Abstract
We describe 3 patients with relapsed/refractory acute myeloid leukemia who developed clinically-apparent differentiation concurrent with clinical response during monotherapy with AG-120, a novel, oral inhibitor of mutant isocitrate dehydrogenase 1. Symptoms included marked leukocytosis and exuberant neutrophil recovery among other clinically-apparent constitutional manifestations. Awareness of the potential for differentiation syndrome with such inhibitors, and prompt identification and intervention, are essential to facilitate clinical resolution.
Background
Cancer-associated isocitrate dehydrogenase (IDH) mutations block normal cellular differentiation via production of the oncometabolite, R-2-hydroxyglutarate. In patients with acute myeloid leukemia (AML) receiving targeted mutant IDH inhibitor therapy, neutrophil recovery within the setting of clinical differentiation syndrome (DS) has been anecdotally described.
Patients and Methods
We describe 3 patients who developed clinically apparent DS while on monotherapy with the mutant IDH1 inhibitor, AG-120, for relapsed/refractory AML.
Results
AG-120-induced differentiation commenced within the first 60 days of treatment, notably in the same timeframe as clinical response, strengthening the purported mechanism of targeted mutant IDH-inhibitor therapy via successful myeloid maturation. Symptoms of DS were non-specific and included culture-negative fever, edema, hypotension, malaise, and pleural and/or pericardial effusions, in addition to marked neutrophil-predominant leukocytosis.
Conclusion
DS can occur during treatment with targeted mutant IDH1 inhibitor therapy. Patients may present with non-specific clinical manifestations often in the setting of leukocytosis related to exuberant neutrophil recovery. Prompt identification and initiation of treatment interventions, including hydroxyurea, corticosteroids and/or consideration of temporary treatment discontinuation, are important to facilitate prompt resolution.
Keywords: AML, Isocitrate dehydrogenase mutation, Myeloid maturation, Retinoic acid syndrome, Targeted therapy
Introduction
Differentiation syndrome (DS) is a potentially fatal complication of effective leukemia treatment first described in patients with acute promyelocytic leukemia (APL) treated with all trans-retinoic acid (ATRA).1 The reported incidence in APL ranges from 2% to 27%,2 likely due to the heterogeneity and range of clinical symptoms, as well as imprecise diagnostic criteria. In APL, signs and symptoms of DS have been described 2−47 days after treatment initiation, and include increasing white blood cell count (WBC) and absolute neutrophil count (ANC), culture-negative fever, weight gain, edema, dyspnea, interstitial infiltrates, pleural effusion, pericardial effusion, hypotension, and renal failure.1,3,4 The underlying pathophysiology remains poorly understood, but is thought to be related to release of inflammatory vasoactive cytokines and tissue infiltration by briskly maturing cells.2,5 Myeloid differentiation and clinical DS have also been described in patients with acute myeloid leukemia (AML) receiving therapy with FLT3 tyrosine kinase inhibitors and hypomethylating agents, including neutrophilic skin infiltrates retaining the aberrant FLT3-ITD mutation in some instances.6,7
Isocitrate dehydrogenase 1 and 2 (IDH1 and IDH2) mutations are noted in approximately 20% of patients with AML.8 Cancer-associated IDH1/2 mutations block normal cellular differentiation and drive tumorigenesis by promoting abnormal reduction of alpha-ketoglutarate (α-KG) to the oncometabolite, R-2-hydroxyglutarate (2-HG).9,10 2-HG accumulation inhibits multiple α-KG-dependent dioxygenases, including histone and DNA demethylases, which regulate cellular epigenetic state.11 The first-in-human phase I clinical study of the novel, oral mutant IDH1 inhibitor, AG-120, is ongoing (ClinicalTrials.gov NCT02074839), and early results in 66 patients indicate that monotherapy is well tolerated, with an overall response rate of 36% by International Working Group (IWG) criteria in a primarily relapsed/refractory AML population.12 Responses occur without a period of bone marrow aplasia, unlike standard cytoreductive therapy.
Neutrophil recovery in the setting of a clinical DS in patients receiving mutant IDH inhibitor therapy has been anecdotally described, but the clinical patterns of differentiation have not been previously reported. Herein, we describe 3 patients treated at our institution who developed clinically apparent differentiation and DS while on AG-120 monotherapy for relapsed/refractory AML.
