Abstract
Background
Hemodialysis patients are potentially susceptible to infection with blood-borne viral agents, especially hepatitis B virus (HBV), hepatitis C virus (HCV), and human immunodeficiency virus (HIV).
Objectives
The aim of this study was to evaluate the prevalence and incidence of HBV, HCV, and HIV infections in hemodialysis patients.
Patients and Methods
This study was carried out in 482 hemodialysis patients who had been referred to eight dialysis centers in the Mazandaran province in Iran from 2012 - 2014. HBs Ag, HCV Ab, HBs Ab and HIV Ab were assessed every three months for two years. The patients’ demographic characteristics, including age, gender, area of residence, and duration of dialysis, were noted. The prevalence of each virus was also determined.
Results
From a total of 482 patients, 253 (52.5%) males and 229 (47.5%) females were evaluated. The mean age of all patients was 54.96 ± 16.1 years, and all participants were HIV negative. One subject had both HBV and HCV infections. HBs Ag and anti-HCV were detected in 10 (2.1%) and 40 (8.27%) patients, respectively. The mean age of HCV-positive patients was 55.4 ± 16.4, while HCV-negative patients were an average of 51.6 ± 10.7 years old (P = 0.002). The incidence of HCV was higher in people from 40–59 years of age (P < 0.001). No patients had HBV, and incidence of HCV was 0.5% in the first year and 0.75 in the second year. No cases of HIV were identified.
Conclusions
The results show that the prevalence rates of HBV and HCV in hemodialysis patients were moderate to low in the Mazandaran province. Based upon the obtained levels of these viruses, these incidence rates are therefore reasonable.
Keywords: Prevalence, Incidence, Hepatitis B, Hepatitis C, Hemodialysis
1. Background
Widespread access to dialysis has significantly increased survival in patients with chronic renal failure. During hemodialysis, the patient’s blood flows through a filter in a dialysis machine. Although this method can be efficient to treat renal failure, it may also lead to the transmission of some blood borne infections, such as HBV, HCV, and HIV (1-3). The prevalence of HBV and HCV among hemodialysis patients is highly variable in different countries and even at different centers in the same locality (4-6). According to various studies conducted at hemodialysis centers in developing countries, a high prevalence of HBV infection (20.2%) has been reported in these patients (7). The modern era since the advent of infection control policies, particularly immunization, and also the separation of hemodialysis patients that are HBs antigen positive, have considerably reduced the spread of HBV in this population. Vaccination against HBV before the patient progresses to end-stage renal failure is the best way to protect against HBV infection in hemodialysis patients (8).
The prevalence of HCV infection in hemodialysis patients differs from 4% to over 70% in some countries (5). The major reasons for this high incidence of infection with hepatitis C are the high prevalence of infection in the general population, a lack of standard methods of prevention and effective vaccination, inadequate disinfection of dialysis machines and other medical equipment, as well as the spread of infection from one patient to another, particularly in dialysis centers (5, 6).
2. Objectives
The aim of this study was therefore to evaluate the prevalence of blood-borne factors (HCV Ab, HBs Ag, and HIV Ab) in hemodialysis patients in the Mazandaran province.
3. Patients and Methods
This study was carried out in 482 hemodialysis patients referred to eight dialysis centers in the Mazandaran province from 2012 - 2013. Data on the patient demographic characteristics, including their age, gender, area of residence (rural vs. urban), and duration of dialysis treatment were gathered from patient files (1). Before dialysis began, all cases were evaluated for HCV, HIV, and HBV and considered positive if anti-HCV and HIV antibodies and HBs Ag were detected. All positive cases were excluded from the study. Then, all HCV, anti-HIV, and HBV-negative cases were re-evaluated at three-month intervals.
Seroconversion to HCV Ab, HIV Ab and HBsAg in those who were negative at the initial evaluation but then seroconverted were considered new cases of infection. New reactive HCV Ab was followed by a PCR as a confirmatory test. HCV genotyping was determined by VERSANT HCV Genotype Assay (LiPA) (Bayer Corporation, Tarrytown, NY, USA). The Amplicor HCV kit and the LiPA were performed according to the manufacturer’s instructions. The ethics and research committees of Babol University of Medical Sciences approved the research protocols, and all patients provided written informed consent to participate in the study.
