Abstract
Although Apicomplexans like the blood stages of Plasmodium and the actively replicating ‘tachyzoite’ stage of Toxoplasma infect very dissimilar host cells, recent studies suggest they share molecular commonalities amongst differences at the parasitophorous vacuolar membrane (PVM) surrounding these intracellular parasites. A protein translocation export (PTEX) complex in the PVM of Plasmodium, is functionally informed by findings in Toxoplasma. Lipids play a role in trafficking to and across the PVM. Toxoplasma exploit an orthologue of a plasmodial secretory aspartyl protease but substrate cleavage yields a signal for targeting to the PVM, rather than directly to the host cell. The studies significantly advance understanding of how trafficking to and across the host-pathogen PVM boundary induces virulence and disease in different host milieu.
Introduction
Plasmodium and Toxoplasma parasites both have complex life cycles in multiple hosts (summarized in Fig. 1). They contain prominent apical secretory organelles (characteristic of the phylum Apicomplexa; Fig. 2a) which discharge when these parasites create a parasitophorous vacuolar membrane (PVM) within which they reside, during intracellular infection. The ‘tachyzoite’ stage of Toxoplasma causes active infection in humans (other mammals and birds). The blood stages of Plasmodium are responsible for all of the symptoms, pathologies and death due to malaria. Although the PVM affords these parasites protection from host cytoplasm, it also presents a barrier for subsequent delivery of host-targeted virulence effectors (Fig. 2b-c). These effectors include parasite (PfEMP1) adhesins delivered to red blood cells infected by Plasmodium falciparum (a species of virulent human malaria) to facilitate parasite sequestration in tissues and (frequent) fatal severe disease [1] (Fig. 2b). Effectors exported by Toxoplasma gondii have also long been known to profoundly modulate the vacuolar environment and the host immune response [2,3] (Fig. 2c). Potentially hundreds of pathogenic effector proteins may be exported to and across the parasite plasma membrane (PM), the surrounding lumen of the parasitophorous vacuole (PV) and the PVM, suggesting apicomplexan parasites utilize complex trafficking mechanisms to remodel the host.
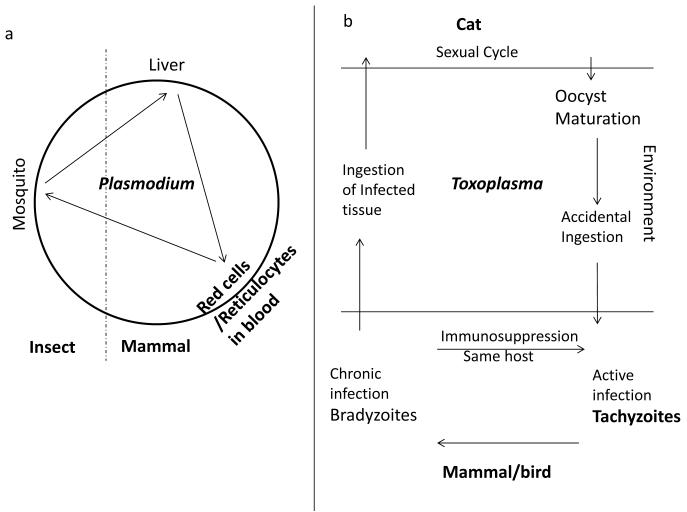
Figure 1. Life cycles of Plasmodium and Toxoplasma.
a Plasmodium. Parasites transmitted by mosquito bite home to the liver where they develop through one proliferative cycle, then infect red blood cells (or reticulocytes) to mature asexually and establish repetitive blood cell infection. A small proportion develop sexually and when taken up by the mosquito undergo complex sexual and asexual development into ‘sporozoites’ which get released into the host blood stream to start a new cycle. b Toxoplasma. Ingestion (from contact with cat feces or uncooked meat) results in active asexual infection by the proliferating ‘tachyzoite’ stage. Chronic infection ensues when tachyzoites convert to bradyzoites (which form cysts found in brain tissue in mice). Ingestion of infected tissue by cats then enables sexual cycle development which culminates in the oocyst stages which are released into the environment to re-initiate the life cycle. Latent to active infection by-passing oocysts can also be achieved by consumption of infected meat (as shown).
Figure 2. Active, intracellular apicomplexan infection and transport across the PVM.
a. Schematic of apicomplexan with characteristic apical organelles as shown, N Nucleus. b Plasmodium falciparum infection of the red cell, establishes the parasitophorous vacuolar member (PVM) surrounding the vacuole (PV) in which the parasite bounded by the parasite plasma membrane (PPM) resides. Other than the apicoplast (not shown) the remaining apical organelles are lost, while membrane structures become prominent in the erythrocyte. Proteins made in the ER (which contains the lipid PI3P and the protease Plasmepsin V, PMV) with HT/PEXEL signal or as PNEPs are exported out across the PPM and PVM through the PTEX (filled blue circle and arrow). Parasite encoded adhesin PfEMP1 family proteins are concentrated and displayed on ‘knobs’ that enable binding to endothelial tissue enabling tissue sequestration, severe malaria disease and death. c. Tachyzoites (causing active Toxoplasma gondii infection) replicate within the PVM which becomes associated with host mitochondria and ER. Dense granule proteins (purple filled circles) are released into the parasitophorous vacuolar (PV) space inducing formation of the membranous nanotubular network (MNN). Dense granule proteins are also detected at the PVM (brown filled circles), where the proteins GRA17 (red) and GRA23 (green) mediate permeation of small molecules across the PVM. Dense granule proteins (yellow) are also detected in host nucleus (grey) where they modulate the host response. T. gondii Asp5 is a Golgi-localized aspartic protease that cleaves an N terminal HT/PEXEL like motif on substrates that include dense granule proteins delivered to the host cytoplasm and nucleus (deep and light yellow). Blue arrow indicates protein translocation across the PVM for which the signals and transport apparatus remain unknown.
