Abstract
Background
Stroke is one of the leading complications during continuous flow-left ventricular assist device (CF-LVAD) support. Risk factors have been well described, though less is known regarding treatment and outcomes. We present a large single center experience on stroke outcome and transplant eligibility by stroke subtype and severity in CF-LVAD patients.
Methods
301 patients underwent CF-LVAD (266 HeartMate II (HM II) and 35 HeartWare (HVAD)) between 1/1/2008 and 4/1/2015. Stroke was defined as a focal neurological deficit with abnormal neuroimaging. Intracerebral hemorrhage (ICH) definition excluded subdural hematoma and hemorrhagic conversion of an ischemic stroke (IS). Treatment in IS included intra-arterial embolectomy (IAE) when appropriate; treatment in ICH included reversal of coagulopathy. Stroke severity was measured using the National Institutes of Health Stroke Scale (NIHSS). Outcomes were in-hospital mortality and transplant status.
Results
40 patients suffered a stroke: 8 ICH (4 HM II, 4 HVAD) and 32 IS (26 HM II, 6 HVAD). Among 8 ICH there were 4 deaths (50%) (NIHSS 18.8±13.7 vs 1.8±1.7 in survivors, p=0.049). Among 32 IS, 12 had hemorrhagic conversion and 5 were treated with IAE. There were 9 deaths (28%) (NIHSS 16.2±10.8 vs 7.0±7.6 in survivors, p=0.011). Among the 32 IS patients, 12 underwent transplant and 1 is awaiting transplant; no ICH patients were transplanted.
Conclusions
In-hospital mortality after stroke is significantly affected by the initial neurological impairment. Patients with IS appear to benefit the most from in-hospital treatment and often make sufficient recovery to be able to progress to transplant.
Introduction
Stroke is one of the leading complications in patients with end-stage heart failure who are treated with a left ventricular assist device (LVAD). The reported incidence of stroke after continuous flow (CF)-LVAD across a variety of studies ranges from 12.1 to 28.7%1–5. Stroke is a leading cause of mortality in LVAD and a contraindication for cardiac transplantation in several centers6. The risk factors for stroke have been well characterized and include among others blood pressure, infection, pump thrombosis, gastrointestinal bleeding and insufficient or excessive anti-thrombotic treatment7–9. The data on stroke after LVAD however includes several gaps. Prior studies do not consistently make a distinction between ischemic and primary hemorrhagic stroke which may have different pathophysiology. These studies rely on chart abstraction and do not have expert adjudication processes to differentiate between hemorrhagic conversion of an ischemic stroke (IS) and primary intracerebral hemorrhage (ICH). Furthermore there has rarely been an adjustment for stroke severity with an impairment measure such as the National Institutes of Health Stroke Scale in studies examining outcomes after stroke. The efficacy of treatment after stroke is also not well known, with small series documenting results after reversing anti-coagulation in intracerebral hemorrhage,10 for example, but not outcomes beyond mortality such as functional statistics. This is notable since stroke can directly lead to death, but more frequently leads to significant disability. The latter can be particularly important to patients with LVADs in whom complications from limited mobility can be substantial, including loss of eligibility for transplantation and infection. We report our large single center experience with treatment of IS and ICH accounting for degree in impairment, with a focus on subsequent discharge disposition and transplantation.
Methods
Patients implanted with a contemporary CF-LVAD at Columbia University Medical Center between January 1, 2008 and April 1, 2015 were identified utilizing our clinical outcomes database. Data presented in this manuscript were collected through April 1, 2015. Clinical care was standardized for all LVAD patients. Heartmate II patients were treated with warfarin for a target INR of 2–2.5 and HVAD patients with a target INR of 2.5–3; both were treated as well as aspirin 81 or 325 mg daily. Data collection and analysis was approved by our Institutional Review Board.
