Summary
As a major site of protein biosynthesis, homeostasis of the endoplasmic reticulum is critical for cell viability. Asparagine linked glycosylation of newly synthesized proteins by the oligosaccharyltransferase plays a central role in ER homeostasis due to the use of protein-linked oligosaccharides as recognition and timing markers for glycoprotein quality control pathways that discriminate between correctly folded proteins and terminally malfolded proteins destined for ER associated degradation. Recent findings indicate how the oligosaccharyltransferase achieves efficient and accurate glycosylation of the diverse proteins that enter the endoplasmic reticulum. In metazoan organisms two distinct OST complexes cooperate to maximize the glycosylation of nascent proteins. The STT3B complex glycosylates acceptor sites that have been skipped by the translocation channel associated STT3A complex.
Introduction
Asparagine linked glycosylation is one of the most common protein modification reactions in eukaryotic cells, occurring upon the majority of proteins that are cotranslationally translocated across or integrated into the rough endoplasmic reticulum during biosynthesis. N-linked oligosaccharides on nascent glycoproteins have multiple roles within the endoplasmic reticulum that are essential for ER homeostasis. After N-linked oligosaccharides are transferred to nascent proteins by the oligosaccharyltransferase (OST), ER resident glucosidases and mannosidases generate a series of glycan trimming intermediates that are specifically recognized by ER-localized lectins to direct the nascent proteins into protein folding, protein degradation or protein export pathways. Accumulation of unfolded glycoproteins in the ER lumen induces the unfolded protein response (UPR) pathway. In this article, we primarily focus upon the features of the mammalian OST that allow efficient and accurate glycosylation of proteins in the endoplasmic reticulum to provide the glycan markers that direct these glycoprotein quality control pathways.
N-linked glycans orchestrate the fate of nascent glycoproteins
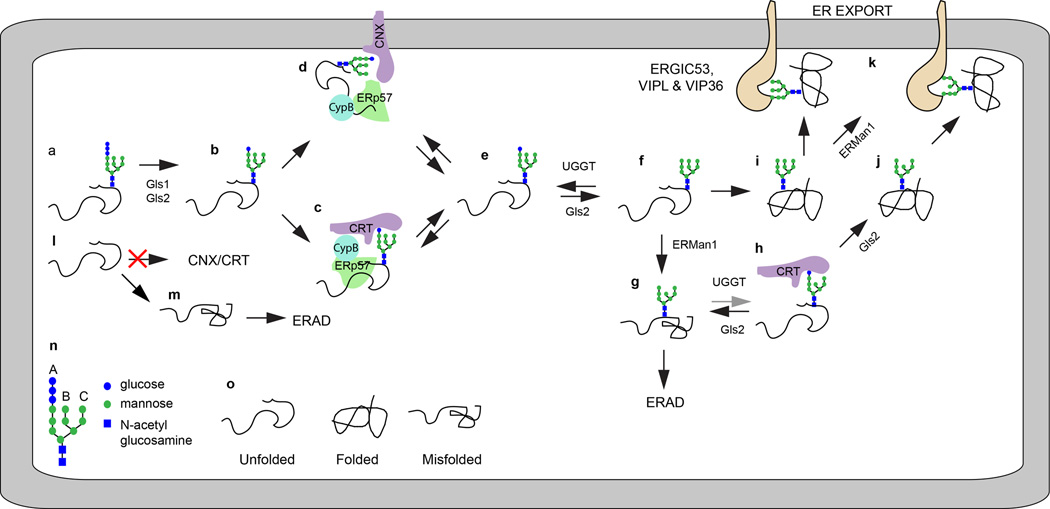
First, we will briefly consider how protein-linked glycans impact ER homeostasis. The CNX/CRT cycle for glycoprotein quality control is the subject of two excellent recent reviews [1,2]. Unfolded nascent glycoproteins have GlcNAc2Man9Glc3 oligosaccharides (abbreviated as GN2M9G3, see Fig. 1n and 1o for the monosaccharide code and protein folding states) attached to asparagine residues (Fig. 1a). Trimming of the oligosaccharide by glucosidase 1 (Gls1) and glucosidase 2 (Gls2) yields the monoglucosylated glycan (GN2M9G1, Fig. 1b) that is recognized by calnexin (CNX) and calreticulin (CRT), two ER localized lectin chaperones (Fig. 1c and 1d). CNX and CRT recruit the oxidoreductase ERp57 and the peptidyl-prolyl isomerase CypB to promote folding of the nascent glycoprotein. Glycan release from CNX and CRT (Fig. 1e) allows cycles of lectin-glycan interaction (Fig. 1c–1f) that are driven by Gls2 trimming of the terminal glucose residue counteracted by reglucosylation of the deglucosylated glycan by the protein folding sensor UDP-glucose glycoprotein glucosyltransferase (UGGT). Trimming of the B-branch α,1–2 linked mannose residue by ER mannosidase 1 (ERMan1, Fig. 1g) insures that terminally malfolded proteins exit the CNX/CRT cycle because UGGT favors oligosaccharide acceptors with the full complement of α,1–2 linked mannose residues (Fig. 1h, GN2M9 > GN2M8 > GN2M7 >>GN2M6).
Figure 1.
Asparagine linked glycosylation allows glycoprotein entry into the CNX/CRT cycle. (a–d) GN2M9G1 dependent binding of unfolded proteins to CNX and CRT. For simplicity, the interaction of ERp57 and CypB with CNX and CRT is only depicted in diagrams c and d. (e, f) Gls2 and UGGT promote cyclic binding and release of glycoproteins form CNX (d) and CRT(c). (g,h) ERMan1 cleavage of the B-branch mannose residue reduces the reglucosylation of the glycan by UGGT. (i, j) Folded glycoproteins that exit the CNX/CRT cycle are trimmed by ERMan1 to yield protein linked GN2M8–9. (k) Secretory cargo proteins can be recognized by the lectins ERGIC53, VIP36 and VIPL. Non-glycosylated glycoproteins (l) do not enter the CNX/CRT cycle and are prone to misfolding (m). (n) The diagram shows the symbol code for saccharide residues in GN2M9G3, and indicates the location of the A, B and C branches. (o) The folding status of glycoproteins in diagrams a–m. The gray arrow in diagram h indicates that the pathway shown is less favored relative to that shown with black arrows.
Folded glycoproteins that exit the CNX/CRT cycle (Fig. 1i, 1j) are trimmed by ER mannosidase I to generate the GN2M8 oligosaccharide that is found on ER resident glycoproteins as well as secretory cargo glycoproteins. ER lectins that bind high mannose oligosaccharides (VIP36, ERGIC-53 and VIPL, Fig. 1k) act as cargo receptors during subsequent formation of ER to Golgi transport vesicles [1]. Defective protein N-glycosylation yields polypeptides that lack the glycans that allow entry into the CNX/CRT cycle (Fig. 1l). One fate for hypoglycosylated proteins is misfolding (Fig. 1m), which results in ER-associated degradation of the polypeptide.
