Abstract
Extracellular vesicles have recently emerged as a novel mode of viral propagation exploited by both enveloped and non-enveloped viruses. In particular non-enveloped viruses utilize the hosts' production of extracellular vesicles to exit from cells non-lytically and to hide and manipulate the immune system. Moreover, challenging the long held idea that viruses behave as independent genetic units, extracellular vesicles enable multiple viral particles and genomes to collectively traffic in and out of cells, which can promote genetic cooperativity among viral quasispecies and enhance the fitness of the overall viral population.
Introduction
A new mode of viral transmission
Many prokaryotic and eukaryotic cells release membrane-bound vesicles into their extracellular environment. Extracellular vesicles are a new form of cellular communication, enabling cells to modulate other cells within their immediate microenvironment as well as at a distance. These vesicles, which can range in size from tens to thousands of nanometers, carry cellular proteins, lipids and nucleic acids including growth factors, cytokines, chemokines, translation regulatory factors, toxins and micro RNAs between cells [1•]. They can be derived from a variety of cellular sources: direct budding from the plasma membrane to form ectosomes; by internalization of cytosol into late endosomes to form a multivesicular body (MVB) which can fuse with the plasma membrane to release small 100 nm vesicles termed exosomes [1•]; and from double-membraned autophagosomes fusing with the plasma membrane to release large (350–500 nm) single membrane vesicles [2,3]. Now a new role is emerging for extracellular vesicles, as transporters of viral particle and viral genome populations to new susceptible host cells (Figure 1).
Figure 1.
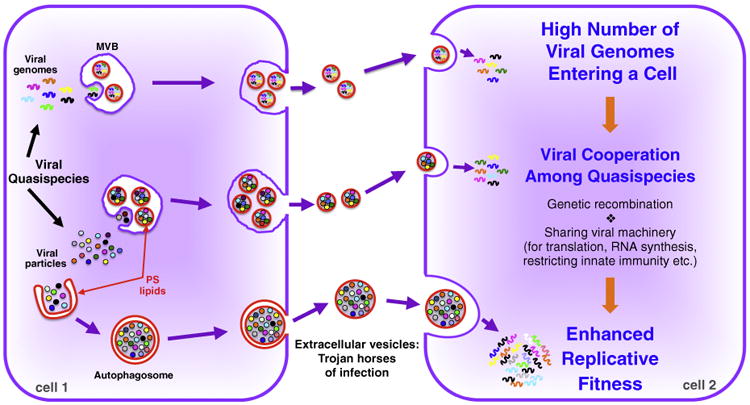
Diagram of extracellular vesicles mediating the non-lytic transmission of populations of virions and viral nucleic acids between two cells. RNA virus replication results in viral progeny that is a mixture of quasispecies. Extracellular vesicles with phosphatidylserine-enriched membranes, derived from multivesicular bodies (MVB) or from autophagosomes, capture populations of quasispecies from the cytoplasm, either in assembled viral particle form (e.g. poliovirus, HAV) or as naked viral RNA (e.g. HCV) (cell 1). These populations of quasispecies in vesicles are delivered to a new host cell (cell 2), giving rise to a high multiplicity of infection. This in turn enhances the replicative fitness of these quasispecies by promoting cooperative interactions among them including recombination among genomes, sharing viral machinery for translation, RNA synthesis, assembly as well as restricting the innate immune defenses of the host cell.
Viruses traffic within vesicles
Historically viral exit from cells has been described to take place essentially by one of two mechanisms. Enveloped viruses exit the cell by budding, thus picking up a host membrane to surround each capsid with. They can bud directly from the plasma membrane (e.g. HIV or Influenza virus) or bud into an exocytic host pathway (e.g. HCV) [4,5]. For this type of viral release the cell does not need to lyse even though it may eventually because of viral stress or immune cytotoxicity. In contrast, cells in culture infected with non-enveloped viruses, such as poliovirus, Coxsackievirus, rhinovirus, norovirus, and Hepatitis A virus (HAV) often lysed and given that electron microscopic and X-ray studies did not find membranes around the capsids [6], it was widely accepted that these viruses must disrupt the plasma membrane in order to exit the cell. This separation between enveloped and non-enveloped viruses became less clear when Lemon and colleagues reported in 2013 finding both enveloped and non-enveloped HAV particles in the extracellular medium of infected liver cells in culture and in the serum of HAV infected humans and monkeys [7••]. The assembled HAV particles were localized to MVBs inside cells and in vitro depletion of ESCRT proteins, which are required for exosome biogenesis [7••], blocked the envelopment and release of the viral particles, strongly suggesting that exosome biogenesis pathways were being exploited by HAV to exit cells. Another non-enveloped hepatitis virus, Hepatitis E virus (HEV), also was reported to exist in an enveloped state by releasing within exosomes [8].