Stroke Adjudication and Treatment
All suspected stroke patients with an LVAD are seen and examined by a board certified vascular neurologist at our institution and undergo neuro-imaging with non-contrast computed tomography at initial encounter. Potential stroke patients undergo a full neurological examination, as well as initial National Institutes of Health Stroke Scale (NIHSS), a well validated measure of initial impairment due to cerebrovascular disease. The NIHSS ranges from 0–42 and captures mental status, cranial nerve, motor, sensory, and cerebellar function; higher scores indicate a greater degree of impairment. The NIHSS at the time of first patient encounter was used in our analyses. Patients are further classified as having ischemic stroke (IS), IS with hemorrhagic conversion, and intracerebral hemorrhage (ICH). Ischemic stroke is defined as any focal neurological deficit regardless of duration of symptoms with an associated infarct noted on neuro-imaging. Transient ischemic attack (TIA) was defined as short lasting symptoms attributable to focal central nervous system dysfunction without evidence of infarction on imaging and without alternative etiologies such as seizures or metabolic disturbance. These definitions are in keeping with the contemporary definition of stroke and transient ischemic attack from the American Heart Association adopted in 200911. Intracerebral hemorrhage is defined as intra-parenchymal bleeding without evidence of significant adjacent hypodensity on CT which would be consistent with hemorrhage into the bed of an infarct (hemorrhagic conversion)12. In contrast to INTERMACS definitions3 we did not include subdural and epidural hematomas in our database since these arise from trauma and the pathological process is not intrinsic to the brain parenchyma and subsequent treatment would be different. For example in patients with a traumatic etiology the risk of recurrence with resuming anticoagulation is lower than for patients with parenchymal hematomas. Sub-arachnoid hemorrhage was included in the definition of stroke in this study if there was no history of antecedent trauma given the concern for hemorrhagic conversion or an underlying mycotic aneurysm. Adjudication of the stroke outcomes was performed by a board certified vascular neurologist (JZW) after review of the neuro-imaging films and clinical data as part of patient care at the time of stroke. Those patients who were not initially cared for by the study neurologist were adjudicated in 2015 by review of imaging and clinical data without knowledge of subsequent outcome.
Patients with stroke were treated according to a published algorithm for the management of strokes7. In brief, acute ischemic stroke patients are not treated with intravenous thrombolysis. Patients however are treated with intra-arterial embolectomy (IAE) if presenting within 8 hours of symptoms with a large vessel occlusion and no evidence of obvious infarction on non-contrast CT head13. Anti-platelet agents and anticoagulation is continued if there is no evidence of hemorrhagic conversion and the infarct involves less than one third of the hemispheric volume in order to prevent hemorrhagic conversion. Hemorrhagic strokes, both primaries or results of an hemorrhagic conversion, are treated with reversal of anticoagulation with vitamin K and prothrombin complex concentrates if the international normalized ratio is greater than 1.5, and platelet transfusion and desmopressin acetate. Anti-platelet agents and anticoagulants are resumed within two weeks if the imaging remains stable in hemorrhagic stroke. All patients undergo at least one set of blood cultures and an investigation for evidence of pump thrombosis, including daily lactate dehydrogenase (LDH) levels.
Clinical Outcomes after Stroke
Patients with LVAD are followed for in-hospital and post-discharge mortality, transplantation, and discharge disposition. After suffering a stroke, all patients undergo in-hospital physical and occupational therapy evaluations in order to determine suitability for discharge home or to an acute inpatient rehabilitation center, depending on the nature of the severity of neurological deficits. In-hospital deaths were classified as due to direct effects of the stroke (such as cerebral herniation or withdrawal of care due to severity of stroke), or non-neurological (such as sepsis and multi-organ failure).
Statistical Analysis
Continuous variables were represented as mean ± standard deviation and compared using independent samples t-test. Categorical variables were represented as proportions and compared using chi-square and Fisher’s exact tests where appropriate. Duration of support was calculated by the difference between date of stroke diagnosis and LVAD implant date in days. Receiver operator characteristic (ROC) curves considering multiple dichotomies of the NIHSS were constructed to verify appropriate NIHSS cut-off for the mortality outcome. Specifically, NIHSS dichotomies based of thresholds of 5, 6, 7, 8, 9, and 10, were evaluated14. A non-parametric approach was used to compare the areas under the curve (AUCs) for the multiple NIHSS cut-offs and to determine the best NIHSS threshold for mortality prediction. We have previously used this methodology for outcome prediction in patients with heart failure14. P-value less than 0.05 was considered significant for all analyses. Statistical analyses were performed using IBM SPSS 22.0 software.