Results
Case 1
A 53-year-old man was found to have incidental anemia during a routine physical examination. Results of complete blood count (CBC), bone marrow aspirate and biopsy, cytogenetic and molecular studies at presentation are shown in Table 1. He received intensive cytarabine-based induction AML chemotherapy, which was associated with complications (Table 1) and end of cycle 1 staging bone marrow demonstrated primary refractory disease with 77% myeloblasts.
Table 1.
Summary of Clinical and Laboratory Parameters in Three Patients with AG-120-induced Differentiation Syndrome
| Case 1 | Case 2 | Case 3 | |
|---|---|---|---|
| Presentation | |||
| CBC | WBC 13.7 × 109/L (4% neutrophils, 14% lymphocytes, 1% monocytes, 81% circulating blasts), Hb 9.2 g/dL, platelets 211 × 109/L | WBC 3.8 × 109/L (30% neutrophils, 37% lymphocytes, 14% monocytes, 16% circulating blasts, ANC 950), Hb 11.1 g/dL, platelets 35 × 109/L | WBC 7.7 × 109/L (0% neutrophils, 73% circulating blasts), Hb 8.9 g/dL, platelets 52 × 109/L |
| Serum parameters | Serum albumin 3.8 g/dL, Cr 1.66 mg/dL, LDH 1495 IU/L | Serum albumin 3.7g/dL, Cr 1.27 mg/dL, LDH 691 IU/L | Serum albumin 1.6 g/dL, Cr 0.47 mg/dL, LDH 953 IU/L |
| BM aspiration and biopsy | 40% myeloblasts positive for CD13+, CD33+, CD34+, CD117+, myeloperoxidase, and HLA-DR, negative for CD14 and CD41, in setting of trilineage dysplasia | 51% myeloblasts positive for CD13, CD33, CD34, CD117, MPO, HLA-DR and negative for CD14 | 70% myeloblasts positive for CD13, CD33, CD34, CD123, MPO (bright), and negative for CD14 and HLA-DR |
| Cytogenetic and molecular analysis | Complex karyotype; 44,XY, add(5)(q13), der(7;17)(p10;q10), inv(9)(p12q13), −15 [20]. Mutations: IDH1-R132C, TP53-R273H | Trisomy 8 and trisomy 9. Mutations in DNMT3A-R882C, IDH1-R132C, IDH2-R140Q, and RUNX1-R166Q | Diploid karyotype. Mutations in DNMT3A (frameshift Y660fs*42), IDH1-R132C |
| First-line therapy | |||
| Regimen | Clofarabine (15 mg/m2 on days 1–5), idarubicin (10 mg/m2 on days 1–3) and cytarabine (1 g/m2 on days 1–5) | Clofarabine (15 mg/m2 on days 1–5), idarubicin (10 mg/m2 on days 1–3), and cytarabine (1 g/m2 on days 1–5) | Cladribine (5 mg/m2 on days 1–5), idarubicin (10 mg/m2 on days 1–3), and cytarabine (1 g/m2 on days 1–5) |
| Complications | Stenotrophomonas and parainfluenza pneumonia, and overall deconditioning | - | Multifocal pneumonia and pansinusitis from parainfluenza 3, hepatic abscess, and pseudomonas bacteremia |
| AG-120 cycle 1 | Given as second-line therapy | Given as fourth-line therapy | Given as second-line therapy |
| Day 1 | WBC 21.7 × 109/L (1% neutrophils, 95% circulating blasts, ANC 220), Hb 9.5 g/dL, platelets 16 × 109/L, LDH 1027 IU/L | WBC 3.1 × 109/L (6% neutrophils, 65% circulating blasts, ANC 190), Hb 11.4 g/dL, platelets 52 × 109/L, LDH 867 IU/L 37% myeloblasts in BM |
WBC 0.5 × 109/L (5% neutrophils, 76% circulating blasts, ANC 20), Hb 8.8 g/dL, platelets 47 × 109/L, LDH 395 IU/L 69% myeloblasts in BM |
| Day 12 | WBC 5.4 × 109/L (62% neutrophils, 26% circulating blasts, ANC 3,360), Hb 10.2 g/dL, platelets 30 × 109/L, LDH 659 IU/L | WBC 25 × 109/L (22% neutrophils, 50% circulating blasts, ANC 5,490), Hb 9.9 g/dL, platelets 144 × 109/L, LDH 1249 IU/L | |