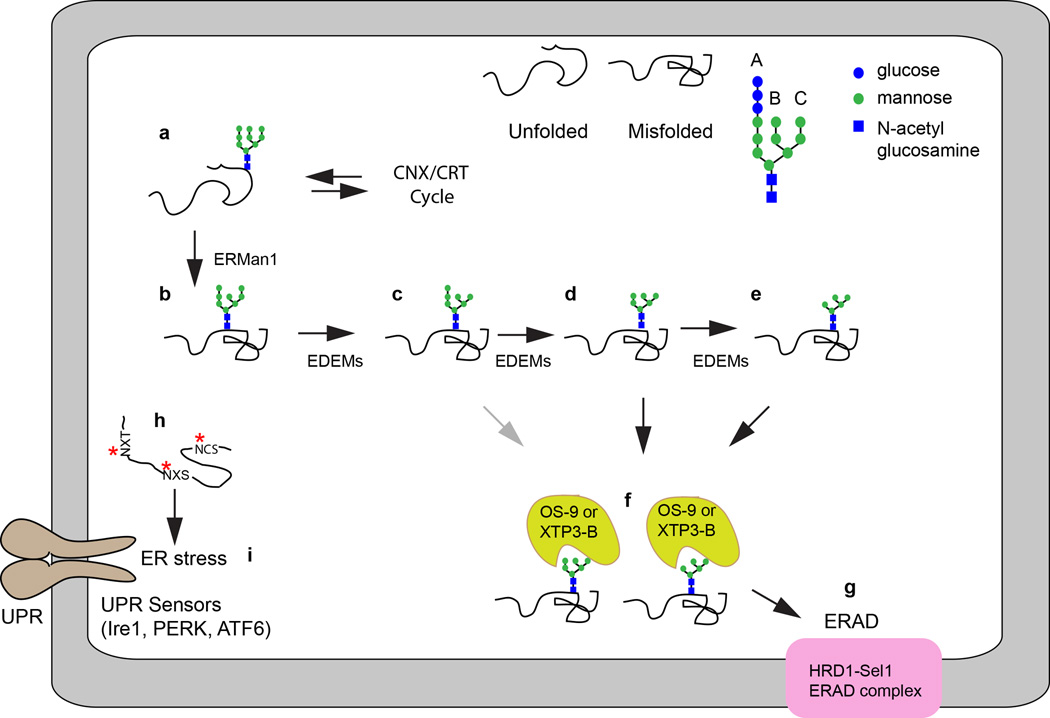
Glycoproteins that exit the CNX/CRT cycle without achieving a folded conformation (Fig. 2a) are trimmed by ERMan1, which is thought to act as a timer to prevent futile CNX/CRT cycles for terminally misfolded proteins (Fig. 2b). The N-linked glycans are sequentially trimmed by the ER degradation enhancing mannosidases (EDEM1, EDEM2 and EDEM3) to expose GN2M5–7 glycans (Fig. 2c–2e). The affinity between these trimmed glycans and the ER lectins OS-9 and XTPB-3 increases as mannose residues are removed (Fig. 2f). OS-9 and XTP3-B promote delivery of unfolded glycoproteins to the HRD1-SEL1 complex (Fig. 2g) for ER associated degradation (ERAD) (as reviewed by [2,3]). Thus, both the protein folding branch and the protein degradation branch of the glycoprotein quality control pathway are dependent upon the accurate and efficient addition of the fully assembled N-linked oligosaccharides to nascent proteins.
Figure 2.
Trimmed N-linked glycans promote ERAD of terminally misfolded proteins. The glycans on unfolded glycoproteins that exit the CNX/CRT cycle (a) are trimmed by ERMan1 (b). Successive processing by α1–2 mannosidase activity of the EDEMs yields protein-linked glycans on misfolded proteins with 5–7 mannose residues (c–e) that are recognized by the ERAD lectins OS-9 and XTP3-B (f) for targeting to the HRD1-Sel1 complex (g) for subsequent retrotranslocation of the misfolded protein into the cytosol. Accumulation of hypoglycosylated misfolded glycoproteins (h, red asterisks indicate skipped sequons) is one cause of ER stress that is sensed by the UPR sensors (i). For simplicity, only a single stress sensor (Ire1, PERK or ATF-6) is shown. The gray arrow in diagram f indicate that the pathway shown is less favored relative to those shown with black arrows.
Given the central role of N-linked glycans in glycoprotein folding pathways, it is not surprising that hypoglycosylation of proteins (Fig. 2h) is one cause of ER protein folding stress that induces the unfolded protein response pathway (UPR, Fig. 2i). Defects in the dolichol oligosaccharide assembly pathway or mutations in oligosaccharyltransferase subunits cause a chronic induction of the UPR pathway. The ER stress sensor proteins, Ire1, PERK, and ATF6 are activated by accumulation of unfolded proteins to up-regulate protein folding machinery and decrease the burden of unfolded proteins in the ER to mitigate ER stress (as reviewed by [4]).
Metazoan cells express two oligosaccharyltransferase complexes
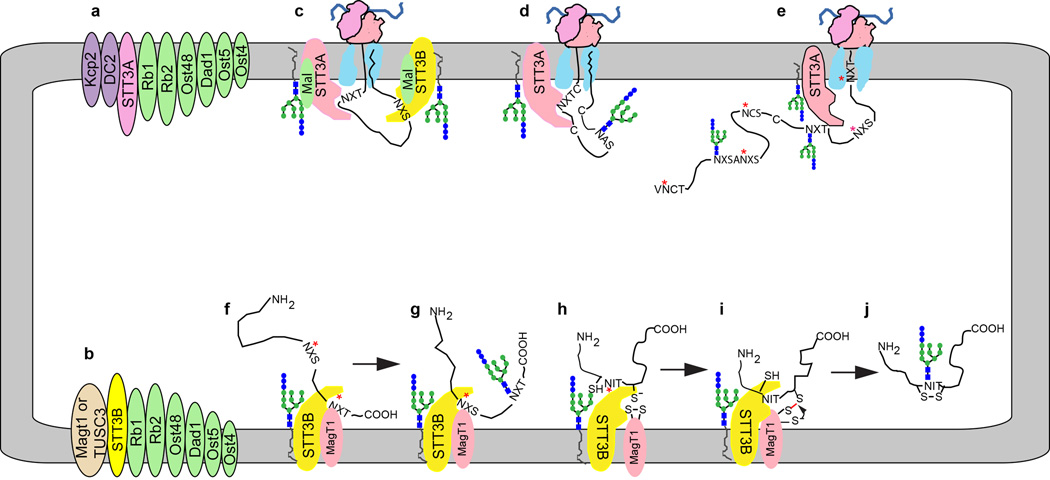
The oligosaccharyltransferase catalyzes the transfer of a preassembled oligosaccharide from a lipid linked oligosaccharide donor onto the asparagine residue of glycosylation acceptor sites (typically N-X-T/S/C where X≠P) or sequons in newly synthesized proteins. Glycoproteomic analysis of cells or tissues from seven model organisms including fungi (S. cerevisiae and S. pombe), plants (A. thaliana) and metazoa (C. elegans, D. rerio, D. melanogaster and M. musculus) indicates that the number of glycoproteins and the total number of modified sequons expanded 10–20 fold during eukaryotic evolution [5,6]. Expansion of the glycoproteome correlates with a duplication of the gene encoding the active site subunit of the OST (STT3) in multicellular plants and metazoa. Most metazoan organisms assemble two OST complexes (Fig. 3a and 3b) that are composed of a catalytic subunit (STT3A or STT3B), a set of shared subunits (ribophorins 1 and 2, OST48, DAD1, OST4 and TMEM258 in mammalian cells) as well as complex-specific accessory subunits that only assemble with STT3A (DC2 and KCP2) or STT3B (MagT1 or TUSC3) [7–9]. TMEM258, which is the long sought human homologue of the yeast Ost5 protein [10], was recently identified as an essential human gene by analysis of a haploid human cell line [11]. TMEM258 co-immunoprecipitates with subunits of both the STT3A and STT3B complexes, and is necessary for normal glycosylation of prosaposin [11]. Alignment of STT3 sequences from diverse eukaryotes as well as phylogenetic analysis of the complex-specific accessory subunits indicate that the OST complex of fungi and certain protists is similar to the metazoan STT3B complex [12]. The in vivo role of the STT3A and STT3B complexes has been the subject of a recent review [13].
