Abstract
Virus-like particles (VLPs) have been utilized as vaccine platforms to increase the immunogenicity of heterologous antigens. A variety of diverse VLP types can serve as vaccine platforms, and research has focused on engineering VLPs to improve their efficacy as vaccines, enhance their stability, and allow for more versatile display of antigens. Here, we review selected VLP vaccine platforms, highlight efforts to improve these platforms through structure-informed rational design, and point to areas of future research that will assist in these efforts.
Introduction
Many viral structural proteins have an intrinsic ability to self-assemble into virus-like particles (VLPs). VLPs structurally resemble the virus from which they were derived and, because of their antigenic similarity to authentic virions and because they possess physical characteristics that are highly immunostimulatory, can serve as effective stand-alone vaccines or vaccine platforms. Most VLPs are between 20–100nm in diameter, a size that allows for free entry into the lymphatic vessels and passive drainage to the subcapsular region of lymph nodes and optimal uptake by professional antigen presenting cells [1]. In some cases, trafficking of VLPs to B cell follicles is facilitated through specific interactions between VLPs and complement components or natural IgM antibodies [2]. VLPs also possess a geometry that contributes to their remarkable ability to activate B cells. Epitopes presented on the multivalent, highly repetitive, and often rigid structures of VLPs can extensively crosslink B cell receptors, leading to strong stimulation of B cells and the induction of robust and long-lasting antibody responses [3–5]. These signals can even override B cell tolerance mechanisms, allowing the induction of potent antibody responses against self-antigens [6–8]. Thus, these two features, size and geometry, are critical for the ability of VLPs to potently activate B cells and elicit strong and long-lasting antibody responses, and are essential to their utility as vaccines.
Over the past several decades it has been increasingly recognized that these structural features of VLPs can be exploited by using VLPs as vaccine platforms. This technique can be used to dramatically increase the immunogenicity of heterologous antigens. Although these inherent physical characteristics are the fundamental basis for the potent immunogenicity of VLP-based immunogens, there are other features desirable in VLPs that have improved their utility as vaccine platforms. Here, we review efforts to engineer VLPs into more stable, versatile, and universal platforms for vaccines. Engineering has led to enhancements in particle stability, the diversity of antigens that can be displayed on VLP surfaces, and the ability to fine-tune immune responses.
Engineering VLPs to display diverse antigens
To maximize the immunogenic potential of particle platform technologies, target antigens must be displayed at high density on the surface of VLPs. A number of different approaches have been used to engineer VLPs so that they can serve as molecular scaffolds for antigen display. Versatility of the VLP platform is a critical feature. In order to maximize utility, VLPs should be able to display foreign antigens of various compositions, sizes, and structures. One approach is to generate recombinant fusions in which foreign antigens are inserted into sites within the viral structural protein so that the foreign antigen is displayed on the resulting VLP’s surface. However, peptide insertions often affect the ability of recombinant proteins to assemble into VLPs. To avoid such problems, pre-formed VLPs can be used as scaffolds to which foreign antigens are attached using various conjugation techniques. Post-production modifications of VLPs can take advantage of naturally occurring sites of conjugation (e.g. amino-groups on surface-exposed lysines) or the VLP can be engineered to have added functionalities.
A variety of VLPs have been engineered to display foreign antigens as fusions with the viral structural protein (selected platforms mentioned in this text are listed in Table 1). One of the best examples is Hepatitis B virus (HBV) core antigen (HBcAg), which forms a 36nm particle consisting of 240 copies of HBcAg, assembled as 120 homodimers. A number of sites for displaying heterologous peptides have been identified on core antigen of HBV and its Hepadnaviridae relatives (e.g. Woodchuck Hepatitis Virus; WHV) [9]. As with many VLPs, the identification of foreign antigen insertion sites in HBcAg VLPs has been a combination of rational design based on structural characteristics (i.e surface exposed loops or disordered regions) and trial-and-error. For instance, using a systematic approach consisting of multiple insertion sites, an array of compensatory mutations, and robust screening methods, Billaud and coworkers vastly enhanced the success rate for producing chimeric WHcAg VLPs [10]. Although general “rules” could be derived from this type of assessment (e.g. that highly basic amino acids in an insert epitope needed to be balanced by the addition of acidic amino acids at flanking sites), these data highlight the empirical nature of much of the VLP vaccine platform engineering efforts.
