Abstract
Surface glycoproteins direct cellular targeting, attachment, and membrane fusion of arenaviruses and are the primary target for neutralising antibodies. Despite significant conservation of the glycoprotein architecture across the arenavirus family, there is considerable variation in the molecular recognition mechanisms used during host cell entry. We review recent progress in dissecting these infection events and describe how arenaviral glycoproteins can be targeted by small-molecule antivirals, the natural immune response and immunoglobulin-based therapeutics. Arenaviral glycoprotein-mediated assembly and infection pathways present numerous opportunities and challenges for therapeutic intervention.
Keywords: Arenavirus, glycoprotein, structure, vaccine, antivirals
Introduction
Pathogenic arenaviruses from the Arenaviridae family can cause a range of human diseases, including severe hemorrhagic fever and fatal transplant-associated infection [1]. Whilst good medical practice [2] and community hygiene [3,4] can limit viral transmission, arenaviruses are under increasing scrutiny due to their categorization as biosecurity risks. Our increasing knowledge of arenavirus structure and pathobiology provides a detailed molecular picture of key events during viral zoonosis and immune resolution of infection. Here, we describe these recent advances and discuss the challenges and opportunities of targeting arenaviruses at that initial stage of host cell entry and viral biogenesis.
Mammalian arenaviruses are predominantly found in the Americas and Africa and are consequently commonly classified as either New or Old World, respectively. While currently no clade distinctions are made amongst the Old World arenaviruses, New World arenaviruses are divided into four clades (A to D) [5]. Mammalian New and Old World arenaviruses reside in a range of rodent species and transmission to humans from these viruses is often associated with perturbation of natural habitats. For example, the causative agent for Bolivian hemorrhagic fever was termed Machupo virus (MACV) after an outbreak in the community around the Machupo river, where it is believed mice had been attracted by newly established grain-based agriculture [6]. Although rodents are the most significant host reservoir, arenaviruses have also been identified in reptiles. Viruses belonging to the new genus Reptarenavirus within the family Arenaviridae, are believed to be the etiological agents of the fatal inclusion body disease in alethinophidian snakes [5,7–9], but their role in human disease, if any, has not been established. Given the diverse nature of these animal reservoirs, it is likely that further host species will be identified.
The arenavirus genome is ambi-sense and consists of two separate segments of RNA, termed L and S. The L segment encodes the RNA-dependent polymerase (L protein) and zinc-binding matrix protein (Z protein) whereas the S segment encodes the nucleoprotein and the glycoprotein precursor (GPC). During the viral glycoprotein maturation, GPC is cleaved into three components; a stable signal peptide (SSP) of an unusual length that is retained as an essential component of the mature complex, an attachment glycoprotein (GP1) and fusion glycoprotein (GP2) [10]. Low resolution electron cryo-microscopy combined with sub-tomogram averaging has revealed that these three components associate to form a three-legged trimeric spike, measuring 9 nm in height and 10 nm in width (Figure 1), which undergoes pH-dependent conformational changes [11].
Figure 1. Cryo-EM analysis of LASV reveals the higher order architecture and receptor-binding properties of the Old World arenavirus SSP-GP1-GP2 spike complex.
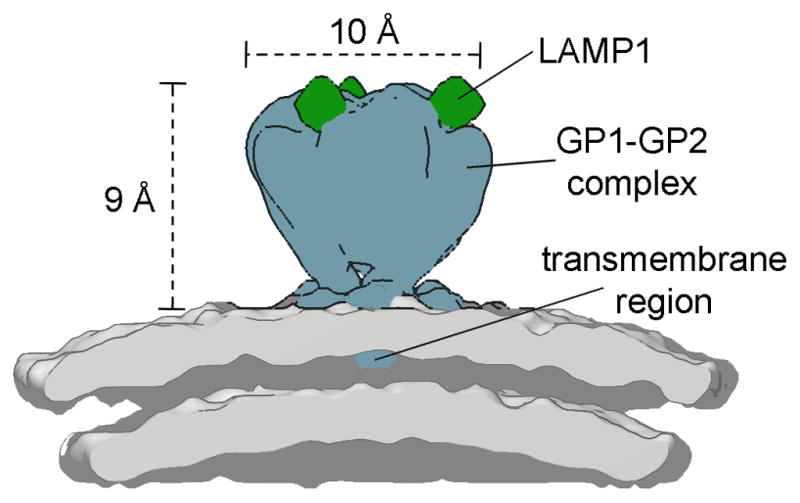
Schematic of the 14 Å-resolution structure of the LASV glycoprotein spike in complex with LAMP receptor at pH 5.0 (EMD accession code EMD-3293). Density corresponding to the GP1-GP2 complex is colored blue, human LAMP1 is green, and the lipid bilayer is gray.
