Abstract
Fungi encounter numerous stresses in a mammalian host, including the immune system, which they must adapt to in order to grow and cause disease. The host immune system tunes its response to the threat level posed by the invading pathogen. We discuss recent findings on how interleukin (IL)-1 signaling is central to tuning the immune response to the virulence potential of invasive fungi, as well as other pathogens. Moreover, we discuss fungal factors that may drive tissue invasion and destruction that regulate IL-1 cytokine release. Moving forward understanding the mechanisms of fungal adaption to the host, together with understanding how the host innate immune system recognizes invading fungal pathogens will increase our therapeutic options for treatment of invasive fungal infections.
INTRODUCTION
Fungi are ubiquitous in nature and, for the most part, are harmless to the majority of individuals. However, it is estimated that 2 million cases of invasive mycoses are reported worldwide each year [1]. These invasive mycoses occur primarily in immunocompromised patients. Thus, in the absence of an adequate innate host defense, these opportunistic pathogens can infect the host and lead to disease. Furthermore, invasive fungal infections continue to be a rapidly emerging and serious threat because of the growing immunocompromised population and the emergence of drug resistance [1].
Candida spp. and Aspergillus spp. are known to cause approximately 30% of all invasive fungal infections [1]. However, the environmental niche filled by Candida spp. and Aspergillus spp. substantially differs, which could drive evolution of distinct adaptation traits necessary for virulence. Candida spp. are found as a normal commensal component of the human skin, gastrointestinal tract and other mucosal surfaces [2], whereas Aspergillus spp. are saprophytic molds found in the environment on decaying organic material [3]. Hundreds of species exist within the Candida and Aspergillus genera, however only a handful have been shown to cause invasive mycoses in humans. Even though mortality rates for invasive fungal infections have significantly decreased in the past decade [4], mortality rates from IC and IA are unacceptably high, ranging anywhere from 20–50% due to limited diagnostic tools and lack of effective treatment options [4–9]. Thus, novel therapeutic targets for anti-fungal drugs are desperately needed. Fungal factors driving adaption and growth in the mammalian host offer great potential as novel anti-fungal targets. In addition, tuning the host inflammatory response to confer optimal host resistance is another exciting avenue for limiting invasive fungal infections.
Innate immunity is essential for resistance against A. fumigatus and C. albicans. Patients with primary immunodeficiencies in the NADPH oxidase complex, STAT3 signaling pathway, CARD9 signaling pathway, IL-17 immunity, leukocyte adhesion deficiencies, and those with severe congenital neutropenia have been shown to be predisposed to developing invasive fungal infections (reviewed in [10] and [11]). Moreover, polymorphisms in numerous innate immune sensing and signaling pathways alter the susceptibility of transplant patients to developing invasive fungal disease (reviewed in [11], [12] and [13]). In this review, we discuss the importance of interleukin-1 (IL-1) in tuning the inflammatory response in the context of invasive fungal disease. Moreover, we highlight the potential importance of this model broadly across the spectrum of infectious diseases. We highlight recent data which demonstrate that the mammalian innate immune system responds in a regulated manner that is tuned to the level of growth, virulence, and pathology induced by the fungal pathogen.
ALARMINS/DAMAGE-ASSOCIATED MOLECULAR PATTERNS (DAMPs) VERSUS MICROBIAL-ASSOCIATED MOLECULAR PATTERNS (PAMPs)
The innate immune system provides an essential early response to microbial infection. Initial sensing of microbes has been well established to be mediated by a series of germline-encoded host pattern-recognition receptor (PRR) families, including Toll-like receptors (TLRs), C-type lectin receptors (CLRs), Nod-like receptors (NLRs), and RIG-I-like receptors (RLRs), which can recognize conserved microbial structures termed microbial-associated molecular patterns (MAMPs). Examples of MAMPs of particular relevance to fungal pathogens include β-1,3-glucan, chitin, mannans, mannoproteins, and unmethylated DNA. However, all fungi whether pathogenic to the host or not will express these MAMPs. To address the conceptual problem of pathogen versus commensal organism, Vance and colleagues have proposed that it is not just the MAMP that is critical for immune cell activation, but also its location within the host and/or cell, which they termed the “patterns of pathogenesis” [14]. These early pathogenic signatures will instigate the inflammatory response following fungal exposure. During the course of invasive fungal infections, fungal growth and invasion into the body, together with the host immune response will cause significant tissue damage, extracellular matrix destruction, and cell death at the site of infection. Tissue destruction and cell death during infection is highly inflammatory due to the release of damage-associated molecular patterns (DAMPs) or alarmins from the dying host cells [15, 16]. These alarmins include both non-protein materials, such as ATP and uric acid, as well as proteins, which include IL-1α, IL-33, S100 proteins, and HMGB1. Thus, during invasive fungal infections initial inflammation will be regulated by PRRs, but if that response is insufficient to prevent invasive growth, alarmin release due to tissue destruction and host cell death will amplify the magnitude of the inflammatory response to attempt to regain control (Figure 1).
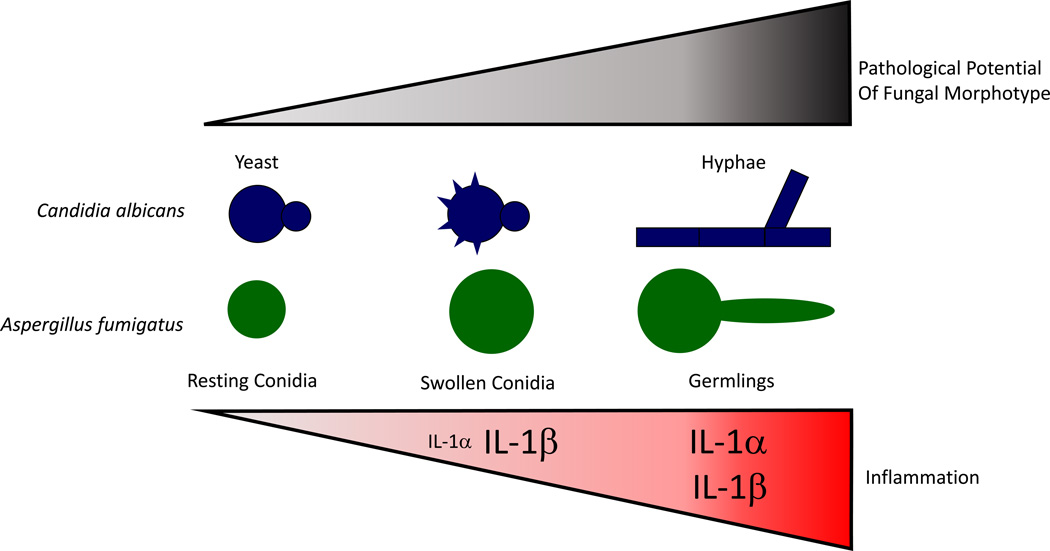
Figure 1. Escalating IL-1 inflammatory response regulates mammalian resistance to invasive, pathological fungal infection.
