Abstract
Distal embolization of microthrombi during stenting for myocardial infarction (MI) causes microvascular obstruction (MVO). We have previously shown that sonoreperfusion (SRP), a microbubble (MB)-mediated ultrasonic (US) therapy, resolves MVO from venous microthrombi in vitro in saline. However, blood is more viscous than saline and arterial thrombi that embolize during stenting are mechanically distinct from venous clot. Therefore, we tested the hypothesis that MVO created with arterial microthrombi are more resistant to SRP therapy compared with venous microthrombi and higher viscosity further increases the US requirement for effective SRP in an in vitro model of MVO. Lipid MB suspended in plasma with adjusted viscosity (1.1 or 4.0 cP) were passed through tubing bearing a mesh with 40 μm pores to simulate a microvascular cross-section; upstream pressure reflected thrombus burden. To simulate MVO, the mesh was occluded with either arterial or venous microthrombi to increase upstream pressure to 40±5 mmHg. Therapeutic long-tone-burst US was delivered to the occluded area for 20 min. MB activity was recorded with a passive cavitation detector (PCD). MVO caused by arterial microthrombi at either blood or plasma viscosity resulted in less effective SRP therapy, compared to venous thrombi. Higher viscosity further reduced the effectiveness of SRP therapy. PCD showed a decrease in inertial cavitation when viscosity was increased while stable cavitation was affected in a more complex manner. Overall, these data suggest that arterial thrombi may require higher acoustic pressure US than venous thrombi to achieve similar SRP efficacy, increased viscosity decreases SRP efficacy, and both inertial and stable cavitation are implicated in observed SRP efficacy.
Keywords: Sonoreperfusion, ultrasound, microbubbles, thrombolysis, microvascular obstruction
INTRODUCTION
Despite successful recanalization of the infarct-related artery by percutaneous coronary intervention following acute myocardial infarction (AMI), there is impaired microvascular perfusion in up to 50% of cases (Niccoli et al. 2009). This clinical problem is termed microvascular obstruction (MVO), and it is associated with poor clinical outcomes and increased mortality (Piana et al. 1994; Abbo et al. 1995). MVO and its sequelae have been reduced in certain patients by treatment with antiplatelet and vasodilatory agents (Marzilli et al. 2000; Ono et al. 2004; Thiele et al. 2008). However, there is currently no consistently effective treatment method for this important clinical problem.
Sonoreperfusion (SRP) is a novel MB-mediated ultrasonic therapy option that may relieve MVO and improve microvascular perfusion. MBs are gas filled microspheres with an outer shell of lipid or polymer. Ultrasound with low acoustic pressures causes expansion and compression of MBs (stable cavitation), resulting in microstreaming in the surrounding fluid (Kooiman et al. 2014). High acoustic pressures lead to MB collapse (inertial cavitation) (Shi et al. 2000; Kiessling et al. 2012) and microjets in the surrounding fluid (Postema et al. 2005). These fluid effects may facilitate mixing and delivery of tissue plasminogen activator (t-PA) by stable cavitation (Shaw et al. 2009) and also cause direct mechanical disruption of a thrombus by inertial cavitation. Using ultra-high-speed imaging, our group has demonstrated that inertial cavitation causes pitting on the surface of a thrombus through direct mechanical effects (Chen et al. 2014). We have also demonstrated successful reperfusion with SRP therapy in an in-vitro model of arteriolar MVO (Leeman et al. 2012) and in a rodent hind limb model of MVO (Pacella et al. 2015). SRP has also been shown to open thrombus occluded venous catheters (Kutty et al. 2010), and coronary arteries in a porcine model of acute myocardial infarction (Xie et al. 2009). However, the viscosity of the surrounding medium has varied in these studies, and venous, or statically formed thrombi, have provided the material for vascular obstruction. The effect of both of these factors, surrounding medium viscosity and thrombus composition, on therapeutic efficacy is not well understood.
It is known that the MB response to ultrasound is affected by the viscosity of the surrounding medium. When the viscosity is increased, there is greater energy dissipation and a dampening effect on MB cavitation (de Jong et al. 2002). Such changes may alter the acoustic response of MBs and thus affect the efficacy of SRP. Accordingly, our first hypothesis is that greater ultrasound energy will be required to attain a given level of SRP efficacy at higher (blood) versus lower (plasma) viscosity. Moreover, thrombus composition varies markedly depending on the site of formation. Arterial thrombi form on vulnerable plaques in a high shear environment where coagulation is heavily dependent on platelets (Roessler et al. 2011). Arterial thrombi are platelet-rich, red blood cell (RBC)-poor, and have a denser fibrin network in comparison to venous thrombi (Roessler et al. 2011; Silvain et al. 2011) and are structurally stiffer. Therefore, our second hypothesis is that, due to their structural and mechanical differences, arterial thrombi require higher ultrasound acoustic energy than venous thrombi to attain a given degree of SRP. We tested our hypotheses in our in-vitro model of arteriolar microvascular obstruction.
MATERIALS AND METHODS
MB Preparation
Lipid MBs were prepared as previously described (Leeman et al. 2012) from a suspension of 20 mg 1,2-distearoyl-sn-glycero-3-phosphocholine (Avanti Polar Lipids, Alabaster, AL, USA), 10 mg 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[amino(polyethylene glycol)-2000] (Avanti Polar Lipids, Alabaster, AL, USA), and 10 mg Polyoxyethylene (40) stearate (Sigma-Aldrich, St. Louis, MO, USA) in chloroform. The chloroform was evaporated using overnight vacuum desiccation and the dried lipids were resuspended in saline. This lipid suspension was sonicated for 70 sec using a 20 kHz probe (Heat Systems Ultrasonics, Newtown, CT, USA) while surrounded by perfluorobutane gas (FluoroMed, L.P., Round Rock, TX, USA). The MBs were then washed with saline twice to remove excess lipid debris. The MBs had an average diameter of 3.0 to 3.5 μm and a concentration of 1×109/ml.
Plasma Preparation
All experimental protocols were approved by the Institutional Animal Care and Use Committee of the University of Pittsburgh. Porcine blood was collected into an acid citrate dextrose (ACD) anticoagulant bag. Freshly drawn blood was centrifuged at 2500g for 15 minutes at 43 C. The precipitant layer containing erythrocytes, leukocytes, and platelets was discarded. The plasma supernatant was immediately stored at −803 C until experimental use.
Viscosity Adjustments
Plasma viscosity was increased using polyvinylpyrrolidone (PVP) (Sigma-Aldrich, St. Louis, MO, USA), a biologically inert polymer (Bolten and Turk 2011). Blood viscosity in vivo is heavily dependent on shear rate. With low shear rates approaching 0 sec−1, blood viscosity is over 100 cP (Simmonds et al. 2013), but decreases as shear rate increases, known as “shear thinning,” plateauing around 3.1 cP for blood with 40% hematocrit (Lipowsky et al. 1980). Viscosity was adjusted to 4.0 cP using 2% w/v PVP to approximate blood viscosity at physiologic shear rates. Viscosity values were confirmed using a capillary viscometer (model C445, Cannon-Manning, State College, PA, USA).
Microthrombi Preparation
Thrombi were prepared by mixing 1 part 0.25 M CaCl2 with 9 parts of ACD anticoagulated venous porcine blood (Lampire Biological Labs, Inc: Ottsville, PA, USA) (Poole 1959). Venous thrombi were formed when blood was allowed to coagulate under static conditions in a 2.5 ml glass vial for 3 hours. Arterial thrombi were prepared by a modified version of the Chandler loop technique (Chandler 1958). Recalcified blood was allowed to coagulate under high shear stress in a vertically rotating loop (11 cm diameter) of PVC tubing (1/8 inch ID; Cole-Parmer, Vernon Hills, IL, USA) at 70 RPM for 1 hour. The linear velocity of the column of blood was 41 cm/sec, creating a shear rate of 1032 sec−1. This approximates the shear rate in human coronary arteries, which can range from 420 sec−1 in small, healthy coronary arteries to as high as 2600 sec−1 in a stenotic vessel (Holme et al. 1997). Thrombi formed in a Chandler loop mimic the structure of in-vivo platelet-rich arterial thrombi (Robbie et al. 1997). Thrombi were fragmented by shaking (4530±100 oscillations/min) in a dental amalgamator (Lantheus Medical Imaging, Inc., Mississauga, Ontario, Canada). Microthrombi were then filtered through a 200 μm nylon mesh (BioDesign Inc., Carmel, NY, USA) to remove remaining large thrombi and produce a size distribution ranging from 10 to 200 μm, the size range responsible for microembolization and subsequent MVO in vivo (Saber et al. 1993; Schwartz et al. 2009; Pacella et al. 2015).