| Day 13 | WBC 4.4 × 109/L (24% neutrophils, 65% circulating blasts, ANC 1,050), Hb 9.4 g/dL, platelets 27 × 109/L, LDH 590 IU/L | aWBC 19.5 × 109/L (26% neutrophils, 28% circulating blasts, ANC 5,070), Hb 11.7 g/dL, platelets 97 × 109/L, LDH 893 IU/L | WBC 33.2 × 109/L (28% neutrophils, 49% circulating blasts, ANC 9,310) Hb 10.0 g/dL, platelets 162 × 109/L, LDH 1249 IU/L |
| Day 14 | WBC 3.1 × 109/L (54% neutrophils, 32% circulating blasts, ANC 1680), Hb 9.8g /dL, platelets 21 × 109/L, LDH 450 IU/L 37% myeloblasts in BM |
aWBC 19.5 × 109/L (26% neutrophils, 28% circulating blasts, ANC 5,070), Hb 11.7 g/dL, platelets 97 × 109/L, LDH 893 IU/L | WBC 29.1 × 109/L (46% neutrophils, 39% circulating blasts, ANC 13,360), Hb 10.3 g/dL, platelets 153 × 109/L, LDH 1448 IU/L |
| Day 15 | WBC 3.1 × 109/L (31% neutrophils, 50% circulating blasts, ANC 960), Hb 9.6 g/dL, platelets 20 × 109/L, LDH 591 IU/L | WBC 19.5 × 109/L (26% neutrophils, 28% circulating blasts, ANC 5,070), Hb 11.7 g/dL, platelets 97 × 109/L, LDH 893 IU/L | WBC 28.7 × 109/L (44% neutrophils, 33% circulating blasts, ANC 12,630), Hb 10.6 g/dL, platelets 172 × 109/L, LDH 1322 IU/L 37% myeloblasts in BM |
| Day 19 | aWBC 2.7 × 109/L (48% neutrophils, 26% circulating blasts, ANC 1,270), Hb 9.7 g/dL, platelets 24 × 109/L, LDH 432 IU/L | WBC 25.4 × 109/L (39% neutrophils, 6% circulating blasts, ANC 9,920), LDH 829 IU/L Max. temp 100.4°F, heart rate 127/min, respiratory rate 20/min, BP 128/68 mmHg, O2 saturation 99% on room air |
WBC 16.9 × 109/L (56% neutrophils, 32% circulating blasts, ANC 9,480), Hb 10.3 g/dL, platelets 125 × 109/L, LDH 1097 IU/L |
| Day 28 | 18% myeloblasts in BM | ||
| AG-120 cycle 2 | |||
| Day 1 | WBC 29 × 109/L (24% neutrophils, 13% circulating blasts, 45% metamyelocytes, ANC 7,020), Hb 10.4 g/dL, platelets 64 × 109/L, LDH 1,672 IU/L | WBC 87.2 × 109/L (40% neutrophils, 6% circulating blasts, ANC 34,870), Hb 11.2 g/dL, platelets 77 × 109/L, LDH 2,930 IU/L 18% myeloblasts in BM |
WBC 23.5 × 109/L (43% neutrophils, 17% circulating blasts, ANC 10,110), Hb 10.8 g/dl, platelets 77 × 109/L, LDH 2040 IU/L |
| Day 5 | WBC 42.5 × 109/L (64% neutrophils, 6% circulating blasts, ANC 27,230), Hb 8.4 g/dL, platelets 138 × 109/L, LDH 2,322 IU/L | aWBC 26.2 × 109/L (33% neutrophils, 12% circulating blasts, ANC 8,660), Hb 8.9 g/dl, platelets 49 × 109/L, LDH 1905 IU/L | |
| Day 8 | WBC 22.3 (41% neutrophils, 4% circulating blasts, ANC 9,140), Hb 8.1 g/dL, platelets 170 × 109/L, LDH 2037 IU/L Heart rate 125, BP 100/59 mmHg |
WBC 39.3 × 109/L (77% neutrophils, 4% circulating blasts, ANC 30,220), Hb 9.6 g/dl, platelets 43 × 109/L, LDH 2009 IU/L | |
| Day 12 | WBC 12.4 × 109/L (76% neutrophils, 0% circulating blasts, ANC 9,390), Hb 8.7 g/dl, platelets 59 × 109/L, LDH 1116 IU/L | aWBC 5.5 × 109/L (76% neutrophils, 0% circulating blasts, ANC 4,200), Hb 8.1 g/dl, platelets 17 × 109/L, LDH 895 IU/L | |
