivity Analyses
Data preprocessing was accomplished using a combination of tools from AFNI (39) and FSL (40) packages. The structural image was skull stripped, segmented, and then registered to standard Montreal Neurological Institute (MNI) space using a nonlinear registration tool (AFNI’s 3dQwarp). Functional image preprocessing began with de-spiking, slice timing correction, motion correction, spatial smoothing (full width at half maximum = 6 mm). The time series were additionally processed to minimize artifacts from head motion, respiration, cardiac pulsation, and hardware using ANATICOR method (41, 42) by performing motion censoring, nuisance regression (motion parameters and averaged signal from eroded local white matter), and band-pass filtering (0.01 – 0.1 Hz) simultaneously in one regression model. Finally, functional images were registered to MNI space via co-registration to the structural image using boundary-based registration (43) within FSL package.
Functional connectivity between the hippocampi and ACC was evaluated by using temporal correlation of time series. Specifically, the preprocessed time series were averaged across voxels within the hippocampus and ACC masks separately. The Pearson’s correlation coefficient was calculated between two averaged time series for each participant. Note that the motion censoring (“scrubbing”) procedure (44) was performed by excluding frames with excessive head motion based on the Euclidian norm of the first time differences of motion estimates (> 0.25 mm) during the analysis. Then, these correlation coefficients were converted to z-values using Fisher’s transformation. Finally, these z-values were entered into second-level random-effects analyses.
To examine associations between structural and functional connectivity, a whole-brain analysis was conducted on the hippocampal functional connectivity. Similarly, the preprocessed time series were averaged across voxels within the hippocampus mask. The Pearson’s correlation coefficient was calculated between the hippocampus seed time series and voxel-wise time series of the whole brain with the motion censoring procedure. After Fisher’s transformation, the association was measured using the cingulum FA as a regressor on the hippocampal functional connectivity. All resulting maps were family-wise error (FWE) corrected at p < 0.05 for the whole brain via Monte-Carlo simulations with AFNI’s 3dClustSim program.
DNA Extraction, Genotyping, Quality Control
The FKBP5 SNP rs1360780 was selected based on our prior studies of FKBP5 polymorphisms within this population (13, 45–47). Genotype data was available for 43 participants (11 with PTSD, 32 controls). Participants were categorized into two groups based on genotype, i.e., how many “risk” alleles comprised their genotype, based on previous studies. For this SNP, individuals were categorized as having either 0/1 or 2 copies of the risk (T) allele. Within this sample, 26 had CC or CT genotype, 17 had TT genotype.
Genotyping methods and quality control have been previously described in detail (13). Briefly, saliva was collected in Oragene vials (DNA Genotek Inc., Ontario Canada). DNA was extracted using the Agencourt DNAdvance extraction kit (Beckman Coulter Inc., Danvers, MA) then quantified using gel electrophoresis and Quantity One software (Bio-Rad, Hercules, CA) or NanoDrop2000 (Thermo Fisher Scientific Inc., Waltham, MA). The DNA was normalized to10 ng/µl and a total amount of 15 ng or 20 ng of dried down DNA per reaction was used for Taqman and Sequenom reactions, respectively. Sequenom reactions were performed using iPlex reagents on the MassARRAY system (Sequenom Inc., San Diego, CA). Additional reactions were performed on the Taqman ViiA7 Real-Time PCR system using Taqman SNP Genotyping Assays as well as Taqman Genotyping Master Mix or Taqman GTXpress Master Mix (Life Technologies Inc., Carlsbad, CA).
Results
Compared to participants without PTSD (controls), those with PTSD had significantly higher frequency and intensity scores on all diagnostic subscales of the CAPS, as expected (see Table 1). These participants also demonstrated comparatively higher exposure to trauma, both over the lifetime and in childhood (p<.05).