Figure 3.
Cooperation between the STT3A and STT3B complexes maximizes protein glycosylation efficiency. (a, b) Subunit composition of the STT3A and STT3B complexes. Ribophorin 1, ribophorin 2 and TMEM258 are abbreviated as Rb1, Rb2 and Ost5, respectively. (c) Cotranslational translocation of a nascent glycoprotein through the Sec61 protein translocation channel allows cotranslational glycosylation of acceptor sequons by the STT3A complex. The malectin-OST association is only shown in this diagram for simplicity. (d) Cotranslational glycosylation of cysteine rich proteins before disulfide bond formation. (e) Examples of acceptor sequons that have been skipped by the STT3A complex including extreme N and C-terminal sites, closely spaced NXS sites, cysteine proximal sites (NCS) and a poorly understood class of non-optimal sequons (designated as NXS). (f, g) Posttranslocational glycosylation of extreme C-terminal sequons (red asterisks) does not occur by an N to C-terminal scanning mechanism but is instead determined by the relative affinity of the STT3B active site for NXT and NXS sequons. (h–j) Posttranslocational glycosylation of cysteine proximal sequons by the STT3B complex can be facilitated by reversible formation of a mixed disulfide between MagT1 (or TUSC3) and the unfolded glycoprotein. Red asterisks in diagrams e–h indicate skipped acceptor sites.
Several ER membrane proteins including malectin and TREX1 interact with OST complexes [14] or overexpressed OST subunits [15]. Malectin is an ER-localized lectin expressed by metazoan organisms that binds the terminal Glc α,1–3 Glc disaccharide in the GN2M9G2 trimming intermediate of protein bound glycans [16,17]. The role of malectin in ER homeostasis is not well understood, in part because the recognition determinant (Glc α,1–3 Glc disaccharide) for malectin is a transient trimming intermediate that precedes formation of the monoglucosylated glycan that is recognized by calnexin and calreticulin [1]. Overexpression of malectin can interfere with glycan trimming reactions and with glycoprotein secretion of both wild type proteins [18], and the terminally misfolded NHK variant of α-1 antitrypsin inhibitor (AT) [19]. Malectin is associated with both the STT3A and STT3B complexes [14] via a direct interaction with ribophorin I subunit [20] (Fig. 3c). A truncated malectin derivative that does not bind ribophorin I does not reduce ATNHK secretion [21]. The preferential association of malectin with ATNHK or misfolded HA conformers has led to the hypothesis that a malectin-glycan interaction can pre-emptively divert proteins into the ERAD pathway [18,19]. Given that malectin is readily detected in OST complexes, it remains unclear how the malectin-glycan interaction could be regulated to prevent ERAD targeting of folding-competent nascent glycoproteins that should enter the CNX/CRT cycle. Further insight into the role of malectin awaits experimental approaches that do not rely upon overexpression of malectin or ribophorin I.
Cotranslational glycosylation by the STT3A complex
Acceptor sites in a nascent polypeptide first have access to the OST active site when the asparagine residue in a sequon is 65–75 residues from the peptidyltransferase site on the translating ribosome [22,23]. The OST active site, which is located approximately 30Å from the lumenal surface of the membrane [24,25], is quite near (~20–30Å) the lumenal face of the protein translocation channel as indicated by a recent mid-resolution structures of native translocons obtained by cryoelectron tomography [26,27]. Proteomic and western-blot analysis of ribosome-associated ER membrane proteins established that the STT3A complex interacts with the Sec61 protein translocation channel [8,28,29], while photocrosslinking experiments established that acceptor sequons in ribosome-bound nascent polypeptides contact STT3A, not STT3B [30], hence the STT3A complex is positioned adjacent to the protein translocation channel (Fig. 3c).
Bioinformatic and biochemical analysis of closely spaced acceptor sites in glycoproteins indicated that acceptor sites in a nascent glycoprotein are recognized by an N-terminal to C-terminal scanning mechanism [31]. STT3A dependent glycosylation of adjacent sites (N-X-T/S-N-X-T/S) or gap-1 sites (N-X-T/S-Z-N-X-T/S) is efficient when both sites have threonine as the +2 residue. Serine at the +2 residue will promote sequon skipping by STT3A, and incomplete modification by STT3B [31]. Based upon the observation that the UPR pathway is strongly induced in cells lacking the STT3A complex [32], we propose that the majority of acceptor sites in human glycoproteins are cotranslationally glycosylated by the STT3A complex as the nascent glycoprotein enters the ER lumen (Fig. 3c).
Human cells that express reduced amounts of STT3A [28] or an HEK293 derived STT3A(−/−) cell line [32] display reduced glycosylation of a subset of glycoproteins. Glycoprotein substrates that are particularly sensitive to STT3A depletion (e.g., prosaposin, progranulin and transferrin) are characterized by a high cysteine content, and for prosaposin and progranulin have multiple independent folding domains [12,28,33] that oxidize rapidly upon entry into the ER lumen [28]. The crystal structures of a bacterial OST (C. lari PglB) in a complex with an acceptor peptide indicates that the asparagine residue in the sequon projects through a narrow porthole in the active site that is closed by a flexible loop between two transmembrane spans [25]. Localization of the STT3A complex adjacent to the translocation channel allows the OST to scan the growing polypeptide chain for acceptor sequons and transfer oligosaccharides cotranslationally before disulfide bond formation will stabilize the protein in a conformation that is incompatible with the entry of the acceptor sequon into the OST active site (Fig. 3d).