In Plasmodium, trafficking has been most extensively studied in P. falciparum-infected red cells. Here, the exported proteins are distinguished by two different types of signals. The first is the host targeting (HT) motif or plasmodial export element (PEXEL) positioned in an N terminal leader (the vacuolar translocation sequence or VTS). Cleavage of the HT/PEXEL releases effectors that are delivered to the cytoplasm, membrane and cytoskeleton of the red cell [4, 5]. Yet PEXEL negative proteins (PNEPs) are also exported, via targeting information likely in their leaders [6]. However, recent studies show that both types of effectors utilize a common protein translocation export (PTEX) machinery across the PVM [7**, 8**] (Fig. 2b). This suggests relatedness in signals and mechanisms of transport between HT/PEXEL and PNEP pathways, explored in two models hypothesized in Fig. 3. In Toxoplasma infection, differences in strain virulence provided the major path to identifying secreted pathogenic effectors, but the signals or machinery involved remained elusive. Recent studies have identified a T. gondii orthologue of a plasmodial protease and an equivalent HT/PEXEL signal [9**, 10**]. Evidence of cleavage of HT-PEXEL signal has enabled identification of substrates and an export pathway to the PVM and beyond (Fig. 4) leading to diverse destinations in the host cell (Fig. 2).
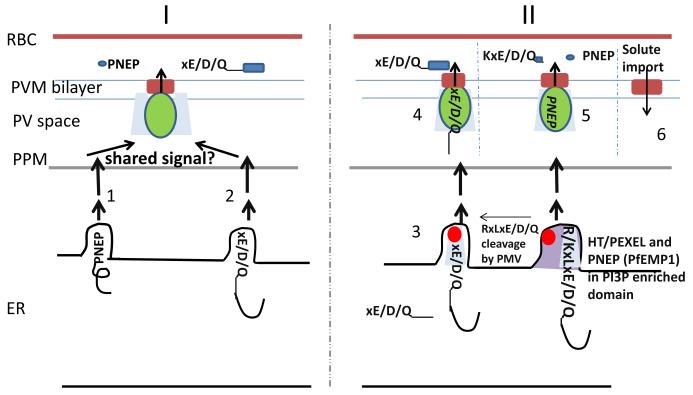
Figure 3. Models hypothesized for PTEX action in Plasmodium: potential signals shared by HT/PEXEL and PNEP proteins and a new role for Exp2.
Model I. Cleaved HT/PEXEL (xE/D/Q) and PNEP proteins exported through distinct routes (1 and 2) at the endoplasmic reticulum (ER) and parasite plasma membrane (PPM) present an unknown shared signal to a minimal PTEX complex comprising of PTEX150 (light blue), HSP101 (green) and Exp2 (brick red) at the PVM. Dark blue circle and rectangle in red blood cell (RBC) show folded polypeptides of PNEP and HT/PEXEL proteins respectively. Model II Vacuolar translocation sequences (VTS) of PNEP proteins (including PfEMP1 bearing KxLxE/D/Q) and uncleaved VTS containing HT/PEXEL motif associate with PI3P enriched domains and PTEX150 cotranslationally in the ER. xE/D/Q exposed by plasmepsin V (PMV) cleaved HT/PEXEL (or generated independently) is proposed to be separately recognized by a determinant (bright red sphere) either in PTEX150 or a second receptor in emergent ER vesicles (3), that are delivered to the PPM and PVM. HSP101 action at PTEX-effector complexes in the PVM enables unfolding and translocation of both HT/PEXEL and PNEP effectors across PVM to the erythrocyte (4, 5). Solute permeation is also mediated by Exp2 at the PVM (6).
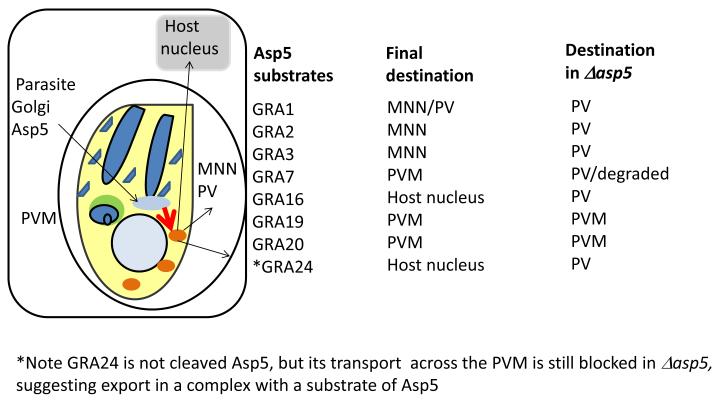
Figure 4. T. gondii Asp5 aspartic protease putative substrates and their location.