Further studies on cells infected with poliovirus, Coxsackievirus and rhinovirus, revealed that these viruses also exited host cells in vesicles and before cell lysis [9,10,11••]. Surprisingly, the origins of these vesicles were not MVBs but rather autophagosomes [9,10,11••,12••]. Autophagosomes, are produced in all eukaryotic cells and autophagy is generally considered to be a bulk degradative pathway, important in times of cellular stress to degrade large quantities of cytoplasm and provide protein, lipid and carbohydrate building blocks to meet the needs of the cell [13]. The double-membraned autophagosome can be generated from a variety of membranes including the endoplasmic reticulum (ER), mitochondria and even the plasma membrane [14]. In the case of poliovirus, the membranes likely originated from the ER as they contained transmembrane resident ER proteins. Hence the intermembrane space of the double membraned structure was the former ER lumen and the encapsulated cytoplasm contained poliovirus [11••]. Notably these autophagosomes did not fuse with lysosomes but instead were targeted to and fused with the plasma membrane to release single membrane-bound vesicles containing viral particles [11••]. This process of ‘secretory autophagy’ has been observed in a variety of uninfected eukaryotic cells [2] and is implicated in the secretion of IL1β [15], synuclein [16] and amyloid β protein [17] as well as entire organelles. Its role in the latter in particular is critical during maturation of reticulocytes into red blood cells [18••].
Both the small exosomes containing HAV, HEV and the larger autophagosome-derived extracellular vesicles containing poliovirus, Coxsackievirus and rhinovirus, when isolated intact from the extracellular environment and added to new susceptible host cells, demonstrated a high degree of infectivity [7••,10,11••,19]. Notably for poliovirus, Coxsackievirus and rhinovirus, infections with vesicles were still receptor-mediated, that is viruses had to bind their respective receptors, suggesting that the vesicle membrane around the viral particles was disrupted after the vesicles engaged with the new host cell [7••,11••]. Moreover the autophagosome-derived vesicles that carried these viruses were all enriched in phosphatidylserine (PS) lipids [11••]. Masking the PS lipids on the vesicles, with specific PS-binding proteins, blocked the infection suggesting that recognition of the lipid by the host cell was an additional factor regulating viral tropism [11••]. A similar role for PS lipids had been previously invoked for bona fide enveloped viruses such as vaccinia during infection [20].
Only after engagement with the host cell did HAV and HEV particles within vesicles become sensitive to neutralizing antibodies, supporting the model of disruption of the vesicle membrane during infection [7••,19]. The mechanism of membrane disruption is currently unknown but recent findings with HEV suggest uptake of vesicles into acidified endosomes where endosomal lipases and lipid-extracting proteins (e.g. Niemann–Pick transporter) permeabilize the membrane [19]. Notably, many non-enveloped virus capsid proteins including the adenovirus protein VI, the rotavirus VP5 and the poliovirus VP4 proteins have intrinsic membrane disrupting activities that can be triggered upon acidification of their environment [21–23]. Hence upon engagement and internalization into an acidified compartment, the viral particles themselves may be able to directly facilitate lysis of their vesicle membrane.
In addition to non-enveloped viruses, authentic enveloped viruses such as Hepatitis C virus (HCV), Pegivirus, Severe Fever with Thrombocytopenia Syndrome (SFTS) virus, Rift Valley Fever virus (RVFV) and even HIV and HTLV-1 appear to exploit extracellular vesicles in novel ways to propagate themselves. HCV, in addition to packaging its genomic RNA in enveloped virions and exporting them out through the secretory pathway, releases full-length naked genomic RNA molecules within exosomes [24•,25,26•,27•]. Although these exosomes are infectious, their infectivity appears to be independent of known HCV receptors such as CD81, SB-RI and the ApoE receptor as the exosomes lack the HCV E1 and E2 envelope proteins [27•]. It is not clear whether packaging of naked viral genomes within exosomes is induced by the virus or is a host response to the virus. Moreover the Ago2-mi122 complex is found to traffic along with the HCV genome, which potentially facilitates replication relative to infection by free HCV particles [26•]. SFTS virus infected cells release entire virions in exosomes and these exosomes are not only infectious but the infection is through a yet to be determined receptor-independent pathway such as direct fusion of exosomes with host cells [28]. Other viruses like RVFV, HIV, HTLV-1 appear to also use exosomes to indirectly favor their transmission to other cells by packaging segments of viral nucleic acids and proteins within exosomes that modulate the survival of neighboring uninfected cells [29•,30,31]. Given these reports of infectivity being independent of virion–host cell surface receptor interactions, it remains to be determined which virus and host factors, including vesicle membrane components (e.g. PS lipids) and lumenal contents (e.g. Ago2-mi122), influence viral and tissue tropism.