Results
Patient Characteristics
During the study period, 301 patients underwent implantation of contemporary CF-LVADs at our center: 266 HeartMate II (HM II, Thoratec, Inc, Pleasanton, California) (88%) and 35 HeartWare left ventricular assist device (HVAD, HeartWare Inc, Framingham, MA) (12%). A total of 40 patients suffered a stroke during the follow up period and one had a subdural hematoma (excluded from the analysis). Patients in the study cohort were followed through April 2015 (last date of follow-up). Median follow-up time on device support was 376 days (IQR: 583.5). Overall stroke rate was 0.094 events per patient-year (EPPY) [event n=40, follow-up time = 424.9 patient-years). Stroke rates for specific device-types were as follows: Heartmate II: 0.078 EPPY [event n=30, follow-up time= 383.0 patient-years] vs. Heartware: 0.239 EPPY [event n=10, follow-up time = 41.9 patient-years] (p = 0.002). Baseline demographics and clinical characteristics of stroke versus stroke-free patients are presented in Table 1. Compared to patients without stroke, those with stroke were significantly more likely to have an HVAD and be diabetic. Overall strokes were more prevalent in patients who had an HVAD (28.6%) than in those who were on HM II support (11.3%) (p=<0.01).
Table 1.
Characteristics of Stroke versus Stroke-free CF-LVAD patients
| Stroke (n=40) | No Stroke (n=261) | p-value | |
|---|---|---|---|
| Pre LVAD history | |||
| Age (years) | 54.7 ± 14.1 | 58.0 ± 13.7 | 0.16 |
| Women | 7 (17.5%) | 56 (21.5%) | 0.57 |
| Body surface area (m2) | 1.99 ± 0.33 | 2.04 ± 0.32 | 0.33 |
| Ischemic | 20 (50.0%) | 141 (54.0%) | 0.64 |
| Hypertension | 22 (55.0%) | 125 (47.9%) | 0.40 |
| Hyperlipidemia | 16 (40.0%) | 104 (39.8%) | 0.98 |
| Diabetes | 21 (52.5%) | 83 (31.8%) | 0.010 |
| Smoking history | 14 (35.0%) | 92 (35.2%) | 0.98 |
| Creatinine (mg/dl) | 1.49 ± 0.69 | 1.48 ± 0.71 | 0.94 |
| Previous TIA | 0 (0.0%) | 11 (4.2%) | 0.370 |
| Previous stroke | 3 (7.5%) | 24 (9.2%) | 0.999 |
| Previous TIA/stroke | 3 (7.5%) | 34 (13.0%) | 0.441 |
| LVAD Type | |||
| HeartMate II | 30 (75.0%) | 236 (90.4%) | 0.005 |
| HVAD | 10 (25.0%) | 25 (32.6%) | |
| LVAD Strategy | |||
| BTT | 24 (60.0%) | 176 (67.4%) | 0.35 |
| DT | 16 (40.0%) | 85 (84.2%) | |
| Post LVAD history | |||
| GI Bleeding | 11 (27.5%) | 77 (29.5%) | 0.795 |
| Device-related infection | 8 (20.0%) | 43 (16.5%) | 0.580 |
| Bacteremia/sepsis | 12 (30.0%) | 47 (18.0%) | 0.075 |
| Pump exchange for thrombosis | 6 (15.0%) | 24 (9.2%) | 0.254 |
Values are n (%).
CF-LVAD = Continuous-Flow Left Ventricular Assist Device; TIA = Transient Ischemic Attack; HVAD = Heartware Left Ventricular Assist Device; BTT = Bridge to Transplant; DT = Destination Therapy; GI = Gastrointestinal.
There were a total of 8 ICH (4 in HM II recipients and 4 in HVAD recipients), no TIA’s, and 32 IS (26 HM II recipients and 6 HVAD recipients). None of our patients were diagnosed with mycotic aneurysms, though one with an initial ischemic stroke and subsequent hemorrhagic conversion had findings on catheter angiography consistent with septic arteritis. Three ICH patients and 7 IS patients were on dipyridamole 75 mg three times per day at time of stroke. Characteristics and outcomes of ICH versus IS patients and of IS with versus without hemorrhagic conversion are presented in Table 2 and 3 respectively. The anticoagulation status of each patient is summarized in table 4. We found no significant differences between the devices given the small sample size. We reviewed the echocardiograms of all patients with stroke in our cohort and did not note any specific abnormalities. The findings on echocardiogram are as follows:
Table 2.
