Abstract
Human Immunodeficiency Virus type 1 (HIV-1) infection induces neurological and neuropsychological deficits, which are associated with dysregulation of the medial prefrontal cortex (mPFC) and other vulnerable brain regions. We evaluated the impact of HIV infection in the mPFC and the therapeutic potential of targeting over-active voltage-gated L-type Ca2+ channels (L-channel) and NMDA receptors (NMDAR), as modeled in HIV-1 transgenic (Tg) rats. Whole-cell patch-clamp recording was used to assess the membrane properties and voltage-sensitive Ca2+ potentials (Ca2+ influx) in mPFC pyramidal neurons. Neurons from HIV-1 Tg rats displayed reduced rheobase, spike amplitude and inwardly-rectifying K+ influx, increased numbers of action potentials, and a trend of aberrant firing compared to those from non-Tg control rats. Neuronal hyper-excitation was associated with abnormally-enhanced Ca2+ influx (independent of NMDAR), which was eliminated by acute L-channel blockade. Combined chronic blockade of over-active L-channels and NMDARs with open-channel blockers abolished HIV effects on spiking, aberrant firing and Ca2+ potential half-amplitude duration, though not the reduced inward rectification. In contrast, individual chronic blockade of over-active L-channels or NMDARs did not alleviate HIV-induced mPFC hyper-excitability. These studies demonstrate that HIV alters mPFC neuronal activity by dysregulating membrane excitability and Ca2+ influx through the L-channels. This renders these neurons more susceptible and vulnerable to excitatory stimuli, and could contribute to HIV-associated neuropathogenesis. Combined targeting of over-active L-channels/NMDARs alleviates HIV-induced dysfunction of mPFC pyramidal neurons, emphasizing a potential novel therapeutic strategy that may effectively decrease HIV-induced Ca2+ dysregulation in the mPFC.
Keywords: HIV, medial prefrontal cortex, calcium, L-channel, NMDAR, pyramidal neuron, calcium channel blockade, HAND
Introduction
The introduction of combined antiretroviral therapy (cART) transformed HIV from a death sentence to a chronic disease with several co-morbid complications, including HIV-Associated Neurocognitive Disorders (HAND) (Antinori et al., 2007). Despite cART, HAND affects ~50% of HIV-infected individuals (Heaton et al., 2010; Simioni et al., 2010) and its prevalence is expected to increase as the HIV-infected population ages. This underscores the need to elucidate cellular/molecular mechanisms driving HIV-mediated neuropathogenesis to devise effective strategies that prevent and/or treat HAND.
HIV affects many brain regions, including the vulnerable medial prefrontal cortex (mPFC) (Ferris et al., 2008), a key regulator of cognition, emotion and motivation-driven behavior, from which glutamatergic pyramidal neurons (80-90% of mPFC neurons) (Yuste, 2005) provide excitatory inputs to the HIV-vulnerable striatum and midbrain (Ferris et al., 2008; Sesack et al., 1989). Although neurons are not infected, HIV-infected leukocytes invade the brain where the virus is transmitted to glial cells, which release neurotoxic viral proteins and inflammatory molecules that cause neuronal dysregulation, injury and loss. Such neuronal dysfunction involves Ca2+ dysregulation, excitotoxicity, oxidative stress, and mitochondrial dysfunction, but the cellular/molecular mechanisms underlying these pathogenic cascades are not fully understood (Mattson et al., 2005).
Because Ca2+ regulates many fundamental signaling and gene expression pathways, severe alterations in Ca2+ homeostasis can be damaging. HIV-1 proteins and HIV-induced inflammatory processes alter Ca2+ homeostasis by excessively increasing intracellular Ca2+ levels ([Ca2+]in) (Chami et al., 2006; Hu, 2015). Much emphasis has been placed on dysfunction of the NMDAR, a Ca2+-permeable ligand-gated ionotropic glutamatergic receptor, which is well-established to participate in HIV-induced Ca2+ dysregulation (Haughey & Mattson, 2002). However, HIV-induced [Ca2+]in increases are also regulated by intracellular Ca2+ release and voltage-gated Ca2+ channels (VGCCs) (Brini et al., 2014; Hu, 2015). In previous clinical trials, individual blockade of NMDARs or voltage-gated L-type Ca2+ channels (L-channels) failed to improve the progression of HIV-associated dementia (Navia et al., 1998; Schifitto et al., 2007).
Our recent research has focused on the impact of HIV on the L-channel, which mediates intrinsic excitability of neurons and regulates Ca2+-induced signal transduction and gene expression (Brini et al., 2014; Ikeda, 2001). We have demonstrated that HIV-1 Tat abnormally increases L-channel activity and expression in the mPFC (Hu, 2015; Napier et al., 2014; 2015a; Wayman et al., 2012), and that chronic exposure to multiple HIV-1 proteins for 6 months increases L-channel expression in vivo (Wayman et al., 2015b). These findings, in combination with the established involvement of NMDAR in HIV neuropathogenesis (Haughey & Mattson, 2002; Mattson et al., 2005), led us to hypothesize that combined repetitive targeting of both over-active L-channels and NMDARs would decrease HIV-induced mPFC neuronal hyper-excitability.
In the present study, we evaluated the impact of combined chronic blockade of over-active L-channels/NMDARs in HAND, as modeled in the HIV-1 Tg rat (Reid et al., 2001). HIV-1 Tg rats express 7 of the 9 HIV-1 genes (gag/pol-deleted) and have been used extensively to model HIV-1 effects on the brain, and recapitulate many features of HAND including neuronal injury and pro-inflammatory responses in the brain (Peng et al., 2010; Royal, III et al., 2012). Here, we assessed HIV-induced functional alterations in the membrane properties and Ca2+ influx of mPFC pyramidal neurons and determined whether combined (or individual) chronic blockade of over-active L-channels and/or NMDAR alleviates these alterations. Two selective (use-dependent) “open-channel” blockers, diltiazem (Dilt, for the L-channel) (Niimi et al., 2003) and memantine (Mem, for the NMDAR) (Chen et al., 1992) were used, which preferentially block over-activated L-channels and NMDARs, respectively, but reserve “house-keeping” channel activity. We report here that (1) mPFC pyramidal neurons in HIV-1 Tg rats are hyper-excitable, exhibiting an abnormal increase of firing and voltage-sensitive Ca2+ influx, due partly to L-channel over-activation, independent of NMDAR, and (2) combined, but not individual, chronic blockade of over-active L-channels and NMDAR significantly reduces these HIV-induced functional deficits. Our studies point to a therapeutic potential for targeting both L-channels and NMDARs to ameliorate and/or reduce HAND.
Materials and Methods
Animals
Male HIV-1 Tg and non-Tg F344 rats, purchased from Envigo (Indianapolis, IN) at 3-4 weeks of age, were housed at the Rush University Comparative Research Center on a 12 hour light/dark cycle with food and water available ad libitum. Rats received daily subcutaneous injections of saline (SAL, 0.1 ml), the selective L-channel blocker, Dilt (15 mg/kg), the selective NMDAR antagonist, Mem (10 mg/kg), or combined Dilt/Mem for 2 weeks, and were used for electrophysiological experiments at 6-7 weeks of age. Animal care and use procedures were conducted with Institutional Animal Care and Use Committee (IACUC) approval and in accordance with NIH, USDA and institutional guidelines.
Whole-cell patch-clamp recording from ex vivo brain slices
Chloral hydrate-sedated rats (400mg/kg, i.p.) were transcardially perfused with ice-cold cutting solution (in mM: 248 sucrose, 2.9 KCl, 2 MgSO4, 1.25 NaH2PO4, 26 NaHCO3, 0.1 CaCl2, 10 glucose, 3 kynurenuc acid, 1 ascorbic acid; pH=7.4-7.45). Brains were removed, immersed in ice-cold cutting solution, then coronally sectioned at 300µm using a vibratome (Leica VT1000S, Buffalo Grove, IL). Slices were incubated in artificial cerebrospinal fluid (aCSF; in mM: 125 NaCl, 2.5 KCl, 25 NaHCO3, 1.25 NaH2PO4, 1 MgCl2, 2 CaCl2, 15 glucose; pH=7.35-7.4, 305-315 mOsm) for 1hr at RT before anchored in an aCSF-perfused recording chamber at ~34ºC. All solutions that brains/brain slices were placed in were oxygenated (95% O2, 5% CO2).
Pyramidal neurons in layers V-VI of the prelimbic area of the mPFC (Paxinos & Watson, 1998) were identified using differential interference contrast microscopy on a Nikon Eclipse E600FN microscope (Nikon Instruments Inc., Melville, NY) for recordings. Recording electrodes were made from glass pipettes using a horizontal pipette puller (p-97 Sutter Instrument Co., Novato, CA) and filled with appropriate internal solution adjusted to pH=7.3-7.35 and 280-285 mOsm [for evoked action potentials (in mM): 120 K-glucontate, 10 HEPES, 0.1 EGTA, 20 KCl, 2 MgCl2, 3 Na2ATP, and 0.3 NaGTP; for voltage-sensitive Ca2+ potentials (in mM): 140 Cs-gluconate, 10 HEPES, 2 MgCl2, 3 Na2ATP and 0.3 NaGTP]. Whole-cell configuration was obtained under voltage-clamp mode settings using internal solution-filled electrodes (4-6 MΩ); then the settings were changed to current-clamp mode for studying changes in the membrane potential (Vm) of neurons. Signals were filtered, amplified and digitized with a Multiclamp 700B amplifier (Molecular Devices, Sunnyvale, CA) and a Digidata 1440 interface (Axon Instruments) and stored on a PC.