In homeostatic conditions when the barrier to mucosal surfaces is intact, C. albicans and A. fumigatus can be found in a resting state on mucosal surfaces and in the airways, respectively. Once this primary immune barrier is breached, fungi can access nutrients necessary for growth. In the case of C. albicans, rearrangement of the cell wall occurs during phagocytosis, while in the case of Aspergillus spp. the cell wall architecture is changed upon conidial swelling. In either case, fungal MAMPs are revealed to the immune system resulting in the activation of an inflammasome-dependent immune responses to clear the fungal threat. If the fungi continue to grow, forming invasive hyphae and expressing hyphal-specific effectors, including GAG, secondary metabolites, and proteases, robust tissue pathology can result. This increased pathology drives elevated levels of pro-inflammatory cytokines and alarmin release, such as IL-1α, which intensify the innate immune response in hope of clearing the infection. Font size for each cytokine is indicative of their relative abundance.
BASICS OF THE IL-1 CYTOKINES IN INFLAMMATION
The IL-1 gene cluster codes for the cytokines IL-1α and IL-1β, as well as the IL-1 receptor antagonist (IL-1ra), all three of which can bind to the IL-1 receptor, type I (IL-1RI) [17]. While IL-1α and IL-1β are pro-inflammatory cytokines, IL-1ra competitively binds the IL-1RI to dampen the immune response [17]. Although IL-1α and IL-1β belong to the same cytokine family, they rely on different proteases and cell death pathways for their secretion. IL-1β is produced as an inactive precursor termed pro-IL-1β that must first be transcriptionally upregulated [18]. For secretion, Pro-IL-1β must then be cleaved by a caspase-1 or caspase-8 containing inflammasome, which is formed following NLR activation [18]. Ultimately, IL-1β is released by pryoptotic cell death, which is mediated by gasdermin D [19–21]. Alternatively, pro-IL-1β can be cleaved by neutrophil-derived proteases, such as elastase, cathepsin G, proteinase 3, and granzyme A, which might be important in neutrophil-rich inflammatory settings, such as those observed during later stages of invasive fungal infections [22]. Unlike IL-1β, pro-IL-1α is constitutively expressed in cells. IL-1α can be released either as pro-IL-1α or mature IL-1α after calpain cleavage, but in either form it can bind to IL-1RI to initiate signaling [17, 23]. IL-1α is typically released during highly pathological situations, including necrotic and necroptotic cell death [17, 24]. Biologically, IL-1α and IL-1β can have different inflammatory activities in certain inflammatory settings [25–27], including during fungal infections [28, 29]. This was most pointedly demonstrated by the work of Rider et al which demonstrated that IL-1α released by cells undergoing necrotic cell death is necessary for neutrophil accumulation to initiate a sterile inflammatory response, while later IL-1β release promotes macrophage accumulation [26]. However, the distinct roles of IL-1α and IL-1β during infections is only beginning to be appreciated.
ROLE OF IL-1α and IL-1β DURING INVASIVE FUNGAL INFECTIONS
Candida albicans
IL-1 signaling is essential for immunity against candidiasis, as immune competent Il1r1-deficient and Il1a/Il1b-deficient mice are both highly susceptible to C. albicans infection [28, 30]. Moreover, treatment of neutropenic mice with either recombinant IL-1α or IL-1β was able to partially protect those mice [31]. Interestingly, IL-1α and IL-1β serve non-redundant roles in the immune response against systemic candidiasis (Table 1) [28]. Specifically, IL-1β is needed for optimal neutrophil recruitment, whereas IL-1α was needed to enhance anti-fungal activity of neutrophils [28].
TABLE 1.
Functions of IL-1α and IL-1β during C. albicans and A. fumigatus infection.
| IL-1α | IL-1β | |
|---|---|---|
| Recombinant IL-1α increases survival in a neutropenic mouse model of invasive candidiasis [31] |
Recombinant IL-1β increases survival in a neutropenic mouse model of invasive candidiasis [31] |
|
| C. albicans | Endogenous IL-1α needed for optimal antifungal activity of neutrophils against pseudohyphae, and Th1 response [28] |
Endogenous IL-1β needed for recruitment of neutrophils, superoxide generation, and Th1 response [28] |
| NLRP3 and NLRC4-dependent IL-1β production [32–35] |
||
| IL-1α needed for neutrophil recruitment [29] | IL-1β needed for optimal antifungal activity of macrophages [29] |
|
| A. fumigatus | NLRP3- and AIM2- dependent IL-1β production [47, 49, 50] |
|
| NLRP3-dependent neutrophil recruitment [47, 49] |
When activated by C. albicans, pro-IL-1β transcription is regulated through a Syk-Card9 pathway and IL-1β release is dependent on the NLRP3 and NLRC4 inflammasomes [32–35]. Mice lacking either inflammasome are highly susceptible to disseminated candidiasis [32–35]. Initially, NLRP3 activation in macrophages was thought to be dependent on the morphological switch of C. albicans from yeast to filamentous form, but is independent of the actual filament because both pseudohyphae and hyphae are capable of inducing IL-1β secretion [34]. More recent data from screening multiple C. albicans genetic mutant libraries demonstrate that filamentation is not sufficient for inflammasome activation to drive IL-1β release and pyroptosis [36–38]. These screens were able to identify both mutants that could filament, but not cause pyroptosis and mutants that were unable to filament, but could still drive pyroptosis. Interestingly, heat-killed C. albicans could not activate macrophage pyroptosis, but if the C. albicans that was previously phagocytized by macrophages for ~1h were heat-killed, they could induce robust pyroptosis [38]. Taken together, these data led Cowen and colleagues to postulate that C. albicans remodels its cell surface in response to macrophage phagocytosis. In an elegant experiment, Cowen and colleagues treated previously phagocytized C. albicans with the Endo H glycosidase prior to heat killing and observed a complete loss of pyroptosis, suggesting highly mannosylated surface proteins were required to induce macrophage pyroptosis [38]. In addition to this early pyroptotic cell death, C. albicans can also induce macrophage cell death in a pyroptosis-independent manner at later times that is highly dependent on the presence of fungal filaments, which likely are mechanically piercing the cell [39]. Whether these two phases of macrophage cell death result in differential activation and release of IL-1α and IL-1β has not been explored.