Histology
Thrombi were fixed in 10% formalin for 48 hours. After fixation, thrombus samples were embedded in paraffin wax and sliced to 5 μm thickness. Samples were stained with hematoxlyin and eosin (H&E) and imaged (4× and 40× magnification).
Scanning Electron Microscopy (SEM)
Thrombi were fixed in 2.5% glutaraldehyde for 1 hour, rinsed in saline, and fixed in 1% osmium tetroxide for 1 hour. Samples were then dehydrated in a graded series of ethyl alcohol solutions. Dehydrated specimens were critical-point dried, mounted on studs, and sputter coated prior to SEM imaging.
In-vitro Model
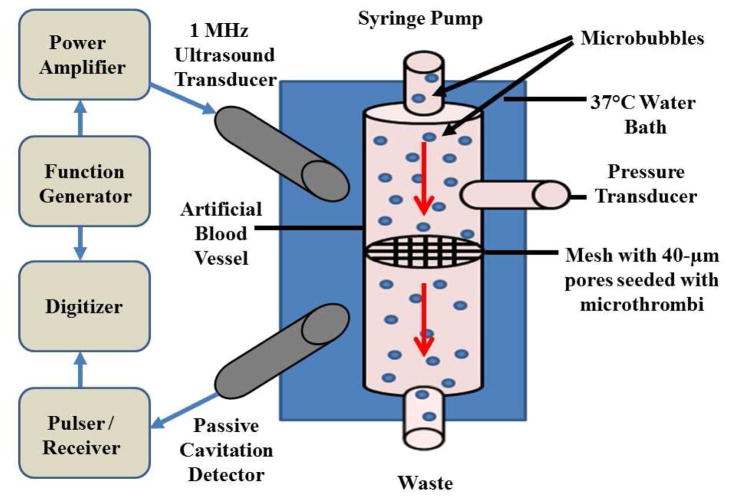
Lipid MBs, suspended in plasma with and without PVP for viscosity adjustment, were passed through a rubber-casted artificial blood vessel with a 4 mm inner diameter using a syringe pump (Harvard Apparatus, Holliston, MA, USA) (Fig 1). The vessel bore a 15 μm thick nylon mesh with 40 μm pores (Fisher Scientific, Hampton, New Hampshire, USA) across the lumen to simulate a microvascular cross-section. The downstream limb of the circuit drained to atmospheric pressure. With a flow rate of 1.5 ml/min, axial velocity through the individual pores was 2 mm/sec, which is approximately the velocity of RBCs through a 40 μm arteriole (Kim et al. 1984). Pressure upstream of this mesh (BD, Franklin Lakes, NJ, USA) during constant flow represented the thrombus burden on the mesh and degree of MVO. The mesh was occluded with microthrombi until upstream pressure reached 40 ±5 mmHg to simulate MVO. Therapeutic ultrasound was driven by a function generator (33250A; Agilent, Santa Clara, CA, USA) with a power amplifier (100A250A; Amplifier Research, Souderton, PA, USA) and was delivered to the mesh with a focused single element immersion transducer (1 MHz, −6 dB beam width of 3.5 mm, A302S-SU-F1.63-PTF, Olympus NDT, Waltham, MA) for 20 minutes. The ultrasound was delivered as a 1 MHz, 5000 cycle tone-burst, with a pulse repetition frequency (PRF) of 0.33 Hz, and a duty cycle of 0.17%, which was previously shown to be efficacious with venous thrombi and saline viscosity (Leeman et al. 2012). Experiments were conducted at peak negative acoustic pressures (P-) of 0.6, 1.0, and 1.5 MPa. Upstream pressure was measured continuously with a fluid-filled pressure transducer and a physiologic monitoring system (Ponemah, Science Inc., Ontario, Canada). MB oscillation in response to ultrasound was recorded with a second, co-localized transducer (3.5 MHz, -6 dB beam width of 1.2 mm, V383-SU-F1.00IN-PTF, Olympus NDT, Waltham, MA, USA) acting as a passive cavitation detector (PCD). The detected signal was amplified by 10 dB using a pulser/receiver (5073PR, Olympus NTD, Waltham, MA), band pass-filtered (2–20 MHz cutoffs) and digitized at 50 MHz sampling frequency with a 12-bit digitizer (Acqiris DP310, Agilent, Santa Clara, CA, USA). Joint time-frequency analysis was performed in MATLAB (The MathWorks, Inc, Natick, MA, USA) using 250 μs windows and a time step of 100 μs. The mean acoustic energy between 3.2–3.8 MHz, excluding the band between 3.4–3.6 MHz, integrated over the whole tone-burst, was defined as inertial cavitation dose (ICD). The mean energy in the peak at the ultraharmonic band (3.48–3.52 MHz) above the broadband signal, integrated over the whole tone-burst, was defined as the stable cavitation dose (SCD). The bandwidth chosen for SCD corresponded to the −6 dB bandwidth in the fundamental peak (Datta et al. 2008).
Figure 1.
Schematic diagram of in-vitro model of microvascular obstruction.
Statistical Analysis
To quantify the change on thrombus burden during the course of SRP therapy, the inverse of the area beneath the 20-minute pressure (clot) vs. time curves was measured and defined as the lytic index. In addition, because the response to therapy occurred most rapidly during the beginning of treatment, we quantified the initial speed of reperfusion by measuring the slope of the pressure vs. time curves during the first 3 minutes of ultrasound. This was defined as the lytic rate. The effect of clot type, viscosity, and acoustic pressure on the lytic rate and lytic index were analyzed using 2-way factorial ANOVA and passed Levene’s equal variance test. Pair-wise differences between two groups were tested using two-tailed t-tests. Statistical significance was defined as p<0.05. These statistical calculations were performed using SPSS statistical software (IBM, Armank, NY, USA).
RESULTS
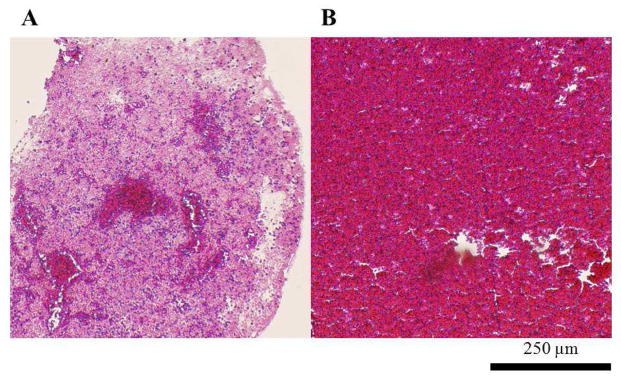
Histology
H&E staining of the arterial thrombi (Fig 2A) demonstrated RBCs (dark-red stain) clustered in the center of the thrombus, and patches of RBCs distributed throughout the remainder of the cross-section. The RBCs are surrounded by a network of pink-staining fibrin and platelets. The venous thrombi (Fig 2B) showed abundant RBCs distributed homogenously throughout the cross-section.
Figure 2.
H&E staining of thrombus (4×). A. Arterial thrombus formed in a Chandler’s loop. B. Venous thrombus formed under static conditions.