Table 1.
VLP platforms discussed in this review with selected references
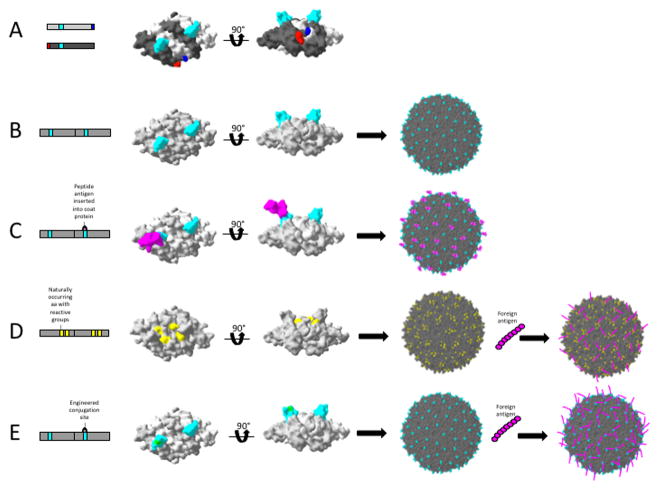
In some cases, engineering more thermodynamically stable versions of the subunits that comprise VLPs can dramatically increase the peptide insertion success rate. In the case of bacteriophage MS2 VLPs, foreign peptides displayed in a surface-exposed loop (the AB-loop) on the wild-type viral coat protein are rarely compatible with protein folding and VLP assembly (Fig. 1) [11]. However, the physical proximity of N- and C- termini of subunits of the coat protein dimer facilitated their genetic fusion into a so-called single-chain dimer [12]. The resulting protein produces VLPs that are indistinguishable from wild-type [13], and much more tolerant of heterologous peptide insertions [12,14]. The vast majority of small peptides (10 amino acids or less) are accepted, but the success of any particular sequence is hard to predict in advance, although hydrophobic peptides tend to cause protein folding defects [14].
Figure 1.
Engineering VLP platforms for improved utility. The bacteriophage MS2 coat protein dimer is shown here as an example of engineering to improve the utility of a VLP platform. (A) The MS2 coat protein dimer is normally assembled from two identical monomers that come together such that their N- and C- termini are juxtaposed (red and blue). However, peptide insertions displayed in the surface exposed AB loop (cyan) are not tolerated, and VLPs generally do not form. (B) Engineering the coat protein monomers into a single-chain dimer (covalent fusion of two monomers) allows proper folding and VLP formation by stabilizing the dimer. (C) Foreign peptides can then be inserted into the AB loop of the down-stream coat protein copy, allowing display on the surface of the VLP (90 copies total). (D) Naturally occurring, surface-exposed amino acids with reactive groups can be utilized for conjugation of foreign antigen (here, surface exposed lysines on MS2 coat protein are shown). (E) Alternatively, the VLP can be engineered to contain amino acids with reactive groups (cysteines or lysines), sortase target sequences, etc. to allow convenient sites for conjugation of antigen post-VLP formation.
Another approach avoids disruption of platform folding and assembly by conjugating antigens to pre-formed VLPs using chemical crosslinkers with various functional groups, click chemistry, sortase-mediated attachment, polyhistidine/NTA-Ni2+, and affinity-tag interactions. Knowledge of VLP structure facilitates identification of sites for conjugation. For instance, the amino groups of surface exposed lysines on bacteriophage Qβ VLPs are convenient sites for conjugation to the thiols of cysteine-containing foreign antigens using bifunctional crosslinkers. The process is compatible with GMP manufacture, and in fact several Qβ VLP-based vaccines have been tested in clinical trials [15–17]. However, VLPs can also be engineered to contain these convenient sites, such as the engineering a surface-exposed cysteine or lysine into the VLP, providing accessible reactive groups [18–20] (Fig. 1). Sortase-mediated conjugation allows for site-directed ligation of cargoes (including peptides and small molecules) bearing a specific amino acid tag (LPETG) to VLPs that have been modified to include a target sequence (a stretch of glycine residues) [21]. Similarly, VLPs have been engineered to display poly-histidine stretches (for non-covalent conjugation to antigens with NTA-Ni2+ moiety) [22], methionine analogues for use with click chemistry [23], or other proteins or tags that allow for non-covalent [24,25] or covalent [26] interactions. These techniques allow VLPs to display diverse antigens, including peptides, full-length proteins, carbohydrates, and other small molecules. Each of these display approaches has the benefit of having little effect on VLP stability, but has the downside of having potential increased cost of production or decreased scalability upon downstream manufacturing of the vaccines.