To measure evoked Vm responses, hyperpolarizing and depolarizing current pulses (−400 to +300pA) were applied for 500ms at 25pA intervals to mimic inhibitory and excitatory inputs. Membrane hyperpolarization was first applied to remove inactivation, thereby allowing activation of low voltage-activated (LVA) Ca2+ channels (Huguenard, 1996). The membrane properties of neurons were determined from the initial action potential evoked by the minimal depolarizing current (rheobase). The input resistance (Rin) was calculated by fitting the Vm response from the −100pA current step with the Boltzman charge-voltage equation. The inclusion criteria for data analysis were (1) a stable resting membrane potential (RMP) more hyperpolarized than −60mV for neurons from non-Tg control rats, and (2) the amplitude of initial Na+-dependent action potential (evoked by rheobase) greater than 60mV and 40mV, respectively, for neurons from non-Tg and HIV-1 Tg rats (Napier et al., 2014; Wayman et al., 2015a,b). The contribution of L-channels to action potential generation (neuronal excitability) was assessed by selectively blocking L-channel activity with acute perfusion of 5μM nifedipine (Nif) (Nasif et al., 2005a).
To isolate VGCC activity, the perfusing aCSF contained inhibitors to block Na+ channels (0.5µM tetrodotoxin), K+ channels (20mM extracellular tetraethylammonium, 140mM intracellular Cs-gluconate), ionotropic glutamate receptors (2.5mM kynurenic acid) and GABAA receptors (100µM picrotoxin). Vm was held at ~-70mV (around average RMP) to compensate for K+ channel blockade-induced depolarization of RMP (Hu et al., 2004). Ca2+ plateau potentials (reflecting Ca2+ influx through voltage-gated Ca2+ channels) were evoked with 40ms rheobase currents. L-channels were selectively blocked with acute perfusion of 5µM Nif and all voltage-gated Ca2+ influx was blocked with acute perfusion of 400µM cadmium (Cd) in the bath. The Ca2+ potential properties were determined after at least 10min of perfusion with aCSF containing ion channel blockers/receptor inhibitors, and when rheobase-evoked Ca2+ spike recordings became consistent. The Ca2+ potential area is the integrated area under the potential (mV x ms), demarcated at the beginning/end by the holding Vm.
Statistical Analysis
Data were analyzed using SigmaPlot (Systat Software Inc., San Jose, CA) and SPSS (IBM Corporation, Armonk, NY). When appropriate, student’s t-tests and two-way ANOVA were used to compare HIV-1 Tg and non-Tg (Ca2+ potential properties and membrane properties, respectively). A Chi-squared test was used for categorical comparison (aberrant firing events). Three-way repeated measures ANOVA (rmANOVA) was performed when three independent variables were present, and in all cases there was no significant three-way interaction. Therefore, data analysis is displayed using the two-way rmANOVA statistics. Two-way rmANOVA was used to compare responses of neurons at different current steps (spike frequency and I-V relationship) or with/without Nif treatment (Ca2+ potentials after L-channel blockade). One-way ANOVA was used to compare effects of chronic treatment with single Ca2+ channel blockers. In all cases, ANOVA was followed by Newman-Keuls post-hoc tests. Data were excluded if criteria were not met and outliers, defined as more than 2-fold the standard deviation from the mean, were removed. Statistical significance was generally set at p≤0.05. When control (i.e., SAL-pretreated) data points were used in multiple analyses, the Bonferroni correction was applied and the statistical significance set point was lowered (specifically for SAL-treated spike frequency comparison: p≤0.017).
Results
mPFC pyramidal neurons in HIV-1 Tg rats display increased supra-threshold excitability, which is partly due to L-channel over-activation
The supra-threshold excitability of mPFC pyramidal neurons was assessed using depolarizing current steps (0-300pA) in brain slices from non-Tg control and HIV-1 Tg rats. The characteristics of action potentials and spike frequency were assessed. We found that neurons from HIV-1 Tg rats (n=25-28 in 10 rats; for Nif-treated spike frequency: n=8 in 4 rats) displayed reduced Rin (p=0.015), rheobase (p=0.037), action potential amplitude (p<0.001)(Table 1), and increased numbers of action potentials evoked by depolarizing currents (two-way rmANOVA: genotype, F(1,144)=4.599, p=0.053; current, F(12,144)=76.146, p≤0.001; interaction, F(12,144)=3.952, p≤0.001)(Figure 1A-B) as compared to neurons from non-Tg rats (n=28-31 in 15-16 rats; for Nif-treated spike frequency: n=7 in 5 rats). Selective L-channel blockade by acute application of Nif in the bath reduced spiking in neurons from both non-Tg and HIV-1 Tg rats, indicating the importance of L-channel activity in the supra-threshold excitability (two-way rmANOVA; non-Tg: treatment, F(1,72)=7.567, p=0.033; current, F(12,72)=33.020, p≤0.001; interaction, F(12,74)=5.944, p≤0.001; HIV-1 Tg: treatment, F(1,84)=11.423, p=0.012; current, F(12,84)=50.176, p≤0.001; interaction, F(12,84)=3.384, p≤0.001). Acute blockade of the L-channels reduced the enhanced evoked spiking in neurons from HIV-1 Tg rats to levels that were similar to those in vehicle-treated control (Ctrl) non-Tg mPFC (p>0.05). However, Nif induced a greater reduction in spiking in neurons from non-Tg rats as compared to that in Nif-treated neurons from HIV-1 Tg rats (two-way rmANOVA: genotype, F(1,156)=4.045, p=0.066; current, F(12,156)=50.899, p≤0.001; interaction, F(12,156)=4.420, p≤0.001)(Figure 1B), suggesting that L-channel over-activation was not solely responsible for the HIV-induced increase in evoked spiking.
Table 1.
Effects of chronic HIV-1 protein expression in vivo on the membrane properties in rat mPFC pyramidal neurons.
| SAL-non-Tg | SAL-HIV-1 Tg | Dilt/Mem-non-Tg | Dilt/Mem-HIV-1 Tg | |
|---|---|---|---|---|
| RMP (mV) | −68.8 ± 0.9 | −67.0 ± 1.0 | −68.8 ± 1.7 | −68.3 ± 1.3 |
|
| ||||
| Rin (MΩ) | 184.4 ± 10.1 | 221.1 ± 10.6* | 169.9 ± 19.9 | 203.1 ± 15.1 |
| Rheobase (pA) | 88.8 ± 6.0 | 70.0 ± 6.5* | 102.8 ± 10.8 | 88.5 ± 9.0 |
|
| ||||
| Threshold (mV) | −40.2 ± 0.6 | −39.9 ± 0.6 | −39.9 ± 1.1 | −39.6 ± 0.9 |
|
| ||||
| 1/2 amplitude duration (ms) | 1.08 ± 0.05 | 1.16 ± 0.05 | 1.20 ± 0.09 | 1.31 ± 0.07 |
|
| ||||
| Amplitude (mV) | 71.1 ± 1.8 | 60.9 ± 1.8*** | 77.4 ± 3.3 | 67.6 ± 2.6*^ |
|
| ||||
| AHP (mV) | −16.1 ± 0.6 | −15.6 ± 0.6 | −16.0 ± 1.0 | −14.6 ± 0.8 |
Two-way ANOVA. HIV-1 Tg vs. non-Tg:
:p≤0.05,
:p≤0.001;
SAL-HIV-1 Tg vs. Dilt/Mem-HIV-1 Tg:
:p≤0.05.
A significant effect of treatment regardless of genotype was observed for 1/2 amplitude duration (dark gray box).
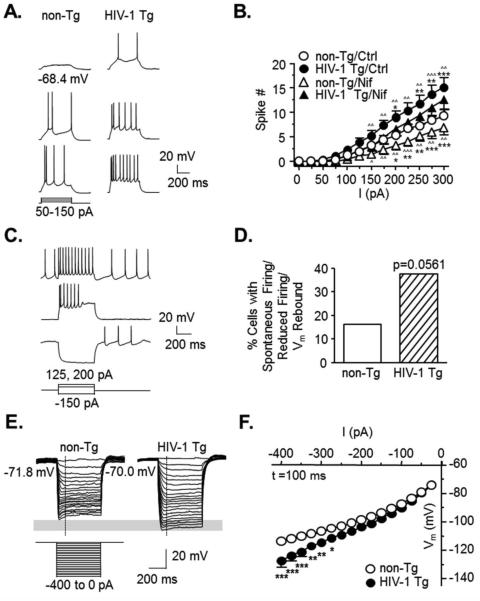
Figure 1. The excitability of mPFC pyramidal neurons is abnormally increased in HIV-1 Tg rats.