Vesicles enable high multiplicity of infection and can facilitate genetic cooperativity
It is generally accepted that viral particles, whether enveloped or non-enveloped, travel and infect as single infectious units, independently of one another. Contrary to this, we recently reported finding viruses traveling and infecting susceptible cells in populations [11••]. Poliovirus, Coxsackievirus and rhinovirus, were each found traveling in large clusters within autophagosome-derived vesicles [11••]. Given the typical discrete size of these vesicles ∼400 nm and the size of an individual poliovirus or rhinovirus particle ∼30 nm, a single vesicle can traffic hundreds to thousands of viral particles. Indeed even smaller vesicles like exosomes (∼100 nm) may have the capacity to fit multiple HAV or HEV particles. This phenomena may not be limited to just RNA viruses as vesicular trafficking of viral populations has also been recently reported for Marseille virus, a large DNA virus of capsid size ∼250 nm, that appears to travel in populations of thousands within giant micrometer size vesicles [32].
This collective transport of multiple viral particles together in a vesicle enables collective simultaneous delivery of multiple viral genomes into a susceptible host cell [11••] (Figure 1), akin to the Trojan horse tactic used to smuggle soldiers into ancient Troy. Significantly this type of delivery yields greater replication efficiency than infections with similar numbers of free (i.e. not in vesicle) viral particles [11••]. This brings up the intriguing possibility that vesicular travel of viral populations may be nature's way of generating high multiplicities of infection in order to enhance viral propagation and survival [33]. In particular for RNA viruses, having a high multiplicity of infection can provide greater opportunities for genetic cooperativity [34•,35•]. Because of rapid replication kinetics combined with an inability to correct errors generated by their own RNA dependent RNA polymerases, the population of RNA viruses within a cell are not identical and instead are a mix of ‘quasispecies’. In this population of quasispecies, each RNA genome can differ from one another in terms of replicative fitness in the next infection cycle, as a result of differences in rates of uncoating, translation, replication, assembly, resistance to innate immune defenses, etc. (Figure 1) [36]. While as free independent viral particles these differences could determine the ultimate survival and propagation of a single quasispecies viral genome, if delivered in a vesicle as part of a population, the cooperative interactions among the quasispecies such as sharing genetic templates, replication machinery and structural proteins and innate immune defense modulators, could yield greater overall survival and long term fitness for multiple quasispecies (Figure 1). For example, a genome encoding a mutation that slows replication but helps evade the immune response could provide a benefit to the population as a whole if helped in replication with cooperative interactions with other population members (e.g. sharing replication machinery). The benefits of cooperative behavior may also extend to exosomes and other extracellular vesicles carrying naked RNA genomes [24•,25,26•,27•]. Furthermore when cells are co-infected with different but closely related RNA viruses, such as poliovirus and Coxsackievirus [37], non-lytic cellular exit by extracellular vesicles would generate vesicles carrying a mix of both viral populations which would then increase the probability of co-infection and genetic interactions that could result in faster emergence of drug resistance and decrease in vaccine efficacy.
Vesicles can help viruses manipulate the immune system
Cell lysis is a highly inflammatory event when it happens in the context of the body, attracting components of the immune system [38]. Hence escape without lysing the host cell would be an advantage for any virus. Moreover, hiding within extracellular vesicles for non-enveloped viruses would be a barrier to neutralizing antibodies [7••]. In addition to these advantages provided by vesicular release, PS lipids enriched within vesicle membranes may play an important role in modulating the immune system. PS lipids are potent anti-inflammatory agents [39•]. When cells undergo apoptosis and PS is flipped from the inner to the outer leaflet of the plasma membrane, it not only becomes an eat me signal for phagocytic uptake by macrophages but also triggers an anti-inflammatory signaling program within the macrophage resulting in release of anti-inflammatory cytokines like TGF-β and IL-10 that block activation of the immune system in the face of normal cell turnover [40]. On the other hand when macrophages encounter pathogens the response is frequently the opposite, with release of inflammatory cytokines and chemokines to alert and recruit other cells including the adaptive immune system [41]. Hence upon uptake, virus containing PS vesicles may short circuit the inflammatory program and attenuate the immune response.
