Abstract
In this era of tremendous technological capabilities and increased focus on improving clinical outcomes, decreasing costs, and increasing precision, there is a need for a more quantitative approach to the field of surgery. Multiscale computational modeling has the potential to bridge the gap to the emerging paradigms of Precision Medicine and Translational Systems Biology, in which quantitative metrics and data guide patient care through improved stratification, diagnosis, and therapy. Achievements by multiple groups have demonstrated the potential for 1) multiscale computational modeling, at a biological level, of diseases treated with surgery and the surgical procedure process at the level of the individual and the population; along with 2) patient-specific, computationally-enabled surgical planning, delivery, and guidance and robotically-augmented manipulation. In this perspective article, we discuss these concepts, and cite emerging examples from the fields of trauma, wound healing, and cardiac surgery.
Keywords: Computer Aided Surgery, Computer-Aided Interventions, Model-Guided Surgery, Mathematical Model, Multiscale Model, Inflammation, Wound Healing, Heart
The Translational Dilemma and Model-Guided Precision Medicine
A central challenge in healthcare is to provide personalized, pre-emptive and predictive medicine121 while containing costs. The attempt to meet this challenge has led to the introduction of the concept of Precision Medicine84. In an era of catchwords, the use of the term “Precision Medicine” must first be clarified. Of course, throughout history medical care has been tailored to individual patients and their specific circumstances, performed within a framework of diagnosis and therapy predicated upon the placement of an individual into defined classifications that are necessarily reflective of groups of patients. The “precision” of the resulting care plans is subject to the current state of medical art and technology as executed, given the expertise of the practitioner. As technology has advanced, so too has the expectation that the resolution of specific classification of an individual patient will become more granular and “precise,” and that there will be a shift from the more subjective “art” of medicine to a quantitative and ostensibly objective intersection between patient state and efficacious therapy. Adding to the potential semantic confusion, if the intent of Precision Medicine was based on the dictionary definition of the words used, it would entail developing the means of engineering specific therapies for a given individual, involving precisely targeting a particular disease state at a specific time. In reality, the goal of “Precision Medicine” as it is currently envisioned is much more modest: it aims to identify some particular set of existing therapies tailored to a patient subset defined by their “omic”/biomarker profile, along with some prior statistical determination of the efficacy of said therapies based on those profiles. Even with this more modest goal, the “Precision Medicine” paradigm offers increased hope for a rational, quantitative description of the dynamic patient state. However, precision Medicine suffers from the often reductionist and non-integrative status quo, as well as paradoxically from the potential data deluge that has affected both basic research and clinical medicine. The former problem has resulted in the Translational Dilemma, namely the lack of effective translation of basic research to the clinic1, 10. The latter has resulted in the nearly synonymous and unanimous association of Big Data with Precision Medicine (as noted by the reduced goals noted above). Indeed, the context in which computational modeling has been proposed as a key technology by which to actualize Precision Medicine is generally that of bioinformatics and other data-driven modeling techniques13, 48, 87, 107.
What is fundamentally missing from this picture, however, is the recognition that human disease initiation and progression are part of a dynamic process driven by fundamentally similar basic mechanisms. The importance of dynamics in determining more “precisely” the state and trajectory of an individual mandates that any computational approach purporting to accomplish precise medical care must account for those generative mechanisms and their resulting dynamic behavior. It is towards this end that we assert the necessity of the role of dynamic, multiscale modeling as a cornerstone for “Precision Medicine.”
Throughout the history of medicine, surgical intervention has served as perhaps the most dramatic and acute of medical treatments, performed under specialized conditions, with specific equipment and within a defined timeframe. In many ways the performance of a surgical procedure represents a microcosm of the overall medical process of diagnosis and therapy, occurring at the intersection of appropriate decision-making, ongoing situational awareness and technical facility. It is not therefore surprising that surgery has traditionally been heavily impacted by technological advances, and so too is it impacted by the current goals of “Precision Medicine.”
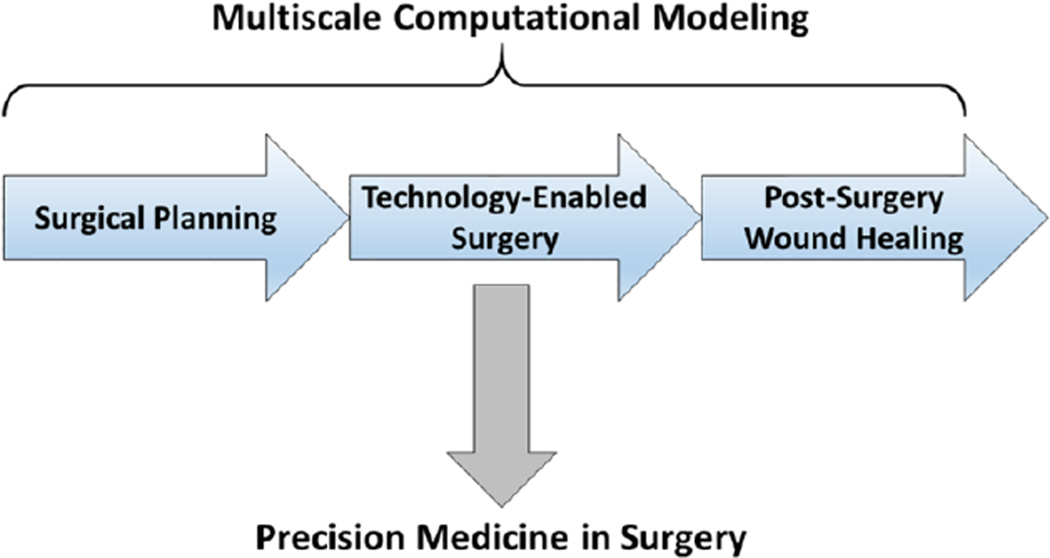
This review will focus on the role of multi-scale dynamic modeling on Precision Medicine by potentially augmenting the three key aspects of surgical disease: 1) pre-operative surgical planning, 2) enhanced situational awareness during an operation specifically related to any alterations in the pre-operative plan, and 3) dealing with the biology of the inevitable recovery from the surgical insult (Figure 1). While there is an extensive and important role of technology aimed at enhancing the connectivity and information flow of the operating room itself, and the impact of those technologies on surgical education, those fields are outside the focus of this review. Rather, herein we focus on the use of modeling and simulation to characterize the target tissues based on their known physical properties and dynamics (using the well-established modeling and simulation methods drawn from the physical sciences and engineering). We give examples of computational modeling aimed at optimizing surgical plans as well as devices for a planned procedure; the integration of dynamic tissue imaging and real-time visualization to aid the “eyes” of the operating surgeon during the case; and at characterizing, predicting, and ultimately reprogramming the important role of surgery-induced inflammation. This latter process is complex and non-linear, and is central to many diseases that require surgical management as well as to the injury and wound healing responses elicited by the surgical intervention itself. We focus especially on how inflammation has been characterized via Translational Systems Biology1, 7, 10, 78, 114, a rational, engineering-oriented approach to guide potential patient-specific modulation of acute inflammation in the peri-operative period.
Figure 1. A synthetic view of Computational Surgery.
Multiscale modeling is envisioned as a binding framework by which to synthesize the currently disparate fields associated with Computational Surgery, leading ultimately to a Precision Medicine framework based on multiscale computational modeling.
Before embarking on this discussion, however, it is useful to list some broad categories of modeling approaches. Computational modeling can be broadly categorized as either data-driven or mechanistic. Statistical models and Big Data algorithms are based on associations and correlations and fall within the realm of data-driven models. In contrast, mechanistic models are based on some level of abstraction of the system being modeled, and thus rely on prior knowledge and assumptions. Within the range of modeling approaches that can be classified as mechanistic (or, more precisely, dynamic and mechanistic) lie ordinary differential equation (ODE) models; partial differential equation models (PDE), which include finite element (FE) models; and agent-based models (ABM). Multiscale models are a class of mechanistic models that span across scales of organization (e.g. from the molecular to the whole organism or population), and usually also span time scales12, 14, 29, 39, 52, 85, 88, 95, 96, 115, 124. These modeling approaches have been reviewed extensively elsewhere (e.g.1, 25, 93, 111).
Before the case: Patient-specific, computationally-enabled surgical planning and enhanced device design
Surgical planning is key to optimize patient care and outcomes in the surgical setting2, 3, 21, 30, 50. The emerging consensus is that patient-specific, computer-based surgical planning, delivery, and guidance is the premise of Precision Medicine and is positioned to dramatically advance therapy21, 30. Computer simulations have revolutionized the fields of engineering (aerospace, automotive industry, civil engineering, etc.). For example, some automotive and aerospace companies now progress directly from simulation to production. We hypothesize that this class of models can be leveraged similarly to drive a quantum improvement in surgery.
Here, we review three examples of patient-specific computational FE models of failing or infarct-injured left ventricles used for surgical planning and therapeutic delivery. Heart failure, a worldwide epidemic that contributes considerably to the overall cost of health care in developed nations, is increasing at an alarming pace - a trend likely to continue as the population ages and life span increases49. Adverse left ventricular (LV) remodeling after myocardial infarction is responsible for nearly 70% of heart failure cases43.