These prior studies focused largely on the interaction of C. albicans with macrophages. C. albicans is a normal member of the mucosal microbiota and, thus, would have to invade the epithelium to cause diseases. Thus, the transition of Candida spp. from the yeast form into the filamentous form can be thought of as an important virulence determinant of the pathogen, enabling it to invade through the epithelium and grow within the host [40, 41], but likely would also cause significant tissue pathology due to destruction of the epithelium barrier and alter the inflammatory environment. Interestingly, in an in vitro oral candidiasis model in which human epithelial cells were infected with Candida spp. or C. albicans mutants that either can or cannot form hyphae, IL-1α expression was increased during the infections with Candida spp. that are able to form hyphae [42–44]. However, a thorough dissection of this oral epithelium model with the C. albicans mutant libraries, as was done in the IL-1β and pyroptosis studies discussed above, has not been undertaken to examine how IL-1α release is controlled. Taken together, these studies support our model that fungal morphotype can influence the release of alarmins, specifically IL-1α, by creating a high-threat environment in which a quick response by neighboring cells is critical, and prevention of collateral damage is not as important as clearing the threat.
Aspergillus fumigatus
IL-1 signaling is essential to control A. fumigatus growth, but controversy still exists on the relative importance of IL-1α and IL-1β following A. fumigatus challenge (Table 1) [29, 45– 48]. Our group has shown that IL-1α was required for neutrophil recruitment after pulmonary challenge with A. fumigatus, while the inflammasome and IL-1β were necessary for optimal fungicidal activity of macrophages [29]. However, others have shown the inflammasome and IL-1β are necessary for neutrophil recruitment following A. fumigatus challenge [47, 49]. Both the NLRP3 and AIM2 inflammasomes work in concert to generate an optimal anti-Aspergillus response [47, 49]. At least in vitro, activation of the NLRP3 inflammasome by A. fumigatus is greatest by hyphal fragments [50]. However, compared to Candida, there is a lack of data concerning how distinct fungal morphotypes of Aspergillus spp. may relate to overall pathology and inflammation. It is important to note that in the above mentioned studies in which discrepancies concerning IL-1 dependency arise, different A. fumigatus strains and morphotypes were used [29, 47, 49]. Interestingly, it is rapidly emerging that different strains of A. fumigatus can induce both dramatically different inflammatory responses [51, 52] and differing levels of virulence in vivo [52–54]. Interestingly, Rizzetto et al showed that the CEA10 isolate of A. fumigatus induced the greatest inflammation, which corresponded with its increased growth in immune competent mice [52]. This raises the possibility that different strains of A. fumigatus might undergo differential growth in the respiratory tract which will induce varying degrees of tissue pathology, resulting in the differential release of IL-1α and IL-1β, or generally alter the inflammatory response.
Invasive aspergillosis is a spectrum of diseases in the clinic. In neutropenic hosts, pathology is driven by excessive fungal growth [55], whereas in hosts immunosuppressed with steroids, patients with chronic granulomatous disease (CGD), or patients with cystic fibrosis, pathology is driven by an inflammatory response that appears out of proportion to hyphal growth in diseased tissues [55–57], which might alter the type of immunotherapeutic intervention one might consider (Figure 2). With this prior observation in mind, van de Veerdonk and colleagues explored whether immunotherapeutic intervention with anakinra (recombinant hIL-1Ra) to decrease inflammation could ameliorate IA in the murine model of X-linked CGD. Interestingly, blockade of IL-1 signaling using anakinra (recombinant hIL-1Ra) in the murine model of CGD significantly blunted IA severity [58]. Similarly, anakinra (recombinant hIL-1Ra) treatment of Cftr−/− mice, which are more susceptible to IA [59], also resulted in an amelioration of Aspergillus-induced mortality that was associated with decreased fungal growth and inflammation [57]. In both cases, the authors have not specifically addressed whether excessive IL-1α and/or IL-1β signaling was responsible for disease caused in these IA models. Interestingly, cells from CGD patients are known to secrete significantly greater amounts of IL-1α and IL-1β in response to inflammatory stimuli [58, 60]. Considering the earlier finding that neutropenic mice infected with C. albicans and subsequently treated with either recombinant IL-1α or IL-1β were partially protected from disease [31], it will be intriguing to assess whether similar cytokine therapies might enhance protective immune responses in neutropenic models of IA. Overall, it is possible that Aspergillus spp. might induce varying degrees of tissue pathology in each of the clinically relevant models of IA, resulting in the differential release of IL-1α and IL-1β and, therefore, may require unique therapeutic interventions (Figure 2).
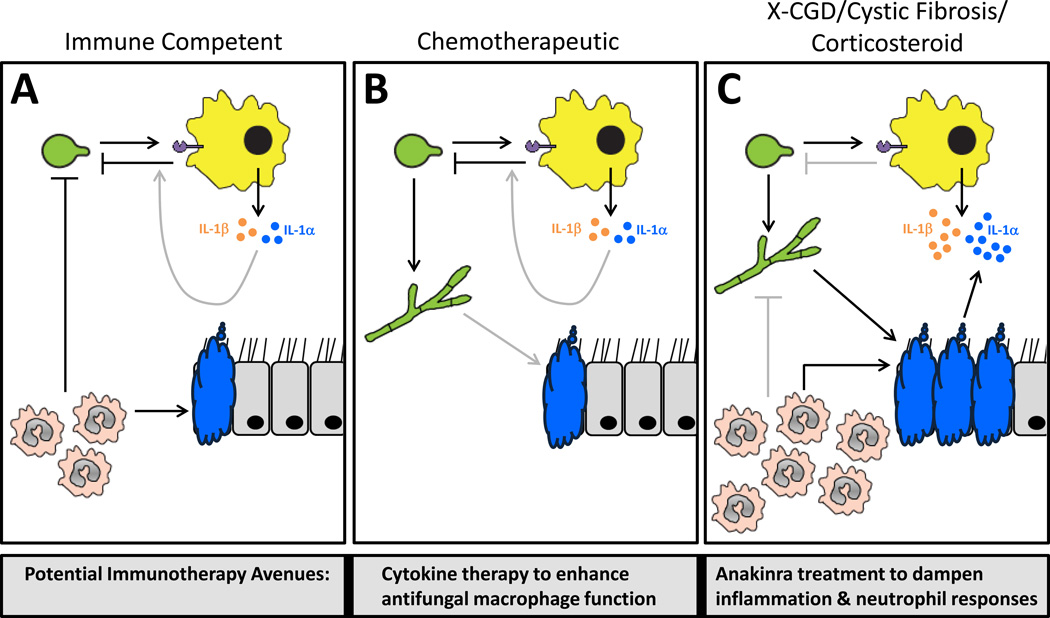
Figure 2. Differential tissue damage and IL-1 mediated inflammation in clinical models of IA will necessitate different immunotherapeutic interventions.