Glycosylation of skipped acceptor sites by the STT3B complex
The major cellular role for the STT3B complex is to maximize sequon occupancy by glycosylating acceptor sites that are skipped by the STT3A complex. STT3B acts either in a cotranslational (Fig. 3c) or posttranslocational mode (Fig. 3f–3i) that likely depends upon the location of the skipped site relative to the protein C-terminus [12,28]. Acceptor sites located in the C-terminal 50 residues of proteins are inaccessible to the STT3A complex until chain termination occurs. Extreme C-terminal acceptor sites are skipped at a high frequency by the STT3A complex, and are posttranslocationally glycosylated by the STT3B complex [12]. Posttranslocational glycosylation does not involve a polypeptide scanning mechanism as extreme C-terminal sites in a single protein are modified at different rates (Fig. 3f, 3g). The rates and efficiency of posttranslocational glycosylation are higher for NXT sequons than for NXS sequons consistent with the relative binding affinity of the OST active site for acceptor peptides (NXT > NXS >>NXC) and the enrichment of NXT glycopeptides among modified extreme C-terminal acceptor sites [12].
Other acceptor sites that have been observed to be skipped by the STT3A complex (Fig. 3e) include the following: (a) sites that are within five residues of the signal sequence cleavage site [14,28], (b) closely spaced NXS sites [31], (c) NXS sites in small type I membrane protein [34–36], (d) internal acceptor sites with non-optimal sequons including the majority of NXC sites, and (e) a subset of acceptor sites that are close to cysteine residues including NCT/S sites [14]. The identification of local sequence features that cause sequon skipping by the STT3A complex will require glycoproteomic analysis of STT3B deficient cell lines. Glycosylation of skipped sites by STT3B is limited by the rates of competing reactions including folding of the nascent glycoprotein and diffusion of the substrate away from the lumenal surface of the ER membrane.
MagT1 and TUSC3 are oxidoreductase subunits of the STT3B complex
MagT1 and TUSC3 were originally proposed to be subunits of the mammalian OST based upon 20% sequence identity to the yeast Ost3 and Ost6 proteins [7]. All four proteins are composed of a conserved lumenal domain followed by four transmembrane spanning segments. The structures of the lumenal domains of TUSC3 and Ost6, as solved by X-ray crystallography [37] and NMR respectively [38], consists of a thioredoxin fold with a conserved active site CXXC motif. Mass spectrometry and co-immunoprecipitation experiments demonstrated that MagT1 is a subunit of the STT3B complex [14]. Analysis of MagT1-depleted HeLa cells indicated that MagT1 is required for glycosylation of all STT3B dependent substrates tested to date [14]. MagT1 is predominantly in the oxidized state in vivo and is thought to form mixed disulfides with free thiols in nascent glycoprotein substrates (Fig. 3h– 3j), thereby delaying disulfide bond formation until nearby acceptor sites have been glycosylated by STT3B [14]. The active site CXXC motif in MagT1, Ost3 and Ost6 is required for full activity of the mammalian STT3B complex and the yeast OST complex [14,38,39]. Given the unexpected finding that MagT1 is necessary for glycosylation of STT3B dependent sequons that lack proximal cysteine residues, we have proposed that the oxidoreductases subunits of the STT3B complex have a role in substrate recruitment [14]. TUSC3 is assembled into the STT3B complex in the HEK293 derived MagT1(−/−) cell line [32] supporting the view that these closely related proteins (73% sequence identity) are functionally redundant [14], at least in tissues or cells where both proteins are expressed.
Despite an evolutionarily conserved role for MagT1 and TUSC3 as oxidoreductase subunits of the oligosaccharyltransferase, there are numerous publications reporting that MagT1 and TUSC3 are cell-surface magnesium transporters [40,41]. However, immunofluorescence staining of MagT1 in HeLa cells [14], T-cells [42], and rat bone marrow osteosarcoma cells [43] is consistent with an intracellular localization for MagT1 in the endoplasmic reticulum. Structurally, MagT1 and TUSC3 are not homologous to any currently identified metal ion transporter. A dual role for MagT1 and TUSC3 as OST subunits and cell surface magnesium transporters is inconsistent with the discovery that MagT1 and TUSC3 are not detectably expressed at the protein level in STT3B(−/−) HEK293 derived cell line [32], as these OST subunits are unstable in the absence of STT3B. The initial evidence that MagT1 mRNA and protein expression was induced in cultured cells exposed to low magnesium in the growth media [40,41] has since been contradicted by studies using other cell types [44,45]. Overexpression of MagT1 alone does not increase the Mg2+ concentration in cells [41]. MagT1 deficient human lymphocytes display altered kinetics of Mg2+ uptake, but have normal cellular levels of Mg2+ [42]. We favor the hypothesis that the link between MagT1 or TUSC3 expression and magnesium ion homeostasis [41,42] likely occurs by an indirect mechanism involving STT3B complex dependent glycosylation of a protein that is needed for Mg2+ transport activity.
The name TUSC3 (tumor suppressor candidate 3) was based upon the initial discovery that TUSC3 is one of several genes that reside within a chromosomal deletion associated with metastatic prostate cancer [46]. A recent reports confirms that TUSC3 is a subunit of the OST complex, and shows that loss of TUSC3 is associated with alterations in ER homeostasis, enhanced migration of cells, and increased tumor formation in nude mouse xenograft model [47].
Cryptic sequons can serve as markers for the ERAD pathway
Interestingly, glycosylation of a normally unutilized sequon (i.e., cryptic sequon) can enhance entry of unfolded proteins into the ERAD pathway. Transthyretin contains a cryptic glycosylation site that undergoes posttranslocational glycosylation by the STT3B complex when terminally misfolded [48]. The ER chaperone GRP94 has six sequons, but is primarily glycosylated at a single site in the folded protein. Hyperglycosylated variants of GRP94 that have nonnative conformations bind to the ER lectin OS-9 and are targeted for degradation by an ERAD independent pathway [49]. It is unclear what features of the internal cryptic sequons in GRP94 favor high frequency sequon skipping by the STT3A complex to yield the native protein. Neuroserpin has a cryptic C-terminal glycosylation site (N401) that is partially glycosylated in the G392E neuroserpin mutant [50]. The point mutation (G392E) in the vicinity of the N401 sequon presumably allows STT3B dependent posttranslocational glycosylation, thereby enhancing delivery of neuroserpin to the ERAD machinery. Taken together, these examples illustrate how hyperglycosylation of proteins on cryptic acceptor sites can serve as a mechanism to enhance N-glycan-dependent protein degradation.