Structure of the arenaviral GP1 attachment glycoprotein
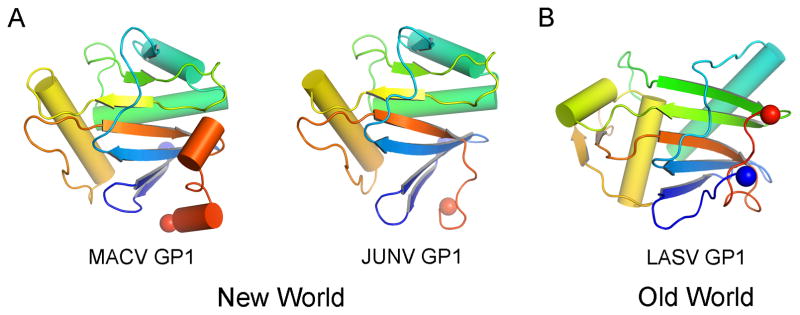
Crystal structures have been determined for the GP1 attachment glycoproteins of Old World Lassa virus (LASV) [12] and the New World arenaviruses, MACV [13] and Junín virus (JUNV) [14] (Figure 2). Despite low sequence conservation between these viral glycoproteins, all three structures display a highly similar compact α/β fold. The domain consists of a twisted β-sheet, which is protected on its convex side by an array of α-helices. The N-linked glycosylation sites cluster around the perimeter of the fold, although bioinformatics analysis revealed that they display only limited conservation in absolute position across the family [13]. The most significant conformational difference between the New World and LASV GP1 structures is the re-positioning of the termini [12]. This has been attributed to a pH-induced conformational change, reflecting the different pH conditions used for crystallization. This hypothesis is consistent with the conformational change that LASV GP1 has been proposed to undergo upon entry into endosomal compartments of the host cell [15]. Nevertheless, this remains to be systematically tested to exclude the effect of primary sequence divergence between the glycoproteins and other experimental factors such as crystal lattice effects.
Figure 2. Comparison of New and Old World arenaviral GP1 attachment glycoprotein folds.
(A) Crystal structures of New World MACV (PDB accession code 2WFO, left) and JUNV (PDB accession code 5EN2, right) GP1 glycoproteins. (B) Crystal structure of Old World LASV GP1 glycoprotein (PDB accession code 4ZJF). Structures are shown in cartoon representation and ramped from blue at the N-terminus to red at the C-terminus. N- and C-termini are shown as blue and red spheres, respectively.
Attachment of New World arenaviruses at the host-cell surface
New and Old World arenaviruses utilize different receptors. The hemorrhagic New World clade B arenaviruses JUNV, MACV, Guanarito (GTOV), Sabia (SABV) and Chapare (CHPV) viruses, as well as the apathogenic arenaviruses of the same clade, use transferrin receptor 1 (TfR1) for cell entry into their natural rodent hosts [16,17]. Pathogenic arenaviruses display protein recognition modes by GP1 that lead to cross-reactivity with human TfR1, providing a rationale for viral zoonosis into human populations. This phenomenon is also observed in North American clade D (formerly known as clade A/B or A/rec) [5] viruses. These viruses also utilize TfR1 orthologs of their respective natural rodent hosts, with one member of the Whitewater Arroyo species complex showing additional ability to utilize human TfR1 [18]. Furthermore, although North American arenaviruses are generally considered non-pathogenic, some have been tentatively implicated in the death of three people in California [19].
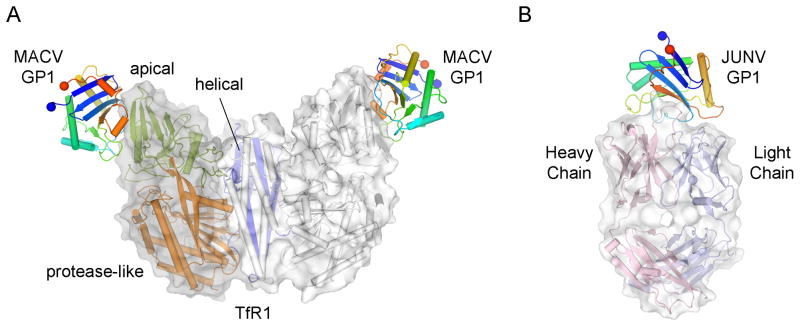
Structural analysis of MACV GP1 in complex with human TfR1 provides a molecular rationale for observed spill over of New World arenaviruses into human populations [20–22] (Figure 3A). MACV GP1 binds to the apical domain of TfR1, at a site not used by either of the physiological ligands transferrin and hereditary hemochromostasis associated protein. Interestingly, it has been suggested antibodies that target TfR1 apical domain may have antiviral potential [23]. However, it is important to note that parameters beyond receptor specificity alone may dictate effective zoonosis. In one extreme example, a recent study on reptarenavirus propagation in cell culture demonstrated ready infection of University of Helsinki virus in human cells although this was only efficient at 30°C and was significantly impeded at mammalian body temperature [24]. Another example is the finding that all known human-pathogenic arenaviruses, but not non-pathogenic arenaviruses, share a Z-protein-mediated innate immune suppression mechanism that blunts the interferon-mediated antiviral responses [25]. These observations underscore that whilst arenavirus-cell surface interactions are necessary for infection, these conditions alone may not be sufficient to establish infection.