Characteristics of intracerebral hemorrhage (ICH) versus ischemic stroke (IS) in CF-LVAD patients
| ICH (n=8) | IS (n=32) | p-value | |
|---|---|---|---|
| Pre LVAD history | |||
| Age (years) | 52.3 ± 13.3 | 55.3 ± 14.5 | 0.591 |
| Women | 2 (25.0%) | 5 (15.6%) | 0.611 |
| Body surface area (m2) | 2.1 ± 0.4 | 2.0 ± 0.3 | 0.452 |
| Ischemic | 3 (37.5%) | 17 (53.1%) | 0.695 |
| Hypertension | 6 (75.0%) | 16 (50.0%) | 0.258 |
| Hyperlipidemia | 2 (25.0%) | 14 (43.8%) | 0.439 |
| Diabetes | 4 (50.0%) | 17 (53.1%) | 0.999 |
| Smoking history | 3 (37.5%) | 11 (34.4%) | 0.999 |
| Creatinine (mg/dl) | 1.59 ± 0.65 | 1.46 ± 0.71 | 0.629 |
| Previous stroke | 1 (12.5%) | 2 (6.3%) | 0.498 |
| Previous TIA/stroke | 1 (12.5%) | 2 (6.3%) | 0.498 |
| Device Type | |||
| HeartMate II | 4 (50.0%) | 26 (81.3%) | 0.089 |
| HVAD | 4 (50.0%) | 6 (18.8%) | |
| Device Strategy | |||
| BTT | 4 (50.0%) | 20 (62.5%) | 0.690 |
| DT | 4 (50.0%) | 12 (37.5%) | |
| Post LVAD history | |||
| GI Bleeding | 2 (25.0%) | 9 (28.1%) | 0.999 |
| Device-related infection | 2 (25.0%) | 6 (18.8%) | 0.650 |
| Sepsis | 3 (37.5%) | 9 (28.1%) | 0.677 |
| Pump exchange for thrombosis | 1 (12.5%) | 5 (15.6%) | 0.999 |
| At the time of stroke | |||
| Duration of support | 284.9 ± 346.1 | 233.8 ± 312.2 | 0.688 |
| INR (for patients on warfarin) | 3.03 ± 1.28 (4 pts) | 2.23 ± 0.6 (24 pts) | 0.301 |
| PTT (for patients on heparin) | 81.1 ± 14.1 (4 pts) | 67.9 ± 20.5 (5 pts) | 0.309 |
| NIHSS | 10.3 ± 12.8 | 9.6 ± 9.4 | 0.170 |
| Outcome post stroke | |||
| Survival to discharge home or rehab | 3 (37.5%) | 23 (71.9%) | 0.102 |
Values are n (%).
ICH = Intracerebral hemorrhage; IS = Ischemic Stroke; CF-LVAD = Continuous-Flow Left Ventricular Assist Device; TIA = Transient Ischemic Attack; HVAD = Heartware Left Ventricular Assist Device; BTT = Bridge to Transplant; DT = Destination Therapy; GI = Gastrointestinal; INR = International Normalized Ratio; PTT = Partial Thromboplastin Time; NIHSS = National Institutes of Health Stroke Scale.
Table 3.
Characteristics of ischemic stroke (IS) patients with or without hemorrhagic conversion in CF-LVAD patients
| Without Hemorrhagic Conversion (n=20) | With Hemorrhagic Conversion (n=12) | p-value | |
|---|---|---|---|
| Pre LVAD history | |||
| Age (years) | 58.4 ± 13.9 | 50.1 ± 14.5 | 0.118 |
| Women | 3 (15.0%) | 2 (16.7%) | 0.999 |
| Body surface area (m2) | 1.9 ± 0.2 | 2.2 ± 0.4 | 0.010 |
| Ischemic | 10 (50.0%) | 7 (53.8%) | 0.647 |
| Hypertension | 11 (55.0%) | 5 (41.7%) | 0.465 |
| Hyperlipidemia | 10 (50.0%) | 4 (33.3%) | 0.471 |
| Diabetes | 11 (55.0%) | 6 (50.0%) | 0.999 |
| Smoking history | 5 (25.0%) | 6 (50.0%) | 0.149 |
| Creatinine (mg/dl) | 1.48 ± 0.65 | 1.43 ± 0.83 | 0.869 |
| Previous stroke | 2 (10.0%) | 0 (0.0%) | 0.516 |
| Previous TIA/stroke | 2 (10.0%) | 0 (0.0%) | 0.516 |
| Device Type | |||
| HeartMate II | 16 (80.0%) | 10 (83.3%) | 0.815 |
| HVAD | 4 (20.0%) | 2 (16.7%) | |
| Device Strategy | |||
| BTT | 9 (45.0%) | 11 (91.7%) | 0.011 |
| DT | 11 (55.0%) | 1 (8.3%) | |
| Post LVAD history | |||
| GI Bleeding | 5 (25.0%) | 4 (33.3%) | 0.696 |
| Device-related infection | 3 (15.0%) | 3 (25.0%) | 0.647 |
| Sepsis | 4 (20.0%) | 5 (41.2%) | 0.240 |
| Pump exchange for thrombosis | 3 (15.0%) | 2 (16.7%) | 0.999 |
| At the time of stroke | |||
| Duration of support | 289.8 ± 363.2 | 140.7 ± 177.9 | 0.132 |
| INR (for patients on warfarin) | 2.2 ± 0.5 | 2.3 ± 0.9 | 0.693 |
| PTT (for patients on heparin) | 68.0 ± 20.6 | 67.7 ± 28.8 | 0.988 |
| NIHSS | 7.2 ± 9.3 | 13.6 ± 8.6 | 0.063 |
| Outcomes post stroke | |||
| Survival to Discharge | 16 (80.0%) | 7 (58.3%) | 0.240 |
Values are n (%).
