Introduction
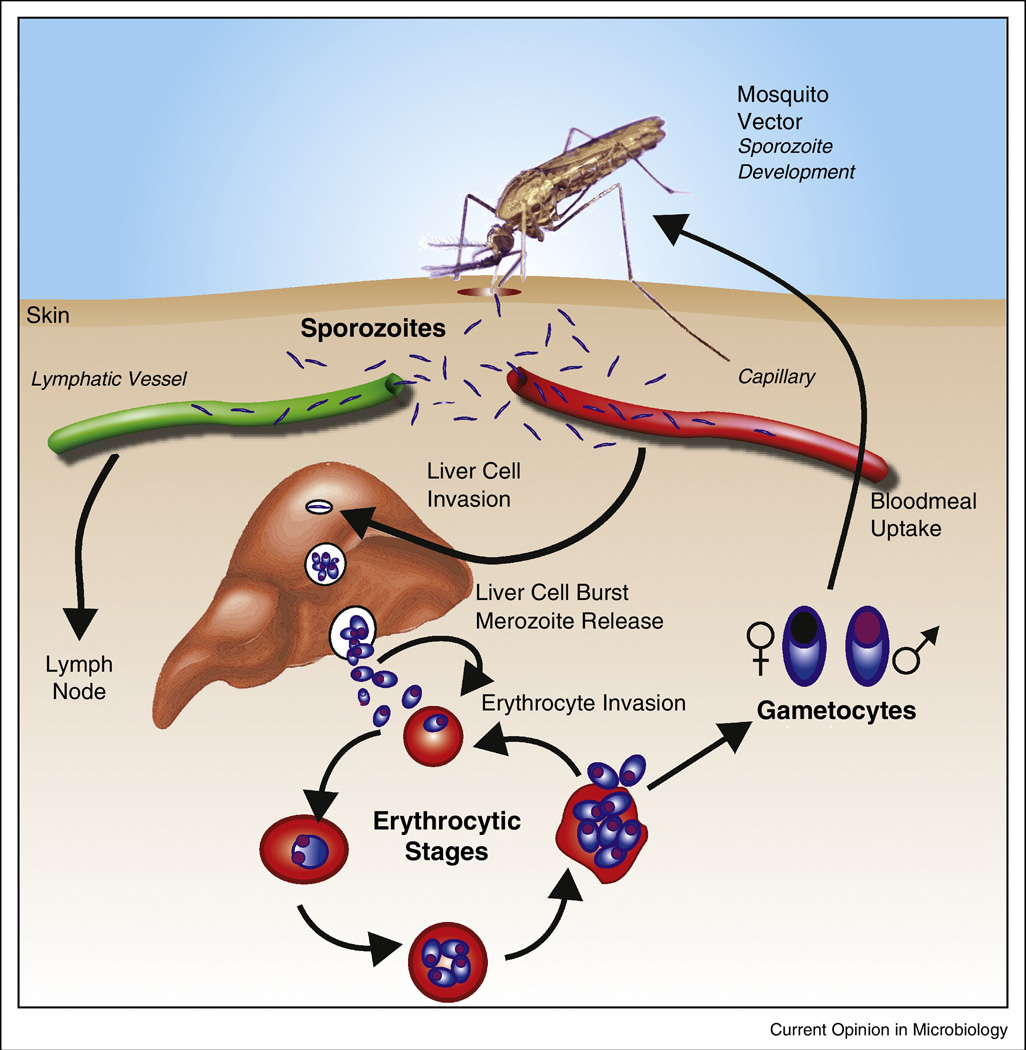
Despite considerable efforts over decades, we do not yet have a highly effective vaccine for any of the five plasmodial species which cause human malaria. While malaria deaths are reported to have declined between 2000 and 2013 [1], further inroads into this global scourge will require new tools, including a vaccine. Development of such a vaccine is complicated by the complex life cycle of the parasite involving both a vertebrate and an invertebrate host (Figure 1). Infectious sporozoites from the Anopheline vector enter the liver, replicate and differentiate there to asexual stage merozoites which can invade erythrocytes. Replication in red cells and accompanying host innate and acquired immune responses are responsible for all the clinical symptoms of disease. Finally small numbers of parasites differentiate into male and female gametocytes which can be picked up by the insect vector and thus complete the cycle. This review summarizes the status of malaria vaccine development, particularly focusing on Plasmodium falciparum since P. vivax vaccine approaches have been recently reviewed ([2], and why it has been so difficult to achieve protective immune responses to the various stages of these fascinating organisms.
Figure 1.
Life Cycle of Malaria Parasites
Sporozoites and Pre-Erythrocytic Stages of Infection
The notion that vaccine-induced immune responses against Plasmodium sporozoites could have a protective effect, inhibiting parasite infectivity in the vertebrate host, arises from early studies indicating that immunization of mice[3] and humans[4] with radiation-attenuated malaria sporozoites conferred sterile protection. Importantly, human vaccine trials demonstrated that sterile immunity induced by exposure to radiation-attenuated Plasmodium falciparum sporozoites was not strain specific and could last for at least 10 months (reviewed in [5]. From these seminal studies two main streams of research developed. One of them has focused on the development of subunit vaccines consisting of well-defined parasite antigens. More recently, a second line of research proposes the development of a vaccine based on the use of whole attenuated sporozoites. Both vaccine candidates have advanced to the point of human clinical trials involving large numbers of volunteers living in endemic areas.