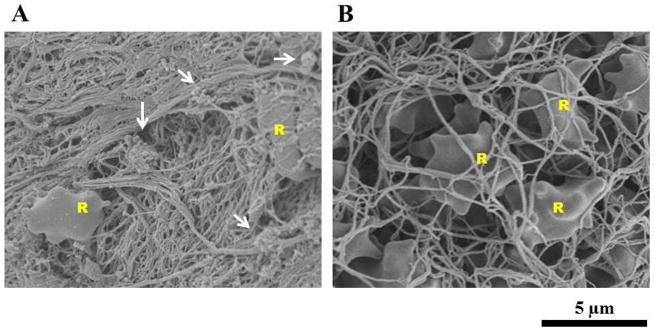
SEM Imaging
SEM imaging of arterial thrombus (Fig 3A) showed fewer RBCs than the venous thrombus, consistent with H&E staining in Figure 2. Clusters of platelets were common throughout the arterial thrombus. Visible platelet clusters were rare in images of venous thrombus. The venous thrombus (Fig 3B) revealed large numbers of RBCs spread homogenously throughout the microscopic field. The fibrin mesh of the venous thrombus is less dense, noted by its “loose” appearance, compared with the fibrin mesh of the arterial thrombus.
Figure 3.
Representative SEM images of thrombus (2000×). A. Arterial thrombus formed in Chandler’s loop. B. Venous thrombus formed under static conditions. Arrows indicate clusters of platelets. R labels indicate RBCs.
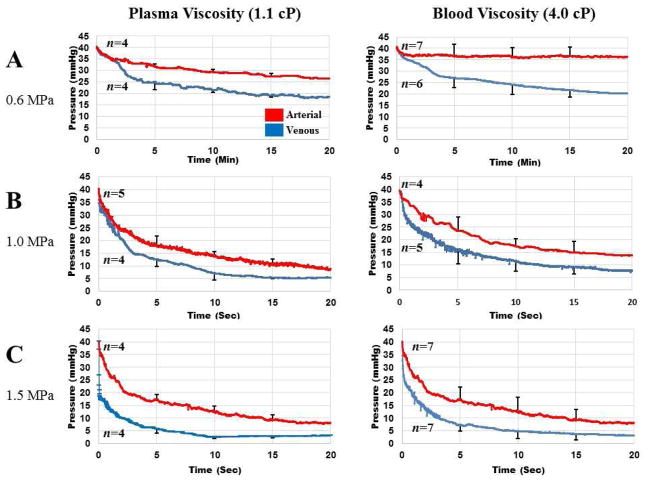
SRP in vitro
Upstream pressure measurements (representing degree of MVO) during the 20 minute SRP treatment are shown in Fig 4 at P-= 0.6, 1.0, and 1.5 MPa (Panels A, B, and C respectively). The left column represents experiments conducted with MBs suspended in plasma (1.1 cP). The right column represents MBs suspended in plasma with PVP, to achieve the same viscosity as blood (4.0 cP). Red curves depict pressure vs time during treatment of MVO caused by arterial microthrombi, and blue curves represent MVO caused by venous microthrombi. There was a trend toward a more rapid and more complete decrease in upstream pressure with venous microthrombi, compared to arterial microthrombi, at each of the acoustic pressures tested, in either plasma or blood viscosity. Reperfusion was rapid during the initial phase of therapy, and then became more gradual and plateaued.
Figure 4.
Pressure upstream of mesh occluded with either arterial (red) or venous thrombi (blue) at plasma viscosity (left column) or blood viscosity (right column) during SRP therapy at A. 0.6 MPa; B. 1.0 MPa; and C. 1.5 MPa.
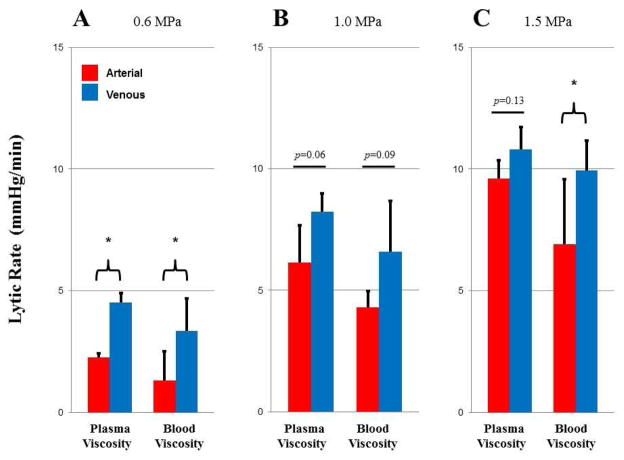
The speed of reperfusion during the initial phase of SRP is represented by the lytic rate, shown in Fig 5. The lytic rate increased with acoustic pressure (ANOVA, p<0.05), was greater for venous compared to arterial type clot (ANOVA, p<0.05) at any given acoustic pressure, and was greater at plasma viscosity compared to blood viscosity (ANOVA p<0.05). There was no significant interaction between the variables of viscosity and thrombus type at each acoustic pressure [(0.6 MPa; p=0.81), (1.0 MPa; p=0.89), (1.5 MPa; p=0.30)], which indicates that viscosity and thrombus type independently had an effect on lytic rate. Pairwise comparisons between venous and arterial type clots at different P- were statistically significant (t-tests, p<0.05) or trended towards statistical significance (t-test, p<0.13) (see Fig.4).
Figure 5.
Lytic rate with arterial (red) and venous (blue) thrombi at plasma or blood viscosity with SRP therapy at A. 0.6 MPa; B. 1.0 MPa; and C. 1.5 MPa. * p<0.05.
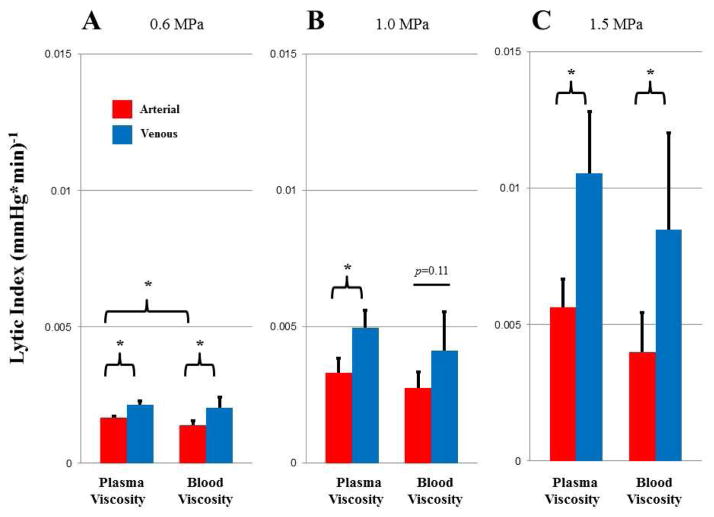
Decrease in overall thrombus burden during SRP therapy is represented by the lytic index in Fig 6. The lytic index, similarly to the lytic rate, increased with acoustic pressure (ANOVA, p<0.05), was higher for venous compared to arterial type clot (ANOVA, p<0.05) at any given acoustic pressure, and was higher at plasma viscosity compared to blood viscosity (ANOVA, p<0.05). Again, as was the case for lytic rate, there was no significant interaction between the variables of viscosity and thrombus type for lytic index [(0.6 MPa; p=0.52), (1.0 MPa; p=0.89), (1.5 MPa; p=0.86)]. Pairwise comparisons between venous and arterial type clots at different acoustic pressures were statistically significant at all pressure and viscosities tested, except at 1.0 MPa at blood viscosity, where there was a trend towards significance (t-test, p=0.11).
Figure 6.
Lytic index with arterial (red) and venous (blue) thrombi at plasma and blood viscosity with SRP therapy at A. 0.6 MPa; B. 1.0 MPa; and C. 1.5 MPa. * p<0.05.
