Abstract
Viruses are pathogens that strictly depend on their host for propagation. Over years of co-evolution viruses have become experts in exploiting the host cell biology and physiology to ensure efficient replication and spread. Here, we will first summarize the concepts that have emerged from in vitro cell culture studies to understand virus spread. We will then review the results from studies in living animals that reveal how viruses exploit the natural flow of body fluids, specific tissue architecture, and patterns of cell circulation and migration to spread within the host. Understanding tissue physiology will be critical for the design of antiviral strategies that prevent virus dissemination.
Introduction
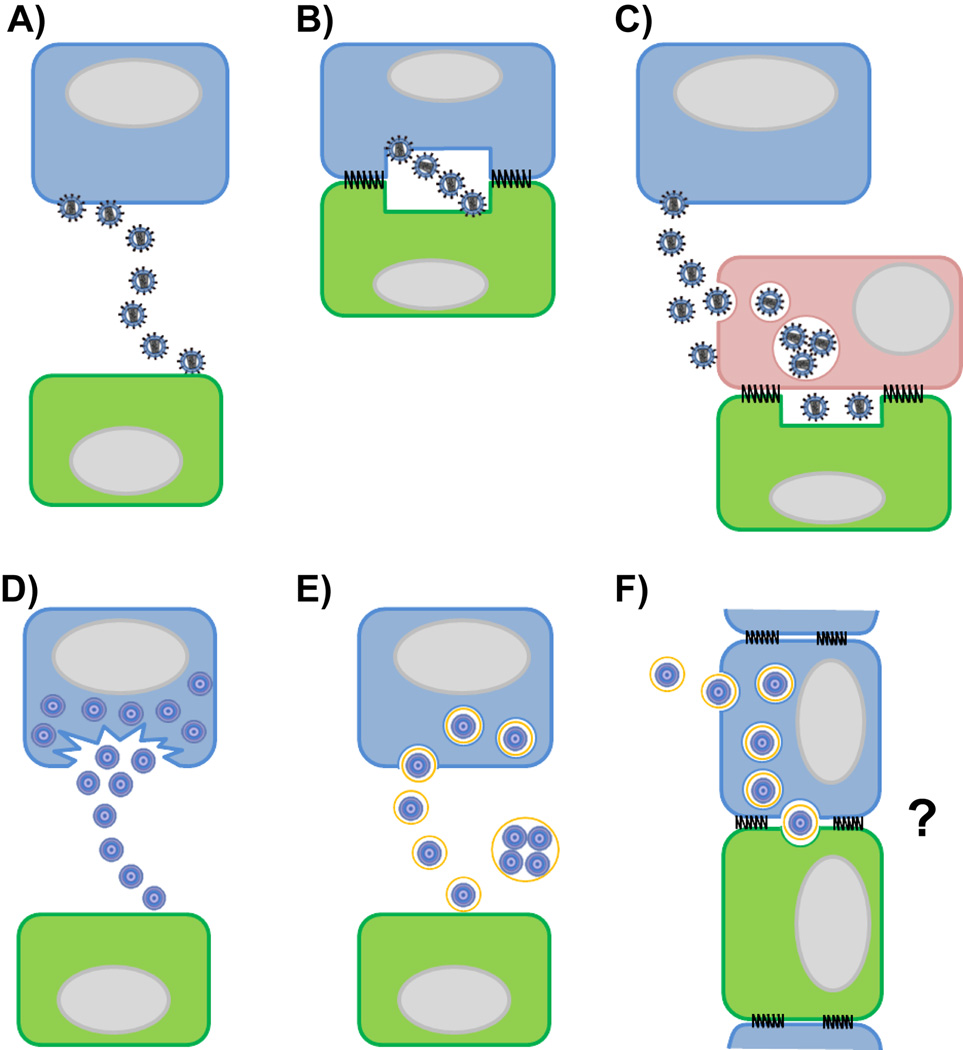
Viruses can be transmitted from infected to non-infected cells by diffusion through the extracellular space. This process is commonly referred to as cell-free transmission (Fig. 1A). Alternatively, the process where cell-surface attached viral particles are delivered to neighboring cells for infection via cell-contacts is defined as virus cell-to-cell transmission (for reviews see [1–5]). Contact-dependent transmission is further classified based on whether the donor cells are infected or not. The ability of productively infected donor cells to establish cell-cell contact with non-infected cells for infection is described by the concept of the virological synapse (Fig. 1B) [6,7]. In contrast, the ability of a non-infected donor cell to capture virus and transfer it to a permissive target cell is designated trans-infection (Fig. 1C) [8,9]. The cell-cell contact formed during trans-infection is also described as the infectious synapse [9]. Contact-dependent transmission has been observed in vitro for many enveloped viruses including the retroviruses human immunodeficiency virus (HIV), human T-lymphotropic virus (HTLV) and murine leukemia virus (MLV) [6,10–12]. The transfer of viral particles has been visualized using live cell microscopy between infected and non-infected fibroblasts, infected and non-infected T cells, between dendritic cells (DCs) and T cells, as well as macrophages and T cells [10–14]. Virological synapses and trans-infection events have now also been documented in living animals suggesting that both processes can contribute to viral spread in vivo [15].