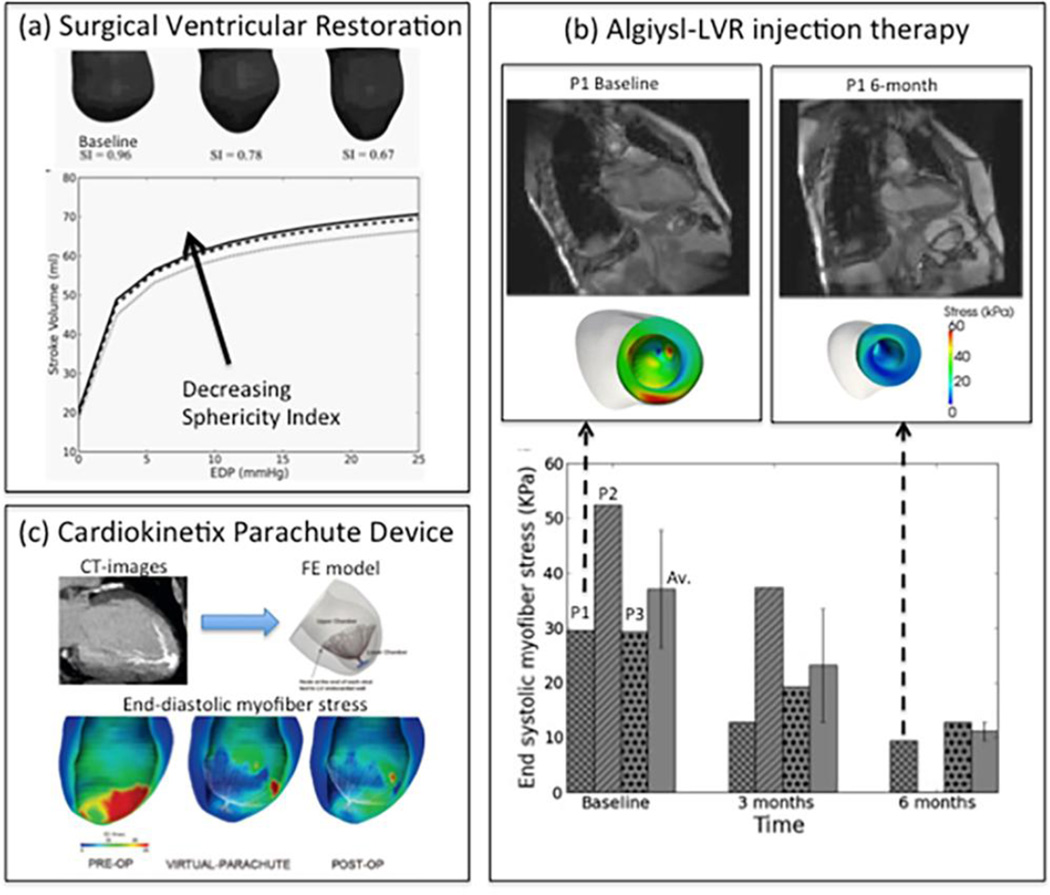
Surgical ventricular restoration (SVR) is a procedure designed to treat heart failure by surgically excluding infarcted tissues from the dilated failing LV. To elucidate and predict the effects of geometrical changes from SVR on cardiac function, Lee et al65 created patient-specific FE LV models before and after surgery using magnetic resonance images. Their results predict that the postsurgical improvement in systolic function was compromised by a decrease in diastolic distensibility in patients. These two conflicting effects typically manifested as a depressed function (stroke volume vs. end-diastolic pressure relation) after surgery. By simulating a restoration of the LV back to its measured baseline sphericity, they showed that both diastolic and systolic function improved. This result confirms that the increase in LV sphericity commonly observed after SVR (endoventricular circular patch plasty) has a negative impact and contributes partly to the depressed function (Figure 2a). On the other hand, peak myofiber stress was reduced substantially (by 50%) after SVR, and the resultant LV myofiber stress distribution became more uniform. This significant reduction in myofiber stress after SVR may help reduce adverse remodeling of the LV. These results are consistent with the speculation proposed in the landmark study Surgical Treatment for Ischemic Heart Failure trial funded by U.S. National Institutes of Health58. The conclusion of the study was a neutral outcome where it was noted that "the lack of benefit seen with surgical ventricular reconstruction is that benefits anticipated from surgical reduction of LV volume (reduced wall stress and improvement in systolic function) are counter-balanced by a reduction in diastolic distensibility." Hence, computer simulations provided the explanation for the observed neutral effect of a cardiac surgical procedure in a large clinical trial.
Figure 2.
Computational modeling of (a): Surgical Ventricular Restoration, (b): Algisyl-LVR injection therapy (c): Cardiokinetix Parachute Device. EDP, P1 and OP represent end diastolic pressure, patient 1 and operation, respectively.
Algisyl-LVR™ (LoneStar Heart, Inc. Laguna Hills, CA) is a medical device under clinical development intended to prevent or reverse progression of heart failure in patients who have a dilated LV. This device consists of a proprietary biopolymer gel that is injected into strategic (i.e., computer predicted/optimized) areas of the heart muscle, where it remains as a permanent implant. The design of the injection patterns as a “belt” in the equator of the heart was determined through computer simulations of cardiac mechanics117. Lee et al66 sought to quantify the effects of Algisyl-LVR™ in combination with coronary artery bypass grafting (Algisyl-LVR™+ CABG) on both LV function and wall stress in heart failure patients. Magnetic resonance images obtained before treatment (n=3), and at 3 months (n=3) and 6 months (n=2) afterwards were used to reconstruct the LV geometry (Figure 2b). Cardiac function was quantified using end-diastolic volume (EDV), end-systolic volume (ESV), regional wall thickness, sphericity index, and regional myofiber stress computed using validated mathematical (finite element) modeling. The LV became more ellipsoidal after treatment, and both EDV and ESV decreased substantially 3 months after treatment in all patients; EDV decreased by 55% and ESV decreased by 47%. Ejection fraction increased 47% during that period. Volumetric-averaged wall thickness increased in all patients by 23% in the 3 month period. These changes were accompanied by an approximately 35% decrease in myofiber stress at end-of-diastole and at end-of-systole (Figure 2b). Post-treatment myofiber stress became more uniform in the LV. These initial results support the concept that computer-aided design of the Algisyl-LVR™+CABG treatment leads to decreased myofiber stress, restores LV geometry, and improves function.
The Parachute(®) (Cardiokinetix, Inc., Menlo Park, CA) is a catheter-based device intended to reverse LV remodeling after antero-apical myocardial infarction. When deployed, the device partitions the LV into upper and lower chambers. To simulate its mechanical effects, Lee et al64 created a FE LV model based on computed tomography (CT) images from a patient before and 6 months after Parachute(®) implantation. Acute mechanical effects were determined by in silico device implantation (VIRTUAL-Parachute). Chronic effects of the device were determined by adjusting the diastolic and systolic material parameters to better match the 6-month post-implantation CT data and LV pressure data at end-diastole (ED) (POST-OP). Regional myofiber stress and pump function were calculated in each case (Figure 2c). The principal finding is that VIRTUAL-Parachute was associated with a 61.2% reduction in the lower chamber myofiber stress at ED. The POST-OP model was associated with a decrease in LV diastolic stiffness and a larger reduction in myofiber stress at the upper (27.1%) and lower chamber (78.4%) at ED. Myofiber stress at end-systole and stroke volume was modestly changed in the POST-OP case. The simulation results suggest that the primary mechanism of Parachute(®) is a reduction in ED myofiber stress, which may reverse eccentric post-infarct LV hypertrophy which is currently under investigation.
Mathematical modeling of the cardiovascular system using FE has become both more powerful and easier to use. FE models of the heart now incorporate constitutive laws based on myocardial architecture that mimic the passive anisotropic non-linear nature of the myocardium that can simulate active contraction. Inverse solutions of patient-specific models now allow the calculation of myocardial material properties and stress. Computational surgical planning is clearly positioned to play an increasing role in the understanding of patient specific (through conventional medical imaging) cardiovascular pathology and in the design of therapies for cardiac surgery.
During the case: Multiscale patient-specific modeling for intra-operative guidance and therapy delivery
Image-guidance in surgery is essential to establish and maintain an accurate patient registration between the image and physical spaces of the anatomy of interest. Computational models play an important role in facilitating patient registration. At the organ level, a geometrical model of the anatomy of interest is often generated from preoperative CT (pCT) or MRI (pMR). For hard tissues such as the spine where vertebrae remain rigid during surgery, a geometrical shape model is often generated to automate the detection and segmentation of the vertebral bodies. These include spinal curve extraction using prior knowledge of shape, gradient, and appearance information models61; training bone-structure edge detection with a coarse-to-fine two-stage registration of a deformable surface model72; fully automatic methods based on deformable fences60; and the use of multi-vertebrae anatomic shape and pose models89. Similarly, vertebral shapes can be extracted from tracked intraoperative images such as ultrasound and stereovision. Image-to-physical space registration is then achieved when the vertebrae are registered between intra- and pre-operative images, using either feature-54, 55, 82 or intensity-based42, 63 techniques. A biomechanical model can also be used to constrain the registration42.
For soft tissues such as the brain, non-rigid deformation or brain shift must be considered to maintain an accurate image-to-patient registration56, 80. A biomechanical model based on the geometry derived from pMR and driven by intraoperative data is an effective means for brain shift compensation. An array of models with differing sophistication, material properties, and choice of validation strategies exist80. They incorporate displacement data often sparsely observed from various intraoperative images either through a direct boundary condition assignment (“forward”) or a data-guided “inversion” scheme57. A model-updated MR can be established by transforming pMR data using the computed whole-brain deformation, which effectively compensates for brain shift and maintains the patient registration accuracy for subsequent guidance56. A pre-computed deformation atlas can also be established before surgery that parametrically samples the deformation driving conditions. This allows efficient model computation intraoperatively, thereby enabling an updated guidance in the operating room18, 33, 105.