Tailoring immune responses by modifying VLPs
Although VLP-based vaccines generally elicit strong immune responses, the relative ability of different VLP types to induce humoral and cell-mediated immunity is likely influenced by a number of factors that may be VLP-type specific. Interactions with specific receptors on or within immune cells [1,27], interactions with molecules that influence trafficking [2], the intrinsic half-life of particles, and compatibility with adjuvants can all have effects on the magnitude and longevity of immune responses, and the specific arms of the immune response that are activated.
For example, VLPs have been modified by altering their nucleic acid content so that they elicit specific antibody isotypes. VLPs are essentially empty spheres, yet many VLPs naturally encapsidate nucleic acid derived from the cell type the VLPs were produced in. For example, when produced recombinantly in E. coli, RNA bacteriophage VLPs naturally encapsidate bacterially-derived ssRNA, a toll-like receptor (TLR) ligand [28]. Mice immunized with RNA-containing bacteriophage VLPs predominantly elicit IgG2a antibodies, indicative of a pro-inflammatory T helper (TH) 1 response, whereas immunization with VLPs in which the ssRNA has been removed predominantly elicit IgG1 antibodies, indicative of a TH2 response [29,30]. VLPs can be deliberately loaded with or linked to adjuvants to enhance their immunogenicity. For example, loading bacteriophage VLPs with CpG oligonucleotides can enhance both cellular and humoral responses to VLPs [31]. Thus, VLPs can be customized as needed, depending on whether inflammatory responses are desired or if there are antibody isotypes with specific activities (neutralization, opsonization, or complement activation) that may be required.
VLPs have also been modified to enhance cell-mediated immune responses. The three-dimensional structure of an antigen can potentially modulate the presentation of CD4 T cell epitopes by changing access of particles to proteolytic enzymes and the MHC class II presentation machinery [32]. While most VLPs are rich in potential TH epitopes, rabbit hemorrhagic disease virus (RHDV) VLPs have been modified to contain the PADRE peptide, a universal TH epitope [33]. An RHDV VLP containing PADRE and targeting a Human Papillomavirus-16 E6 peptide elicited enhanced anti-tumor T-cell responses in an HPV-tumor mouse model [33]. The use of universal TH epitopes may be advantageous for antigen presentation by diverse HLA molecules.
Engineering more stable VLPs
Although VLPs are generally highly stable, VLP types that can withstand variations in temperature and other conditions will likely be advantageous both during manufacturing and distribution. Engineering VLPs to withstand storage or transport at diverse temperatures can facilitate the logistics of vaccine deployment, particularly in areas of the developing world where it is difficult to maintain a cold-chain. Additionally, improving the stability of VLPs reduces the cost of vaccines by allowing a longer shelf-life and also reduces energy costs associated with cold-storage. In some cases VLPs have been modified in order to enhance structural stability. These modifications have been used to facilitate the engineering of recombinant VLPs (as described above) but also have been undertaken with these other downstream applications in mind. One common technique to improve particle thermostability is the introduction of intersubunit disulfide bonds. For instance, mutations that introduce cysteines at the 5-fold symmetry axes increase the stability of MS2 VLPs [34,35]. Similarly, Hepatitis B core antigen VLPs with enhanced stability were produced by identifying amino acid pairs of interacting dimers with the shortest distances between side chains and substituting cysteines that could form disulfide bonds, creating a disulfide-bond network stabilizing the particle [36]. Post-production methods, such as lyophilization or spray-drying of VLPs, can also enhance thermostability and allow for long-term storage [37,38].
Future directions for structural engineering of VLP vaccine platforms
As we’ve described, numerous efforts have been undertaken to improve on the stability, versatility, and universality of VLP vaccine platforms. Moving forward, two major areas of research will drive this field forward toward more efficient and adaptable vaccines: (1) identifying new molecular scaffolds to increase the diversity of antigens that can be displayed on VLPs and (2) adapting VLP platforms to vaccine discovery efforts.