Partial correlation results revealed that cingulum connectivity was not significantly associated with childhood maltreatment (CTQ total and subscale scores; p>.05) or adult trauma (TEI adult; p>.05) after controlling for age and current depressive symptoms. After accounting for age and depressive symptoms, ANCOVA results (F1,50=6.77, p=.012) revealed that the PTSD group (Mean FA=.28, SE = .01) demonstrated poorer cingulum connectivity compared to controls (Mean FA=.31, SE = .01). The relationship between PTSD and cingulum connectivity remained significant after also covarying total trauma exposure (F1,49=5.2, p=.027).
A secondary analysis was conducted to examine potential interactions of PTSD and FKBP5 on cingulum connectivity. After covarying age and depressive symptoms, factorial ANCOVA results indicated a significant interaction of genotype and PTSD diagnosis (F1,37=4.52, p=.04; see Figure 2). Individuals with a PTSD diagnosis who carried 2 FKBP5 risk alleles (TT genotype with PTSD, n=6)) demonstrated the lowest cingulum FA values compared to individuals with this genotype without PTSD (n=11), and CC/CT genotypes both with (n=5) and without PTSD (n=21).
Figure 2.
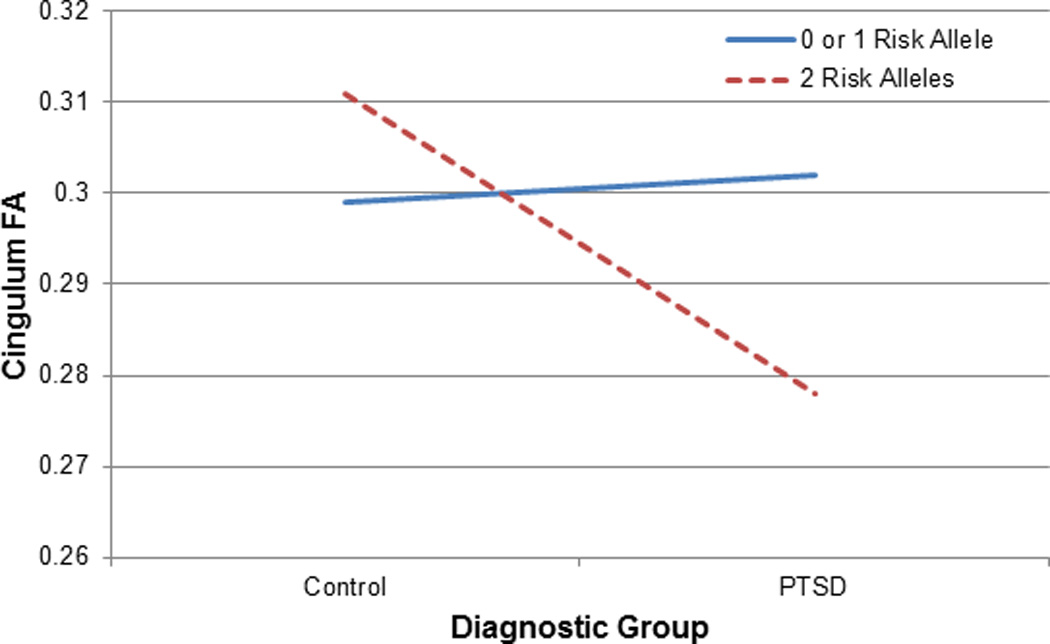
Participants with two copies of the (T) risk allele for an FKBP5 polymorphism (rs1360780) and a diagnosis of PTSD demonstrate poorer cingulum connectivity compared to the other genotype and diagnostic groups.