Pyramidal neurons exposed to HIV are hyper-excitable, partially due to L-channel activity. (A) Representative traces showing evoked neuronal firing from a non-Tg (left) or HIV-1 Tg (right) rat. (B) The current-response (spike number) relationships show evoked firings in mPFC neurons from non-Tg or HIV-1 Tg rats before (circles, Ctrl) and after (triangles) acute selective L-channel blockade with nifedipine (Nif; non-Tg, open shapes, n=7 neurons in 5 rats; HIV-1 Tg, filled shapes, n=8 neurons in 4 rats). Note that neurons from HIV-1 Tg rats displayed significantly greater spike numbers compared to those from non-Tg rats, and that acute L-channel blockade reduced spiking in HIV-1 Tg neurons to levels similar to those observed in non-Tg/Ctrl neurons. Significance is denoted by * for differences between HIV-1 Tg and non-Tg within acute treatment, and by ^ for differences between Ctrl and Nif within genotype (*:p≤0.017, except Nif-Tg vs. Nif-non-Tg, *,^:p≤0.05, **,^^:p≤0.01, ***,^^^:p≤0.001). HIV-1 Tg rats displayed a trend of increased aberrant firing properties in these neurons. (C) Representative traces displaying deformed action potential properties that were mainly observed in neurons from HIV-1 Tg rats, including (1) spontaneous firing with depolarized RMP (top trace), (2) overactivation-induced loss of spiking (middle trace), and (3) post-hyperpolarization rebound-associated firing (bottom trace). (D) The percentage of neurons that displayed aberrant firing patterns from each group showing a trend (p=0.056) of increased aberrant firing events in neurons from HIV-1 Tg rats compared to those from non-Tg rats (non-Tg, open bar, 16.13%, n=5/31 neurons in 16 rats; HIV-1 Tg, hatched bar, 37.5%, n=12/32 neurons in 11 rats). The inward rectification was reduced in neurons from HIV-1 Tg rats. (E) Representative traces from a non-Tg (left) or HIV-1 Tg rat (right) showing membrane hyperpolarization (downward traces from the resting level) in response to negative current stimuli (−400pA to −25pA). Early state Vm was measured at the time point (100ms) indicated by the vertical, dashed lines. The gray rectangle indicates the difference in Vm response between neurons from HIV-1 Tg and non-Tg rats. (F) The current-voltage relationships (I-V curves) are graphed (non-Tg, n=30 neurons in 16 rats; HIV-1 Tg, n=27 neurons in 10 rats). Neurons from HIV-1 Tg rats (filled circles) displayed more hyperpolarized Vm in response to negative current steps compared to those from non-Tg rats (open circles), indicating reduced inwardly flowing cations. Significance is denoted as follows: *:p≤0.025, **:p≤0.01, ***:p≤0.001.
A trend towards increased aberrant firing was also observed in neurons from HIV-1 Tg rats (n=12/32, 37.5%, n=11 rats) as compared to those from non-Tg rats (n=5/31, 16.13%, n=16 rats, χ2(1)=3.650, p=0.056)(Figure 1C-D). This was similar to that observed in our previous studies induced by bath application of HIV-1 Tat (Napier et al., 2014; Wayman et al., 2015a), and in adult HIV-1 Tg rats (Wayman et al., 2015b). The properties of such abnormal firing included (1) spontaneous firing with depolarized RMP, (2) reduced firing with deformed action potentials in response to moderate depolarizing currents (~150pA, suggesting over-activation-induced inactivation), and (3) post-hyperpolarization rebound-associated firing (suggesting a possible increase of LVA-Ca2+ channel activity) (Figure 1C). The RMP, threshold, half-amplitude duration of action potential and afterhyperpolarization (AHP) were not significantly altered in neurons from HIV-1 Tg rats as compared to those from non-Tg rats (Table 1). These findings demonstrate that (1) mPFC pyramidal neurons in HIV-1 Tg rats were more excitable and therefore more vulnerable even to moderate excitatory stimuli, and (2) increased L-channel activity plays a critical role in this increased supra-threshold excitability of mPFC pyramidal neurons.
Sub-threshold excitability of mPFC pyramidal neurons is altered in HIV-1 Tg rats
We next evaluated the sub-threshold excitability of mPFC pyramidal neurons from non-Tg and HIV-1 Tg rats in brain slices using hyperpolarizing current steps (−400pA to −25pA) (Figure 1E-F). We found that more hyperpolarized current steps (≤−275pA) induced greater Vm hyperpolarization in neurons from HIV-1 Tg rats (n=10 rats) compared to those from non-Tg rats (n=30 in 16 rats) at early states (100ms, n=27 HIV-1 Tg neurons; two-way rmANOVA: genotype, F(1,825)=5.864, p=0.019; current, F(15,825)=357.486, p≤0.001; interaction, F(15,825)=7.552, p≤0.001)(Figure 1F). Similar results were also found at steady states (450ms, n=28 HIV-1 Tg neurons; data not shown, two-way rmANOVA: genotype, F(1,810)=3.697, p=0.060; current, F(15,810)=604.423, p≤0.001; interaction, F(15,810)=6.334, p≤0.001). These data indicate that the voltage-sensitive inward flow of cations elicited by membrane hyperpolarization (i.e. the inward rectification) was significantly reduced in mPFC pyramidal neurons from HIV-1 Tg rats compared to those from non-Tg rats. This reduction could reflect HIV-induced alterations in the mPFC sub-threshold excitability in response to inhibitory inputs. Importantly, this dysregulation, if resulting from reduced K+ influx through inwardly rectifying K+ (Kir) channels, could consequently lead to an increase in local extracellular K+ levels, which could facilitate membrane depolarization of nearby neurons and thereby promote their firing.
Increased Ca2+ influx is mediated by over-activation of voltage-gated L-channels, independent of NMDAR, in neurons from HIV-1 Tg rats
We assessed the contribution of Ca2+ channel dysregulation, in an NMDAR-independent fashion, to the increased excitability of neurons in the context of HIV by comparing Ca2+ influx through VGCCs in the mPFC of HIV-1 Tg and non-Tg rats (Figure 2A-C). To assure Ca2+ influx selectively through VGCCs, glutamate- and GABA-mediated excitatory and inhibitory inputs, as well as voltage-sensitive Na+ and K+ channels, were blocked. We found that Ca2+ influx was significantly enhanced, reflected by the increased Ca2+ potential duration (t(51)=2.340, p=0.0232) (Figure 2B) and Ca2+ potential area (t(49)=2.687, p=0.0098) (Figure 2C) in neurons from HIV-1 Tg rats (n=34-35 neurons in 18-19 rats) compared to those from non-Tg rats (n=17-18 neurons in 9 rats). Other Ca2+ potential properties, including the rheobase (183.5 ± 6.9pA vs. 175.6 ± 10.3pA), threshold (−22.5 ± 0.9mV vs. −23.9 ± 1.1mV), and amplitude (64.6 ± 1.3mV vs. 62.6 ± 2.0mV) were not significantly altered (p>0.05) in neurons from HIV-1 Tg rats as compared to those from non-Tg rats, respectively.
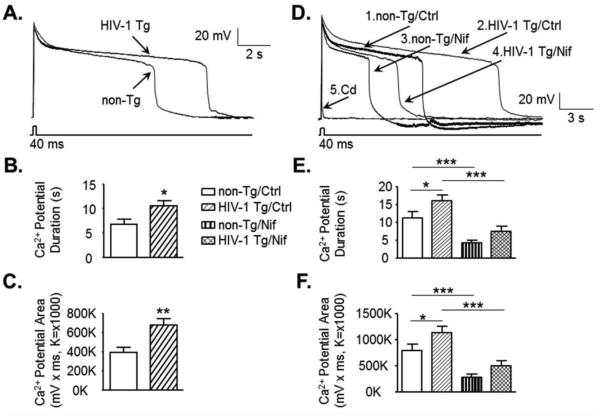
Figure 2. Ca2+ influx through the voltage-sensitive L-channels is abnormally increased in mPFC pyramidal neurons from HIV-1 Tg rats, independent of NMDAR.
(A) Representative neuronal Ca2+ potential traces from a non-Tg or HIV-1 Tg rat. (B, C) The bar graphs indicate an increase in the Ca2+ potential duration and area, reflecting increased Ca2+ influx through VGCCs in neurons from HIV-1 Tg rats compared to those from non-Tg rats (non-Tg, open bars, n=17-18 neurons in 9 rats; HIV-1 Tg, hatched bars, n=34-35 neurons in 18-19 rats). (D) Representative neuronal Ca2+ potential traces from a non-Tg or HIV-1 Tg rat before (Ctrl) and after acute treatment with nifedipine (Nif, 5µM) or cadmium (Cd, 400µM). Acute treatment with Nif reduced Ca2+ potentials, and Cd completely suppressed Ca2+ potentials. (E-F) The bar graphs indicate that the increased duration and area of Ca2+ potentials in neurons from HIV-1 Tg rats were significantly reduced by selective blockade of the L-channels with acute application of Nif in the bath (non-Tg, n=9 neurons in 8 rats; HIV-1 Tg, n=15 neurons in 11 rats). There was no significant difference in the Ca2+ potential duration or area between neurons from non-Tg/Ctrl and HIV-1 Tg/Nif. Significance is denoted as follows: *:p≤0.05, **:p≤0.01, ***:p≤0.001.
To assess the contribution of L-channel activity to the enhanced Ca2+ influx in neurons from HIV-1 Tg rats, the L-channels were selectively blocked with acute perfusion of Nif in the bath. L-channel blockade significantly reduced Ca2+ influx duration and area in neurons from both non-Tg (n=9 in 8 rats) and HIV-1 Tg rats (n=15 in 11 rats) (Figure 2D-F). In neurons from HIV-1 Tg rats, we found that Nif significantly reduced Ca2+ influx to Ctrl levels in neurons from non-Tg rats. There was also no significant difference in bath Nif-treated Ca2+ influx between neurons from non-Tg rats and those from HIV-1 Tg rats (two-way ANOVA; Duration: genotype, F(1,22)=3.886, p=0.061; treatment, F(1,22)=63.766, p≤0.001; interaction, F(1,22)=0.548, p=0.467; Area: genotype, F(1,22)=3.592, p=0.071; treatment, F(1,22)=65.002, p≤0.001; interaction, F(1,22)=0.720, p=0.405)(Figure 2D-F). These findings suggest that over-activated L-channels are mainly responsible for the enhanced voltage-sensitive Ca2+ influx in mPFC neurons from HIV-1 Tg rats. All Ca2+ influx was completely blocked with acute perfusion of Cd (400µM) in neurons from HIV-1 Tg (n=4 neurons in 4 rats) or non-Tg rats (n=3 neurons in 3 rats)(Figure 2D).