Data were analyzed using the Statistical Package for the Social Sciences (SPSS) version 22. Chi-square, independent samples T-test, and Fisher’s exact test were employed for the qualitative and quantitative variables where appropriate. Statistical significance was defined by P ≤ 0.05.
4. Results
Out of the 482 enrolled patients, 253 (52.5%) were men and 229 (47.5%) were women. The mean age was 54.96 ± 16.1 years. The mean duration of dialysis was 50.6 ± 36.2 months.
All patients were negative for HIV antibodies during their dialysis treatment. Forty (8.3%) patients were positive for the HCV Ab. The mean age of these patients was 51.6 ± 10.7 years, which was statistically significant compared to the mean age of patients without hepatitis C (55.4 ± 16.4) (P = 0.002). Ten (2%) patients were positive for HBs Ag with a mean age of 58.5 ± 14.1 years, while the mean age of those who were negative for the antigen was 55.0 ± 16.1 years. No significant difference was observed between the mean age of these two groups (P = 0.54). The mean duration of dialysis in patients with HBV was 45.6 ± 37.2 months, while the average length of dialysis in the HBs Ag-negative patients was 49.2 ± 40.8 months (P = 0.19).
In the first evaluation, one patient was positive for both HBV and HCV. This patient became negative for the HBs Ag during the study period. Furthermore, new cases of hepatitis B were not identified, while the HBs Ag-positive patients became negative. Overall, the seroconversion rate of HCV was estimated as 0.63% (5 out of 400 patients). Two out of the 5 seroconversions occurred during 2012 among the 398 negative cases, and 3 of these 5 cases were identified during 2013 in 402 negative patients. The incidence rates for HCV infection in 2012 and 2013 were 0.5% and 0.75%, respectively.
Results obtained from anti-HBs antibody titration showed that 53.35% of vaccinated patients had sufficient amounts of antibodies against HBV. In the five new HCV Ab-positive cases, HCV RNA was detected in four. Genotypes 1 and 2 were seen in two and one cases, respectively, and dual genotypes of both 1 and 2 were noted in one case (Table 1 and Figure 1).
Table 1. A comparison of the Demographic Characteristics Among Hemodialysis Patients With and Without HCV and HBV Infectionsa.
| Variable | HCV Ab | P Value | HBs Ag | P Value | ||
|---|---|---|---|---|---|---|
| Positive | Negative | Positive | Negative | |||
| Age, y | < 0.001 | 0.71 | ||||
| < 39 | 5 (12.5) | 89 (20.2) | 1 (1.1) | 94 (98.9) | ||
| 59 - 40 | 28 (70) | 161 (36.5) | 4 (2.1) | 198 (97.9) | ||
| > 60 | 7 (17.5) | 191 (43.5) | 5 (2.5) | 193 (97.5) | ||
| Sex | 0.73 | 0.87 | ||||
| Male | 20 (50) | 247 (56) | 5 (2) | 248 (98) | ||
| Female | 20 (50) | 194 (44) | 5 (2.2) | 224 (97.8) | ||
| Residence | 0.67 | 0.72 | ||||
| Rural | 21 (52.5) | 247 (56) | 5 (1.9) | 263 (98.1) | ||
| Urban | 19 (47.5) | 194 (44) | 5 (2.3) | 209 (97.7) | ||
| Duration of dialysis, y | 0.61 | 0.8 | ||||
| ≤ 1 | 44 (10) | 44 (10) | 0 | 0 | ||
| 1 - 3 | 15 (37.5) | 165 (37.4) | 4 (40) | 4 (40) | ||
| 3 ≥ | 21 (52.5) | 232 (52.6) | 6 (60) | 247 (52.5) | ||
aValues are expressed as No. (%).
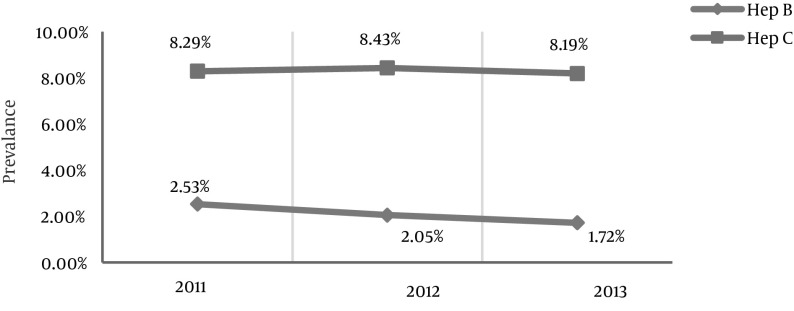
Figure 1. The Prevalence of HBV and HCV Each Year.
