Abstract
Background
A new post–myocardial infarction (MI) therapy is injection of high-water content polymeric biomaterial gels (hydrogels) into damaged myocardium to modulate cardiac negative remodeling and preserve heart function.
Methods
We investigated the therapeutic potential of a novel gelatinized alginate hydrogel with a unique microstructure of uniform capillary-like channels (termed Capgel). Shortly (48 hours) after induced anterior MI, Sprague Dawley rats received intramyocardial injection of Capgel directly into the antero-septal wall at the infarct border zone (n=12) or no injection (n=10, controls). Echocardiograms were performed at 48h (week 0) and 4 weeks (week 4) to evaluate left ventricular function.
Results
Echocardiograms showed 27% improvement of left ventricular systolic function over time with gel injection: fractional shortening increased from 26±3% at week 0 to 33±2% at week 4 (p=0.001). Capgel was present at the injection site after 4 weeks, but was minimal at 8 weeks. The remaining gel was heavily populated by CD68+ macrophages with CD206+ clusters and blood vessels. An in vitro experiment was performed to assess Angiotensin-(1-7) released from Capgel. Angiotensin-(1-7) was released from the Capgel in a sustained manner for 90 days.
Conclusions
Use of Capgel, a degradable, bioactive hydrogel composed of gelatinized capillary-alginate gel, appears safe for intramyocardial injection, is associated with improved left ventricular function after MI in rats, and may provide a long-term supply of Angiotensin-(1-7).
Keywords: gelatinized alginate hydrogel, capillary-like channels, myocardial infarction
INTRODUCTION
Despite advancement in pharmacological therapies for ischemic heart disease, post-myocardial infarction (MI) management remains challenging. Among novel therapeutic strategies developed to improve tissue salvage and attenuate adverse remodeling after MI, biomaterial has demonstrated great potential for cardiac tissue repair and regeneration [1]. Alginate, an algae-derived polysaccharide, appears feasible due to its biocompatible, non-toxic, and non-immunogenic characteristics and has been approved by Food and Drug Administration for various medical applications [2]. At present, two modalities of injectable alginate-based gels with encouraging preclinical results have reached clinical trials. Alginate-based solutions that gel in-situ and administer via intracoronary injection [3,4] are currently under clinical investigation in patients with acute MI [5]; however, 6 months after injection the alginate-based solution did not show superior beneficial effects compared with a saline injection to prevent left ventricular (LV) remodeling [Zeymer et al. PRESERVATION I: bioabsorbable cardiac matrix for the prevention of remodeling of the ventricle after large ST-segment elevation myocardial infarction. Abstract presented at European Society of Cardiology 2015 Congress; 2015 Sept 1; London, UK]. A different modality of injectable alginate hydrogel, Algisyl-LVR™, in solid form and administered via intramyocardial injection improves heart function and prevents progressive remodeling in dogs with advanced heart failure [6]. Clinical benefits of Algisyl-LVR™ suggesting its ability to modify remodeling were reported among patients with severe heart failure in the AUGMENT-HF trial [7]. Early preclinical and clinical trial results support the promising therapeutic potential of alginate-based approaches for myocardial repair and regeneration.
We have recently developed a novel hydrogel composed of gelatinized alginate with parallel capillary-like channels (hereafter termed Capgel). The present study evaluated the safety and therapeutic potential of Capgel as an injectable system to modify LV function in a rat model of MI. This hydrogel was designed to provide a substrate for endogenous cell homing and growth, as well as a possible reservoir to deliver therapeutic cells or agents. Accordingly, we hypothesized that the unique microstructure of continuous, parallel tubular channels will serve as a 3D matrix to facilitate repair by enhancing cell penetration, tissue formation, and vehicles to deliver therapeutics.
MATERIALS and METHODS
All experiments were performed under the approval of the University of Florida IACUC and in accordance with National Institutes of Health guidelines. All authors have read and agree with the contents of the manuscript.