(A) In immune competent hosts, germinating Aspergillus conidia are rapidly recognized by tissue-resident leukocytes, which results in the release of IL-1α and IL-1β and the recruitment of neutrophils to the site of infection. Neutrophils then exert their powerful antifungal effects to limit Aspergillus growth. Additionally, IL-1α/β may work to enhance the antifungal functions of macrophages. (B) In neutropenic hosts, typically due to cyclophosphamide treatment, the lack of neutrophils allows extensive fungal growth. In these hosts, macrophages will still exert their antifungal function, but this is obviously not sufficient to prevent IA. In this population it would likely be advantageous to treat with inflammatory cytokines to further enhance monocyte/macrophage recruitment to the respiratory tract and/or their antifungal effector functions. (C) In CGD patients, cystic fibrosis patients, and individuals treated with high-doses of corticosteroids there is significant inflammation, neutrophil recruitment, and tissue damage associated with IA. However, due likely to impaired antifungal activity of the monocytes/macrophages and neutrophils in these populations, the host is unable to effectively clear the Aspergillus leading to continued inflammation. In these populations it has been shown to be advantageous to treat with anti-inflammatory compounds, such as anakinra, in order to limit inflammation, neutrophil recruitment, and tissue damage to the respiratory tract.
FUNGAL TARGETS THAT CONTRIBUTE TO TUNING HOST PATHOLOGY, CELL DEATH, AND IL-1 IMMUNITY
During invasive fungal infections, the initial fungal morphotype that establishes an infection within the mammalian host likely is not the most inflammatory fungal morphotype. This is especially true with A. fumigatus as the conidia have a hydrophobin layer that is immunologically inert and is responsible for hiding the underlying carbohydrate cell wall from the immune system [61]. Upon seeding the environmentally exposed mucosa of the mammalian host, both A. fumigatus and C. albicans encounter the mucosal epithelium which provides a barrier against growth inside the host. It is recognized that invasion of the host is typically mediated by a transition to filamentous growth, which also corresponds with immunogenicity of the fungi. Thus, understanding both the molecular switch(s) for filamentous growth and its resultant changes in fungal biology will be essential for limiting tissue pathology and inflammation associated with invasive growth.
During the transition to filamentous growth, rearrangement of cell wall components is critical for immune activation through CLRs and NLRP3 inflammasome activation [34, 38, 50]. β-1,3-glucans have been shown to induce both pro-IL1β expression, as well as induce its maturation by the NLRP3 inflammasome [62]. More recently, highly mannosylated cell wall proteins in C. albicans were determined to induce macrophage pyroptosis through inflammasome activation [38]. Specific to A. fumigatus, a novel cell wall carbohydrate, galactosaminogalactan (GAG), has emerged as an important virulence determinant that can also regulate IL-1 signaling and cell death [58, 63–65]. GAG is not expressed by resting conidia, but is highly expressed on germtubes and hyphae [65]. GAG also exists in both acetylated and deacetylated forms in the cell wall [66]. GAG has several immunomodulatory functions. First, it masks β-1,3-glucans from immune recognition in the hyphal cell wall [63, 67]. Second, GAG can induce IL-1ra, which inhibits IL-1 signaling and ultimately impairs neutrophil recruitment to the site of infection [58], though others have not observed this [64]. The reason for these discrepant findings remains to be elucidated, but the experimental design differed significantly between those studies. Specifically, Latgé and colleagues have exogenously treated mice with urea-soluble GAG [58, 65], while Sheppard and colleagues expressed the A. fumigatus Uge3 enzyme in Aspergillus nidulans, which typically cannot make GAG [64]. Third, GAG contributes to resistance against NADPH-oxidase induced neutrophil extracellular traps (NETs) [64]. Finally, GAG induces cell death in both epithelial cells and peripheral blood neutrophils, but the mechanism(s) of cell death induced remains inconclusive [63, 65]. GAG is critical for A. fumigatus adherence to epithelial cells, which is likely important for its induction of cell death [63, 67]. Moreover, it has recently been shown that the deacetylated form of GAG is necessary for adhesion and virulence [66]. Thus, a greater understanding of fungal cell wall carbohydrate and glycoprotein expression, biosynthesis, and exposure on distinct fungal morphotypes is necessary for understanding cell adhesion, invasive growth, cell death, and production of the IL-1 cytokine family during invasive fungal infections.
The founding infectious fungi must also rapidly adapt to the environmental and nutritional stresses of its mammalian niche to grow. Among all eukaryotic organisms, cAMP and MAP kinase pathways are conserved and required for the transcriptional regulation of cell growth and differentiation processes in response to specific extracellular stimuli. In both A. fumigatus and C. albicans it is well established that MAP kinase and cAMP signaling are necessary for full virulence [68]. Adaptation to neutral-alkaline pH environments that are encountered within the mammalian host is one environmental stress invasive fungi most overcome, and this has been shown to occur through Rim101/PacC signaling. The Rim101 mutant of C. albicans was unable to undergo the morphological change from yeast to hyphal growth when grown on basic media, and has been shown to have defects in cellular and tissue invasion in vitro and in vivo [69–72]. In an in vitro oral epithelium model of C. albicans infection, a Rim101 mutant was unable to induce as much cell damage, which corresponded with decreased expression of IL-1α, TNFα, and IL-8 compared to its parental or complemented strains [72]. Interestingly, the PacC mutant of A. fumigatus did not have a defect in hyphal growth, but PacC-mediated signaling was crucial for in vitro epithelial cell destruction and tissue invasion in a neutropenic murine model of IA [73]. In vitro, epithelial cell destruction by A. fumigatus occurred in a biphashic manner in which the early cell death (<16h) was dependent on direct fungal-epithelial cell contact, whereas later cell death (>16h) was partially dependent on a secreted protease that is antipain sensitive [73]. This later finding with the PacC-null mutant of A. fumigatus demonstrates that secreted factors that are expressed during hyphal growth are likely critical in host pathology and cell death, which will likely skew the inflammatory environment, including the IL-1 cytokines. In support of this, deletion of the transcription factor PrtT, which controls the expression of multiple Aspergillus proteases, resulted in an A. fumigatus mutant strain that was less cytotoxic to lung epithelial cells in in vitro assays [74]. While our understanding of the role of proteases in IA is limited, much more is known about the role of Aspergillus proteases in chronic airway disease and inflammation. Fungal proteases are sufficient to induce inflammation and allergic airway diseases [75]. A protease isolated from Aspergillus melleus was sufficient to induce allergic airway diseases through a unique TLR4-dependent inflammatory pathway that included increased expression of both IL-1α and IL-1β [75]. During invasive C. albicans infections, secreted aspartyl proteinases (SAPs) have been identified and are associated with hyphal formation, tissue damage, and virulence [76]. Moreover, internalized Sap2 and Sap6 could mediate NLRP3 inflammasome activation in human monocytes leading to IL-1β release [77]. Sap2 was also sufficient to drive IL-1β-dependent inflammation in a vaginitis model and contributed to the inflammation observed during C. albicans-induced vaginitis [78]. Thus, many more studies examining the role of extracellular proteases, as well as other secreted factors, are needed to understand invasive fungal disease, tissue pathology, cell death, and inflammation.