| Day 15 | aWBC 13.4 × 109/L (53% neutrophils, 0% circulating blasts, ANC 7,090), Hb 8.9 g/dL, platelets 38 × 109/L, LDH 1103 IU/L | WBC 29.7 × 109/L (35% neutrophils, 6% circulating blasts, ANC 10,400), Hb 10.1 g/dl, platelets 11 × 109/L, LDH 1833 IU/L | WBC 6.5 × 109/L (85% neutrophils, 0% circulating blasts, ANC 5,500), Hb 11.3 g/dl, platelets 13 × 109/L, LDH 715 IU/L |
| Day 28 | aWBC 1.8 × 109/L (78% neutrophils, 0% circulating blasts, ANC 1,420), Hb 8.5 g/dL, platelets 16 × 109/L, LDH 334 IU/L | Complete response | |
| AG-120 cycle 3 | |||
| Day 1 | WBC 1.8 × 109/L (78% neutrophils, 0% circulating blasts, ANC 1,420), Hb 8.5 g/dL, platelets 16 × 109/L, LDH 334 IU/L | WBC 4.4 × 109/L 3% myeloblasts in a hypercellular marrow IWG complete response with incomplete platelet recovery |
WBC 2.9 × 109/L ANC 2,250 Matched unrelated donor stem cell transplant |
| Day 28 | IWG complete response Successful matched related donor allogeneic SCT |
Abbreviations: ANC = absolute neutrophil count; BM = bone marrow; CBC = complete blood count; Cr = creatinine; Hb = hemoglobin; HLA-DR = Human Leukocyte Antigen - antigen D Related; IWG = international working group; LDH = lactate dehydrogenase; WBC = white blood cell count;
± 2 days
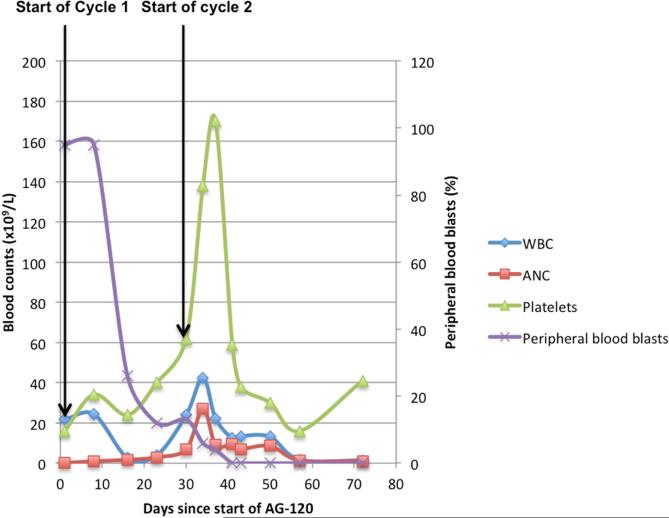
Given the presence of an IDH1-R132C mutation, the patient was transitioned to AG-120 500 mg/day orally on 28-day continuous cycles. His CBC on day 1 of therapy demonstrated a WBC of 21.7 × 109/L, with 1% neutrophils and 95% circulating blasts (Table 1, Figure 1A). A day 14 bone marrow aspiration demonstrated persistent AML, with 37% myeloblasts and at completion of cycle 1, blasts had reduced to 18% and he started cycle 2 without dose adjustments or treatment interruption (Table 1). On cycle 2, day 1, his WBC was 29 × 109/L with 24% neutrophils and 13% circulating blasts. On cycle 2, day 5, he reported a mild backache, denying fever, chills, cough, shortness of breath, nausea, vomiting, diarrhea, or urinary symptoms. Physical exam was normal except for mild 1+ non-tender bilateral pedal edema. The CBC demonstrated a rise in WBC to 42.5 × 109/L, with 64% neutrophils and 6% circulating blasts (Table 1, Figure 1A). There were no signs or symptoms of infection and he was maintained on empiric antimicrobial prophylaxis and started on hydroxyurea at 2 g/day. On cycle 2, day 8, he described symptoms of mild vertigo, poor appetite, and anorexia, and was found to be tachycardic and mildly hypotensive (100/59 mmHg) with an otherwise unremarkable physical exam. An EKG confirmed sinus tachycardia with no ST/T wave changes. An infectious evaluation including urine, sputum, and blood cultures was negative at 7 days. Hydroxyurea was reduced to 1 g/day. On cycle 2, day 12, the WBC was 12.4 × 109/L (76% neutrophils and 0% circulating blasts; Table 1). Hydroxyurea was discontinued 8 days after initiation. In the absence of any infectious etiology, the rise in leukocyte count with a predominance of mature neutrophils, concomitant reduction in circulating myeloblasts, platelet recovery, and mild clinical symptoms of hypotension, tachycardia, malaise, vertigo and lower back pain that spontaneously resolved, were suggestive of DS.