Exploring new molecular structures as scaffolds for the display of diverse antigens on the surface of VLP platforms will increase the versatility of VLP platforms. Generally, VLP-display has been limited to short peptide antigens, although there are some exceptions in which larger molecules have been targeted [39–41]. Nevertheless, as the repertoire of solved molecular structures is expanded and our understanding of how these structures form improves, rational design of VLPs that include these molecular structures will expand. This is highlighted by recent efforts to add coiled-coil domains to P22 bacteriophage VLPs to aid in folding and display of large foreign antigens [20]. Rationally designing hybrid protein structures based on the building blocks found in nature will drive the field toward more stable and versatile VLP vaccine platforms and recent efforts to use computational modeling to predict whether peptides are compatible with VLP assembly may also improve success rates [42–46]. Synthetic multi-subunit structures that are modeled and engineered to be built from simple protein subunits are already being made [47,48], and as computational approaches for protein design improve, this holds promise for eventually generating new synthetic VLP platforms that are only tangentially based on viruses found in nature.
Adapting VLP vaccine platforms for vaccine discovery efforts is another major area of research. Bacteriophage MS2 and PP7 VLPs have been adapted to allow affinity selection against monoclonal antibodies of interest as well a patient serum and have yielded vaccine candidates [14,45,49–54]. RNA bacteriophage VLPs are particularly well-suited to be used in vaccine discovery efforts due to their simple structures made from a single coat protein, ability to encapsidate their own coding RNA (i.e. a linkage of genotype-phenotype), and the ability to accept a diversity of foreign peptides. Other VLP vaccine platforms similarly could be adapted into vaccine discovery platforms, and because each VLP platform has its own unique capabilities (i.e. immunogenicity, size of antigens that can be displayed), it would be beneficial to have more options. For instance, the MS2 and PP7 VLP vaccine discovery platforms are limited to relatively short peptide inserts (<10 aa is ideal). Recent efforts have attempted to couple the affinity selection features of MS2 VLP platform with computational structural modeling approaches to further enhance its vaccine discovery capabilities [45]. We used the predictive structural modeling tool Phyre2 [55] to generate models of VLPs displaying foreign peptides and then tried to identify conformational mimics of an antigen of interest [45]. As described by Joshi et al., first “building” a VLP computationally is another way to improve vaccine discovery efforts, and can provide a much needed rational approach that can increase the number of VLP vaccine candidate successes, save money and time, and ultimately provide the tools needed to make VLPs much more versatile and adaptable going forward [44]. We are currently limited by our lack of knowledge of the biophysical properties that drive protein folding, but the field is set to expand rapidly as computational power increases and predictive modeling tools improve. New areas of VLP engineering will emerge that will fuel in silico VLP vaccine discovery efforts and limit the trial-and-error nature of VLP vaccine design.
One VLP platform will never meet all vaccination needs. The simple fact of preexisting immunity to many of the viruses will preclude their universal application. Additionally, some VLP platforms may have specific features, such as the ability to display large foreign antigens or the ability to display multiple epitopes, that will make them uniquely suited to a particular vaccine need. As our knowledge of protein folding dynamics and immunology increases, the versatility of VLPs as vaccine platforms will advance.
Highlights.