Fig. 1.
In vitro pathways of virus cell transmission. (A–C) Enveloped viruses have evolved with the host cell to efficiently spread from an infected cell (depicted in blue) to a non-infected cell (depicted in green). Cell-free transmission of enveloped viruses by diffusion through the extracellular environment after budding from an infected cell (A). Productively infected cell transfer virus particles across a virological synapse for cis-infection (B). For trans-infection, cell-free virus particles are captured by a cell that itself does not get infected (depicted in pink) and then presented to a target cell at a cell-cell contact designated infectious synapse (C). (D–E) Non-enveloped viruses can be released from an infected cell after cell-lysis (D) or non-lytically by acquisition of temporary host membrane to infect susceptible target cells via cell-free transmission (E). Panel (F) depicts a hypothesis for cell-to-cell transmission of non-enveloped viruses with acquired host membrane after polarized release at cell contact sites. Grey ovals represent cell nuclei.
Virus cell-to-cell transmission at the virological synapse
Some viruses evolved to utilize existing cell–cell contacts, such as synaptic contacts in order to spread between neurons [16,17]. Alternatively, viruses can initiate the formation of new cell-cell contacts or stabilize transient interactions between cells for transmission. Herpes simplex virus-infected cells actively attract nerve endings and induce skin cell migration for cell-contact formation and virus transmission [18,19]. Retrovirus-infected cells express the envelope glycoprotein to stabilize transient cell interactions between migratory immune cells for virus transfer [6,7,20].
Imaging techniques such as time-lapse confocal microscopy have been fundamental to characterize virus transmission across cell-cell contacts between virus-producing cells and non-infected cells [21]. Virological synapses were first described in mixed cultures of HTLV- and HIV-infected with non-infected T cells [6,7,22]. Similar cell-cell contacts have also been observed for other viruses [10,23,24]. Tight cell contacts are rapidly initiated through interactions of the virus glycoprotein with the target cell receptor leading to an accumulation of viral proteins and cellular factors at the cell-cell contact [7,10,20,25]. Similar to the supramolecular organization of immune and neuronal synapses [26,27], virological synapses of HIV-infected cells reveal a characteristic accumulation of the viral proteins Gag and Env together with the cellular receptors CD4 and CXCR4, surrounded by an adhesive contact of intercellular adhesion molecule-1 (ICAM-1) and lymphocyte function-associated antigen 1 (LFA-1) [11,25,28,29]. Signaling pathways are induced in target cells that partially resemble the T cell activation seen in immunological synapses [27]. Binding of HIV gp120 to CD4 and ICAM-1 to LFA-1 partially activates T cell receptor (TCR) signaling pathways resulting in reduced cell migration and polarization [28–32]. Virus assembly and release is then polarized towards cell-cell contact sites. In the case of MLV, virus budding is polarized to areas on the plasma membrane where the clustering of Env at the cell-cell interface initiates the recruitment of Gag [12,33]. In contrast, HIV assembly is directed towards sites of cell-cell contact by polarization of the cytoskeleton and the secretory machinery [34,35], as well as spatial clustering of organelles such as mitochondria [36]. A structural analysis of the virological synapse between HIV-infected and non-infected T cells or astrocytes reveals a complex membrane organization with cell-type specific differences in the cell contact architecture and the distribution of sites for virus budding and release [37,38]. Mechanistic details of Gag polarization and virus release at the cell-cell interface comes from a recent study of the immunological synapse [39]. Electron microscopy of cell contacts between T cells and antigen presenting cells revealed the formation of numerous microvesicles at the contact center and transfer of TCR-containing vesicles. The endosomal sorting complexes required for transport (ESCRT) machinery components tumor susceptibility gene 101 (Tsg101) and vacuolar protein sorting-associated protein 4 (Vps4) were essential for cargo sorting and microvesicle scission from the plasma membrane, respectively. Strikingly, the HIV polyprotein Gag was shown to co-opt this pathway for Env-independent budding at the cell-contact site with TCR ligation-directed polarization. This study indicates that HIV can spread between immune cells by exploiting the fundamental properties of the immunological synapse for material transfer to other cells.