RTS,S, the most advanced subunit malaria vaccine now known as Mosquirix™, contains the conserved central repeat and C-terminal regions of the P. falciparum Circumsporozoite Protein (CSP) which is expressed on sporozoites and in early liver stages. Phase III clinical trials in over 15,000 African children demonstrated that the vaccine efficacy of RTS,S ranges from 25–55%, depending on the age of the children and the intensity of transmission[6], and that this protection appears to be mediated by antibodies and perhaps also CD4+ T cells[7]. The partial efficacy of the RTS,S vaccine wanes over time with a significant reduction by 3 years post-immunization [6]. Thus, while these results are encouraging, it is generally accepted that improvements in the efficacy of this vaccine will be needed.
The limited efficacy of this vaccine may be due, at least in part, to the narrow breadth of the immune responses induced by RTS,S, which consists mostly of antibodies against the repeat domain of the CSP, which are known to neutralize sporozoite infectivity. While this vaccine also induces some antibody and CD4+ T cell responses against the C-terminal region [7], the anti-parasite effect of these immune mechanisms has not been demonstrated. Thus, the protective capacity of the RTS,S-induced immune responses may be quite limited, relying mostly on the recognition of few or perhaps single epitopes. The efficacy of this vaccine might be significantly improved if additional antigens were included in this construct. This would broaden the specificity of the anti-parasite immune response and help achieve additive anti-parasite effects from responses specific for the other antigens. In fact there are a number of sporozoite and liver stage antigens that have been identified, some of them several years ago, that could be part of a more complex vaccine construct. Unfortunately, very few studies have been done to evaluate their potential as components of a multi-antigen vaccine. In addition, optimization of the vaccination schedule might elicit more effective antibody responses, as had been seen in an early Phase I trial of this vaccine [8, 9].
Another significant limitation of the RTS,S vaccine is the fact that it does not induce CD8+ T cell responses, which represent an efficient anti-parasite mechanism that eliminates malaria liver stages in rodent model systems (reviewed in [10]. Development of vaccines capable of inducing CD8+ T cell responses in addition to antibodies may require major changes in the design of the vaccine construct and will also necessitate the identification of a number of CD8+ T cell epitopes presented by class I MHC molecules in hepatocytes expressed in most if not all individuals living in malaria endemic areas. Finally, a recent study indicates that RTS,S-induced responses may not be fully effective against P. falciparum strains expressing CSP alleles different from that used to develop RTS,S [11]. If confirmed, such allele-specific response may explain in part the reduced RTS,S efficacy, and most importantly, it raises the issue as to whether this vaccine may select for resistant strains and therefore gradually lose efficacy.
Vaccination with whole sporozoites, attenuated by irradiation or genetic manipulation, has recently acquired a new impetus. This new approach is based on the use of cryopreserved parasites instead of exposure of individuals to the bites of infected mosquitos as was done in the original studies. Limited clinical trials with volunteers from non-endemic areas demonstrated the development of sterile immunity in all immunized volunteers, after 5 immunizations with irradiated sporozoites administered intravenously [12]. It was shown that immunized individuals developed strong antibody responses to sporozoites as well as T cell responses. Recent trials in adults in malaria endemic areas also showed induction of sterile immunity, but only in 20–30 percent of vaccinees. It is unclear what causes the difference in efficacy, but it is conceivable that the semi-immune status of individuals living in endemic areas may adversely affect the development of a fully efficacious immune response. It is also possible that due to the labile nature of sporozoites, and the fact that only live parasites can induce protective immunity, the logistical conditions present in endemic areas may negatively affect parasite viability and reduce appropriate immunogenicity. In addition, the difficulties in producing enormous numbers of sporozoites as well as the current intravenous route of immunization remain as challenges to this approach, especially in children.
Another variation of the whole sporozoite immunization platform is sporozoite infection under the cover of chloroquine treatment, which allows infection of the liver but prevents progression of blood-stage parasites to the point of clinical illness [13]. Naïve volunteers immunized by this chemoprophylaxis and sporozoite immunization (CPS) strategy involving very few mosquitoes develop efficient sterile immunity against subsequent challenge infection by mosquitoes carrying homologous parasites. In contrast, when the immunized volunteers are challenged with blood-stage parasites, they are not protected, suggesting that this immunity is directed to preerythrocytic stages ([14]. In addition, this controlled human challenge model has enabled investigation of immune activation during infection (recently reviewed in [15]).