Virus-like particles are important vaccine platforms that have been engineered to enhance their immunogenicity, stability, and versatility
VLPs can be engineered to facilitate chemical modification or genetic insertion
Altering nucleic acid content and engineering T-cell epitopes into the VLP platform provides the ability to tailor the immune response elicited by the vaccine
Future improvement in VLP vaccines will be driven by enhanced predictive structural modeling tools and adapting VLPs for vaccine discovery
Acknowledgments
This work has been funded by the National Institutes of Health (R01 AI083305 to BC and R01 GM042901 to DSP).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- •1.Manolova V, Flace A, Bauer M, Schwarz K, Saudan P, Bachmann MF. Nanoparticles target distinct dendritic cell populations according to their size. Eur J Immunol. 2008;38:1404–1413. doi: 10.1002/eji.200737984. [DOI] [PubMed] [Google Scholar]
- •2.Link A, Zabel F, Schnetzler Y, Titz A, Brombacher F, Bachmann MF. Innate immunity mediates follicular transport of particulate but not soluble protein antigen. J Immunol. 2012;188:3724–3733. doi: 10.4049/jimmunol.1103312. These two manuscripts describe how VLPs interact with various components of the immune system to provoke strong responses. [DOI] [PubMed] [Google Scholar]
- 3.Zabel F, Kündig TM, Bachmann MF. Virus-induced humoral immunity: on how B cell responses are initiated. Curr Opin Virol. 2013;3:357–362. doi: 10.1016/j.coviro.2013.05.004. [DOI] [PubMed] [Google Scholar]
- •4.Bachmann MF, Jennings GT. Vaccine delivery: a matter of size, geometry, kinetics and molecular patterns. Nat Rev Immunol. 2010;10:787–796. doi: 10.1038/nri2868. This review highlights the key structural features of potent antigens. [DOI] [PubMed] [Google Scholar]
- 5.Jennings GT, Bachmann MF. The coming of age of virus-like particle vaccines. Biol Chem. 2008;389:521–536. doi: 10.1515/bc.2008.064. [DOI] [PubMed] [Google Scholar]
- 6.Bachmann MF, Zinkernagel RM. Neutralizing antiviral B cell responses. Annu Rev Immunol. 1997;15:235–270. doi: 10.1146/annurev.immunol.15.1.235. [DOI] [PubMed] [Google Scholar]
- 7.Chackerian B, Durfee MR, Schiller JT. Virus-like display of a neo-self antigen reverses B cell anergy in a B cell receptor transgenic mouse model. J Immunol. 2008;180:5816–5825. doi: 10.4049/jimmunol.180.9.5816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bachmann MF, Rohrer UH, Kündig TM, Bürki K, Hengartner H, Zinkernagel RM. The influence of antigen organization on B cell responsiveness. Science. 1993;262:1448–1451. doi: 10.1126/science.8248784. [DOI] [PubMed] [Google Scholar]
- 9.Whitacre DC, Lee BO, Milich DR. Use of hepadnavirus core proteins as vaccine platforms. Expert Rev Vaccines. 2009;8:1565–1573. doi: 10.1586/erv.09.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••10.Billaud JN, Peterson D, Barr M, Chen A, Sallberg M, Garduno F, Goldstein P, McDowell W, Hughes J, Jones J, et al. Combinatorial approach to hepadnavirus-like particle vaccine design. J Virol. 2005;79:13656–13666. doi: 10.1128/JVI.79.21.13656-13666.2005. This paper lays out an impressively comprehensive combinatorial approach to display peptides on Woodchuck Hepatitis Virus core antigen VLPs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Caldeira JC, Peabody DS. Thermal stability of RNA phage virus-like particles displaying foreign peptides. J Nanobiotechnology. 2011;9:22. doi: 10.1186/1477-3155-9-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peabody DS. Subunit fusion confers tolerance to peptide insertions in a virus coat protein. Arch Biochem Biophys. 1997;347:85–92. doi: 10.1006/abbi.1997.0312. [DOI] [PubMed] [Google Scholar]
- 13.Plevka P, Tars K, Liljas L. Structure and stability of icosahedral particles of a covalent coat protein dimer of bacteriophage MS2. Protein Sci. 2009;18:1653–1661. doi: 10.1002/pro.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••14.Peabody DS, Manifold-Wheeler B, Medford A, Jordan SK, do Carmo Caldeira J, Chackerian B. Immunogenic display of diverse peptides on virus-like particles of RNA phage MS2. J Mol Biol. 2008;380:252–263. doi: 10.1016/j.jmb.2008.04.049. Construction of a single-chain dimer version of the MS2 bacteriophage coat protein dramatically increases its utility as a display platform. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tissot AC, Maurer P, Nussberger J, Sabat R, Pfister T, Ignatenko S, Volk HD, Stocker H, Muller P, Jennings GT, et al. Effect of immunisation against angiotensin II with CYT006-AngQb on ambulatory blood pressure: a double-blind, randomised, placebo-controlled phase IIa study. Lancet. 2008;371:821–827. doi: 10.1016/S0140-6736(08)60381-5. [DOI] [PubMed] [Google Scholar]