Combined chronic blockade of Ca2+ influx through over-active L-channels and NMDARs alleviates HIV-1 associated hyper-excitability of mPFC pyramidal neurons
Because we demonstrated that the L-channels are functionally involved in HIV-induced hyper-excitability, in an NMDAR-independent fashion, and because NMDAR dysfunction is well-established in HIV-induced Ca2+ dysregulation (Haughey & Mattson, 2002), although not the only source of HIV-induced Ca2+ dysregulation (Hu, 2015), we assessed whether combined chronic blockade of over-active L-channels and NMDARs could ameliorate or reduce HIV-induced hyper-excitability. We pretreated HIV-1 Tg and non-Tg rats daily for 2 weeks with SAL or two open-channel blockers, Dilt and Mem (Dilt/Mem), which target the L-channel and NMDAR, respectively. We then assessed the effects of combined chronic blockade of Ca2+-permeable channels on neuronal excitability and evoked Ca2+ plateau potentials in mPFC pyramidal neurons (Figure 3).
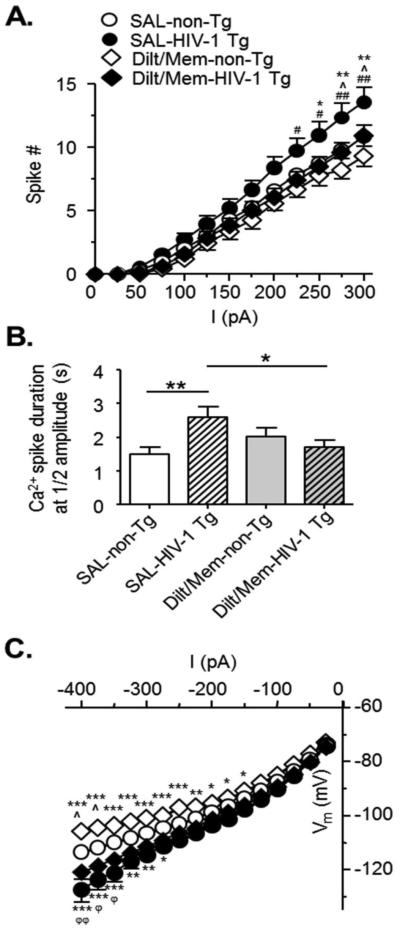
Figure 3. Combined chronic blockade of over-active L-channels and NMDARs ameliorates hyper-excitability of mPFC pyramidal neurons from HIV-1 Tg rats.
(A) The current-spike response curves show that combined chronic blockade of Ca2+ channels with diltiazem and memantine (Dilt/Mem) for 2 weeks abolished the excessive spiking in neurons from HIV-1 Tg rats (SAL, circles; SAL-non-Tg, n=28 neurons in 15 rats; SAL-HIV-1 Tg, n=22 neurons in 9 rats; Dilt/Mem, diamonds; Dilt/Mem-non-Tg, n=9 neurons in 5 rats; Dilt/Mem-HIV-1 Tg, n=12 neurons in 4 rats). Note that neurons from Dilt/Mem-treated HIV-1 Tg rats displayed similar spiking numbers to neurons from SAL-treated non-Tg rats. Significance is denoted by * for differences between HIV-1 Tg and non-Tg within treatment, by ^ for differences between SAL and Dilt/Mem within genotype, and by # for differences between SAL-HIV-1 Tg and Dilt/Mem-Non-Tg. *,#,^:p≤0.017, **,##:p≤0.01. (B) The bar graphs show that combined chronic blockade of over-active L-channel/NMDAR alleviated the abnormal increase of Ca2+ influx in neurons from HIV-1 Tg rats. This improvement was indicated by a reduction in the half amplitude of Ca2+ spikes, which returned to levels similar to that in neurons from SAL-pretreated non-Tg rats (SAL-non-Tg, open bars, n=22 neurons in 13 rats; SAL-HIV-1 Tg, open hatched bars, n=40 neurons in 19 rats; Dilt/Mem-non-Tg, gray bars, n=18 neurons in 9 rats; Dilt/Mem-HIV-1 Tg, gray hatched bars, n=22 neurons in 7 rats). Significance is denoted as follows: *:p≤0.05, **:p≤0.01. (C) The current-voltage (I-V) relationships indicate that combined chronic blockade of L-channel/NMDAR did not alleviate the reduced inward rectification in neurons from HIV-1 Tg rats. The I-V curve is graphed for the early state (100ms; SAL-non-Tg, n=30 neurons in 16 rats; SAL-HIV-1 Tg, n=27 neurons in 10 rats; Dilt/Mem-non-Tg, n=8 neurons in 4 rats; Dilt/Mem-HIV-1 Tg, n=13 neurons in 4 rats). Neurons from Dilt/Mem-pretreated HIV-1 Tg rats (filled diamonds) still displayed more hyperpolarized Vm in response to negative currents compared to those from SAL-pretreated non-Tg rats (open circles). Significance is denoted by * for differences between HIV-1 Tg and non-Tg within treatment, by ^ for differences between SAL and Dilt/Mem within genotype, and by ϕ for differences between SAL-non-Tg and Dilt/Mem-HIV-1 Tg. *,^,ϕ:p≤0.025, **,ϕϕ:p≤0.01, ***:p≤0.001.
We found that combined chronic blockade of the two Ca2+-permeable ion channels (Dilt/Mem) abolished the abnormal increase of action potentials in neurons from HIV-1 Tg rats (Dilt/Mem: n=12 in 4 rats; SAL: n=22 in 9 rats; two-way rmANOVA: treatment, F(1,384)=2.677, p=0.112; current, F(12,384)=168.125, p≤0.001; interaction, F(12,384)=2.122, p=0.015). The reduced firing levels were similar to those in neurons from SAL-pretreated non-Tg rats (n=28 in 15 rats, p>0.05)(Figure 3A). In contrast, the spike frequency in neurons from non-Tg rats was not significantly altered by combined chronic Dilt/Mem pretreatment (n=9 in 5 rats).
We also found that some characteristics of action potentials were affected by combined chronic Dilt/Mem pretreatment in pyramidal neurons from both HIV-1 Tg (n=13-14 in 4 rats) and non-Tg (n=8-9 in 4-5 rats) rats (Table 1). In both genotypes, combined Dilt/Mem pretreatment resulted in a trend towards an increased rheobase (two-way ANOVA: genotype, F(1,72)=3.966, p=0.05; treatment, F(1,72)=3.809, p=0.055; interaction, F(1,72)=0.0725, p=0.788), a significantly-increased amplitude (two-way ANOVA: genotype, F(1,78)=16.6437, p<0.001; treatment, F(1,78)=6.930, p=0.01; interaction, F(1,78)=0.00554, p=0.94), and an increased 1/2 amplitude duration (two-way ANOVA: genotype, F(1,74)=2.056, p=0.156; treatment, F(1,74)=4.057, p=0.048; interaction, F(1,74)=0.0673, p=0.796). Neurons from HIV-1 Tg rats exhibited increased action potential amplitude (p=0.042) after combined Dilt/Mem pretreatment, which was similar to that in neurons from SAL-pretreated non-Tg rats (p>0.05). Interestingly, combined Dilt/Mem pretreatment also induced an increase in the 1/2 amplitude duration of evoked action potentials in neurons from both non-Tg and HIV-1 Tg rats as compared to those from SAL-pretreated non-Tg and HIV-1 Tg rats. This increase in the 1/2 amplitude duration might result from non-specific inhibitory effects of Dilt and/or Mem on voltage-gated K+ channels and Ca2+-activated K+ channels, respectively (Bukanova & Solntseva, 1997; Ito et al., 2010; Kahlfuss et al., 2014). Combined chronic Dilt/Mem pretreatment did not significantly affect the RMP, threshold, and AHP (Table 1). These findings suggest that combined chronic blockade of L-channel/NMDAR reduces hyper-excitability induced by HIV.
Associated with the decreased firing, we also found that combined chronic blockade of over-active L-channels and NMDARs reduced the abnormal increase of Ca2+ influx in neurons from HIV-1 Tg rats. In HIV-1 Tg rats, this improvement in Ca2+ regulation was indicated by a significant reduction in the Ca2+ potential duration measured at the ½ amplitude level (SAL: n=40 neurons in 19 rats, Dilt/Mem: n=22 neurons in 7 rats, p≤0.05); this Ca2+ influx in neurons from Dilt/Mem-pretreated HIV-1 Tg rats was similar (p>0.05) to that in neurons from SAL-pretreated non-Tg rats (n=22 in 13 rats; two-way ANOVA: genotype, F(1,97)=1.399, p=0.240; treatment, F(1,97)=0.183, p=0.670; interaction, F(1,97)=5.963, p=0.016)(Figure 3B). In addition to the observed effects on the half amplitude duration, a trend of improvements was also found in the entire Ca2+ potential duration (p=0.063), where duration was reduced in neurons from HIV-1 Tg rats pretreated with combined Dilt/Mem compared to those pretreated with SAL (9.3±1.2s vs 11.9±0.8s; data not shown; two-way ANOVA; genotype, F(1,113)=1.619, p=0.206; treatment, F(1,113)=0.00007, p=0.979; interaction, F(1,113)=0.548, p=0.015). Nevertheless, the whole Ca2+ potential area was not significantly affected by combined Dilt/Mem pretreatments.