IS THERE A UNIVERSAL IMPORTANCE OF SENSING PATHOLOGICAL POTENTIAL OF PATHOGENS: LESSONS FROM BACTERIA AND VIRUSES?
Bacteria
This idea of an escalating immune response based on the level of threat posed to the immune system has also been demonstrated in the context of bacterial infections. Both Pseudomonas aeruginosa and Staphylococcus aureus can cause highly inflammatory pulmonary disease resulting in substantial damage and destruction of the lung tissue. Highly virulent strains of P. aeruginosa possess a cytotoxin named ExoU that is known to cause epithelial damage and necrotic cell death [79, 80]. Infection with exoU-encoding strains results in neutrophil recruitment that is dependent on IL-1α, but when the exoU gene was deleted, neutrophil recruitment switched to be dependent on IL-1β signaling [81]. Similarly, during infection with the highly virulent USA300 strain of S. aureus, it has been shown that bacterial toxins induce necroptosis of host cells, which largely contributes to the highly inflammatory pathology seen during S. aureus-induced pneumonia [82]. Thus, it appears that highly pathological bacterial strains that encode cytotoxic exotoxins can drive rapid necrotic host cell death which correlated with IL-1α release from the dying cells.
Viruses
New viral virions can bud from infected cells either in a cytopathic (lytic) or non-cytopathic manner. During cutaneous infection with cytopathic herpes simplex virus-1 (HSV-1), IL-1α is essential for leukocyte recruitment and restriction of viral dissemination [83]. Likewise, in a model of disseminated adenovirus infection, cooperation between IL-1α signaling and the complement system were shown to induce neutrophil recruitment and subsequent clearance of virus-containing macrophages [84]. In this particular system, the presence of a mutated viral protease p23, in which the virus cannot induce endosomal rupture, IL-1α levels were significantly lower resulting in an overall reduced inflammatory response [85]. Finally, in pigs experimentally infected with the cytopathic porcine reproductive and respiratory syndrome virus (PPRSV), the degree of lung pathology observed 7 days after challenge correlated with IL-1α expression [86]. This suggests that host cell damage and IL-1α release is a central part of activating the antiviral immune response to viruses that have cytopathic effects.
CONCLUDING REMARKS
As highlighted in this review, IL-1 signaling is a critical signaling hub in host defense against infectious disease, spanning fungi, bacteria and viruses. In the context of innate anti-fungal immunity, we propose a model in which the immune system responds in an escalating manner, based on the threat posed by the specific invading pathogen in accordance to its virulence and pathological potential (Figure 1). The first level of protection lies in the physical barrier functions of the epithelial layer, which prevents the pathogen from gaining access to the body. Additionally, tissue sentinel immune cells patrol the epithelial barrier and can phagocytose and kill microbes with limited virulence potential, in an inflammasome and IL-1β-dependent manner. However, when a pathogen takes on a highly virulent phenotype, in which it can rapidly adapt to the new environment, it can cause epithelial cell destruction, tissue damage, and cell death. In this latter scenario, IL-1α will be released from host cells to act as a rapid danger or alarmin signal to surrounding cells. In this “last resort” scenario, limiting the amount of tissue damage caused by the immune system is less important than clearing the threat posed by the pathogen to the host. Overall, by understanding all the factors that contribute to increased virulence and high levels of damage to the host, we can discover novel therapeutic strategies to combat a number of infectious diseases.
HIGHLIGHTS.
IL-1α and IL-1β have non-redundant role immunity against invasive fungal infections
Pathogens that induce greater tissue damage tend to drive IL-1α release
Fungal effectors driving tissue pathology and damage largely remain unidentified
Manipulation of the host immune response may be a potential avenue to treat invasive fungal infections
Acknowledgments
This work was supported in part by institutional start-up funds and in part through the Dartmouth Lung Biology Center for Molecular, Cellular, and Translational Research Grant P30-GM106394 (PI: Bruce A. Stanton) and Center for Molecular, Cellular, and Translational Immunological Research Grant P30-GM103415 (PI: William R. Green).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Brown GD, et al. Hidden killers: human fungal infections. Sci Transl Med. 2012;4(165):165rv13. doi: 10.1126/scitranslmed.3004404. [DOI] [PubMed] [Google Scholar]
- 2.Underhill DM, Iliev ID. The mycobiota: interactions between commensal fungi and the host immune system. Nat Rev Immunol. 2014;14(6):405–416. doi: 10.1038/nri3684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Park SJ, Mehrad B. Innate Immunity to Aspergillus Species. Clin Microbiol Rev. 2009;22(4):535–551. doi: 10.1128/CMR.00014-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Upton A, et al. Invasive Aspergillosis following Hematopoietic Cell Transplantation: Outcomes and Prognostic Factors Associated with Mortality. Clinical Infectious Diseases. 2007;44(4):531–540. doi: 10.1086/510592. [DOI] [PubMed] [Google Scholar]
- 5.Marr KA, et al. Combination Antifungal Therapy for Invasive AspergillosisA Randomized TrialCombination Therapy for Invasive Aspergillosis. Annals of Internal Medicine. 2015;162(2):81–89. doi: 10.7326/M13-2508. [DOI] [PubMed] [Google Scholar]
- 6.Gudlaugsson O, et al. Attributable Mortality of Nosocomial Candidemia, Revisited. Clinical Infectious Diseases. 2003;37(9):1172–1177. doi: 10.1086/378745. [DOI] [PubMed] [Google Scholar]
- 7.González de Molina FJ, et al. Assessment of candidemia-attributable mortality in critically ill patients using propensity score matching analysis. Crit Care. 2012;16(3):R105. doi: 10.1186/cc11388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maertens JA, et al. Isavuconazole versus voriconazole for primary treatment of invasive mould disease caused by <em>Aspergillus</em> and other filamentous fungi (SECURE): a phase 3, randomised-controlled, non-inferiority trial. The Lancet. 387(10020):760–769. doi: 10.1016/S0140-6736(15)01159-9. [DOI] [PubMed] [Google Scholar]
- 9.Steinbach WJ, et al. Clinical epidemiology of 960 patients with invasive aspergillosis from the PATH Alliance registry. Journal of Infection. 65(5):453–464. doi: 10.1016/j.jinf.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 10.Lanternier F, et al. Primary immunodeficiencies underlying fungal infections. Curr Opin Pediatr. 2013;25(6):736–747. doi: 10.1097/MOP.0000000000000031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wojtowicz A, Bochud PY. Host genetics of invasive Aspergillus and Candida infections. Semin Immunopathol. 2015;37(2):173–186. doi: 10.1007/s00281-014-0468-y. [DOI] [PubMed] [Google Scholar]
- 12.Romani L. Immunity to fungal infections. Nat Rev Immunol. 2011;11(4):275–288. doi: 10.1038/nri2939. [DOI] [PubMed] [Google Scholar]
- 13.Smeekens SP, et al. Genetic susceptibility to Candida infections. EMBO Mol Med. 2013;5(6):805–813. doi: 10.1002/emmm.201201678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Vance RE, Isberg RR, Portnoy DA. Patterns of pathogenesis: discrimination of pathogenic and non-pathogenic microbes by the innate immune system. Cell Host Microbe. 2009;6(1):10–21. doi: 10.1016/j.chom.2009.06.007. **Well written review ariticle to presents a novel conceptual framework, the "Patterns of Pathogenesis, which enables us to better understand how the innate system potentially distinguishes pathogencity organism from the plethora of microbes typical found in and on a host.
