Abstract
The Epidermal Growth Factor Receptor (EGFR) is a transmembrane receptor tyrosine kinase with critical implications in cell proliferation, migration, wound healing and the regulation of apoptosis. However, the EGFR has been shown to be hyper-expressed in a number of human malignancies. The MDA-MB-468 metastatic breast cell line is one example of this. This particular cell line hyper-expresses the EGFR and undergoes EGFR-mediated apoptosis in response to EGF ligand. The goal of this study was to identify the kinases that could be potential intermediates for the EGFR-mediated induction of apoptosis intracellularly. After identifying Cyclic GMP-dependent Protein Kinase G (PKG) as a plausible intermediate, we wanted to determine the temporal relationship of these two proteins in the induction of apoptosis. We observed a dose-dependent decrease in MDA-MB-468 cell viability, which was co-incident with increased PKG activity as measured by VASPSer239 phosphorylation. In addition, we observe a dose dependent decrease in cell viability, as well as an increase in apoptosis, in response to two different PKG agonists, 8-Bromo-cGMP and 8-pCPT-cGMP. MDA-MB-468 cells with reduced PKG activity exhibited had attenuated EGFR-mediated apoptosis. These findings indicate that PKG does not induce cell death via transphosphorylation of the EGFR. Instead, PKG activity occurs following EGFR activation. Together, these data indicate PKG as an intermediary in EGFR-mediated cell death, likely via apoptotic pathway.
Keywords: Epidermal Growth Factor Receptor, Protein Kinase G, Apoptosis, MDA-MB-468 Cells
INTRODUCTION
The Epidermal Growth Factor Receptor (EGFR) is a cell surface receptor that is expressed in almost every tissue of the body, and plays critical roles in development and tissue homeostasis [1, 2]. In addition to these physiological roles, many cancers, such as lung [3], breast [4], and colon [5] malignancies, are characterized by hyper-activated EGFR signaling, either due to overexpression of the receptor or somatic activating mutations of the receptor [4, 6]. Perturbations in EGFR expression and/or activation are associated with poor patient prognosis. There are a number of FDA-approved therapies that target the EGFR and attenuate cancer growth. These include small molecular inhibitors such as Erlotinib and Gefitinib, which has been approved for treatment of lung [7] and colon [8] cancers, and humanized monoclonal antibodies such as Cetuximab, which has been approved for treating colorectal cancers [9].
Despite the progress in using EGFR inhibitors as a mode of cancer treatment, these therapies are not failsafe. These drugs can have off-target effects (i.e. dermatitis, colitis, and corneal erosions) and some cancers become refractory to the drugs over time [10–12]. EGFR inhibition can also trigger autophagy in cancer cells, a degradative process that actually helps cancer cells withstand nutrient-poor conditions [13]. Thus, despite the therapeutic benefits of EGFR-inhibition, there remains a need for more aggressive and/or targeted treatments that will arrest cancer growth and progression.
A more comprehensive understanding of how EGFR signaling is regulated will facilitate the design of new agents. We hypothesize that one key to new therapies might lie within the cell itself. Paradoxically, cells that overexpress the EGFR naturally (i.e. A431 and MDA-MB-468 cells [14, 15]) or through bioengineering [16], undergo apoptosis in response to EGF treatment. By utilizing this endogenous signaling mechanism we predict that this will specifically target EGFR-overexpressing cells with minimal side-effects to healthy cells. However, to most effectively utilize these pathways, we need to fully understand the mechanisms that regulate their signaling.
The goal of this study was to determine the signaling events that occur downstream of EGFR activation that lead to apoptosis. By determining how of EGFR-mediated apoptosis occurs in cell lines that overexpress the EGFR, we predict this pathway can be used to halt cancers that express high levels of EGFR. These studies were conducted primarily in MDA-MB-468 cell line, a metastatic, breast, epithelial cell line [17]. These cells express approximately 1.3 × 106 EGFR per cell [18], and have a well documented induction of apoptosis in response to EGF ligand (≥ 10 ng/mL) [4]
One candidate regulator of EGFR-mediated apoptosis is Cyclic guanosine monophosphate (cGMP)-dependent protein kinase (PKG). PKG exists in two functionally indistinguishable forms: PKG I and PKG II. PKG I is localized within the cytoplasm whereas PKG II is generally associated with the cell membrane [19]. Additionally, there are two isoforms of the type I PKG homologue: PKGIβ and PKGIα. These two isoforms are closely related, except in the N-terminal domain of the PKGIα isoform, which has 16 fewer residues than the PKGIβ isoform [19]. PKG1 has been demonstrated to be sufficient to induce apoptosis in MDA-MB-468 cells [20]. PKG is activated by cGMP, a second messenger produced by the conversion of GTP by guanylyl cyclase. PKG mediates downstream signaling through interactions with intracellular phosphoproteins [21], including VASP [22] and Phosphodiesterase type 5 (PDE5) [23]. The activity of these proteins includes regulating smooth muscle tone, bone growth, platelet aggregation, and electrolyte and fluid homeostasis. [24–26].
Given that stimulation of PKGI but not PKGII was sufficient to induce apoptosis [27], we developed the hypothesis that the EGFR mediates apoptosis though PKGI activity. We find that MDA-MB-468 cells have a reduction in cell viability and an increase in apoptosis in response to both EGF and PKG agonists. Further, stimulation of the EGFR leads to enhanced PKG activity in MDA-MB-468, A431, and HeLa cell lines, as measured by Vasodilator Stimulated Phosphoprotein (VASP) phosphorylation. PKG stimulation does not enhance EGFR phosphorylation in MDA-MB-468 cells, indicative that PKG is downstream of the EGFR. Further, knock down of PKG decreases the dose dependent EGFR-mediated cell viability and reduces apoptotic pathways. From these data, we conclude that in MDA-MB-468 cells, the EGFR utilizes PKG to promote cell death via apoptotic pathways.
MATERIALS AND METHODS
Cell Lines
MDA-MB-468 and A431 cell lines were acquired from the American Type Culture Collection (ATCC). Both cell lines were maintained in growth media [Dulbecco’s Modified Eagle Medium (DMEM)] supplemented with 10% Fetal Bovine Serum (FBS), 1% penicillin, 1% streptomycin, and 2 mM glutamine. The cells were maintained at incubation conditions of 37 °C in 5% CO2.
HeLa cells cell lines were acquired from the American Type Culture Collection (ATCC). They were maintained in growth media [Dulbecco’s Modified Eagle Medium (DMEM)] supplemented with 5% Fetal Bovine Serum (FBS), 1% penicillin, 1% streptomycin, and 2 mM glutamine. The cells were maintained at incubation conditions of 37 °C in 5% CO2.