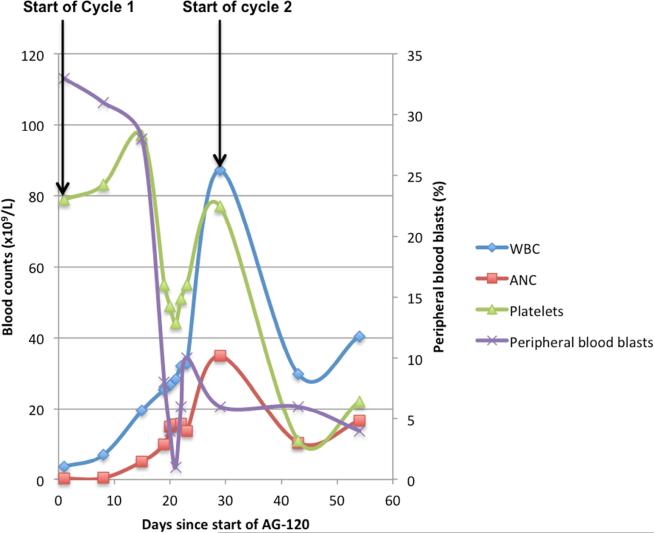
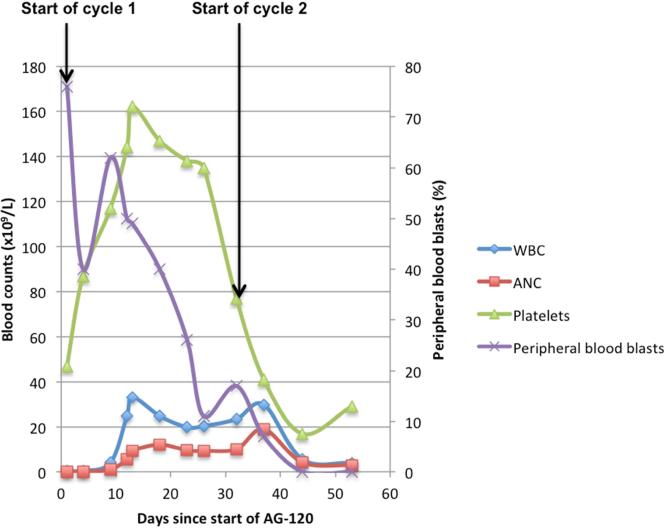
Figure 1.
Trends of Cell Counts and Blasts with Start of AG-120 Treatment in (A) Patient 1; (B) Patient 2; (C) Patient 3.
Abbreviations: ANC = absolute neutrophil count; WBC = white blood cell count.
The patient achieved a complete response after 3 cycles of AG-120 and transitioned to a matched related donor allogeneic stem cell transplant, and remains in an ongoing complete remission 2 months after transplantation with successful engraftment.
Case 2
Laboratory parameters for a 45-year-old man presenting with fatigue are shown in Table 1. He was treated with front-line AML induction therapy (Table 1). Due to primary refractory disease after cycle 1, he was treated with AG-221 (mutant IDH2 inhibitor) 100 mg daily for 4 cycles without evidence of response. He then received azacitidine (75 mg/m2 IV on days 2–8) and nivolumab (3 mg/kg on days 1 and 14) on a clinical trial for 4 cycles without response.