Protein hypoglycosylation causes a family of human diseases
Mutations in the proteins that are responsible for the biosynthesis and processing of N-linked and O-linked glycans cause a family of diseases known as congenital disorders of glycosylation [51]. CDG categories that impact ER homeostasis include (a) defects in the synthesis of sugar nucleotides, (b) defects in enzymes responsible for Dol-PP-GN2M9G3 synthesis and (c) defects in subunits of the OST or the translocation channel associated TRAP complex. Notably, defects in Dol-PP-GN2M9G3 assembly pathway negatively impact ER homeostasis not only by causing hypoglycosylation of proteins due to the presence of the non-optimal donor substrate, but in addition the nascent glycoproteins carry glycans with structures that bypass initial events in the CNX/CRT cycle. Here, we will focus on OST and TRAP mutations that cause CDG, as defects in donor substrate biosynthesis have been reviewed extensively [52,53]. A mutation in the OST subunit OST48 (DDOST) causes a general defect in N-glycosylation [54], while mutations in the STT3A or STT3B genes cause selective hypoglycosylation of STT3A or STT3B specific substrates [33]. Mutations in SSR4, a subunit of the TRAP complex, causes hypoglycosylation of proteins [55,56], presumably because the TRAP complex interacts with the Sec61 translocation channel and the STT3A complex [8,29].
Mutations in the TUSC3 gene have been identified in individuals with nonsyndromic autosomal recessive mental retardation (ARMR), a disease primarily characterized by intellectual impairment [57–60]. Glycosylation of serum transferrin, the standard diagnostic marker for CDG, is normal in patients with a TUSC3 deficiency [57,59] and was near-normal in the patient with the STT3B deficiency [33], consistent with the evidence that transferrin is an STT3A dependent substrate [12]. Consequently, isoelectric focusing of serum transferrin is not a satisfactory diagnostic marker for patients with mutations in the STT3B, MagT1 or TUSC3 genes. The observation that a TUSC3 deficiency or a MagT1 deficiency (see below) does not cause the multi-systemic developmental defects typical for most CDG variants is likely explained by the apparent functional redundancy of MagT1 and TUSC3 and the substantial overlap in tissue-specific expression. MagT1 is more widely expressed, while TUSC3 expression is more restricted [41,57].
Mutations in the human MagT1 gene cause XMEN syndrome, an X-linked immunodeficiency with Mg+2 defect, chronic Epstein-Barr virus (EBV) infection, and neoplasia (as reviewed in [61]). Of the 10 reported patients to date [62–64] all are male and the frequency of female carriers has yet to be determined [42,61]. XMEN patients have normal growth and development indicating that the previous hypothesis that mutations in MagT1 are one cause of X-linked intellectual disability [57] was premature [65]. In our opinion, further insight into the etiology of XMEN disease could be obtained by exploring the hypothesis that the proximal cause of XMEN is a defect in protein N-glycosylation while the alteration in magnesium homeostasis is one of several secondary consequences of the N-glycosylation defect.
The fully assembled oligosaccharide donor (Dol-PP-GN2M9G3) is more stringently selected by the STT3A complex than the STT3B complex [7]. Accurate donor substrate selection insures that newly synthesized glycoproteins carry the correct glycans for entry into the glycoprotein quality control pathways. Analysis of the CHO derived MI8-5 cell line, which assembles Dol-PP-GN2M9 as the oligosaccharide donor due to a premature stop codon in the ALG6 gene, revealed that a reduction in expression of STT3B acts synergistically with the ALG6 deficiency to exacerbate hypoglycosylation of proteins [66]. We propose that OST subunit genes should be considered as likely modifier genes that will influence the disease severity of patients with variants of CDG that impact the dolichol-oligosaccharide assembly pathway.
Future perspectives
Efficient and accurate glycosylation of proteins in the endoplasmic reticulum by the STT3A and STT3B complexes is crucial for normal cellular growth and development. In this review we have summarized how N-glycosylation is achieved by expression of two distinct OST complexes with exclusive accessory subunits. We have highlighted recent progress in the investigation of a family of human diseases caused by mutations in OST subunit genes. Further investigation into protein homeostasis in the endoplasmic reticulum will likely reveal unanticipated links between the various pathways that control the fate of newly synthesized proteins in the endoplasmic reticulum.
Acknowledgments
This work was supported by a research grant from the National Institutes of Health [GM43768].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
③ Of special interest
③③ Of outstanding interest
- 1. Tannous A, Pisoni GB, Hebert DN, Molinari M. N-linked sugar-regulated protein folding and quality control in the ER. Semin Cell Dev Biol. 2015;41:79–89. doi: 10.1016/j.semcdb.2014.12.001. ③③ A recent comprehensive review of the calnexin/calreticulin cycle. Particular attention is focused upon the roles of chaperones (PDI and peptidyl-prolyl isomerase families) that promote folding of CNX and CRT-bound proteins and the ER enzymes (UGGT, glucosidases and mannosidases) that regulate glycoprotein entry into and exit from the CNX/CRT cycle.
- 2.Caramelo JJ, Parodi AJ. A sweet code for glycoprotein folding. FEBS Lett. 2015;589:3379–3387. doi: 10.1016/j.febslet.2015.07.021. [DOI] [PubMed] [Google Scholar]
- 3.Benyair R, Ogen-Shtern N, Lederkremer GZ. Glycan regulation of ER-associated degradation through compartmentalization. Semin Cell Dev Biol. 2015;41:99–109. doi: 10.1016/j.semcdb.2014.11.006. [DOI] [PubMed] [Google Scholar]
- 4.Gardner BM, Pincus D, Gotthardt K, Gallagher CM, Walter P. Endoplasmic reticulum stress sensing in the unfolded protein response. Cold Spring Harb Perspect Biol. 2013;5:a013169. doi: 10.1101/cshperspect.a013169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zielinska DF, Gnad F, Schropp K, Wisniewski JR, Mann M. Mapping N-glycosylation sites across seven evolutionarily distant species reveals a divergent substrate proteome despite a common core machinery. Mol. Cell. 2012;46:542–548. doi: 10.1016/j.molcel.2012.04.031. [DOI] [PubMed] [Google Scholar]
- 6.Zielinska DF, Gnad F, Wisniewski JR, Mann M. Precision mapping of an in vivo N-glycoproteome reveals rigid topological and sequence constraints. Cell. 2010;141:897–907. doi: 10.1016/j.cell.2010.04.012. [DOI] [PubMed] [Google Scholar]
- 7.Kelleher DJ, Karaoglu D, Mandon EC, Gilmore R. Oligosaccharyltransferase isoforms that contain different catalytic STT3 subunits have distinct enzymatic properties. Mol. Cell. 2003;12:101–111. doi: 10.1016/s1097-2765(03)00243-0. [DOI] [PubMed] [Google Scholar]
- 8.Shibatani T, David LL, McCormack AL, Frueh K, Skach WR. Proteomic analysis of mammalian oligosaccharyltransferase reveals multiple subcomplexes that contain Sec61, TRAP, and two potential new subunits. Biochemistry. 2005;44:5982–5992. doi: 10.1021/bi047328f. [DOI] [PubMed] [Google Scholar]
- 9.Roboti P, High S. The oligosaccharyltransferase subunits OST48, DAD1 and KCP2 function as ubiquitous and selective modulators of mammalian N-glycosylation. J. Cell Sci. 2012;125:3474–3484. doi: 10.1242/jcs.103952. [DOI] [PubMed] [Google Scholar]
- 10.Reiss G, te Heesen S, Gilmore R, Zufferey R, Aebi M. A specific screen for oligosaccharyltransferase mutations identifies the 9 kDa OST5 protein required for optimal activity in vivo and in vitro. Embo J. 1997;16:1164–1172. doi: 10.1093/emboj/16.6.1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Blomen VA, Majek P, Jae LT, Bigenzahn JW, Nieuwenhuis J, Staring J, Sacco R, van Diemen FR, Olk N, Stukalov A, et al. Gene essentiality and synthetic lethality in haploid human cells. Science. 2015;350:1092–1096. doi: 10.1126/science.aac7557. ③ TMEM258 was identified as an essential human gene that encodes a subunit of oligosaccharyltransferase (yeast Ost5 homologue). TMEM258 co-immunoprecipitates with subunits of the STT3A and STT3B complexes and its depletion reduced in vivo glycosylation of the glycoprotein prosaposin.