Agonists
The following agonists of PKG, 8-Bromoguanosine 3′,5′-cyclic monophosphate (8-Br-cGMP) (Sigma-Aldrich), 8-(4-Chlorophenylthio)-guanosine 3′,5′-cyclic monophosphate (8-pCPT-cGMP) (Biolog Life Science Institute, Bremen, Germany), and 8- (4- Chlorophenylthio)guanosine- 3′, 5′-cyclic monophosphate, acetoxymethyl ester (8-pCPT-cGMP-AM) (Biolog Life Science Institute, Bremen, Germany), were acquired from the locations indicated, and were used at concentrations specified in each assay. EGF ligand was acquired from ProSpec Protein Specialist (East Brunswick, NJ) and Staurosporine (STS) was acquired from Sigma-Aldrich.
Cell Viability Analyses
MTT assay
3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide (MTT) reagent was acquired from Sigma Aldrich (St. Louis, MO). MTT assays were used to assess cell viability as described previously [28, 29]. Briefly, cells were plated at 5,000–10,000 cells/well in growth media in a 96 well plate. After 48 hours, cells were washed with phosphate buffered saline pH 7.3 (PBS) and serum starved in serum free (SF) DMEM for 3 hours. A 15 mM 8-Br-cGMP stock was prepared in de-ionized water. This 15 mM 8-Br-cGMP stock was diluted in serum free DMEM. Staurosporine (STS) was made as 1 mM stock in DMSO that was diluted in serum free DMEM and used at a final concentration of 168 nM. After a 48-hour incubation period, 25 μl of MTT (5 mg/mL in PBS) was added to each well, and cells were incubated for 2 hours at 37°C in the absence of CO2. MTT that had been converted to formazan was extracted by a 30-minute incubation at 37°C in extraction buffer (20% sodium dodecyl sulfate, 50% N-dimethylformamide, 50% ddH2O, 80% Acetic Acid, 1M HCl) was then added to all samples of the 96-well plate in order to extract and solubilize the formazan crystals. The 96-well plate was then analyzed after 24 hours with a BioTek Synergy HT plate reader and Gen5 BioTek software, at a wavelength of 570 nm.
Alamarblue assay
Alamarblue reagent was acquired from ThermoScientific (Waltham, MA). Alamarblue assays were performed according to manufacturer’s directions. MDA-MB-468 cells were plated in growth media at a density of 5,000–10,000 cells/well in a 96-well place. After a 48-hour incubation, the cells were washed with PBS and serum starved overnight in 50 μl/well of DMEM. 8-pCPT-cGMP-AM was made as a 50mM stock in Dimethylformamide (DMF) and diluted in serum free DMEM. EGF was made according to manufacturer’s instructions at 1 μg/ml stock and diltured in serum free DMEM before addition to the cells. Staurosporine (STS) was added as described for MTT assays. After a 48-hour incubation period, The Alamarblue reagent was then added at 10% of the total sample volume (10 μl) and incubated for 2 hours. Fluorescence was measured in HT plate reader with Gen5 BioTek software, at wavelengths of 530 nm (excitation) and 590 nm (emission).
Dose Response Assays (Western Blot Analyses)
MDA-MB-468 cells were plated into two 6-well dishes at a density of 600,000 cells/well. After 24-hours, the cells were rinsed with PBS and serum starved overnight. Cells were treated with the indicated concentration of 8-Br-cGMP, 8-pCPT-cGMP-AM, 8-pCPT-cGMP-AM or EGF for 1 hour prior to the harvesting of each cell lysate.
Cell Lysate Preparation and Immunoblot Analyses
Cell lysates were acquired by washing the cells twice in PBS prior to the equilibration to 4 °C and addition of ice cold RIPA lysis buffer for cell solubilization (150 mm NaCl, 1% Nonidet P-40, 0.5% deoxycholate, 0.1% SDS, 50 mm Tris (pH 8.0), 10 mm sodium pyrophosphate, 100 mm sodium fluoride, 2 mm phenylmethyl sulfonyl fluoride). The lysis buffer/cell mixture was then rotated end-over-end for 10 min at 4 °C, insoluble material was removed by 10 minutes at 15,000 × g in a microcentrifuge at 4 °C. The protein concentration was then assessed by a BCA assay (Pierce), and diluted 1:3 in 6XSDS sample buffer. Equivalent amounts of protein (20–60 μg) were separated by the indicated percentage SDS-PAGE and transferred to nitrocellulose. Individual antibodies [total EGFR and GAPDH (Santa Cruz Biotechnology, Dallas, TX); pVASP (Ser239), total VASP, PKGI, pY1045, Cleaved-Caspase 3, and PARP (Cell Signaling Technology, Danvers, MA)] were used with the appropriate HRP-conjugated secondary antibody according to the manufacturer’s instructions. Immunoreactive bands were detected by ECL and visualized using a Fotodyne imaging system. All western blot data were analyzed and quantified using ImageJ software.
siRNA knockdown of PKG
siRNA targeting PKG were acquired from Dharmacon (SMARTpool 5 nmol) and reconstituted into 20 μM aliquots. Our scramble siRNA control (siCON) was acquired from IDTDNA (Coralville, IA). MDA-MB-468 cells were seeded at 1 million cells/60 mm dish and transfected with final concentrations of 200 nM PKG siRNA or siCON (200 nM) with INTERFERin (Polyplus Transfection) according to manufacturer’s instructions. On the following day (24 hours post-transfection), cells were split and plated into two, 96-well dishes. Forty-eight hours post-transfection, one of the 96-well dishes was serum starved for 2 hours, and each treatment group (siCON and PKG siRNA transfected cells) was treated with either DMEM, EGF, or STS. The plate was incubated for an additional 24 or 48-hours. Cell viability was assessed via Alamarblue analysis, as described previously.
Statistical Analyses
A student-t test was used to determine statistical significance within each experiment. A p value of less than 0.1 is designated significant, and is indicated by a single asterisk (*). A p value of less than 0.05 is designated significant, and is indicated by two asterisks (**). A p value of less than 0.01 is designated very significant, and is indicated by three asterisks (***). A p value of less than 0.001 is designated extremely significant, and is indicated by four asterisks (****).