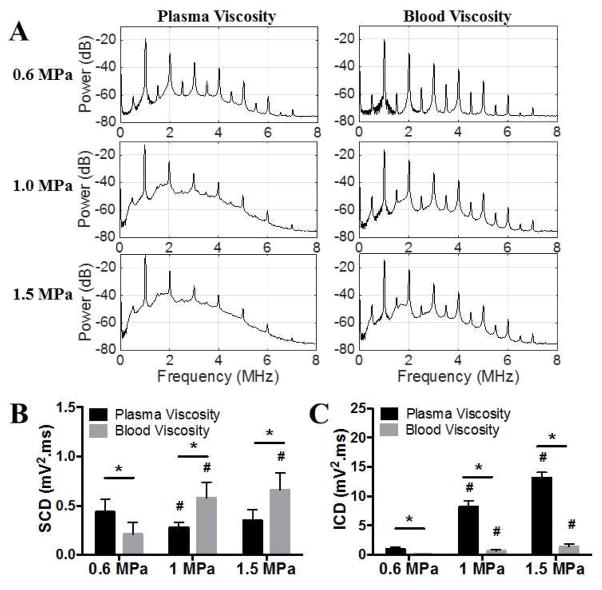
Passive Cavitation Detection
The power spectra of the PCD data between 0.1 ms and 1.1 ms of ultrasound pulses are plotted in Fig 7A. Subharmonic (0.5 MHz) and ultraharmonic (1.5, 2.5, 3.5 MHz) peaks characteristic of stable MB cavitation are more prevalent at blood viscosity compared to plasma viscosity. The SCD and ICD at plasma viscosity and blood viscosity are compared in Fig 7B and 7C. Quantitatively, SCD (Fig 7B) did not vary significantly with US pressure at plasma viscosity (0.44 ± 0.13 mV2•ms at 0.6 MPa, 0.28 ± 0.05 mV2•ms at 1.0 MPa, and 0.35 ± 0.11 mV2•ms at 1.5 MPa, respectively) but increased with US pressure at blood viscosity (from 0.21 ± 0.12 mV2•ms at 0.6 MPa to 0.58 ± 0.16 mV2•ms at 1 MPa, and 0.66 ± 0.17 mV2•ms at 1.5 MPa, p<0.05). SCD differed significantly between plasma and blood viscosity at all US pressures (p<0.05). SCD was lower in blood viscosity than in plasma viscosity at 0.6 MPa (0.21 ± 0.12 mV2•ms vs 0.44 ± 0.13 mV2•ms, p<0.05) but higher in blood viscosity than in plasma viscosity at 1.0 MPa (0.58 ± 0.16 mV2•ms vs 0.28 ± 0.05 mV2•ms, p<0.05) and 1.5 MPa (0.66 ± 0.17 mV2•ms vs 0.35 ± 0.11 mV2•ms, p<0.05). On the other hand, ICD (Fig 7C) increased significantly with US pressure at either plasma viscosity (from 1.00 ± 0.31 mV2•ms at 0.6 MPa to 8.21± 0.97 mV2•ms at 1 MPa, and 13.15 ± 0.92 mV2•ms at 1.5 MPa, respectively, p<0.05) or blood viscosity (from 0.09 ± 0.02 mV2•ms at 0.6 MPa to 0.65± 0.26 mV2•ms at 1 MPa, and 1.41 ± 0.44 mV2•ms at 1.5 MPa, respectively, p<0.05). ICD was lower with blood viscosity than with plasma viscosity at each US pressure tested (p<0.05), demonstrating that increased viscosity dampened the inertial cavitation of MBs in response to US.
Figure 7.
PCD results from MB oscillation activities at plasma and blood viscosity at various acoustic pressures. A. Average power spectra between 0.1 ms and 1.1 ms of ultrasound pulses. B. Comparison of stable cavitation dose (SCD). C. Comparison of inertial cavitation doses (ICD). * p<0.05, # p<0.05 vs 0.6 MPa.
DISCUSSION
Current therapy for acute myocardial infarction hinges on immediately restoring epicardial coronary artery patency with percutaneous coronary intervention (PCI), such as with primary stenting. However, even after recanalization of the epicardial artery, there is a failure of microvascular perfusion in many patients (MVO) (Ito et al. 1996). PCI itself causes microembolization of atherothrombotic material from the stented site of plaque rupture into the microcirculation of the infarct bed (Mauri et al. 2006), accounting for a significant portion of MVO after PCI (Porto et al. 2012). Even in patients with angiographically restored epicardial coronary artery flow, MVO incidence varies between 17% (Wu et al. 1998) to over 80% (Costantini et al. 2004). MVO severely limits the success of reperfusion therapies (Tanaka et al. 2009; Goldstein et al. 2011; Isshiki et al. 2011; Montorsi et al. 2011; Prasad and Herrmann 2011), is associated with additional myonecrosis, and is linked to worse prognosis, including death (Ito et al. 1996; Gibson 2003; Khumri et al. 2006). Mechanical obstruction by embolized and in situ microvascular thrombi (Jaffe et al. 2010) is a major component of MVO, which confers worse prognosis (Jaffe et al. 2010), and suggests the need for therapies directed against MVO. SRP may represent a novel therapeutic approach to attain microvascular patency after PCI.
Much of the literature using SRP focuses on the clearing of venous thrombi that form in a static or low flow environment. However, arterial thrombi that form under high shear conditions are mechanically stiffer, due to a tighter fibrin network. The potential effects of these differences on the efficacy of SRP are poorly understood. Furthermore, it is known that when the viscosity of a MB suspension is increased, that MB cavitation is dampened, which is corroborated by our findings (Fig.7). However, whether or not this change in cavitation translates to an effect on SRP therapy is also not yet known. The purpose of the present investigation was to explore the effects of thrombus composition and perfusate viscosity on the efficacy of SRP in an in vitro model of MVO.
Cavitation induced by US is thought to be the major mechanism for US-induced clot disruption, and is greatly enhanced by adding MBs, which lower the acoustic energy threshold for cavitation. MBs are shell-encapsulated gas microspheres that are used as intravascular US contrast agents in diagnostic echocardiography (Villanueva et al. 1993b; Villanueva et al. 1993a; Villanueva et al. 1996; Villanueva et al. 2002; Wei et al. 2005). Although MBs may vary in shell composition, all expand and contract (oscillate) when insonified by US at specific frequencies and acoustic pressures. MB behavior ranges from “stable cavitation” where the MB oscillates non-linearly at harmonic multiples of the transmitted US frequency, to “inertial cavitation”, where the oscillating MB rapidly collapses. It has been hypothesized that such mechanical events, when occurring in fluids in proximity to thrombus, induce: (1) local perturbations in fluid dynamics – microstreaming and microjetting – that facilitate clot dissolution through a mechanical “chiseling” effect, including disruption of fibrin strands; (2) improved local mixing of fresh tissue plasminogen activator (tPA) (endogenous or exogenous), resulting in improved enzymatic transport; (3) greater washout of fibrin degradation products; (4) and/or exposure of new binding sites for tPA (Everbach and Francis 2000; Pfaffenberger et al. 2005; Porter 2009; Acconcia et al. 2013).
Effects of thrombus composition on SRP efficacy
The results of our model of MVO caused by either arterial or venous microthrombi suggest that thrombus origin is a significant factor in therapeutic efficacy. We demonstrated that for any set of acoustic parameters, greater lytic efficacy was observed with venous microthrombi vs arterial microthrombi. We posit that this is due to the fact that thrombi forming within the arterial system are stiffer than venous thrombi, responsible for deep venous thrombosis and pulmonary embolism, which form under much lower shear stress. Arterial thrombi are platelet-rich, red blood cell (RBC)-poor, and have a denser fibrin network in comparison to venous thrombi (Roessler et al. 2011; Silvain et al. 2011). Platelet contractile proteins result in thrombus contraction and increased fibrin density. Furthermore, factor XIII, released by activated platelets, facilitates fibrin cross-linking and increases thrombus stability (Janbain et al. 2015). Arterial thrombi have more plasminogen activator inhibitor-1 (PAI-1) (Tynngard et al. 2006), which inactivates tPA, allowing less endogenous lysis versus venous thrombi. These structural changes make arterial thrombi stiffer than venous thrombi, which is supported by the finding that the linear Young’s moduli of thrombi formed in the arterial system (Hinnen et al. 2007) are higher than the Young’s moduli reported from venous thrombi (R. Aglyamov et al. 2007; Mfoumou et al. 2014). Accordingly, to optimize SRP therapy for treatment of MVO caused by arterial microthrombi after AMI, higher peak acoustic pressures will be required to attain equally effective SRP as seen previously with venous microthrombi (Leeman et al. 2012; Pacella et al. 2015). Thus, SRP therapy and operating parameters appear to depend on the thrombus type.
