Abstract
Purpose
To demonstrate the feasibility of using DNP hyperpolarized [1-13C]-pyruvate to measure early response to temozolomide (TMZ) therapy using an orthotopic human glioblastoma xenograft model.
Materials and Methods
Twenty athymic rats with intracranial implantation of human glioblastoma cells were divided into two groups: one group received an oral administration of 100 mg/kg TMZ (n = 10) and the control group received vehicle only (n = 10). 13C 3D magnetic resonance spectroscopic imaging (MRSI) data were acquired following injection of 2.5 mL (100 mM) hyperpolarized [1-13C]-pyruvate using a 3T scanner prior to treatment (day D0), at D1 (days from treatment) or D2.
Results
Tumor metabolism as assessed by the ratio of lactate to pyruvate (Lac/Pyr) was significantly altered at D1 for the TMZ-treated group but tumor volume did not show a reduction until D5 to D7. The percent change in Lac/Pyr from baseline was statistically different between the two groups at D1 and D2 (P < 0.008), while percent tumor volume was not (P > 0.2).
Conclusion
The results from this study suggest that metabolic imaging with hyperpolarized [1-13C]-pyruvate may provide a unique tool that clinical neuro-oncologists can use in the future to monitor tumor response to therapy for patients with brain tumors.
Keywords: dynamic nuclear polarization, hyperpolarized 13C MRSI, brain tumor, response to temozolomide
The general course of treatment for patients with brain tumors consists of surgery, radiation therapy, and chemotherapy with the goal of stopping tumor growth by inhibiting cell proliferation or inducing cell death. Temozolomide (TMZ) is one of the most frequently used chemotherapeutic drugs for treating brain tumors, especially high-grade gliomas. It is an alkylating agent and works by damaging DNA and thus triggering the death of tumor cells.
The current standard for monitoring brain tumor response to therapy is a combination of clinical symptoms and Macdonald criteria, which are based on the change in contrast enhancement from magnetic resonance imaging (MRI) or computed tomography (CT) scans (1). Advanced MR techniques such as 1H magnetic resonance spectroscopy (MRS), perfusion MRI, and diffusion-weighted MRI have also been used for monitoring brain tumor response to therapy (2–7), but may take several weeks to detect response to therapy. Early detection of brain tumor response to treatment would be valuable in defining criteria for continuing or modifying treatment strategies.
Positron emission tomography (PET) is an imaging modality that has been widely used in the clinic for predicting treatment effects. Although the ability to detect altered 18F-fluoro-dexoyglucose (FDG) uptake in cancerous tissue has made this technique useful in some areas of oncology (8), its use in neuro-oncology presents unique challenges due to the high background signal from normal gray matter (9).
Dynamic nuclear polarization (DNP) and the recent development of a dissolution process that retains polarization in liquid state have allowed for the acquisition of 13C MRS data with more than 10,000-fold increase in sensitivity (10). Preclinical 13C MRS of hyperpolarized [1-13C]-pyruvate has been applied to the investigation of in vivo metabolism in subcutaneous lymphoma (11), prostate cancer (12,13), cardiac ischemia (14), and liver cancer (15). A recent study has demonstrated that this technique can be used for differentiating brain tumors from normal tissue in an animal model of brain cancer (16). The purpose of this study was to demonstrate the feasibility of using DNP-hyperpolarized [1-13C]-pyruvate to measure response to chemotherapy using TMZ in an orthotopic human glioblastoma xenograft model. Our emphasis was to establish the time frame of changes in 13C imaging parameters and to compare the results with alterations in tumor volume over time.
MATERIALS AND METHODS
Cell Culture
U-87 MG human glioblastoma multiforme (GBM) cell lines obtained from the Tissue Bank in our institution were maintained as exponentially growing monolayers in complete medium consisting of Eagle’s minimal essential medium with 10% fetal calf serum and 1% nonessential amino acids. Cells were cultured at 37°C in a humidified atmosphere consisting of 95% air and 5% CO2. Cells were harvested by trypsinization, washed once with Hanks’ Balanced Salt Solution, and resuspended in Hanks’ Balanced Salt Solution for tumor implantation.