RESULTS
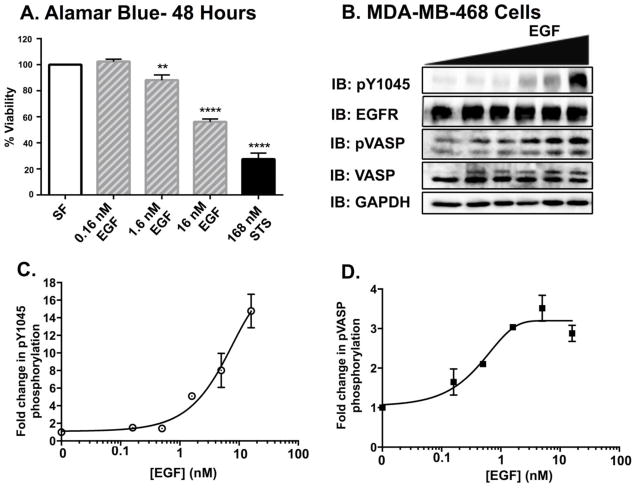
It is well documented that cell lines that hyperexpress the EGFR, such as MDA-MB-468 cells [4, 30, 31], undergo EGFR-mediated apoptosis. This is demonstrated with the dose-dependent decrease in MDA-MB-468 cell viability (Fig 1A). How a mitogenic growth factor receptor mediates cell death has studied for a number of years, with no clear resolution of the molecular mechanism. Determining the effectors that are necessary for EGFR-mediated apoptosis is a critical first step understanding the underlying molecular mechanism.
Figure 1. Increases in EGF ligand concentration elicit a dose dependent increase in pVASPSer239 phosphorylation in MDA-MB-468 cells.
A. MDA-MB-468 cells were seeded into 96-well dishes prior to being serum starved overnight. The cells were treated for 48 hours prior to AlamarBlue, cell viability analyses. Data are reported as the mean ± SEM (n=3). B. Serum-starved MDA-MB-468 cells were treated with varying concentrations of EGF (0, 0.16, 0.5, 1.6, 5 and 16 nM) for 30 minutes. Cell lysates were prepared, and equivalent amounts of protein (20 μg) were resolved by 12% SDS-PAGE and transferred to nitrocellulose. Membranes were probed for EGFR phosphorylated at tyrosine 1045 (pY1045), total EGFR (EGFR), VASP phosphorylated at serine 239 (pVASP), total VASP (VASP), and GAPDH as a loading control. Quantification of EGFR phosphorylation (pY1045) (C.) and VASP phosphorylation (pVASP) (D.) immunoblots using ImageJ software. Data are plotted as the mean ± Standard Error of the Mean (SEM) (n=3).
Based on previous studies linking Protein kinase G (PKG) activity to apoptosis in MDA-MB-468 cells, we examined whether PKG was downstream of EGFR activity (Fig 1B). Following treatment with EGF, there was a dose-dependent increase in EGFR phosphorylation [measured as a function of phosphorylation of tyrosine 1045 (pY1045)] (Fig 1C). Active PKG phosphorylates VASP specifically at Serine239 [32]. Serine phosphorylation of VASP is accompanied by a slowed electrophoretic mobility of the protein on SDS-PAGE resulting in two bands on both phosphorylated VASP (pVASP) and total VASP immunoblots [33–35]. Therefore, the differences observed in total VASP levels are a reflection of phosphorylation-dependent changes in protein electrophoretic mobility.
Using an phosphoVASP immunoblot to monitor activation of PKG, we found that, co-incident with receptor phosphorylation, there was a dose-dependent increase in PKG activity (Fig 1B). Comparison of the EC50 of EGF-mediated EGFR and VASP phosphorylation (4.7 nM and 0.49 nM, respectively) indicates that the processes are tightly coupled; only low levels of EGFR activity are needed to stimulate PKG.
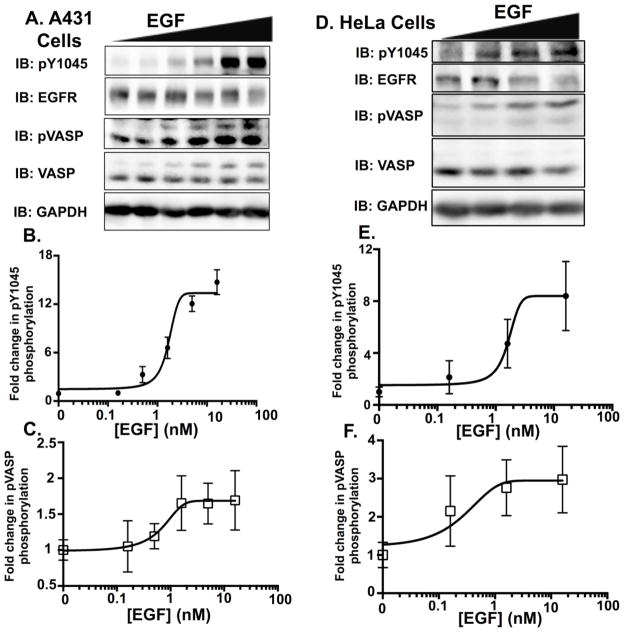
EGFR:PKG communication is not unique to MDA-MB-468 cells [36]. A431 cells are a metastatic epidermoid cell line that also undergoes EGF-dependent apoptosis [31], and hyperexpresses EGFRs at levels (1.5 × 106 EGFR/cell [37]) comparable to MDA-MB-468 cells [18]. When treated with EGF, A431 cells had a similar dose-dependent induction of EGFR and VASP activity (Fig 2A). EGF induced EGFR phosphorylation in A431 cells with a similar efficacy and potency as seen in MDA-MB-468 cells (Fig 2B). A431 and MDA-MB-468 cells had comparable levels of pVASP activity (~2–3-fold over basal), and comparable EC50’s to stimulate pVASP (0.56 nM and 0.66 nM, respectively). Further, in HeLa cells that expresses much lower levels of EGFR (~50,000 EGFRs/cells [38], despite a smaller dynamic range of EGFR phosphorylation (Fig 2D), the EC50 was comparable (0.66 nM). Thus, in multiple EGFR-expressing cell lines, there was an EGF-dependent PKG activation.
Figure 2. Increases in EGF ligand concentration elicit a dose dependent increase in pVASPSer239 phosphorylation in A431 and HeLa cell lines.
A. A dose response was conducted in A431 cells using 0, 0.16, 0.5, 1.6, 5 and 16 nM concentrations of EGF ligand. Cells were serum starved for 2 hours, and then exposed to the various ligand concentrations for 60 minutes. Forty μg of protein per sample were then resolved on a 10% SDS-PAGE. Western blot data of pY1045 (B.) and pVASPSer239 (C.) from three independent dose response experiments in A431 cells were quantified to blots of total protein (EGFR and VASP, respectively) using ImageJ software. Error bars are expressed as ± SEM. D. A dose response was conducted in HeLa cells using 0, 0.16, 1.6 and 16 nM concentrations of EGF ligand. Cells were serum starved for 2 hours, and then exposed to the various ligand concentrations for 60 minutes. HeLa cell lysates (60 μg) were then resolved on a 10% SDS-PAGE. Western blot data of pY1045 (E.) and pVASPSer239 (F.) from three independent dose response experiments in HeLa cells were quantified to blots of total protein (EGFR and VASP, respectively) using ImageJ software. Error bars are expressed as ± SEM (n=3).