Figure 3. Crystal structures of receptor- and antibody-bound New World arenavirus GP1 complexes.
(A) Crystal Structure of MACV GP1 bound to the TfR1 ectodomain (PDB accession code 3KAS). The TfR1 homodimer is shown as a cartoon with a transparent van der Waals surface. One protomer of the dimer is colored according to domain (apical domain colored green, protease-like domain colored orange, and helical domain colored blue) and the other white. MACV GP1 is colored as in Figure 2. (B) Crystal structure of JUNV GP1 bound to the fAb fragment of a neutralizing monoclonal antibody derived from a mouse (PDB accession code 5EN2). The Fab fragment heterodimer is shown as a cartoon with a surrounding van der Waals surface. The heavy chain of the Fab fragment is colored pink and the light chain colored blue. JUNV GP1 is colored as in Figure 2.
Old World arenavirus attachment factors
No Old World arenaviruses are known to utilize the TfR1-mediated host-cell entry pathway. Instead, Old World arenaviruses such as LASV, lymphocytic choriomeningitis virus (LCMV), Mobala, as well as clade C New World viruses Oliveros and Latino, have been shown to functionally recognize the α subunit of dystroglycan (α-dystroglycan)[26–31]. Dystroglycan is widely expressed and is integral in linking the extracellular matrix to the cytoskeleton [32,33]. Interestingly, LASV recognition is dependent on the structure of the conjugated O-glycans displayed on α-dystroglycan. This leads to a dependency for O-glycan biosynthesis factors such as the transferase LARGE, which is important in elaborating O-mannosyl initiated glycans on α-dystroglycan [30,34,35]. In addition to these entry mechanisms, lectin- and other protein-mediated pathways may contribute to LASV infection of certain cell types [36].
During endocytosis and exposure to the acidic interior of late endosomes (pH 5–5.5), LASV binds to lysosomal associated membrane protein 1 (LAMP-1) [15] at the tip of the viral spike (Figure 1) [11]. This pH-dependent interaction [15] is controlled by a histidine-switch at the putative binding face of LASV GP1 [12] and is dependent on both α2,3-linked sialylation and the presence of a single N-linked glycan on LAMP-1 [15]. Although the molecular bases for LASV GP1 dystroglycan and GP1 LAMP-1 interactions are yet to be elucidated, the shared dependency on glycans for both complexes is suggestive of related modes of interaction. Immunologically, Jae et al. make the interesting observation that the upper and lower human respiratory tract is dominated by α2,6-linked and α2,3-lined sialylated glycans, respectively, which may impede LASV infection [15].
LAMP-1 dependency does not appear to be shared across Old World arenaviruses and, for example, is not required for LCMV infection [15]. The variable dependency on the lysosomal factors would seem to suggest that we have an incomplete picture of this critical stage of the infection cycle.
Viral membrane fusion with the host
Following arenaviral endocytosis, the GP1 is shed and membrane fusion is mediated by GP2. It is suggested that GP1 may act as a molecular shield preventing premature conformational changes [11]. Whilst pre-fusion crystal structures of the GP2 have yet to be determined, the structures of LCMV and Guanarito virus GP2 in their post-fusion conformation reveal a well-conserved, class I fusion protein architecture common to a number of enveloped viruses such as the Orthomyxoviridae, Filoviridae, and Paramyxoviridae [37,38]. Class I fusion glycoproteins are characterised by an α-helical architecture and form an extended coiled-coil structure upon fusion. Upon cleavage of the GPC, the GP2 is rendered metastable and exposure to endosomal pH drives consequent rearrangements and membrane merger [10]. Interestingly, small molecule inhibitors of arenavirus cell entry are believed to target the pH-sensitive molecular interface between SSP and GP2, resulting in inhibition of pH-induced fusion [10,39].
Arenaviral glycoproteins are targeted by neutralizing antibodies
The high mortality rate arising from infection by some arenaviruses, such as 15–30% mortality in JUNV infection, indicates that the human immune system can fail to control infection [40]. However, passive transfer of immunoglobulins from convalescent patients is an effective treatment for acute human JUNV infections [41–43]. The molecular basis for viral neutralization has elegantly been revealed through the elucidation of the crystal structure of the JUNV GP1 in complex with a neutralising IgG2a Fab [14] (Figure 3B). The originating antibody was raised in mice by immunization with inactivated JUNV [44]. The antibody achieves neutralization through the recognition of the TfR1 binding interface. The interaction mimics a central GP1-receptor contact by inserting a tyrosine residue into a pocket on the GP1. Thus, neutralization is most probably achieved by interfering with binding to the host cell receptor. Consistent with this hypothesis, Mahmutovic et al. demonstrate that the receptor binding site is also a targeted by antibodies contained in the polyclonal immunoglobulin pool of JUNV survivors [14]. Given the practical limitations of convalescent serum therapy [41], the GP1-TfR1 interface provides a promising target for rational development of alternative antibody and small-molecule antivirals [45]. An example of promising antibody therapy is a glycoprotein-specific IgG cocktail, produced by DNA vaccination, that has been demonstrated to protect experimental animals against lethal JUNV and GTOV challenge. Encouragingly, some, albeit limited, cross-neutralization was observed between the JUNV and MACV glycoprotein-specific sera [46].