- 12. Shrimal S, Trueman SF, Gilmore R. Extreme C-terminal sites are posttranslocationally glycosylated by the STT3B isoform of the OST. J. Cell Biol. 2013;201:81–95. doi: 10.1083/jcb.201301031. ③③ Glycosylation sequons that are located in the last 50 residues of a protein sequence are skipped at a high frequency by the STT3A complex. The STT3B complex mediates posttranslocational glycosylation of extreme C-terminal sequons by a non-scanning mechanism.
- 13.Shrimal S, Cherepanova NA, Gilmore R. Cotranslational and posttranslocational N-glycosylation of proteins in the endoplasmic reticulum. Semin. Cell Dev. Biol. 2015;41:71–78. doi: 10.1016/j.semcdb.2014.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cherepanova NA, Shrimal S, Gilmore R. Oxidoreductase activity is necessary for N-glycosylation of cysteine-proximal acceptor sites in glycoproteins. J. Cell Biol. 2014;206:525–539. doi: 10.1083/jcb.201404083. ③③ This paper demonstrated that MagT1 is an ER-localized subunit of the STT3B complex. The active site CXXC motif in the thioredoxin domain of MagT1 is required for posttranslocational N-glycosylation of a panel of STT3B-dependent glycosylation sites including extreme C-terminal sites and cysteine-proximal acceptor sites.
- 15.Hasan M, Fermaintt CS, Gao N, Sakai T, Miyazaki T, Jiang S, Li QZ, Atkinson JP, Morse HC, 3rd, Lehrman MA, et al. Cytosolic Nuclease TREX1 Regulates Oligosaccharyltransferase Activity Independent of Nuclease Activity to Suppress Immune Activation. Immunity. 2015 doi: 10.1016/j.immuni.2015.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schallus T, Feher K, Sternberg U, Rybin V, Muhle-Goll C. Analysis of the specific interactions between the lectin domain of malectin and diglucosides. Glycobiology. 2010;20:1010–1020. doi: 10.1093/glycob/cwq059. [DOI] [PubMed] [Google Scholar]
- 17.Schallus T, Jaeckh C, Feher K, Palma AS, Liu Y, Simpson JC, Mackeen M, Stier G, Gibson TJ, Feizi T, et al. Malectin: a novel carbohydrate-binding protein of the endoplasmic reticulum and a candidate player in the early steps of protein N-glycosylation. Mol. Biol. Cell. 2008;19:3404–3414. doi: 10.1091/mbc.E08-04-0354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Galli C, Bernasconi R, Solda T, Calanca V, Molinari M. Malectin participates in a backup glycoprotein quality control pathway in the mammalian ER. PLoS One. 2011;6:e16304. doi: 10.1371/journal.pone.0016304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen Y, Hu D, Yabe R, Tateno H, Qin SY, Matsumoto N, Hirabayashi J, Yamamoto K. Role of malectin in Glc(2)Man(9)GlcNAc(2)-dependent quality control of alpha1-antitrypsin. Mol. Biol. Cell. 2011;22:3559–3570. doi: 10.1091/mbc.E11-03-0201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qin SY, Hu D, Matsumoto K, Takeda K, Matsumoto N, Yamaguchi Y, Yamamoto K. Malectin forms a complex with ribophorin I for enhanced association with misfolded glycoproteins. J. Biol. Chem. 2012;287:38080–38089. doi: 10.1074/jbc.M112.394288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takeda K, Qin SY, Matsumoto N, Yamamoto K. Association of malectin with ribophorin I is crucial for attenuation of misfolded glycoprotein secretion. Biochem Biophys Res Commun. 2014;454:436–440. doi: 10.1016/j.bbrc.2014.10.102. [DOI] [PubMed] [Google Scholar]
- 22.Whitley P, Nilsson IM, von Heijne G. A nascent secretory protein may traverse the ribosome/endoplasmic reticulum translocase complex as an extended chain. J. Biol. Chem. 1996;271:6241–6244. doi: 10.1074/jbc.271.11.6241. [DOI] [PubMed] [Google Scholar]
- 23.Deprez P, Gautschi M, Helenius A. More than one glycan is needed for ER glucosidase II to allow entry of glycoproteins into the calnexin/calreticulin cycle. Mol. Cell. 2005;19:183–195. doi: 10.1016/j.molcel.2005.05.029. [DOI] [PubMed] [Google Scholar]
- 24.Nilsson I, von Heijne G. Determination of the distance between the oligosaccharyltransferase active site and the endoplasmic reticulum membrane. J. Biol. Chem. 1993;268:5798–5801. [PubMed] [Google Scholar]
- 25.Lizak C, Gerber S, Numao S, Aebi M, Locher KP. X-ray structure of a bacterial oligosaccharyltransferase. Nature. 2011;474:350–355. doi: 10.1038/nature10151. [DOI] [PubMed] [Google Scholar]
- 26.Pfeffer S, Burbaum L, Unverdorben P, Pech M, Chen Y, Zimmermann R, Beckmann R, Forster F. Structure of the native Sec61 protein-conducting channel. Nat Commun. 2015;6:8403. doi: 10.1038/ncomms9403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pfeffer S, Dudek J, Gogala M, Schorr S, Linxweiler J, Lang S, Becker T, Beckmann R, Zimmermann R, Forster F. Structure of the mammalian oligosaccharyl-transferase complex in the native ER protein translocon. Nat. Commun. 2014;5:3072. doi: 10.1038/ncomms4072. ③ Cryoelectron tomography of the native protein translocation channel in ER membranes provides evidence that an OST complex is positioned adjacent to the Sec61 complex. The observed protein density was absent in membranes from cells treated with siRNA for Rb-1 or Rb-2, which are present in both the STT3A and STT3B complexes.