- 16.Ambühl PM, Tissot AC, Fulurija A, Maurer P, Nussberger J, Sabat R, Nief V, Schellekens C, Sladko K, Roubicek K, et al. A vaccine for hypertension based on virus-like particles: preclinical efficacy and phase I safety and immunogenicity. J Hypertens. 2007;25:63–72. doi: 10.1097/HJH.0b013e32800ff5d6. [DOI] [PubMed] [Google Scholar]
- 17.Klimek L, Willers J, Hammann-Haenni A, Pfaar O, Stocker H, Mueller P, Renner WA, Bachmann MF. Assessment of clinical efficacy of CYT003-QbG10 in patients with allergic rhinoconjunctivitis: a phase IIb study. Clin Exp Allergy. 2011;41:1305–1312. doi: 10.1111/j.1365-2222.2011.03783.x. [DOI] [PubMed] [Google Scholar]
- 18.Peabody DS. A Viral Platform for Chemical Modification and Multivalent Display. J Nanobiotechnology. 2003;1:5. doi: 10.1186/1477-3155-1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chatterji A, Ochoa WF, Paine M, Ratna BR, Johnson JE, Lin T. New addresses on an addressable virus nanoblock; uniquely reactive Lys residues on cowpea mosaic virus. Chem Biol. 2004;11:855–863. doi: 10.1016/j.chembiol.2004.04.011. [DOI] [PubMed] [Google Scholar]
- •20.Servid A, Jordan P, O’Neil A, Prevelige P, Douglas T. Location of the bacteriophage P22 coat protein C-terminus provides opportunities for the design of capsid-based materials. Biomacromolecules. 2013;14:2989–2995. doi: 10.1021/bm400796c. The authors use several approaches to modify the C-terminus of the P22 coat protein so that it can serve as a site for chemical attachments. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schoonen L, Pille J, Borrmann A, Nolte RJ, van Hest JC. Sortase A-Mediated N-Terminal Modification of Cowpea Chlorotic Mottle Virus for Highly Efficient Cargo Loading. Bioconjug Chem. 2015 doi: 10.1021/acs.bioconjchem.5b00485. [DOI] [PubMed] [Google Scholar]
- 22.Koho T, Ihalainen TO, Stark M, Uusi-Kerttula H, Wieneke R, Rahikainen R, Blazevic V, Marjomäki V, Tampé R, Kulomaa MS, et al. His-tagged norovirus-like particles: A versatile platform for cellular delivery and surface display. Eur J Pharm Biopharm. 2015;96:22–31. doi: 10.1016/j.ejpb.2015.07.002. [DOI] [PubMed] [Google Scholar]
- •23.Patel KG, Swartz JR. Surface functionalization of virus-like particles by direct conjugation using azide-alkyne click chemistry. Bioconjug Chem. 2011;22:376–387. doi: 10.1021/bc100367u. Using a cell-free expression approach, the authors produce bacteriophage VLPs displaying methionine analogues that can be targeted using click chemistry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Venter PA, Dirksen A, Thomas D, Manchester M, Dawson PE, Schneemann A. Multivalent display of proteins on viral nanoparticles using molecular recognition and chemical ligation strategies. Biomacromolecules. 2011;12:2293–2301. doi: 10.1021/bm200369e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thrane S, Janitzek CM, Agerbæk M, Ditlev SB, Resende M, Nielsen MA, Theander TG, Salanti A, Sander AF. A Novel Virus-Like Particle Based Vaccine Platform Displaying the Placental Malaria Antigen VAR2CSA. PLoS One. 2015;10:e0143071. doi: 10.1371/journal.pone.0143071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••26.Brune KD, Leneghan DB, Brian IJ, Ishizuka AS, Bachmann MF, Draper SJ, Biswas S, Howarth M. Plug-and-Display: decoration of Virus-Like Particles via isopeptide bonds for modular immunization. Sci Rep. 2016;6:19234. doi: 10.1038/srep19234. Expression of the SpyCatcher peptide on AP205 bacteriophage VLPs allows the modular covalent attachment of targets displaying the SpyTag peptide sequence. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Storni T, Lechner F, Erdmann I, Bächi T, Jegerlehner A, Dumrese T, Kündig TM, Ruedl C, Bachmann MF. Critical role for activation of antigen-presenting cells in priming of cytotoxic T cell responses after vaccination with virus-like particles. J Immunol. 2002;168:2880–2886. doi: 10.4049/jimmunol.168.6.2880. [DOI] [PubMed] [Google Scholar]