No differences in resting-state connectivity were found between participants with and without PTSD (p>.05). However, t-test results indicated that carriers of the CC/CT genotypes (n=24) demonstrated significantly greater functional coupling between the hippocampi and ACC compared to carriers of the TT genotype (p<.05). Factorial ANCOVA was not conducted due to the limited numbers of participants with PTSD in this subset (n=7). Results of a correlational analysis indicated that cingulum FA positively correlated with resting state functional connectivity between the hippocampi and regions of the ACC (r=.5, p=.005; Figure 3). A whole-brain analysis was conducted to examine functional connectivity of the hippocampus with other brain regions. When cingulum FA was used as a regressor, there was a significant association between the cingulum FA and the hippocampal functional connectivity at rest in the ACC but not other regions, after correcting for multiple comparisons (p<.05, corrected).
Figure 3.
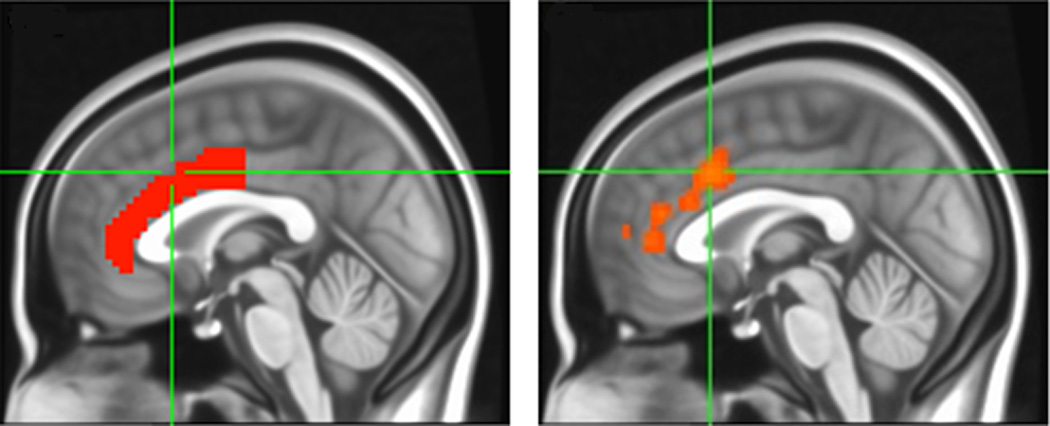
When cingulum FA was used as a regressor in a whole-brain, seed-based analysis, the hippocampus demonstrated significant functional connectivity to dorsal regions of the anterior cingulate, exclusively (p<.05, FWE corrected across the whole brain). Left panel depicts atlas-based ACC mask; right panel depicts regions of ACC activation corresponding with hippocampal activation (unmasked image).
Discussion
The primary objective of the present study was to examine associations between PTSD and connectivity of a major white matter tract implicated in PTSD, the cingulum bundle, which serves as a primary connection between the hippocampus and ACC. We also examined associations between cingulum connectivity, functional connectivity (using resting state fMRI) and FKBP5 genotype.
As predicted, findings indicated that participants with PTSD demonstrated significantly poorer cingulum connectivity, even after accounting for differences in age, depressive symptoms, and trauma exposure. In previous voxel-wise analyses, we observed that individuals with PTSD demonstrated poorer microstructural integrity in posterior aspects of the cingulum (PC) (10). The findings from our prior ROI-based study of the PC suggested that differences between the diagnostic groups may be due in part to genetic factors, particularly, the presence of one or more risk alleles for an FKBP5 SNP that has been closely associated with PTSD risk (rs1360780) (13); findings indicated that those with 2 risk alleles had significantly lower PC FA values as compared to individuals who carried 0 or 1 risk allele.