- 28.Ruiz-Canada C, Kelleher DJ, Gilmore R. Cotranslational and posttranslational N-glycosylation of polypeptides by distinct mammalian OST isoforms. Cell. 2009;136:272–283. doi: 10.1016/j.cell.2008.11.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Conti BJ, Devaraneni PK, Yang Z, David LL, Skach WR. Cotranslational stabilization of Sec62/63 within the ER Sec61 translocon is controlled by distinct substrate-driven translocation events. Mol Cell. 2015;58:269–283. doi: 10.1016/j.molcel.2015.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nilsson I, Kelleher DJ, Miao Y, Shao Y, Kreibich G, Gilmore R, Von Heijne G, Johnson AE. Photocross-linking of nascent chains to the STT3 subunit of the oligosaccharyltransferase complex. J. Cell Biol. 2003;161:715–725. doi: 10.1083/jcb.200301043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shrimal S, Gilmore R. Glycosylation of closely spaced acceptor sites in human glycoproteins. J. Cell Sci. 2013;126:5513–5523. doi: 10.1242/jcs.139584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cherepanova NA, Gilmore R. Mammalian cells lacking either the cotranslational or posttranslocational oligosaccharyltransferase complex display substrate-dependent defects in asparagine linked glycosylation. Scientific Reports. 2016 doi: 10.1038/srep20946. in press. ③ The CRISPR/Cas9 gene editing was used to generate viable HEK293 derived cell lines that do not express STT3A or STT3B or both oxidoreductase subunits (MagT1 and TUSC3). MagT1 and TUSC3 are both unstable in cells that do not express STT3B. Glycosylation of a panel of glycoprotein substrates in the knockout cell lines showed that certain glycosylation sites can be efficiently glycosylated by either the STT3A or STT3B complex.
- 33.Shrimal S, Ng BG, Losfeld ME, Gilmore R, Freeze HH. Mutations in STT3A and STT3B cause two congenital disorders of glycosylation. Hum. Mol. Genet. 2013;22:4638–4645. doi: 10.1093/hmg/ddt312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bas T, Gao GY, Lvov A, Chandrasekhar KD, Gilmore R, Kobertz WR. Post-translational N-glycosylation of type I transmembrane KCNE1 peptides: implications for membrane protein biogenesis and disease. J. Biol. Chem. 2011;286:28150–28159. doi: 10.1074/jbc.M111.235168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Malaby HL, Kobertz WR. Molecular determinants of co- and post-translational N-glycosylation of type I transmembrane peptides. Biochem. J. 2013;453:427–434. doi: 10.1042/BJ20130028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Malaby HL, Kobertz WR. The middle×residue influences cotranslational N-glycosylation consensus site skipping. Biochemistry. 2014;53:4884–4893. doi: 10.1021/bi500681p. ③ The frequency of acceptor site skipping of NXS sequons by the STT3A complex is increased by unfavorable X residues (W, L, F, I and E). Frequently skipped sequons (e.g., NWS) are also non-optimal acceptor sites for posttranslocational glycosylation by the STT3B complex.
- 37. Mohorko E, Owen RL, Malojcic G, Brozzo MS, Aebi M, Glockshuber R. Structural basis of substrate specificity of human oligosaccharyl transferase subunit N33/Tusc3 and its role in regulating protein N-glycosylation. Structure. 2014;22:590–601. doi: 10.1016/j.str.2014.02.013. ③③ The X-ray crystal structure of the lumenal domain of TUSC3 has a thioredoxin fold. Structures of TUSC3 with disulfide linked model substrate peptides reveal a peptide binding groove containing the active site CXXC motif. TUSC3 is proposed to delay glycoprotein folding to allow glycosylation of cysteine-proximal acceptor sites.
- 38.Schulz BL, Stirnimann CU, Grimshaw JP, Brozzo MS, Fritsch F, Mohorko E, Capitani G, Glockshuber R, Grutter MG, Aebi M. Oxidoreductase activity of oligosaccharyltransferase subunits Ost3p and Ost6p defines site-specific glycosylation efficiency. Proc. Natl. Acad. Sci. USA. 2009;106:11061–11066. doi: 10.1073/pnas.0812515106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schulz BL, Aebi M. Analysis of glycosylation site occupancy reveals a role for Ost3p and Ost6p in site-specific N-glycosylation efficiency. Mol. Cell. Proteomics. 2009;8:357–364. doi: 10.1074/mcp.M800219-MCP200. [DOI] [PubMed] [Google Scholar]
- 40.Goytain A, Quamme GA. Identification and characterization of a novel mammalian Mg2+ transporter with channel-like properties. BMC Genomics. 2005;6:48. doi: 10.1186/1471-2164-6-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhou H, Clapham DE. Mammalian MagT1 and TUSC3 are required for cellular magnesium uptake and vertebrate embryonic development. Proc. Natl. Acad. Sci. USA. 2009;106:15750–15755. doi: 10.1073/pnas.0908332106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li FY, Chaigne-Delalande B, Kanellopoulou C, Davis JC, Matthews HF, Douek DC, Cohen JI, Uzel G, Su HC, Lenardo MJ. Second messenger role for Mg2+ revealed by human T-cell immunodeficiency. Nature. 2011;475:471–476. doi: 10.1038/nature10246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zheng J, Mao X, Ling J, Chen C, Zhang W. Role of Magnesium Transporter Subtype 1 (MagT1) in the Osteogenic Differentiation of Rat Bone Marrow Stem Cells. Biol Trace Elem Res. 2015 doi: 10.1007/s12011-015-0459-4. [DOI] [PubMed] [Google Scholar]
- 44.Schweigel M, Kolisek M, Nikolic Z, Kuzinski J. Expression and functional activity of the Na/Mg exchanger, TRPM7 and MagT1 are changed to regulate Mg homeostasis and transport in rumen epithelial cells. Magnes Res. 2008;21:118–123. [PubMed] [Google Scholar]
- 45.Wolf FI, Trapani V, Simonacci M, Mastrototaro L, Cittadini A, Schweigel M. Modulation of TRPM6 and Na(+)/Mg(2+) exchange in mammary epithelial cells in response to variations of magnesium availability. J Cell Physiol. 2010;222:374–381. doi: 10.1002/jcp.21961. [DOI] [PubMed] [Google Scholar]
- 46.MacGrogan D, Levy A, Bova GS, Isaacs WB, Bookstein R. Structure and methylation-associated silencing of a gene within a homozygously deleted region of human chromosome band 8p22. Genomics. 1996;35:55–65. doi: 10.1006/geno.1996.0322. [DOI] [PubMed] [Google Scholar]
- 47.Horak P, Tomasich E, Vanhara P, Kratochvilova K, Anees M, Marhold M, Lemberger CE, Gerschpacher M, Horvat R, Sibilia M, et al. TUSC3 loss alters the ER stress response and accelerates prostate cancer growth in vivo. Sci. Rep. 2014;4:3739. doi: 10.1038/srep03739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sato T, Sako Y, Sho M, Momohara M, Suico MA, Shuto T, Nishitoh H, Okiyoneda T, Kokame K, Kaneko M, et al. STT3B-dependent posttranslational N-glycosylation as a surveillance system for secretory protein. Mol. Cell. 2012;47:99–110. doi: 10.1016/j.molcel.2012.04.015. [DOI] [PubMed] [Google Scholar]
- 49. Dersh D, Jones SM, Eletto D, Christianson JC, Argon Y. OS-9 facilitates turnover of nonnative GRP94 marked by hyperglycosylation. Mol Biol Cell. 2014;25:2220–2234. doi: 10.1091/mbc.E14-03-0805. ③ Hyperglycosylated variants of GRP94 are in a non-native conformation and are recognized by the OS-9 lectin. GRP94 is an example of a protein that has multiple cryptic glycosylation sites that are not glycosylated in the native protein.