Intracerebral Tumor Implantation
The details of intracerebral implantation procedure have been described elsewhere (16). In brief, 7-week-old male athymic rats (rnu/rnu, homozygous) purchased from Harlan (Indianapolis, IN) were housed under aseptic conditions with filtered air and sterilized food, water, bedding, and cages. For implantation, rats were anesthetized with an intraperitoneal injection of ketamine (60 mg/kg) and xylazine (7.5 mg/kg). Ten μL of cell suspension (5 × 106 cells) were slowly injected into the right caudate-putamen of rat brain using an implantable guide-screw system. All protocols for animal studies were approved by the Institutional Animal Care and Use Committee in our institution.
Animal Population and Imaging Study Scheme
Twenty male athymic rats (median weight 267 g) with human GBM xenograft tumors (U-87 MG cell lines) were included in this study. Rats were randomly assigned to TMZ treatment (Temodar, Schering, Kenilworth, NJ) or vehicle control (Ora-Plus, Paddock Laboratories, North Minneapolis, MN) groups. All rats received treatment when the size of the tumor monitored by T2-weighted axial images was at least the size of one spectroscopic voxel, between the 9th to 15th day from the tumor implantation (Table 1). The TMZ-treated group (n = 10) received a single dose of 100 mg/kg TMZ by oral gavage while the control group (n = 10) received 1 mL of vehicle only. TMZ was dissolved in vehicle using a homogenizer and delivered to the animals in approximately 1 mL of vehicle. All animals underwent 13C and 1H imaging study before treatment (day D0), at D1 (days from treatment) and/or D2 and at several subsequent timepoints. The D0 scan served as a baseline. Five treated and five control rats were sacrificed at D2 and the tumor tissue from their brains analyzed for immunohistochemistry. Two treated and five control rats were monitored until they exhibited neurologic symptoms indicative of deteriorating body condition and were then euthanized. Three treated rats were sacrificed between D7 and D16. Table 1 shows a summary of the animals included in this study, the timing of their imaging studies, and the endpoint for each animal.
Table 1.
Summary of Rats Included in the Study
| Rat ID | Group | Tx date from tumor implantation (days) | Weight at Tx (g) | Imaging time points between D0 and D7 (days from Tx) | Study endpoint (days from Tx) |
|---|---|---|---|---|---|
| T1 | Treated | 14 | 220 | D0, D1, D3, D6 | D38 |
| T2 | Treated | 15 | 290 | D0, D1 | D34 |
| T3 | Treated | 10 | 260 | D0, D1, D2, D5, D7 | D7 |
| T4 | Treated | 10 | 270 | D0, D1, D4, D6 | D14 |
| T5 | Treated | 10 | 300 | D0, D1, D2 | D2 |
| T6 | Treated | 12 | 240 | D0, D1, D2, D4, D6 | D16 |
| T7 | Treated | 13 | 310 | D0, D1, D2 | D2 |
| T8 | Treated | 15 | 280 | D0, D1, D2 | D2 |
| T9 | Treated | 14 | 300 | D0, D1, D2 | D2 |
| T10 | Treated | 10 | 250 | D0, D2 | D2 |
| C1 | Control | 14 | 200 | D0, D2, D5, D7 | D8 |
| C2 | Control | 10 | 270 | D0, D1, D4, D6 | D6 |
| C3 | Control | 12 | 270 | D0, D1, D2 | D2 |
| C4 | Control | 11 | 230 | D0, D1, D2 | D2 |
| C5 | Control | 13 | 260 | D0, D1, D2 | D2 |
| C6 | Control | 12 | 280 | D0, D1, D2 | D2 |
| C7 | Control | 9 | 290 | D0, D1, D2, D5 | D5 |
| C8 | Control | 10 | 320 | D0, D1, D5 | D5 |
| C9 | Control | 9 | 250 | D0, D2, D5, D6 | D6 |
| C10 | Control | 13 | 250 | D0, D2 | D2 |
Tx, treatment.