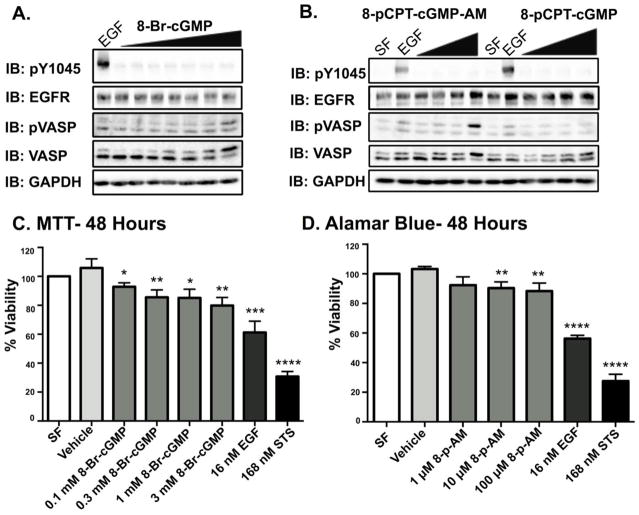
Having established that EGFR could activate PKG, we wanted to determine if direct activation of PKG was sufficient to induce cell death. MDA-MB-468 cells were treated with three commercially available agonists of PKG – 8-Bromoguanosine 3′,5′-cyclic monophosphate (8-Br-cGMP), 8-(4-Chlorophenylthio)-guanosine 3′,5′-cyclic monophosphate (8-pCPT-cGMP), and 8- (4- Chlorophenylthio) guanosine- 3′,5′-cyclic monophosphate, acetoxymethyl ester (8-pCPT-cGMP-AM). 8-Br-cGMP is a brominated derivative cGMP analog and is cell permeant [39]. 8-pCPT-cGMP and 8-pCPT-cGMP-AM are both PKG agonists that only differ by the presence of an acetoxymethyl ester; the addition of the acetoxymethyl ester makes 8-pCPT-cGMP-AM more cell permeant, and resistant to metabolic turnover [40]. MDA-MB-468 cells were treated with varying concentrations of these compounds and found to activate PKG (measured by VASP phosphorylation) (Fig 3A and 3B), treatment with 8-pCPT-cGMP did not produce detectable levels of pVASP, likely due to limited cell permeability, and was not used in cell viability experiments. Importantly, none of the PKG activators were able to induce detectable levels of EGFR phosphorylation (Fig 3A and 3B).
Figure 3. PKG agonists do not elicit EGFR phosphorylation, but do induce dose dependent decreases in MDA-MB-468 cell viability.
A. The PKG agonist, 8-Br- cGMP, was used at 0.1, 0.3, 1, 1.3, 2 and 3 mM concentrations. This treatment was accompanied with a positive control for EGFR phosphorylation, 16 nM EGF. The cells were exposed to the various conditions for 1 hour prior to the harvesting of each cell lysate. Forty μg of protein per sample were then resolved on a 12% SDS-PAGE. B. The PKG agonist, 8-pCPT-cGMP-AM (8-p-AM), was employed at 1, 10, and 100 μM concentrations. The other PKG agonist in use, 8-pCPT-cGMP, was employed at 100, 250, and 500 μM concentrations. Cells treated with serum free DMEM (SF) served as a negative control for both pVASP and EGFR phosphorylation. Cells treated with 16 nM EGF served as a positive control for EGFR and pVASP-SER239 phosphorylation. C. Results from an MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) cell viability assay. Cells were serum starved overnight, and then subjected to each experimental condition for 48 hours total prior to MTT analyses. A vehicle control of 6.7% deionized water in DMEM was used to accompany the 3 mM 8-Br-cGMP treatment. Data are expressed as means of percent viability (all to the serum free control) ± SEM (n=3). D. Results from an AlamarBlue cell viability assay. Cells were serum starved overnight, and then subjected to each experimental condition for 48 hours total prior to AlamarBlue analyses. Cells treated with the PKG agonist, 8-p-AM, were quantified to their respective vehicle control (cells treated with 0.2% DMF). All other data were quantified to the serum free control. All data are plotted as the average of percent viability ± SEM (n=4).
Next, the effect of the PKG agonists on cell viability was examined. Both 8-Br-cGMP and 8-pCPT-cGMP-AM caused a modest, dose-dependent decrease in cell viability (Fig 3C and 3D).
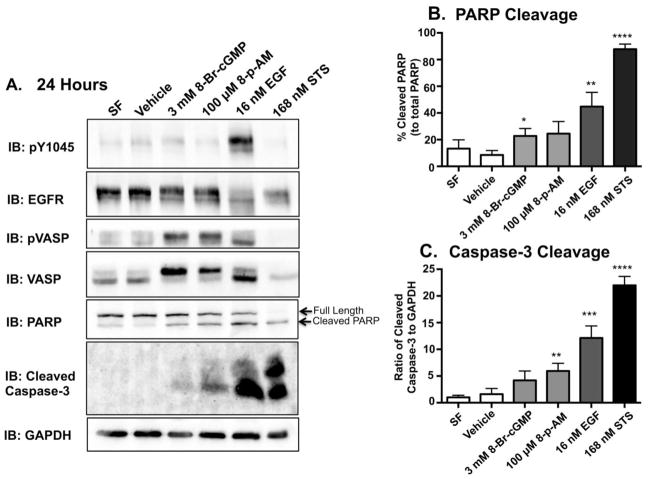
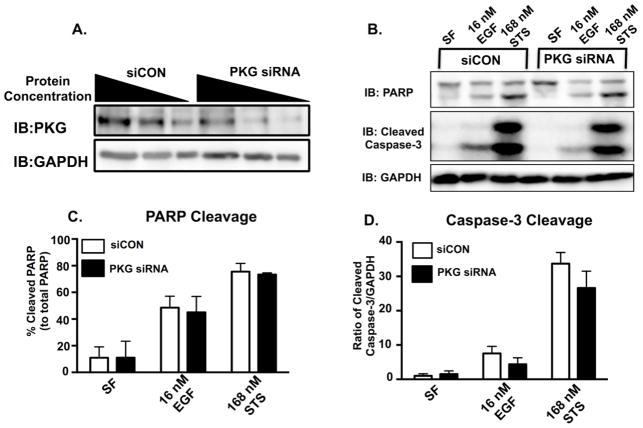
To determine if the observed decreases in cell viability were a consequence of apoptosis, we examined caspase-3 and poly ADP-ribose polymerase (PARP) cleavage via immunoblot analysis. Caspase-3 is a critical executioner of apoptosis [41]; when active, executioner caspases cleave and activate various substrates, such as poly ADP-ribose polymerase (PARP), which ultimately cause the morphological changes associated with apoptotic cells [42, 43]. MDA-MB-468 cells were treated with 8-Br-cGMP, 8-pCPT-cGMP-AM (8-p-AM) and 16 nM EGF for 24 hours. Despite the robust induction of EGFR phosphorylation with relatively short durations of EGF treatments (Figs 1A, 2A, and 2D), the induction of EGFR-mediated apoptosis requires a longer duration of ligand/agonist exposure. Based on experimental data, a 24-hour time point was used when examining apoptotic pathways (Fig 4A). Staurosporine (STS) is an alkaloid that robustly induces apoptosis after 3 hours; longer treatments caused catastrophic damage to the cell. STS was used as a positive control for the presence of cleaved PARP and caspase 3 by immunoblot (Figure 4). Cells were incubated in serum free media (SF) and vehicle alone as a negative control for apoptosis, and with staurosporine (STS) as a positive control. The PKG activators and EGF induced PARP and caspase-3 cleavage (Fig 4A). Consistent with the cell viability assays, EGF treatment was more efficacious in inducing apoptosis than PKG activation (Fig 4B and 4C). It should be noted that at 24 hours, MDA-MB-468 cells exposed to EGF and STS appear to exhibit a slight decrease in total EGFR levels (Figure 4A). These decreases are likely due to ligand-mediated receptor down-regulation (in EGF-treated cells) or from increased proteolysis in the apoptotic cell (EGF and STS-treated cells).