Effects of perfusate viscosity on SRP efficacy
Human blood viscosity varies considerably, depending on hematocrit, shear rate, and vessel size, and various disease states (Schabitz 1982). For example, in the microcirculation, hematocrit and viscosity decrease as the vessel size is reduced (Toksvang and Berg 1931) and during MVO, there is a reduction in shear rate and a subsequent increase in blood viscosity. Blood viscosity will also increase in disease states including polycythemia vera, leukemia, and sickle cell anemia (Somer and Meiselman 1993). We demonstrated that SRP efficacy is directly related to perfusate viscosity, showing a reduced lytic rate and lytic index for blood vs plasma viscosity. Moreover, the effect of perfusate viscosity was independent the effect of thrombus type. Hence, these data suggest that the viscosity of a patient’s blood is an important parameter to consider in the optimization of acoustic parameters for clinical translation of SRP therapy.
Effect of perfusate viscosity on MB cavitation
The passive cavitation detection data demonstrated damping of inertial cavitation in the setting of increased viscosity Fig 7C, where SRP demonstrated less reperfusion efficacy (Figures 5 and 6). ICD increased with US pressure at either plasma or blood viscosity but decreased with viscosity at each US pressure (Fig 7C). Higher ICD in plasma vs blood viscosity resulted in greater SRP efficacy (higher lytic rates and lytic indices) at all pressures tested. This supports that inertial cavitation of MBs is an important mechanism for in vitro SRP. MB inertial cavitation has been directly shown using high-speed optical imaging to cause pitting on the surface of a thrombus (Chen et al. 2014), which likely facilitates mechanical fragmentation of thrombi.
The effect of viscosity on microbubble behavior at various US pressures (P-=0.25–1.5 MPa) have been investigated previously, using a combination of ultra-high speed microscopy (Chen et al. 2013) recordings of individual microbubbles and PCD on a population of microbubbles (Helfield et al. 2016a). It was found that microbubble fragmentation propensity was lower in 4 cP media as compared to microbubbles in 1 cP fluid. PCD data revealed that the inertial cavitation strength was much lower in 4 cP media than that in 1 cP media and stable cavitation strength was generally higher in 4 cP media, except for P-=0.25 MPa. Additional study demonstrated that the oscillations of phospholipid encapsulated MBs were affected by fluid viscosity in a size- and pressure-dependent manner, resulting in behavior not entirely predicted by current MB models (Helfield et al. 2016b).
However, in our dataset, the relative decrease in inertial cavitation dose with high viscosity was greater than the decrease in therapeutic efficacy observed. This observation could be explained by stable cavitation, which may also be playing a role in SRP. Indeed, SCD significantly increased in blood viscosity from plasma viscosity at 1.0 and 1.5 MPa (Fig 7B) and may have contributed to the observed SRP efficacy in these conditions. Stable cavitation has been associated with microstreaming and radiation force, which could be generating shear forces on the clots. At blood viscosity, SCD increased with US pressure and the highest SCD was achieved at 1.5 MPa. However, this was not associated with the greatest SRP efficacy (Figs 4 and 5). Moreover, SCD did not increase significantly with US pressure at plasma viscosity, yet SRP efficacy dramatically increased with US pressure. These results suggest that SCD alone cannot explain our findings.
It appears that both ICD and SCD are implicated in effective SRP. Interestingly, it has been recently shown that a combination of moderate and high US pressure pulses was most efficacious at causing MB penetration into a fibrin mesh (Acconcia et al. 2014).
SRP and pharmacological thrombolysis
When tPA is exogenously administered, previous studies have shown that the stable cavitation of MB with low acoustic pressures has enhanced its effect (Shaw et al. 2009; Leeman et al. 2012; Bader et al. 2015). This may be mediated through microstreaming and improved mixing of the fluid surrounding the thrombus (Hitchcock et al. 2011). These stable cavitation regimes would allow for the use of ultrasound with lower peak negative acoustic pressure, and minimize off target effects of higher pressure ultrasound, such as damage to the endothelium (Chen et al. 2012), but appear to lack mechanical efficacy in the absence of tPA at plasma viscosity (Leeman et al. 2012). Whether a combined regime will result in the safest and most efficacious SRP remains to be determined.
Limitations
In this study, the effect of perfusate viscosity on SRP efficacy was studied by adding PVP to plasma. Whether our findings would hold true with whole blood as the perfusate remains to be established. Indeed, adding PVP to plasma cannot fully recapitulate the mechanical (non-Newtonian properties) and biophysical (platelets, white blood cells) properties of whole blood. Figure S1 demonstrates that MB activity is dampened in plasma+PVP compared to plasma and further dampened in blood. Adding PVP to plasma is not a perfect substitute for blood but does partially recapitulate the dampening of MB oscillations as would be expected in a perfusate with higher viscosity.
During plaque rupture, coronary artery shear rates will decrease as blood flow diminishes. In vivo distributions of microthrombi causing MVO likely span a spectrum of compositions between fibrin rich arterial type (high shear) and red blood cell rich venous type (stasis). The effect of this heterogeneity was not investigated in this study, but is likely that SRP efficacy using a mixture of venous and arterial microthrombi or a clot formed under intermediate shear rate would fall between our findings with arterial and venous microthrombi.
SRP was investigated in this study using lipid encapsulated perfluorobutane microbubbles. Microbubble size, shell composition and type of gas are known to affect microbubble stability and cavitation (stable and inertial) and therefore using different MB could have an effect on SRP efficacy.
It should be mentioned that, in the absence of tPA, our in vitro model of MVO exclusively investigates the mechanical effects of SRP. Biological effects on the endothelium, which are likely important in vivo, are not studied. In addition, our arterial thrombi did not incorporate atherosclerotic plaques, which would be present in vivo and may have an effect on therapeutic efficacy. Furthermore, the coronary microcirculation is a complex system of vessels with variable diameter and flow, which is not fully replicated by our mesh with only 40 μm pores. However, this model provides an opportunity to investigate the purely mechanical effects of MB and US on SRP efficacy, which might be ultimately required to optimize this therapeutic approach.
SUMMARY
The effects of both thrombus type and viscosity on sonoreperfusion efficacy were investigated using our in vitro model of microvascular obstruction. Our results showed that arterial thrombi and increased viscosity of the perfusate decreased therapeutic efficacy of sonoreperfusion. The cavitation activities of MBs recorded at different viscosities provided further mechanistic insights. At higher viscosity, there was a decrease in the amount of inertial cavitation, suggesting that inertial cavitation is an important mechanism for non-pharmacologic sonoreperfusion. Stable cavitation appeared to play a role in high viscosity media when inertial cavitation was reduced. Therefore, in designing an optimal sonoreperfusion regimen, consideration should be given to the origin of the occlusive thrombi and patient’s blood rheology, as these factors may have a significant impact on the optimal acoustic requirements
Supplementary Material
Power spectra of passively detected cavitation signal at 1 MHz and excitation pressures of 0.25, 0.5, 1.0 and 1.5 MPa in plasma, plasma+2%PVP (4cP) and blood (porcine RBC at 40% hematocrit suspended in PBS). The system was maintained at 37°C. Transmit (and receive transducers were confocally aligned on flowing lipid encapsulated microbubbles (mean size of 3.1 μm) at a concentration of 5×104 MB/mL. MB cavitation activity was dampened in plasma+PVP and was further dampened in blood at 0.25 and 0.5 MPa. Compared to plasma, inertial cavitation level was reduced by ~10 dB in plasma+PVP and in blood at 0.5 MPa; Compared to plasma+PVP, stable cavitation was reduced in blood by 10 dB at 0.25 MPa and by ~5 dB at 0.5 MPa. These data support that adjusting plasma viscosity to 4 cP with PVP partly recapitulates the damping effect of blood on MB oscillations under the ultrasound conditions tested.