Polarization Procedure
A mixture of 32 μL (approximately 40 mg) [1-13C]-pyruvate (Isotec, Miamisburg, OH) and 15 mM OX63 trityl radical, along with 0.5 mM (approximately 0.47 μL) of Prohance gadolinium for polarization enhancement (17), was hyperpolarized using a HyperSense DNP polarizer (Oxford Instruments, Abingdon, UK) in a field of 3.35T at approximately 1.4°K by irradiation with 94.1 GHz microwaves using methods described previously (10). After approximately 60 minutes of microwave irradiation, the hyperpolarized pyruvic acid was rapidly dissolved in a saline solution with 5.96 g/L Tris (40 mM), 4.00 g/L NaOH (100 mM), and 0.1 mg/L Na2 EDTA (ethylenediaminetetraacetic acid). The final dissolved solution had a concentration of 100 mM, pH approximately 7.5, and a polarization of approximately 25%. The degree of polarization was measured by taking a small aliquot and injecting it into a custom-built polarimeter approximately 15 seconds after dissolution. Approximately 2.8 mL of this solution was then injected into the tail vein of a rat within 10 seconds of removal from the polarizer. Taking into account the volume of the catheter, which is 0.3 mL, approximately 2.5 mL of the dissolved solution was delivered to the animals. The injection lasted 10 seconds.
1H and 13C MR Imaging
Experiments were performed using a 3T GE Signa scanner (GE Healthcare, Milwaukee, WI) equipped with the multinuclear spectroscopy (MNS) hardware package. The radiofrequency (RF) coil used in these experiments was a dual-tuned 1H-13C coil with a quadrature 13C channel and linear 1H channel that had an 8 cm inner coil diameter and 9 cm length constructed based on an earlier design (18).
Before each imaging experiment, the rat was placed on a heated pad and anesthetized with isoflurane (2%–3%). A catheter was placed into the tail vein for the intravenous administration of hyperpolarized pyruvate solution. The rat was transferred to a heated pad positioned in the RF coil in the MR scanner. Anesthesia was continued with a constant delivery of isoflurane (1%–2%) through a long tube to a cone placed over the rat’s nose and mouth while the rat was in the scanner. The body temperature was maintained at 37°C throughout the imaging procedures by maintaining a flow of heated water through the pad underneath the rat.
Prior to each 13C imaging, high-resolution T2-weighted anatomical images were obtained in the axial plane using a fast spin-echo (FSE) sequence. These axial images were acquired in 13 minutes with an 8 cm field of view (FOV), 192 × 192 matrix, 1.5 mm slice thickness, and 8 NEX (TE/TR = 60/4000 msec). The 13C 3D magnetic resonance spectroscopic imaging (MRSI) data were acquired using a double spin-echo sequence, which has been described in detail previously (19). In brief, the sequence consists of a slice-selective variable small flip angle excitation pulse and a pair of nonlocalized 180° hyperbolic secant refocusing pulses. A 10 × 8 × 1 phase encoding matrix with fly-back readout on the z-axis (10 × 8 × 16 effective matrix) with 40 mm × 32 mm × 86.4 mm FOV rendered a 4 mm × 4 mm × 5.4 mm spatial resolution (0.086 cm3 voxel resolution). The data were acquired in 17 seconds with TE/TR = 140/215 msec, 581 Hz spectral bandwidth, and 59 spectral points. The MRSI acquisition began 20 seconds after the start of the injection of hyperpolarized 13C-pyruvate in order to obtain data while the hyperpolarized 13C-lactate was at a maximum (16). Centric k-space encoding and variable flip angle acquisition schemes were used to enable the efficient use of the hyperpolarized magnetization (20).
Anatomical images were obtained using an axial T1-weighted spin-echo sequence after the injection of 0.1 mL gadolinium (Gd)-DTPA (approximately 0.2 mmol/kg, diluted 1:2 with saline). These were acquired in 13.5 minutes with an 8 cm FOV, 320 × 192 matrix, 1.2 mm slice thickness, and 6 NEX (TE/TR = 10/700 msec).
Data Analysis
The methods for processing 13C MRSI data have been described previously (12,21). The raw readout data were reordered into a 4D array. Only the k-space data from flat parts of the flyback trajectory were selected. The time domain signal was apodized by a 16-Hz Gaussian filter and zero-filled to 256 points. A 4D Fourier transform was used to produce a 3D spatial array of spectra. An additional linear phase correction was applied in the flyback dimension to correct for the offset of individual k-space points (22).