Virus transmission through trans-infection
Technological advances in microscopy, such as time-lapse confocal microscopy and electron tomography, have enabled researchers to gain insight into the organization of infectious synapses during virus trans-infection. Monocyte-derived DCs (MDDCs) were observed to bind HIV particles in vitro and subsequently form infectious synapses with virus receptor-expressing T cells [9,40]. Virus particles are attached to or internalized into virus-containing compartments by MDDCs through the interaction with C-type lectins [41,42] on immature MDDCs or the I-type lectin CD169/Siglec-1 on mature MDDCs [43–47]. CD169-dependent trans-infection of HIV and MLV has also been observed in macrophages and monocytes [15,48–50]. After the cell contact is initiated, reorientation of the virus-containing compartment to the contact site is accompanied by an accumulation of cellular receptors and cell adhesion molecules to form long-lasting contacts for virus transfer [9,41,51,52]. The cortical actin cytoskeleton and membrane sorting pathways facilitate virus transmission to target cells [40,53–55]. Sheet-like dendrites that are derived from the plasma membranes of MDDCs form a shielded cell contact region. Within this microenvironment, filopodia protrusions emanating from CD4+ T cells make contact with HIV particles within surface-accessible virus-containing compartments for infection [40,56]. Live cell microscopy confirms the highly dynamic nature of infectious synapses [51]. The distinction of virus cell-to-cell transmission into virological synapses largely observed in T cells and trans-infection routes mediated by antigen-presenting cells can also be more blurred. Certain HIV isolates are able to productively infect macrophages [57–59]. In addition, macrophages can engulf HIV-infected T cells that lead to their efficient infection and subsequent virus cell-to-cell spread [60,61].
Transmission of non-enveloped viruses
The concepts of contact-dependent virus transmission have been developed for enveloped viruses that bud from cellular membranes. The general belief that non-enveloped viruses are exclusively released as a consequence of cell lysis (Fig. 1D) has recently been challenged [62]. HepA, HepE, and poliovirus were shown to escape from intact cells by acquiring a temporary membrane (Fig. 1E) [63–66]. Older reports demonstrate the non-lytic release of poliovirus and SV40 from the apical side of polarized cells without loss of cell viability [67,68]. Mechanistically, the autophagic pathway and the ESCRT machinery have been identified to play a role in temporary membrane acquisition and non-lytic release of some non-enveloped viruses [64,66,69–71]. With the recent observations of non-lytic, polarized release, future studies should explore if contact-dependent cell-to-cell transmission plays a role in the spread of non-enveloped viruses (Fig. 1F).