- 15.Bianchi ME. DAMPs, PAMPs and alarmins: all we need to know about danger. Journal of Leukocyte Biology. 2007;81(1):1–5. doi: 10.1189/jlb.0306164. [DOI] [PubMed] [Google Scholar]
- 16.Stephenson HN, Herzig A, Zychlinsky A. Beyond the grave: When is cell death critical for immunity to infection? Current Opinion in Immunology. 2016;38:59–66. doi: 10.1016/j.coi.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 17.Garlanda C, Dinarello CA, Mantovani A. THE INTERLEUKIN-1 FAMILY: BACK TO THE FUTURE. Immunity. 2013;39(6):1003–1018. doi: 10.1016/j.immuni.2013.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Latz E, Xiao TS, Stutz A. Activation and regulation of the inflammasomes. Nat Rev Immunol. 2013;13(6):397–411. doi: 10.1038/nri3452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.He W, et al. Gasdermin D is an executor of pyroptosis and required for interleukin-1β secretion. Cell Res. 2015;25(12):1285–1298. doi: 10.1038/cr.2015.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kayagaki N, et al. Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature. 2015;526(7575):666–671. doi: 10.1038/nature15541. [DOI] [PubMed] [Google Scholar]
- 21.Shi J, et al. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature. 2015;526(7575):660–665. doi: 10.1038/nature15514. [DOI] [PubMed] [Google Scholar]
- 22.Netea MG, et al. Inflammasome-Independent Regulation of IL-1-Family Cytokines. Annual Review of Immunology. 2015;33(1):49–77. doi: 10.1146/annurev-immunol-032414-112306. [DOI] [PubMed] [Google Scholar]
- 23.Kim B, et al. The Interleukin-1α Precursor is Biologically Active and is Likely a Key Alarmin in the IL-1 Family of Cytokines. Front Immunol. 2013;4 doi: 10.3389/fimmu.2013.00391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wallach D, et al. Programmed necrosis in inflammation: Toward identification of the effector molecules. Science. 2016;352(6281) doi: 10.1126/science.aaf2154. [DOI] [PubMed] [Google Scholar]
- 25.Chen CJ, et al. Identification of a key pathway required for the sterile inflammatory response triggered by dying cells. Nat Med. 2007;13(7):851–856. doi: 10.1038/nm1603. [DOI] [PubMed] [Google Scholar]
- 26. Rider P, et al. IL-1alpha and IL-1beta recruit different myeloid cells and promote different stages of sterile inflammation. J Immunol. 2011;187(9):4835–4843. doi: 10.4049/jimmunol.1102048. **Important study demonstrating a differential roles of IL-1α and IL-1β in the inflammatory leukocyte response to necrotic cells.
- 27.Barry KC, et al. IL-1alpha signaling initiates the inflammatory response to virulent Legionella pneumophila in vivo. J Immunol. 2013;190(12):6329–6339. doi: 10.4049/jimmunol.1300100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vonk AG, et al. Endogenous interleukin (IL)-1 alpha and IL-1 beta are crucial for host defense against disseminated candidiasis. J Infect Dis. 2006;193(10):1419–1426. doi: 10.1086/503363. [DOI] [PubMed] [Google Scholar]
- 29. Caffrey AK, et al. IL-1α Signaling Is Critical for Leukocyte Recruitment after Pulmonary Aspergillus fumigatus Challenge. PLoS Pathog. 2015;11(1) doi: 10.1371/journal.ppat.1004625. *References 28 & 29 demonstrate that IL-1α and IL-1β have non-redundent function in the innate immune response to fungal pathogens.
- 30.Bellocchio S, et al. The Contribution of the Toll-Like/IL-1 Receptor Superfamily to Innate and Adaptive Immunity to Fungal Pathogens In Vivo. The Journal of Immunology. 2004;172(5):3059–3069. doi: 10.4049/jimmunol.172.5.3059. [DOI] [PubMed] [Google Scholar]
- 31.Van't Wout JW, et al. Protection of neutropenic mice from lethal Candida albicans infection by recombinant interleukin 1. European Journal of Immunology. 1988;18(7):1143–1146. doi: 10.1002/eji.1830180728. [DOI] [PubMed] [Google Scholar]
- 32.Gross O, et al. Syk kinase signalling couples to the Nlrp3 inflammasome for anti-fungal host defence. Nature. 2009;459(7245):433–436. doi: 10.1038/nature07965. [DOI] [PubMed] [Google Scholar]
- 33.Hise AG, et al. An essential role for the NLRP3 inflammasome in host defense against the human fungal pathogen Candida albicans. Cell Host Microbe. 2009;5(5):487–497. doi: 10.1016/j.chom.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Joly S, et al. Cutting edge: Candida albicans hyphae formation triggers activation of the Nlrp3 inflammasome. J Immunol. 2009;183(6):3578–3581. doi: 10.4049/jimmunol.0901323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tomalka J, et al. A novel role for the NLRC4 inflammasome in mucosal defenses against the fungal pathogen Candida albicans. PLoS Pathog. 2011;7(12):e1002379. doi: 10.1371/journal.ppat.1002379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wellington M, Koselny K, Krysan DJ. Candida albicans Morphogenesis Is Not Required for Macrophage Interleukin 1β Production. mBio. 2013;4(1) doi: 10.1128/mBio.00433-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wellington M, et al. Candida albicans Triggers NLRP3-Mediated Pyroptosis in Macrophages. Eukaryot Cell. 2014;13(2):329–340. doi: 10.1128/EC.00336-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. O’Meara TR, et al. Global analysis of fungal morphology exposes mechanisms of host cell escape. Nat Commun. 6 doi: 10.1038/ncomms7741. **Elegant us of a C. albicans mutant library screen to identify novel mutant that cannot activate the inflammasome and pyroptosis. Importantly, elegant follow-up studies on hits in the mutant library demonstrate that mannoprotein exposure, likely through cell wall rearrangement, upon phagocytosis is critical for inflammasome activation to drive pyroptosis
- 39. Uwamahoro N, et al. The Pathogen Candida albicans Hijacks Pyroptosis for Escape from Macrophages. mBio. 2014;5(2) doi: 10.1128/mBio.00003-14. *This work demonstrates that C. albicans not only drives early pyroptotic cell death in macrophages (which can be independent of filamentation), but can also induce a later necrotic cell death likely due to the mechical rupturing of the cells by hyphae.