Prospects for arenaviral vaccines
Although cell-mediated immunity is likely to play an important role, the ready neutralization of JUNV by antibodies is consistent with the development of the only currently available arenavirus vaccine Candid#1, which is composed of live-attenuated JUNV and is licensed in Argentina for the vaccination of populations within high-risk areas [47]. The successful development of a JUNV vaccine has stimulated considerable interest in the development of vaccines to other arenaviruses and potentially cross-protective vaccines [46,48–51].
The success in vaccine development and the efficacy of survivor plasma transfusion in the treatment of JUNV-mediated haemorrhagic fever has, however, not been mirrored in LASV vaccinology [52,53]. Delayed and weak neutralizing antibody responses have been observed both in natural infection [54] and in candidate vaccine trials [55,56]. Interestingly, the higher glycan density on the LASV GP1 compared to JUNV GP1 (seven and four N-linked glycosylation sites, respectively) does seem to shield the LASV envelope against efficient antibody-mediated neutralization [57]. Despite these observations, the discovery of antibodies that can bind to and penetrate the glycan shield of the attachment glycoprotein of the human immunodeficiency virus gives hope that these glycan effects are not insurmountable [58–60]. Nevertheless, it is currently uncertain whether successful arenaviral vaccines will be dependent on potent antibodies or whether they will rely predominantly on a strong cellular component.
Antivirals targeting glycoprotein folding
Although ribavirin has been found somewhat effective for the treatment of haemorrhagic fevers caused by arenaviruses such as LASV and JUNV [61], there are currently no virus-specific small-molecule antiviral agents approved for the treatment of arenaviruses. However, arenaviruses have been shown to be vulnerable to drugs that target viral glycoprotein folding by inhibiting host cell ER α-glucosidases, such as iminosugars. These small molecule drugs have demonstrated in vitro efficacy against Pichinde virus, JUNV, and Tacaribe virus [62]. The initial success of these compounds highlights the differential dependency of the glycan-mediated folding pathway between self and viral glycoprotein biosynthesis and suggests a promising window for therapeutic control of infection.
Perspectives
Recent structural analyses of arenavirus glycoproteins, alone and in complex with receptors and antibodies, have revealed that these surface-exposed molecules are both key determinants of species tropism and clear targets for therapeutic intervention. We anticipate that further structural and mechanistic studies will further help to define features of the arenaviral life cycle that can be targeted in next generation cross-protective vaccines and therapies.
Highlights.
Arenavirus entry into a host cell is orchestrated by virion-encoded glycoproteins
Arenaviral glycoproteins form trimeric spikes and interact with host cell receptors
Neutralizing antibodies target arenaviral glycoproteins
Arenaviruses are vulnerable to drugs that target viral glycoprotein folding
Acknowledgments
We thank Daniel Pinschewer and Jack Nunberg for helpful comments and discussions. A.Z. is supported by a Marie Curie Fellowship (658363), T.A.B. is supported by the MRC (MR/J007897/1 and MR/N002091/1), and M.C. is supported by the Center for HIV/AIDS Vaccine Immunology and Immunogen Discovery Grant (UM1AI100663) and the International AIDS Vaccine Initiative through the Neutralizing Antibody Consortium and Bill and Melinda Gates Center for Vaccine Discovery. The Wellcome Trust Centre for Human Genetics is supported by Wellcome Trust Centre grant 090532/Z/09/Z. N.Z. is a Fellow of Merton College, Oxford, and receives a personal consultancy from Unither Virology. NZ is a named inventor on patent application describing the antiviral use of iminosugars against arenaviruses (US 2010/0222384 A1).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Shao J, Liang Y, Ly H. Human hemorrhagic Fever causing arenaviruses: molecular mechanisms contributing to virus virulence and disease pathogenesis. Pathogens. 2015;4:283–306. doi: 10.3390/pathogens4020283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fisher-Hoch SP, Tomori O, Nasidi A, Perez-Oronoz GI, Fakile Y, Hutwagner L, McCormick JB. Review of cases of nosocomial Lassa fever in Nigeria: the high price of poor medical practice. BMJ. 1995;311:857–859. doi: 10.1136/bmj.311.7009.