IS = Ischemic Stroke; CF-LVAD = Continuous-Flow Left Ventricular Assist Device; TIA = Transient Ischemic Attack; HVAD = Heartware Left Ventricular Assist Device; BTT = Bridge to Transplant; DT = Destination Therapy; GI = Gastrointestinal; INR = International Normalized Ratio; PTT = Partial Thromboplastin Time; NIHSS = National Institutes of Health Stroke Scale.
Table 4.
Anticoagulation status at the time of stoke by stroke subtype and device.
| Device | #patients Warfarin | INR (mean) | #patients Heparin | PTT (mean) | # patients out of range (proportion INR below and above range) | |
|---|---|---|---|---|---|---|
| ICH | HVAD | 2 | 2.8 | 2 | 89.5 | 2 (1.6, 4) |
| HMII | 2 | 3.3 | 2 | 72.8 | 1 (4.2) | |
| IS | HVAD | 5 | 2.5 | 1 | 52.7 | 2 (1.5, 2.3) |
| HMII | 21 | 2.2 | 4 | 71.7 | 11 (4 above, 7 below) |
ICH patients: 1 mild-moderate aortic insufficiency (AI), 1 mild AI, 2 trace AI, 4 no AI. In 2 patients the aortic valve (AV) opened with every cycle, in 3 the AV closed, and in 3 AV intermittently opened. One patient had mild aortic root dilation and one had AV thrombus.
IS patients: 3 mild-moderate AI, 2 mild AI, 16 trace/trivial AI, 11 no AI. In 4 the AV opened, 2 had the AV intermittently/minimally opening, and in 26 the AV closed. None of IS patients had AV thrombus or aortic dilation.
Hospital Course and Clinical Outcomes after Stroke
The in-hospital mortality was highest for ICH compared to ischemic strokes. There were 4 in-hospital deaths in the 8 patients with ICH (50%), of which 3 were neurological and 1 non-neurological (multi-organ failure). None of the patients with in-hospital death after ICH were restarted on antithrombotics. Patients with ICH had wide variability in stroke severity, though the NIHSS was significantly higher at the time of stroke in those who died compared to those who did not (NIHSS 18.8±13.7 vs 1.8±1.7 in survivors, p=0.049). These findings reflect that patients with more severe hemorrhages are more likely to die in-hospital; none of the patients with ICH had surgical evacuation. Average timing of death post-stroke was 23.25 days in these patients; 1 patient was discharged to Hospice 506 days after stroke. In the 4 survivors, anti-thrombotic medications were started on average 7.2 days after stroke. Among 4 ICH survivors, 2 were discharged to home, 1 to nursing home, and 1 to hospice (device explanted). Only 1 of the 3 survivors discharged to home or nursing home remained listed as bridge to transplant (BTT), while the other 2 patients were destination therapy (DT). At the time of analysis the BTT patient was not transplanted. These 3 patients remained on anticoagulation without a lower INR target, and aspirin.