Acknowledgments
We thank Linda Lavery, David Fischer, Regeant Panday, Jianjun Wang, Andrew Carson, and Jonathan Franks for providing valuable technical assistance. SEM imaging was performed with equipment graciously provided by Dr. Simon Watkins at the Center for Biological Imaging (CBI) at the University of Pittsburgh. This work was funded in part by the Center for Ultrasound Molecular Imaging and Therapeutics at the University of Pittsburgh. John Black received research support from the Howard Hughes Research Institute (HHMI). Dr. Pacella was funded by the Dean’s Bridge Funding program of the University of Pittsburgh. Dr. Villanueva was funded in part by the National Institutes of Health (R01EB016516-01A1). This publication was made possible by Grant Number UL1 RR024153 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH. Information on NCRR is available at http://www.ncrr.nih.gov/. Information on Re-engineering the Clinical Research Enterprise can be obtained from http://nihroadmap.nih.gov/clinicalresearch/overview-translational.asp.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbo KM, Dooris M, Glazier S, O'Neill WW, Byrd D, Grines CL, Safian RD. Features and outcome of no-reflow after percutaneous coronary intervention. Am J Cardiol. 1995;75:778–82. doi: 10.1016/s0002-9149(99)80410-x. [DOI] [PubMed] [Google Scholar]
- Acconcia C, Leung BYC, Hynynen K, Goertz DE. Interactions between ultrasound stimulated microbubbles and fibrin clots. Applied Physics Letters. 2013;103:053701. [Google Scholar]
- Acconcia C, Leung BYC, Manjunath A, Goertz DE. Interactions between Individual Ultrasound-Stimulated Microbubbles and Fibrin Clots. Ultrasound in Medicine & Biology. 2014;40:2134–50. doi: 10.1016/j.ultrasmedbio.2014.03.008. [DOI] [PubMed] [Google Scholar]
- Bader KB, Bouchoux G, Peng T, Klegerman ME, McPherson DD, Holland CK. Thrombolytic efficacy and enzymatic activity of rt-PA-loaded echogenic liposomes. J Thromb Thrombolysis. 2015;40:144–55. doi: 10.1007/s11239-015-1204-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolten D, Turk M. Experimental study on the surface tension, density, and viscosity of aqueous poly(vinylpyrrolidone) solutions. J Chem Eng Data. 2011;56:582–8. [Google Scholar]
- Chandler AB. In vitro thrombotic coagulation of the blood; a method for producing a thrombus. Lab Invest. 1958;7:110–4. [PubMed] [Google Scholar]
- Chen H, Brayman AA, Evan AP, Matula TJ. Preliminary Observations on the Spatial Correlation between Short-Burst Microbubble Oscillations and Vascular Bioeffects. Ultrasound Med Biol. 2012;38:2151–62. doi: 10.1016/j.ultrasmedbio.2012.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Leeman JE, Wang J, Pacella JJ, Villanueva FS. New insights into mechanisms of sonothrombolysis using ultra-high-speed Imaging. Ultrasound Med Biol. 2014;40:258–62. doi: 10.1016/j.ultrasmedbio.2013.08.021. [DOI] [PubMed] [Google Scholar]
- Chen X, Wang J, Versluis M, de Jong N, Villanueva FS. Ultra-fast bright field and fluorescence imaging of the dynamics of micrometer-sized objects. Review of Scientific Instruments. 2013;84:063701. doi: 10.1063/1.4809168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costantini CO, Stone GW, Mehran R, Aymong E, Grines CL, Cox DA, Stuckey T, Turco M, Gersh BJ, Tcheng JE, Garcia E, Griffin JJ, Guagliumi G, Leon MB, Lansky AJ. Frequency, correlates, and clinical implications of myocardial perfusion after primary angioplasty and stenting, with and without glycoprotein IIb/IIIa inhibition, in acute myocardial infarction. J Am Coll Cardiol. 2004;44:305–12. doi: 10.1016/j.jacc.2004.03.058. [DOI] [PubMed] [Google Scholar]
- Datta S, Coussios CC, Ammi AY, Mast TD, de Courten-Myers GM, Holland CK. Ultrasound-enhanced thrombolysis using Definity as a cavitation nucleation agent. Ultrasound Med Biol. 2008;34:1421–33. doi: 10.1016/j.ultrasmedbio.2008.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong N, Bouakaz A, Frinking P. Basic acoustic properties of microbubbles. Echocardiography. 2002;19:229–40. doi: 10.1046/j.1540-8175.2002.00229.x. [DOI] [PubMed] [Google Scholar]
- Everbach EC, Francis CW. Cavitational mechanisms in ultrasound-accelerated thrombolysis at 1 MHz. Ultrasound Med Biol. 2000;26:1153–60. doi: 10.1016/s0301-5629(00)00250-7. [DOI] [PubMed] [Google Scholar]
- Gibson CM. Has my patient achieved adequate myocardial reperfusion? Circulation. 2003;108:504–7. doi: 10.1161/01.CIR.0000082932.69023.74. [DOI] [PubMed] [Google Scholar]
- Goldstein JA, Maini B, Dixon SR, Brilakis ES, Grines CL, Rizik DG, Powers ER, Steinberg DH, Shunk KA, Weisz G, Moreno PR, Kini A, Sharma SK, Hendricks MJ, Sum ST, Madden SP, Muller JE, Stone GW, Kern MJ. Detection of lipid-core plaques by intracoronary near-infrared spectroscopy identifies high risk of periprocedural myocardial infarction. Circ Cardiovasc Interv. 2011;4:429–37. doi: 10.1161/CIRCINTERVENTIONS.111.963264. [DOI] [PubMed] [Google Scholar]
- Helfield B, Black JJ, Qin B, Pacella J, Chen X, Villanueva FS. Fluid viscosity affects the cavitation of lipid encapsulated microbubbles for therapeutic applications. Ultrasound Med Biol. 2016a;42:782–94. doi: 10.1016/j.ultrasmedbio.2015.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helfield B, Chen X, Qin B, Villanueva FS. Individual lipid encapsulated microbubble radial oscillations: effects of fluid viscosity. J Acoust Soc Am. 2016b;139:204–14. doi: 10.1121/1.4939123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinnen JW, Rixen DJ, Koning OH, van Bockel JH, Hamming JF. Development of fibrinous thrombus analogue for in-vitro abdominal aortic aneurysm studies. J Biomech. 2007;40:289–95. doi: 10.1016/j.jbiomech.2006.01.010. [DOI] [PubMed] [Google Scholar]
- Hitchcock KE, Ivancevich NM, Haworth KJ, Caudell Stamper DN, Vela DC, Sutton JT, Pyne-Geithman GJ, Holland CK. Ultrasound-enhanced rt-PA thrombolysis in an ex vivo porcine carotid artery model. Ultrasound Med Biol. 2011;37:1240–51. doi: 10.1016/j.ultrasmedbio.2011.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holme PA, Orvim U, Hamers MJ, Solum NO, Brosstad FR, Barstad RM, Sakariassen KS. Shear-induced platelet activation and platelet microparticle formation at blood flow conditions as in arteries with a severe stenosis. Arterioscler Thromb Vasc Biol. 1997;17:646–53. doi: 10.1161/01.atv.17.4.646. [DOI] [PubMed] [Google Scholar]
- Isshiki T, Kozuma K, Kyono H, Suzuki N, Yokoyama N, Yamamoto Y. Initial clinical experience with distal embolic protection using “Filtrap”, a novel filter device with a self-expandable spiral basket in patients undergoing percutaneous coronary intervention. Cardiovasc Interv Ther. 2011;26:12–7. doi: 10.1007/s12928-010-0027-y. [DOI] [PubMed] [Google Scholar]