Asexual Blood Stages of Infection
The progressive development of naturally acquired immunity to the erythrocytic stages of Plasmodium falciparum malaria with age has been recognized for many years [16], and the contributions of antibodies to this immunity are due in part to a groundbreaking study showing that passive transfer of IgG from adult Africans long exposed to malaria could drive down erythrocytic malaria parasite levels in children with this disease [17]. This finding was later extended to a study demonstrating that African immunoglobulin could also reduce parasites in adults with malaria in Thailand, establishing that the antibodies could recognize conserved determinants on parasites half a world away [18]. What we still don’t know is the targets of the effector antibodies and the mechanisms by which they function in vivo. More recent studies of antibody populations in endemic areas using array technologies have highlighted the complexity of the human antibody response to the erythrocytic stages of malaria infection [19].Nevertheless, these findings have served as a rationale for development of vaccines designed to reduce morbidity and mortality.
Many blood-stage vaccine candidates have been identified based on studies in mouse malaria parasites, and in some cases protective immunity to these proteins has also been demonstrated in non-human primates (reviewed in [20, 21]. In general these antigens are involved in merozoite invasion of red cells, and experience with model systems has shown that high concentrations of specific antibodies are necessary for protection in vivo; the levels required (perhaps 80–100ug/ml) challenge human vaccinology in terms of platforms which could generate and maintain such levels. With respect to merozoites, there are a number of candidates which have reached human efficacy trials [20], but results have generally been disappointing. Partial efficacy was seen with P. falciparum AMA1 (apical membrane antigen-1) tested in Malian children but primarily against parasites with homologous AMA1 sequences (8% of total episodes)[22]. Allelic diversity seen with AMA1 and other merozoite proteins presumably reflects the strong selective pressure of the host immune system on important parasite proteins involved in merozoite invasion of the red cell, and it continues to present a major challenge to merozoite vaccine development. Disappointing Phase II trials with several candidates have provided impetus for identification of new possible candidates and recent interest has focused on PfRh5 (reticulocyte-binding protein homologue), a crucial and relatively conserved merozoite protein which binds to basigin on the surface of erythrocytes [23]. Antibodies to PfRh5 display high levels of in vitro growth inhibitory activity (GIA) and immunization of non-human primates elicits protective immune responses against heterologous parasites [24]; human clinical trials are in progress. Other merozoite antigens involved in erythrocyte binding have also been examined but the redundant invasion pathways employed by the parasite may allow escape from host immune responses (reviewed [25]).
Assays to evaluate functionality of immune responses to merozoites are complicated by the lack of understanding of effector mechanisms in naturally acquired immunity and the lack of efficacy in clinical vaccine trials. Thus there are no definitive surrogate markers of protective immunity. Of course, CD4+ T cells are required for antibody production but may have other effector functions as well including production of cytokines. Limited attention has been given to CD8+ T cells since human red cells lack Class I molecules, and roles of other cell types have not been well characterized. Given the established importance of B cell products, most tests measure antibodies and these include ELISA assays, GIA assays, antibody dependent cell-mediated inhibition (ADCI), antibody dependent respiratory burst assays with neutrophils (ADRB)[26], complement-dependent GIA to merozoites [27], and opsonophagocytosis of infected red cells. The ADCI assay has been extremely difficult to reproduce in various laboratories so its utility is unclear, and the ADRB, cGIA, and opsonophagocytosis assays have not been extensively evaluated. The GIA has been studied in most detail and standardized, but it only evaluates the ability of antibodies to inhibit invasion of merozoites into red cells and subsequent parasite growth, and there are likely other important effector functions which it does not measure. In addition, sera from African adults has been shown to inhibit this reaction in vitro, a finding which may suggest possible host adaptive immune system interference with other antibodies which inhibit parasite growth [28].
A very recent study has been informative with respect to mechanisms of protection and GIA using controlled human malaria infection (CHMI) with P. falciparum after immunization with AMA1, one of the best studied and most promising of the merozoite vaccine candidates [29]. The immunization of malaria naïve individuals resulted in relatively high levels of antibody (median nearly 100ug/ml prior to challenge) and GIA (median 59.5%), and it might be expected that these antibodies would have a negative effect on the in vivo growth rate of parasites after a low level challenge with the homologous parasite. However, the observed growth rates were indistinguishable in those that received AMA1 or only the parasite challenge. In this case there is no pre-existing exposure to malaria to modulate results so this raises questions as to our understanding of what is required in vivo to achieve partially protective responses. Questions include the necessary level of antibodies, antibody specificity, possible requirements for additional merozoite antigens in the vaccine, inadequate cellular responses, or the possibility that higher parasitemias are required to activate other host effector systems such as phagocytosis.