The ratio of lactate over pyruvate (Lac/Pyr) was estimated from the 13C magnitude spectra in order to assess an early treatment response. Lac/Pyr was calculated from a voxel containing Gd-enhanced brain tissue in the T1-weighted post-Gd image of the rat brains. The carbon spectra were voxel-shifted in order to minimize partial volume effects by applying linear phase to the k-space data as described by the Fourier shift theorem (23). In order to estimate the variation of 13C metabolism over time, a percent Lac/Pyr change from baseline was calculate using the following formula:
| [1] |
where (Lac/Pyr)Di and (Lac/Pyr)D0 are Lac/Pyr at a certain timepoint i and at baseline, respectively. The percent change in Lac/Pyr from baseline at D1 and D2 were compared between the rats in the treated and control groups using a Mann–Whitney rank-sum test.
In order to estimate tumor volume, the 3D volume of the contrast enhancing lesion was calculated from the axial T1 post-Gd slices using the method described previously (21). At each time point a percent tumor volume change from baseline was calculated using the same method as the Eq. [1].
Immunohistochemistry
The brains were routinely fixed in phosphate-buffered 10% formalin, dehydrated by graded ethanols, and embedded in Paraplast Plus wax (McCormick Scientific). Tissue sections were incubated with 0.8 μg/mL of rabbit polyclonal cleaved caspase-3 (Asp175) antibody (Cell Signaling Technology, Beverly, MA) for 32 minutes at 37°C. Antigen retrieval for cleaved cas-pase-3 was performed for 8 minutes in Tris buffer (pH 8) at 90°C. Sections were subsequently treated with a 3% methanol-hydrogen peroxide solution at 22°C for 16 minutes. Nuclei were counterstained with hematoxylin. All immunohistochemistry assays were performed on the Benchmark XT (Ventana Medical Systems, Tucson, AZ) using the iView detection system.
RESULTS
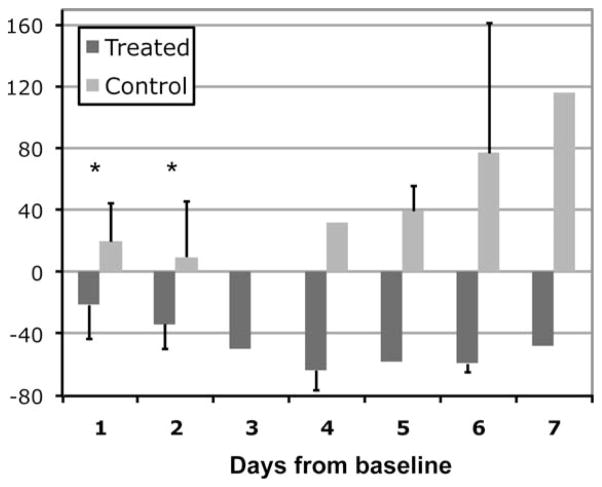
For the treated group the tumor metabolism as measured by the 13C metabolic ratio was altered as early as 1 day after TMZ treatment (Fig. 1). At baseline, both the treated and control rats exhibited elevated levels of Lac/Pyr with mean Lac/Pyr of 1.1 (SD = 0.5). For the group treated with TMZ, Lac/Pyr showed a mean 21% reduction at D1 (SD = 22) and a mean 34% reduction at D2 (SD = 16) compared to baseline values (Fig. 1). In contrast, Lac/Pyr of the control rats was continuously elevated following administration of vehicle, showing a mean 20% (SD = 25) and 16% (SD = 34) increase compared to baseline values at D1 and D2, respectively (Fig. 1). Lac/Pyr was statistically different between the treated (n = 9) and control (n = 7) groups at D1 (P < 0.008). This pattern continued at D2 (P < 0.002) between the two groups (n = 7 for treated rats and n = 8 for control rats). Lac/Pyr for the control group was progressively elevated over time, while levels of Lac/Pyr stabilized for the treated group after D5 (Fig. 1).
Figure 1.
Percent change in Lac/Pyr from baseline for the treated and control groups between 1 to 7 days following treatment. The 13C metabolism measured by this parameter showed a significant difference between the two groups at days 1 and 2 (*P < 0.008). The statistical test was confined to days 1 and 2 due to the lack of data at other timepoints.