Figure 4. PKG agonists induce apoptosis in the MDA-MB-468 cell line.
A. MDA-MB-468 cells were serum starved overnight. With the exception of Staurosproine (STS), the cells were then subjected to each experimental condition for 24 hours. MDA-MB-468 cells were treated with STS for 3 hours prior to being harvested. This was done in order to induce a robust response without inducing catastrophic damage to the cell. The induction of apoptosis was determined by using PARP and Cleaved Caspase-3 antibodies. With the exception of STS, 40 μg of protein per sample were then resolved on a 15% SDS-PAGE. Fifteen μg of protein from the STS sample were resolved on the 15% SDS-PAGE. This was done in order to to ensure that our positive control was within the dynamic range. Quantification of western blot data from cleaved PARP (B.) and Caspase-3 (C.) from three independent experiments. Band intensities were determined using Image J software and plotted relative to total PARP (% cleaved PARP) or GAPDH (ratio of cleaved Caspase-3/GAPDH), respectively. Data are plotted as the average ± SEM (n=3)
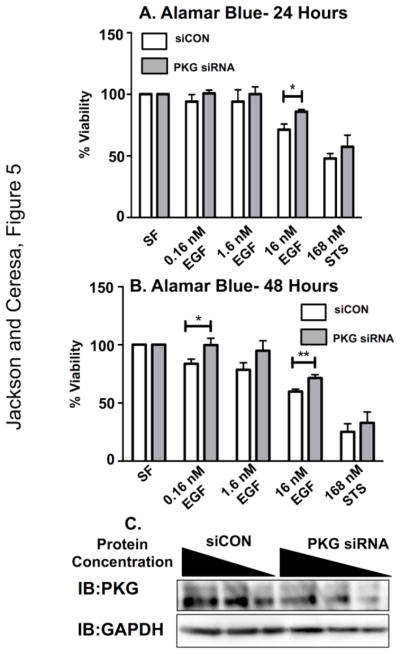
Finally, to determine if PKG is a signaling intermediate in EGFR-mediated apoptosis, we examined EGFR-mediated signaling with attenuated PKG expression (Fig 5). Cells were transfected with control (siCON) or PKG specific siRNA, treated with EGF for 24 or 48 hours, and assayed for cell viability (Fig 5A and 5B). PKG knockdown was verified by immunoblot (Fig 5C) and was quantified using Image J software to be 55%, consistent with previous reports [44]. In the absence of PKG, EGF treatment was not as effective at decreasing cell viability. This was statistically significant after 48 hours of EGF treatment and at high EGF doses (i.e. 16 nM), however, this trend is observed at all three EGF concentrations. When PKG knock down cells were treated with EGF, there was a trend of reduced activity in apoptotic markers (PARP and Caspase-3 cleavage) (Fig 6B–D), but this was not statistically significant.
Figure 5. Reduction in PKG activity yields a significant increase in MDA-MB-468 cell viability upon exposure to EGF ligand.
MDA-MB-468 cells were subjected to either 200 nM control siRNA (siCON) or 200 nM of siRNA targeting PKG for a total of 96 hours. The day after initial siRNA transfection, MDA-MB-468 cells were seeded into 96-well dishes and were then subjected to various concentrations of EGF ligand for either 24 hours (A) or 48 hours (B). Data are plotted as the average of percent viability ± SEM (n=3) C. Representative western blot image of 200 nM siCON and 200 nM PKG siRNA exposed MDA-MB-468 cells at 96 hours. Cell lysates were loaded in volumes of decreasing protein concentration (20, 10, and 5 μg) and then resolved on a 12% SDS-PAGE. Blots were then quantified using ImageJ software in order to determine the percentage of PKG knockdown (55%).
Figure 6. Reduction in PKG activity yields less apoptosis in MDA-MB-468 cells.
A. Representative western blot image of 200 nM siCON and 200 nM PKG siRNA exposed MDA-MB-468 cells at 96 hours. Cell lysates were loaded in volumes of decreasing protein concentration (20, 10, and 5 μg) and then resolved on a 12% SDS-PAGE. Blots were then quantified using ImageJ software in order to determine the percentage of PKG knockdown (55%). B. Representative western blot image of western blot analyses of PKG knockdown and siCON MDA-MB-468 cells. Cells were treated with 200 nM siCON or 200 nM PKG siRNA for 96 hours. The cells were also exposed to either serum free media (SF), EGF, or Staurosporine (STS) for 48 hours. Cell lysates were then harvested and resolved on a 15% SDS PAGE. Immunoblot images for PARP and Cleaved Caspase-3 were used as indicators of apoptosis. Quantification of western blot data from cleaved PARP (C.) and Caspase-3 (D.) from three independent experiments. Band intensities were determined using Image J software and plotted relative to total PARP (% cleaved PARP) or GAPDH (ratio of cleaved Caspase-3/GAPDH), respectively. Data are plotted as the average ± SEM (n=3).