Preparation of injectable Capgel and characterization of Capgel mechanical properties
Keltone® LVCR sodium alginate was kindly provided by ISP Alginates (now FMC Bioploymers, Philadelphia, PA). All other chemicals used were purchased from Fisher Scientific (Pittsburgh, PA) and Sigma-Aldrich (St. Louis, MO). Capgel was synthesized as previously described [8]. Briefly, a solution of 2% (w/v) alginate and 2.6% (w/v) oligo-gelatin was poured into an alginate-coated glass petri dish; a primary membrane was formed on the top of the gel using a Kimwipe® soaked in 0.5M CuSO4. The dish was then submerged in a tank of 0.5M CuSO4 and undisturbed for at least 36h. The gel was then removed, rinsed extensively in distilled water, sectioned and crosslinked using N-ethyl-N′-(3-dimethylamino propyl) carbodiimide hydrochloride (EDC, Sigma-Aldrich) and N-hydroxysulfosuccinimide (Sulfo-NHS, Pierce) chemistry at a mole ratio of 1:2:2 alginate/EDC/Sulfo-NHS in phosphate-buffered saline at 4°C for 21h. The crosslinked scaffolds were then rinsed extensively with sodium citrate followed by normal saline rinsing. Finally, these scaffolds were autoclaved in saline (121°C, 20 min) and then maintained at 4°C prior to animal applications. For the in vitro mechanical analysis, the gels were stored at 37°C in EBM2 basal media for 24h before testing. Mechanical properties of Capgel and heart slices collected from Sprague Dawley rats were measured using indentation technique as previously described [9].
Intramyocardial hydrogel injection in healthy rat
A total of 10 normal male Sprague Dawley (~300g) rats received intramyocardial injection (28G insulin needle) of either 1) saline (n = 5) or 2) Capgel (~20 μL, n = 5). Rats were anesthetized (2–3% isoflurane:oxygen mixture), intubated by mouth, placed on mechanical ventilation connected to a rodent ventilator (Harvard Apparatus model 683, Holliston, MA), and maintained on a 2–3% isoflurane:oxygen mixture. Under electrocardiographic monitoring, the chest of mechanically ventilated animals was then opened by left thoracotomy and the heart was exposed. Injections were performed in the mid-lateral anterior portion of LV wall. The chest was then closed, and the animals were allowed to recover, and then kept alive for an additional 4 weeks.
Rat myocardial infarction model and hydrogel injection
A total of 39 male Sprague Dawley rats (~300g) were anesthetized (2–3% isoflurane:oxygen mixture), then intubated by mouth, placed on mechanical ventilation connected to a rodent respirator (Harvard Apparatus model 683, Holliston, MA), and maintained on a 2–3% isoflurane:oxygen mixture. Acute MI was performed via permanent ligation of the left anterior descending (LAD) coronary artery [10]. Briefly, via left thoracotomy the pericardial sac was removed to expose the heart. A small suture anchor was made at the bottom of the cardiac apex to facilitate placement of a ligature around the LAD. After induced anterior MI (~15–20 min), animals assigned to the treatment group received a single injection of Capgel (~20 μL) into the antero-septal wall in the infarct border zone via a 28G needle. The infarct border zone was targeted for Capgel injection with the thought of limiting scar expansion and supporting stunned/hibernating myocardium via neovascularization and cell recruitment stimulated. Animals assigned to the control group were handled in an identical fashion but did not receive any injection because prior studies in this model in our lab showed that a saline control injection using the same volume (20 μL) was not associated with myocardial alterations at 4 weeks. Overall, 39 rats were included in the study. Early mortality (during or shortly after surgery), but before treatment assignment, was 35.9% (n=14). Two other rats died within 24h after surgery: one assigned to Capgel and the other assigned to the control group. Another animal was subsequently excluded when no evidence of MI-related LV dysfunction (fractional shortening >45%) was present at week 0 echo. Thus, 22 animals with post-MI LV dysfunction were available for echo evaluation at week 4 and comprise the data presented below: 12 were in the Capgel group and 10 in the control group.
Echocardiographic assessment
Echocardiographic data (echo) were collected using methodology as previously described [10]. To examine LV diastolic function, mitral inflow velocity was obtained by pulsed-wave Doppler interrogation in an apical view. Image acquisition and data analysis were performed by an experienced technician and analyzed by a cardiologist expert in echocardiography masked to group assignment. For normal (healthy heart) rats, all animals were initially examined (pre-surgery) to establish baseline LV function and at 4 weeks post-procedure. For infarcted rats (MI model), echocardiographic data were collected 48h post-MI (week 0) and again 4 weeks later (week 4). All animals were sacrificed after final echo assessment. Echo-derived measurements from at least three consecutive cardiac cycles were averaged to quantify each variable.
Histological assessment
After sacrifice, all hearts were rapidly removed, fixed (4% paraformaldehyde), embedded in paraffin and sectioned (5μm) for staining. Staining included H&E for overall morphology, Trichrome for collagen, and a-smooth muscle actin (mouse anti-SMA, 1:600 Sigma-Aldrich, St. Louis, MO) immunostaining for blood vessels and myofibroblasts. Whole-slide images were captured using Aperio CS ScanScope® (Aperio, Vista, CA). Macrophage (Mac) populations were characterized with immunofluorescent staining using primary antibodies to CD68 (mouse anti-Pan Mac, 1:300, Abcam, Cambridge, MA) [10], CD86 (anti-rabbit M1 activated, 1:100, Abcam, Cambridge, MA), and CD206 (rabbit anti-M2 activated, 1:450, Abcam, Cambridge, MA) Mac markers. For optimal staining, CD68 required heat retrieval using Trilogy Solution (Cell Marque, Rocklin, CA) while CD206 performed best with Citra pH6.0 retrieval.