Conclusions
Recent findings of how viruses exploit extracellular vesicles are revising the useful but simple models we have of how viruses enter, replicate and exit cells. Instead a more complex picture of viral propagation and transmission is emerging where vesicles enable non-enveloped and enveloped virions as well as naked genomes, host and viral components to be agents and modulators of infectivity and replicative fitness. Extracellular vesicles enable viruses to infect cells in both receptor-dependent and receptor-independent mechanisms; to modulate the immune response; and by transporting populations of viral particles and genomes increase multiplicities of infection to facilitate cooperative interactions and enhance viral replicative fitness.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1•.Tkach M, Théry C. Communication by extracellular vesicles: where we are and where we need to go. Cell. 2016;164:1226–1232. doi: 10.1016/j.cell.2016.01.043. [DOI] [PubMed] [Google Scholar]
- 2.Ponpuak M, Mandell MA, Kimura T, Chauhan S, Cleyrat C, Deretic V. Secretory autophagy. Curr Opin Cell Biol. 2015;35:106–116. doi: 10.1016/j.ceb.2015.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang M, Kenny SJ, Ge L, Xu K, Schekman R. Translocation of interleukin-1β into a vesicle intermediate in autophagy-mediated secretion. Elife. 2015;4 doi: 10.7554/eLife.11205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hurley JH. ESCRTs are everywhere. EMBO J. 2015;34:2398–2407. doi: 10.15252/embj.201592484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lindenbach BD. Virion assembly and release. Curr Top Microbiol Immunol. 2013;369:199–218. doi: 10.1007/978-3-642-27340-7_8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rossmann MG. Structure of viruses: a short history. Q Rev Biophys. 2013;46:133–180. doi: 10.1017/S0033583513000012. [DOI] [PubMed] [Google Scholar]
- 7••.Feng Z, Hensley L, McKnight KL, Hu F, Madden V, Ping L, Jeong SH, Walker C, Lanford RE, Lemon SM. A pathogenic picornavirus acquires an envelope by hijacking cellular membranes. Nature. 2013;496:367–371. doi: 10.1038/nature12029. This is the first report of a non-enveloped virus, HAV, also existing in an enveloped form, both in vitro and in vivo. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nagashima S, Jirintai S, Takahashi M, Kobayashi T, Tanggis, Nishizawa T, Kouki T, Yashiro T, Okamoto H. Hepatitis E virus egress depends on the exosomal pathway, with secretory exosomes derived from multivesicular bodies. J Gen Virol. 2014;95:2166–2175. doi: 10.1099/vir.0.066910-0. [DOI] [PubMed] [Google Scholar]
- 9.Bird SW, Maynard ND, Covert MW, Kirkegaard K. Nonlytic viral spread enhanced by autophagy components. Proc Natl Acad Sci U S A. 2014;111:3081–3086. doi: 10.1073/pnas.1401437111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Robinson SM, Tsueng G, Sin J, Mangale V, Rahawi S, McIntyre LL, Williams W, Kha N, Cruz C, Hancock BM, Nguyen DP, Sayen MR, Hilton BJ, Doran KS, Segall AM, Wolkowicz R, Cornell CT, Whitton JL, Gottlieb RA, Feuer R. Coxsackievirus B exits the host cell in shed microvesicles displaying autophagosomal markers. PLoS Pathog. 2014;10:e1004045. doi: 10.1371/journal.ppat.1004045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11••.Chen YH, Du W, Hagemeijer MC, Takvorian PM, Pau C, Cali A, Brantner CA, Stempinski ES, Connelly PS, Ma HC, Jiang P, Wimmer E, Altan-Bonnet G, Altan-Bonnet N. Phosphatidylserine vesicles enable efficient en bloc transmission of enteroviruses. Cell. 2015;160:619–630. doi: 10.1016/j.cell.2015.01.032. This is the first report showing that RNA viruses are using vesicles to traffic in populations (as opposed to single viral particles) and thereby achieve high multiplicities of infection of host cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12••.Jackson WT, Giddings TH, Jr, Taylor MP, Mulinyawe S, Rabinovitch M, Kopito RR, Kirkegaard K. Subversion of cellular autophagosomal machinery by RNA viruses. PLoS Biol. 2005;3:e156. doi: 10.1371/journal.pbio.0030156. This was the first report showing that the autophagy pathway is regulating poliovirus release from infected cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feng Y, Yao Z, Klionsky DJ. How to control self-digestion: transcriptional, post-transcriptional, and post-translational regulation of autophagy. Trends Cell Biol. 2015:25354–25363. doi: 10.1016/j.tcb.2015.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cuervo AM. The plasma membrane brings autophagosomes to life. Nat Cell Biol. 2010;12:735–737. doi: 10.1038/ncb0810-735. [DOI] [PubMed] [Google Scholar]