Given the persistence of his IDH1-R132C mutation, he started AG-120, 500 mg daily in the phase I trial. Laboratory parameters on cycle 1, day 1 are shown in Table 1 and included a WBC of 3.1 × 109/L with 6% neutrophils and 65% circulating blasts. Over the first 2 weeks on AG-120, peripheral myeloblasts steadily declined from 65% to 23% (Figure 1B). On cycle 1, day 19, he was hospitalized for acute onset chest pain and shortness of breath with exertion, without cough or upper respiratory complaints (vital signs and CBC shown in Table 1, and included a WBC of 25.4 × 109/L with 39% neutrophils and 6% circulating blasts). An EKG showed sinus tachycardia and diffuse ST segment elevation concerning for pericarditis. A 2-view chest X-ray identified left lower lung opacities representing possible atelectasis or early pneumonia, with a small left pleural effusion and enlarged cardiac silhouette suggestive of pericardial effusion. The patient was admitted to the intensive care unit and started on empiric broad-spectrum antibiotics for possible pneumonia, prednisone 1 mg/kg for 3 days for pericarditis, and furosemide diuresis. An echocardiogram on day 2 demonstrated a sustained left ventricular ejection fraction of 59%, with a moderate to large pericardial effusion with no right ventricular chamber collapse. A repeat echocardiogram 2 days later showed significant improvement in pericardial effusion with medical management. His clinical status improved and he was discharged after 5 days (cycle 1, day 23) with a further 1-week tapering course of steroids. AG-120 was continued during hospitalization and upon hospital discharge. All cultures during hospitalization were negative.
By cycle 2, day 1 of AG-120, the WBC had increased to 87.2 × 109/L with 40% neutrophils and 6% circulating blasts (Table 1, Figure 1B) and bone marrow blast count was stable (18%). Review of systems and physical exam were unremarkable. He received a 5-day course of hydroxyurea (2 g twice daily) to control leukocytosis. The WBC decreased over the next 2 weeks, normalizing by cycle 3, day 1 (Table 1). At that time, repeat assessment identified a complete remission with incomplete platelet recovery, with 3% myeloblasts in a hypercellular marrow with no morphologic evidence of residual leukemia, and full neutrophil and Hb recovery. In the absence of infection, the pericardial effusion followed by a dramatic rise in leukocyte count with predominantly mature neutrophils was highly suggestive of a clinically-significant DS.
Case 3
A 51-year-old man with fever and fatigue was diagnosed with AML. A CBC on admission showed WBC of 7.7 × 109/L (0% neutrophils and 73% circulating blasts; Table 1). Following induction therapy, which was associated with multiple complications (Table 1), he had primary refractory disease.
The patient was then treated with AG-120 1200 mg daily in the phase I trial. In cycle 1, the WBC improved from 0.5 × 109/L on day 1 with 5% neutrophils and 76% circulating blasts, to 25 × 109/L on day 12, with 22% neutrophils and 50% circulating blasts (Table 1). He was started on hydroxyurea 2 g/day orally. On day 15 the bone marrow blast count had decreased from 69% to 37% (Table 1, Figure 1C). The rapid rise in WBC count was associated with an increase in ANC and falling peripheral blast and bone marrow blast percentages, coinciding with treatment response and laboratory evidence of differentiation. Full count recovery with absence of leukemic blasts in the bone marrow and peripheral blood, leading to an IWG-defined complete response, was obtained after 2 cycles of AG-120. Leukocytosis was managed with 2 weeks of tapering hydroxyurea, which was discontinued at the start of cycle 3. The WBC was 2.9 × 109/L and ANC 2,250 at that time. After 3 cycles of AG-120, he underwent a matched unrelated donor stem cell transplant.
Discussion
The discovery of recurrent pathogenic IDH mutations and the advent of mutant IDH inhibitors has been one of the most significant translational advances in the management of AML in the past decade. AG-120 inhibits the mutant IDH1 enzyme and reduces aberrant serum 2-HG levels, which induces differentiation of leukemia cells.13
The pathophysiology of DS in AML remains incompletely understood. In APL, ATRA and arsenic trioxide (ATO) are thought to induce release of cytokines from differentiating myeloid cells, leading to excessive inflammatory response,2,5 and may increase expression of cell-surface integrins, which could increase adhesion of myeloid cells to vascular endothelium, thereby facilitating extravasation.2,5 The DS observed in patients treated with mutant IDH inhibitor therapy may be due to similar phenomena when treatment removes the differentiation block in the malignant myeloid clone, leading to a rapid increase in differentiated neutrophils.