DISCUSSION
The purpose of this study was to identify effectors downstream of EGFR activation that are actively mediating apoptosis in MDA-MB-468 cells. Using a combination of immunoblot assays, pharmacological activators, and RNAi, we tested the hypothesis that the EGFR is signaling via PKG to induce apoptosis. The ligand-dependent activation of the EGFR corresponded with phosphorylation of the PKG substrate VASP (Fig 1B). It is notable that both EGFR and PKG agonists induce apoptosis with much slower kinetics than more aggressive agents, such as staurosporine. The directionality of this signaling was confirmed by the observation that direct activation of PKG with the agonists 8-Br-cGMP and 8-p-AM (Fig 3A and 3B) failed to stimulate the EGFR. Finally, when PKG was knocked down, there was a statistically significant reduction in EGFR-mediated cell viability and a decrease in caspase-3 cleavage.
PKG is not normally associated with a downstream effector of the EGFR. However, it is not unprecedented; EGF stimulation in an ovarian cancer cell line, OV2008, leads to an EGFR-dependent VASP phosphorylation as well [36]. In addition to MDA-MB-468 cells, EGF treatment of A431 and HeLa cells, leads to a dose-dependent induction of phosphoVASP (Fig 2). It should be noted that in cell lines which express physiological levels of EGFR, exposure to higher levels of ligand often elicit total receptor degradation [29] (Fig 2D). EGFR:PKG communication can occur independent of receptor expression. However, the cytotoxicity associated with EGFR-mediated PKG activity may be a function of the kinase’s subcellular distribution, the duration of receptor:effector communication, or the presence of downstream substrates.
While A431 cells undergo EGFR-mediated apoptosis [31, 45], EGF is not as potent in its induction of apoptosis as what has been observed in MDA-MB-468 cells. For instance, it has been reported that A431 cells are capable of surviving EGF treatment for several days, and can become resistant to the induction of apoptosis in the presence of EGF [46]. Further, the initial reports of PKG-mediated apoptosis were in MDA-MB-468 cells [20, 27]. We therefore elected to pursue subsequent experiments in a cell line with the greatest dynamic range.
PKG activity is often associated with cytoprotective biology (i.e. heart cells [47], neurons, and glia cells [48, 49]) rather than a mediator of apoptosis. However, there is other evidence linking PKG to other proapoptotic effects in breast [27], ovarian [36], and colon cancer. Activation of PKGI in colon cancer cells induces apoptosis in a c-Jun NH2 NH2-terminal kinase 1 (JNK1) pathway dependent manner [50]. Many of the roles of cGMP and PKG signaling are well understood; however, a lack of clarity remains in terms of the role of PKG in cancer. This research aims to provide more information about PKG and it’s association with EGFR, and other receptor tyrosine kinases with implications in human malignancies.
By most accounts, apoptosis is an aberrant EGFR-mediated signaling event for a receptor that typical promotes cell growth. It is a challenge to decipher the molecular events that specifically promote apoptosis in the context of receptor- and cell stress-mediated pro-growth signaling. As a result, the molecular details of EGFR-mediated apoptosis remain controversial. The signal transducers and activators of transcription (STAT) pathway is one of the most intensively studied mechanism entailed in EGFR-mediated apoptosis in cancer [51]. Studies have shown that in cell lines that hyper-express EGFR, the EGFR activates STAT1, which recognizes responsive elements of and upregulates p21 [52, 53]. When active, p21 is a cyclin-dependent kinase inhibitor that suppresses reduces cell proliferation and induces apoptosis [54]. More recent studies have shown that in cell lines with elevated EGFR expression, EGF stimulation suppresses activity within the phosphoinositide 3-kinase/protein kinase B/mammalian target of rapamycin (PI3K/AKT/mTOR) pathway, suggesting that inhibition of the PI3K/AKT/mTOR pathway might be required for EGFR-mediated apoptosis to occur [55].
This report provides data indicating that in MDA-MB-468 cells, PKG contributes to EGFR-mediated cell death, likely through apoptotic signaling pathways. Although the contribution of PKG to EGFR-mediated apoptosis appears modest, this is likely a consequence of partial PKG knock down (~55% knock down). It remains unclear how a mitogenic growth factor receptor and a cytoprotective effector collaborate to induce apoptosis. One possible explanation is that hyperstimulation of cell viability pathways leads to cell catastrophe over time. Several pieces of evidence support this model. First, cell lines that have low levels of EGFR and more rapid receptor turnover do not induce apoptosis [56]. Second, low levels of EGF ligand do not induce MDA-MDA-468 cell apoptosis [4]. Third, the slowed kinetics of cell death following EGFR or PKG activation argue that the induction of apoptosis does not occur as a direct activation of an enzymatic pathway. Nevertheless, the EGFR:PKG communication shows promise as a new pathway for selectively mediating cytotoxicity in cells that overexpress the EGFR.