- 28.Pickett GG, Peabody DS. Encapsidation of heterologous RNAs by bacteriophage MS2 coat protein. Nucleic Acids Res. 1993;21:4621–4626. doi: 10.1093/nar/21.19.4621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tumban E, Peabody J, Peabody DS, Chackerian B. A universal virus-like particle-based vaccine for human papillomavirus: longevity of protection and role of endogenous and exogenous adjuvants. Vaccine. 2013;31:4647–4654. doi: 10.1016/j.vaccine.2013.07.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jegerlehner A, Maurer P, Bessa J, Hinton HJ, Kopf M, Bachmann MF. TLR9 signaling in B cells determines class switch recombination to IgG2a. J Immunol. 2007;178:2415–2420. doi: 10.4049/jimmunol.178.4.2415. [DOI] [PubMed] [Google Scholar]
- 31.Schwarz K, Meijerink E, Speiser DE, Tissot AC, Cielens I, Renhof R, Dishlers A, Pumpens P, Bachmann MF. Efficient homologous prime-boost strategies for T cell vaccination based on virus-like particles. Eur J Immunol. 2005;35:816–821. doi: 10.1002/eji.200425755. [DOI] [PubMed] [Google Scholar]
- 32.Carmicle S, Steede NK, Landry SJ. Antigen three-dimensional structure guides the processing and presentation of helper T-cell epitopes. Mol Immunol. 2007;44:1159–1168. doi: 10.1016/j.molimm.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 33.Jemon K, Young V, Wilson M, McKee S, Ward V, Baird M, Young S, Hibma M. An enhanced heterologous virus-like particle for human papillomavirus type 16 tumour immunotherapy. PLoS One. 2013;8:e66866. doi: 10.1371/journal.pone.0066866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fiedler JD, Higginson C, Hovlid ML, Kislukhin AA, Castillejos A, Manzenrieder F, Campbell MG, Voss NR, Potter CS, Carragher B, et al. Engineered mutations change the structure and stability of a virus-like particle. Biomacromolecules. 2012;13:2339–2348. doi: 10.1021/bm300590x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ashcroft AE, Lago H, Macedo JM, Horn WT, Stonehouse NJ, Stockley PG. Engineering thermal stability in RNA phage capsids via disulphide bonds. J Nanosci Nanotechnol. 2005;5:2034–2041. doi: 10.1166/jnn.2005.507. [DOI] [PubMed] [Google Scholar]
- 36.Lu Y, Chan W, Ko BY, VanLang CC, Swartz JR. Assessing sequence plasticity of a virus-like nanoparticle by evolution toward a versatile scaffold for vaccines and drug delivery. Proc Natl Acad Sci U S A. 2015;112:12360–12365. doi: 10.1073/pnas.1510533112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tumban E, Muttil P, Escobar CA, Peabody J, Wafula D, Peabody DS, Chackerian B. Preclinical refinements of a broadly protective VLP-based HPV vaccine targeting the minor capsid protein, L2. Vaccine. 2015;33:3346–3353. doi: 10.1016/j.vaccine.2015.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lang R, Winter G, Vogt L, Zurcher A, Dorigo B, Schimmele B. Rational design of a stable, freeze-dried virus-like particle-based vaccine formulation. Drug Dev Ind Pharm. 2009;35:83–97. doi: 10.1080/03639040802192806. [DOI] [PubMed] [Google Scholar]
- 39.Kratz PA, Böttcher B, Nassal M. Native display of complete foreign protein domains on the surface of hepatitis B virus capsids. Proc Natl Acad Sci U S A. 1999;96:1915–1920. doi: 10.1073/pnas.96.5.1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tissot AC, Renhofa R, Schmitz N, Cielens I, Meijerink E, Ose V, Jennings GT, Saudan P, Pumpens P, Bachmann MF. Versatile virus-like particle carrier for epitope based vaccines. PLoS One. 2010;5:e9809. doi: 10.1371/journal.pone.0009809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Spohn G, Schori C, Keller I, Sladko K, Sina C, Guler R, Schwarz K, Johansen P, Jennings GT, Bachmann MF. Preclinical efficacy and safety of an anti-IL-1β vaccine for the treatment of type 2 diabetes. Mol Ther Methods Clin Dev. 2014;1:14048. doi: 10.1038/mtm.2014.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Joshi H, Cheluvaraja S, Somogyi E, Brown DR, Ortoleva P. A molecular dynamics study of loop fluctuation in human papillomavirus type 16 virus-like particles: a possible indicator of immunogenicity. Vaccine. 2011;29:9423–9430. doi: 10.1016/j.vaccine.2011.10.039. [DOI] [PubMed] [Google Scholar]