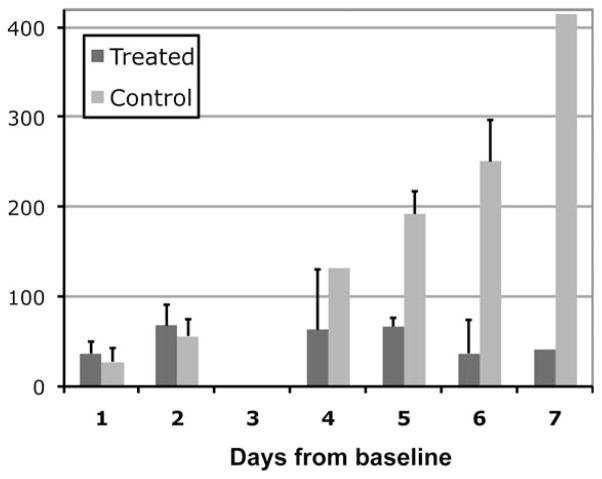
In contrast to the findings from 13C MRSI data, both the treated and control rats showed a similar increase in tumor volume for the first few time points (Fig. 2). The treated group showed a mean 37% (SD = 13) and 68% (SD = 23) increase in their tumor volume at D1 and D2 while the control group showed a mean increase of 28% (SD = 15) and 56% (SD = 19) at D1 and D2. There were no significant differences in the change of tumor volume between the two groups either at D1 (P > 0.1) or at D2 (P > 0.2). The volume of tumor in the treated rats showed a steady increase in the first 2 days and then started to decrease at approximately the 5th day after the initiation of treatment. In contrast, the tumor volume of the control rats continued to increase (Fig. 2).
Figure 2.
Percent tumor volume change from baseline for the treated and control groups between 1 to 7 days following treatment. Both groups showed an increase in their tumor volume at days 1 and 2 (P > 0.2). The statistical test was confined to days 1 and 2 due to the lack of data at other timepoints.
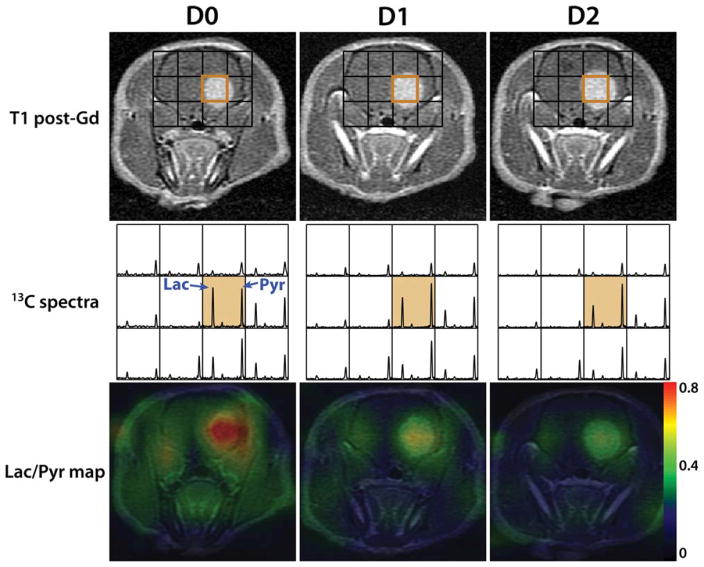
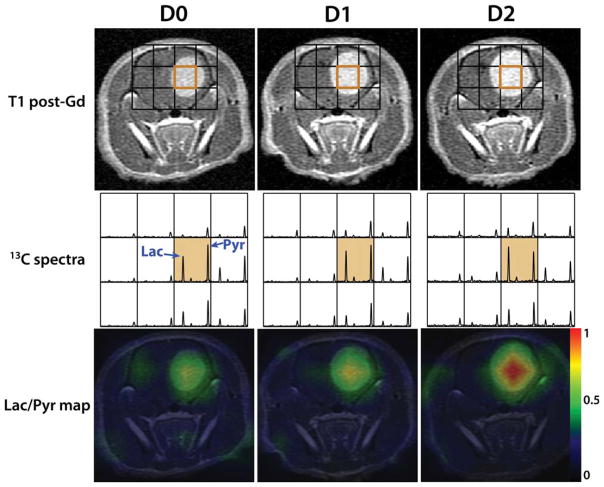
Figures 3 and 4 are examples from a treated and a control rat, depicting the morphological and metabolic changes that occurred shortly after treatment. T1 post-Gd images, 13C spectra and Lac/Pyr overlay map from D0, D1, and D2 scans are displayed. Lac/Pyr overlay maps were generated by spatially interpolating Lac/Pyr values to the resolution of anatomical images using a linear kernel and overlaying it on the anatomical images. In both examples the tumor volume continues to increase over time. The lactate peak in the orange voxel, which contained the region of tumors, decreased shortly after the treatment for the treated rat, while the pyruvate peak remained almost constant (Fig. 3). In contrast, the lactate peak of the control rat showed a relative increase after receiving vehicle (Fig. 4) and Lac/Pyr progressively increased. The Lac/Pyr maps reiterated these findings for both groups.