Benefits of cell-to-cell transmission for virus pathogenesis
Multiple studies suggest that contact-dependent transmission provides advantages for virus spread and thus play a role in pathogenesis. Early studies demonstrated that cell contact-dependent transmission can be orders of magnitude more efficient than infection through cell-free virus [72,73]. A comprehensive study comparing HIV cell-to-cell and cell-free transmission shows that contact-dependent spread of HIV is the result of specific donor and target cell features [74]. Contact-dependent HIV infection has been shown to overcome multiple barriers to cell-free virus that were experimentally imposed on the donor or target cell. For example, poor virus transmission rates because of low receptor expression levels or cellular restriction factors are compensated by cell-to-cell but not cell-free infection [74–77]. Contact-dependent virus transfer across virological or infectious synapses also enables viruses to evade certain neutralizing antibodies [47,74,78–81]. Several studies found that cell-to-cell transmission of HIV resulted in a higher proviral content of infected target cells [74,82,83]. As a result, HIV-1 was able to overcome individual anti-retroviral drugs but not combinations of drugs through cell-to-cell transmission suggesting that the ability to suppress high viral multiplicity of infection is a feature of effective ART [84,85]. Interestingly, high multiplicity of infection results in bystander death through apoptosis and/or pyroptosis of the target cell, an effect that required HIV cell-to-cell transmission [86–89].
Virus transmission in vivo
In vitro studies of virus cell-to-cell transmission, discussed above, have revealed many basic insights and mechanistic details of virus transmission. However, to what extent these processes contribute to virus spread in vivo remains largely unclear. Live animal and tissue explant studies are essential for our understanding of virus spread and the development of antiviral strategies. Similar to the impact that time-lapse confocal microscopy had in visualizing virus transmission in tissue culture, intravital imaging techniques such as in vivo bioluminescence imaging and multi-photon microscopy are now opening up new avenues to follow virus dissemination directly in living animals.
Systemic virus spread by cell-free virus and migratory cells
Only few studies have started to address how viruses spread within complex tissues of living organisms. Many viruses enter the host at mucosal surfaces or skin and subsequently spread based on their cell tropism to different tissues for replication and host-to-host transmission. This systemic dissemination is closely linked to the physiology of the host as most tissues are connected through a system of extracellular fluid consisting of interstitial fluid, lymph and blood [90]. The interstitial fluid surrounds all cells of a tissue and provides essential nutrition as well as environmental cues necessary for survival. It originates from capillary-filtered blood plasma and, thus, has a similar composition. After leaving the tissue, interstitial fluid is collected in primitive vessels of the lymphatic system that become larger and more complex. The collected interstitial fluid is thereafter named lymph. Large lymphatic vessels collect lymph from various areas of the body and drain into the systemic blood circulation at the subclavian veins to close the loop.
The systemic flow and the positioning of lymphoid tissue along vessels permit tissue surveillance by the immune system to protect against pathogens and provide a network for immune cell migration. However, the continuous flow of extracellular fluid also provides an efficient system for viruses to spread over long distances within the host. Under experimental conditions simulating virus transmission through arthropod vectors, scratching or wounding during biting, several subcutaneously injected cell-free viruses arrived through the afferent lymph within minutes at tissue-draining lymph nodes to infect immune and neuronal cells (Figure 2A) [91–97]. Consequently, cell-free virus spread after budding from an infected cell in peripheral tissue is by all means realistic although it has not been directly shown in vivo.