In vitro release studies of Angiotensin-(1-7) from the Capgel
0.9μg and 9μg of Angiotensin-(1-7) [Ang-(1-7)] was mixed with 20μl of Capgel, the mixture was incubated with 1.2ml PBS in a tube at 37°C. 10 μL of the solution was removed at 1 day and 90 days to quantify the Ang-(1-7) released from the Capgel and then Ang-(1-7) level was quantified using ELISA kit [11].
Statistical analysis
Data are expressed as mean ± SE and were analyzed by t test, one-way or two-way ANOVA where appropriate. Values of p <0.05 were considered statistically significant. All of the data were analysed using GraphPad Prism 5 software (GraphPad Prism Institute Inc).
RESULTS
Structure of capillary-alginate gel
The gelatin-alginate composition of Capgel, unlike the amorphous gel materials currently under study elsewhere for post-MI treatment [12,13,3,4,14], holds the microstructure of the capillary-like channels (Fig. 1A,B), which provides a large surface area for cell adhesion, penetration, tissue ingrowth, and a reservoir to retain therapeutic agents. Because cells actively sense and respond to their mechanical environment, the mechanical properties of Capgel must also be considered. Using a custom indentation system [9], we characterized polymerized Capgel and transverse ventricular slices of 12-week-old Sprague Dawley rats. We found that Capgel samples have a steady-state elastic modulus of 3.16 ± 0.60 kPa, while control Sprague Dawley rats have a steady-state elastic modulus of 26.10 ± 7.10 kPa (Fig. 1C).
Figure 1.
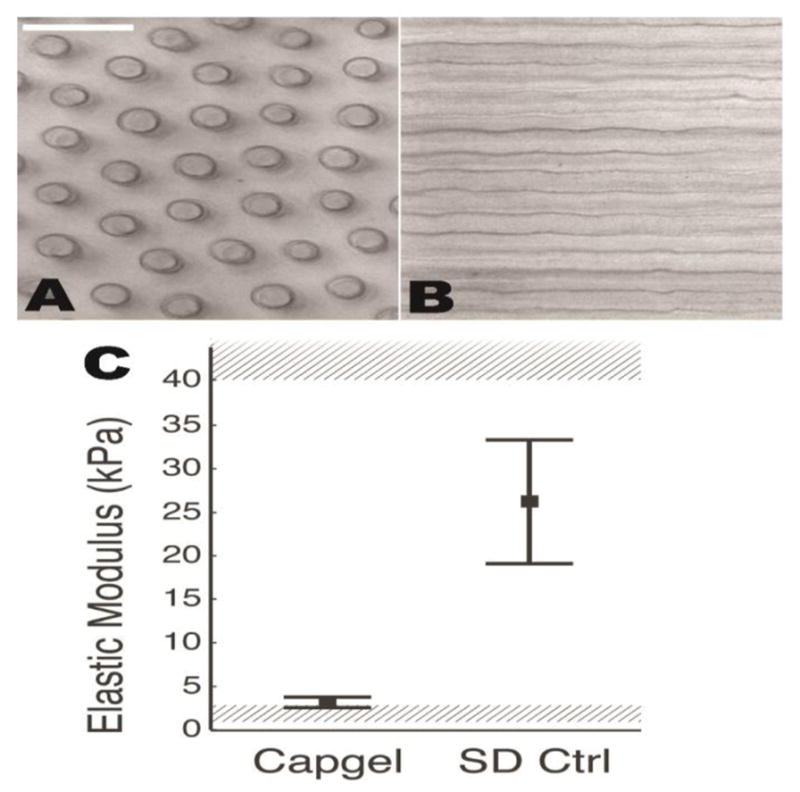
Image series of Capgel and cells cultured within the scaffold at different time points. (A,B) Phase-contrast images perpendicular (A) and parallel (B) to channel long axis. (C) Mechanical properties of Capgel are above baseline modulus shown to support cardiomyocyte function (1–3 kPa, lower shaded region,(Bhana et al., 2010; Engler et al., 2008; Jacot et al., 2008; Bajaj et al., 2010)and below fibrotic modulus shown to induce dysfunction (40–70 kPa, upper shaded region, Bhana et al., 2010; Engler et al., 2008; Jacot et al., 2008). Mean and standard deviation for polymerized Capgel (n = 49 indentations) and 12-week-old Sprague Dawley normal ventricular myocardium (16 indentations) are shown.