In all 3 patients, manifestations of AG-120-induced DS occurred concurrently with early evidence of clinical response, suggesting that DS is related to treatment response and successful myeloid maturation. Further analyses are needed to confirm this association. In case 1, laboratory and clinical symptoms were first noted on cycle 2, day 5, including leukocytosis, mild pedal edema, malaise, asymptomatic hypotension, and tachycardia, without fever or other constitutional manifestations, and hydroxyurea alone was used with gradual resolution of symptoms. In case 2, signs and symptoms began on cycle 1, day 19 with low-grade fever, leukocytosis, and pericardial and pleural effusions. Tapering prednisone resulted in complete resolution of pericarditis, pericardial effusion and clinical symptoms. A second discrete episode of differentiation seemed to begin at the start of cycle 2, with extreme leukocytosis that stabilized with hydroxyurea alone. In case 3, laboratory parameters consistent with myeloblast differentiation were first noted on cycle 1, day 12 without apparent clinical manifestations. Hydroxyurea was initiated for a short tapering course due to the rapid leukocytosis, and AG-120 was not held.
Signs and symptoms of DS are not specific. They can include fever, edema, weight gain, leukocytosis, rash, hypotension, renal dysfunction, and pleural and pericardial effusions. A rising leukocyte count, comprising increasing neutrophils with a parallel decrease in leukemic blasts, was the most consistent finding among our patients experiencing AG-120-related DS. We did not observe significant weight gain or renal failure. In relapsed/refractory AML, many of these non-specific clinical findings can also be related to systemic infections or progressive leukemia, and care must be taken to accurately identify and appropriately manage mutant IDH inhibitor-induced DS. We recommend prompt initiation of hydroxyurea (suggested dose 2–4 g/day orally, titrated daily as needed to control leukocytosis), corticosteroids (suggested dose 10 mg dexamethasone every 12 hours for 3 days or until improvement), furosemide (40–80 mg/day) in patients with clinically apparent effusions, and consideration of temporary hold of mutant IDH inhibitor therapy until clinical improvement.
Conclusion
DS can occur during treatment with the mutant IDH1 inhibitor, AG-120. The timing of onset and the signs and symptoms can vary, and may present with dramatic clinical manifestations in addition to marked leukocytosis and exuberant neutrophil recovery. DS should be considered in all patients treated with AG-120 experiencing rapid neutrophil-predominant leukocytosis, culture-negative fever, constitutional symptoms, and pleural or pericardial effusions. Steroid therapy appears successful in clinically significant DS, and oral hydroxyurea can mitigate rapid leukocytosis. Ongoing research will improve the identification of risk factors and better define this syndrome in patients treated with targeted “differentiating” therapy.
Clinical Practice Points.
Differentiation syndrome (DS) is a potentially fatal complication of effective leukemia treatment, and the underlying pathophysiology is poorly understood.
Isocitrate dehydrogenase (IDH) mutations are found in ~20% of patients with acute myeloid leukemia (AML), and promote tumorigenesis via a block in normal cellular differentiation.
Mutant-IDH inhibitors are in clinical development, with early results from the first-in-human clinical study of the novel, oral, mutant-IDH1 inhibitor, AG-120, demonstrating clinical benefit in patients with IDH1-mutant AML.
In patients with IDH1-mutant AML receiving targeted mutant-IDH1 inhibitor therapy, neutrophil recovery in the setting of a clinical DS has been anecdotally described.
We describe 3 patients with relapsed/refractory IDH1-mutant AML who developed clinically apparent DS concurrent with clinical response on AG-120 monotherapy, supporting release of differentiation block as its mode of action.
Clinical manifestations of DS were non-specific but included constitutional symptoms, culture-negative fevers and pleural/pericardial effusions, in addition to marked leukocytosis and exuberant neutrophil recovery.
Steroids and hydroxyurea were successful in mitigating symptoms; temporary AG-120 discontinuation may also be necessary in some instances.
Awareness that DS may occur during treatment with mutant IDH inhibitors, and prompt identification and initiation of interventions, is essential for optimal patient care.
Ongoing research will permit better characterization of this syndrome and identify risk factors in patients treated with targeted “differentiation” therapy.