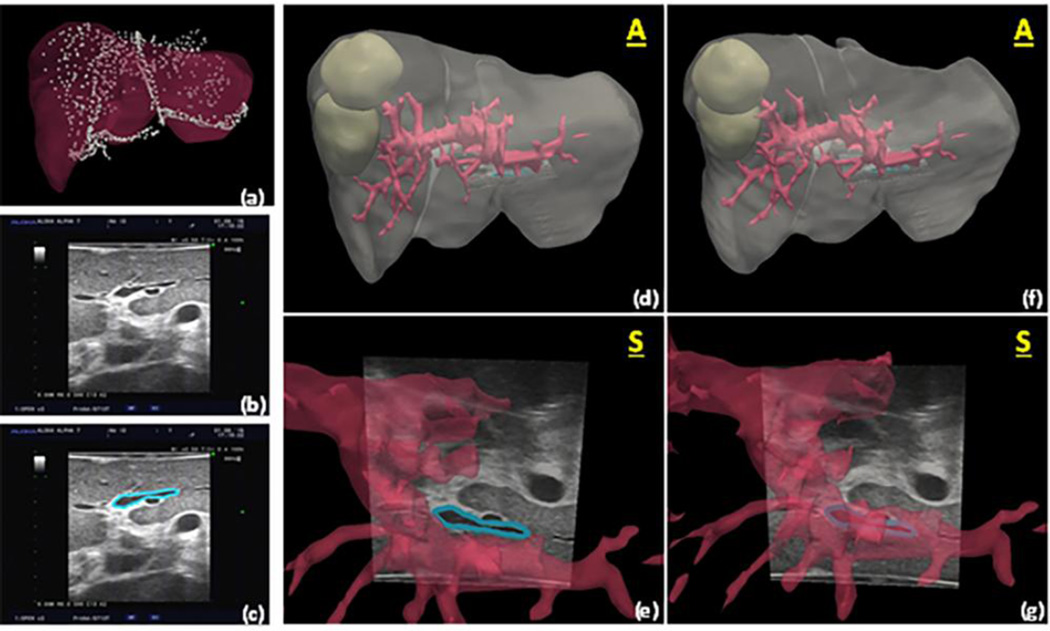
Although the developments above that integrate computational modeling and image guidance are important steps forward, what is equally exciting is that the framework has begun to translate to information-driven surgeries in other soft tissue organs as well as towards therapeutics. For example, with the former, a considerable body of work is beginning to emerge with respect to extending these approaches to open and laparoscopic liver resection. As these new surgical goals are realized, the paradigms of traditional image guidance are becoming re-realized to accommodate new challenges. For example, in the case of open liver surgery, the organ is presented for surgery in a configuration that is quite different than its preoperatively imaged counterpart. At the procedural time when observation and measurement of the organ can usually be achieved, the liver has been separated from its surrounding ligaments and packed for surgical presentation, and in the process has experienced considerable deformation and gross shape change. As a result, reference targets attached to the physical patient as in traditional image-guided neurosurgery are not relevant. Deformation is volumetrically present at the earliest stages of presentation; consequently, it is necessary for guidance environments to be capable of dynamic continuous organ-based image-to-physical registration, e.g.22. Equally necessary, preoperative segmentation and planning capabilities98, computer vision-based measurement techniques74, and computational modeling to account for non-rigid deformations92 are becoming essential components to these novel integrated systems, as illustrated in Figure 3. Briefly, Figure 3a, d, f displays a liver volume facilitated by a commercial software, Scout Liver (Pathfinder Technologies Inc. Nashville, TN), which uses state-of-the-art liver segmentation techniques. From segmented volumes, three-dimensional surfaces can be extracted by standard image processing methods. A custom tetrahedral mesh generator is then used to generate a finite element grid. Lastly, using sparse intraoperative data such as the intraoperatively swabbed points shown in Figure 3a, a custom, nonrigid image-to-physical registration process can be used to correct for deformation, as shown when comparing the alignment of the segment III portal pedicle shown in Figure 3e, and 3g. Going further, we should note that similar to the variation in surgical approaches to different organ systems, the translation of the type of alignment approaches shown here also requires equal specificity in order to include the adaptation to less invasive environments such as in laparoscopic procedures47. Nevertheless, while more work is still needed, it is encouraging to see investigators translating new directions in the field of model-driven computational surgery; e.g., areas such as prostate6, kidney5, lung15, and breast24 to name several.
Figure 3.
Using methods from Rucker et al.92, (a) preoperative liver model and registered swabbed point cloud acquired from intraoperative liver surface are shown with ultrasound image of segment III portal pedicle in (b), and (c) showing manual segmentation of structure. In (d) we see the planned model cloud with ultrasound slice (white arrow) showing alignment of pedicle based on rigid registration, and (e) is the close-up. Notice how inferior vessel region does not align with corresponding vascular structure. In (f), a deformation correction driven by the closest point mismatch in data shown in (a) has been performed, and (g) shows the new location of the structure as well as modified shapes to vasculature. It should be noted that in this example ultrasound data was used for validation only, i.e. no localized ultrasound structures were used within the alignment algorithm, only data shown in (a) was used. We should further note that the alignment error of this feature after rigid registration was estimated at 5.5 +/− 2.6 mm (10.9 mm maximum error). The error was reduced to 2.4 +/− 1.5 mm (5.4 mm maximum error) after model-based deformation correction.
Lastly, in addition to the work in other soft-tissue organs as described above, advances to simulate therapeutic interactions with diseased or dysfunctional tissue are rapidly being realized. The potential for using multiscale models to provide information regarding therapy delivery would represent an exciting barrier to breech for surgical intervention. For example, one of the areas that has had considerable development is in the area of thermal ablation, with computational modeling examples in the literature simulating radio frequency17, microwave19, and cryo-ablation59. As an example17, investigators have crossed length scales to bring together tissue-scale power deposition and thermal transport models with a cell-scale damage integral index, all cast within an optimization framework for determining the best delivery of ablation therapy. The proposed example advances a multiscale modeling effort to link delivery and mechanism across length scales for the optimization of treatment. As modeling efforts in other therapies continue to emerge such as irreversible electroporation41, convection-enhanced drug delivery102, interventional therapies such as chemo-125 and radio-therapy53, and work in neuromodulation16, it is exciting to speculate what new capabilities computational multi-scale modeling will provide in real-time to the surgical and interventional suites of the future.
After the case: Dealing with the aftermath via Multiscale Modeling of Surgical Wound Healing
Surgical interventions invariably involve management of wounds, either as the reason for an operation, or as a consequence of the surgical procedure. Wound-related complications are a major source of post-operative morbidity, and have a significant impact on healthcare costs23, 108. Therefore, a greater understanding of the mechanistic processes involved in wound healing and how those processes can become impaired could play an important role in developing Precision Medicine interventions to improve surgical care. Given that wound healing is an inherently multiscale process in which various cellular responses are coordinated over multiple length and time scales to close the wound and repair the tissue, wound healing is a natural target for the application of multiscale modeling.
Wound healing is generally subdivided into the three overlapping and mechanistically-interrelated phases of inflammation, proliferation, and remodeling that proceed after hemostasis is achieved37, 122. Immediately following tissue injury, platelets adhere to the exposed extracellular matrix (ECM), degranulate, and release damage-associated molecular pattern (DAMP) molecules, chemokines, cytokines, and other biochemical mediators that initiate hemostasis through platelet aggregation and the formation of a provisional fibrin matrix. Next, inflammatory cells emigrate into the damaged tissue over a time-scale of days, and begin the process of removing infectious agents, cellular debris, and damaged ECM proteins. During the proliferation phase, which occurs on the scale of weeks, fibroblasts migrate into the wound and rapidly increase in number in response to the release of growth factors and chemoattractants released by the inflammatory cells. The fibroblasts contract the matrix in an effort to draw the wound margins closer, and then begin the process of synthesizing ECM proteins75. Simultaneously, a vascularized bed of granulation tissue forms, and keratinocytes re-epithelialize the wound. During the remodeling phase, which can continue over a period months to years, the tissue remodeling is completed and scar tissue is formed. The presence of microbes can affect and interfere with this process at multiple stages, ranging from overt and dramatic wound and tissue infection to more subtle signaling events and intrinsic ecological dynamics present in any cohabitating microbial communities81, 83, 126.
The wound healing process is well-studied in animal systems, but species-specific differences with humans also exist, providing another reason for the Translational Dilemma32, 71, 104. To overcome these differences, new experimental methodologies, particularly with regard to imaging, are emerging that may allow the time courses of wound healing to be studied more rigorously in humans71. Nevertheless, the collection of a time course of primary samples in humans suffering from chronic wound healing diseases remains a significant hurdle due to the possibility of compromising the healing wound. Even in the event that such measurements can be made, the cell-cell and cell-matrix processes involved are inherently complex, multiscale, and produce emergent behaviors that cannot be easily understood using reductionist approaches. As such, clinically-realistic and mechanistic computational models that can incorporate data and observations from a host of different experiments executed at varying temporal and spatial scales into a unified picture should provide new insights into improving and accelerating patient wound healing. Once validated experimentally or clinically, these multiscale models could be used to predict the healing (or non-healing) trajectories that might be associated with a given surgical procedure (e.g., SVR and patient-specific surgical plans) and with the “omic”/biomarker profile of the patient. In turn, these models could be connected to models based on imaging of the tissues to be manipulated surgically in order to better predict the likely outcome of a given surgical procedure (as noted above in the section on patient specific guidance and therapy planning).
A number of modeling strategies have been explored for their utility in accurately simulating and explaining the wound healing process. Models employing differential equations and balance laws are the most standard, classical method utilized in modeling biological processes, including inflammation and wound healing7, 112, 113. Continuum-based models have been used to explore all phases of wound healing, from inflammation118, 119, to wound closure11, 120, to tissue remodeling76. This modeling framework, while extremely useful from a basic, mechanistic point of view, cannot be used readily to create tissue-realistic simulations that involve stochastic biological effects.
Nonetheless, the compendium of continuum-based models has resulted in important insights into the wound healing response, including illuminating relationships amongst various cell populations, inflammatory mediators, and other chemicals118, 119, the role of physical constraints in guiding re-epithelialization11, 120, and parameters of the healing wound that control the pattern of collagen deposition77. Using these techniques, computational models have been used to suggest and simulate therapies to modulate wound healing76, 119.
More recently, agent-based models (ABM) have been applied to wound healing problems8, 31, 70, 79, 90, 97, 99, 106, 127 because they offer several advantages in terms of temporal and spatial flexibility, and for integrating and synthesizing cellular and molecular-level data. ABM represent discrete processes and provide a useful translational tool for examining stochastic and spatial aspects of inflammation and wound healing8. These models often predict complex patterns, structures, behaviors, and self-organizing principles that “emerge” from a set of simple rules that govern the agent’s behaviors and its interactions with other agents and the environment73. The ability of ABMs to represent spatial relationships and tissue patterning effects makes them an appealing approach for modeling wound healing biology. Most of these ABM, however, have been focused on delineating intracellular detail as an adjunct to basic research20, 100, 115, 116. We propose that ABM are also well-suited to a translational role to bridge basic science knowledge and the rational development of clinically applicable strategies7, 9, 40, 114. We have developed a series of ABM with this specific goal in mind in order to model diabetic wound healing79, host-pathogen interactions in surgical wound healing44, 101, patient-specific inflammation following phonotrauma injury to the vocal folds67, 69, surgical injury in rats68, and chronic, non-healing pressure ulcers in human spinal cord injury patients99, 127.
The mechanical properties and functionality of the healing tissue are derived, in part, from multiscale mechanical interactions that balance the distribution of macroscale tissue loads and cell traction forces with microstructural deformations in the ECM and global tissue reorganization. In addition, these multiscale mechanical interactions supply important mechanical signals that help regulate various cellular processes in the healing wound, particularly with respect to driving fibroblast remodeling and scar formation34, 38, 51. As such, many models of wound healing incorporate mechanical features into the modeling framework, including some of the continuum-based models and ABM already cited11, 76.