- 40.Thompson DS, Carlisle PL, Kadosh D. Coevolution of Morphology and Virulence in Candida Species. Eukaryot Cell. 2011;10(9):1173–1182. doi: 10.1128/EC.05085-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang W, et al. Fungal invasion of epithelial cells. Microbiological Research. 2014;169(11):803–810. doi: 10.1016/j.micres.2014.02.013. [DOI] [PubMed] [Google Scholar]
- 42.Schaller M, et al. Infection of human oral epithelia with Candida species induces cytokine expression correlated to the degree of virulence. J Invest Dermatol. 2002;118(4):652–657. doi: 10.1046/j.1523-1747.2002.01699.x. [DOI] [PubMed] [Google Scholar]
- 43.Jayatilake JA, et al. IL-1alpha, IL-1ra and IL-8 are differentially induced by Candida in experimental oral candidiasis. Oral Dis. 2007;13(4):426–433. doi: 10.1111/j.1601-0825.2007.01318.x. [DOI] [PubMed] [Google Scholar]
- 44.Villar CC, Kashleva H, Dongari-Bagtzoglou A. Role of Candida albicans polymorphism in interactions with oral epithelial cells. Oral Microbiol Immunol. 2004;19(4):262–269. doi: 10.1111/j.1399-302X.2004.00150.x. [DOI] [PubMed] [Google Scholar]
- 45. Jhingran A, et al. Compartment-specific and sequential role of MyD88 and CARD9 in chemokine induction and innate defense during respiratory fungal infection. PLoS Pathog. 2015;11(1):e1004589. doi: 10.1371/journal.ppat.1004589. **Elegant study that demonstrates there are two waves of neutrophil recruitment which are necessary for resistance against invasive aspergillosis. The early wave is IL-1RI/MyD88-dependent, while the late wave is CARD9-dependent.
- 46.Bretz C, et al. MyD88 Signaling Contributes to Early Pulmonary Responses to Aspergillus fumigatus. Infect Immun. 2008;76(3):952–958. doi: 10.1128/IAI.00927-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Karki R, et al. Concerted activation of the AIM2 and NLRP3 inflammasomes orchestrates host protection against Aspergillus infection. Cell Host Microbe. 2015;17(3):357–368. doi: 10.1016/j.chom.2015.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Leal SM, et al. Distinct Roles for Dectin-1 and TLR4 in the Pathogenesis of Aspergillus fumigatus Keratitis. PLoS Pathog. 2010;6(7) doi: 10.1371/journal.ppat.1000976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gresnigt MS, et al. A polysaccharide virulence factor from Aspergillus fumigatus elicits anti-inflammatory effects through induction of Interleukin-1 receptor antagonist. PLoS Pathog. 2014;10(3):e1003936. doi: 10.1371/journal.ppat.1003936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Said-Sadier N, et al. Aspergillus fumigatus stimulates the NLRP3 inflammasome through a pathway requiring ROS production and the Syk tyrosine kinase. PLoS One. 2010;5(4):e10008. doi: 10.1371/journal.pone.0010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Amarsaikhan N, et al. Isolate-Dependent Growth, Virulence, and Cell Wall Composition in the Human Pathogen Aspergillus fumigatus. PLoS One. 2014;9(6) doi: 10.1371/journal.pone.0100430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Rizzetto L, et al. Strain Dependent Variation of Immune Responses to A. fumigatus: Definition of Pathogenic Species. PLoS One. 2013;8(2) doi: 10.1371/journal.pone.0056651. **Important study that demonstrates that inflammatory response to different A. fumigatus strains/isolates can be dramatic different. This is foundational to future mechanistic studies.
- 53.Mondon P, et al. Variation in virulence of Aspergillus fumigatus strains in a murine model of invasive pulmonary aspergillosis. Journal of Medical Microbiology. 1996;45(3):186–191. doi: 10.1099/00222615-45-3-186. [DOI] [PubMed] [Google Scholar]
- 54.Alshareef F, Robson GD. Genetic and virulence variation in an environmental population of the opportunistic pathogen Aspergillus fumigatus. Microbiology. 2014;160(4):742–751. doi: 10.1099/mic.0.072520-0. [DOI] [PubMed] [Google Scholar]
- 55. Balloy V, et al. Differences in Patterns of Infection and Inflammation for Corticosteroid Treatment and Chemotherapy in Experimental Invasive Pulmonary Aspergillosis. Infect Immun. 2005;73(1):494–503. doi: 10.1128/IAI.73.1.494-503.2005. *Bit older paper, but demonstrates an important clinically relavant observation that invasive aspergillosis in neutropenic host or those on corticosteroid differ dramatically.
- 56.Romani L, et al. Defective tryptophan catabolism underlies inflammation in mouse chronic granulomatous disease. Nature. 2008;451(7175):211–215. doi: 10.1038/nature06471. [DOI] [PubMed] [Google Scholar]
- 57.Iannitti RG, et al. IL-1 receptor antagonist ameliorates inflammasome-dependent inflammation in murine and human cystic fibrosis. Nat Commun. 2016;7 doi: 10.1038/ncomms10791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. de Luca A, et al. IL-1 receptor blockade restores autophagy and reduces inflammation in chronic granulomatous disease in mice and in humans. Proc Natl Acad Sci U S A. 2014;111(9):3526–3531. doi: 10.1073/pnas.1322831111. **References 57 & 58 are both important studies that show that X-CGD and Cftr−/− mice have exagerrated inflammatory responses to A. fumigatus. In both cases, blockade of IL-1 signaling using anakinra could partially ameriolate IA.