Some data has suggested that naturally acquired immunity to malaria is primarily dependent on immune responses to parasite-encoded antigens found on the surface of the infected red cell rather than on the merozoite; antibodies to these antigens accumulate over time with progressive exposure to various parasite strains [30]. Since they are displayed on the surface for long periods and accessible to blood components, they are prime targets for vaccines. The most prominent of these are encoded the PfEMP1 (P. falciparum erythrocyte membrane protein-1) proteins encoded by the large var gene family, with approximately 60 members in each genome (reviewed in [31–33]. These large proteins (approximately 300kDa) with multiple variable domains and ability to switch expression from one to another have been a great challenge for vaccine developers. One exception is the PfEMP1 gene encoding VAR2CSA which has been associated with placental malaria (reviewed in [34, 35]), and its domains are being investigated in human trials for a vaccine against pregnancy associated malaria. The recent remarkable finding in two individuals of monoclonal antibodies in two individuals that are broadly reactive to the surfaces of infected red cells points to a potential conserved antigenic target on the red cell surface and may provide new insight to this area [36].
The general goal of blood stage vaccines in the past has been to reduce morbidity and mortality to malaria as opposed to eliminating parasites and this remains a laudable objective. In addition to probing for conserved epitopes on the infected erythrocyte, additional utilization of CHMI rather than expensive and laborious field trials in children, more comprehensive analysis of correlates of protection in humans, defined concentrations of functional human antibodies, and exploration of the activity of defined mixtures of antibodies to various parasite antigens will be areas to be explored in the future.
Sexual and Mosquito Stages of Malaria
The recent inroads made in malaria have primarily been due to control of the Anopheline vectors of this disease, employing insecticide treated bednets, insecticide spraying, treatment of larval breeding sites, etc. However, new insights into the biology of sexual stage parasites [37] and to the insect vector will be necessary to decrease transmission, perhaps including a transmission blocking vaccine (TBV). Since the female Anopheline mosquito picks up human plasma at the same time as she feeds on blood containing gametocytes, antibodies from the vertebrate blood are also transferred to the mosquito midgut and can function there to block transmission. This strategy derives from original observations on avian models of malaria [38] which led to experiments showing that chickens immunized with inactivated P. gallinaceum gametocytes produced a transmission blocking activity in the serum that inhibited parasite development in the mosquito [39, 40]. Since the vaccine recipient does not directly and immediately benefit, this approach was originally considered to be an “altruistic vaccine”. However, this limited view has been replaced by the recognition that the recipient’s family, neighbors, and community would benefit from a successful TBV and eventually the recipient would as well.
Most efforts to develop a TBV have utilized parasite molecules unique to the sexual or mosquito stages such as components of gametes, zygotes, or ookinetes. Construction of monoclonal antibodies which had TB activity facilitated the identification of candidate antigens ([41]; the major proteins that have been investigated were recently reviewed [42, 43]. Those that are the best studied include Pfs25 found primarily on ookinetes, and Pfs230 and Pfs48/45 found primarily on gametes and zygotes and involved in fertilization. Clinical trials in humans of these proteins are in early stages, but indications are that high antibody titers are required for reduction of transmission in laboratory assays. A significant advantage of most of these molecules is that they are minimally exposed to the vertebrate immune system and consequently are less subject to immune selective pressures. The counter to this advantage of more sequence conservation is that malaria infection of the vertebrate host may not boost a vaccine-elicited immune response to these antigens. Given this situation, it will be challenging to maintain the high titers of antibodies likely required to limit transmission of gametocytes to the mosquito over long periods of time.
Evaluating the functionality of TBV candidates has been somewhat simpler, partly because the main focus has been on the role of antibodies, in some cases with the participation of complement. To mimic this process, the Standard Membrane Feeding Assay (SMFA) was developed in which cultured P. falciparum gametocytes are incubated with antibodies, then fed to mosquitoes, and one week later the oocysts on the mosquito midgut are enumerated. While termed the standard assay, its difficulty was in the lack of reproducibility and variable application of the two readouts of the assay, viz., inhibition of oocyst development and inhibition of prevalence of infected mosquitoes. The latter is important since current evidence suggests that a single oocyst may allow transmission by the mosquito [44]. Recent studies have systematically examined the SMFA and have made computer modeling and statistical evaluation of SMFA results possible [45];[46]. Standardization of this difficult assay has enhanced comparative evaluation of TBV candidates.
Another problem for TBV vaccine candidates is actually testing them in the field. While the SMFA and other assays provide an indication of the potential of antibodies to prevent mosquito infection in the laboratory, the actual measure of a TBV is at the community level (reviewed in [47]. The standard epidemiologic method for such evaluation is a cluster randomized trial, involving a number of villages, some villages receiving TBV vaccine and others receiving a control vaccine, with the readout being malaria cases. However, this approach is difficult, time-consuming, and costly, so surrogate measures are being sought that should reflect declines in malaria.
A variant of the transmission blocking vaccine approach is to use antigens derived from the vector as vaccine candidates rather than from the parasite (reviewed in [42, 43]. These are usually components of the insect midgut; it may be that important mosquito-encoded receptors for specific parasite components can elicit antibodies with more potential to inhibit infection of the vector. As an example, it has been proposed that binding of Pfs47 to its receptor in the mosquito allows the parasite to evade the destructive innate immune system of the vector, and different haplotypes of this protein would permit successful infection of various Anopheline species around the world [48].