In an early model by Tranquillo and Murray, the role of fibroblast traction forces in wound closure was explored by tracking fibroblast concentration, ECM concentration, and ECM displacements109. Here, the cell traction forces, which propel the migrating fibroblasts into the wound from the surrounding dermis, also induced local ECM reorganization and deformation. The summed cellular-level local deformations resulted in a successful prediction of tissue-level macroscopic closure of the wound. Dallon and colleagues also investigated the role of multiscale mechanical interactions between cells, diffusible molecules, and ECM in directing the remodeling phase of wound healing25, 26, 76. These hybrid models indicate that the initial organization of fibrin in the wound controls the final organization of collagen in the scar.
Discrete cell-matrix mechanical interactions have also been simulated using ABM90, 91. These models provide a more detailed view of how cell traction forces on individual fibers crosslinked into fiber networks can induce dramatic local structural remodeling and facilitate long-distance mechanical communication with other cells. These local changes in ECM structure are also directed in part by simultaneous global restructuring via multiscale mechanical interactions. This behavior has been predicted by an image-based multiscale FE model that couples centimeter-scale tissue properties to micrometer-scale ECM restructuring4, 94, 103. These models have been benchmarked against in vitro time-lapse imaging experiments on fibroblast remodeled fibrin gels and demonstrate that the multiscale interplay between macroscopic domain geometry, boundary conditions, and cell traction forces drives short-term remodeling and the development of fibrin fiber alignment, which could be deterministic of the extent of scarring27, 28. An attractive feature of these models is that deterministic and stochastic fiber-based rules can be inserted into the modeling framework so that concepts such as strain stabilized enzymatic matrix remodeling46 and gross tissue-level failure45 can be explored as multiscale phenomena, which may have relevance for understanding fibrosis and wound strength, respectively.
An ABM representation of cells and their interactions with the ECM may also be added to such models. For example, in one study ABM was used to produce a tissue-realistic model of a set of liver lobules. It incorporated inflammation, fibrosis, and the impact of these mechanisms of tissue stiffness as a macroscopic, scale-spanning property of the liver35. Coupling this model with a more detailed view of multiscale mechanical interactions can further illuminate the role mechanics plays in tissue fibrosis, for example in the case of liver resection described above. The healing tissue’s mechanical properties and functionality are directly dependent on the organization and alignment of the tissue microstructure that proceeds from these interactions. Thus, insights on how environmental parameters of the wound site can be manipulated to shift tissue remodeling from producing the collagen alignment observed in fibrotic tissue towards the organization observe in healthy tissue would be of tremendous value.
Similar ideas on the role of multiscale mechanic have been explored in the context of a thrombus formation and fibrinolysis (see Xu et al. for a review124) and angiogenesis and neovessel growth36, 110. Each of these mechanisms play important roles in determine the fate of the healing wound, and thus will require the development of hybrid modeling strategies that incorporate both continuum and discrete representations of the salient temporal and spatial scales using physics to predict the healing outcome. Finally, it should be noted that several other mechanical models of wounds have been developed that focus on larger scales, such as pressure ulcers99, 127, and that there are many opportunities to address these types of problems from a multiscale perspective.
An understanding of the means by which cells control and modify their behavior in response to their physical environment is of critical importance in the eventual engineering of precision interventions, since the vast majority (if not all) of those interventions will involve molecular factors intended to modulate the signaling and regulatory pathways that govern cellular behaviors. The multiscale modeling transformation required at this point involves moving from the physics of wound healing to the biology of wound healing. It is here that the principle of multiscale abstraction takes precedence, for while the underlying physical milieu of the wound provides the environmental inputs and outputs for the cellular actors that govern biology, the accessibility of those physical measurements become untenable in the clinical arena. Therefore, knowledge about the physical-mechanical drivers of wound biology developed and tested in experimental systems can now be abstracted into input/output variables for the cellular populations that actually heal the wound within clinically relevant multiscale models. As noted above, ABM are particularly well-suited for representing the aggregate behavior of cellular populations, and their ability to encapsulate mechanistic knowledge within their rules allows the instantiation of imputed biological control structures. More importantly, the multiscale, spatially explicit nature of ABM permits the generation of clinically relevant and accessible metrics related to cell population features and tissue-level inflammatory mediator dynamics44, 67–69, 79, as well as macroscopic visual features of the healing wound itself99, 127. These types of metrics are potentially retrievable from human clinical wounds, including sequentially without fundamentally disturbing the wound, thereby allowing the personalized calibration of such dynamic computational models. The promise of this strategy can be seen in the ABM of pressure ulcer generation and healing (the Pressure Ulcer ABM [PUABM])127. In this work, the spatial representation of the developing decubitus ulcer in the ABM can be matched to digital photographs of existing wounds, and be used to predict the trajectory of such a wound’s development and potential for healing (Figure 4). The development of an iterative process of tuning the PUABM to known inflammatory responsiveness for a particular patient, the generation of multiple simulation trajectories, and repeated recalibration to visual images and re-simulation for subsequent trajectories, would help achieve the goals of predictive, personalized multiscale dynamic modeling of the healing surgical wound.
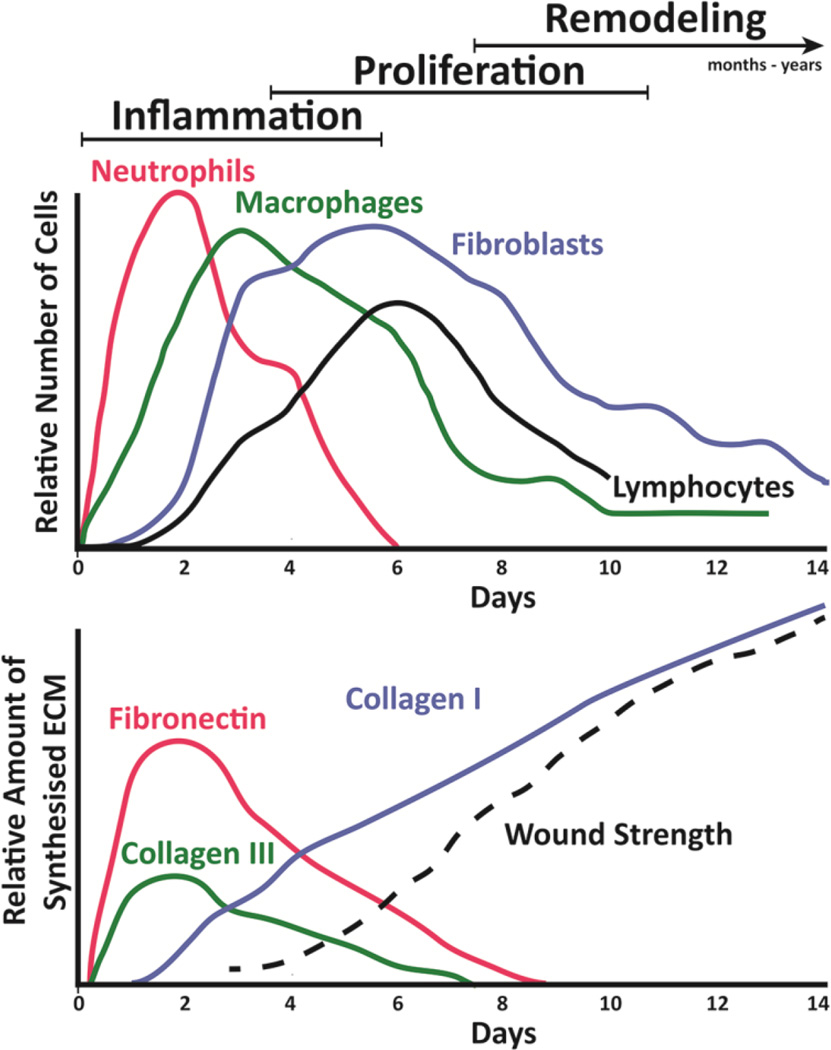
Figure 4.
Time Scales Involved in the Wound Healing Process. (A) Relative number of primary cell types involved in the three passes of wound healing. The inflammatory phase initiates within minutes of wounding and continues for days as first neutrophils and then macrophages move into the provisional fibrin matrix established during hemostasis. Over the next several days, fibroblasts and vascular endothelial cells (not shown) rapidly expand in number and then begin to remodel the wound site with newly synthesized ECM that continues for many months. (B) The corresponding temporal pattern of ECM production in the wound. Fibronectin and type III collagen is synthesized initially. Later, ECM production by fibroblasts transitions predominantly to type I collagen and results in an increase in the wound breaking strength. Adapted from Witte et al.123
Putting it All Together: Technologically enhanced precision and personalized surgical care
We have described a series of currently disparate multiscale modeling efforts in the arenas of surgical planning, surgical imaging, and wound healing. These studies share two main aspects: they are focused on clinically relevant and related problems, and they share a common general methodology. Without a doubt, these efforts will continue on separate tracks, and may lead to novel insights and therapies individually. As we move forward, workflow, and instrumentation in today’s operating theatres will be critical considerations in the realization and translation of multiscale model-enhanced therapies, similar to the many benchtop discoveries which face considerable translational obstacles. The workflow within operating rooms is quite complex and represents a concert of events that has competing goals. For example, the requirement of aseptic technique alone requires fundamental compromises to what is typically considered efficient engineering design. Going further, the ability to measure patient-specific variables during surgery to control multiscale model-enhanced therapies is considerable, and the localization and accuracy of those measurements is equally challenging. Only relatively recently have investigators begun to look at the full concert of activities within the operating room theatre and attempted to quantify procedural medicine62, 86. Finally, we must also realize that post-procedural biological process and care are important considerations to complete this picture. Wound healing models must interrelate inflammation, cell migration, adhesion, force generation and wound contraction, matrix synthesis, angiogenesis, and tissue remodeling, and how these processes are controlled/influenced by the mechano-chemical environment and coordinated across multiple length and time scales in the healing wound.
It has long been recognized that surgical interventions represent a paradox to Hippocrates’ charge to “do no harm;” surgery is exactly the infliction of tissue damage (“harm”) with the promise that such harm provides a shorter path towards the restoration of patient health. As such, surgeons have long incorporated the cost-benefit analysis necessary for surgical decision making into their training and practice. However, the exponential increase in our understanding of the multiple factors that can potentially affect the consequences of a particular surgical intervention for a particular patient challenges the ability of surgeons to decide on an optimal course. We assert that a rational response to this challenge requires the systematic integration of these recognized, inherently multi-scale factors in the context of the surgical workflow, including planning, technical factors and biological response, to allow surgeons to evaluate and compare multiple scenarios and trajectories. We recognize the asymmetric nature of our knowledge and understanding of the mechanistic underpinnings of the various scales involved in surgery (i.e. we will have more confidence in our understanding of one level than another). Indeed, the modular, multi-scale approach we have proposed is intended to account for a persistence of epistemological uncertainty. Our goal is to aid in the recognition of how technological advances in multi-scale modeling and simulation impacts the delivery and consequences of surgical care, and recognize that the implementation of such a program must intrinsically incorporate a process of iterative refinement that integrates the future promise of greater capability with the need to provide the best possible care for our patients today.