Combined chronic Dilt/Mem pretreatment did not affect the HIV-1-associated reduction in inward rectification in response to membrane hyperpolarization in mPFC pyramidal neurons
We also assessed the subthreshold excitability by subjecting pyramidal neurons to hyperpolarizing current steps (−400pA to −25pA) and evaluating the corresponding changes in Vm. Combined pretreatment with Dilt/Mem did not affect the reduced inward rectification in neurons from SAL-pretreated HIV-1 Tg rats (SAL: n=27 in 10 rats, Dilt/Mem: n=13 in 4 rats, p>0.05). Neurons from Dilt/Mem-pretreated HIV-1 Tg rats still showed significantly reduced inward rectification (reflecting decreased inflowing cations in response to membrane hyperpolarization) at more hyperpolarized current steps (≤325pA) when compared to those from SAL-pretreated non-Tg rats (n=30 in 16 rats; two-way rmANOVA: genotype, F(1,615)=2.244, p=0.142; current, F(15,615)=515.294, p≤0.001; interaction, F(15,615)=3.559, p≤0.001)(Figure 3C). Collectively, these data show that combined chronic L-channel/NMDAR blockade did not affect HIV-induced decreases in cation influx during membrane hyperpolarization.
Individual chronic blockade of over-active L-channels or NMDARs did not alleviate HIV-1 associated hyper-excitability of mPFC pyramidal neurons
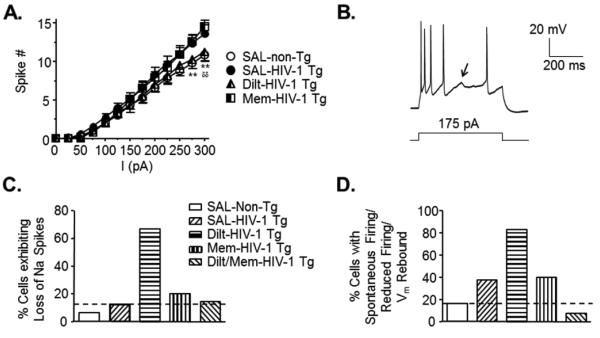
To compare the effects of individual chronic Ca2+ channel blockade with those of combined Dilt/Mem pretreatments on Ca2+ dysregulation, HIV-1 Tg rats were given daily s.c. injection of either Dilt or Mem alone for two weeks prior to evaluation (Figure 4). The two individual blockers for Ca2+-permeable channels had different effects on spike frequency. Individual chronic pretreatment with Mem did not affect the increased spiking in neurons from HIV-1 Tg rats (n=10 in 4 rats) when compared to those from SAL-pretreated non-Tg rats (n=28 in 15 rats). Individual chronic pretreatment of HIV-1 Tg rats with Dilt (n=8 neurons in 4 rats) induced a reduction in spike numbers that was not significantly different from those in neurons from SAL-pretreated non-Tg or SAL-pretreated HIV-1 Tg rats (n=22 neurons in 9 rats; two-way rmANOVA: treatment, F(3,768)=1.230, p=0.306; current, F(12,768)=339.448, p≤0.001; interaction, F(36,768)=1.960, p≤0.001)(Figure 4A). However, this effect of individual Dilt pretreatment on reduced spiking was likely due to a unique aberrant firing event, which was identified mainly in neurons from HIV-1 Tg rats pretreated with Dilt (66.7%, n=8/12 in 4 rats), but rarely in neurons from rats that received other pretreatments (SAL-non-Tg: 6.5%, n=2/31 in 16 rats; SAL-HIV-1 Tg: 12.5%, n=4/32 in 11 rats; Mem-HIV-1 Tg: 20%, n=3/15 in 4 rats; Dilt/Mem-HIV-1 Tg: 16.7%, n=2/12 in 4 rats; χ2(4)=23.87, p<0.0001)(Figure 4B-C). This additional abnormal spiking event was characterized by an apparent and inconsistent loss of action potentials, even though the threshold was apparently achieved (Figure 4B). In addition, the other aberrant firing events that were first described in neurons from HIV-1 Tg rats pretreated with SAL in Figure 1C-D (i.e., defined as spontaneous firing with depolarized RMP, over-activation-induced loss of spiking, and post-hyperpolarization-associated firing) were also observed in neurons from Dilt- or Mem-pretreated HIV-1 Tg rats. When compared to neurons from HIV-1 Tg rats pretreated with SAL, these abnormal firing events occurred more often in neurons from Dilt-pretreated rats, and to a similar extent in neurons from Mem-pretreated rats. Conversely, combined chronic Dilt/Mem pretreatment actually reduced the abnormal firing events in neurons from HIV-1 Tg rats (8.3%, n=1/12 in 4 rats) as compared to those from SAL-pretreated HIV-1 Tg rats (SAL-HIV-1 Tg: 37.5%, n=12/32 in 11 rats; Mem-HIV-1 Tg: 40%, n=6/15 in 4 rats; Dilt-HIV-1 Tg: 83.3%, n=10/12 in 4 rats; SAL-non-Tg: 16.1%, n=5/31 in 16 rats; χ2(4)=24.51, p<0.0001)(Figure 4D).
Figure 4. Individual blockade of over-active L-channels or NMDARs did not alleviate hyper-excitability of mPFC pyramidal neurons from HIV-1 Tg rats.
(A) The current-spike response curves for neurons from individual memantine (Mem)- or diltiazem (Dilt)-pretreated HIV-1 Tg rats compared to those form SAL-pretreated HIV-1 Tg and non-Tg rats. Mem did not induce any significant change in the number of evoked spiking when compared to neurons from HIV-1 Tg rats pretreated with SAL. Although there was an apparent reduction in the spike number after individual Dilt pretreatment, it likely resulted from the aberrant loss of action potentials, displayed in Figure 4B (SAL-non-Tg, open circles, n=28 neurons in 15 rats; SAL-HIV-1 Tg, filled circles, n=22 neurons in 9 rats; Mem-HIV-1 Tg, n=10 neurons in 4 rats; Dilt-HIV-1 Tg, n=8 neurons in 4 rats). Significance is denoted by * for differences between SAL-HIV-1 Tg and SAL-non-Tg, and by δ for differences between SAL-non-Tg and Mem-HIV-1 Tg. **,δδ:p≤0.01. (B) A representative trace displaying a unique aberrant firing event characterized specifically by an apparent and inconsistent loss of Na+ spiking although firing threshold was achieved (denoted by the arrow). (C) The bar graph shows that this apparent loss of Na+ spiking occurred mainly in neurons from HIV-1 Tg rats after Dilt-pretreatment (non-Tg, 6.45%, n=2/31 neurons in 16 rats; HIV-1 Tg, 12.5%, n=4/32 neurons in 11 rats; Dilt-HIV-1 Tg, 66.67%, n=8/12 neurons in 4 rats; Mem-HIV-1 Tg, 20%, n=3/15 neurons in 4 rats; Dilt/Mem-HIV-1 Tg, 16.67%, n=2/12 neurons in 4 rats). (D) The bar graph shows the percentage of neurons that displayed aberrant firing patterns which were first described in Figure 1 (i.e., spontaneous firing, overactivation-induced loss of spiking, and post-hyperpolarization-associated firing), showing again that Dilt-treated HIV-1 Tg neurons mainly exhibited these aberrant events (non-Tg, 16.13%, n=5/31 neurons in 16 rats; HIV-1 Tg, 37.5%, n=12/32 neurons in 11 rats; Dilt-HIV-1 Tg, 83.33%, n=10/12 neurons in 4 rats; Mem-HIV-1 Tg, 40%, n=6/15 neurons in 4 rats; Dilt/Mem-HIV-1 Tg, 8.33%, n=1/12 neurons in 4 rats). Note that combined Ca2+ channel blockade (Dilt/Mem) abolished these aberrant firing events.
Differential effects on action potential and membrane properties were also found after individual pretreatment with the two blockers for Ca2+-permeable channels. Individual pretreatment with either blocker induced RMP depolarization when compared to neurons from SAL-pretreated non-Tg rats (F(2,52)=5.705, p=0.006; Mem-HIV-1 Tg: −64.4 ± 1.7 mV, n=15 in 4 rats; Dilt-HIV-1 Tg: −64.3 ± 1.7 mV, n=12 in 4 rats; SAL-non-Tg: −68.8 ± 3.1 mV, n=28 in 15 rats). This change in RMP likely resulted from some non-specific effects of the two blockers on ion channels other than the L-channel and NMDAR, including but not limited to the two-pore-domain K+ (K2P) channels (He & Bausch, 2014; Takahira et al., 2005), which regulate outflow of K+ ions at resting states to maintain RMP at normal levels (Lesage & Lazdunski, 2000). In the case of Mem-pretreatment alone, a trend towards a reduced action potential amplitude was seen (Mem-HIV-1 Tg: 63.4 ± 3.0 mV, n=15 neurons in 4 rats; Dilt-HIV-1 Tg: 68.6 ± 4.2 mV, n=12 neurons in 4 rats; SAL-non-Tg: 71.1 ± 1.4 mV, n=28 neurons in 15 rats, F(2,55)=2.777, p=0.071). Other membrane properties, including Rin, rheobase, threshold, half amplitude duration and AHP, were not significantly altered in comparison to neurons from SAL-treated non-Tg rats (data not show). Together, these novel findings show that, unlike combined chronic Dilt/Mem treatments, individual blockade of L-channels or NMDARs did not ameliorate HIV-induced hyper-excitability in mPFC pyramidal neurons.