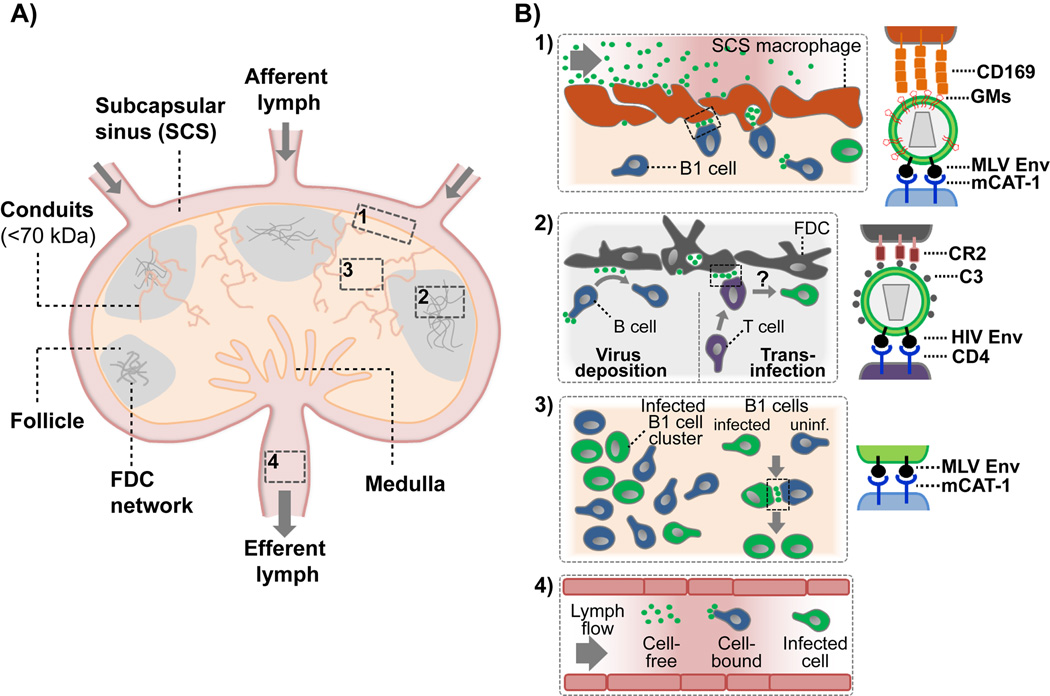
Fig. 2.
Model depicting the structural organization of a lymph node (A) and in vivo pathways of virus transmission (local tissue, systemic) (B). (A) Lymph arrives at draining lymph nodes through afferent lymphatic vessels and enters at the lymph node subcapsular sinus (SCS). Small molecules (<70 kDa) access the lymph node cortex via conduits for subsequent filtration by immune cells [98–100]. Sinus-lining macrophages and DCs surveil the lymph for antigen, immune complexes and pathogens. Filtered lymph is collected at the medulla and leaves the lymph node through the efferent lymphatics to enter secondary lymph nodes. B cell follicles with a stromal cell network of follicular dendritic cells (FDC) are in close contact with the SCS floor. Examples of in vivo virus transmission (Boxes 1–4) are summarized in (B). (B) Pathways of retrovirus transmission within lymphoid tissue and for systemic spread. (1) CD169-expressing SCS macrophages capture lymph-derived MLV and HIV by recognition of gangliosides (GMs) embedded in the virus lipid bilayer. In the case of MLV, SCS macrophages then form stable contacts with MLV receptor (mouse cationic amino acid transporter-1, mCAT-1) expressing B-1 cells to trans-infect these cells. (2) B cells can deposit HIV particles on FDCs within B cell follicles for subsequent trans-infection of T cells. Binding depends on the complement protein C3 and complement receptors 2 (CR2). (3) MLV-infected B1 cells are found in clusters within infected popliteal lymph nodes. Infected cells form mCAT1-dependent virological synapses with uninfected cells. (4) Long-distance spread of HIV within the lymph can be mediated by either cell-free, cell-bound or migration of HIV-infected cells. Viruses are depicted as green spheres.
An alternative pathway for virus long-distance spread is through migratory cells. Productively infected cells can function as vehicles and, thus, contribute to systemic virus dissemination. HIV-infected T cells were shown to exit peripheral lymph nodes and significantly contribute to the systemic infection of humanized mice (Figure 2A and 2B, box 4) [98]. Blocking leukocyte egress from lymphoid tissue in HIV-infected humanized mice significantly reduces virus dissemination [98]. Similarly, mouse cytomegalovirus-infected blood monocytes can disseminate virus from local infection sites to salivary glands and promote latency [99].
Finally, based on their described function in antigen delivery to draining lymph nodes [100,101], mucosal tissue DCs are suspected of transporting viruses such as HIV and varicella zoster virus to draining lymph nodes for infection of T cells [102]. A clear contribution of this pathway to virus dissemination in living animals remains to be determined.