Among the 32 IS patients, 12 had hemorrhagic conversion and 5 were treated with IAE and none had a craniotomy. There were 9 deaths (28%), with a greater initial neurological impairment noted in those with in-hospital mortality versus survivors (NIHSS 16.2±10.8 vs 7.0±7.6, p=0.011); average timing of death for 9 patients was 100.6 days after stroke. There were 5 deaths (42%) in the 12 patients with hemorrhagic conversion. We noted 5 of the 9 overall deaths to occur due to neurological reasons: 4 due to cerebral edema from hemorrhagic conversion, and 1 from malignant cerebral edema; the remaining 4 deaths were non-neurological (three multi-organ failure and one hemorrhagic shock from disseminated intravascular coagulation). None of the hemorrhagic conversion patients had been restarted on antithrombotic treatment before the event. In the 23 IS survivors, 7 were transferred to acute inpatient rehabilitation,14 were discharged to home, 1 was transferred to an outside hospital for further transplant evaluation, and 1 to nursing home. A majority of IS patients who survived to discharge were BTT (13 out of 23, 56.6%). In the IS patients 2 pump exchanges occurred after IS, 4 occurred before IS with a range from 1–317 days beforehand (mean 96.8 days beforehand). Of these 13 patients, 10 underwent transplant, 1 remains actively listed, 1 was delisted due to hypercoagulability with multiple thromboembolic complications, and 1 was successfully explanted without need for CF-LVAD support. Two additional patients underwent transplant, 1 who was originally DT and 1 who died prior to discharge secondary to complications from primary graft failure. In total, 12 patients in the IS group were transplanted with 11 out of 12 patients remaining alive one year after transplant. Among the 12 transplanted patients 2 had undergone IAE and 5 had their stroke complicated by hemorrhagic conversion. None of the patients had an in-hospital stroke after undergoing cardiac transplantation, while all stroke patients underwent imaging with at least one non-contrast head CT before transplantation. Head imaging was performed on 8 patients after transplant, and none had new neurological events post-operatively; 7 patients had brain MRI’s, 1 had head CT. There was no evidence of new acute infarcts on any post-transplant brain imaging.
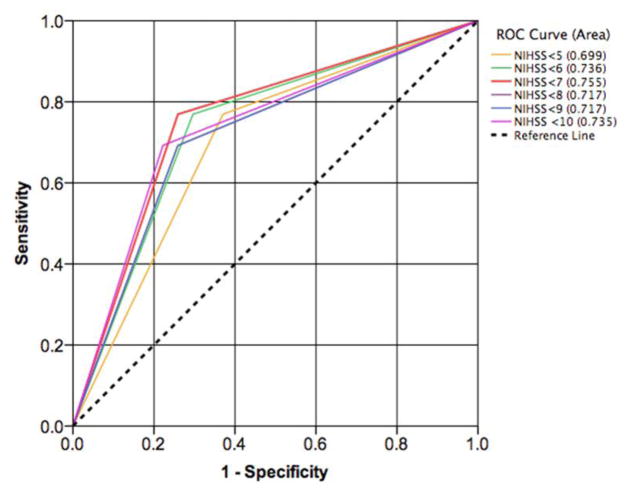
An ROC analysis identified a NIHSS cut-off of <7 as an appropriate threshold for creating a dichotomous NIHSS variable for the prediction of survival to hospital discharge in stroke patients with a sensitivity of 77% and specificity of 74%. AUC for NIHSS<7 was 0.775 (Figure 1). Of note, none of the 5 stroke patients with an NIHSS ≥22 survived to discharge.
FIGURE 1.
Receiver operator characteristic curves (ROC) for comparison of multiple dichotomies of National Institutes of Health Stroke Scale (NIHSS) in the prediction of survival to hospital discharge in stroke patients.
ROC predicting survival to discharge
Discussion
In this study including outcomes in a large sample of strokes after CF-LVAD placement we found differences in among stroke subtypes. In particular we noted poor outcomes in ICH patients, especially when associated with a large neurologic deficit at presentation, while good outcomes were still possible in the context of smaller deficits. Furthermore we found that while mortality was also significant for IS, a high proportion of these patients were able to have enough recovery to undergo cardiac transplantation. Similar to the ICH patients, the initial neurological impairment significantly predicted in-hospital outcomes. We also found that patients with hemorrhagic conversion had a high mortality (42%), but overall still lower than those with ICH (50%). Additionally, some of the IS patients with hemorrhagic conversion were able to undergo transplantation. The findings in our study highlight the importance of systematically categorizing the type of neurological injury the patient sustained, including the stroke subtype and presence of hemorrhagic conversion. In our study we also demonstrated the importance of capturing and describing the degree of initial neurological injury as a means of assessing the severity of stroke.