The findings from the present study, which employed probabilistic tractography as well as resting state fMRI, extend our prior research in several ways. The method and region under investigation was different for our earlier study (13), which used FA extraction from a small portion of the cingulum tract; mean FA was extracted from a circumscribed area, the posterior cingulum, which is proximal to the hippocampus . In this study we employed a probabilistic tractography method that measures connectivity of the entire cingulum, rather than a specific region of this tract, and examined associations between structural and functional connectivity. Unlike our earlier analytic method, probabilistic tractography is sensitive to multi-fiber orientations at the voxel level and individual differences in tract anatomy. Also unlike our earlier studies (10, 13), the present study examined how differences in structure influence function. Consistent with our prior research (10) we found significant effects of PTSD on the cingulum; here, poorer cingulum connectivity was associated with a PTSD diagnosis. In addition, our prior studies (10, 13) used a self-report PTSD measure (PTSD Symptom Scale; (48). Here we employed a clinician-administered, gold-standard measure of PTSD, the CAPS (28). Given the excellent psychometric properties of this measure, as well as the fact that it is clinician-administered, use of this measure may decrease the likelihood of over-estimating PTSD rates in our sample.
Further, the present study also included data on functional connectivity. To our knowledge, this is the first study to examine associations between white matter and resting state connectivity and FKBP5 genotype. Here, we observed a significant interaction between FKBP5 genotype and PTSD diagnosis; individuals who carried two risk alleles for a putatively functional FKBP5 SNP (rs1360780) and met criteria for a PTSD diagnosis demonstrated relatively poorer cingulum connectivity compared to the other genotype and diagnostic groups. Further, we found that this risk genotype affected functional connectivity at rest. Although statistical power precluded our ability to explore gene by diagnosis interactions in the resting state data, we observed that those individuals who carried two copies of the risk allele demonstrated significantly poorer functional connectivity between the hippocampi and ACC. Remarkably, when cingulum FA was used as a regressor in a seed-based, whole-brain correlational analysis, only the ACC emerged as being functionally coupled with the hippocampus, even after correcting for family-wise error for the whole brain. Similarly, cingulum FA and hippocampal-ACC functional connectivity were found to be highly correlated.
Our findings suggest that, following trauma exposure, carriers of two FKBP5 risk alleles may be more likely to develop decrements in this tract, and these decrements appear to influence functional connectivity. It is likely that these functional alterations, in turn, influence the development of the psychological and cognitive manifestations that are characteristic of PTSD. The cingulum is a major association fiber bundle that extends from the entorhinal cortex to the anterior cingulate cortex, connecting the parahippocampal gyrus, aspects of the parietal lobe, supplementary motor areas, medial and dorsolateral aspects of the prefrontal cortex (49). Given that it connects these areas, it is likely that abnormalities in cingulum connectivity adversely affect a number of relevant cognitive and emotional processes. One process is extinction of learned fear, a type of learning that is thought to be impaired in PTSD, and which is thought to rely on the quality of connections between medial prefrontal and limbic (i.e., amygdala and hippocampal) regions (50). In fact, emerging evidence suggests that cingulum connectivity is inversely associated with the magnitude of fear-potentiated startle response during extinction learning (51). However, the role of hippocampal-prefrontal connectivity has also been highlighted in working memory, contextual memory, and reward learning (52), all of which are functions known to be altered in psychiatric disorders such as PTSD, depression, and schizophrenia. The importance of hippocampal connectivity in PTSD cannot be understated; a burgeoning literature underscores the relevance of abnormal hippocampal-frontal connectivity in this disorder (e.g., Chen & Etkin, 2013). FKBP5 is likely to have an impact on this connectivity, given its role in glucocorticoid signaling. A recent review describes the relevance of glucocorticoids in shaping hippocampal-prefrontal connectivity via dendritic modeling and changing postsynaptic spine plasticity (Hall 2015),
A primary limitation of the present study is the sample size, which is likely to have reduced power to detect between-group differences in resting state connectivity and associations with FKBP5 genotype. We chose to restrict our analyses to the cingulum, given that this tract has demonstrated associations with cognitive and affective features that characterize PTSD (9, 53); however, it is possible that connectivity of other white matter tracts, including the corpus callosum and fornix, would be relevant to these phenomena. Further studies that examine other promising tracts of interest are certainly warranted to establish the presence of other intermediate neural phenotypes for PTSD. Further, the sample size was small for genotype by diagnosis analyses, which raises the possibility of false positive findings; thus, replication is warranted with a larger sample size.