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kelly JD, Barrie MB, Ross RA, Temple BA, Moses LM, Bausch DG. Housing equity for health equity: a rights-based approach to the control of Lassa fever in post-war Sierra Leone. BMC Int Health Hum Rights. 2013;13:2. doi: 10.1186/1472-698X-13-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bonwitt J, Kelly AH, Ansumana R, Agbla S, Sahr F, Saez AM, Borchert M, Kock R, Fichet-Calvet E. Rat-atouille: A Mixed Method Study to Characterize Rodent Hunting and Consumption in the Context of Lassa Fever. Ecohealth. 2016 doi: 10.1007/s10393-016-1098-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Radoshitzky SR, Bao Y, Buchmeier MJ, Charrel RN, Clawson AN, Clegg CS, DeRisi JL, Emonet S, Gonzalez JP, Kuhn JH, et al. Past, present, and future of arenavirus taxonomy. Arch Virol. 2015;160:1851–1874. doi: 10.1007/s00705-015-2418-y. [DOI] [PubMed] [Google Scholar]
- 6.Kuns ML. Epidemiology of Machupo virus infection. II. Ecological and control studies of hemorrhagic fever. Am J Trop Med Hyg. 1965;14:813–816. doi: 10.4269/ajtmh.1965.14.813. [DOI] [PubMed] [Google Scholar]
- 7**.Stenglein MD, Sanders C, Kistler AL, Ruby JG, Franco JY, Reavill DR, Dunker F, Derisi JL. Identification, characterization, and in vitro culture of highly divergent arenaviruses from boa constrictors and annulated tree boas: candidate etiological agents for snake inclusion body disease. MBio. 2012;3:e00180–00112. doi: 10.1128/mBio.00180-12. These papers report the existence of reptilian-borne arenaviruses, revealing a wider range of host organisms than previously appreciated. This is important in our understanding of both the evolutionary origins of arenaviruses and their continued potential for spill-over into humans. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8**.Bodewes R, Kik MJ, Raj VS, Schapendonk CM, Haagmans BL, Smits SL, Osterhaus AD. Detection of novel divergent arenaviruses in boid snakes with inclusion body disease in The Netherlands. J Gen Virol. 2013;94:1206–1210. doi: 10.1099/vir.0.051995-0. These papers report the existence of reptilian-borne arenaviruses, revealing a wider range of host organisms than previously appreciated. This is important in our understanding of both the evolutionary origins of arenaviruses and their continued potential for spill-over into humans. [DOI] [PubMed] [Google Scholar]
- 9**.Hetzel U, Sironen T, Laurinmaki P, Liljeroos L, Patjas A, Henttonen H, Vaheri A, Artelt A, Kipar A, Butcher SJ, et al. Isolation, identification, and characterization of novel arenaviruses, the etiological agents of boid inclusion body disease. J Virol. 2013;87:10918–10935. doi: 10.1128/JVI.01123-13. These papers report the existence of reptilian-borne arenaviruses, revealing a wider range of host organisms than previously appreciated. This is important in our understanding of both the evolutionary origins of arenaviruses and their continued potential for spill-over into humans. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nunberg JH, York J. The curious case of arenavirus entry, and its inhibition. Viruses. 2012;4:83–101. doi: 10.3390/v4010083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11**.Li S, Zhaoyang S, Pryce R, Parsy M, Fehling SK, Schlie K, Siebert CA, Garten W, Bowden TA, Strecker T, et al. Acidic pH-induced Conformations and LAMP1 binding of the Lassa Virus Glycoprotein Spike. PLoS Pathog. 2016;12:e1005418. doi: 10.1371/journal.ppat.1005418. Using electron cryo-tomography and sub-tomogram averaging, this paper reports the low resolution ultrastructure of the LASV GP at pH 7, 5, and 3, revealing a trimeric globular structure that loses a putative GP1 subunit upon acidification to pH 3. This work also identifies the putative LAMP-1 receptor-binding site, at the interface between two spike protomers. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12*.Cohen-Dvashi H, Cohen N, Israeli H, Diskin R. Molecular Mechanism for LAMP1 Recognition by Lassa Virus. J Virol. 2015;89:7584–7592. doi: 10.1128/JVI.00651-15. This paper reports the crystal structure of the LASV GP1 glycoprotein, revealing a shared overall fold with New World arenaviruses. This work also identifies the putative recognition site for LAMP-1 receptor binding. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bowden TA, Crispin M, Graham SC, Harvey DJ, Grimes JM, Jones EY, Stuart DI. Unusual molecular architecture of the machupo virus attachment glycoprotein. J Virol. 2009;83:8259–8265. doi: 10.1128/JVI.00761-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14**.Mahmutovic S, Clark L, Levis SC, Briggiler AM, Enria DA, Harrison SC, Abraham J. Molecular Basis for Antibody-Mediated Neutralization of New World Hemorrhagic Fever Mammarenaviruses. Cell Host Microbe. 