Another approach that is being discussed for malaria as well as other mosquito transmitted diseases is the generation and dissemination of genetically modified vectors. For example, the gene encoding the variable region of a monoclonal antibody directed to a malaria protein such as PfCSP or Pfs25 could be introduced into the Anopheles genome; this would be equivalent to a passive protection model in the vector. The problem has always been how to drive this genomic modification through the vector population. One recent study has proposed introducing CRISPR/cas9 information into the mosquito genome and allowing that system to facilitate the gene drive [49]. The over 70 species of Anopheline mosquitoes which are capable of transmitting malaria will make this a significant challenge but new approaches to these vectors are now possible.
Conclusions
The positive results with the Phase III trial of RTS,S have established that a vaccine against malaria can provide partial protection to children in endemic areas, but its limited efficacy and relatively short window of protection mandate that new generations of more efficacious vaccines must be sought. Supporting evidence shows that anti-parasite immune responses can control infection against other stages as well, but translating these experimental findings into vaccines for blood stages has been disappointing and efforts to test a TBV are just beginning. Difficulties include the biological complexity of the organism with an array of stage-specific genes many of which in the erythrocytic stages are antigenically diverse. In addition, it appears necessary to elicit high and long-lasting antibody titers, account for redundant pathways of merozoite invasion, and still seek surrogate markers of protective immunity and better model systems. Most vaccine studies have focused on a single or a few antigens with an apparent functional role, but this is likely to be too restrictive, and broad, multi-antigen, multi-stage immune responses based on an understanding of biological responses are needed. Finally, novel tools and developments involving parasite sexual stages and the mosquito vector should provide new avenues for reducing or blocking malaria transmission.
Highlights.
The positive clinical results with RTS,S in a large Phase III trial in African children have established that a malaria vaccine can be developed, but the partial efficacy and the limited life-span of the protection will require new generations of more efficacious vaccines.
Blood stage vaccines have generally been disappointing and transmission blocking vaccines are in early stages of clinical testing.
Additional candidates for all stages need to be evaluated and more multi-antigen, multi-stage combinations need to be tested without Phase II efficacy trials. This will require surrogate markers of protective immunity and other models such as Controlled Human Malaria Infections and better animal models.
Investigations into the biology of plasmodial sexual stages and the Anopheline vectors of malaria may provide new approaches to reducing malaria transmission.
Acknowledgments
This review was supported in part by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases, National Institutes of Health to CAL, and F. Z. thanks the Bloomberg Family Foundation and PATH-MVI for continued support.
The authors gratefully acknowledge all the scientists and study volunteers over many decades who have contributed to our knowledge of immune responses in malaria and regret that the citations do not include the important work of many investigators.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Organization WH. World Malaria Report 2014. http://wwwwhoint/malaria/publications/world_malaria_report_2014/en/2014. [Google Scholar]
- 2.Mueller I, Shakri AR, Chitnis CE. Development of vaccines for Plasmodium vivax malaria. Vaccine. 2015;33(52):7489–7495. doi: 10.1016/j.vaccine.2015.09.060. [DOI] [PubMed] [Google Scholar]
- 3.Nussenzweig RS, Vanderberg J, Most H, Orton C. Protective immunity produced by the injection of x-irradiated sporozoites of plasmodium berghei. Nature. 1967;216(5111):160–162. doi: 10.1038/216160a0. [DOI] [PubMed] [Google Scholar]
- 4.Clyde DF. Immunization of man against falciparum and vivax malaria by use of attenuated sporozoites. Am J Trop Med Hyg. 1975;24(3):397–401. doi: 10.4269/ajtmh.1975.24.397. [DOI] [PubMed] [Google Scholar]
- 5.Hoffman SL, Goh LM, Luke TC, Schneider I, Le TP, Doolan DL, Sacci J, de la Vega P, Dowler M, Paul C, et al. Protection of humans against malaria by immunization with radiation-attenuated Plasmodium falciparum sporozoites. J Infect Dis. 2002;185(8):1155–1164. doi: 10.1086/339409. [DOI] [PubMed] [Google Scholar]
- 6. Rts,S CTP. Efficacy and safety of RTS,S/AS01 malaria vaccine with or without a booster dose in infants and children in Africa: final results of a phase 3, individually randomised, controlled trial. Lancet. 2015;386:31–45. doi: 10.1016/S0140-6736(15)60721-8. ** Final report on the efficacy and safety of the RTS,S/AS01 malaria vaccine candidate, in a phase 3 trial involving more than 15000 children. Describes its overall efficacy and its decreased efficacy with time.