- Ito H, Maruyama A, Iwakura K, Takiuchi S, Masuyama T, Hori M, Higashino Y, Fujii K, Minamino T. Clinical implications of the 'no reflow' phenomenon. A predictor of complications and left ventricular remodeling in reperfused anterior wall myocardial infarction. Circulation. 1996;93:223–8. doi: 10.1161/01.cir.93.2.223. [DOI] [PubMed] [Google Scholar]
- Jaffe R, Dick A, Strauss BH. Prevention and treatment of microvascular obstruction-related myocardial injury and coronary no-reflow following percutaneous coronary intervention: a systematic approach. JACC Cardiovasc Interv. 2010;3:695–704. doi: 10.1016/j.jcin.2010.05.004. [DOI] [PubMed] [Google Scholar]
- Janbain M, Nugent DJ, Powell JS, St-Louis J, Frame VB, Leissinger CA. Use of Factor XIII (FXIII) concentrate in patients with congenital FXIII deficiency undergoing surgical procedures. Transfusion. 2015;55:45–50. doi: 10.1111/trf.12784. [DOI] [PubMed] [Google Scholar]
- Khumri TM, Nayyar S, Idupulapati M, Magalski A, Stoner CN, Kusnetzky LL, Kosiborod M, Spertus JA, Main ML. Usefulness of myocardial contrast echocardiography in predicting late mortality in patients with anterior wall acute myocardial infarction. Am J Cardiol. 2006;98:1150–5. doi: 10.1016/j.amjcard.2006.05.045. [DOI] [PubMed] [Google Scholar]
- Kiessling F, Fokong S, Koczera P, Lederle W, Lammers T. Ultrasound microbubbles for molecular diagnosis, therapy, and theranostics. J Nucl Med. 2012;53:345–8. doi: 10.2967/jnumed.111.099754. [DOI] [PubMed] [Google Scholar]
- Kim S, Lipowsky HH, Usami S, Chien S. Arteriovenous distribution of hemodynamic parameters in the rat dental pulp. Microvasc Res. 1984;27:28–38. doi: 10.1016/0026-2862(84)90039-6. [DOI] [PubMed] [Google Scholar]
- Kooiman K, Vos HJ, Versluis M, de Jong N. Acoustic behavior of microbubbles and implications for drug delivery. Adv Drug Deliv Rev. 2014;72C:28–48. doi: 10.1016/j.addr.2014.03.003. [DOI] [PubMed] [Google Scholar]
- Kutty S, Xie F, Gao S, Drvol LK, Lof J, Fletcher SE, Radio SJ, Danford DA, Hammel JM, Porter TR. Sonothrombolysis of intra-catheter aged venous thrombi using microbubble enhancement and guided three-dimensional ultrasound pulses. J Am Soc Echocardiogr. 2010;23:1001–6. doi: 10.1016/j.echo.2010.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leeman JE, Kim JS, Yu FTH, Chen X, Kim K, Wang J, Chen X, Villanueva FS, Pacella JJ. Effect of acoustic conditions on microbubble-mediated microvascular sonothrombolysis. Ultrasound Med Biol. 2012;38:1589–98. doi: 10.1016/j.ultrasmedbio.2012.05.020. [DOI] [PubMed] [Google Scholar]
- Lipowsky HH, Usami S, Chien S. In vivo measurements of "apparent viscosity" and microvessel hematocrit in the mesentery of the cat. Microvasc Res. 1980;19:297–319. doi: 10.1016/0026-2862(80)90050-3. [DOI] [PubMed] [Google Scholar]
- Marzilli M, Orsini E, Marraccini P, Testa R. Beneficial effects of intracoronary adenosine as an adjunct to primary angioplasty in acute myocardial infarction. Circulation. 2000;101:2154–9. doi: 10.1161/01.cir.101.18.2154. [DOI] [PubMed] [Google Scholar]
- Mauri L, Rogers C, Baim DS. Devices for distal protection during percutaneous coronary revascularization. Circulation. 2006;113:2651–6. doi: 10.1161/CIRCULATIONAHA.105.551770. [DOI] [PubMed] [Google Scholar]
- Mfoumou E, Tripette J, Blostein M, Cloutier G. Time-dependent hardening of blood clots quantitatively measured in vivo with shear-wave ultrasound imaging in a rabbit model of venous thrombosis. Thromb Res. 2014;133:265–71. doi: 10.1016/j.thromres.2013.11.001. [DOI] [PubMed] [Google Scholar]
- Montorsi P, Caputi L, Galli S, Ciceri E, Ballerini G, Agrifoglio M, Ravagnani P, Trabattoni D, Pontone G, Fabbiocchi F, Loaldi A, Parati E, Andreini D, Veglia F, Bartorelli AL. Microembolization during carotid artery stenting in patients with high-risk, lipid-rich plaque. A randomized trial of proximal versus distal cerebral protection. J Am Coll Cardiol. 2011;58:1656–63. doi: 10.1016/j.jacc.2011.07.015. [DOI] [PubMed] [Google Scholar]
- Niccoli G, Burzotta F, Galiuto L, Crea F. Myocardial no-reflow in humans. J Am Coll Cardiol. 2009;54:281–92. doi: 10.1016/j.jacc.2009.03.054. [DOI] [PubMed] [Google Scholar]
- Ono H, Osanai T, Ishizaka H, Hanada H, Kamada T, Onodera H, Fujita N, Sasaki S, Matsunaga T, Okumura K. Nicorandil improves cardiac function and clinical outcome in patients with acute myocardial infarction undergoing primary percutaneous coronary intervention: role of inhibitory effect on reactive oxygen species formation. Am Heart J. 2004;148:E15. doi: 10.1016/j.ahj.2004.05.014. [DOI] [PubMed] [Google Scholar]
- Pacella JJ, Brands J, Schnatz FG, Black JJ, Chen X, Villanueva FS. Treatment of microvascular micro-embolization using microbubbles and long-tone-burst ultrasound: an in vivo study. Ultrasound Med Biol. 2015;41:456–64. doi: 10.1016/j.ultrasmedbio.2014.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaffenberger S, Devcic-Kuhar B, Kastl SP, Huber K, Maurer G, Wojta J, Gottsauner-Wolf M. Ultrasound thrombolysis. Thromb Haemost. 2005;94:26–36. doi: 10.1160/TH04-12-0818. [DOI] [PubMed] [Google Scholar]
- Piana RN, Paik GY, Moscucci M, Cohen DJ, Gibson CM, Kugelmass AD, Carrozza JP, Jr, Kuntz RE, Baim DS. Incidence and treatment of 'no-reflow' after percutaneous coronary intervention. Circulation. 1994;89:2514–8. doi: 10.1161/01.cir.89.6.2514. [DOI] [PubMed] [Google Scholar]
- Poole JC. A study of artificial thrombi produced by a modification of Chandler's method. Q J Exp Physiol Cogn Med Sci. 1959;44:377–84. doi: 10.1113/expphysiol.1959.sp001419. [DOI] [PubMed] [Google Scholar]
- Porter TR. The utilization of ultrasound and microbubbles for therapy in acute coronary syndromes. Cardiovasc Res. 2009;83:636–42. doi: 10.1093/cvr/cvp206. [DOI] [PubMed] [Google Scholar]
- Porto I, Biasucci LM, De Maria GL, Leone AM, Niccoli G, Burzotta F, Trani C, Tritarelli A, Vergallo R, Liuzzo G, Crea F. Intracoronary microparticles and microvascular obstruction in patients with ST elevation myocardial infarction undergoing primary percutaneous intervention. Eur Heart J. 2012;33:2928–38. doi: 10.1093/eurheartj/ehs065. [DOI] [PubMed] [Google Scholar]
- Postema M, van Wamel A, ten Cate FJ, de Jong N. High-speed photography during ultrasound illustrates potential therapeutic applications of microbubbles. Medical Physics. 2005;32:3707–11. doi: 10.1118/1.2133718. [DOI] [PubMed] [Google Scholar]
- Prasad A, Herrmann J. Myocardial infarction due to percutaneous coronary intervention. N Engl J Med. 2011;364:453–64. doi: 10.1056/NEJMra0912134. [DOI] [PubMed] [Google Scholar]
- Aglyamov RSR, Skovoroda A, Xie H, Kim KM, Rubin J, O'Donnell MW, Wakefield T, Myers DY, Emelianov S. Model-based reconstructive elasticity imaging using ultrasound. Int J Biomed Imaging. 2007;2007:35830. doi: 10.1155/2007/35830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbie LA, Young SP, Bennett B, Booth NA. Thrombi formed in a Chandler loop mimic human arterial thrombi in structure and RAI-1 content and distribution. Thromb Haemost. 1997;77:510–5. [PubMed] [Google Scholar]