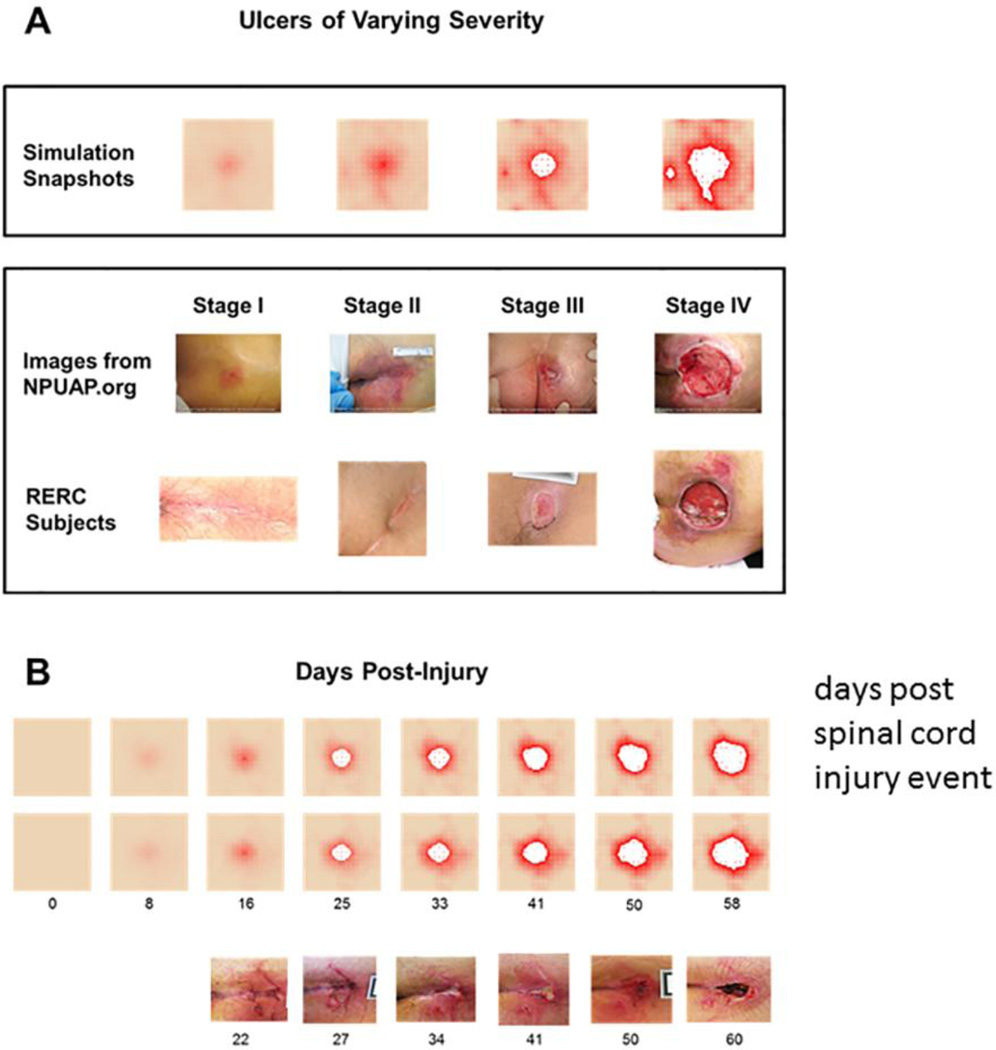
Figure 5. Simulations of the PUABM Match Key Features of Clinical Images.
(A) Simulations achieved visual appearances with characteristics similar to each stage of PU development. The first row of clinical images come from the National Pressure Ulcer Advisory Panel (copyright npuap.org, used with permission) and are of different subjects. Images in the second row are from people with SCI enrolled in a prospective study of PU at each stage. Irregular shapes and increasing nearby damage are observed in both sets of clinical data. (B) Numbers indicate days post-injury. Simulated ulcers evolve with visual characteristics that match PU progression observed in people with SCI. Two simulation time courses are matched against one patient from our study. We match key features: irregular shapes, nearby satellite ulcers (open arrows), jagged edges (solid arrows), and decreasing tissue health across the field. Reprinted from127.
Acknowledgments
YV would like to acknowledge funding by the National Institutes of Health (grants P50-GM-5378, UO1-DK-072146, RO1-GM-107231-01A1); Department of Defense (grants W911QY-14-1-0003 and W81XWH-14-DMRDP-CRMRP-RTRA); National Institute on Disability Rehabilitation Research grant H133E070024; and a Shared University Research Award from IBM, Inc. GA would like to acknowledge funding by the National Institutes of Health (grants RO1-GM-115839-01 and P30-DK-42086). GK and JG would like to acknowledge funding by the National Institutes of Health (grants R01-HL-118627, U01-HL-119578). JG would also like to acknowledge funding by the National Institutes of Health (grant R01-HL-077921). ES would like to acknowledge funding from the National Science Foundation (grant CAREER CMMI 1452728). MIM would like to acknowledge funding by the National Institutes of Health (grants R01-NS-049251, R21-NS-087796, and R01-CA-162477). SJ would like to acknowledge funding by the National Institutes of Health (grant R01-NS-092853 and R21-NS-078607), and the Dartmouth Clinical and Translational Science Institute under Award KL2-TR-001088 from the National Center for Advancing Translational Sciences of the NIH.
ABBREVIATIONS
- ABM
agent-based models
- CABG
coronary artery bypass grafting
- CT
computed tomography
- DAMP
damage-associated molecular pattern
- ECM
extracellular matrix
- ED
end-diastole
- EDV
end-diastolic volume
- ESV
end-systolic volume
- LV
left ventricular
- ODE
ordinary differential equation
- PDE
partial differential equation
- PDGF
platelet-derived group factor
- pCT
preoperative CT
- pMR
preoperative magnetic resonance imaging [MRI]
- PUABM
Pressure Ulcer ABM
- SVR
Surgical ventricular restoration
- TGF-β1
transforming growth factor-β1
REFERENCES
- 1.Complex Systems and Computational Biology Approaches to Acute Inflammation. New York, NY: Springer; 2013. [Google Scholar]
- 2.Image-Guided Interventions: Technology and Applications. New York, NY: Springer; 2008. [Google Scholar]
- 3.Medical Image Registration. Boca Raton, FL: CRC Press; 2001. [Google Scholar]
- 4.Aghvami M, Barocas VH, Sander EA. Multiscale mechanical simulations of cell compacted collagen gels. J Biomech Eng. 2013;135:71004. doi: 10.1115/1.4024460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Altamar HO, Ong RE, Glisson CL, Viprakasit DP, Miga MI, Herrell SD, Galloway RL. Kidney Deformation and Intraprocedural Registration: A Study of Elements of Image-Guided Kidney Surgery. Journal of Endourology. 2011;25:511–517. doi: 10.1089/end.2010.0249. [DOI] [PubMed] [Google Scholar]
- 6.Alterovitz R, Goldberg K, Pouliot J, Hsu ICJ, Kim Y, Noworolski SM, Kurhanewicz J. Registration of MR prostate images with biomechanical modeling and nonlinear parameter estimation. Medical Physics. 2006;33:446–454. doi: 10.1118/1.2163391. [DOI] [PubMed] [Google Scholar]
- 7.An G, Faeder J, Vodovotz Y. Translational systems biology: Introduction of an engineering approach to the pathophysiology of the burn patient. J. Burn Care Res. 2008;29:277–285. doi: 10.1097/BCR.0b013e31816677c8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.An G, Mi Q, Dutta-Moscato J, Solovyev A, Vodovotz Y. Agent-based models in translational systems biology. WIRES. 2009;1:159–171. doi: 10.1002/wsbm.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.An GC. Translational systems biology using an agent-based approach for dynamic knowledge representation: An evolutionary paradigm for biomedical research. Wound Rep. Reg. 2010;18:8–12. doi: 10.1111/j.1524-475X.2009.00568.x. [DOI] [PubMed] [Google Scholar]
- 10.An GVY. Translational Systems Biology: Concepts and Practice for the Future of Biomedical Research. New York, NY: Elsevier; 2014. [Google Scholar]
- 11.Arciero JC, Mi Q, Branca MF, Hackam DJ, Swigon D. Continuum model of collective cell migration in wound healing and colony expansion. Biophys J. 2011;100:535–543. doi: 10.1016/j.bpj.2010.11.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ayton GS, Noid WG, Voth GA. Multiscale modeling of biomolecular systems: in serial and in parallel. Curr. Opin. Struct. Biol. 2007;17:192–198. doi: 10.1016/j.sbi.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 13.Baldock AL, Rockne RC, Boone AD, Neal ML, Hawkins-Daarud A, Corwin DM, Bridge CA, Guyman LA, Trister AD, Mrugala MM, Rockhill JK, Swanson KR. From patient-specific mathematical neuro-oncology to precision medicine. Front Oncol. 2013;3:62. doi: 10.3389/fonc.2013.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Belfiore M, Pennisi M. In Silico Modeling of the Immune System: Cellular and Molecular Scale Approaches. 2014;2014:371809. doi: 10.1155/2014/371809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brock KK, Dawson LA, Sharpe MB, Moseley DJ, Jaffray DA. Feasibility of a novel deformable image registration technique to facilitate classification, targeting, and monitoring of tumor and normal tissue. International Journal of Radiation Oncology Biology Physics. 2006;64:1245–1254. doi: 10.1016/j.ijrobp.2005.10.027. [DOI] [PubMed] [Google Scholar]
- 16.Butson CR, Cooper SE, Henderson JM, McIntyre CC. Patient-speciftic analysis of the volume of tissue activated during deep brain stimulation. Neuroimage. 2007;34:661–670. doi: 10.1016/j.neuroimage.2006.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen CC, Miga MI, Galloway RL., Jr Optimizing electrode placement using finite-element models in radiofrequency ablation treatment planning. IEEE Trans Biomed Eng. 2009;56:237–245. doi: 10.1109/TBME.2008.2010383. [DOI] [PubMed] [Google Scholar]
- 18.Chen I, Coffey AM, Ding SY, Dumpuri P, Dawant BM, Thompson RC, Miga MI. Intraoperative Brain Shift Compensation: Accounting for Dural Septa. Ieee Transactions on Biomedical Engineering. 2011;58:499–508. doi: 10.1109/TBME.2010.2093896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chiang J, Birla S, Bedoya M, Jones D, Subbiah J, Brace CL. Modeling and Validation of Microwave Ablations With Internal Vaporization. Ieee Transactions on Biomedical Engineering. 2015;62:657–663. doi: 10.1109/TBME.2014.2363173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Christley S, Lee B, Dai X, Nie Q. Integrative multicellular biological modeling: a case study of 3D epidermal development using GPU algorithms. BMC Syst Biol. 2010;4:107. doi: 10.1186/1752-0509-4-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cleary K, Peters TM. Image-guided interventions: technology review and clinical applications. Annu Rev Biomed Eng. 2010;12:119–142. doi: 10.1146/annurev-bioeng-070909-105249. [DOI] [PubMed] [Google Scholar]
- 22.Clements LW, Chapman WC, Dawant BM, Galloway RL, Miga MI. Robust surface registration using salient anatomical features for image-guided liver surgery: Algorithm and validation. Medical Physics. 2008;35:2528–2540. doi: 10.1118/1.2911920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Collins TC, Daley J, Henderson WH, Khuri SF. Risk factors for prolonged length of stay after major elective surgery. Ann Surg. 1999;230:251–259. doi: 10.1097/00000658-199908000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Conley RH, Meszoely IM, Weis JA, Pheiffer TS, Arlinghaus LR, Yankeelov TE, Miga MI. Realization of a biomechanical model assisted image guidance system for breast cancer surgery using supine MRI. International Journal of Computer Assisted Radiology and Surgery. 2015 doi: 10.1007/s11548-015-1235-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dallon JC. Multiscale modeling of cellular systems in biology. Current Opinion in Colloid & Interface Science. 2010;15:24–31. [Google Scholar]
- 26.Dallon JC, Sherratt JA, Maini PK. Mathematical modelling of extracellular matrix dynamics using discrete cells: fiber orientation and tissue regeneration. J Theor. Biol. 1999;199:449–471. doi: 10.1006/jtbi.1999.0971. [DOI] [PubMed] [Google Scholar]