- 7.Kester KE, Cummings JF, Ofori-Anyinam O, Ockenhouse CF, Krzych U, Moris P, Schwenk R, Nielsen RA, Debebe Z, Pinelis E, et al. Randomized, double-blind, phase 2a trial of falciparum malaria vaccines RTS,S/AS01B and RTS,S/AS02A in malaria-naive adults: safety, efficacy, and immunologic associates of protection. J Infect Dis. 2009;200(3):337–346. doi: 10.1086/600120. [DOI] [PubMed] [Google Scholar]
- 8.Ajua A, Lell B, Agnandji ST, Asante KP, Owusu-Agyei S, Mwangoka G, Mpina M, Salim N, Tanner M, Abdulla S, et al. The effect of immunization schedule with the malaria vaccine candidate RTS,S/AS01E on protective efficacy and anti-circumsporozoite protein antibody avidity in African infants. Malar J. 2015;14:72. doi: 10.1186/s12936-015-0605-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stoute JA, Slaoui M, Heppner DG, Momin P, Kester KE, Desmons P, Wellde BT, Garcon N, Krzych U, Marchand M. A preliminary evaluation of a recombinant circumsporozoite protein vaccine against Plasmodium falciparum malaria. RTS,S Malaria Vaccine Evaluation Group. N Engl J Med. 1997;336(2):86–91. doi: 10.1056/NEJM199701093360202. [DOI] [PubMed] [Google Scholar]
- 10.Radtke AJ, Tse SW, Zavala F. From the draining lymph node to the liver: the induction and effector mechanisms of malaria-specific CD8+ T cells. Semin Immunopathol. 2015;37(3):211–220. doi: 10.1007/s00281-015-0479-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Neafsey DE, Juraska M, Bedford T, Benkeser D, Valim C, Griggs A, Lievens M, Abdulla S, Adjei S, Agbenyega T, et al. Genetic Diversity and Protective Efficacy of the RTS,S/AS01 Malaria Vaccine. N Engl J Med. 2015;373(21):2025–2037. doi: 10.1056/NEJMoa1505819. *This is an in depth sieve analysis of parasites infecting children in the RTS,S trial (reference 6) suggesting that some of the immunity is allele-specific and broader coverage of different alleles might enhance protection.
- 12. Seder RA, Chang LJ, Enama ME, Zephir KL, Sarwar UN, Gordon IJ, Holman LA, James ER, Billingsley PF, Gunasekera A, et al. Protection against malaria by intravenous immunization with a nonreplicating sporozoite vaccine. Science. 2013;341(6152):1359–1365. doi: 10.1126/science.1241800. *Describes a successful vaccine trial in which volunteers from nonendemic areas were vaccinated with attenuated sporozoites administered intravenously.
- 13.Roestenberg M, McCall M, Hopman J, Wiersma J, Luty AJ, van Gemert GJ, van de Vegte-Bolmer M, van Schaijk B, Teelen K, Arens T, et al. Protection against a malaria challenge by sporozoite inoculation. N Engl J Med. 2009;361(5):468–477. doi: 10.1056/NEJMoa0805832. [DOI] [PubMed] [Google Scholar]
- 14. Bijker EM, Bastiaens GJ, Teirlinck AC, van Gemert GJ, Graumans W, van de Vegte-Bolmer M, Siebelink-Stoter R, Arens T, Teelen K, Nahrendorf W, et al. Protection against malaria after immunization by chloroquine prophylaxis and sporozoites is mediated by preerythrocytic immunity. Proc Natl Acad Sci U S A. 2013;110(19):7862–7867. doi: 10.1073/pnas.1220360110. *Reports that the immunity induced by chemoprophylaxis and sporozotie immunization is directed to the preerythrocytic stages, not the asexual stages of infection.
- 15.Scholzen A, Sauerwein RW. Immune activation and induction of memory: lessons learned from controlled human malaria infection with Plasmodium falciparum. Parasitology. 2016;143(2):224–235. doi: 10.1017/S0031182015000761. [DOI] [PubMed] [Google Scholar]
- 16.Marsh K, Kinyanjui S. Immune effector mechanisms in malaria. Parasite Immunol. 2006;28(1–2):51–60. doi: 10.1111/j.1365-3024.2006.00808.x. [DOI] [PubMed] [Google Scholar]
- 17.Cohen S, Mc GI, Carrington S. Gamma-globulin and acquired immunity to human malaria. Nature. 1961;192:733–737. doi: 10.1038/192733a0. [DOI] [PubMed] [Google Scholar]
- 18.Sabchareon A, Burnouf T, Ouattara D, Attanath P, Bouharoun-Tayoun H, Chantavanich P, Foucault C, Chongsuphajaisiddhi T, Druilhe P. Parasitologic and clinical human response to immunoglobulin administration in falciparum malaria. Am J Trop Med Hyg. 1991;45(3):297–308. doi: 10.4269/ajtmh.1991.45.297. [DOI] [PubMed] [Google Scholar]