Safety and histology assessment after intramyocardial injection of Capgel in normal rats
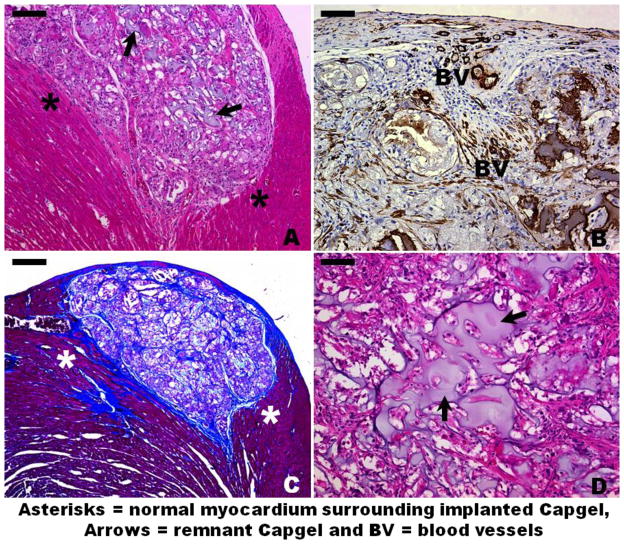
In healthy, non-infarcted rats Capgel appeared safe for intramyocardial injection. Intramyocardial injection did not result in arrhythmic events and all animals survived after Capgel injection. Capgel injection did not change either systolic or diastolic LV function indices at 4 weeks compared with the saline control group (Fig. 2), no significant differences in fractional shortening or mitrial vavle E/A ratio). Remnant gel could be observed at the injection site after 4 weeks (black arrows, Fig. 3A,D). Gomori’s Trichrome Staining showed web-like collagenous formations integrated throughout the Capgel implant area with the entire implant site enveloped by a continuous collagen rich capsule (blue, Fig. 3C). Blood vessels were observed both within and at the border of the gel at the implant site (BV, Fig. 3B). Myocardium bordering implanted gel appeared normal and the mild inflammatory response appeared well organized and very localized to the site of gel injection (asterisks, Fig. 3A,C). Results from this safety study therefore show that Capgel can be safely injected into the myocardium without apparent acute toxicity, tissue necrosis, mortality, or loss of function over time.
Figure 2.
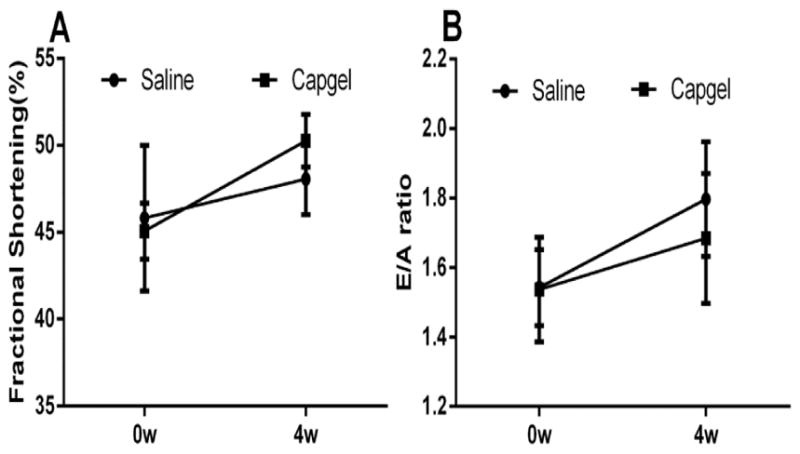
Intramyocardial administration of Capgel or saline did not change the heart function. Systolic funciton is assessed by fractional shortening (A) and diastolic funciton by mitral valve E/A ratio (B).
Figure 3.
Histological and immunohistochemical staining of Capgel implant sites in the LV myocardium at 4 weeks. (A) H&E staining of a subepicardial Capgel implant site. (B) Smooth muscle actin (SMA) staining within implanted Capgel. (C) Trichrome staining of a subepicardial Capgel implant site. Note the blue staining (collagen) to the left and below the Capgel implant site indicating some minor myocardial damage caused by the needle during injection. (D) Higher magnification H&E image showing well-cellularized remnants of implanted Capgel. Scale bar = 100 μm for A, 50 μm for B and D, and 200 μm for C.
Capgel improved cardiac function in rat model of MI
After MI, the control and Capgel injected animals had similar cardiac function at week 0 (Fig. 4A,B). However, the Capgel-treated rats showed 27% improvement in systolic function over time, as assessed by fractional shortening (from 26±3% to 33±2%, p<0.05, Fig. 4A). Capgel treatment did not alter the diastolic function as measured by mitral valve E/A ratio (Fig. 4B).