Acknowledgments
This work was supported in part by NIH grant GM092874 (B.P.C.) and Southern Regional Education Board – State Doctoral Scholars Program (N.M.J.). We thank members of the Ceresa lab for their critical insights on this work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Schneider MR, et al. Beyond wavy hairs: the epidermal growth factor receptor and its ligands in skin biology and pathology. Am J Pathol. 2008;173(1):14–24. doi: 10.2353/ajpath.2008.070942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol. 2001;2(2):127–37. doi: 10.1038/35052073. [DOI] [PubMed] [Google Scholar]
- 3.Paez JG, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304(5676):1497–500. doi: 10.1126/science.1099314. [DOI] [PubMed] [Google Scholar]
- 4.Armstrong DK, et al. Epidermal growth factor-mediated apoptosis of MDA-MB-468 human breast cancer cells. Cancer Res. 1994;54(20):5280–3. [PubMed] [Google Scholar]
- 5.Zhang X, et al. Mutations of epidermal growth factor receptor in colon cancer indicate susceptibility or resistance to gefitinib. Oncol Rep. 2008;19(6):1541–4. [PubMed] [Google Scholar]
- 6.Brabyn CJ, Kleine LP. EGF causes hyperproliferation and apoptosis in T51B cells: involvement of high and low affinity EGFR binding sites. Cell Signal. 1995;7(2):139–50. doi: 10.1016/0898-6568(94)00073-k. [DOI] [PubMed] [Google Scholar]
- 7.Sordella R, et al. Gefitinib-sensitizing EGFR mutations in lung cancer activate anti-apoptotic pathways. Science. 2004;305(5687):1163–7. doi: 10.1126/science.1101637. [DOI] [PubMed] [Google Scholar]
- 8.Lou YF, et al. Combination of gefitinib and DNA methylation inhibitor decitabine exerts synergistic anti-cancer activity in colon cancer cells. PLoS One. 2014;9(5):e97719. doi: 10.1371/journal.pone.0097719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wong SF. Cetuximab: an epidermal growth factor receptor monoclonal antibody for the treatment of colorectal cancer. Clin Ther. 2005;27(6):684–94. doi: 10.1016/j.clinthera.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 10.Janjigian YY, et al. Dual inhibition of EGFR with afatinib and cetuximab in kinase inhibitor-resistant EGFR-mutant lung cancer with and without T790M mutations. Cancer Discov. 2014;4(9):1036–45. doi: 10.1158/2159-8290.CD-14-0326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maemondo M, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362(25):2380–8. doi: 10.1056/NEJMoa0909530. [DOI] [PubMed] [Google Scholar]
- 12.Oxnard GR, et al. New strategies in overcoming acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors in lung cancer. Clin Cancer Res. 2011;17(17):5530–7. doi: 10.1158/1078-0432.CCR-10-2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fung C, et al. EGFR tyrosine kinase inhibition induces autophagy in cancer cells. Cancer Biol Ther. 2012;13(14):1417–24. doi: 10.4161/cbt.22002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stanton P, et al. Epidermal growth factor receptor expression by human squamous cell carcinomas of the head and neck, cell lines and xenografts. Br J Cancer. 1994;70(3):427–33. doi: 10.1038/bjc.1994.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davidson NE, et al. Epidermal growth factor receptor gene expression in estrogen receptor-positive and negative human breast cancer cell lines. Mol Endocrinol. 1987;1(3):216–23. doi: 10.1210/mend-1-3-216. [DOI] [PubMed] [Google Scholar]
- 16.Hognason T, et al. Epidermal growth factor receptor induced apoptosis: potentiation by inhibition of Ras signaling. FEBS Lett. 2001;491(1–2):9–15. doi: 10.1016/s0014-5793(01)02166-4. [DOI] [PubMed] [Google Scholar]
- 17.Cailleau R, Olive M, Cruciger QV. Long-term human breast carcinoma cell lines of metastatic origin: preliminary characterization. In Vitro. 1978;14(11):911–5. doi: 10.1007/BF02616120. [DOI] [PubMed] [Google Scholar]
- 18.Filmus J, et al. MDA-468, a human breast cancer cell line with a high number of epidermal growth factor (EGF) receptors, has an amplified EGF receptor gene and is growth inhibited by EGF. Biochem Biophys Res Commun. 1985;128(2):898–905. doi: 10.1016/0006-291x(85)90131-7. [DOI] [PubMed] [Google Scholar]
- 19.Wall ME, et al. Mechanisms associated with cGMP binding and activation of cGMP-dependent protein kinase. Proc Natl Acad Sci U S A. 2003;100(5):2380–5. doi: 10.1073/pnas.0534892100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fallahian F, et al. Cyclic GMP induced apoptosis via protein kinase G in oestrogen receptor-positive and -negative breast cancer cell lines. FEBS J. 2011;278(18):3360–9. doi: 10.1111/j.1742-4658.2011.08260.x. [DOI] [PubMed] [Google Scholar]
- 21.Francis SH, et al. cGMP-dependent protein kinases and cGMP phosphodiesterases in nitric oxide and cGMP action. Pharmacol Rev. 2010;62(3):525–63. doi: 10.1124/pr.110.002907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Walter U, Gambaryan S. cGMP and cGMP-dependent protein kinase in platelets and blood cells. Handb Exp Pharmacol. 2009;(191):533–48. doi: 10.1007/978-3-540-68964-5_23. [DOI] [PubMed] [Google Scholar]
- 23.Wyatt TA, et al. ANF elicits phosphorylation of the cGMP phosphodiesterase in vascular smooth muscle cells. Am J Physiol. 1998;274(2 Pt 2):H448–55. doi: 10.1152/ajpheart.1998.274.2.H448. [DOI] [PubMed] [Google Scholar]
- 24.Corbin JD, Francis SH. Cyclic GMP phosphodiesterase-5: target of sildenafil. J Biol Chem. 1999;274(20):13729–32. doi: 10.1074/jbc.274.20.13729. [DOI] [PubMed] [Google Scholar]
- 25.Lohmann SM, et al. Distinct and specific functions of cGMP-dependent protein kinases. Trends Biochem Sci. 1997;22(8):307–12. doi: 10.1016/s0968-0004(97)01086-4. [DOI] [PubMed] [Google Scholar]
- 26.Sausbier M, et al. Mechanisms of NO/cGMP-dependent vasorelaxation. Circ Res. 2000;87(9):825–30. doi: 10.1161/01.res.87.9.825. [DOI] [PubMed] [Google Scholar]
- 27.Fallahian F, Karami-Tehrani F, Salami S. Induction of apoptosis by type Ibeta protein kinase G in the human breast cancer cell lines MCF-7 and MDA-MB-468. Cell Biochem Funct. 2012;30(3):183–90. doi: 10.1002/cbf.1831. [DOI] [PubMed] [Google Scholar]
- 28.Hansen MB, Nielsen SE, Berg K. Re-examination and further development of a precise and rapid dye method for measuring cell growth/cell kill. J Immunol Methods. 1989;119(2):203–10. doi: 10.1016/0022-1759(89)90397-9. [DOI] [PubMed] [Google Scholar]