Schematic of Toxoplasma tachyzoite stage with apical organelles (blue as defined in Fig. 2) and Aspartic protease 5 (Asp5) in the Golgi, that cleaves an HT/PEXEL like motif on proteins prior to their transport (red arrow) to the dense granules, from where they are delivered to the parasitophorous vacuole (PV), membranous nanotubular network (MNN), parasitophorous vacuolar membrane (PVM) and host cell (nucleus). Failure to cleave, effected in Δasp5 mutants results in accumulation of multiple Asp5 substrates in the PV. GRA19 and GRA20 remain at PVM, but since their molecular interactions are likely to be altered, it may be that HT/PEXEL like motif in Toxoplasma targets effectors to a specialized site/complex in the PV/PVM, at which additional (but presently unknown) signals and transporters (likely distinct from Plasmodial PTEX) mediate translocation across the T. gondii PVM.
A molecular protein translocon of export (PTEX) mechanism in Plasmodium, modulated by insights from Toxoplasma
Five parasite protein components of Plasmodial PTEX have been identified [7**-8**, 11]. Inducible knock out/expression systems yield that two, PTEX150 (an integral transmembrane protein) and Hsp101 belonging to Clp/HSP100 family of AAA1 ATPases, are essential and individually needed for protein export [7,8]. Other members TRX-2 and PTEX88 are non-essential [12, 13], but may potentially be needed to maintain high efficiency and wide substrate range of export. Exp2 the fifth component is proposed to be ‘a pore’ (based similarity to E. coli hemolysin in modeling studies [11]). Yet orthologues of Exp2 in Toxoplasma are the GRA17 and GRA23 proteins, that mediate permeation of small molecules, rather than protein export across the PVM [14**] (Fig. 2). Exp2 functionally complements solute transport reduced in T. gondii Δgra17 mutants. Further when Exp2 is expressed in Xenopus oocysts, its activity is consistent with forming a large membrane pore [14**]. In Plasmodial infection of reticulocytes, substantial levels of PTEX/Exp2 are detected in vesicles in the host cells apparently detached from the PVM [15*, 16]. While there is no doubt of co-association of Exp2 with atleast a subset of PTEX components, knock downs of Exp2 in blood stage Plasmodium are needed to definitively establish its function protein export in the PVM.
Whither PTEX-recognition signals, and how many on different cargo?
Multiple studies suggest that exposure of sequences downstream of RxL motif in the ER triggers plasmodial protein export to the erythrocyte, regardless of whether this occurs through cleavage by the protease PMV [17, 18], signal peptidase [19] or by an ectopic protease (completely independent of Plasmepsin V or signal peptidase) [20]. Therefore sequences downstream of the HT/PEXEL motif may reasonably present recognition sites for PTEX at the PVM (Fig. 3, Model I). But, PTEX also recognizes PNEPs that by definition do not contain cleaved HT/PEXEL motifs. Another limitation of Model I is the assumption that PTEX150 and Hsp101 jointly encounter effectors only as they try to cross the PVM. This predicts that knock downs in either would lead to effector accumulation in the PV. Yet knock down of PTEX150 appears to result in effector accumulation in the parasite [7*] rather than the PV, suggesting PTEX150 may associate with cargo at an internal secretory site (Fig. 3 Model II). Further in its ‘off/inactive’ state when HSP101 disassociates from PTEX complex, the effectors remain bound to PTEX150 independent of HSP101 [8*]. Finally, knock down or an ‘off/inactive’ state of HSP101 results in effector accumulation in the parasite PV [7**, 8**], consistent with the interpretation that HSP101 binds to unfold PTEX150-associated effectors at the PVM.
But this begs the question of how PTEX150 recognizes both PNEPs and HT/PEXEL proteins within the parasite? A clue may come from the PNEP PfEMP1 family of virulence determinants that contain N terminal leaders with generalized KxLxE/D/Q motifs. This precludes cleavage by PMV (which recognizes RxL but not KxL), but K to R is a conservative substitution and it is reasonable to consider that PfEMP1 leaders with K motifs have capacity to interact with the same ER machinery as ‘R’ containing (HT/PEXEL) motifs do prior to their cleavage (on newly translating precursors). PfEMP1 leaders also bind phosphatidylinositol-3-phosphate (PI3P), a lipid that is found in the malarial ER [19]. Indeed the PI3P binding property is also shared by all uncleaved HT/PEXEL leaders (VTS) of proteins belonging to the RIFIN, STEVOR families, HSP40 and HRPII [19], suggesting a HT/PEXEL recognition mechanism shared with at least one (large) family of PENP proteins.