Our findings of high mortality associated with ICH compared to other samples6,10, is likely due to the methods by which ICH was defined and captured. In our study we excluded patients with subdural and epidural hematoma where the primary inciting process would be trauma rather than a process intrinsic to the cerebral circulation. In addition we categorized patients with hemorrhagic conversion as IS since the primary underlying process was an initial arterial occlusion and infarction with subsequent recanalization. In patients with IS and hemorrhagic conversion anti-platelet agents and anticoagulants are started earlier than in ICH patients7, and they appeared more likely to be discharged home and undergo transplantation than patients with primary ICH, further validating our classification. The findings on outcomes among patients with IS are also notable. Overall we found that treatment with IAE was feasible and safe in patients with IS and that comprehensive inpatient care aimed at reducing neurological and medical complications could make patients eligible for cardiac transplantation.
This study has some important limitations that should be considered. We included data from a single high volume LVAD center with a dedicated stroke neurology service immersed in a broader LVAD multi-disciplinary team and our findings may not be generalizable to other centers. The analysis is retrospective in nature, though all of the data was obtained from reviews of comprehensive electronic medical records and review of actual neuro-imaging to classify stroke subtypes. Similarly we did not have access to aspects of care provided for the patients who first presented to their local hospital. We did not have data available on outpatient functional status with the modified Rankin scale at 3 months, which is the most common outcome in stroke clinical trials13. In our study, however, we had comprehensive follow up for in-hospital death, as well as eventual transplantation. We did not collect systematic data on blood pressure at the time of or before the stroke, which has been recently demonstrated to be a possible risk factor for stroke15,16. It is not known, however, if blood pressure would affect stroke severity, particularly in hemorrhagic stroke17. Due to lack of data from hospitals where the patients first presented we are not able to comment on the immediate triggers of stroke, such as hypertensive crisis. In future studies we plan on collecting this data to analyze this important question. Our study has some important strengths, including a standardized approach to stroke care throughout the years of follow up allowing for the results to be independent of the standards of care depending on the year. We are one of the first studies to report on comprehensive outcomes after stroke, notably in-hospital mortality, and suitability for transplantation.
In summary, strokes after LVAD placement do not affect all patients equally and transplantation is still a feasible therapeutic option for stroke survivors. The differences in mortality and transplantation rates in our study based on initial stroke severity and stroke subtype highlight the importance of standardized and comprehensive assessment and management of all strokes after LVAD. Further understanding of the functional status after stroke, and the associated risk factors for good outcomes is an important gap that should be explored in future research. It appears also warranted to investigate whether faster care of stroke may favorably impact neurological impairment at presentation and thereby clinical outcomes. This research could have implications for the care of neurological complications in mechanical circulatory support.
Supplementary Material
Footnotes
Disclosures
JZW (NINDS K23 NS 073104) and PCC (NHLBI R01 HL092144) are funded by the National Institutes of Health for research. JZW has received a consulting fee from Heartware Incorporated (< $ 10,000) regarding mechanisms of stroke and is on the Clinical Endpoint committee for the ReliantHeart BTT clinical trial (no reimbursement provided).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Yuan N, Arnaoutakis GJ, George TJ, et al. The spectrum of complications following left ventricular assist device placement. J Card Surg. 2012 Sep;27(5):630–638. doi: 10.1111/j.1540-8191.2012.01504.x. [DOI] [PubMed] [Google Scholar]