- 19.Crompton PD, Kayala MA, Traore B, Kayentao K, Ongoiba A, Weiss GE, Molina DM, Burk CR, Waisberg M, Jasinskas A, et al. A prospective analysis of the Ab response to Plasmodium falciparum before and after a malaria season by protein microarray. Proc Natl Acad Sci U S A. 2010;107(15):6958–6963. doi: 10.1073/pnas.1001323107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miura K. Progress and prospects for blood-stage malaria vaccines. Expert Rev Vaccines. 2016:1–17. doi: 10.1586/14760584.2016.1141680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beeson JG, Drew DR, Boyle MJ, Feng G, Fowkes FJ, Richards JS. Merozoite surface proteins in red blood cell invasion, immunity and vaccines against malaria. FEMS Microbiol Rev. 2016 doi: 10.1093/femsre/fuw001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thera MA, Doumbo OK, Coulibaly D, Laurens MB, Ouattara A, Kone AK, Guindo AB, Traore K, Traore I, Kouriba B, et al. A field trial to assess a blood-stage malaria vaccine. N Engl J Med. 2011;365(11):1004–1013. doi: 10.1056/NEJMoa1008115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Crosnier C, Bustamante LY, Bartholdson SJ, Bei AK, Theron M, Uchikawa M, Mboup S, Ndir O, Kwiatkowski DP, Duraisingh MT, et al. Basigin is a receptor essential for erythrocyte invasion by Plasmodium falciparum. Nature. 2011;480(7378):534–537. doi: 10.1038/nature10606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Douglas AD, Baldeviano GC, Lucas CM, Lugo-Roman LA, Crosnier C, Bartholdson SJ, Diouf A, Miura K, Lambert LE, Ventocilla JA, et al. A PfRH5-based vaccine is efficacious against heterologous strain blood-stage Plasmodium falciparum infection in aotus monkeys. Cell Host Microbe. 2015;17(1):130–139. doi: 10.1016/j.chom.2014.11.017. * This paper provides supporting evidence for a human clinical trial with PfRh5 and shows efficacy in non-human primates against a heterologous challenge.
- 25.Wright GJ, Rayner JC. Plasmodium falciparum erythrocyte invasion: combining function with immune evasion. PLoS Pathog. 2014;10(3):e1003943. doi: 10.1371/journal.ppat.1003943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Llewellyn D, Miura K, Fay MP, Williams AR, Murungi LM, Shi J, Hodgson SH, Douglas AD, Osier FH, Fairhurst RM, et al. Standardization of the antibody-dependent respiratory burst assay with human neutrophils and Plasmodium falciparum malaria. Sci Rep. 2015;5:14081. doi: 10.1038/srep14081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boyle MJ, Reiling L, Feng G, Langer C, Osier FH, Aspeling-Jones H, Cheng YS, Stubbs J, Tetteh KK, Conway DJ, et al. Human antibodies fix complement to inhibit Plasmodium falciparum invasion of erythrocytes and are associated with protection against malaria. Immunity. 2015;42(3):580–590. doi: 10.1016/j.immuni.2015.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miura K, Perera S, Brockley S, Zhou H, Aebig JA, Moretz SE, Miller LH, Doumbo OK, Sagara I, Dicko A, et al. Non-apical membrane antigen 1 (AMA1) IgGs from Malian children interfere with functional activity of AMA1 IgGs as judged by growth inhibition assay. PLoS One. 2011;6(6):e20947. doi: 10.1371/journal.pone.0020947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Payne RO, Milne KH, Elias SC, Edwards NJ, Douglas AD, Brown RE, Silk SE, Biswas S, Miura K, Roberts R, et al. Demonstration of the Blood-Stage Controlled Human Malaria Infection Model to Assess Efficacy of the Plasmodium falciparum AMA1 Vaccine FMP2.1/AS01. J Infect Dis. 2016 doi: 10.1093/infdis/jiw039. *A controlled human malaria challenge infection with AMA1 shows no change in parasite replication rate and raises questions as to the mechanisms involved in efficacy.
- 30.Bull PC, Lowe BS, Kortok M, Molyneux CS, Newbold CI, Marsh K. Parasite antigens on the infected red cell surface are targets for naturally acquired immunity to malaria. Nat Med. 1998;4(3):358–360. doi: 10.1038/nm0398-358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kirkman LA, Deitsch KW. Antigenic variation and the generation of diversity in malaria parasites. Curr Opin Microbiol. 2012;15(4):456–462. doi: 10.1016/j.mib.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hviid L, Jensen AT. PfEMP1 - A Parasite Protein Family of Key Importance in Plasmodium falciparum Malaria Immunity and Pathogenesis. Adv Parasitol. 2015;88:51–84. doi: 10.1016/bs.apar.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 33.Chan JA, Fowkes FJ, Beeson JG. Surface antigens of Plasmodium falciparum-infected erythrocytes as immune targets and malaria vaccine candidates. Cell Mol Life Sci. 2014;71(19):3633–3657. doi: 10.1007/s00018-014-1614-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ataide R, Mayor A, Rogerson SJ. Malaria, primigravidae, and antibodies: knowledge gained and future perspectives. Trends Parasitol. 2014;30(2):85–94. doi: 10.1016/j.pt.2013.12.007. [DOI] [PubMed] [Google Scholar]
- 35.Tuikue-Ndam N, Deloron P. Developing vaccines to prevent malaria in pregnant women. Expert Opin Biol Ther. 2015;15(8):1173–1182. doi: 10.1517/14712598.2015.1049595. [DOI] [PubMed] [Google Scholar]
- 36. Tan J, Pieper K, Piccoli L, Abdi A, Foglierini M, Geiger R, Tully CM, Jarrossay D, Ndungu FM, Wambua J, et al. A LAIR1 insertion generates broadly reactive antibodies against malaria variant antigens. Nature. 2016;529(7584):105–109. doi: 10.1038/nature16450. * This fascinating finding in two individuals of monoclonal antibodies with broad reactivity to infected red cell surfaces opens a new window into exploring a conserved epitope on the surface.