After HT/PEXEL cleavage, the exposed downstream sequences may also be recognized by PTEX150 (or a protein bound to it, red dot Figure 3 Model II) in the emergent ER vesicle explaining why generation of xE/D/Q can be mediated by multiple protease systems and bypass PI3P in export. In absence of cleavage, PI3P binding may be sufficient for export: PI3P domains are difficult to predict on the basis of linear signals and may be functionally supported by very different primary sequences. But there may well be domains with shared secondary and tertiary function that are not necessarily highly conserved at the level of primary sequence and therefore remain elusive in other PNEPs. Whether PNEPs other than PfEMP1 bind to ER-PI3P (or associated factors there), additional effector lipids or PTEX150, are all highly amenable to experimental testing. However a recent study [21] has questioned the role of ER-PI3P in export to the erythrocytes, suggesting the need to discuss the data against and in support of a role of this lipid in protein trafficking to the host erythrocyte.
A role for phosphoinositides in plasmodial protein export
Boddey et al. 2016 [21] have challenged the conclusions of Bhattacharjee et al. 2012 [19] that PI(3)P from the malaria parasite ER enables export of HT/PEXEL motif containing proteins to the host red cell. Notably, there are many important differences in design and reagents between these two studies. In [21], p40phox the control PI3P binding protein shows an apparent Kd of binding of 195nM and 196 mM compared to 10 nM in [19]. Further, p40phox was found to bind to control lipids in sedimentation assays and recombinant parasite-effector leaders showed aggregation [21], suggesting failure to recapitulate prior standards established by multiple laboratories [19, 22- 27]. With a lower relative Kd for the positive control and extensive aggregation, malarial effector proteins too would show one to two log weaker and/or lack of affinity for PI3P. When not occupied by a lipid ligand, the protein-lipid binding site is in an aqueous environment and may be prone to losing proper conformation [28], which cannot be distinguished by GFP fluorescence alone. Lipid strips/other methods utilized by Boddey et al. 2016 [21] cannot be fully interpreted in absence of high fidelity SPR and sedimentation assays.
In another point of departure when Boddey et al 2016 [21] failed to detected export of GFP mediated by a Phytophthora leader [19] they used constructs that lack critical amino acids between the signal sequence and the Phytophthora leader and downstream DEER sequences needed for export (e.g. HRPIIssPH001D5 compared to SS-Nuk10-GFP reported by [19]). Other studies have shown that oomycete validated RxLR and DEER export motif and the P. falciparum HT/PEXEL signals are equivalent in Phytophthora [29]. It should be noted that configuring RxLR…DEER can be challenging because the DEER are significantly downstream of the RxLR.
Since the highest quality of protein folding critical for localizing protein-lipid interaction in the ER were not achieved in Boddey et al 2016 [21] in biochemical, cellular localization assays or export assays, they do not invalidate the findings of Bhattacharjee et al. 2012 [19]. In eukaryotes, PI3P in the ER lumen is not well known but recent studies in yeast show that PI3P is found in the lumenal leaflet of the autophagosome [30*]. PI3P is enriched in the ER of HeLa and BT6 cells [31] and implicated in ER structure-function in muscle [32]. The need for a lumenal PI3K enzyme (to account for lumenal lipid) is also not likely necessary as lipids can flip bi-directionally across the ER membrane [33] or be co-translationally recruited into the ER lumen [34]. For a second phosphoinositide phosphatidylinositol-4-phosphate (PI4P), blocking parasite phosphatidylinositol-4-kinase (PfPI4KIIIβ) decreases vesicular pools of PI4P [35] and protein export to the PVM in the liver stages [36*], as well as potently kills intracellular parasites [35], but mechanisms of protein export across the liver stage PVM remain poorly understood. Indeed, there is still a lot to be investigated and learned as to the role of ER PI3P as well as other phosphoninositides in malarial trafficking, signaling and disease processes.
A Plasmepsin protease in Toxoplasma with substrates secreted to and across the PVM
In Toxoplasma, secretion of dense granule proteins have been known to underlie development of the membranous nanotubular network (MNN) in the PV as well as alter host cell functions [ 2, 3, 37-40] (Fig. 1-2). For instance, GRA15 influences host NF-kB nuclear translocation and transcription of cytokines [38], GRA16 acts in the nucleus to target the cell cycle and the p53 tumor suppressor pathway [39] and GRA24 promotes p38 MAPK activation and immune responses to infection [40]. Nonetheless the signals that mediate transport of these (and other) dense granule proteins across the PVM remain enigmatic. A T. gondii HT/ PEXEL like motif and evidence for its cleavage was reported in GRA19, GRA20 and GRA21, granule proteins of the MNN and PVM, but these proteins were not transported to the host cell [41]. But recent identification of T. gondii Asp5 (an orthologue of plasmodial PMV) and its functional analyses through deletion of TgAsp5 or chemical blocking, prevented cleavage of the TgHT/PEXEL like motif and relocated numerous GRA proteins from the MNN and PVM to the PV (9**, 10**, 42*; with examples summarized in Fig. 4). Notably in Δasp5 mutants, GRA16 and GRA24 were no longer exported across the PVM but retained in the PV. It should be noted that GRA24 has no recognizable HT/PEXEL like motif (but it may complex with a protein that does). Together these data suggest that cleavage of the TgHT/PEXEL targets from the lumen of the PV to the PVM where proteins like GRA16 and 24 may interact with a translocon to exit the PVM.