Cell-to-cell transmission of lymph-derived virus in lymphoid tissue
Cell-free virus is transported via extracellular fluid until it reaches a susceptible cell population. Physical barriers at the fluid-tissue interface restrict virus access to target cells localized within tissues. For example, secondary lymphoid tissues such as lymph nodes are designed to efficiently filter the lymph (Figure 2A). Only small molecules (<70kDa, <5nm) can passively enter the lymph node interior through conduits for direct contact with immune cells [103–105]. Larger particles remain in the lymph or interact with immune cells at the interface. The cell sieve between the lymph node sinus and cortex is organized by a layer of tissue-specific resident macrophages and lymphatic endothelial cells that play an important role in the immune surveillance of the lymph [106,107]. Sinus-lining macrophages can capture pathogens to block their systemic spread, present immune complexes to immune cells and orchestrate immune responses by recruiting effector cells to the subcapsular sinus (SCS) floor [108]. Analogue tissue architecture is found at the marginal zone in the spleen and allows the body to similarly survey the blood [109].
Viruses have evolved mechanisms to overcome this barrier and access host tissue for the infection of permissive lymphocytes in the subjacent tissue. Fluid-derived retroviruses MLV and HIV are filtered by sinus-lining macrophages of the draining lymph node and spleen in vivo (Fig. 2B, box 1) [15]. Virus capture is mediated by the I-type lectin CD169 through the recognition of gangliosides within the retrovirus membrane [15], as previously demonstrated in vitro [43–46]. As such, retroviruses appear to exploit the inherent function of CD169+ macrophages to capture exosomes that similarly carry gangliosides [110,111]. MLV was found in deep plasma membrane invaginations of SCS macrophages in vivo as has been observed previously in monocyte-derived DCs and macrophages in vitro [47,51,112–114]. Using intravital microscopy, MLV transfer from SCS macrophages to B1 cells could be directly visualized [15]. After trans-infection, B cells formed Env-dependent virological synapses with susceptible cells in vivo to amplify the infection (Fig. 2B, box 3) [15,115]. These studies suggest that viruses could use fluid-based spread for long-distance travel followed by the exploitation of CD169-mediated capture of viral particles for efficient trans-infection of permissive lymphocytes for subsequent spreading in lymphoid tissues.
In addition, the complement system might facilitate virus tissue access and subsequent transport within tissue. Lymph-derived HIV particles accumulate on follicular dendritic cells in B cell follicles of lymph nodes (Fig. 2B, box 2) [116,117]. Interestingly, the transport of lymph-derived HIV particles into B cell follicles is species independent and occurs in the absence of HIV-specific antibodies [117,118]. Mechanistically, HIV was shown to fix complement factors such as C3 on its surface to mediate cell binding through complement receptor 2 (CD21) [119–122]. CD21-expressing B cells and follicular dendritic cells can bind complement-opsonized HIV for transfer to T cells in vitro [120,123,124]. Blocking of CD21 interferes with HIV accumulation on follicular dendritic cells and B cells in vitro and in vivo [123–125]. Similar transport pathways were recently described for immune complexes, vesicular stomatitis virus and soluble HIV gp120 [126–129]. After B cell-mediated transport, immune complexes are retained intact on follicular dendritic cells within a periodically cycling compartment for long-term antigen presentation [130]. Similarly, the follicular dendritic cell network in B cell follicles can store HIV particles for a long time and, thus, is considered to function as a reservoir [116–118,131]. Since follicular dendritic cells lack CD4 expression and are not infected by HIV [124], they transmit HIV to susceptible T cells via trans-infection, a process that may also occur in vivo (Fig. 2B, box 2).
Conclusions
In vitro studies of virus transmission have been fundamental to characterize the mechanism of virus cell-to-cell spread between defined cell types. Visualization of retrovirus cell-to-cell transmission helped to define the basic concepts of the virological and infectious synapse, and provided dynamic subcellular details about the individual steps of synapse formation and transfer. Importantly, the results of in vitro studies set the stage for the challenging task to study virus spreading in living animals. Initial in vivo studies have revealed how local and systemic virus transmission is critically influenced by the tissue physiology. The mechanism of virus transmission is shaped by the tissue context and influenced by physical barriers such as the fluid-tissue interfaces (lymph/lymph node, blood/spleen), local cell populations with limited exchange with the systemic cell pool or spatially restricted cell migration and cell-cell interaction. In vivo studies will be critical for the understanding of viral transmission since each tissue is a composite of specific cell subsets that depend on tissue specific cues from neighboring cells for cell development, homeostasis and function that can often not be reconstituted in vitro. Continued advances in in vivo imaging technologies together with high-resolution in vitro imaging studies will continue to provide critical insights into the mechanism of virus spread and how this knowledge can be harnessed for antiviral strategies that interfere with virus dissemination.