To conclude, we found that, compared to similarly traumatized individuals without PTSD, those with PTSD demonstrated significantly poorer structural and functional connectivity in a hippocampal-prefrontal pathway previously demonstrated to be critical to basic emotion on as well as cognitive functioning (Sexton et. al., 2009). Participants with PTSD who carried two risk alleles for a putatively functional FKBP5 polymorphism demonstrated the poorest cingulum connectivity compared to the other diagnostic and genotype groups. This is of clinical interest; given that the cingulum is a highly heritable white matter pathway (14), altered connectivity for this tract may serve as a marker of risk for the development of anxious psychopathology. In summary, our findings indicate that cingulum connectivity is an attractive intermediate neural phenotype for PTSD, as well as other mood and anxiety disorders. Abnormalities in this pathway are likely to be heritable, and may predict vulnerability for the development of this disorder following exposure to a traumatic stressor.
Acknowledgments
Funding: This work was primarily supported by National Institutes of Mental Health (R01 MH071537, M01RR00039 and P20RR16435 to KJR, MH101380 to NF, MH098212 and MH092576 to TJ). Support was also received from Howard Hughes Medical Institute, PHS Grant UL1 RR025008 from the Clinical and Translational Science Award program, National Center for Research Resources, and the Burroughs Wellcome Fund (KJR).
Footnotes
Disclosures: The authors have no financial conflicts of interest to disclose.
References
- 1.Sexton CE, Mackay CE, Ebmeier KP. A systematic review of diffusion tensor imaging studies in affective disorders. Biol Psychiatry. 2009;66(9):814–823. doi: 10.1016/j.biopsych.2009.05.024. [DOI] [PubMed] [Google Scholar]
- 2.de Diego-Adelino J, Pires P, Gomez-Anson B, Serra-Blasco M, Vives-Gilabert Y, Puigdemont D, et al. Microstructural white-matter abnormalities associated with treatment resistance, severity and duration of illness in major depression. Psychol Med. 2013:1–12. doi: 10.1017/S003329171300158X. [DOI] [PubMed] [Google Scholar]
- 3.Henderson SE, Johnson AR, Vallejo AI, Katz L, Wong E, Gabbay V. A preliminary study of white matter in adolescent depression: relationships with illness severity, anhedonia, and irritability. Front Psychiatry. 2013;4:152. doi: 10.3389/fpsyt.2013.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Duffy SL, Paradise M, Hickie IB, Lewis SJ, Naismith SL, Lagopoulos J. Cognitive impairment with and without depression history: an analysis of white matter microstructure. J Psychiatry Neurosci. 2014;39(2):135–143. doi: 10.1503/jpn.130079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Byun MS, Choi JS, Yoo SY, Kang DH, Choi CH, Jang DP, et al. Depressive Symptoms and Brain Metabolite Alterations in Subjects at Ultra-high Risk for Psychosis: A Preliminary Study. Psychiatry Investig. 2009;6(4):264–271. doi: 10.4306/pi.2009.6.4.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kazlouski D, Rollin MD, Tregellas J, Shott ME, Jappe LM, Hagman JO, et al. Altered fimbria-fornix white matter integrity in anorexia nervosa predicts harm avoidance. Psychiatry Res. 2011;192(2):109–116. doi: 10.1016/j.pscychresns.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yau WY, Bischoff-Grethe A, Theilmann RJ, Torres L, Wagner A, Kaye WH, et al. Alterations in white matter microstructure in women recovered from anorexia nervosa. Int J Eat Disord. 2013;46(7):701–708. doi: 10.1002/eat.22154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kubicki M, Westin CF, Nestor PG, Wible CG, Frumin M, Maier SE, et al. Cingulate fasciculus integrity disruption in schizophrenia: a magnetic resonance diffusion tensor imaging study. Biol Psychiatry. 2003;54(11):1171–1180. doi: 10.1016/s0006-3223(03)00419-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Daniels JK, Lamke JP, Gaebler M, Walter H, Scheel M. White matter integrity and its relationship to PTSD and childhood trauma--a systematic review and meta-analysis. Depress Anxiety. 2013;30(3):207–216. doi: 10.1002/da.22044. [DOI] [PubMed] [Google Scholar]