Unlike plasmodial PMV, deletion of Asp5 is not lethal to T. gondii, but it is associated with loss of parasite fitness, loss of the MNN, dramatic modulation of the host immune response as well as impairment in forming the cyst wall when actively proliferating tachyzoites convert to the dormant bradyzoite form. Further although Plasmodium PMV is localized to the parasite ER, T. gondii Asp5 resides the Golgi. Since the Golgi is the major site for sorting cargo in eukaryotes, TgAsp5 may cleave effectors enroute to many destinations, which are understandably more numerous in nucleated host cells infected by Toxoplasma, compared to the terminally differentiated red cell infected by Plasmodium, which has a rudimentary Golgi especially early in intracellular infection [43]. In yeast and mammalian Golgi, Kex2 like proteases cleave substrates whose pro-forms carry conformational information important for folding and targeting [44] Notably T. gondii Gra17 and 23 (of the solute permeant pathway) are not substrates for TgAsp5 (and their plasmodial orthologue Exp2 also lacks an HT/PEXEL motif). Therefore export of many key effectors to PVM and possibly across it occur independent of Δasp5, and in this regard they may be considered PNEPs of Toxoplasma.
Concluding remarks
Although the five amino HT/PEXEL motif rapidly identified hundreds of effectors in Plasmodium, effector function, molecular machinery and precise signals recognized in export are still emerging areas. This review largely summarizes one or more mechanisms in the Plasmodium and Toxoplasma ER and Golgi, their relatedness and impact on protein translocation to and across the PVM, based on breakthrough findings reported over the past two years. Not discussed here, the parasites also induce assembly of export machinery beyond the PVM in the host cell, but far less is understood of the mechanistic basis, other than it is likely to largely be in post translational in nature. Further, despite commonalities in apicomplexan trafficking, Plasmodial PTEX150 has no direct orthologues in Toxoplasma (yet), suggesting that simple comparative analogies may not readily reveal mechanisms of polypeptide translocation across the T. gondii PVM. But as a first step, sequences exposed downstream of the TgHT/PEXEL-like cleavage site may enable access to specialized PVM sites and thereby lead to development of tools to discover new pathogenic transport mechanisms. Finally, conservation of eukaryotic secretion machinery in both organisms assures that orthologous processes in each apicomplexan will better illuminate the functional range of protein trafficking mechanisms evolved in both, to elicit powerful virulence phenotypes adapted to different host niche.
Acknowledgments
Malaria research in the Haldar lab is funded by grants from the National Institutes of Health R01HL069630 and R01HL130330. I thank members of my laboratory, collaborator Dr. Robert Stahelin and members of his laboratory, for helpful discussions on trafficking in apicomplexans.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Miller LH, Ackerman HC, Su X, Wellems TE. Malaria biology and disease pathogenesis: insights for new treatments. Nature Medicine. 2013;19:156–167. doi: 10.1038/nm.3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mercier C, Dubremetz JF, Rauscher B, Lecordier L, Sibley LD, Cesbron-Delauw MF. Biogenesis of nanotubular network in Toxoplasma parasitophorous vacuole induced by parasite proteins. Molecular Biology of the Cell. 2002;13:2397–409. doi: 10.1091/mbc.E02-01-0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rosowski EE, Lu D, Julien L, Rodda L, Gaiser RA, Jensen KD, et al. Strain-specific activation of the NF-kappaB pathway by GRA15, a novel Toxoplasma gondii dense granule protein. J Exp Med. 2011;208:195–212. doi: 10.1084/jem.20100717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hiller NL, Bhattacharjee S, van Ooij C, Liolios K, Harrison T, Lopez-Estrano C, Haldar K. A host-targeting signal in virulence proteins reveals a secretome in malarial infection. Science. 2004;306:1934–1937. doi: 10.1126/science.1102737. [DOI] [PubMed] [Google Scholar]
- 5.Marti M, Good RT, Rug M, Knuepfer E, Cowman AF. Targeting malariavirulence and remodeling proteins to the host erythrocyte. Science. 2004;306:1930–1933. doi: 10.1126/science.1102452. [DOI] [PubMed] [Google Scholar]
- 6.Grüring C, Heiber A, Kruse F, Flemming S, Franci G, Colombo SF, Fasana E, Schoeler H, Borgese N, Stunnenberg HG, Przyborski JM, Gilberger T, Spielmann T. Uncovering common principles in protein export of malaria parasites. Cell Host Microbe. 2012;12:717–29. doi: 10.1016/j.chom.2012.09.010. [DOI] [PubMed] [Google Scholar]
- 7 **.Elsworth B, Matthews K, Nie CQ, Kalanon M, Charnaud SC, Sanders PR, Chisholm SA, Counihan NA, Shaw PJ, Pino P, Chan JA, Azevedo MF, Rogerson SJ, Beeson JG, Crabb BS, Gilson PR, de Koning-Ward TF. PTEX is an essential nexus for protein export in malaria parasites. Nature. 2014;511:587–591. doi: 10.1038/nature13555. This study developed conditional knock downs to definitively establish that PTEX150 is required for both HT/PEXEL protein and PNEP export by the human malaria Plasmodium falciparum to the mature host erythrocyte as well as that HSP101 is required for export in Plasmodium berghei infections in a murine model. It definitively established the essential nature of both PTEX components in remodeling the host cell and the conservation of PTEX function across parasite species.