Acknowledgments
Funding
This work was supported in part by the MD Anderson Cancer Center Support Grant (CCSG) CA016672 and by the generous philanthropic contributions to MD Anderson's MDS/AML Moon Shot Program. All patients were enrolled on the phase I clinical trial of AG-120 (NCT02074839) sponsored by Agios Pharmaceuticals, Inc., who performed a courtesy review of this manuscript prior to submission.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure
CDD has received research support from, and has served on an advisory board for Agios Pharmaceuticals, Inc.
Formatting assistance was provided by Helen Varley, PhD, CMPP, Excel Scientific Solutions, Horsham, UK, and supported by Agios.
References
- 1.Frankel SR, Eardley A, Heller G, et al. All-trans retinoic acid for acute promyelocytic leukemia. Results of the New York Study. Ann Intern Med. 1994;120:278–86. doi: 10.7326/0003-4819-120-4-199402150-00004. [DOI] [PubMed] [Google Scholar]
- 2.Luesink M, Jansen JH. Advances in understanding the pulmonary infiltration in acute promyelocytic leukaemia. Br J Haematol. 2010;151:209–20. doi: 10.1111/j.1365-2141.2010.08325.x. [DOI] [PubMed] [Google Scholar]
- 3.De Botton S, Dombret H, Sanz M, et al. Incidence, clinical features, and outcome of all trans-retinoic acid syndrome in 413 cases of newly diagnosed acute promyelocytic leukemia. The European APL Group. Blood. 1998;92:2712–8. [PubMed] [Google Scholar]
- 4.Tallman MS, Andersen JW, Schiffer CA, et al. Clinical description of 44 patients with acute promyelocytic leukemia who developed the retinoic acid syndrome. Blood. 2000;95:90–5. [PubMed] [Google Scholar]
- 5.Luesink M, Pennings JL, Wissink WM, et al. Chemokine induction by all-trans retinoic acid and arsenic trioxide in acute promyelocytic leukemia: triggering the differentiation syndrome. Blood. 2009;114:5512–21. doi: 10.1182/blood-2009-02-204834. [DOI] [PubMed] [Google Scholar]
- 6.Sexauer A, Perl A, Yang X, et al. Terminal myeloid differentiation in vivo is induced by FLT3 inhibition in FLT3/ITD AML. Blood. 2012;120:4205–14. doi: 10.1182/blood-2012-01-402545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fathi AT, Le L, Hasserjian RP, Sadrzadeh H, Levis M, Chen YB. FLT3 inhibitor-induced neutrophilic dermatosis. Blood. 2013;122:239–42. doi: 10.1182/blood-2013-01-478172. [DOI] [PubMed] [Google Scholar]
- 8.DiNardo CD, Ravandi F, Agresta S, et al. Characteristics, clinical outcome, and prognostic significance of IDH mutations in AML. Am J Hematol. 2015;90:732–6. doi: 10.1002/ajh.24072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dang L, White DW, Gross S, et al. Cancer-associated IDH1 mutations produce 2-hydroxyglutarate. Nature. 2009;462:739–44. doi: 10.1038/nature08617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ward PS, Patel J, Wise DR, et al. The common feature of leukemia-associated IDH1 and IDH2 mutations is a neomorphic enzyme activity converting alpha-ketoglutarate to 2-hydroxyglutarate. Cancer Cell. 2010;17:225–34. doi: 10.1016/j.ccr.2010.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heuser M, Araujo Cruz MM, Goparaju R, Chaturvedi A. Enigmas of IDH mutations in hematology/oncology. Exp Hematol. 2015;43:685–97. doi: 10.1016/j.exphem.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 12.DiNardo C, de Botton S, Pollyea DA, et al. Molecular profiling and relationship with clinical response in patients with IDH1 mutation-positive hematologic malignancies receiving AG-120, a first-in-class potent inhibitor of mutant IDH1, in addition to data from the completed dose escalation portion of the phase 1 study. Blood. 2015;126:A1306. [Google Scholar]
- 13.Fan B, Le K, Manyak E, et al. Longitudinal pharmacokinetic/pharmacodynamic profile of AG-120, a potent inhibitor of the IDH1 mutant protein, in a phase 1 study of IDH1-mutant advanced hematologic malignancies. Blood. 2015;126:A1310. [Google Scholar]