- Roessler FC, Ohlrich M, Marxsen JH, Stellmacher F, Sprenger A, Dempfle CE, Seidel G. The platelet-rich plasma clot: a standardized in-vitro clot formation protocol for investigations of sonothrombolysis under physiological flows. Blood Coagul Fibrinolysis. 2011;22:407–15. doi: 10.1097/MBC.0b013e3283468a60. [DOI] [PubMed] [Google Scholar]
- Saber RS, Edwards WD, Bailey KR, McGovern TW, Schwartz RS, Holmes DR., Jr Coronary embolization after balloon angioplasty or thrombolytic therapy: an autopsy study of 32 cases. J Am Coll Cardiol. 1993;22:1283–8. doi: 10.1016/0735-1097(93)90531-5. [DOI] [PubMed] [Google Scholar]
- Schabitz J. The importance of hemorheology in internal medicine. Z Gesamte Inn Med. 1982;37:372–8. [PubMed] [Google Scholar]
- Schwartz RS, Burke A, Farb A, Kaye D, Lesser JR, Henry TD, Virmani R. Microemboli and microvascular obstruction in acute coronary thrombosis and sudden coronary death: relation to epicardial plaque histopathology. Journal of the American College of Cardiology. 2009;54:2167–73. doi: 10.1016/j.jacc.2009.07.042. [DOI] [PubMed] [Google Scholar]
- Shaw GJ, Meunier JM, Huang SL, Lindsell CJ, McPherson DD, Holland CK. Ultrasound-enhanced thrombolysis with tPA-loaded echogenic liposomes. Thromb Res. 2009;124:306–10. doi: 10.1016/j.thromres.2009.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi WT, Forsberg F, Tornes A, Ostensen J, Goldberg BB. Destruction of contrast microbubbles and the association with inertial cavitation. Ultrasound Med Biol. 2000;26:1009–19. doi: 10.1016/s0301-5629(00)00223-4. [DOI] [PubMed] [Google Scholar]
- Silvain J, Collet JP, Nagaswami C, Beygui F, Edmondson KE, Bellemain-Appaix A, Cayla G, Pena A, Brugier D, Barthelemy O, Montalescot G, Weisel JW. Composition of coronary thrombus in acute myocardial infarction. J Am Coll Cardiol. 2011;57:1359–67. doi: 10.1016/j.jacc.2010.09.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmonds MJ, Meiselman HJ, Baskurt OK. Blood rheology and aging. J Geriatr Cardiol. 2013;10:291–301. doi: 10.3969/j.issn.1671-5411.2013.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somer T, Meiselman HJ. Disorders of blood viscosity. Ann Med. 1993;25:31–9. doi: 10.3109/07853899309147854. [DOI] [PubMed] [Google Scholar]
- Tanaka A, Imanishi T, Kitabata H, Kubo T, Takarada S, Tanimoto T, Kuroi A, Tsujioka H, Ikejima H, Komukai K, Kataiwa H, Okouchi K, Kashiwaghi M, Ishibashi K, Matsumoto H, Takemoto K, Nakamura N, Hirata K, Mizukoshi M, Akasaka T. Lipid-rich plaque and myocardial perfusion after successful stenting in patients with non-ST-segment elevation acute coronary syndrome: an optical coherence tomography study. Eur Heart J. 2009;30:1348–55. doi: 10.1093/eurheartj/ehp122. [DOI] [PubMed] [Google Scholar]
- Thiele H, Schindler K, Friedenberger J, Eitel I, Furnau G, Grebe E, Erbs S, Linke A, Mobius-Winkler S, Kivelitz D, Schuler G. Intracoronary compared with intravenous bolus abciximab application in patients with ST-elevation myocardial infarction undergoing primary percutaneous coronary intervention: the randomized Leipzig immediate percutaneous coronary intervention abciximab IV versus IC in ST-elevation myocardial infarction trial. Circulation. 2008;118:49–57. doi: 10.1161/CIRCULATIONAHA.107.747642. [DOI] [PubMed] [Google Scholar]
- Toksvang L, Berg R. The viscosity of the blood in narrow capillary tubes. Am J Physiol. 1931;96:562–8. [Google Scholar]
- Tynngard N, Lindahl T, Ramstrom S, Berlin G. Effects of different blood components on clot retraction analysed by measuring elasticity with a free oscillating rheometer. Platelets. 2006;17:545–54. doi: 10.1080/09537100600759238. [DOI] [PubMed] [Google Scholar]
- Villanueva FS, Abraham JA, Schreiner GF, Csikari M, Fischer D, Mills JD, Schellenberger U, Koci BJ, Lee JS. Myocardial contrast echocardiography can be used to assess the microvascular response to vascular endothelial growth factor-121. Circulation. 2002;105:759–65. doi: 10.1161/hc0602.103634. [DOI] [PubMed] [Google Scholar]
- Villanueva FS, Camarano G, Ismail S, Goodman NC, Sklenar J, Kaul S. Coronary reserve abnormalities in the infarcted myocardium. Assessment of myocardial viability immediately versus late after reflow by contrast echocardiography. Circulation. 1996;94:748–54. doi: 10.1161/01.cir.94.4.748. [DOI] [PubMed] [Google Scholar]
- Villanueva FS, Glasheen WP, Sklenar J, Kaul S. Assessment of risk area during coronary occlusion and infarct size after reperfusion with myocardial contrast echocardiography using left and right atrial injections of contrast. Circulation. 1993a;88:596–604. doi: 10.1161/01.cir.88.2.596. [DOI] [PubMed] [Google Scholar]
- Villanueva FS, Glasheen WP, Sklenar J, Kaul S. Characterization of spatial patterns of flow within the reperfused myocardium by myocardial contrast echocardiography. Implications in determining extent of myocardial salvage. Circulation. 1993b;88:2596–606. doi: 10.1161/01.cir.88.6.2596. [DOI] [PubMed] [Google Scholar]
- Wei K, Tong KL, Belcik T, Rafter P, Ragosta M, Wang XQ, Kaul S. Detection of coronary stenoses at rest with myocardial contrast echocardiography. Circulation. 2005;112:1154–60. doi: 10.1161/CIRCULATIONAHA.104.513887. [DOI] [PubMed] [Google Scholar]
- Wu KC, Zerhouni EA, Judd RM, Lugo-Olivieri CH, Barouch LA, Schulman SP, Blumenthal RS, Lima JA. Prognostic significance of microvascular obstruction by magnetic resonance imaging in patients with acute myocardial infarction. Circulation. 1998;97:765–72. doi: 10.1161/01.cir.97.8.765. [DOI] [PubMed] [Google Scholar]
- Xie F, Lof J, Matsunaga T, Zutshi R, Porter TR. Diagnostic ultrasound combined with glycoprotein IIb/IIIa-targeted microbubbles improves microvascular recovery after acute coronary thrombotic occlusions. Circulation. 2009;119:1378–85. doi: 10.1161/CIRCULATIONAHA.108.825067. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Power spectra of passively detected cavitation signal at 1 MHz and excitation pressures of 0.25, 0.5, 1.0 and 1.5 MPa in plasma, plasma+2%PVP (4cP) and blood (porcine RBC at 40% hematocrit suspended in PBS). The system was maintained at 37°C. Transmit (and receive transducers were confocally aligned on flowing lipid encapsulated microbubbles (mean size of 3.1 μm) at a concentration of 5×104 MB/mL. MB cavitation activity was dampened in plasma+PVP and was further dampened in blood at 0.25 and 0.5 MPa. Compared to plasma, inertial cavitation level was reduced by ~10 dB in plasma+PVP and in blood at 0.5 MPa; Compared to plasma+PVP, stable cavitation was reduced in blood by 10 dB at 0.25 MPa and by ~5 dB at 0.5 MPa. These data support that adjusting plasma viscosity to 4 cP with PVP partly recapitulates the damping effect of blood on MB oscillations under the ultrasound conditions tested.