- 37.Nilsson SK, Childs LM, Buckee C, Marti M. Targeting Human Transmission Biology for Malaria Elimination. PLoS Pathog. 2015;11(6):e1004871. doi: 10.1371/journal.ppat.1004871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huff CG, Marchbank DF, Shiroishi T. Changes in infectiousness of malarial gametocytes. II. Analysis of the possible causative factors. Exp Parasitol. 1958;7(4):399–417. doi: 10.1016/0014-4894(58)90036-5. [DOI] [PubMed] [Google Scholar]
- 39.Gwadz RW. Successful immunization against the sexual stages of Plasmodium gallinaceum. Science. 1976;193(4258):1150–1151. doi: 10.1126/science.959832. [DOI] [PubMed] [Google Scholar]
- 40.Carter R, Chen DH. Malaria transmission blocked by immunisation with gametes of the malaria parasite. Nature. 1976;263(5572):57–60. doi: 10.1038/263057a0. [DOI] [PubMed] [Google Scholar]
- 41.Rener J, Graves PM, Carter R, Williams JL, Burkot TR. Target antigens of transmission-blocking immunity on gametes of plasmodium falciparum. J Exp Med. 1983;158(3):976–981. doi: 10.1084/jem.158.3.976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nikolaeva D, Draper SJ, Biswas S. Toward the development of effective transmission-blocking vaccines for malaria. Expert Rev Vaccines. 2015;14(5):653–680. doi: 10.1586/14760584.2015.993383. [DOI] [PubMed] [Google Scholar]
- 43.Wu Y, Sinden RE, Churcher TS, Tsuboi T, Yusibov V. Development of malaria transmission-blocking vaccines: from concept to product. Adv Parasitol. 2015;89:109–152. doi: 10.1016/bs.apar.2015.04.001. [DOI] [PubMed] [Google Scholar]
- 44.Stone WJ, Eldering M, van Gemert GJ, Lanke KH, Grignard L, van de Vegte-Bolmer MG, Siebelink-Stoter R, Graumans W, Roeffen WF, Drakeley CJ, et al. The relevance and applicability of oocyst prevalence as a read-out for mosquito feeding assays. Sci Rep. 2013;3:3418. doi: 10.1038/srep03418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Miura K, Deng B, Tullo G, Diouf A, Moretz SE, Locke E, Morin M, Fay MP, Long CA. Qualification of standard membrane-feeding assay with Plasmodium falciparum malaria and potential improvements for future assays. PLoS One. 2013;8(3):e57909. doi: 10.1371/journal.pone.0057909. *This and the following reference provide a technical advance in the SMFA, long held to be not reproducible. These two publications are now permitting reliable evaluation of antibodies to sexual/mosquito stage candidates.
- 46.Churcher TS, Blagborough AM, Delves M, Ramakrishnan C, Kapulu MC, Williams AR, Biswas S, Da DF, Cohuet A, Sinden RE. Measuring the blockade of malaria transmission--an analysis of the Standard Membrane Feeding Assay. Int J Parasitol. 2012;42(11):1037–1044. doi: 10.1016/j.ijpara.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 47.Nunes JK, Woods C, Carter T, Raphael T, Morin MJ, Diallo D, Leboulleux D, Jain S, Loucq C, Kaslow DC, et al. Development of a transmission-blocking malaria vaccine: progress, challenges, and the path forward. Vaccine. 2014;32(43):5531–5539. doi: 10.1016/j.vaccine.2014.07.030. [DOI] [PubMed] [Google Scholar]
- 48. Molina-Cruz A, Canepa GE, Kamath N, Pavlovic NV, Mu J, Ramphul UN, Ramirez JL, Barillas-Mury C. Plasmodium evasion of mosquito immunity and global malaria transmission: The lock-and-key theory. Proc Natl Acad Sci U S A. 2015;112(49):15178–15183. doi: 10.1073/pnas.1520426112. * Interesting proposal about the role of different variants of Pfs47 in evading the innate immune system of the mosquito and raises possibilities for targets of intervention.
- 49. Gantz VM, Jasinskiene N, Tatarenkova O, Fazekas A, Macias VM, Bier E, James AA. Highly efficient Cas9-mediated gene drive for population modification of the malaria vector mosquito Anopheles stephensi. Proc Natl Acad Sci U S A. 2015;112(49):E6736–E6743. doi: 10.1073/pnas.1521077112. *Demonstration of passive transfer of a monoclonal antibody to mosquitoes and application of the Cas9 system for driving this modification through the mosquito population.