- 59.Iannitti RG, et al. Th17/Treg Imbalance in Murine Cystic Fibrosis Is Linked to Indoleamine 2,3-Dioxygenase Deficiency but Corrected by Kynurenines. American Journal of Respiratory and Critical Care Medicine. 2013;187(6):609–620. doi: 10.1164/rccm.201207-1346OC. [DOI] [PubMed] [Google Scholar]
- 60.Bagaitkar J, et al. NADPH oxidase controls neutrophilic response to sterile inflammation in mice by regulating the IL-1α/G-CSF axis. Blood. 2015;126(25):2724–2733. doi: 10.1182/blood-2015-05-644773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Aimanianda V, et al. Surface hydrophobin prevents immune recognition of airborne fungal spores. Nature. 2009;460(7259):1117–1121. doi: 10.1038/nature08264. [DOI] [PubMed] [Google Scholar]
- 62.Kankkunen P, et al. (1,3)-β-Glucans Activate Both Dectin-1 and NLRP3 Inflammasome in Human Macrophages. The Journal of Immunology. 2010;184(11):6335–6342. doi: 10.4049/jimmunol.0903019. [DOI] [PubMed] [Google Scholar]
- 63.Gravelat FN, et al. Aspergillus Galactosaminogalactan Mediates Adherence to Host Constituents and Conceals Hyphal β-Glucan from the Immune System. PLoS Pathog. 2013;9(8) doi: 10.1371/journal.ppat.1003575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lee MJ, et al. The Fungal Exopolysaccharide Galactosaminogalactan Mediates Virulence by Enhancing Resistance to Neutrophil Extracellular Traps. PLoS Pathog. 2015;11(10) doi: 10.1371/journal.ppat.1005187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Fontaine T, et al. Galactosaminogalactan, a New Immunosuppressive Polysaccharide of Aspergillus fumigatus. PLoS Pathog. 2011;7(11) doi: 10.1371/journal.ppat.1002372. **References 63–65 are an important series of studies that demonstrate a critical role of the newly identified GAG cell wall carbohydrate in virulence through modulation of the host innate immune response.
- 66.Lee MJ, et al. Deacetylation of Fungal Exopolysaccharide Mediates Adhesion and Biofilm Formation. mBio. 2016;7(2) doi: 10.1128/mBio.00252-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Beaussart A, et al. Nanoscale biophysical properties of the cell surface galactosaminogalactan from the fungal pathogen Aspergillus fumigatus. Nanoscale. 2015;7(36):14996–15004. doi: 10.1039/c5nr04399a. [DOI] [PubMed] [Google Scholar]
- 68.Shapiro RS, Robbins N, Cowen LE. Regulatory Circuitry Governing Fungal Development, Drug Resistance, and Disease. Microbiol Mol Biol Rev. 2011;75(2):213–267. doi: 10.1128/MMBR.00045-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bensen ES, et al. Transcriptional profiling in Candida albicans reveals new adaptive responses to extracellular pH and functions for Rim101p. Mol Microbiol. 2004;54(5):1335–1351. doi: 10.1111/j.1365-2958.2004.04350.x. [DOI] [PubMed] [Google Scholar]
- 70.Davis D, et al. Candida albicans RIM101 pH Response Pathway Is Required for Host-Pathogen Interactions. Infect Immun. 2000;68(10):5953–5959. doi: 10.1128/iai.68.10.5953-5959.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sanchez AA, et al. Relationship between Candida albicans Virulence during Experimental Hematogenously Disseminated Infection and Endothelial Cell Damage In Vitro. Infect Immun. 2004;72(1):598–601. doi: 10.1128/IAI.72.1.598-601.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Villar CC, et al. Invasive Phenotype of Candida albicans Affects the Host Proinflammatory Response to Infection. Infect Immun. 2005;73(8):4588–4595. doi: 10.1128/IAI.73.8.4588-4595.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Bertuzzi M, et al. The pH-Responsive PacC Transcription Factor of Aspergillus fumigatus Governs Epithelial Entry and Tissue Invasion during Pulmonary Aspergillosis. PLoS Pathog. 2014;10(10) doi: 10.1371/journal.ppat.1004413. *Important study demonstrating the PacC transcription factor of A. fumigatus is necessary for fungal adaption to the host microenvironment of the respiratory tract. Furthermore, it was necessary specifically for tissue invasive, induction of epithelial cell destruction, and fungal virulence.
- 74.Sharon H, Hagag S, Osherov N. Transcription factor PrtT controls expression of multiple secreted proteases in the human pathogenic mold Aspergillus fumigatus. Infect Immun. 2009;77(9):4051–4060. doi: 10.1128/IAI.00426-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Millien VO, et al. Cleavage of Fibrinogen by Proteinases Elicits Allergic Responses Through Toll-Like Receptor 4. Science. 2013;341(6147):792–796. doi: 10.1126/science.1240342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Naglik JR, Challacombe SJ, Hube B. Candida albicans secreted aspartyl proteinases in virulence and pathogenesis. Microbiol Mol Biol Rev. 2003;67(3):400–428. doi: 10.1128/MMBR.67.3.400-428.2003. table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pietrella D, et al. Secreted aspartic proteases of Candida albicans activate the NLRP3 inflammasome. European Journal of Immunology. 2013;43(3):679–692. doi: 10.1002/eji.201242691. [DOI] [PubMed] [Google Scholar]
- 78.Pericolini E, et al. Secretory Aspartyl Proteinases Cause Vaginitis and Can Mediate Vaginitis Caused by Candida albicans in Mice. mBio. 2015;6(3) doi: 10.1128/mBio.00724-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Finck-Barbancon V, et al. ExoU expression by Pseudomonas aeruginosa correlates with acute cytotoxicity and epithelial injury. Mol Microbiol. 1997;25(3):547–557. doi: 10.1046/j.1365-2958.1997.4891851.x. [DOI] [PubMed] [Google Scholar]
- 80.Hauser AR, Engel JN. Pseudomonas aeruginosa Induces Type-III-Secretion-Mediated Apoptosis of Macrophages and Epithelial Cells. Infect Immun. 1999;67(10):5530–5537. doi: 10.1128/iai.67.10.5530-5537.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Al Moussawi K, Kazmierczak BI. Distinct contributions of interleukin-1alpha (IL-1alpha) and IL-1beta to innate immune recognition of Pseudomonas aeruginosa in the lung. Infect Immun. 2014;82(10):4204–4211. doi: 10.1128/IAI.02218-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kitur K, et al. Toxin-induced necroptosis is a major mechanism of Staphylococcus aureus lung damage. PLoS Pathog. 2015;11(4):e1004820. doi: 10.1371/journal.ppat.1004820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Milora KA, et al. Interleukin-1α released from HSV-1 infected keratinocytes acts as a functional alarmin in the skin. Nat Commun. 2014;5:5230. doi: 10.1038/ncomms6230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Di Paolo NC, et al. IL-1alpha and complement cooperate in triggering local neutrophilic inflammation in response to adenovirus and eliminating virus-containing cells. PLoS Pathog. 2014;10(3):e1004035. doi: 10.1371/journal.ppat.1004035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Di Paolo NC, et al. Virus sensing at the Plasma Membrane Triggers Interleukin-1α–Mediated Pro-inflammatory Macrophage Response in vivo. Immunity. 2009;31(1):110–121. doi: 10.1016/j.immuni.2009.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Amarilla SP, et al. A comparative study of the local cytokine response in the lungs of pigs experimentally infected with different PRRSV-1 strains: Upregulation of IL-1α in highly pathogenic strain induced lesions. Veterinary Immunology and Immunopathology. 2015;164(3–4):137–147. doi: 10.1016/j.vetimm.2015.02.003. [DOI] [PubMed] [Google Scholar]