- 27.De Jesus AM, Aghvami M, Sander EA. A Combined In Vitro Imaging and Multi-scale Modeling System for Studying the Role of Cell Matrix Interactions in Cutaneous Wound Healing. PLoS One. 2016 doi: 10.1371/journal.pone.0148254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.De Jesus AM, Sander EA. Observing and quantifying fibroblast-mediated fibrin gel compaction. J Vis Exp. 2014:e50918. doi: 10.3791/50918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dick TE, Molkov Y, Nieman G, Hsieh Y, Jacono FJ, Doyle J, Scheff J, Calvano SE, Androulakis IP, An G, Vodovotz Y. Linking inflammation and cardiorespiratory variability in sepsis via computational modeling. Front Physiol. 2012;3:222. doi: 10.3389/fphys.2012.00222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dimaio S, Kapur T, Cleary K, Aylward S, Kazanzides P, Vosburgh K, Ellis R, Duncan J, Farahani K, Lemke H, Peters T, Lorensen WB, Gobbi D, Haller J, Clarke LL, Pizer S, Taylor R, Galloway R, Jr, Fichtinger G, Hata N, Lawson K, Tempany C, Kikinis R, Jolesz F. Challenges in image-guided therapy system design. Neuroimage. 2007;37(Suppl 1):S144–S151. doi: 10.1016/j.neuroimage.2007.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dokukina IV, Gracheva ME. A model of fibroblast motility on substrates with different rigidities. Biophys J. 2010;98:2794–2803. doi: 10.1016/j.bpj.2010.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dorsett-Martin WA. Rat models of skin wound healing: a review. Wound. Repair Regen. 2004;12:591–599. doi: 10.1111/j.1067-1927.2004.12601.x. [DOI] [PubMed] [Google Scholar]
- 33.Dumpuri P, Thompson RC, Dawant BM, Cao A, Miga MI. An atlas-based method to compensate for brain shift: Preliminary results. Medical Image Analysis. 2007;11:128–145. doi: 10.1016/j.media.2006.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Duscher D, Maan ZN, Wong VW, Rennert RC, Januszyk M, Rodrigues M, Hu M, Whitmore AJ, Whittam AJ, Longaker MT, Gurtner GC. Mechanotransduction and fibrosis. J Biomech. 2014;47:1997–2005. doi: 10.1016/j.jbiomech.2014.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dutta-Moscato J, Solovyev A, Mi Q, Nishikawa T, Soto-Gutierrez A, Fox IJ, Vodovotz Y. A multiscale agent-based in silico model of liver fibrosis progression. Frontiers in Bioengineering and Biotechnology. 2014;2:1–10. doi: 10.3389/fbioe.2014.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Edgar LT, Sibole SC, Underwood CJ, Guilkey JE, Weiss JA. A computational model of in vitro angiogenesis based on extracellular matrix fibre orientation. Comput Methods Biomech Biomed Engin. 2013;16:790–801. doi: 10.1080/10255842.2012.662678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eming SA. Biology of Wound Healing. In: Bolognia JL, Jorizzo JL, Schaffer JV, editors. Dermatology. Philadephia: Elsevier Saunders; 2012. [Google Scholar]
- 38.Evans ND, Oreffo RO, Healy E, Thurner PJ, Man YH. Epithelial mechanobiology, skin wound healing, and the stem cell niche. J Mech Behav Biomed Mater. 2013;28:397–409. doi: 10.1016/j.jmbbm.2013.04.023. [DOI] [PubMed] [Google Scholar]
- 39.Faeder JR. Toward a comprehensive language for biological systems. BMC. Biol. 2011;9:68. doi: 10.1186/1741-7007-9-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Foteinou PT, Calvano SE, Lowry SF, Androulakis IP. Translational potential of systems-based models of inflammation. Clin. Transl. Sci. 2009;2:85–89. doi: 10.1111/j.1752-8062.2008.00051.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Garcia PA, Rossmeisl JH, Neal RE, Ellis TL, Davalos RV. A Parametric Study Delineating Irreversible Electroporation from Thermal Damage Based on a Minimally Invasive Intracranial Procedure. Biomedical Engineering Online. 2011;10:21. doi: 10.1186/1475-925X-10-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gill S, Abolmaesumi P, Fichtinger G, Boisvert J, Pichora D, Borshneck D, Mousavi P. Biomechanically constrained groupwise ultrasound to CT registration of the lumbar spine. Medical Image Analysis. 2012;16:662–674. doi: 10.1016/j.media.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 43.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Judd SE, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Mackey RH, Magid DJ, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER, 3rd, Moy CS, Mussolino ME, Neumar RW, Nichol G, Pandey DK, Paynter NP, Reeves MJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Wong ND, Woo D, Turner MB. Heart disease and stroke statistics--2014 update: a report from the American Heart Association. Circulation. 2014;129:e28–e292. doi: 10.1161/01.cir.0000441139.02102.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gopalakrishnan V, Kim M, An G. Using an Agent-Based Model to Examine the Role of Dynamic Bacterial Virulence Potential in the Pathogenesis of Surgical Site Infection. Adv Wound Care (New Rochelle) 2013;2:510–526. doi: 10.1089/wound.2012.0400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hadi MF, Sander EA, Barocas VH. Multiscale model predicts tissue-level failure from collagen fiber-level damage. J Biomech Eng. 2012;134:091005. doi: 10.1115/1.4007097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hadi MF, Sander EA, Ruberti JW, Barocas VH. Simulated remodeling of loaded collagen networks via strain-dependent enzymatic degradation and constant-rate fiber growth. Mech Mater. 2012;44:72–82. doi: 10.1016/j.mechmat.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hammill CW, Clements LW, Stefansic JD, Wolf RF, Hansen PD, Gerber DA. Evaluation of a Minimally Invasive Image-Guided Surgery System for Hepatic Ablation Procedures. Surgical Innovation. 2014;21:419–426. doi: 10.1177/1553350613508019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hansen J, Iyengar R. Computation as the mechanistic bridge between precision medicine and systems therapeutics. Clin. Pharmacol. Ther. 2013;93:117–128. doi: 10.1038/clpt.2012.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Heidenreich PA, Albert NM, Allen LA, Bluemke DA, Butler J, Fonarow GC, Ikonomidis JS, Khavjou O, Konstam MA, Maddox TM, Nichol G, Pham M, Pina IL, Trogdon JG. Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ Heart Fail. 2013;6:606–619. doi: 10.1161/HHF.0b013e318291329a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Henak CR, Anderson AE, Weiss JA. Subject-specific analysis of joint contact mechanics: application to the study of osteoarthritis and surgical planning. J Biomech Eng. 2013;135:021003. doi: 10.1115/1.4023386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hinz B. The myofibroblast: paradigm for a mechanically active cell. J Biomech. 2010;43:146–155. doi: 10.1016/j.jbiomech.2009.09.020. [DOI] [PubMed] [Google Scholar]
- 52.Hunt CA, Ropella GE, Lam T, Gewitz AD. Relational grounding facilitates development of scientifically useful multiscale models. Theor Biol Med Model. 2011;8:35. doi: 10.1186/1742-4682-8-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jackson PR, Juliano J, Hawkins-Daarud A, Rockne RC, Swanson KR. Patient-Specific Mathematical Neuro-Oncology: Using a Simple Proliferation and Invasion Tumor Model to Inform Clinical Practice. Bulletin of Mathematical Biology. 2015;77:846–856. doi: 10.1007/s11538-015-0067-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ji S, Fan X, Paulsen K, Roberts D, Mirza SK, Lollis SS. Patient Registration Using Intraoperative Stereovision in Image-guided Open Spinal Surgery. IEEE Trans. Biomed. Eng. 2015;62:2177–2186. doi: 10.1109/TBME.2015.2415731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ji S, Fan X, Paulsen KD, Roberts DW, Mirza SK, Lollis SS. Intraoperative CT as a registration benchmark for intervertebral motion compensation in image-guided open spinal surgery. Int. J. Comput. Assist. Radiol. Surg. 2015 doi: 10.1007/s11548-015-1255-5. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ji S, Fan X, Roberts DW, Hartov A, Schaewe TJ, Simon DA, Paulsen KD. Brain Shift Compensation via Intraoperative Imaging and Data Assimilation. In: Neu C, Genin G, editors. CRC Handbook of Imaging in Biological Mechanics. CRC Press and Taylor & Francis; 2014. pp. 229–240. [Google Scholar]
- 57.Ji SB, Hartov A, Roberts D, Paulsen K. Data assimilation using a gradient descent method for estimation of intraoperative brain deformation. Medical Image Analysis. 2009;13:744–756. doi: 10.1016/j.media.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jones RHVE, Michler RE, Sopko G, Oh JK, O'Connor CM, Hill JA, Menicanti L, Sadowski Z, Desvigne-Nickens P, Rouleau JL, Lee KL. STICH Hypothesis 2 Investigators. Coronary bypass surgery with or without surgical ventricular reconstruction. N Engl J Med. 2009:1705–1717. doi: 10.1056/NEJMoa0900559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kim C, O'Rourke AP, Will JA, Mahvi DM, Webster JG. Finite-element analysis of hepatic cryoablation around a large blood vessel. Ieee Transactions on Biomedical Engineering. 2008;55:2087–2093. doi: 10.1109/TBME.2008.919837. [DOI] [PubMed] [Google Scholar]