5. Discussion
Infection with HBV and HCV is a major problem in patients undergoing hemodialysis. Viral transmission occurs through the internal contamination of devices used for hemodialysis due to an insufficient cleaning system used between dialysis sessions, equipment sharing, and the use of common vials to prepare and inject drugs (5).
Despite the accessibility of serologic tests, a vaccine, and global prevention standards, the risk of hepatitis B infection in dialysis patients is still a serious problem. However, the prevalence of infection with hepatitis B is very low in comparison with hepatitis C (6). The prevalence of HBV was 2.53% in 2011 and remained at a similar level during the following years. Improvements in hemodialysis devices and more stringent hygienic rules have significantly decreased the number of hepatitis B cases in this population. The incidence of hepatitis B was 3.23% in India and was higher than that obtained by our study (9); this difference could be due to the higher population in India compared with Iran and the therefore subsequently higher number of hemodialysis cases.
In another study performed in India, the prevalence of HBV was greater (10.2%) and was also higher than the report of Prakash et al. as was the case in our study (8, 9). However, it should be noted that both of the Indian studies were conducted in different geographic areas with distinct populations and environmental conditions. In the present study, we found that the HCV prevalence reached its peak in 2012 (8.43%); the prevalence in 2011 and in the years after 2012 decreased. The significant increase in the hepatitis C prevalence seen in 2011 could be due to many factors, such as the breakdown of hemodialysis devices that may have caused overcrowding of patients.
In 2015, Chebrolu et al. reported a 2.1% hepatitis C prevalence (10). The rates of overt hepatitis C infection and occult hepatitis C were reported to be 2.4% and 0.25%, respectively, in Baid-Agrawal et al.’s 2014 study, which were lower than the results of our study (11). This difference could have resulted from more meticulous hygiene in developed countries versus that in our country. In 2008, Fabrizi et al. reported the prevalence of HBV in developing countries as 2% - 20%, which was higher than that found in developed ones (12). Thus, this finding could also be generalized to hepatitis C.
Mittal et al. reported a hepatitis C prevalence of 16.1%, while in Prakash et al.’s study, it was found to be 6.99% (8). Although these two studies had different circumstances, over time, the prevalence of HCV decreased in both. An increased commitment of personnel to the preventive rules set forth by the WHO could be one explanation for this decreased prevalence.
Alavian et al. showed that the incidences of hepatitis C in a hemodialysis unit in the Mazandaran province in 2003 and 2008 were 12% and 11.8%, respectively, and in comparison with our study (13, 14). According to Mohtasham-Amiri et al. the prevalence of hepatitis C in the Guilan province was 24.8% in 2003 (15). In 2011, Joukar et al. reported that the prevalence of hepatitis C was 11.9% in the same province (16). These findings indicate a decline in the prevalence of hepatitis C in the Guilan province, which is similar to the result of the current study and mirrors the decrease in the prevalence of hepatitis C in the Mazandaran province. In 2012, the hepatitis C prevalence in hemodialysis patients was reportedly 2.5% and 7% in the Isfahan and Kerman provinces, respectively (17, 18). In 2010, Alavian et al. estimated the incidence of HCV in hemodialysis patients to be from 4.5% to 26.4% in different provinces. According to other studies, the prevalence of hepatitis C in the Mazandaran province was reported to be less than the national average (14).
In our study, the prevalence of hepatitis C was less than 15%, which was consistent with the results of the Su et al. study; fortunately, the prevalence of hepatitis C in our country is less than that in developing countries (19). In agreement with Katayama et al. the prevalence of HBV was 6.2% and the annual incidence was zero, which are similar to the results obtained by our study (20). The incidence of HBV in the present study was also zero, but only in 2011. In 2012 and 2013, the prevalence rates of HCV were 0.5% and 0.75%, respectively. In spite of the peak HCV prevalence in 2012, its incidence was at the peak in 2013.