Figure 4.
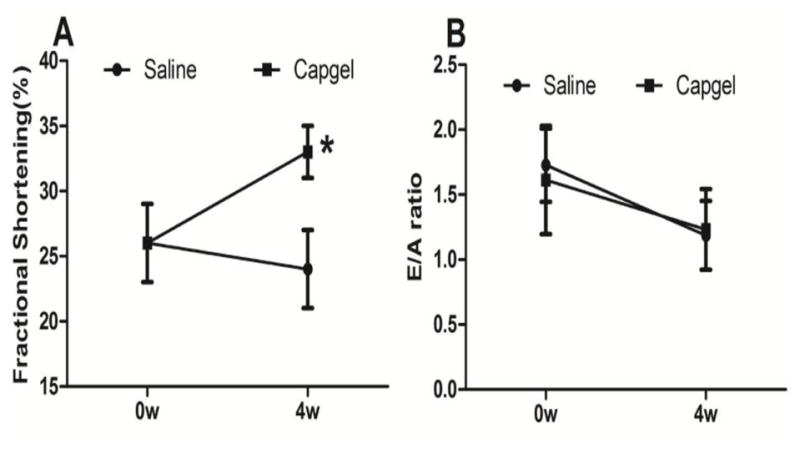
Capgel improved cardiac function. (A) fractional shortening and (B) Mitral valve E/A ratio were measured at 4 weeks after injection. *p<0.05.
Histological analysis of infarcted hearts
At the injection site in the anterior wall, Capgel was still observable after 4 weeks, but was present at much lower amounts at 8 weeks (Fig. 5A,B). The remaining gel was also heavily populated by CD68+ macrophages with CD206+ macrophage clusters and relatively fewer CD86+ macrophages (Fig. 5C–H). Blood vessels were observable within the gel areas at both 4-week (not shown) and 8-week (Fig. 5F–H) time points.
Figure 5.
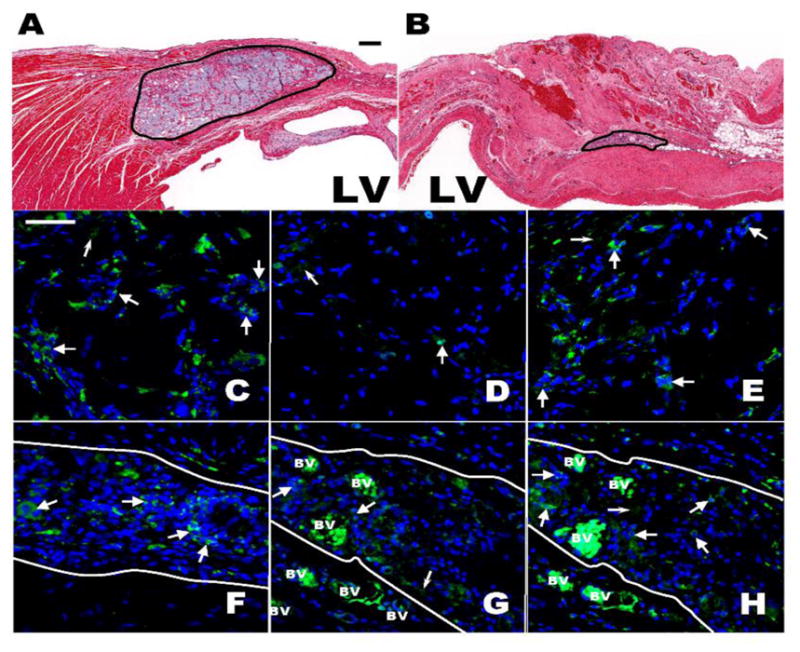
Histological and Immunofluorescent assesment 4 and 8 weeks post Capgel injection into the antero-septal LV Wall. (A,B) Brightfield micrograph of H&E stained antero-septal LV wall showing the Capgel area after 4 (A) and 8 (B) weeks; Scale bar = 200 μm for both. (C–H) Immunofluorescent staining (green) of macrophages populating the Capgel area 4 (C–E) and 8 (F–H) weeks post gel injection: CD68 (C,F), CD86 (D,G) and CD206 (E,H). Capgel area is between solid white lines (F–H). Large white arrow heads point to positively stained cells/clusters of cells for a given antibody; small white arrow heads point to nonspecific staining and/or autoflourescences of the injected Capgel. Blue = DAPI stained cell nuclei and BV = blood vessel; note the intense autofluorescence of RBCs within the vessels. Scale bar = 50 μm for all.