- 43.Joshi H, Singharoy A, Sereda YV, Cheluvaraja SC, Ortoleva PJ. Multiscale simulation of microbe structure and dynamics. Prog Biophys Mol Biol. 2011;107:200–217. doi: 10.1016/j.pbiomolbio.2011.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •44.Joshi H, Lewis K, Singharoy A, Ortoleva PJ. Epitope engineering and molecular metrics of immunogenicity: a computational approach to VLP-based vaccine design. Vaccine. 2013;31:4841–4847. doi: 10.1016/j.vaccine.2013.07.075. This article proposes using in silico design of VLPs and antigens to more rationally design VLP-based vaccines. [DOI] [PubMed] [Google Scholar]
- 45.Crossey E, Frietze K, Narum DL, Peabody DS, Chackerian B. Identification of an Immunogenic Mimic of a Conserved Epitope on the Plasmodium falciparum Blood Stage Antigen AMA1 Using Virus-Like Particle (VLP) Peptide Display. PLoS One. 2015;10:e0132560. doi: 10.1371/journal.pone.0132560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang L, Tang R, Bai S, Connors NK, Lua LH, Chuan YP, Middelberg AP, Sun Y. Energetic changes caused by antigenic module insertion in a virus-like particle revealed by experiment and molecular dynamics simulations. PLoS One. 2014;9:e107313. doi: 10.1371/journal.pone.0107313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••47.King NP, Bale JB, Sheffler W, McNamara DE, Gonen S, Gonen T, Yeates TO, Baker D. Accurate design of co-assembling multi-component protein nanomaterials. Nature. 2014;510:103–108. doi: 10.1038/nature13404. Using a computational design approach, the authors design protein subunits that can assemble into specific particle architectures. This approach could radically change the way that nanoparticle-based vaccines are designed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •48.Burkhard P, Lanar DE. Malaria vaccine based on self-assembling protein nanoparticles. Expert Rev Vaccines. 2015;14:1525–1527. doi: 10.1586/14760584.2015.1096781. The authors review their use of a self-assembling repetitive antigen display technology to produce malaria vaccines. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ord RL, Caldeira JC, Rodriguez M, Noe A, Chackerian B, Peabody DS, Gutierrez G, Lobo CA. A malaria vaccine candidate based on an epitope of the Plasmodium falciparum RH5 protein. Malar J. 2014;13:326. doi: 10.1186/1475-2875-13-326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Caldeira J, Bustos J, Peabody J, Chackerian B, Peabody DS. Epitope-Specific Anti-hCG Vaccines on a Virus Like Particle Platform. PLoS One. 2015;10:e0141407. doi: 10.1371/journal.pone.0141407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chackerian B, do Caldeira JC, Peabody J, Peabody DS. Peptide epitope identification by affinity selection on bacteriophage MS2 virus-like particles. J Mol Biol. 2011;409:225–237. doi: 10.1016/j.jmb.2011.03.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.do Caldeira JC, Medford A, Kines RC, Lino CA, Schiller JT, Chackerian B, Peabody DS. Immunogenic display of diverse peptides, including a broadly cross-type neutralizing human papillomavirus L2 epitope, on virus-like particles of the RNA bacteriophage PP7. Vaccine. 2010;28:4384–4393. doi: 10.1016/j.vaccine.2010.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Frietze KM, Roden RB, Lee JH, Shi Y, Peabody DS, Chackerian B. Identification of anti-CA125 antibody responses in ovarian cancer patients by a novel deep sequence-coupled biopanning platform. Cancer Immunol Res. 2015 doi: 10.1158/2326-6066.CIR-15-0165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •54.O’Rourke JP, Peabody DS, Chackerian B. Affinity selection of epitope-based vaccines using a bacteriophage virus-like particle platform. Curr Opin Virol. 2015;11:76–82. doi: 10.1016/j.coviro.2015.03.005. We review a bacteriophage VLP platform technology that can also be used for vaccine discovery. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •55.Kelley LA, Mezulis S, Yates CM, Wass MN, Sternberg MJ. The Phyre2 web portal for protein modeling, prediction and analysis. Nat Protoc. 2015;10:845–858. doi: 10.1038/nprot.2015.053. The Phyre2 server is a freely available tool for homology modeling that is one of several such tools that can be useful in in silico design of VLPs. [DOI] [PMC free article] [PubMed] [Google Scholar]