Sypsa et al. reported a 6.2% incidence of HCV in 2005 that was higher than that of our study in 2013 (21). The highest prevalence of hepatitis C was identified in the age group from 40 - 59 years (P < 0.001). Prakash et al. found a 7.8% hepatitis C prevalence in patients from 14 - 60 years that was higher than any other age group (9). In the studies of Joukar et al. and Kalantari et al. and Zahedi et al. there were no associations between age and the prevalence of hepatitis C (16-18). In addition, a higher prevalence of hepatitis C has also been seen with increasing dialysis duration times (P = 0.61). In this study, we found a significant relationship between the duration of hemodialysis and the prevalence of HCV, which was similar to the results of Joukar et al.’s survey but differed from Kalantari’s and Zahedi’s studies. The result was in agreement with the investigations by Mohtasham Amiri and Joukar (15, 16), but this information was not reported in the studies of Kalantari and Zahedi et al. (16, 18), so no comparison could be made.
In Prakash et al. study, the mean duration of dialysis for patients both with and without hepatitis C was 17 and 12 months, respectively, and was significantly meaningful (9). In contrast, longer dialysis times increase the probability of hepatitis C infection. Joukar et al. found a significant relationship between gender and the hepatitis C prevalence, while in our study the incidence of this infection was not significant between genders (16). Although 52.5% of the hemodialysis patients in the present study were male, the numbers of male and female patients with hepatitis B and C were equal. Several studies have reported that hepatitis is more common in men. For example, 66.9% of the patients in Mittal et al.’s investigation were male. In addition, 11.4% of hepatitis B and 32.9% of hepatitis C patients were male (8). In Prakash’s study, 2.6% of patients with hepatitis B and 9.4% of patients with hepatitis C were male (9). Although none of these differences was statistically significant, the results are in line with our study. In the Joukar et al. study, this association was significant, and the difference in sample size and variations in the characteristics of the study population could be the reasons for these distinctions (16). The HIV prevalence was zero in our study, which was similar to the Zahedi et al. survey (18).
5.1. Conclusions
Our results show that the prevalence of HBV and HCV in hemodialysis patients was moderate to low in the Mazandaran province. Based upon the obtained prevalence information regarding these viruses, the incidence rates were therefore reasonable.
Acknowledgments
The authors would like to extend their thanks to the clinical research development unit of Rohani hospital at the Babol University of Medical Sciences.
Footnotes
Funding/Support:Funding was provided by the research vice chancellor of the Babol University of Medical Sciences.
References
- 1.Saha D, Agarwal SK. Hepatitis and HIV infection during haemodialysis. J Indian Med Assoc. 2001;99(4):203. [PubMed] [Google Scholar]
- 2.Yousefi Abdolmalehi E, Seyfi S. Although maintenance dialysis prevents death from uremia, patient survival and quality of life remains an important issue. J Mazandaran Univ Med Sci. 2012;22(86) [Google Scholar]
- 3.Mallamaci F, Tripepi G, Cutrupi S, Malatino LS, Zoccali C. Prognostic value of combined use of biomarkers of inflammation, endothelial dysfunction, and myocardiopathy in patients with ESRD. Kidney Int. 2005;67(6):2330–7. doi: 10.1111/j.1523-1755.2005.00338.x. [DOI] [PubMed] [Google Scholar]
- 4.Longo D, Fauci A, Kasper D, Hauser S, Larry Jameson J, Loscalzo J. Harrison's principles of internal medicine. Vol. 2. McGraw-Hill Medical New York; 2008. [Google Scholar]
- 5.Thongsawat S, Maneekarn N, Kuniholm MH, Pantip C, Thungsuputi A, Lumlertkul D, et al. Occult hepatitis C virus infection during an outbreak in a hemodialysis unit in Thailand. J Med Virol. 2008;80(5):808–15. doi: 10.1002/jmv.21126. [DOI] [PubMed] [Google Scholar]