Discussion
Our study provides compelling evidence that points to the potential benefit of combined blockade of over-active L-channels and NMDARs as a promising therapeutic strategy for HAND. Here, we first characterized HIV-induced functional impairments in mPFC pyramidal neurons, focusing on voltage-gated Ca2+ influx, and then assessed the improvements following combined chronic blockade of over-active L-channels and NMDARs. We found that pyramidal neurons in HIV-1 Tg rats were hyper-excitable in response to moderate excitatory stimuli, reflected by increased rheobase, spike frequency and Ca2+ influx, showed a trend of aberrant spiking phenomenon, and exhibited reduced cation influx in response to membrane hyperpolarization. Increased L-channel activity, independent of NMDAR, contributed to this HIV-induced mPFC neuronal hyper-excitability. More importantly, combined chronic blockade of over-active L-channels/NMDARs alleviated HIV-induced hyper-excitability, by which abnormally-increased spiking, Ca2+ potential duration at half-amplitude, and aberrant firing events were abolished. Conversely, individual chronic blockade of L-channel or NMDAR did not relieve neurons from abnormally-increased spiking and/or aberrant firing.
Alterations in the membrane properties of mPFC neurons from HIV-1 Tg rats were determined first. Increased supra-threshold excitability was found, displayed by reduced rheobase, increased action potentials, and enhanced Ca2+ potentials, which contributed to mPFC neuronal hyper-excitability and could be mediated by different types of voltage-sensitive ion channels. Voltage-gated Na+ channels were unlikely to contribute to this hyper-excitability because the firing threshold was unaffected and spike amplitude was reduced, as seen in our previous studies after Tat exposure (Napier et al., 2014; Wayman et al., 2015a), suggesting decreased Na+ currents in mPFC neurons in HIV-1 Tg rats. Therefore, the increased firing could be attributed to dysfunction of K+ channels and/or Ca2+ channels. We found that over-active L-channels contribute to mPFC hyper-excitability, but only partly, suggesting the involvement of K+ channels and/or non-L-type Ca2+ channels in HIV-induced alterations (Brailoiu et al., 2014; Clark, III et al., 2005; Piekarz et al., 2012). In fact, a likelihood of reduced K2P channel function was indicated by the increased Rin in neurons from HIV-1 Tg rats. Importantly, the HIV-induced increase in spiking was abolished by combined chronic blockade of L-channels/NMDARs. The reduced Na+ channel activity was also interestingly normalized after combined chronic blockade of L-channel/NMDAR. Further studies are needed to determine the mechanism(s) that underlies such change.
Enhanced voltage-sensitive Ca2+ influx clearly indicates that over-activation of VGCCs was associated with increased mPFC neuronal excitability, independent of NMDARs. VGCCs couple membrane depolarization with voltage-activated (not ligand-activated) influx of Ca2+, which acts as a messenger that participates in diverse intracellular signaling. The current study revealed enhanced Ca2+ influx through the high voltage-activated (HVA)-Ca2+ channels in neurons form HIV-1 Tg rats, which was abolished by selective L-channel blockade. This suggests a key role of the L-channel in HIV-induced Ca2+ dysregulation in mPFC pyramidal neurons, although possible effects of non-L-type (P/Q, N and R-type) Ca2+ channels (Brini et al., 2014) are not excluded (Brailoiu et al., 2014; Clark, III et al., 2005; Piekarz et al., 2012). The L-channel includes LVA-Cav1.3 and HVA-Cav1.2 subtypes (Lipscombe, 2002), and is critical in regulating Ca2+-mediated signal transduction and gene expression in neurons (Brini et al., 2014; Ikeda, 2001). Here, we determined that the HVA L-channel is the major contributor to the HIV-enhanced neuronal Ca2+ influx, reflecting disruption of the L-channel kinetics (e.g., increased open probability and/or time) and expression. Furthermore, our previous studies also have revealed that acute Tat alters Ca2+ homeostasis by increasing L-channel activity ex vivo and L-channel expression in vivo in mPFC pyramidal neurons (Hu, 2015; Napier et al., 2014; 2015a; Wayman et al., 2012), and that the mPFC of adult HIV-1 Tg rats (6-7 months of age) displays increased L-channel expression (Wayman et al., 2015b). Additionally, increased LVA-Ca2+ channel activity was also implied by the occurrence of post-hyperpolarization-associated firing found in many mPFC neurons from HIV-1 Tg rats, which was rarely seen in those from non-Tg rats. Importantly, the enhanced HVA-Ca2+ influx was reduced by combined chronic L-channel/NMDAR blockade, along with normalization of firing in mPFC neurons.
Direct and indirect effects of HIV-1 proteins could also contribute to the mPFC neuronal hyper-excitation. HIV-1 Tg rats express low level HIV-1 proteins, including Tat, gp41, gp120, Vpr, Nef, Rev and Vpu (Reid et al., 2001), which could contribute to L-channel and NMDAR over-activation. In fact, gp120, gp160 and Tat directly increase neuronal L-channel and NMDAR activity (Haughey & Mattson, 2002; Lannuzel et al., 1995). HIV-1 protein exposure also indirectly induces neurotoxicity by disrupting macrophage, microglia and astrocyte function, leading to release of inflammatory cytokines/chemokines, reactive oxygen species, matrix-degrading enzymes and glutamate (Henderson et al., 2012; Mattson et al., 2005). These processes also could ultimately alter neuronal Ca2+ homeostasis (Mattson et al., 2005). Moreover, Tat, gp120 and gp41 increase extracellular glutamate levels by disrupting astrocytic glutamate transporters, and gp120 and Tat can promote release of neurotoxic cytokines from monocytes and glia (Haughey & Mattson, 2002; Mattson et al., 2005). These dysregulations can result in pathogenic cascades, and when associated with L-channel/NMDAR dysfunction, could render mPFC neurons more susceptible and vulnerable to excitatory stimuli.
The most important, clinically relevant finding of the present study is that combined chronic targeting of both over-active L-channel/NMDAR alleviates HIV-induced hyper-excitability in mPFC pyramidal neurons. Individual targeting of NMDARs or L-channels has previously been tested in clinical trials as a therapeutic strategy to treat HAND, but neither was successful due to the inability to halt or reverse the HIV-associated dementia complex (Navia et al., 1998; Schifitto et al., 2007). Here, we demonstrate that chronically-combined open-channel (use-dependent) blockade significantly reduced excessive Ca2+ influx and associated hyper-excitability of mPFC pyramidal neurons in young HIV-1 Tg rats, and abolished HIV-induced aberrant firing. The improvements in voltage-sensitive Ca2+ influx were partial, in which the Ca2+ potential duration at half amplitude was normalized, though the entire duration only showed a trend towards recovery and the entire Ca2+ potential area was not significantly affected. These findings suggest a likely reduction of Ca2+ influx that occurred mainly in the cell soma, but not in the dendrites. Given that we administered combined treatment for a relatively short period of time (two weeks) in this study, longer term treatments are expected to produce more significant improvements in this HIV-induced Ca2+ dysregulation. Collectively, our novel findings emphasize the therapeutic potential of combined long-term blockade of over-activated L-channel/NMDAR as a strategy to ameliorate and/or reduce HAND (Figure 5). Future studies are needed to determine whether combined chronic L-channel/NMDAR blockade improves deficits in mPFC-related behavior, that have been reported in HIV-1 Tg rats (Nesil et al., 2015; Reid et al., 2016; Repunte-Canonigo et al., 2014).
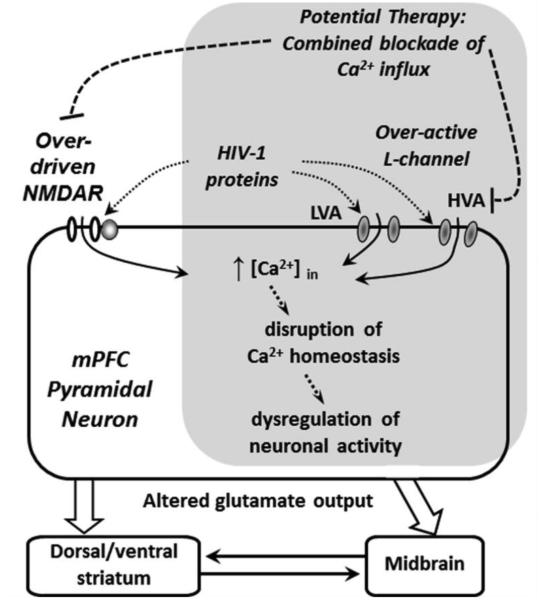
Figure 5. A hypothesized mechanism by which hyper-excitability of mPFC pyramidal neurons induced by HIV in a HIV-1 Tg rat model is alleviated by combined chronic treatments of L-channel blocker and NMDAR antagonist.
Over-activation of the L-channel and NMDAR contribute to HIV-induced hyper-excitation of pyramidal neurons in the mPFC, which could alter excitatory glutamatergic outputs from the mPFC. This change could consequently affect the function of other vulnerable cortical and subcortical brain regions altered in neuroAIDS. Combined chronic treatments with selective L-channel blocker and NMDAR antagonist that preferentially target over-activated Ca2+ permeable ion channels alleviate the mPFC neuronal hyper-excitability in HIV-1 Tg rats. This novel finding from the present study suggests that combined targeting of over-active L-channels and NMDARs may be necessary and could provide more effective therapeutic potential for treating HIV-induced neurocognitive and neuropsychiatric deficits. The gray box indicates the novel findings from the current study.