- 50.Moriconi C, Ordonez A, Lupo G, Gooptu B, Irving JA, Noto R, Martorana V, Manno M, Timpano V, Guadagno NA, et al. Interactions between N-linked glycosylation and polymerisation of neuroserpin within the endoplasmic reticulum. FEBS J. 2015;282:4565–4579. doi: 10.1111/febs.13517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hennet T, Cabalzar J. Congenital disorders of glycosylation: a concise chart of glycocalyx dysfunction. Trends Biochem Sci. 2015;40:377–384. doi: 10.1016/j.tibs.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 52.Freeze HH. Understanding human glycosylation disorders: biochemistry leads the charge. J. Biol. Chem. 2013;288:6936–6945. doi: 10.1074/jbc.R112.429274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Haeuptle MA, Hennet T. Congenital disorders of glycosylation: an update on defects affecting the biosynthesis of dolichol-linked oligosaccharides. Hum. Mutat. 2009;30:1628–1641. doi: 10.1002/humu.21126. [DOI] [PubMed] [Google Scholar]
- 54.Jones MA, Ng BG, Bhide S, Chin E, Rhodenizer D, He P, Losfeld ME, He M, Raymond K, Berry G, et al. DDOST mutations identified by whole-exome sequencing are implicated in congenital disorders of glycosylation. Am. J. Hum. Genet. 2012;90:363–368. doi: 10.1016/j.ajhg.2011.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Losfeld ME, Ng BG, Kircher M, Buckingham KJ, Turner EH, Eroshkin A, Smith JD, Shendure J, Nickerson DA, Bamshad MJ, et al. A new congenital disorder of glycosylation caused by a mutation in SSR4, the signal sequence receptor 4 protein of the TRAP complex. Hum. Mol. Genet. 2014;23:1602–1605. doi: 10.1093/hmg/ddt550. ③ A mutation in a subunit of the TRAP complex (SSR4) causes a novel form of congenital disorders of glycosylation (SSR4-CDG). Mutations in the SSR4 likely cause hypoglycosylation of proteins due to the previously established interaction between the protein translocation channel, the TRAP complex and the STT3A complex.
- 56.Ng BG, Raymond K, Kircher M, Buckingham KJ, Wood T, Shendure J, Nickerson DA, Bamshad MJ, University of Washington Center for Mendelian G. Wong JT, et al. Expanding the Molecular and Clinical Phenotype of SSR4-CDG. Hum Mutat. 2015;36:1048–1051. doi: 10.1002/humu.22856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Molinari F, Foulquier F, Tarpey PS, Morelle W, Boissel S, Teague J, Edkins S, Futreal PA, Stratton MR, Turner G, et al. Oligosaccharyltransferase-subunit mutations in nonsyndromic mental retardation. Am. J. Hum. Genet. 2008;82:1150–1157. doi: 10.1016/j.ajhg.2008.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Garshasbi M, Kahrizi K, Hosseini M, Nouri Vahid L, Falah M, Hemmati S, Hu H, Tzschach A, Ropers HH, Najmabadi H, et al. A novel nonsense mutation in TUSC3 is responsible for non-syndromic autosomal recessive mental retardation in a consanguineous Iranian family. Am. J. Med. Genet. A. 2011;155A:1976–1980. doi: 10.1002/ajmg.a.34077. [DOI] [PubMed] [Google Scholar]
- 59.El Chehadeh S, Bonnet C, Callier P, Beri M, Dupre T, Payet M, Ragon C, Mosca-Boidron AL, Marle N, Mugneret F, et al. Homozygous Truncating Intragenic Duplication in TUSC3 Responsible for Rare Autosomal Recessive Nonsyndromic Intellectual Disability with No Clinical or Biochemical Metabolic Markers. JIMD Rep. 2015;20:45–55. doi: 10.1007/8904_2014_390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang MJ, Xing LX, Cui M, Yang X, Shi JG, Li J, Zhang KJ, Zheng ZJ, Zhang FC, Li JL, et al. Association of TUSC3 gene polymorphisms with non-syndromic mental retardation based on nuclear families in the Qinba mountain area of China. Genet Mol Res. 2015;14:5022–5030. doi: 10.4238/2015.May.12.5. [DOI] [PubMed] [Google Scholar]
- 61. Ravell J, Chaigne-Delalande B, Lenardo M. X-linked immunodeficiency with magnesium defect, Epstein-Barr virus infection, and neoplasia disease: a combined immune deficiency with magnesium defect. Curr Opin Pediatr. 2014;26:713–719. doi: 10.1097/MOP.0000000000000156. ③③ This is a detailed review summarizing multiple reports on the XMEN disease caused by mutations in the MagT1 gene. The review highlights recent progress in understanding disease pathogenesis and its link to Mg2+ homeostasis and Mg2+ signaling pathways.
- 62.Dhalla F, Murray S, Sadler R, Chaigne-Delalande B, Sadaoka T, Soilleux E, Uzel G, Miller J, Collins GP, Hatton CS, et al. Identification of a novel mutation in MAGT1 and progressive multifocal leucoencephalopathy in a 58-year-old man with XMEN disease. J Clin Immunol. 2015;35:112–118. doi: 10.1007/s10875-014-0116-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chaigne-Delalande B, Li FY, O'Connor GM, Lukacs MJ, Jiang P, Zheng L, Shatzer A, Biancalana M, Pittaluga S, Matthews HF, et al. Mg2+ regulates cytotoxic functions of NK and CD8 T cells in chronic EBV infection through NKG2D. Science. 2013;341:186–191. doi: 10.1126/science.1240094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Patiroglu T, Haluk Akar H, Gilmour K, Unal E, Akif Ozdemir M, Bibi S, Burns S, Chiang SC, Schlums H, Bryceson YT, et al. A case of XMEN syndrome presented with severe auto-immune disorders mimicking autoimmune lymphoproliferative disease. Clin Immunol. 2015;159:58–62. doi: 10.1016/j.clim.2015.04.015. ③ This paper describes a new XMEN patient with severe autoimmune disorders, thereby expanding the spectrum of diseases associated with XMEN syndrome. This article contains clinical summary for 10 XMEN patients described in the literature so far.
- 65.Piton A, Redin C, Mandel JL. XLID-causing mutations and associated genes challenged in light of data from large-scale human exome sequencing. Am. J. Hum. Genet. 2013;93:368–383. doi: 10.1016/j.ajhg.2013.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shrimal S, Gilmore R. Reduced expression of the oligosaccharyltransferase exacerbates protein hypoglycosylation in cells lacking the fully assembled oligosaccharide donor. Glycobiology. 2015;25:774–783. doi: 10.1093/glycob/cwv018. [DOI] [PMC free article] [PubMed] [Google Scholar]