In vitro release of Angiotensin-(1-7) from the Capgel
Ang-(1-7) has been previously shown to be cardioprotective for the infarcted myocardium [15,16]. Ang-(1-7) is a small peptide and has short half-life after administered into animals [16]. Thus, temporally and spatially controlled delivery of Ang-(1-7) or other therapeutic compounds using Capgel could sustain local molecule release, prolong molecule bioactivity, and reduce the off-target complications. Capgel prolongs the Ang-(1-7) release at stable rates (1 day for 0.9 μg 180ng/ml, 1 day for 9 μg 200ng/ml, 90 days for 0.9 μg 600ng/ml, 90 days for 9 μg 4250ng/ml; Fig. 6).
Figure 6.
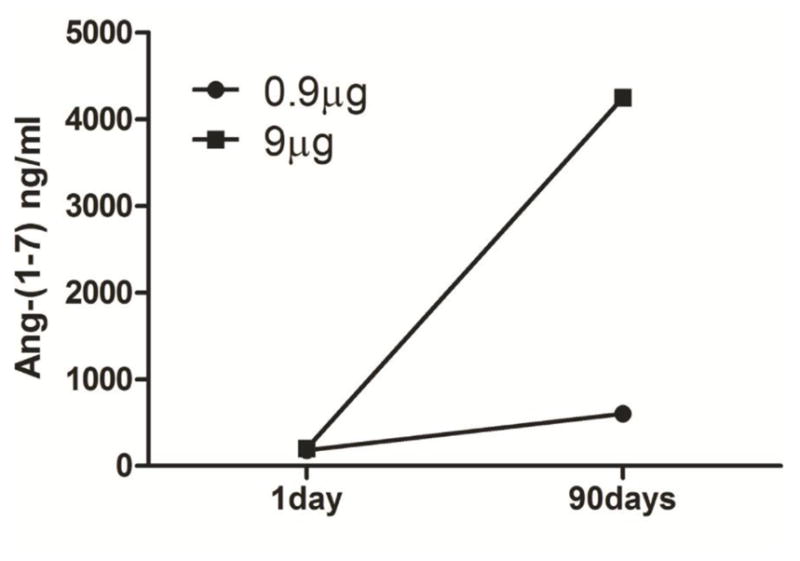
Release of Angiotensin-(1-7) from Capgel overtime. Ang-(1-7) concentration in the incubating solution was monitored at 1day and 90days.
DISCUSSION
In the present study, we investigated for the first time the effects of Capgel, a novel gelatinized capillary-alginate hydrogel, as an injectable tissue scaffolding system in a rat acute MI model. Capgel injected into the infarct border zone appeared safe and was not associated with increased mortality or morbidity over 4 weeks follow-up. Intramyocardial injection of Capgel after MI preserved infarct wall thickness and was associated with improved contractile function at week 4. Furthermore, this small amount of Capgel (20 μL) did not appear to alter LV diastolic properties (E/A ratio >1).
Utilizing our custom indentation system, we spatially characterized the Capgel and compared its mechanical properties to heart ventricle tissue. Capgel modulus is higher than gels previously demonstrated to support neonatal cardiomyocyte sarcomere organization and beating [17–19], suggesting mechanical properties of Capgel are adequate to support surrounding cell function. Both Capgel and normal SD heart moduli are well below published values for post-infarct myocardium of 40–70 kPa [18]. The stiffness and dysfunction of fibrotic, remodeled myocardium has been corroborated by in vitro studies showing decrease in cardiomyocyte sarcomere organization and function on substrates over ~50 kPa [20,17–19]. Thus, Capgel appears rigid enough to support cell function while soft enough to avoid fibrotic signals.
Previous studies have suggested that intracoronary or intramyocardial injection of alginate into the infarcted myocardium significantly reduces negative LV remodeling via passively thickening scar [12,21,13,3,4,22,14] and thereby reducing LV wall stress. There is however some question about the benefits over the long term as the material is cleared from the healing myocardium [22]. Others report that injecting decellularized ECM materials into ischemia-damaged LV has the potential to preserve LV function by promoting neovascularization and enhancing endogenous reparative cell recruitment, including macrophages and c-Kit+ cells [21,23]. Our histologic results indicate that Capgel becomes vascularized and is also populated by CD68+macrophages at both 4 and 8 weeks (Fig. 3C,F), suggesting a similar bioactivity to decellularized matrices. Macrophages populating the injected Capgel also appear to be largely CD206+ (Fig. 3E,H) as opposed to CD86+ (Fig 3D,G), indicating M2-type activation. This M2 (alternative) activation is more consistent with a regenerative healing response rather than classic inflammatory response, which is often adverse to reparative healing [14].