- 10.Fani N, King TZ, Jovanovic T, Glover EM, Bradley B, Choi K, et al. White matter integrity in highly traumatized adults with and without post-traumatic stress disorder. Neuropsychopharmacology. 2012;37(12):2740–2746. doi: 10.1038/npp.2012.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sanjuan PM, Thoma R, Claus ED, Mays N, Caprihan A. Reduced white matter integrity in the cingulum and anterior corona radiata in posttraumatic stress disorder in male combat veterans: a diffusion tensor imaging study. Psychiatry Res. 2013;214(3):260–268. doi: 10.1016/j.pscychresns.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Keedwell PA, Chapman R, Christiansen K, Richardson H, Evans J, Jones DK. Cingulum White Matter in Young Women at Risk of Depression: The Effect of Family History and Anhedonia. Biol Psychiatry. 2012 doi: 10.1016/j.biopsych.2012.01.022. [DOI] [PubMed] [Google Scholar]
- 13.Fani N, King TZ, Reiser E, Binder EB, Jovanovic T, Bradley B, et al. FKBP5 Genotype and Structural Integrity of the Posterior Cingulum. Neuropsychopharmacology. 2014;39(5):1206–1213. doi: 10.1038/npp.2013.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jahanshad N, Kochunov PV, Sprooten E, Mandl RC, Nichols TE, Almasy L, et al. Multi-site genetic analysis of diffusion images and voxelwise heritability analysis: a pilot project of the ENIGMA-DTI working group. NeuroImage. 2013;81:455–469. doi: 10.1016/j.neuroimage.2013.04.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hosang GM, Shiles C, Tansey KE, McGuffin P, Uher R. Interaction between stress and the BDNF Val66Met polymorphism in depression: a systematic review and meta-analysis. BMC Med. 2014;12:7. doi: 10.1186/1741-7015-12-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang L, Benedek DM, Fullerton CS, Forsten RD, Naifeh JA, Li XX, et al. PTSD risk is associated with BDNF Val66Met and BDNF overexpression. Mol Psychiatry. 2014;19(1):8–10. doi: 10.1038/mp.2012.180. [DOI] [PubMed] [Google Scholar]
- 17.Hemmings SM, Martin LI, Klopper M, van der Merwe L, Aitken L, de Wit E, et al. BDNF Val66Met and DRD2 Taq1A polymorphisms interact to influence PTSD symptom severity: a preliminary investigation in a South African population. Prog Neuropsychopharmacol Biol Psychiatry. 2013;40:273–280. doi: 10.1016/j.pnpbp.2012.10.011. [DOI] [PubMed] [Google Scholar]
- 18.Carballedo A, Amico F, Ugwu I, Fagan AJ, Fahey C, Morris D, et al. Reduced fractional anisotropy in the uncinate fasciculus in patients with major depression carrying the met-allele of the Val66Met brain-derived neurotrophic factor genotype. Am J Med Genet B Neuropsychiatr Genet. 2012;159B(5):537–548. doi: 10.1002/ajmg.b.32060. [DOI] [PubMed] [Google Scholar]
- 19.Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. NeuroImage. 2006;31(4):1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 20.Bookstein FL. "Voxel-based morphometry" should not be used with imperfectly registered images. NeuroImage. 2001;14(6):1454–1462. doi: 10.1006/nimg.2001.0770. [DOI] [PubMed] [Google Scholar]
- 21.Behrens TE, Woolrich MW, Jenkinson M, Johansen-Berg H, Nunes RG, Clare S, et al. Characterization and propagation of uncertainty in diffusion-weighted MR imaging. Magn Reson Med. 2003;50(5):1077–1088. doi: 10.1002/mrm.10609. [DOI] [PubMed] [Google Scholar]