Figure 3.
An example of a treated rat, showing its T1 post-Gd images, 13C spectra zoomed-in around brain, and Lac/Pyr overlay map at D0 (pre-treatment), D1 (1 day after the initiation of treatment) and D2 scan. The lactate peak decreased shortly after the treatment, resulting in a drastic drop in Lac/Pyr.
Figure 4.
An example of a control rat, showing its T1 post-Gd images, 13C spectra zoomed-in around brain and Lac/Pyr overlay map at D0 (pre-treatment), D1 (1 day after the treatment initiation) and D2 scan. The lactate peak continued to increase relatively to the pyruvate peak after the treatment, resulting in a steep increase in Lac/Pyr.
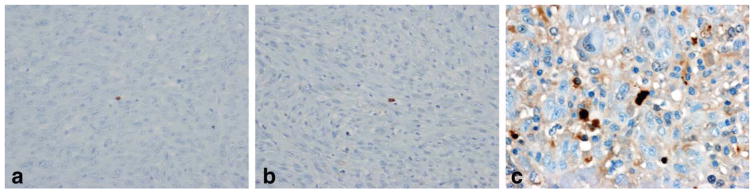
The results from the immunohistochemical analysis of tumor tissue showed different levels of caspase-3 staining for the rats sacrificed at different time points (Fig. 5). Figure 5a,b are caspase-3 stained slices from a control rat (C3) and a treated rat (T9) sacrificed at D2, respectively. A very small amount of stained cells appeared at these tumors. In contrast, there were a large number of stained cells for the slice from a treated rat (T3) that was sacrificed at D7, indicating that a greater degree of apoptosis had been induced by DNA alkylation (Fig. 5c).
Figure 5.
Immunohistochemical staining of caspase-3 for a control rat sacrificed at D2 (a, 20× magnification), a treated rat sacrificed at D2 (b, 20× magnification) and a treated rat sacrificed at D7 (c, 40× magnification).
DISCUSSION
This study demonstrated the feasibility of detecting early response to TMZ treatment in human glioblastoma orthotopic xenografts using hyperpolarized 13C MRSI with [1-13C]-pyruvate as a substrate. The 13C data from the treated rats showed the ability to detect altered tumor metabolism as early as 1 day after TMZ therapy initiation (Fig. 1), while their tumor volume from T1 post-Gd imaging did not show a reduction until 5 to 7 days after treatment (Fig. 2). The percent change in Lac/Pyr from baseline was statistically different between the two groups at D1 and D2 (P < 0.008), while the percent tumor volume change from baseline was not (P > 0.1).
Seven of the 9 treated rats exhibited a reduction in Lac/Pyr at day 1 after the initiation of treatment. The other two rats showed an increase of 6% and 16% at day 1 but a decrease of −11% and −27% at day 2 compared to baseline, respectively. Lac/Pyr of all control rats (n = 10) exhibited an increase over time (Fig. 1). The substantial difference in 13C metabolism between the two groups at this early stage following treatment suggests that this method has great potential for early monitoring response to TMZ chemotherapy in brain tumors.
Early detection of treatment response is critical for determining whether patients should remain on a specific therapy or not. Effective treatment is often complicated for patients with GBM by its infiltrative nature, which constrains the ability of surgeons to safely perform a complete resection. TMZ is typically given to patients after surgery in an attempt to kill the remaining tumor cells. The heterogeneous nature of GBM makes it difficult to predict the outcome of TMZ therapy (24,25). The ability to noninvasively detect tumor response to TMZ therapy may therefore assist in providing more personalized treatment strategies and therefore improve the management of patients with GBM.