2015;18:705–713. doi: 10.1016/j.chom.2015.11.005. This paper reports the crystal structure of JUNV GP1 in complex with a neutralizing antibody. This work reveals that viral neutralization may occur through direct interference with TfR1 recognition. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15**.Jae LT, Raaben M, Herbert AS, Kuehne AI, Wirchnianski AS, Soh TK, Stubbs SH, Janssen H, Damme M, Saftig P, et al. Virus entry. Lassa virus entry requires a trigger-induced receptor switch. Science. 2014;344:1506–1510. doi: 10.1126/science.1252480. This paper reports the identification of a pH-dependent mechanism for LAMP-1 recognition by LASV. This has emphasized the intricate endosomal pathways exploited by some arenaviruses. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Radoshitzky SR, Abraham J, Spiropoulou CF, Kuhn JH, Nguyen D, Li W, Nagel J, Schmidt PJ, Nunberg JH, Andrews NC, et al. Transferrin receptor 1 is a cellular receptor for New World haemorrhagic fever arenaviruses. Nature. 2007;446:92–96. doi: 10.1038/nature05539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abraham J, Kwong JA, Albarino CG, Lu JG, Radoshitzky SR, Salazar-Bravo J, Farzan M, Spiropoulou CF, Choe H. Host-species transferrin receptor 1 orthologs are cellular receptors for nonpathogenic new world clade B arenaviruses. PLoS Pathog. 2009;5:e1000358. doi: 10.1371/journal.ppat.1000358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zong M, Fofana I, Choe H. Human and host species transferrin receptor 1 use by North American arenaviruses. J Virol. 2014;88:9418–9428. doi: 10.1128/JVI.01112-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Enserink M. Emerging diseases. New arenavirus blamed for recent deaths in California. Science. 2000;289:842–843. doi: 10.1126/science.289.5481.842. [DOI] [PubMed] [Google Scholar]
- 20.Abraham J, Corbett KD, Farzan M, Choe H, Harrison SC. Structural basis for receptor recognition by New World hemorrhagic fever arenaviruses. Nat Struct Mol Biol. 2010;17:438–444. doi: 10.1038/nsmb.1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abraham J, Kwong JA, Albarino CG, Lu JG, Radoshitzky SR, Salazar-Bravo J, Farzan M, Spiropoulou CF, Choe H. Host-species transferrin receptor 1 orthologs are cellular receptors for nonpathogenic new world clade B arenaviruses. PLoS Pathog. 2009;5:e1000358. doi: 10.1371/journal.ppat.1000358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Radoshitzky SR, Kuhn JH, Spiropoulou CF, Albarino CG, Nguyen DP, Salazar-Bravo J, Dorfman T, Lee AS, Wang E, Ross SR, et al. Receptor determinants of zoonotic transmission of New World hemorrhagic fever arenaviruses. Proc Natl Acad Sci USA. 2008;105:2664–2669. doi: 10.1073/pnas.0709254105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abraham J, Corbett KD, Farzan M, Choe H, Harrison SC. Structural basis for receptor recognition by New World hemorrhagic fever arenaviruses. Nat Struct Mol Biol. 2010;17:438–444. doi: 10.1038/nsmb.1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hepojoki J, Kipar A, Korzyukov Y, Bell-Sakyi L, Vapalahti O, Hetzel U. Replication of boid inclusion body disease-associated arenaviruses is temperature sensitive in both boid and mammalian cells. J Virol. 2015;89:1119–1128. doi: 10.1128/JVI.03119-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xing J, Ly H, Liang Y. The Z proteins of pathogenic but not nonpathogenic arenaviruses inhibit RIG-I-like receptor-dependent interferon production. J Virol. 2015;89:2944–2955. doi: 10.1128/JVI.03349-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cao W, Henry MD, Borrow P, Yamada H, Elder JH, Ravkov EV, Nichol ST, Compans RW, Campbell KP, Oldstone MB. Identification of alpha-dystroglycan as a receptor for lymphocytic choriomeningitis virus and Lassa fever virus. Science. 1998;282:2079–2081. doi: 10.1126/science.282.5396.2079. [DOI] [PubMed] [Google Scholar]
- 27.Spiropoulou CF, Kunz S, Rollin PE, Campbell KP, Oldstone MB. New World arenavirus clade C, but not clade A and B viruses, utilizes alpha-dystroglycan as its major receptor. J Virol. 2002;76:5140–5146. doi: 10.1128/JVI.76.10.5140-5146.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kunz S, Sevilla N, McGavern DB, Campbell KP, Oldstone MB. Molecular analysis of the interaction of LCMV with its cellular receptor [alpha]-dystroglycan. J Cell Biol. 2001;155:301–310. doi: 10.1083/jcb.200104103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kunz S, Rojek JM, Perez M, Spiropoulou CF, Oldstone MB. Characterization of the interaction of lassa fever virus with its cellular receptor alpha-dystroglycan. J Virol. 2005;79:5979–5987. doi: 10.1128/JVI.79.10.5979-5987.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rojek JM, Kunz S. Cell entry by human pathogenic arenaviruses. Cell Microbiol. 2008;10:828–835. doi: 10.1111/j.1462-5822.2007.01113.x. [DOI] [PubMed] [Google Scholar]