- 8 **.Beck JR, Muralidharan V, Oksman A, Goldberg DE. PTEX component HSP101 mediates export of diverse malaria effectors into host erythrocytes. Nature. 2014;511:592–5. doi: 10.1038/nature13574. Using a destabilizing domain approach, this study showed that HSP101 is required for HT/PEXEL protein and PNEP export by the human malaria Plasmodium falciparum to the mature host erythrocyte. It also provided evidence that HSP101 engages with PTEX150 to enable export or disengages to block export and reported on substrate binding by each of these PTEX components during this process thereby providing the most advanced mechanistic analysis available of export by PTEX.
- 9 **.Hammoudi P-M, Jacot D, Mueller C, Di Cristina M, Dogga SK, Marq J-B, Romano J, Tosetti N, Dubrot J, Emre Y, Lunghi M, Coppens I, Yamamoto M, Sojka D, Pino P, Soldati-Favre D. Fundamental Roles of the Golgi-Associated Toxoplasma Aspartyl Protease, ASP5, at the Host-Parasite Interface. PLoS Pathog. 2015;11(10):e1005211. doi: 10.1371/journal.ppat.1005211. Provided comprehensive analyses of the consequences genetic knock out of TgASP5 on export of dense granule proteins and the host response as well as tachyzoite to bradyzoite transition.
- 10 **.Coffey MJ, Sleebs BE, Uboldi AD, Garnham A, Franco M, Marino ND, Panas MW, Ferguson DJ, Enciso M, O'Neill MT, Lopaticki S, Stewart RJ, Dewson G, Smyth GK, Smith BJ, Masters SL, Boothroyd JC, Boddey JA, Tonkin CJ. An aspartyl protease defines a novel pathway for export of Toxoplasma proteins into the host cell. Elife. 2015;4:pii: e10809. doi: 10.7554/eLife.10809. (2015) Provided comprehensive analyses of genetically and chemically blocking TgASP5 and the consequences for export of dense granule proteins and the host cell response.
- 11.de Koning-Ward TF, Gilson PR, Boddey JA, Rug M, Smith BJ, Papenfuss AT, Sanders PR, Lundie RJ, Maier AG, Cowman AF, Crabb BS. A newly discovered protein export machine in malaria parasites. Nature. 2009;459:945–9. doi: 10.1038/nature08104. (2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matthews K, Kalanon M, Chisholm SA, Sturm A, Goodman CD, Dixon MW, Sanders PR, Nebl T, Fraser F, Haase S, McFadden GI, Gilson PR, Crabb BS, de Koning-Ward TF. The Plasmodium translocon of exported proteins (PTEX) component thioredoxin-2 is important for maintaining normal blood-stage growth. Mol Microbiol. 2013;89:1167–86. doi: 10.1111/mmi.12334. [DOI] [PubMed] [Google Scholar]
- 13.Matz JM, Matuschewski K, Kooij TW. Two putative protein export regulators promote Plasmodium blood stage development in vivo. Mol Biochem Parasitol. 2013;191:44–52. doi: 10.1016/j.molbiopara.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 14 **.Gold DA, Kaplan AD, Lis A, Bett GC, Rosowski EE, Cirelli KM, Bougdour A, Sidik SM, Beck JR, Lourido S, Egea PF, Bradley PJ, Hakimi MA, Rasmusson RL, Saeij JP. The Toxoplasma Dense Granule Proteins GRA17 and GRA23 Mediate the Movement of Small Molecules between the Host and the Parasitophorous Vacuole. Cell Host Microbe. 2015;17:642–52. doi: 10.1016/j.chom.2015.04.003. This study provided the first molecular definition for permeation of small molecules an important nutrient uptake process in apicomplexan infection. It also established that Exp2 a Plasmodial PTEX component hypothesized to function in protein export provides a pore for small molecules.
- 15 *.Matz JM, Goosmann C, Brinkmann V, Grützke J, Ingmundson A, Matuschewski K, Kooij TW. The Plasmodium berghei translocon of exported proteins reveals spatiotemporal dynamics of tubular extensions. Sci Rep. 2015;5:12532. doi: 10.1038/srep12532. Provides evidence of dynamics of PTEX and therefore mechanisms of export in a murine model of malaria.