- 15.Jiang S, Dupont N, Castillo EF, Deretic V. Secretory versus degradative autophagy: unconventional secretion of inflammatory mediators. J Innate Immun. 2013;5:471–479. doi: 10.1159/000346707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ejlerskov P, Rasmussen I, Nielsen TT, Bergström AL, Tohyama Y, Jensen PH, Vilhardt F. Tubulin polymerization-promoting protein (TPPP/p25α) promotes unconventional secretion of α-synuclein through exophagy by impairing autophagosome–lysosome fusion. J Biol Chem. 2013;288:17313–17335. doi: 10.1074/jbc.M112.401174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nilsson P, Loganathan K, Sekiguchi M, Matsuba Y, Hui K, Tsubuki S, Tanaka M, Iwata N, Saito T, Saido TC. Aβ secretion and plaque formation depend on autophagy. Cell Rep. 2013;5:61–69. doi: 10.1016/j.celrep.2013.08.042. [DOI] [PubMed] [Google Scholar]
- 18••.Mankelow TJ, Griffiths RE, Trompeter S, Flatt JF, Cogan NM, Massey EJ, Anstee DJ. Autophagic vesicles on mature human reticulocytes explain phosphatidylserine-positive red cells in sickle cell disease. Blood. 2015;126:1831–1834. doi: 10.1182/blood-2015-04-637702. Using microscopy, the authors report on secretory autophagy being utilized by reticulocytes for mitochondrial extrusion during maturation into red blood cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yin X, Ambardekar C, Lu Y, Feng Z. Distinct entry mechanisms for nonenveloped and quasi-enveloped hepatitis E viruses. J Virol. 2016;90:4232–4242. doi: 10.1128/JVI.02804-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Amara A, Mercer J. Viral apoptotic mimicry. Nat Rev Microbiol. 2015;13:461–469. doi: 10.1038/nrmicro3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Denisova E, Dowling W, LaMonica R, Shaw R, Scarlata S, Ruggeri F, Mackow ER. Rotavirus capsid protein VP5* permeabilizes membranes. J Virol. 1999;73:3147–3153. doi: 10.1128/jvi.73.4.3147-3153.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Panjwani A, Strauss M, Gold S, Wenham H, Jackson T, Chou JJ, Rowlands DJ, Stonehouse NJ, Hogle JM, Tuthill TJ. Capsid protein VP4 of human rhinovirus induces membrane permeability by the formation of a size-selective multimeric pore. PLoS Pathog. 2014;10:e1004294. doi: 10.1371/journal.ppat.1004294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wiethoff CM, Nemerow GR. Adenovirus membrane penetration: tickling the tail of a sleeping dragon. Virology. 2015;479–480:591–599. doi: 10.1016/j.virol.2015.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24•.Longatti A, Boyd B, Chisari FV. Virion-independent transfer of replication-competent hepatitis C virus RNA between permissive cells. J Virol. 2015;89:2956–2961. doi: 10.1128/JVI.02721-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Patman G. Hepatitis: exosomal route of HCV transmission exists in patients. Nat Rev Gastroenterol Hepatol. 2014;11(12):704. doi: 10.1038/nrgastro.2014.179. [DOI] [PubMed] [Google Scholar]
- 26•.Bukong TN, Momen-Heravi F, Kodys K, Bala S, Szabo G. Exosomes from hepatitis C infected patients transmit HCV infection and contain replication competent viral RNA in complex with Ago2-miR122-HSP90. PLoS Pathog. 2014;10:e1004424. doi: 10.1371/journal.ppat.1004424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27•.Ramakrishnaiah V, Thumann C, Fofana I, Habersetzer F, Pan Q, de Ruiter PE, Willemsen R, Demmers JA, Stalin Raj V, Jenster G, Kwekkeboom J, Tilanus HW, Haagmans BL, Baumert TF, van der Laan LJ. Exosome-mediated transmission of hepatitis C virus between human hepatoma Huh7.5 cells. Proc Natl Acad Sci U S A. 2013;110:13109–13113. doi: 10.1073/pnas.1221899110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Silvas JA, Popov VL, Paulucci-Holthauzen A, Aguilar PV. Extracellular vesicles mediate receptor-independent transmission of novel tick-borne bunyavirus. J Virol. 