In addition to the local mechanical stabilization and recruitment of endogenous cells for repair, Capgel may be an excellent delivery vehicle for cells, biologics, and drugs. Gelatin incorporated into the formulation enables cell adhesion, and this new formulation cross-linked using EDC chemistry was implemented as an injectable stem cell delivery/retention system [23,24]. Capgel was previously utilized by our group for neuroregenerative tissue scaffolding and was also shown to support growth of mouse embryonic stem cells [8]. Capgel could be useful to deliver stem cells for cardiac repair or molecules that limit cell death, promote vessel formation, or recruit stem cells for repair [23]. Furthermore, our in vitro experiment had shown that Ang-(1-7) was conserved in the capillary structure of Capgel and a sustained Ang-(1-7) release from the Capgel continued for 90 days. These results further suggest that local delivery of Ang-(1-7) or other growth factors using Capgel may achieve a long-term supply of this cardioprotective peptide or growth factors that would induce better healing in vivo.
In conclusion, we have developed a new material, Capgel, for cardiac applications and established the safety and efficacy of Capgel in rat model of MI. This is a first application of this gel in cardiomyocardium environment. Capgel has a unique capillary structure and promotes healing in MI. Furthermore, Capgel is capable of providing a long-term supply of small peptides. Further studies to investigate the potential of Capgel to deliver stem cells and therapeutic compounds are warranted.
Acknowledgments
Sources of Funding
Drs. Carl Pepine and Chris Cogle are supported, in part, by the National Institutes of Health (NIH)/National Heart, Lung and Blood Institute award UM1 HL087366 to the Cardiovascular Cell Therapy Research Network, and the NIH/National Center for Advancing Translational Sciences award UL1 TR001427 to the University of Florida. Dr B Byrne is supported by University of Florida Center for Cell and Gene Therapy.
We thank Marda Jorgensen and McKnight Brain Institute Cell & Tissue Analysis Core (MBI-CTAC) for histology and staining services, and Mohan K. Raizada, PhD, Sergio Li Calzi, PhD, and Maria B Grant, MD, for logistical support.
Footnotes
Disclosures
The authors report no relationships that could be construed as a conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Venugopal JR, Prabhakaran MP, Mukherjee S, Ravichandran R, Dan K, Ramakrishna S. Biomaterial strategies for alleviation of myocardial infarction. J R Soc Interface. 2012;9:1–19. doi: 10.1098/rsif.2011.0301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ruvinov E, Cohen S. Alginate biomaterial for the treatment of myocardial infarction: Progress, translational strategies, and clinical outlook: From ocean algae to patient bedside. Adv Drug Deliv Rev. 2016;96:54–76. doi: 10.1016/j.addr.2015.04.021. [DOI] [PubMed] [Google Scholar]
- 3.Landa N, Miller L, Feinberg MS, et al. Effect of injectable alginate implant on cardiac remodeling and function after recent and old infarcts in rat. Circulation. 2008;117:1388–1396. doi: 10.1161/CIRCULATIONAHA.107.727420. [DOI] [PubMed] [Google Scholar]
- 4.Leor J, Tuvia S, Guetta V, et al. Intracoronary injection of in situ forming alginate hydrogel reverses left ventricular remodeling after myocardial infarction in Swine. J Am Coll Cardiol. 2009;54:1014–1023. doi: 10.1016/j.jacc.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 5.Rao SV, Zeymer U, Douglas PS, et al. A randomized, double-blind, placebo-controlled trial to evaluate the safety and effectiveness of intracoronary application of a novel bioabsorbable cardiac matrix for the prevention of ventricular remodeling after large ST-segment elevation myocardial infarction: Rationale and design of the PRESERVATION I trial. Am Heart J. 2015;170:929–937. doi: 10.1016/j.ahj.2015.08.017. [DOI] [PubMed] [Google Scholar]
- 6.Sabbah HN, Wang M, Gupta RC, et al. Augmentation of left ventricular wall thickness with alginate hydrogel implants improves left ventricular function and prevents progressive remodeling in dogs with chronic heart failure. JACC Heart Fail. 2013;1:252–258. doi: 10.1016/j.jchf.2013.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.LoneStar Heart, Inc. A Randomized, Controlled Study to Evaluate Algisyl-LVR™ as a Method of Left Ventricular Augmentation for Heart Failure (AUGMENT-HF) [Accessed 01/26/2016];Study NCT01311791. Available at: http://www.ClinicalTrials.gov/show/NCT01311791.