To the best of our knowledge, this is the first report of a noninvasive imaging modality that detected response to TMZ therapy 1 day after the initiation of the treatment. A recent study used the same xenograft model of rat brain (U-87 MG) to evaluate TMZ therapy using anatomical MRI, diffusion MRI, and 1H MRS (26). Authors from that study reported that there was a 34% increase in apparent diffusion coefficient (ADC) of the treated group compared to the control group at 11 days after the initiation of the treatment. They also reported a 3-fold increase in NAA-to-total choline ratio (NAA/tCho) from the treated group and a 5-fold decrease in NAA/tCho from the control group that occurred between the 12th and 23rd day from baseline. The tumor volume measured from T2-weighted MRI showed a reduction at D7, which is consistent with our study, where the tumor volume measured from T1-weighted post-Gd images started to shrink between D5 to D7 (Fig. 2). Morphological evidence of tumor reduction at these time points was consistent with the finding from immunohistochemical analysis of a rat sacrificed at D7, which exhibited widespread apoptotic cells (Fig. 5).
In conclusion, the results from this study suggest that metabolic imaging with hyperpolarized [1-13C]-pyruvate may provide a unique tool that clinical neuro-oncologists can use in the future to monitor tumor response to therapy for patients with brain tumors. Future studies will examine the application of this technology to human subjects and will investigate whether similar results can be found using other treatments.
Acknowledgments
The authors thank Kristen Scott and Myriam Chaumeil for assistance with the experiments.
Contract grant sponsor: Academic-industry partnership grant from the UC Discovery program in conjunction with GE Healthcare; Contract grant number: K08 NS063456-03 (to J.J.P.).
References
- 1.Macdonald DR, Cascino TL, Schold SC, Jr, Cairncross JG. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol. 1990;8:1277–1280. doi: 10.1200/JCO.1990.8.7.1277. [DOI] [PubMed] [Google Scholar]
- 2.Walecki J, Tarasow E, Kubas B, et al. Hydrogen-1 MR spectroscopy of the peritumoral zone in patients with cerebral glioma: assessment of the value of the method. Acad Radiol. 2003;10:145–153. doi: 10.1016/s1076-6332(03)80038-7. [DOI] [PubMed] [Google Scholar]
- 3.Balmaceda C, Critchell D, Mao X, et al. Multisection 1H magnetic resonance spectroscopic imaging assessment of glioma response to chemotherapy. J Neurooncol. 2006;76:185–191. doi: 10.1007/s11060-005-5261-2. [DOI] [PubMed] [Google Scholar]
- 4.Leimgruber A, Ostermann S, Yeon EJ, et al. Perfusion and diffusion MRI of glioblastoma progression in a four-year prospective temozolomide clinical trial. Int J Radiat Oncol Biol Phys. 2006;64:869–875. doi: 10.1016/j.ijrobp.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 5.Akella NS, Twieg DB, Mikkelsen T, et al. Assessment of brain tumor angiogenesis inhibitors using perfusion magnetic resonance imaging: quality and analysis results of a phase I trial. J Magn Reson Imaging. 2004;20:913–922. doi: 10.1002/jmri.20202. [DOI] [PubMed] [Google Scholar]
- 6.Tomura N, Narita K, Izumi J, et al. Diffusion changes in a tumor and peritumoral tissue after stereotactic irradiation for brain tumors: possible prediction of treatment response. J Comput Assist Tomogr. 2006;30:496–500. doi: 10.1097/00004728-200605000-00024. [DOI] [PubMed] [Google Scholar]
- 7.Hamstra DA, Rehemtulla A, Ross BD. Diffusion magnetic resonance imaging: a biomarker for treatment response in oncology. J Clin Oncol. 2007;25:4104–4109. doi: 10.1200/JCO.2007.11.9610. [DOI] [PubMed] [Google Scholar]
- 8.Gambhir SS. Molecular imaging of cancer with positron emission tomography. Nat Rev Cancer. 2002;2:683–693. doi: 10.1038/nrc882. [DOI] [PubMed] [Google Scholar]
- 9.Wong TZ, van der Westhuizen GJ, Coleman RE. Positron emission tomography imaging of brain tumors. Neuroimaging Clin N Am. 2002;12:615–626. doi: 10.1016/s1052-5149(02)00033-3. [DOI] [PubMed] [Google Scholar]