- 16.Meibalan E, Comunale MA, Lopez AM, Bergman LW, Mehta A, Vaidya AB, Burns JM., Jr. Host erythrocyte environment influences the localization of exported protein 2, an essential component of the Plasmodium translocon. Eukaryot Cell. 2015;14:371–84. doi: 10.1128/EC.00228-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boddey JA, Hodder AN, Günther S, Gilson PR, Patsiouras H, Kapp EA, Pearce JA, de Koning-Ward TF, Simpson RJ, Crabb BS, Cowman AF. An aspartyl protease directs malaria effector proteins to the host cell. Nature. 2010;463:627–31. doi: 10.1038/nature08728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Russo I, Babbitt S, Muralidharan V, Butler T, Oksman A, Goldberg DE. Plasmepsin V licenses Plasmodium proteins for export into the host erythrocyte. Nature. 2010;463:632–6. doi: 10.1038/nature08726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bhattacharjee S, Stahelin RV, Speicher KD, Speicher DW, Haldar K. Endoplasmic reticulum PI(3)P lipid binding targets malaria proteins to the host cell. Cell. 2012;148:201–212. doi: 10.1016/j.cell.2011.10.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tarr SJ, Cryar A, Thalassinos K, Haldar K, Osborne AR. The C-terminal portion of the cleaved HT motif is necessary and sufficient to mediate export of proteins from the malaria parasite into its host cell. Mol Microbiol. 2013;87:835–850. doi: 10.1111/mmi.12133. (2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boddey JA, O'Neill MT, Lopaticki S, Carvalho TG, Hodder AN, Nebl T, Wawra S, van West P, Ebrahimzadeh Z, Richard D, Flemming S, Spielmann T, Przyborski J, Babon JJ, Cowman AF. Export of malaria proteins requires co-translational processing of the PEXEL motif, independent of phosphatidylinositol-3-phosphate binding. Nat Commun. 2016 Feb 1;7:10470. doi: 10.1038/ncomms10470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bravo J, Karathanassis D, Pacold CM, Pacold ME, Ellson CD, Anderson KE, Butler PJ, Lavenir I, Perisic O, Hawkins PT, Stephens L, Williams RL. The crystal structure of the PX domain from p40(phox) bound to phosphatidylinositol 3-phosphate. Mol Cell; 2001;8:829–839. doi: 10.1016/s1097-2765(01)00372-0. [DOI] [PubMed] [Google Scholar]
- 23.Ellson CD, Gobert-Gosse S, Anderson KE, Davidson K, Erdjument-Bromage H, Tempst P, Thuring JW, Cooper MA, Lim ZY, Holmes AB, Gaffney PR, Coadwell J, Chilvers ER, Hawkins PT, Stephens LR. PtdIns(3)P regulates the neutrophil oxidase complex by binding to the PX domain of p40(phox) Nat Cell Biol; 2001;3:679–682. doi: 10.1038/35083076. [DOI] [PubMed] [Google Scholar]
- 24.Kanai F, Liu H, Field SJ, Akbary H, Matsuo T, Brown GE, Cantley LC, Yaffe MB. The PX domains of p47phox and p40phox bind to lipid products of PI(3)K. Nat Cell Biol. 2001;3:675–678. doi: 10.1038/35083070. [DOI] [PubMed] [Google Scholar]
- 25.Zhan Y, Virbasius JV, Song X, Pomerleau DP, Zhou GW. The p40phox and p47phox PX domains of NADPH oxidase target cell membranes via direct and indirect recruitment by phosphoinositides. J Biol Chem. 2002;277:4512–4518. doi: 10.1074/jbc.M109520200. [DOI] [PubMed] [Google Scholar]
- 26.Zhao X, Bey EA, Wientjes FB, Cathcart MK. Cytosolic phospholipase A2 (cPLA2) regulation of human monocyte NADPH oxidase activity. cPLA2 affects translocation but not phosphorylation of p67 (phox) and p47 (phox) J Biol Chem. 2002;277:25385–25392. doi: 10.1074/jbc.M203630200. [DOI] [PubMed] [Google Scholar]
- 27.Stahelin RV, Burian A, Bruzik KS, Murray D, Cho W. Membrane binding mechanisms of the PX domains of NADPH oxidase p40phox and p47phox. J Biol Chem. 2003;278:14469–14479. doi: 10.1074/jbc.M212579200. [DOI] [PubMed] [Google Scholar]
- 28.Moravcevic K, Oxley CL, Lemmon MA. Conditional peripheral membrane proteins: facing up to limited specificity. Structure. 2012;20:15–27. doi: 10.1016/j.str.2011.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dou D, Kale SD, Wang X, Jiang RH, Bruce NA, Arredondo FD, Zhang X, Tyler BM. RXLR-mediated entry of Phytophthora sojae effector Avr1b into soybean cells does not require pathogen-encoded machinery. Plant Cell. 2008;20:1930–1947. doi: 10.1105/tpc.107.056093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30 *.Cheng J, Fujita A, Yamamoto H, Tatematsu T, Kakuta S, Obara K, Ohsumi Y, Fujimoto T. Yeast and mammalian autophagosomes exhibit distinct phosphatidylinositol 3-phosphate asymmetries. Nature Commun. 2014;5:3207. doi: 10.1038/ncomms4207. This provides immunoelectron microscopy evidence for the presence of lipid phosphatidylinositol 3-phosphate (PI3P) in ER lumen.