- 8.Willenberg BJ, Zheng T, Meng FW, et al. Gelatinized copper-capillary alginate gel functions as an injectable tissue scaffolding system for stem cell transplants. J Biomater Sci Polym Ed. 2011;22:1621–1637. doi: 10.1163/092050610X519453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rubiano A, Qi Y, Guzzo D, Rowe K, Pepine C, Simmons C. Stem cell therapy restores viscoelastic properties of myocardium in rat model of hypertension. J Mech Behav Biomed Mater. 2015;59:71–77. doi: 10.1016/j.jmbbm.2015.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qi Y, Zhang J, Cole-Jeffrey CT, et al. Diminazene aceturate enhances angiotensin-converting enzyme 2 activity and attenuates ischemia-induced cardiac pathophysiology. Hypertension. 2013;62:746–752. doi: 10.1161/HYPERTENSIONAHA.113.01337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qi YF, Zhang J, Wang L, et al. Angiotensin-converting enzyme 2 inhibits high-mobility group box 1 and attenuates cardiac dysfunction post-myocardial ischemia. J Mol Med (Berl) 2015 doi: 10.1007/s00109-015-1356-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Christman KL, Fok HH, Sievers RE, Fang Q, Lee RJ. Fibrin glue alone and skeletal myoblasts in a fibrin scaffold preserve cardiac function after myocardial infarction. Tissue Eng. 2004;10:403–409. doi: 10.1089/107632704323061762. [DOI] [PubMed] [Google Scholar]
- 13.Dai W, Wold LE, Dow JS, Kloner RA. Thickening of the infarcted wall by collagen injection improves left ventricular function in rats: a novel approach to preserve cardiac function after myocardial infarction. J Am Coll Cardiol. 2005;46:714–719. doi: 10.1016/j.jacc.2005.04.056. [DOI] [PubMed] [Google Scholar]
- 14.Singelyn JM, Sundaramurthy P, Johnson TD, et al. Catheter-deliverable hydrogel derived from decellularized ventricular extracellular matrix increases endogenous cardiomyocytes and preserves cardiac function post-myocardial infarction. J Am Coll Cardiol. 2012;59:751–763. doi: 10.1016/j.jacc.2011.10.888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee VC, Lloyd EN, Dearden HC, Wong K. A systematic review to investigate whether Angiotensin-(1-7) is a promising therapeutic target in human heart failure. Int J Pept. 2013;2013:260346. doi: 10.1155/2013/260346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qi Y, Shenoy V, Wong F, et al. Lentivirus-mediated overexpression of angiotensin-(1-7) attenuated ischaemia-induced cardiac pathophysiology. Exp Physiol. 2011;96:863–874. doi: 10.1113/expphysiol.2011.056994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bhana B, Iyer RK, Chen WL, et al. Influence of substrate stiffness on the phenotype of heart cells. Biotechnol Bioeng. 2010;105:1148–1160. doi: 10.1002/bit.22647. [DOI] [PubMed] [Google Scholar]
- 18.Engler AJ, Carag-Krieger C, Johnson CP, et al. Embryonic cardiomyocytes beat best on a matrix with heart-like elasticity: scar-like rigidity inhibits beating. J Cell Sci. 2008;121:3794–3802. doi: 10.1242/jcs.029678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jacot JG, McCulloch AD, Omens JH. Substrate stiffness affects the functional maturation of neonatal rat ventricular myocytes. Biophys J. 2008;95:3479–3487. doi: 10.1529/biophysj.107.124545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bajaj P, Tang X, Saif TA, Bashir R. Stiffness of the substrate influences the phenotype of embryonic chicken cardiac myocytes. J Biomed Mater Res A. 2010;95:1261–1269. doi: 10.1002/jbm.a.32951. [DOI] [PubMed] [Google Scholar]
- 21.Dai W, Gerczuk P, Zhang Y, et al. Intramyocardial injection of heart tissue-derived extracellular matrix improves postinfarction cardiac function in rats. J Cardiovasc Pharmacol Ther. 2013;18:270–279. doi: 10.1177/1074248412472257. [DOI] [PubMed] [Google Scholar]
- 22.Segers VF, Lee RT. Biomaterials to enhance stem cell function in the heart. Circ Res. 2011;109:910–922. doi: 10.1161/CIRCRESAHA.111.249052. [DOI] [PubMed] [Google Scholar]
- 23.Della Rocca DG, Willenberg BJ, Ferreira LF, et al. A degradable, bioactive, gelatinized alginate hydrogel to improve stem cell/growth factor delivery and facilitate healing after myocardial infarction. Med Hypotheses. 2012;79:673–677. doi: 10.1016/j.mehy.2012.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Willenberg BJ, Hamazaki T, Meng FW, Terada N, Batich C. Self-assembled copper-capillary alginate gel scaffolds with oligochitosan support embryonic stem cell growth. J Biomed Mater Res A. 2006;79:440–450. doi: 10.1002/jbm.a.30942. [DOI] [PubMed] [Google Scholar]