- 2.Kirklin JK, Naftel DC, Kormos RL, et al. Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) analysis of pump thrombosis in the HeartMate II left ventricular assist device. J Heart Lung Transplant. 2014 Jan;33(1):12–22. doi: 10.1016/j.healun.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 3.Kirklin JK, Naftel DC, Pagani FD, et al. Sixth INTERMACS annual report: a 10,000-patient database. J Heart Lung Transplant. 2014 Jun;33(6):555–564. doi: 10.1016/j.healun.2014.04.010. [DOI] [PubMed] [Google Scholar]
- 4.Lazar RM, Shapiro PA, Jaski BE, et al. Neurological events during long-term mechanical circulatory support for heart failure: the Randomized Evaluation of Mechanical Assistance for the Treatment of Congestive Heart Failure (REMATCH) experience. Circulation. 2004 May 25;109(20):2423–2427. doi: 10.1161/01.CIR.0000129414.95137.CD. [DOI] [PubMed] [Google Scholar]
- 5.Pagani F, Milano CA, Tatooles A, et al. HeartWare HVAD for the treatment of pateints with advanced heart failure ineligible for cardiac transplantation: results from the ENDURANCE destination therapy trial. The Journal of Heart and Lung Transplantation. 2015;34:S9. [Google Scholar]
- 6.Patlolla V, Mogulla V, DeNofrio D, Konstam MA, Krishnamani R. Outcomes in patients with symptomatic cerebrovascular disease undergoing heart transplantation. J Am Coll Cardiol. 2011 Aug 30;58(10):1036–1041. doi: 10.1016/j.jacc.2011.04.038. [DOI] [PubMed] [Google Scholar]
- 7.Willey JZ, Demmer RT, Takayama H, Colombo PC, Lazar RM. Cerebrovascular disease in the era of left ventricular assist devices with continuous flow: risk factors, diagnosis, and treatment. J Heart Lung Transplant. 2014 Sep;33(9):878–887. doi: 10.1016/j.healun.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 8.Harvey L, Holley C, Roy SS, et al. Stroke After Left Ventricular Assist Device Implantation: Outcomes in the Continuous-Flow Era. Ann Thorac Surg. 2015 Jun 9; doi: 10.1016/j.athoracsur.2015.02.094. [DOI] [PubMed] [Google Scholar]
- 9.Morgan JA, Brewer RJ, Nemeh HW, et al. Stroke while on long-term left ventricular assist device support: incidence, outcome, and predictors. ASAIO J. 2014 May-Jun;60(3):284–289. doi: 10.1097/MAT.0000000000000074. [DOI] [PubMed] [Google Scholar]
- 10.Wilson TJ, Stetler WR, Jr, Al-Holou WN, Sullivan SE, Fletcher JJ. Management of intracranial hemorrhage in patients with left ventricular assist devices. J Neurosurg. 2013 May;118(5):1063–1068. doi: 10.3171/2013.1.JNS121849. [DOI] [PubMed] [Google Scholar]
- 11.Easton JD, Saver JL, Albers GW, et al. Definition and evaluation of transient ischemic attack: a scientific statement for healthcare professionals from the American Heart Association/American Stroke Association Stroke Council; Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Nursing; and the Interdisciplinary Council on Peripheral Vascular Disease. The American Academy of Neurology affirms the value of this statement as an educational tool for neurologists. Stroke. 2009 Jun;40(6):2276–2293. doi: 10.1161/STROKEAHA.108.192218. [DOI] [PubMed] [Google Scholar]
- 12.Morgenstern LB, Hemphill JC, 3rd, Anderson C, et al. Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2010 Sep;41(9):2108–2129. doi: 10.1161/STR.0b013e3181ec611b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jauch EC, Saver JL, Adams HP, Jr, et al. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013 Mar;44(3):870–947. doi: 10.1161/STR.0b013e318284056a. [DOI] [PubMed] [Google Scholar]
- 14.Dini FL, Demmer RT, Simioniuc A, et al. Right ventricular dysfunction is associated with chronic kidney disease and predicts survival in patients with chronic systolic heart failure. Eur J Heart Fail. 2012 Mar;14(3):287–294. doi: 10.1093/eurjhf/hfr176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lampert BC, Eckert C, Weaver S, et al. Blood Pressure Control in Continuous Flow Left Ventricular Assist Devices: Efficacy and Impact on Adverse Events. Ann Thorac Surg. 2014 Jan;97(1):139–146. doi: 10.1016/j.athoracsur.2013.07.069. [DOI] [PubMed] [Google Scholar]
- 16.Najjar SS, Slaughter MS, Pagani FD, et al. An analysis of pump thrombus events in patients in the HeartWare ADVANCE bridge to transplant and continued access protocol trial. J Heart Lung Transplant. 2014 Jan;33(1):23–34. doi: 10.1016/j.healun.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 17.Wasson LT, Yuzefpolskaya M, Wakabayashi M, et al. Hypertension: an unstudied potential risk factor for adverse outcomes during continuous flow ventricular assist device support. Heart Fail Rev. 2014 Oct 5; doi: 10.1007/s10741-014-9458-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.