- 22.Mukherjee P, Berman JI, Chung SW, Hess CP, Henry RG. Diffusion tensor MR imaging and fiber tractography: theoretic underpinnings. AJNR American journal of neuroradiology. 2008;29(4):632–641. doi: 10.3174/ajnr.A1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mukherjee P, Chung SW, Berman JI, Hess CP, Henry RG. Diffusion tensor MR imaging and fiber tractography: technical considerations. AJNR American journal of neuroradiology. 2008;29(5):843–852. doi: 10.3174/ajnr.A1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen AC, Etkin A. Hippocampal network connectivity and activation differentiates post-traumatic stress disorder from generalized anxiety disorder. Neuropsychopharmacology. 2013;38(10):1889–1898. doi: 10.1038/npp.2013.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bluhm RL, Williamson PC, Osuch EA, Frewen PA, Stevens TK, Boksman K, et al. Alterations in default network connectivity in posttraumatic stress disorder related to early-life trauma. J Psychiatry Neurosci. 2009;34(3):187–194. [PMC free article] [PubMed] [Google Scholar]
- 26.Hall BS, Moda RN, Liston C. Glucocorticoid Mechanisms of Functional Connectivity Changes in Stress-Related Neuropsychiatric Disorders. Neurobiology of stress. 2015;1:174–183. doi: 10.1016/j.ynstr.2014.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Binder EB. The role of FKBP5, a co-chaperone of the glucocorticoid receptor in the pathogenesis and therapy of affective and anxiety disorders. Psychoneuroendocrinology. 2009;34(Suppl 1):S186–S195. doi: 10.1016/j.psyneuen.2009.05.021. [DOI] [PubMed] [Google Scholar]
- 28.Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, Charney DS. The development of a clinician-administered PTSD scale. Journal of Traumatic Stress. 1995;8:75–90. doi: 10.1007/BF02105408. [DOI] [PubMed] [Google Scholar]
- 29.Bernstein DP, Fink L. Childhood Trauma Questionnaire: a retrospective self-report manual. New York: The Psychological Corporation; 1998. [Google Scholar]
- 30.Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Med Image Anal. 2001;5(2):143–156. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- 31.Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, et al. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage. 2004;23(Suppl 1):S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- 32.Heberlein KA, Hu X. Simultaneous acquisition of gradient-echo and asymmetric spin-echo for single-shot z-shim: Z-SAGA. Magn Reson Med. 2004;51(1):212–216. doi: 10.1002/mrm.10680. [DOI] [PubMed] [Google Scholar]
- 33.Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17(3):143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mori S, Wakana S, Nagae-Poetscher L, Van Zijl PC. In: MRI Atlas of Human White Matter. Elsevier, editor. Amsterdam, the Netherlands: 2005. [Google Scholar]
- 35.Fox RJ, Sakaie K, Lee JC, Debbins JP, Liu Y, Arnold DL, et al. A Validation Study of Multicenter Diffusion Tensor Imaging: Reliability of Fractional Anisotropy and Diffusivity Values. AJNR American journal of neuroradiology. 2011 doi: 10.3174/ajnr.A2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lebel C, Gee M, Camicioli R, Wieler M, Martin W, Beaulieu C. Diffusion tensor imaging of white matter tract evolution over the lifespan. NeuroImage. 2012;60(1):340–352. doi: 10.1016/j.neuroimage.2011.11.094. [DOI] [PubMed] [Google Scholar]