- 31.Kunz S, Campbell KP, Oldstone MB. Alpha-dystroglycan can mediate arenavirus infection in the absence of beta-dystroglycan. Virology. 2003;316:213–220. doi: 10.1016/j.virol.2003.07.002. [DOI] [PubMed] [Google Scholar]
- 32.Ibraghimov-Beskrovnaya O, Ervasti JM, Leveille CJ, Slaughter CA, Sernett SW, Campbell KP. Primary structure of dystrophin-associated glycoproteins linking dystrophin to the extracellular matrix. Nature. 1992;355:696–702. doi: 10.1038/355696a0. [DOI] [PubMed] [Google Scholar]
- 33.Barresi R, Campbell KP. Dystroglycan: from biosynthesis to pathogenesis of human disease. J Cell Sci. 2006;119:199–207. doi: 10.1242/jcs.02814. [DOI] [PubMed] [Google Scholar]
- 34.Kunz S, Rojek JM, Kanagawa M, Spiropoulou CF, Barresi R, Campbell KP, Oldstone MB. Posttranslational modification of alpha-dystroglycan, the cellular receptor for arenaviruses, by the glycosyltransferase LARGE is critical for virus binding. J Virol. 2005;79:14282–14296. doi: 10.1128/JVI.79.22.14282-14296.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rojek JM, Spiropoulou CF, Campbell KP, Kunz S. Old World and clade C New World arenaviruses mimic the molecular mechanism of receptor recognition used by alpha-dystroglycan’s host-derived ligands. J Virol. 2007;81:5685–5695. doi: 10.1128/JVI.02574-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shimojima M, Stroher U, Ebihara H, Feldmann H, Kawaoka Y. Identification of cell surface molecules involved in dystroglycan-independent Lassa virus cell entry. J Virol. 2012;86:2067–2078. doi: 10.1128/JVI.06451-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Parsy ML, Harlos K, Huiskonen JT, Bowden TA. Crystal structure of Venezuelan hemorrhagic fever virus fusion glycoprotein reveals a class 1 postfusion architecture with extensive glycosylation. J Virol. 2013;87:13070–13075. doi: 10.1128/JVI.02298-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Igonet S, Vaney MC, Vonhrein C, Bricogne G, Stura EA, Hengartner H, Eschli B, Rey FA. X-ray structure of the arenavirus glycoprotein GP2 in its postfusion hairpin conformation. Proc Nat Acad Sci USA. 2011;108:19967–19972. doi: 10.1073/pnas.1108910108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.York J, Dai D, Amberg SM, Nunberg JH. pH-induced activation of arenavirus membrane fusion is antagonized by small-molecule inhibitors. J Virol. 2008;82:10932–10939. doi: 10.1128/JVI.01140-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Harrison LH, Halsey NA, McKee KT, Jr, Peters CJ, Barrera Oro JG, Briggiler AM, Feuillade MR, Maiztegui JI. Clinical case definitions for Argentine hemorrhagic fever. Clin Infect Dis. 1999;28:1091–1094. doi: 10.1086/514749. [DOI] [PubMed] [Google Scholar]
- 41.Maiztegui JI, Fernandez NJ, de Damilano AJ. Efficacy of immune plasma in treatment of Argentine haemorrhagic fever and association between treatment and a late neurological syndrome. Lancet. 1979;2:1216–1217. doi: 10.1016/s0140-6736(79)92335-3. [DOI] [PubMed] [Google Scholar]
- 42.Enria DA, Briggiler AM, Fernandez NJ, Levis SC, Maiztegui JI. Importance of dose of neutralising antibodies in treatment of Argentine haemorrhagic fever with immune plasma. Lancet. 1984;2:255–256. doi: 10.1016/s0140-6736(84)90299-x. [DOI] [PubMed] [Google Scholar]
- 43.Ruggiero HA, Perez Isquierdo F, Milani HA, Barri A, Val A, Maglio F, Astarloa L, Gonzalez Cambaceres C, Milani HL, Tallone JC. Treatment of Argentine hemorrhagic fever with convalescent’s plasma. 4433 cases. Presse Med. 1986;15:2239–2242. [PubMed] [Google Scholar]
- 44.Sanchez A, Pifat DY, Kenyon RH, Peters CJ, McCormick JB, Kiley MP. Junin virus monoclonal antibodies: characterization and cross-reactivity with other arenaviruses. J Gen Vir. 1989;70(Pt 5):1125–1132. doi: 10.1099/0022-1317-70-5-1125. [DOI] [PubMed] [Google Scholar]
- 45.Radoshitzky SR, Kuhn JH, de Kok-Mercado F, Jahrling PB, Bavari S. Drug discovery technologies and strategies for Machupo virus and other New World arenaviruses. Expert Opin Drug Discov. 2012;7:613–632. doi: 10.1517/17460441.2012.687719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Golden JW, Maes P, Kwilas SA, Ballantyne J, Hooper JW. Glycoprotein-specific antibodies produced by DNA vaccination protect guinea pigs from lethal Argentine and Venezuelan hemorrhagic fever. J Virol. 2016 doi: 10.1128/JVI.02969-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Maiztegui JI, McKee KT, Jr, Barrera Oro JG, Harrison LH, Gibbs PH, Feuillade MR, Enria DA, Briggiler AM, Levis SC, Ambrosio AM, et al. Protective efficacy of a live attenuated vaccine against Argentine hemorrhagic fever. AHF Study Group. J Infect Dis. 1998;177:277–283. doi: 10.1086/514211. [DOI] [PubMed] [Google Scholar]