- 6.Khedmat H, Amini M, Ghamar-Chehreh ME, Agah S. Hepatitis C virus infection in dialysis patients. Saudi J Kidney Dis Transpl. 2014;25(1):1–8. doi: 10.4103/1319-2442.124455. [DOI] [PubMed] [Google Scholar]
- 7.Telaku S, Fejza H, Elezi Y, Bicaj T. Hepatitis B and C in dialysis units in Kosova. Virol J. 2009;6:72. doi: 10.1186/1743-422X-6-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mittal G, Gupta P, Thakuria B, Mukhiya GK, Mittal M. Profile of hepatitis B virus, hepatitis C virus, hepatitis d virus and human immunodeficiency virus infections in hemodialysis patients of a tertiary care hospital in uttarakhand. J Clin Exp Hepatol. 2013;3(1):24–8. doi: 10.1016/j.jceh.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prakash S, Jain A, Sankhwar SN, Usman K, Prasad N, Saha D, et al. Prevalence of hepatitis B & C viruses among patients on hemodialysis in Lucknow, Uttar Pradesh. Clin Epidemiol Glob Health. 2014;2(1):19–23. [Google Scholar]
- 10.Chebrolu P, Colombo RE, Baer S, Gallaher TR, Atwater S, Kheda M, et al. Bacteremia in hemodialysis patients with hepatitis C. Am J Med Sci. 2015;349(3):217–21. doi: 10.1097/MAJ.0000000000000391. [DOI] [PubMed] [Google Scholar]
- 11.Baid-Agrawal S, Schindler R, Reinke P, Staedtler A, Rimpler S, Malik B, et al. Prevalence of occult hepatitis C infection in chronic hemodialysis and kidney transplant patients. J Hepatol. 2014;60(5):928–33. doi: 10.1016/j.jhep.2014.01.012. [DOI] [PubMed] [Google Scholar]
- 12.Fabrizi F, Messa P, Martin P. Hepatitis B virus infection and the dialysis patient. Semin Dial. 2008;21(5):440–6. doi: 10.1111/j.1525-139X.2008.00437.x. [DOI] [PubMed] [Google Scholar]
- 13.Alavian SM, Ardeshiri A, Hajarizadeh B. Prevalence of HCV, HBV and HIV infections among Hemophiliacs. Hakim Res J. 2003;2(6):45–51. [Google Scholar]
- 14.Alavian SM, Kabir A, Ahmadi AB, Lankarani KB, Shahbabaie MA, Ahmadzad-Asl M. Hepatitis C infection in hemodialysis patients in Iran: a systematic review. Hemodial Int. 2010;14(3):253–62. doi: 10.1111/j.1542-4758.2010.00437.x. [DOI] [PubMed] [Google Scholar]
- 15.Mohtasham Amiri Z, Jafari shakib A, Torchi Rodsari M. Prevalence Hepatitis C and risk factors in hemodialysis patients. Payesh. 2003;2(4):291–5. [Google Scholar]
- 16.Joukar F, Besharati S, Mirpour H, Khoshsarvar M, Mansorghanaei F. Serum prevalence of hepatitis B and C on patients hemodialysis, Gilan. J Infect Dis Trop Med Assoc Infect Dis Special. 2010;15(50):19–23. [Google Scholar]
- 17.Kalantari H, Ebadi S, Yaran M, Maracy MR, Shahshahan Z. Prevalence and risk factors of hepatitis B and C viruses among hemodialysis patients in Isfahan, Iran. Adv Biomed Res. 2014;3:73. doi: 10.4103/2277-9175.125869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zahedi MJ, Darvish Moghaddam S, Alavian SM, Dalili M. Seroprevalence of Hepatitis Viruses B, C, D and HIV Infection Among Hemodialysis Patients in Kerman Province, South-East Iran. Hepat Mon. 2012;12(5):339–43. doi: 10.5812/hepatmon.5969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Su Y, Norris JL, Zang C, Peng Z, Wang N. Incidence of hepatitis C virus infection in patients on hemodialysis: a systematic review and meta-analysis. Hemodial Int. 2013;17(4):532–41. doi: 10.1111/j.1542-4758.2012.00761.x. [DOI] [PubMed] [Google Scholar]
- 20.Katayama K, Sato T, Do SH, Yamada H, Tabuchi A, Komiya Y, et al. Hepatitis B virus infection in hemodialysis patients in Japan: Prevalence, incidence and occult hepatitis B virus infection. Hepatol Res. 2015;45(12):1211–9. doi: 10.1111/hepr.12492. [DOI] [PubMed] [Google Scholar]
- 21.Sypsa V, Touloumi G, Papatheodoridis GV, Tassopoulos NC, Ketikoglou I, Vafiadis I, et al. Future trends of HCV-related cirrhosis and hepatocellular carcinoma under the currently available treatments. J Viral Hepat. 2005;12(5):543–50. doi: 10.1111/j.1365-2893.2005.00588.x. [DOI] [PubMed] [Google Scholar]