- 29.Hyatt DC, Ceresa BP. Cellular localization of the activated EGFR determines its effect on cell growth in MDA-MB-468 cells. Exp Cell Res. 2008;314(18):3415–25. doi: 10.1016/j.yexcr.2008.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kottke TJ, et al. Comparison of paclitaxel-, 5-fluoro-2′-deoxyuridine-, and epidermal growth factor (EGF)-induced apoptosis. Evidence for EGF-induced anoikis. J Biol Chem. 1999;274(22):15927–36. doi: 10.1074/jbc.274.22.15927. [DOI] [PubMed] [Google Scholar]
- 31.Gill GN, Lazar CS. Increased phosphotyrosine content and inhibition of proliferation in EGF-treated A431 cells. Nature. 1981;293(5830):305–7. doi: 10.1038/293305a0. [DOI] [PubMed] [Google Scholar]
- 32.Smolenski A, et al. Analysis and regulation of vasodilator-stimulated phosphoprotein serine 239 phosphorylation in vitro and in intact cells using a phosphospecific monoclonal antibody. J Biol Chem. 1998;273(32):20029–35. doi: 10.1074/jbc.273.32.20029. [DOI] [PubMed] [Google Scholar]
- 33.Dhayade S, et al. Sildenafil Potentiates a cGMP-Dependent Pathway to Promote Melanoma Growth. Cell Rep. 2016;14(11):2599–610. doi: 10.1016/j.celrep.2016.02.028. [DOI] [PubMed] [Google Scholar]
- 34.Doppler HR, et al. Protein kinase D1-mediated phosphorylations regulate vasodilator-stimulated phosphoprotein (VASP) localization and cell migration. J Biol Chem. 2013;288(34):24382–93. doi: 10.1074/jbc.M113.474676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Knauer O, et al. Differential phosphoproteome profiling reveals a functional role for VASP in Helicobacter pylori-induced cytoskeleton turnover in gastric epithelial cells. Cell Microbiol. 2008;10(11):2285–96. doi: 10.1111/j.1462-5822.2008.01207.x. [DOI] [PubMed] [Google Scholar]
- 36.Leung EL, et al. Protein kinase G type Ialpha activity in human ovarian cancer cells significantly contributes to enhanced Src activation and DNA synthesis/cell proliferation. Mol Cancer Res. 2010;8(4):578–91. doi: 10.1158/1541-7786.MCR-09-0178. [DOI] [PubMed] [Google Scholar]
- 37.Krupp MN, Connolly DT, Lane MD. Synthesis, turnover, and down-regulation of epidermal growth factor receptors in human A431 epidermoid carcinoma cells and skin fibroblasts. J Biol Chem. 1982;257(19):11489–96. [PubMed] [Google Scholar]
- 38.Berkers JA, van Bergen en Henegouwen PM, Boonstra J. Three classes of epidermal growth factor receptors on HeLa cells. J Biol Chem. 1991;266(2):922–7. [PubMed] [Google Scholar]
- 39.Zhuo M, et al. Role of guanylyl cyclase and cGMP-dependent protein kinase in long-term potentiation. Nature. 1994;368(6472):635–9. doi: 10.1038/368635a0. [DOI] [PubMed] [Google Scholar]
- 40.Schwede F, et al. Cyclic nucleotide analogs as biochemical tools and prospective drugs. Pharmacol Ther. 2000;87(2–3):199–226. doi: 10.1016/s0163-7258(00)00051-6. [DOI] [PubMed] [Google Scholar]
- 41.Fernandes-Alnemri T, Litwack G, Alnemri ES. CPP32, a novel human apoptotic protein with homology to Caenorhabditis elegans cell death protein Ced-3 and mammalian interleukin-1 beta-converting enzyme. J Biol Chem. 1994;269(49):30761–4. [PubMed] [Google Scholar]
- 42.Elmore S. Apoptosis: a review of programmed cell death. Toxicol Pathol. 2007;35(4):495–516. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Waring P, Mullbacher A. Cell death induced by the Fas/Fas ligand pathway and its role in pathology. Immunol Cell Biol. 1999;77(4):312–7. doi: 10.1046/j.1440-1711.1999.00837.x. [DOI] [PubMed] [Google Scholar]
- 44.Yang J, et al. Sildenafil potentiates bone morphogenetic protein signaling in pulmonary arterial smooth muscle cells and in experimental pulmonary hypertension. Arterioscler Thromb Vasc Biol. 2013;33(1):34–42. doi: 10.1161/ATVBAHA.112.300121. [DOI] [PubMed] [Google Scholar]
- 45.Kawamoto T, et al. Relation of epidermal growth factor receptor concentration to growth of human epidermoid carcinoma A431 cells. J Biol Chem. 1984;259(12):7761–6. [PubMed] [Google Scholar]
- 46.Tikhomirov O, Carpenter G. Ligand-induced, p38-dependent apoptosis in cells expressing high levels of epidermal growth factor receptor and ErbB-2. J Biol Chem. 2004;279(13):12988–96. doi: 10.1074/jbc.M311655200. [DOI] [PubMed] [Google Scholar]
- 47.Oldenburg O, et al. Bradykinin induces mitochondrial ROS generation via NO, cGMP, PKG, and mitoKATP channel opening and leads to cardioprotection. Am J Physiol Heart Circ Physiol. 2004;286(1):H468–76. doi: 10.1152/ajpheart.00360.2003. [DOI] [PubMed] [Google Scholar]
- 48.Nakamizo T, et al. Phosphodiesterase inhibitors are neuroprotective to cultured spinal motor neurons. J Neurosci Res. 2003;71(4):485–95. doi: 10.1002/jnr.10483. [DOI] [PubMed] [Google Scholar]
- 49.Thippeswamy T, et al. Glial-mediated neuroprotection: evidence for the protective role of the NO-cGMP pathway via neuron-glial communication in the peripheral nervous system. Glia. 2005;49(2):197–210. doi: 10.1002/glia.20105. [DOI] [PubMed] [Google Scholar]
- 50.Soh JW, et al. Cyclic GMP mediates apoptosis induced by sulindac derivatives via activation of c-Jun NH2-terminal kinase 1. Clin Cancer Res. 2000;6(10):4136–41. [PubMed] [Google Scholar]
- 51.Quesnelle KM, Boehm AL, Grandis JR. STAT-mediated EGFR signaling in cancer. J Cell Biochem. 2007;102(2):311–9. doi: 10.1002/jcb.21475. [DOI] [PubMed] [Google Scholar]
- 52.Chin YE, et al. Cell growth arrest and induction of cyclin-dependent kinase inhibitor p21 WAF1/CIP1 mediated by STAT1. Science. 1996;272(5262):719–22. doi: 10.1126/science.272.5262.719. [DOI] [PubMed] [Google Scholar]
- 53.Bhatia K, et al. A mutant p21 cyclin-dependent kinase inhibitor isolated from a Burkitt’s lymphoma. Cancer Res. 1995;55(7):1431–5. [PubMed] [Google Scholar]
- 54.Sheikh MS, Rochefort H, Garcia M. Overexpression of p21WAF1/CIP1 induces growth arrest, giant cell formation and apoptosis in human breast carcinoma cell lines. Oncogene. 1995;11(9):1899–905. [PubMed] [Google Scholar]
- 55.Kim K, Wu HG, Jeon SR. Epidermal growth factor-induced cell death and radiosensitization in epidermal growth factor receptor-overexpressing cancer cell lines. Anticancer Res. 2015;35(1):245–53. [PubMed] [Google Scholar]
- 56.Rush JS, et al. Endosomal accumulation of the activated epidermal growth factor receptor (EGFR) induces apoptosis. J Biol Chem. 2012;287(1):712–22. doi: 10.1074/jbc.M111.294470. [DOI] [PMC free article] [PubMed] [Google Scholar]