In contrast to the effects of combined chronic treatment, we found that individual chronic blockade of over-active L-channels or NMDARs did not relieve neurons from HIV-induced hyper-excitation. The present study revealed that pretreatment with the NMDAR blocker Mem alone did not reduce abnormally-increased spiking or aberrant firing. Intriguingly, we identified a unique aberrant spiking event associated with individual Dilt pretreatment. We also found non-specific effects of Mem and Dilt on K2P, Na+, voltage-gated K+ and Ca2+-activated K+ channels, as reported by previous studies (Bukanova & Solntseva, 1997; He & Bausch, 2014; Ito et al., 2010; Kahlfuss et al., 2014; Kimura et al., 1983; Takahira et al., 2005). Further investigations are needed to determine if such non-Ca2+ channel effects of the two blockers also actually contribute to the aberrant firing events.
The present study also revealed alterations in the subthreshold excitability in mPFC neurons of HIV-1 Tg rats, which could alter extracellular K+ level, and consequentially alter RMP and neuronal excitability. This change was reflected by a reduction in the inward rectification, which implies decreased activity of the channels that are activated by membrane hyperpolarization and conduct inwardly flowing K+ and other cations, including the Kir and hyperpolarization-activated cation current (Ih) channels (Hille, 2001). Reduced Kir channel activity and expression could result in increased extracellular K+ levels, which could facilitate RMP depolarization and consequently increase firing. It is worth noting that the reduction of inwardly rectifying K+ (and other cations) in mPFC pyramidal neurons in HIV-1 Tg rats was not alleviated by combined chronic blockade of over-activated L-channel/NMDAR. Further studies could determine if a modified combinatorial treatment, including a Kir channel activator, can improve the subthreshold excitability of mPFC neurons.
Our current studies have focused on the dysregulation of NMDAR/L-channel-mediated Ca2+-related processes by HIV in pyramidal neurons from the mPFC. Although we have noted some HIV-related effects on other ion channels (including K+ and Na+ channels), such channels are not a specific focus of the current NMDAR/L-channel-based studies. However, it is important to note that HIV also affects many other ion channels and receptors that likely participate in pathogenic processes related to HAND and that may also be affected in the current model/treatment regimen. For example, HIV reduces inhibitory GABA neurotransmission (Buzhdygan et al., 2016) and enhances the activity of another ionotropic glutamate receptor, the AMPAR (Epstein, 1998; Zhou et al., 2016). Reduced inhibitory neurotransmission (GABA) and increased excitatory stimulation of glutamatergic ionotropic receptors (AMPA and NMDAR) also contributes to membrane depolarizations that, if large enough, could ultimately activate VGCCs, including the L-channel, and promote excessive Ca2+ influx. It is likely that the observed improvements after combined chronic Ca2+ channel blockade result from effects directly at the pyramidal neurons as well as on other systems that provide inputs to these neurons or affect their external environment. Future studies are needed to elucidate the exact mechanism(s) of NMDAR/L-channel blockade, which could include determination of excitatory and inhibitory inputs to the mPFC, as well as additive or synergistic effects on Ca2+ influx.
Collectively, our findings demonstrate that the excitability of mPFC pyramidal neurons is abnormally increased in HIV-1 Tg rats, which is due in part to over-activation of the L-channel. This hyper-excitability renders these neurons more susceptible and vulnerable to excitatory stimuli that could ultimately lead to injury or cell death. This mPFC neuronal hyper-excitability is ameliorated by combined, but not individual, chronic blockade of over-activated L-channel/NMDAR, which is reflected by normalized firing, significantly-reduced Ca2+ influx, and decreased aberrant firing. In combination with previous studies focusing on NMDAR-mediated excitotoxicity induced by HIV (Haughey & Mattson, 2002; Hu, 2015; Mattson et al., 2005), our novel findings reveal that the pathophysiological effects of HIV on both the L-channel and NMDAR contribute to excessive Ca2+ influx, which dysregulates Ca2+ homeostasis, disturbs Ca2+ signaling, and causes mPFC neuronal hyper-excitation. The improvements in mPFC neuronal activity following combined chronic blockade of over-activated L-channel/NMDAR not only emphasize the impact of these hyper-active Ca2+-permeable ion channels on HIV-induced dysregulation of mPFC neuronal activity, but also highlight a potentially promising novel therapeutic strategy.
Highlights.
Excitability of mPFC pyramidal neurons is abnormally increased in HIV-1 Tg rats.
This is due in part to L-channel over-activation, independent of NMDAR.
Combined L-channel/NMDAR blockade ameliorates this neuronal hyper-excitability.
Individual L-channel/NMDAR blockade does not ameliorate this hyper-excitability.
Combined targeting of Ca2+ channels may improve HIV-induced neurological deficits.
Acknowledgements
This work was supported by the National Institutes of Health [grant numbers NS084817 (to X-TH), DA033966 & NS060632 (to LA)]. We would like to thank Dr. Wesley N. Wayman (Dept. of Pharmacology, Rush University Medical Center) for technical advice and support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: The authors have no conflicts to disclose.
References
- Antinori A, Arendt G, Becker JT, Brew BJ, Byrd DA, Cherner M, et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69:1789–1799. doi: 10.1212/01.WNL.0000287431.88658.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brailoiu E, Deliu E, Sporici RA, Benamar K, Brailoiu GC. HIV-1-Tat excites cardiac parasympathetic neurons of nucleus ambiguus and triggers prolonged bradycardia in conscious rats. Am J Physiol Regul Integr Comp Physiol. 2014;306:R814–R822. doi: 10.1152/ajpregu.00529.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brini M, Cali T, Ottolini D, Carafoli E. Neuronal calcium signaling: function and dysfunction. Cell Mol Life Sci. 2014;71:2787–2814. doi: 10.1007/s00018-013-1550-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukanova YV, Solntseva EI. The calcium channel antagonist diltiazem effectively blocks two types of potassim channels in the neuronal membrane. Bulletin of Experimental Biology and Medicine. 1997;9:858–861. Ref Type: Abstract. [Google Scholar]
- Buzhdygan T, Lisinicchia J, Patel V, Johnson K, Neugebauer V, Paessler S, et al. Neuropsychological, Neurovirological and Neuroimmune Aspects of Abnormal GABAergic Transmission in HIV Infection. J Neuroimmune Pharmacol. 2016;11:279–293. doi: 10.1007/s11481-016-9652-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chami M, Oules B, Paterlini-Brechot P. Cytobiological consequences of calcium-signaling alterations induced by human viral proteins. Biochim Biophys Acta. 2006;1763:1344–1362. doi: 10.1016/j.bbamcr.2006.09.025. [DOI] [PubMed] [Google Scholar]
- Chen HS, Pellegrini JW, Aggarwal SK, Lei SZ, Warach S, Jensen FE, et al. Open-channel block of N-methyl-D-aspartate (NMDA) responses by memantine: therapeutic advantage against NMDA receptor-mediated neurotoxicity. J Neurosci. 1992;12:4427–4436. doi: 10.1523/JNEUROSCI.12-11-04427.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark JP, III, Sampair CS, Kofuji P, Nath A, Ding JM. HIV protein, transactivator of transcription, alters circadian rhythms through the light entrainment pathway. Am J Physiol Regul Integr Comp Physiol. 2005;289:R656–R662. doi: 10.1152/ajpregu.00179.2005. [DOI] [PubMed] [Google Scholar]
- Epstein LG. HIV neuropathogenesis and therapeutic strategies. Acta Paediatr Jpn. 1998;40:107–111. doi: 10.1111/j.1442-200x.1998.tb01892.x. [DOI] [PubMed] [Google Scholar]
- Ferris MJ, Mactutus CF, Booze RM. Neurotoxic profiles of HIV, psychostimulant drugs of abuse, and their concerted effect on the brain: Current status of dopamine system vulnerability in NeuroAIDS. Neurosci Biobehav Rev. 2008;32:883–909. doi: 10.1016/j.neubiorev.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haughey NJ, Mattson MP. Calcium dysregulation and neuronal apoptosis by the HIV-1 proteins Tat and gp120. J Acquir Immune Defic Syndr. 2002;31(Suppl 2):S55–S61. doi: 10.1097/00126334-200210012-00005. [DOI] [PubMed] [Google Scholar]