- 48.Olschlager S, Flatz L. Vaccination strategies against highly pathogenic arenaviruses: the next steps toward clinical trials. PLoS Pathog. 2013;9:e1003212. doi: 10.1371/journal.ppat.1003212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kotturi MF, Botten J, Sidney J, Bui HH, Giancola L, Maybeno M, Babin J, Oseroff C, Pasquetto V, Greenbaum JA, et al. A multivalent and cross-protective vaccine strategy against arenaviruses associated with human disease. PLoS Pathog. 2009;5:e1000695. doi: 10.1371/journal.ppat.1000695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cheng BY, Ortiz-Riano E, de la Torre JC, Martinez-Sobrido L. Arenavirus Genome Rearrangement for the Development of Live Attenuated Vaccines. J Virol. 2015;89:7373–7384. doi: 10.1128/JVI.00307-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cheng BY, Ortiz-Riano E, Nogales A, de la Torre JC, Martinez-Sobrido L. Development of live-attenuated arenavirus vaccines based on codon deoptimization. J Virol. 2015;89:3523–3533. doi: 10.1128/JVI.03401-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McCormick JB, King IJ, Webb PA, Scribner CL, Craven RB, Johnson KM, Elliott LH, Belmont-Williams R. Lassa fever. Effective therapy with ribavirin. N Engl J Med. 1986;314:20–26. doi: 10.1056/NEJM198601023140104. [DOI] [PubMed] [Google Scholar]
- 53.Clayton AJ. Lassa immune serum. Bull World Health Organ. 1977;55:435–439. [PMC free article] [PubMed] [Google Scholar]
- 54.Jahrling PB, Frame JD, Rhoderick JB, Monson MH. Endemic Lassa fever in Liberia. IV. Selection of optimally effective plasma for treatment by passive immunization. Trans R Soc Trop Med Hyg. 1985;79:380–384. doi: 10.1016/0035-9203(85)90388-8. [DOI] [PubMed] [Google Scholar]
- 55.Lukashevich IS, Carrion R, Jr, Salvato MS, Mansfield K, Brasky K, Zapata J, Cairo C, Goicochea M, Hoosien GE, Ticer A, et al. Safety, immunogenicity, and efficacy of the ML29 reassortant vaccine for Lassa fever in small non-human primates. Vaccine. 2008;26:5246–5254. doi: 10.1016/j.vaccine.2008.07.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fisher-Hoch SP, Hutwagner L, Brown B, McCormick JB. Effective vaccine for lassa fever. J Virol. 2000;74:6777–6783. doi: 10.1128/jvi.74.15.6777-6783.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sommerstein R, Flatz L, Remy MM, Malinge P, Magistrelli G, Fischer N, Sahin M, Bergthaler A, Igonet S, Ter Meulen J, et al. Arenavirus Glycan Shield Promotes Neutralizing Antibody Evasion and Protracted Infection. PLoS Pathog. 2015;11:e1005276. doi: 10.1371/journal.ppat.1005276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Crispin M, Doores KJ. Targeting host-derived glycans on enveloped viruses for antibody-based vaccine design. Curr Opin Virol. 2015;11:63–69. doi: 10.1016/j.coviro.2015.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pejchal R, Doores KJ, Walker LM, Khayat R, Huang PS, Wang SK, Stanfield RL, Julien JP, Ramos A, Crispin M, et al. A potent and broad neutralizing antibody recognizes and penetrates the HIV glycan shield. Science. 2011;334:1097–1103. doi: 10.1126/science.1213256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Crispin M, Bowden TA. Antibodies expose multiple weaknesses in the glycan shield of HIV. Nat Struct Mol Biol. 2013;20:771–772. doi: 10.1038/nsmb.2627. [DOI] [PubMed] [Google Scholar]
- 61**.Snell NJ. Ribavirin--current status of a broad spectrum antiviral agent. Expert Opin Pharmacother. 2001;2:1317–1324. doi: 10.1517/14656566.2.8.1317. This paper reports the glycan dependence of the antibody response against LASV. The presence of individual glycans in LASV impede the development of a robust antibody response. This has important implication for guiding the development of effective vaccines against arenaviruses. [DOI] [PubMed] [Google Scholar]
- 62*.Chang J, Warren TK, Zhao X, Gill T, Guo F, Wang L, Comunale MA, Du Y, Alonzi DS, Yu W, et al. Small molecule inhibitors of ER alpha-glucosidases are active against multiple hemorrhagic fever viruses. Antiviral Res. 2013;98:432–440. doi: 10.1016/j.antiviral.2013.03.023. This paper describes the antiviral properties of small molecule drugs targeting viral biogenesis at the stage of glycoprotein folding. [DOI] [PMC free article] [PubMed] [Google Scholar]