- 31.Sarkes D, Rameh LE. A novel HPLC-based approach makes possible the spatial characterization of cellular PtdIns5P and other phosphoinositides. Biochem J. 2010;428:375–384. doi: 10.1042/BJ20100129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Amoasii L, Hnia K, Chicanne G, Brech A, Cowling BS, Muller MM, Schwab Y, Koebel P, Ferry A, Payrastre B, Laporte J. Myotubularin and PtdIns3P remodel the sarcoplasmic reticulum in muscle in vivo. J Cell Sci. 2013;126:1806–1819. doi: 10.1242/jcs.118505. [DOI] [PubMed] [Google Scholar]
- 33.van Meer G. Cellular lipidomics. EMBO J. 2005;24:3159–3165. doi: 10.1038/sj.emboj.7600798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Romisch K. Diversion at the ER: How Plasmodium falciparum exports proteins into host erythrocytes. F1000Research. 2012;1:1–12. doi: 10.12688/f1000research.1-12.v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McNamara CW, Lee MC, Lim CS, Lim SH, Roland J, Nagle A, Simon O, Yeung BK, Chatterjee AK, McCormack SL, Manary MJ, Zeeman AM, Dechering KJ, Kumar TR, Henrich PP, Gagaring K, Ibanez M, Kato N, Kuhen KL, Fischli C, Rottmann M, Plouffe DM, Bursulaya B, Meister S, Rameh L, Trappe J, Haasen D, Timmerman M, Sauerwein RW, Suwanarusk R, Russell B, Renia L, Nosten F, Tully DC, Kocken CH, Glynne RJ, Bodenreider C, Fidock DA, Diagana TT, Winzeler EA. Targeting Plasmodium PI(4)K to eliminate malaria. Nature. 2013;504:248–53. doi: 10.1038/nature12782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36 *.Hanson KK, Ressurreição AS, Buchholz K, Prudêncio M, Herman-Ornelas JD, Rebelo M, Beatty WL, Wirth DF, Hänscheid T, Moreira R, Marti M, Mota MM. Torins are potent antimalarials that block replenishment of Plasmodium liver stage parasitophorous vacuole membrane proteins. Proc Natl Acad Sci U S A. 2013;110:2838–47. doi: 10.1073/pnas.1306097110. Provided evidence that chemical inhibition of PI4K blocked export of Exp2 to the liver stage PVM.
- 37.Michelin A, Bittame A, Bordat Y, Travier L, Mercier C, Dubremetz JF, Lebrun M. GRA12, a Toxoplasma dense granule protein associated with the intravacuolar membranous nanotubular network. Int J Parasitol. 2009;39:299–306. doi: 10.1016/j.ijpara.2008.07.011. [DOI] [PubMed] [Google Scholar]
- 38.Shastri AJ, Marino ND, Franco M, Lodoen MB, Boothroyd JC. GRA25 is a novel virulence factor of Toxoplasma gondii and influences the host immune response. Infect Immun. 2014;82:2595–605. doi: 10.1128/IAI.01339-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bougdour A, Durandau E, Brenier-Pinchart MP, Ortet P, Barakat M, Kieffer S, Curt-Varesano A, Curt-Bertini RL, Bastien O, Coute Y, Pelloux H, Hakimi MA. Host cell subversion by Toxoplasma GRA16, an exported dense granule protein that targets the host cell nucleus and alters gene expression. Cell Host Microbe. 2013;13:489–500. doi: 10.1016/j.chom.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 40.Braun L, Brenier-Pinchart MP, Yogavel M, Curt-Varesano A, Curt-Bertini RL, Hussain T, Kieffer-Jaquinod S, Coute Y, Pelloux H, Tardieux I, Sharma A, Belrhali H, Bougdour A, Hakimi MA. A Toxoplasma dense granule protein, GRA24, modulates the early immune response to infection by promoting a direct and sustained host p38 MAPK activation. J Exp Med. 2013;210:2071–86. doi: 10.1084/jem.20130103. An important study showing that a dense granule protein can alter host signaling.
- 41.Hsiao CH, Luisa Hiller N, Haldar K, Knoll LJ. A HT/PEXEL motif in Toxoplasma dense granule proteins is a signal for protein cleavage but not export into the host cell. Traffic. 2013;14:519–31. doi: 10.1111/tra.12049. An early study providing evidence of HT/PEXEL motif in dense granule proteins exported to the PVM (although cleavage of the motif did not relocate these proteins).
- 42 *.Curt-Varesano A, Braun L, Ranquet C, Hakimi MA, Bougdour A. The aspartyl protease TgASP5 mediates the export of the Toxoplasma GRA16 and GRA24 effectors into host cells. Cell Microbiol. 2015;18:151–67. doi: 10.1111/cmi.12498. This study delineated the role of TgAP5 in the export of two key substrate effectors GRA16 and GRA24 to host cells.
- 43.Elmendorf HG, Haldar K. Identification and localization of ERD2 in the malaria parasite Plasmodium falciparum: separation from sites of sphingomyelin synthesis and implications for organization of the Golgi. EMBO J. 1993;12:4763–73. doi: 10.1002/j.1460-2075.1993.tb06165.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rockwell NC, Krysan DJ, Komiyama T, Fuller RS. Precursor processing by kex2/furin proteases. Chem Rev. 2002;102:4525–4548. doi: 10.1021/cr010168i. [DOI] [PubMed] [Google Scholar]