- He S, Bausch SB. Synaptic plasticity in glutamatergic and GABAergic neurotransmission following chronic memantine treatment in an in vitro model of limbic epileptogenesis. Neuropharmacology. 2014;77:379–386. doi: 10.1016/j.neuropharm.2013.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Clifford DB, Franklin DR, Jr., Woods SP, Ake C, Vaida F, et al. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology. 2010;75:2087–2096. doi: 10.1212/WNL.0b013e318200d727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson L, Sharma A, Monaco MCG, Major EO, Al-Harthi L. Human immunodeficiency virus type 1 (HIV-1) Tansactivator of transcription through its intact core and cysteine-rich domains inhibits Wnt/beta-catenin signaling in astrocytes: relevance to HIV neuropathogenesis. J Neuroscie. 2012;32:16306–16313. doi: 10.1523/JNEUROSCI.3145-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B. Ionic Channels of Excitable Membranes. 3rd Skyscrape, Inc.; New York: 2001. [Google Scholar]
- Hu X-T. HIV-1 Tat-induced calcium dysregulation and neuronal dysfunction in vulnerable brain regions. Current Drug Targets. 2015 doi: 10.2174/1389450116666150531162212. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X-T, Basu S, White FJ. Repeated cocaine administration suppresses HVA-Ca2+ potentials and enhances activity of K+ channels in rat nucleus accumbens neurons. J Neurophysiol. 2004;92:1597–1607. doi: 10.1152/jn.00217.2004. [DOI] [PubMed] [Google Scholar]
- Huguenard JR. Low-threshold calcium currents in central nervous system neurons. Annu Rev Physiol. 1996;58:329–348. doi: 10.1146/annurev.ph.58.030196.001553. [DOI] [PubMed] [Google Scholar]
- Ikeda SR. Signal transduction. Calcium channels--link locally, act globally. Science. 2001;294:318–319. doi: 10.1126/science.1066160. [DOI] [PubMed] [Google Scholar]
- Ito T, Nuriya M, Yasui M. Regulation of Kv2.1 phosphorylation in an animal model of anoxia. Neurobiol Dis. 2010;38:85–91. doi: 10.1016/j.nbd.2010.01.002. [DOI] [PubMed] [Google Scholar]
- Kahlfuss S, Simma N, Mankiewicz J, Bose T, Lowinus T, Klein-Hessling S, et al. Immunosuppression by N-methyl-D-aspartate receptor antagonists is mediated through inhibition of Kv1.3 and KCa3.1 channels in T cells. Mol Cell Biol. 2014;34:820–831. doi: 10.1128/MCB.01273-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura S, Nakaya H, Kanno M. Electrophysiological effects of diltiazem, nifedipine and Ni2+ on the subepicardial muscle cells of canine heart under the condition of combined hypoxia, hyperkalemia and acidosis. Naunyn Schmiedebergs Arch Pharmacol. 1983;324:228–232. doi: 10.1007/BF00503900. [DOI] [PubMed] [Google Scholar]
- Lannuzel A, Lledo PM, Lamghitnia HO, Vincent JD, Tardieu M. HIV-1 envelope proteins gp120 and gp160 potentiate NMDA-induced [Ca2+]i increase, alter [Ca2+]i homeostasis and induce neurotoxicity in human embryonic neurons. Eur J Neurosci. 1995;7:2285–2293. doi: 10.1111/j.1460-9568.1995.tb00649.x. [DOI] [PubMed] [Google Scholar]
- Lesage F, Lazdunski M. Molecular and functional properties of two-pore-domain potassium channels. Am J Physiol Renal Physiol. 2000;279:F793–F801. doi: 10.1152/ajprenal.2000.279.5.F793. [DOI] [PubMed] [Google Scholar]
- Lipscombe D. L-type calcium channels - Highs and new lows. Circ Res. 2002;90:933–935. doi: 10.1161/01.res.0000019740.52306.92. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Haughey NJ, Nath A. Cell death in HIV dementia. Cell Death Differ. 2005;12(Suppl 1):893–904. doi: 10.1038/sj.cdd.4401577. [DOI] [PubMed] [Google Scholar]
- Napier TC, Chen L, Kashanchi F, Hu X-T. Repeated cocaine treatment enhances HIV-1 Tat-induced cortical excitability via over-activation of L-type calcium channels. J Neuroimmune Pharmacol. 2014 doi: 10.1007/s11481-014-9524-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasif FJ, Hu XT, White FJ. Repeated cocaine administration increases voltage-sensitive calcium currents in response to membrane depolarization in medial prefrontal cortex pyramidal neurons. J Neurosci. 2005a;25:3674–3679. doi: 10.1523/JNEUROSCI.0010-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasif FJ, Sidiropoulou K, Hu XT, White FJ. Repeated cocaine administration increases membrane excitability of pyramidal neurons in the rat medial prefrontal cortex. J Pharmacol Exp Ther. 2005b;312:1305–1313. doi: 10.1124/jpet.104.075184. [DOI] [PubMed] [Google Scholar]
- Navia BA, Dafni U, Simpson D, Tucker T, Singer E, McArthur JC, et al. A phase I/II trial of nimodipine for HIV-related neurologic complications. Neurology. 1998;51:221–228. doi: 10.1212/wnl.51.1.221. [DOI] [PubMed] [Google Scholar]
- Nesil T, Cao J, Yang Z, Chang SL, Li MD. Nicotine attenuates the effect of HIV-1 proteins on the neural circuits of working and contextual memories. Mol Brain. 2015;8:43. doi: 10.1186/s13041-015-0134-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niimi Y, Hino N, Ochi R. Diltiazem facilitates inactivation of single L-type calcium channels in guinea pig ventricular myocytes. Jpn Heart J. 2003;44:1005–1014. doi: 10.1536/jhj.44.1005. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 4 Academic Press; New York: 1998. [Google Scholar]
- Peng J, Vigorito M, Liu X, Zhou D, Wu X, Chang SL. The HIV-1 transgenic rat as a model for HIV-1 infected individuals on HAART. J Neuroimmunol. 2010;218:94–101. doi: 10.1016/j.jneuroim.2009.09.014. [DOI] [PubMed] [Google Scholar]
- Piekarz AD, Due MR, Khanna M, Wang B, Ripsch MS, Wang R, et al. CRMP-2 peptide mediated decrease of high and low voltage-activated calcium channels, attenuation of nociceptor excitability, and anti-nociception in a model of AIDS therapy-induced painful peripheral neuropathy. Mol Pain. 2012;8:54. doi: 10.1186/1744-8069-8-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid W, Sadowska M, Denaro F, Rao S, Foulke J, Jr., Hayes N, et al. An HIV-1 transgenic rat that develops HIV-related pathology and immunologic dysfunction. Proc Natl Acad Sci U S A. 2001;98:9271–9276. doi: 10.1073/pnas.161290298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid WC, Casas R, Papadakis GZ, Muthusamy S, Lee DE, Ibrahim WG, et al. Neurobehavioral Abnormalities in the HIV-1 Transgenic Rat Do Not Correspond to Neuronal Hypometabolism on 18F-FDG-PET. PLoS ONE. 2016;11:e0152265. doi: 10.1371/journal.pone.0152265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repunte-Canonigo V, Lefebvre C, George O, Kawamura T, Morales M, Koob GF, et al. Gene expression changes consistent with neuroAIDS and impaired working memory in HIV-1 transgenic rats. Mol Neurodegener. 2014;9:26. doi: 10.1186/1750-1326-9-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royal W, III, Zhang L, Guo M, Jones O, Davis H, Bryant JL. Immune activation, viral gene product expression and neurotoxicity in the HIV-1 transgenic rat. J Neuroimmunol. 2012;247:16–24. doi: 10.1016/j.jneuroim.2012.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schifitto G, Navia BA, Yiannoutsos CT, Marra CM, Chang L, Ernst T, et al. Memantine and HIV-associated cognitive impairment: a neuropsychological and proton magnetic resonance spectroscopy study. AIDS. 2007;21:1877–1886. doi: 10.1097/QAD.0b013e32813384e8. [DOI] [PubMed] [Google Scholar]
- Sesack SR, Deutch AY, Roth RH, Bunney BS. Topographical organization of the efferent projections of the medial prefrontal cortex in the rat: An anterograde tract-tracing study with phaseolus vulgaris leucoagglutinin. J comp Neurol. 1989;290:213–242. doi: 10.1002/cne.902900205. [DOI] [PubMed] [Google Scholar]
- Simioni S, Cavassini M, Annoni JM, Rimbault AA, Bourquin I, Schiffer V, et al. Cognitive dysfunction in HIV patients despite long-standing suppression of viremia. AIDS. 2010;24:1243–1250. doi: 10.1097/QAD.0b013e3283354a7b. [DOI] [PubMed] [Google Scholar]
- Takahira M, Sakurai M, Sakurada N, Sugiyama K. Fenamates and diltiazem modulate lipid-sensitive mechano-gated 2P domain K(+) channels. Pflugers Arch. 2005;451:474–478. doi: 10.1007/s00424-005-1492-5. [DOI] [PubMed] [Google Scholar]
- Wayman WN, Chen L, Napier TC, Hu X-T. Cocaine self-administration enhances excitatory responses of pyramidal neurons in the rat medial prefrontal cortex to HIV-1 Tat. Eur J Neurosci. 2015a;41:1195–1206. doi: 10.1111/ejn.12853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayman WN, Chen L, Hu XT, Napier TC. HIV-1 transgenic rat prefrontal cortex hyper-excitability is enhanced by cocaine self-administration. Neuropsychopharmacology. 2015b doi: 10.1038/npp.2015.366. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayman WN, Dodiya HB, Persons AL, Kashanchi F, Kordower JH, Hu X-T, et al. Enduring cortical alterations after a single in vivo treatment of HIV-1 Tat. Neuroreport. 2012;23:825–829. doi: 10.1097/WNR.0b013e3283578050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuste R. Origin and classification of neocortical interneurons. Neuron. 2005;48:524–527. doi: 10.1016/j.neuron.2005.11.012. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Tang H, Xiong H. Chemokine CCL2 enhances NMDA receptor-mediated excitatory postsynaptic current in rat hippocampal slices-a potential mechanism for HIV-1-associated neuropathy? J Neuroimmune Pharmacol. 2016;11:306–315. doi: 10.1007/s11481-016-9660-2. [DOI] [PMC free article] [PubMed] [Google Scholar]