2015;90:873–886. doi: 10.1128/JVI.02490-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29•.Ahsan NA, Sampey GC, Lepene B, Akpamagbo Y, Barclay RA, Iordanskiy S, Hakami RM, Kashanchi F. Presence of viral RNA and proteins in exosomes from cellular clones resistant to rift valley fever virus infection. Front Microbiol. 2016;11(7):139. doi: 10.3389/fmicb.2016.00139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kadiu I, Narayanasamy P, Dash PK, Zhang W, Gendelman HE. Biochemical and biologic characterization of exosomes and microvesicles as facilitators of HIV-1 infection in macrophages. J Immunol. 2012;189:744–754. doi: 10.4049/jimmunol.1102244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jaworski E, Narayanan A, Van Duyne R, Shabbeer-Meyering S, Iordanskiy S, Saifuddin M, Das R, Afonso PV, Sampey GC, Chung M, Popratiloff A, Shrestha B, Sehgal M, Jain P, Vertes A, Mahieux R, Kashanchi F. Human T-lymphotropic virus type 1-infected cells secrete exosomes that contain Tax protein. J Biol Chem. 2014;289:22284–22305. doi: 10.1074/jbc.M114.549659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arantes TS, Rodrigues RA, Silva LK, Oliveira GP, de Souza HL, Khalil BJY, Oliveira DB, Torres AA, da Silva LL, Colson P, Kroon EG, da Fonseca FG, Bonjardim CA, La Scola B, Abrahão JS. The large marseillevirus explores different entry pathways by forming giant infectious vesicles. J Virol. 2016 doi: 10.1128/JVI.00177-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Altan-Bonnet N, Chen YH. Intercellular transmission of viral populations with vesicles. J Virol. 2015;89:12242–12244. doi: 10.1128/JVI.01452-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34•.Bordería AV, Isakov O, Moratorio G, Henningsson R, Agüera-González S, Organtini L, Gnädig NF, Blanc H, Alcover A, Hafenstein S, Fontes M, Shomron N, Vignuzzi M. Group selection and contribution of minority variants during virus adaptation determines virus fitness and phenotype. PLoS Pathog. 2015;11(5):e1004838. doi: 10.1371/journal.ppat.1004838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35•.Combe M, Garijo R, Geller R, Cuevas JM, Sanjuán R. Single-cell analysis of RNA virus infection identifies multiple genetically diverse viral genomes within single infectious units. Cell Host Microbe. 2015;18:424–432. doi: 10.1016/j.chom.2015.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Andino R, Domingo E. Viral quasispecies. Virology. 2015;479–480:46–51. doi: 10.1016/j.virol.2015.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Muslin C, Joffret ML, Pelletier I, Blondel B, Delpeyroux F. Evolution and emergence of enteroviruses through intra- and inter-species recombination: plasticity and phenotypic impact of modular genetic exchanges in the 5′ untranslated region. PLoS Pathog. 2015;11(11):e1005266. doi: 10.1371/journal.ppat.1005266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fink SL, Cookson BT. Apoptosis, pyroptosis, and necrosis: mechanistic description of dead and dying eukaryotic cells. Infect Immun. 2005;73:1907–1916. doi: 10.1128/IAI.73.4.1907-1916.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39•.Birge RB, Boeltz S, Kumar S, Carlson J, Wanderley J, Calianese D, Barcinski M, Brekken RA, Huang X, Hutchins JT, Freimark B, Empig C, Mercer J, Schroit AJ, Schett G, Herrmann M. Phosphatidylserine is a global immunosuppressive signal in efferocytosis, infectious disease, and cancer. Cell Death Differ. 2016 doi: 10.1038/cdd.2016.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Henson PM, Bratton DL. Antiinflammatory effects of apoptotic cells. J Clin Invest. 2013;123:2773–2774. doi: 10.1172/JCI69344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brubaker SW, Bonham KS, Zanoni I, Kagan JC. Innate immune pattern recognition: a cell biological perspective. Annu Rev Immunol. 2015;33:257–290. doi: 10.1146/annurev-immunol-032414-112240. [DOI] [PMC free article] [PubMed] [Google Scholar]