- 37.Ly M, Canu E, Xu G, Oh J, McLaren DG, Dowling NM, et al. Midlife measurements of white matter microstructure predict subsequent regional white matter atrophy in healthy adults. Hum Brain Mapp. 2013 doi: 10.1002/hbm.22311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang HH, Zhang ZJ, Tan QR, Yin H, Chen YC, Wang HN, et al. Psychopathological, biological, and neuroimaging characterization of posttraumatic stress disorder in survivors of a severe coalmining disaster in China. J Psychiatr Res. 2010;44(6):385–392. doi: 10.1016/j.jpsychires.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 39.Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29(3):162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 40.Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TEJ, Johansen-Berg H, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl 1):S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- 41.Jo HJ, Gotts SJ, Reynolds RC, Bandettini PA, Martin A, Cox RW, et al. Effective Preprocessing Procedures Virtually Eliminate Distance-Dependent Motion Artifacts in Resting State FMRI. Journal of applied mathematics. 2013;2013:1–9. doi: 10.1155/2013/935154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jo HJ, Saad ZS, Simmons WK, Milbury LA, Cox RW. Mapping sources of correlation in resting state FMRI, with artifact detection and removal. Neuroimage. 2010;52(2):571–582. doi: 10.1016/j.neuroimage.2010.04.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Greve DN, Fischl B. Accurate and robust brain image alignment using boundary-based registration. Neuroimage. 2009;48(1):63–72. doi: 10.1016/j.neuroimage.2009.06.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012;59(3):2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Binder EB, Bradley RG, Liu W, Epstein MP, Deveau TC, Mercer KB, et al. Association of FKBP5 polymorphisms and childhood abuse with risk of posttraumatic stress disorder symptoms in adults. JAMA. 2008;299(11):1291–1305. doi: 10.1001/jama.299.11.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Klengel T, Mehta D, Anacker C, Rex-Haffner M, Pruessner JC, Pariante CM, et al. Allele-specific FKBP5 DNA demethylation mediates gene-childhood trauma interactions. Nat Neurosci. 2013;16(1):33–41. doi: 10.1038/nn.3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fani N, Gutman D, Tone EB, Almli L, Mercer KB, Davis J, et al. FKBP5 and Attention Bias for Threat: Associations With Hippocampal Function and Shape. JAMA Psychiatry. 2013;70(4):392–400. doi: 10.1001/2013.jamapsychiatry.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Foa EB, Riggs DS, Dancu CV, Rothbaum BO. Reliability and validity of a brief instrument for assessing post-traumatic stress disorder. Journal of Traumatic Stress. 1993;6:459–473. [Google Scholar]
- 49.Schmahmann JD, Pandya DN, Wang R, Dai G, D'Arceuil HE, de Crespigny AJ, et al. Association fibre pathways of the brain: parallel observations from diffusion spectrum imaging and autoradiography. Brain. 2007;130(Pt 3):630–653. doi: 10.1093/brain/awl359. [DOI] [PubMed] [Google Scholar]
- 50.Hartley CA, Phelps EA. Changing fear: the neurocircuitry of emotion regulation. Neuropsychopharmacology. 2010;35(1):136–146. doi: 10.1038/npp.2009.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fani N, King TZ, Brewster R, Srivastava A, Stevens JS, Glover EM, et al. Fear-potentiated startle during extinction is associated with white matter microstructure and functional connectivity. Cortex. 2015;64:249–259. doi: 10.1016/j.cortex.2014.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Godsil BP, Kiss JP, Spedding M, Jay TM. The hippocampal-prefrontal pathway: the weak link in psychiatric disorders? European neuropsychopharmacology : the journal of the European College of Neuropsychopharmacology. 2013;23(10):1165–1181. doi: 10.1016/j.euroneuro.2012.10.018. [DOI] [PubMed] [Google Scholar]
- 53.Phan KL, Orlichenko A, Boyd E, Angstadt M, Coccaro EF, Liberzon I, et al. Preliminary evidence of white matter abnormality in the uncinate fasciculus in generalized social anxiety disorder. Biol Psychiatry. 2009;66(7):691–694. doi: 10.1016/j.biopsych.2009.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]