- 60.Kim Y, Kim D. A fully automatic vertebra segmentation method using 3D deformable fences. Computerized Medical Imaging and Graphics. 2009;33:343–352. doi: 10.1016/j.compmedimag.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 61.Klinder T, Ostermann J, Ehm M, Franz A, Kneser R, Lorenz C. Automated model-based vertebra detection, identification, and segmentation in CT images. Medical Image Analysis. 2009;13:471–482. doi: 10.1016/j.media.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 62.Lalys F, Jannin P. Surgical process modelling: a review. Int J Comput Assist Radiol Surg. 2014;9:495–511. doi: 10.1007/s11548-013-0940-5. [DOI] [PubMed] [Google Scholar]
- 63.Lang A, Mousavi P, Gill S, Fichtinger G, Abolmaesumi P. Multi-modal registration of speckle-tracked freehand 3D ultrasound to CT in the lumbar spine. Medical Image Analysis. 2012;16:675–686. doi: 10.1016/j.media.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 64.Lee LCGL, Zhang Z, Pease M, Nikolic SD, Mishra R, Ratcliffe MB, Guccione JM. Patient-specific finite element modeling of the Cardiokinetix Parachute(®) device: effects on left ventricular wall stress and function. Med Biol Eng Comput. 2014;52:557–566. doi: 10.1007/s11517-014-1159-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lee LC, Wenk JF, Zhong L, Klepach D, Zhang Z, Ge L, Ratcliffe MB, Zohdi TI, Hsu E, Navia JL, Kassab GS, Guccione JM. Analysis of patient-specific surgical ventricular restoration: importance of an ellipsoidal left ventricular geometry for diastolic and systolic function. J Appl Physiol (1985) 2013;115:136–144. doi: 10.1152/japplphysiol.00662.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lee LCWS, Klepach D, Ge L, Zhang Z, Lee RJ, Hinson A, Gorman JH, 3rd, Gorman RC, Guccione JM. Algisyl-LVR™ with coronary artery bypass grafting reduces left ventricular wall stress and improves function in the failing human heart. Int J Cardiol. 2013b;168:2022–2028. doi: 10.1016/j.ijcard.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li NYK, Verdolini K, Clermont G, Mi Q, Hebda PA, Vodovotz Y. A patient-specific in silico model of inflammation and healing tested in acute vocal fold injury. PLoS ONE. 2008;3:e2789. doi: 10.1371/journal.pone.0002789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li NYK, Vodovotz Y, Hebda PA, Verdolini K. Biosimulation of inflammation and healing in surgically injured vocal folds. Ann. Otol. Rhinol. Laryngol. 2010;119:412–423. doi: 10.1177/000348941011900609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li NYK, Vodovotz Y, Kim KH, Mi Q, Hebda PA, Verdolini Abbott K. Biosimulation of acute phonotrauma: an extended model. Laryngoscope. 2011;121:2418–2428. doi: 10.1002/lary.22226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Li X, Upadhyay AK, Bullock AJ, Dicolandrea T, Xu J, Binder RL, Robinson MK, Finlay DR, Mills KJ, Bascom CC, Kelling CK, Isfort RJ, Haycock JW, MacNeil S, Smallwood RH. Skin stem cell hypotheses and long term clone survival--explored using agent-based modelling. Sci Rep. 2013;3:1904. doi: 10.1038/srep01904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lindblad WJ. Considerations for selecting the correct animal model for dermal wound-healing studies. J. Biomater. Sci. Polym. Ed. 2008;19:1087–1096. doi: 10.1163/156856208784909390. [DOI] [PubMed] [Google Scholar]
- 72.Ma J, Lu L, Zhan YQ, Zhou XA, Salganicoff M, Krishnan A. Hierarchical Segmentation and Identification of Thoracic Vertebra Using Learning-Based Edge Detection and Coarse-to-Fine Deformable Model. Medical Image Computing and Computer-Assisted Intervention - Miccai 2010, Pt I. 2010;6361:19–27. doi: 10.1007/978-3-642-15705-9_3. [DOI] [PubMed] [Google Scholar]
- 73.Macal CM, North MJ. Tutorial on agent-based modeling and simulation; Proceedings of the 37th conference on Winter simulation Winter Simulation Conference; 2005. pp. 2–15. [Google Scholar]
- 74.Maier-Hein L, Mountney P, Bartoli A, Elhawary H, Elson D, Groch A, Kolb A, Rodrigues M, Sorger J, Speidel S, Stoyanov D. Optical techniques for 3D surface reconstruction in computer-assisted laparoscopic surgery. Medical Image Analysis. 2013;17:974–996. doi: 10.1016/j.media.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 75.Martin P. Wound healing--aiming for perfect skin regeneration. Science. 1997;276:75–81. doi: 10.1126/science.276.5309.75. [DOI] [PubMed] [Google Scholar]
- 76.McDougall S, Dallon J, Sherratt J, Maini P. Fibroblast migration and collagen deposition during dermal wound healing: mathematical modelling and clinical implications. Philos. Transact. A Math. Phys. Eng Sci. 2006;364:1385–1405. doi: 10.1098/rsta.2006.1773. [DOI] [PubMed] [Google Scholar]
- 77.McDougall S, Dallon J, Sherratt J, Maini P. Fibroblast migration and collagen deposition during dermal wound healing: mathematical modelling and clinical implications. Philos Trans A Math Phys Eng Sci. 2006;364:1385–1405. doi: 10.1098/rsta.2006.1773. [DOI] [PubMed] [Google Scholar]
- 78.Mi Q, Li NYK, Ziraldo C, Ghuma A, Mikheev M, Squires R, Okonkwo DO, Verdolini Abbott K, Constantine G, An G, Vodovotz Y. Translational systems biology of inflammation: Potential applications to personalized medicine. Personalized Medicine. 2010;7:549–559. doi: 10.2217/pme.10.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mi Q, Rivière B, Clermont G, Steed DL, Vodovotz Y. Agent-based model of inflammation and wound healing: insights into diabetic foot ulcer pathology and the role of transforming growth factor-β1. Wound Rep. Reg. 2007;15:617–682. doi: 10.1111/j.1524-475X.2007.00271.x. [DOI] [PubMed] [Google Scholar]
- 80.Miga MI. Computational Modeling for Enhancing Soft Tissue Image Guided Surgery: An Application in Neurosurgery. Ann. Biomed. Eng. 2015 doi: 10.1007/s10439-015-1433-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Misic AM, Gardner SE, Grice EA. The Wound Microbiome: Modern Approaches to Examining the Role of Microorganisms in Impaired Chronic Wound Healing. Adv Wound Care (New Rochelle) 2014;3:502–510. doi: 10.1089/wound.2012.0397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Muratore D, J R, Dawant B, Galloway RJ. Three-dimensional image registration of phantom vertebrae for image-guided surgery: a preliminary study. Comput Aided Surg. 2002;7:342–352. doi: 10.1002/igs.10055. [DOI] [PubMed] [Google Scholar]
- 83.Najjar PA, Smink DS. Prophylactic antibiotics and prevention of surgical site infections. Surg Clin North Am. 2015;95:269–283. doi: 10.1016/j.suc.2014.11.006. [DOI] [PubMed] [Google Scholar]
- 84.National Research Council (U.S.) Toward precision medicine : building a knowledge network for biomedical research and a new taxonomy of disease. Washington, D.C: National Academies Press; 2011. Committee on A Framework for Developing a New Taxonomy of Disease; pp. xiii–128. [PubMed] [Google Scholar]
- 85.Newman SA, Christley S, Glimm T, Hentschel HG, Kazmierczak B, Zhang YT, Zhu J, Alber M. Multiscale models for vertebrate limb development. Curr. Top. Dev. Biol. 2008;81:311–340. doi: 10.1016/S0070-2153(07)81011-8. [DOI] [PubMed] [Google Scholar]
- 86.Padoy N, Blum T, Ahmadi SA, Feussner H, Berger MO, Navab N. Statistical modeling and recognition of surgical workflow. Medical Image Analysis. 2012;16:632–641. doi: 10.1016/j.media.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 87.Peterson TA, Doughty E, Kann MG. Towards precision medicine: advances in computational approaches for the analysis of human variants. J. Mol. Biol. 2013;425:4047–4063. doi: 10.1016/j.jmb.2013.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Qutub AA, Mac GF, Karagiannis ED, Vempati P, Popel AS. Multiscale models of angiogenesis. IEEE Eng Med Biol Mag. 2009;28:14–31. doi: 10.1109/MEMB.2009.931791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rasoulian A, Rohling R, Abolmaesumi P. Lumbar Spine Segmentation Using a Statistical Multi-Vertebrae Anatomical Shape + Pose Model. IEEE Trans. Med. Imaging. 2013;32:890–1900. doi: 10.1109/TMI.2013.2268424. [DOI] [PubMed] [Google Scholar]
- 90.Reinhardt JW, Krakauer DA, Gooch KJ. Complex Matrix Remodeling and Durotaxis Can Emerge From Simple Rules for Cell-Matrix Interaction in Agent-Based Models. J Biomech Eng. 2013;135:071003. doi: 10.1115/1.4024463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rouillard AD, Holmes JW. Coupled agent-based and finite-element models for predicting scar structure following myocardial infarction. Prog Biophys Mol Biol. 2014;115:235–243. doi: 10.1016/j.pbiomolbio.2014.06.010. [DOI] [PubMed] [Google Scholar]