- 10.Ardenkjaer-Larsen JH, Fridlund B, Gram A, et al. Increase in signal-to-noise ratio of > 10,000 times in liquid-state NMR. Proc Natl Acad Sci U S A. 2003;100:10158–10163. doi: 10.1073/pnas.1733835100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Day SE, Kettunen MI, Gallagher FA, et al. Detecting tumor response to treatment using hyperpolarized 13C magnetic resonance imaging and spectroscopy. Nat Med. 2007;13:1382–1387. doi: 10.1038/nm1650. [DOI] [PubMed] [Google Scholar]
- 12.Chen AP, Albers MJ, Cunningham CH, et al. Hyperpolarized C-13 spectroscopic imaging of the TRAMP mouse at 3T-initial experience. Magn Reson Med. 2007;58:1099–1106. doi: 10.1002/mrm.21256. [DOI] [PubMed] [Google Scholar]
- 13.Albers MJ, Bok R, Chen AP, et al. Hyperpolarized 13C lactate, pyruvate, and alanine: noninvasive biomarkers for prostate cancer detection and grading. Cancer Res. 2008;68:8607–8615. doi: 10.1158/0008-5472.CAN-08-0749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Golman K, Petersson JS, Magnusson P, et al. Cardiac metabolism measured noninvasively by hyperpolarized 13C MRI. Magn Reson Med. 2008;59:1005–1013. doi: 10.1002/mrm.21460. [DOI] [PubMed] [Google Scholar]
- 15.Hu S, Lustig M, Balakrishnan A, et al. 3D compressed sensing for highly accelerated hyperpolarized (13)C MRSI with in vivo applications to transgenic mouse models of cancer. Magn Reson Med. 2010;63:312–321. doi: 10.1002/mrm.22233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Park I, Larson PE, Zierhut ML, et al. Hyperpolarized 13C magnetic resonance metabolic imaging: application to brain tumors. Neuro Oncol. 2010;12:133–144. doi: 10.1093/neuonc/nop043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ardenkjaer-Larsen JH, Macholl S, Johannesson H. Dynamic nuclear polarization with trityls at 1.2 K. Appl Magn Reson. 2008;34:509–522. [Google Scholar]
- 18.Derby K, Tropp J, Hawryszko C. Design and evaluation of a novel dual-tuned resonator for spectroscopic imaging. J Magn Reson. 1990;86:645–651. [Google Scholar]
- 19.Cunningham CH, Chen AP, Albers MJ, et al. Double spin-echo sequence for rapid spectroscopic imaging of hyperpolarized 13C. J Magn Reson. 2007;187:357–362. doi: 10.1016/j.jmr.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 20.Zhao L, Mulkern R, Tseng CH, et al. Gradient-echo imaging considerations for hyperpolarized 129Xe MR. J Magn Reson B. 1996;113:179–183. [PubMed] [Google Scholar]
- 21.Nelson SJ. Analysis of volume MRI and MR spectroscopic imaging data for the evaluation of patients with brain tumors. Magn Reson Med. 2001;46:228–239. doi: 10.1002/mrm.1183. [DOI] [PubMed] [Google Scholar]
- 22.Cunningham CH, Vigneron DB, Chen AP, et al. Design of flyback echo-planar readout gradients for magnetic resonance spectroscopic imaging. Magn Reson Med. 2005;54:1286–1289. doi: 10.1002/mrm.20663. [DOI] [PubMed] [Google Scholar]
- 23.Haacke EM, Brown RW, Thompson MR, Venkatesan R. Magnetic Resonance Imaging: physical principles and sequence design. 1. New York: Wiley-Liss; 1999. [Google Scholar]
- 24.Huang F, Kavan P, Guiot MC, Markovic Y, Roberge D. When temozolomide alone fails: adding procarbazine in salvage therapy of glioma. Can J Neurol Sci. 2008;35:192–197. doi: 10.1017/s0317167100008623. [DOI] [PubMed] [Google Scholar]
- 25.Brandes AA, Tosoni A, Basso U, et al. Second-line chemotherapy with irinotecan plus carmustine in glioblastoma recurrent or progressive after first-line temozolomide chemotherapy: a phase II study of the Gruppo Italiano Cooperativo di Neuro-Oncologia (GICNO) J Clin Oncol. 2004;22:4779–4786. doi: 10.1200/JCO.2004.06.181. [DOI] [PubMed] [Google Scholar]
- 26.Walker P, Tizon X, Parfait S, et al. Evaluation of early response to temozolomide and radiotherapy with magnetic resonance imaging and proton magnetic resonance spectroscopy in human glioma models in nude rats. Proceedings of the 99th Annual Meeting of AACR; San Diego. 2008; abstract 3733. [Google Scholar]