- 92.Rucker DC, Wu Y, Clements LW, Ondrake JE, Pheiffer TS, Simpson AL, Jarnagin WR, Miga MI. A Mechanics-Based Nonrigid Registration Method for Liver Surgery Using Sparse Intraoperative Data. Ieee Transactions on Medical Imaging. 2014;33:147–158. doi: 10.1109/TMI.2013.2283016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sander E, Stein A, Swickrath M, Barocas V. Trends in Computational Nanomechanics. Netherlands: Springer; 2010. Out of many, one: modeling schemes for biopolymer and biofibril networks; pp. 557–602. [Google Scholar]
- 94.Sander EA, Stylianopoulos T, Tranquillo RT, Barocas VH. Image-based multiscale modeling predicts tissue-level and network-level fiber reorganization in stretched cell-compacted collagen gels. Proc Natl Acad Sci U S A. 2009;106:17675–17680. doi: 10.1073/pnas.0903716106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sanga S, Frieboes HB, Zheng X, Gatenby R, Bearer EL, Cristini V. Predictive oncology: a review of multidisciplinary, multiscale in silico modeling linking phenotype, morphology and growth. Neuroimage. 2007;37(Suppl 1):S120–S134. doi: 10.1016/j.neuroimage.2007.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Scheff JD, Mavroudis PD, Foteinou PT, An G, Calvano SE, Doyle J, Dick TE, Lowry SF, Vodovotz Y, Androulakis IP. A multiscale modeling approach to inflammation: A case study in human endotoxemia. Shock. 2013;244:279–289. [Google Scholar]
- 97.Schluter DK, Ramis-Conde I, Chaplain MA. Computational modeling of single-cell migration: the leading role of extracellular matrix fibers. Biophys J. 2012;103:1141–1151. doi: 10.1016/j.bpj.2012.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Simpson AL, Geller DA, Hemming AW, Jarnagin WR, Clements LW, D'Angelica MI, Dumpuri P, Goenen M, Zendejas I, Miga MI, Stefansic JD. Liver Planning Software Accurately Predicts Postoperative Liver Volume and Measures Early Regeneration. Journal of the American College of Surgeons. 2014;219:199–207. doi: 10.1016/j.jamcollsurg.2014.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Solovyev A, Mi Q, Tzen Y-T, Brienza D, Vodovotz Y. Hybrid equation- / agent-based model of ischemia-induced hyperemia and pressure ulcer formation predicts greater propensity to ulcerate in subjects with spinal cord injury. PLoS Comp. Biol. 2013;9:e1003070. doi: 10.1371/journal.pcbi.1003070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Stern JR, Christley S, Zaborina O, Alverdy JC, An G. Integration of TGF-beta- and EGFR-based signaling pathways using an agent-based model of epithelial restitution. Wound Repair Regen. 2012;20:862–863. doi: 10.1111/j.1524-475X.2012.00852.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Stern JR, Olivas AD, Valuckaite V, Zaborina O, Alverdy JC, An G. Agent-based model of epithelial host-pathogen interactions in anastomotic leak. J Surg Res. 2013;184:730–738. doi: 10.1016/j.jss.2012.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Stoverud KH, Darcis M, Helmig R, Hassanizadeh SM. Modeling Concentration Distribution and Deformation During Convection-Enhanced Drug Delivery into Brain Tissue. Transport in Porous Media. 2012;92:119–143. [Google Scholar]
- 103.Stylianopoulos T, Barocas VH. Volume-averaging theory for the study of the mechanics of collagen networks. Computer Methods in Applied Mechanics and Engineering. 2007;196:2981–2990. [Google Scholar]
- 104.Sullivan TP, Eaglstein WH, Davis SC, Mertz P. The pig as a model for human wound healing. Wound Repair Regen. 2001;9:66–76. doi: 10.1046/j.1524-475x.2001.00066.x. [DOI] [PubMed] [Google Scholar]
- 105.Sun K, Pheiffer TS, Simpson AL, Weis JA, Thompson RC, Miga MI. Near Real-Time Computer Assisted Surgery for Brain Shift Correction Using Biomechanical Models. IEEE J Transl Eng Health Med. 2014;2 doi: 10.1109/JTEHM.2014.2327628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sun T, Adra S, Smallwood R, Holcombe M, MacNeil S. Exploring hypotheses of the actions of TGF-beta1 in epidermal wound healing using a 3D computational multiscale model of the human epidermis. PLoS One. 2009;4:e8515. doi: 10.1371/journal.pone.0008515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Tang D, Yang C, Zheng J, Canton G, Bach R, Hatsukami T, Wang L, Billiar K, Yang D, Yuan C. Image-Based Modeling and Precision Medicine: Patient-Specific Carotid and Coronary Plaque Assessment and Predictions. IEEE Trans. Biomed Eng. 2013 doi: 10.1109/TBME.2013.2242891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Taylor GD, Kirkland TA, McKenzie MM, Sutherland B, Wiens RM. The effect of surgical wound infection on postoperative hospital stay. Can J Surg. 1995;38:149–153. [PubMed] [Google Scholar]
- 109.Tranquillo RT, Murray JD. Continuum model of fibroblast-driven wound contraction: inflammation-mediation. J Theor Biol. 1992;158:135–172. doi: 10.1016/s0022-5193(05)80715-5. [DOI] [PubMed] [Google Scholar]
- 110.Underwood CJ, Edgar LT, Hoying JB, Weiss JA. Cell-generated traction forces and the resulting matrix deformation modulate microvascular alignment and growth during angiogenesis. Am J Physiol Heart Circ Physiol. 2014;307:H152–H164. doi: 10.1152/ajpheart.00995.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Vermolen FJ, Gefen A. Multiscale Computer Modeling in Biomechanics and Biomedical Engineering. Springer; 2013. Wound healing: multi-scale modeling; pp. 321–345. [Google Scholar]
- 112.Vodovotz Y, An G. Systems Biology and Inflammation. In: Yan Q, editor. Systems Biology in Drug Discovery and Development: Methods and Protocols. Totowa, NJ: Springer Science & Business Media; 2009. pp. 181–201. [Google Scholar]
- 113.Vodovotz Y, Clermont G, Chow C, An G. Mathematical models of the acute inflammatory response. Curr. Opin. Crit Care. 2004;10:383–390. doi: 10.1097/01.ccx.0000139360.30327.69. [DOI] [PubMed] [Google Scholar]
- 114.Vodovotz Y, Csete M, Bartels J, Chang S, An G. Translational systems biology of inflammation. PLoS. Comput. Biol. 2008;4:1–6. doi: 10.1371/journal.pcbi.1000014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Walker DC, Georgopoulos NT, Southgate J. From pathway to population--a multiscale model of juxtacrine EGFR-MAPK signalling. BMC. Syst. Biol. 2008;2:102. doi: 10.1186/1752-0509-2-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Walker DC, Hill G, Wood SM, Smallwood RH, Southgate J. Agent-based computational modeling of wounded epithelial cell monolayers. IEEE Trans. Nanobioscience. 2004;3:153–163. doi: 10.1109/tnb.2004.833680. [DOI] [PubMed] [Google Scholar]
- 117.Wall STWJ, Healy KE, Ratcliffe MB, Guccione JM. Theoretical impact of the injection of material into the myocardium: a finite element model simulation. Circulation. 2006;114:2627–2635. doi: 10.1161/CIRCULATIONAHA.106.657270. [DOI] [PubMed] [Google Scholar]
- 118.Waugh HV, Sherratt JA. Macrophage dynamics in diabetic wound dealing. Bull. Math. Biol. 2006;68:197–207. doi: 10.1007/s11538-005-9022-3. [DOI] [PubMed] [Google Scholar]
- 119.Waugh HV, Sherratt JA. Modeling the effects of treating diabetic wounds with engineered skin substitutes. Wound. Repair Regen. 2007;15:556–565. doi: 10.1111/j.1524-475X.2007.00270.x. [DOI] [PubMed] [Google Scholar]
- 120.Wearing HJ, Sherratt JA. Keratinocyte growth factor signalling: a mathematical model of dermal-epidermal interaction in epidermal wound healing. Math. Biosci. 2000;165:41–62. doi: 10.1016/s0025-5564(00)00008-0. [DOI] [PubMed] [Google Scholar]
- 121.Weston AD, Hood L. Systems biology, proteomics, and the future of health care: toward predictive, preventative, and personalized medicine. J. Proteome. Res. 2004;3:179–196. doi: 10.1021/pr0499693. [DOI] [PubMed] [Google Scholar]
- 122.Witte MB, Barbul A. General principles of wound healing. Surg Clin North Am. 1997;77:509–528. doi: 10.1016/s0039-6109(05)70566-1. [DOI] [PubMed] [Google Scholar]
- 123.Witte MB, Barbul A. General principles of wound healing. Surg. Clin. North Am. 1997;77:509–528. doi: 10.1016/s0039-6109(05)70566-1. [DOI] [PubMed] [Google Scholar]
- 124.Xu Z, Kim O, Kamocka M, Rosen ED, Alber M. Multiscale models of thrombogenesis. Wiley Interdiscip Rev Syst Biol Med. 2012;4:237–246. doi: 10.1002/wsbm.1160. [DOI] [PubMed] [Google Scholar]
- 125.Yankeelov TE, Atuegwu N, Hormuth D, Weis JA, Barnes SL, Miga MI, Rericha EC, Quaranta V. Clinically Relevant Modeling of Tumor Growth and Treatment Response. Science Translational Medicine. 2013;5 doi: 10.1126/scitranslmed.3005686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zhao G, Usui ML, Lippman SI, James GA, Stewart PS, Fleckman P, Olerud JE. Biofilms and Inflammation in Chronic Wounds. Adv Wound Care (New Rochelle) 2013;2:389–399. doi: 10.1089/wound.2012.0381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ziraldo C, Solovyev A, Allegretti A, Krishnan S, Henzel MK, Sowa GA, Brienza D, An G, Mi Q, Vodovotz Y. A Computational, Tissue-Realistic Model of Pressure Ulcer Formation in Individuals with Spinal Cord Injury. PLoS Comput Biol. 2015;11:e1004309. doi: 10.1371/journal.pcbi.1004309. [DOI] [PMC free article] [PubMed] [Google Scholar]