Acknowledgments
We thank Pradeep Uchil for critical reading of this manuscript. This work was supported by the NIH grant CA098727 to WM and the Leopoldina Fellowship LPDS2009-21 to XS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Sattentau Q. Avoiding the void: cell-to-cell spread of human viruses. Nat Rev Microbiol. 2008;6:815–826. doi: 10.1038/nrmicro1972. [DOI] [PubMed] [Google Scholar]
- 2.Mothes W, Sherer NM, Jin J, Zhong P. Virus cell-to-cell transmission. J Virol. 2010;84:8360–8368. doi: 10.1128/JVI.00443-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jolly C. T cell polarization at the virological synapse. Viruses. 2010;2:1261–1278. doi: 10.3390/v2061261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fackler OT, Murooka TT, Imle A, Mempel TR. Adding new dimensions: towards an integrative understanding of HIV-1 spread. Nat Rev Microbiol. 2014;12:563–574. doi: 10.1038/nrmicro3309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alvarez RA, Barria MI, Chen BK. Unique features of HIV-1 spread through T cell virological synapses. PLoS Pathog. 2014;10:e1004513. doi: 10.1371/journal.ppat.1004513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Igakura T, Stinchcombe JC, Goon PK, Taylor GP, Weber JN, Griffiths GM, Tanaka Y, Osame M, Bangham CR. Spread of HTLV-I between lymphocytes by virus-induced polarization of the cytoskeleton. Science. 2003;299:1713–1716. doi: 10.1126/science.1080115. • Together with Jolly et al. [7], this study establishes the concept of the virological synapse.
- 7. Jolly C, Kashefi K, Hollinshead M, Sattentau QJ. HIV-1 cell to cell transfer across an Env-induced, actin-dependent synapse. J Exp Med. 2004;199:283–293. doi: 10.1084/jem.20030648. • Together with Igakura et al. [6], this study establishes the concept of the virological synapse.
- 8. Cameron PU, Freudenthal PS, Barker JM, Gezelter S, Inaba K, Steinman RM. Dendritic cells exposed to human immunodeficiency virus type-1 transmit a vigorous cytopathic infection to CD4+ T cells. Science. 1992;257:383–387. doi: 10.1126/science.1352913. • Together with McDonald et al. [9], this study introduces the concept of trans-infection.
- 9. McDonald D, Wu L, Bohks SM, KewalRamani VN, Unutmaz D, Hope TJ. Recruitment of HIV and its receptors to dendritic cell-T cell junctions. Science. 2003;300:1295–1297. doi: 10.1126/science.1084238. • Together with Cameron et al. [8], this study introduces the concept of trans-infection and establishes the model of the infectious synapse.
- 10. Sherer NM, Lehmann MJ, Jimenez-Soto LF, Horensavitz C, Pypaert M, Mothes W. Retroviruses can establish filopodial bridges for efficient cell-to-cell transmission. Nat Cell Biol. 2007;9:310–315. doi: 10.1038/ncb1544. • Together with Hubner et al. [11], this study visualizes the transfer of retrovirus particles across cell-cell contacts in tissue culture cells.
- 11. Hubner W, McNerney GP, Chen P, Dale BM, Gordon RE, Chuang FY, Li XD, Asmuth DM, Huser T, Chen BK. Quantitative 3D video microscopy of HIV transfer across T cell virological synapses. Science. 2009;323:1743–1747. doi: 10.1126/science.1167525. • Together with Sherer et al. [10], this study visualizes the transfer of retrovirus particles across cell-cell contacts in tissue culture cells.
- 12. Jin J, Sherer NM, Heidecker G, Derse D, Mothes W. Assembly of the murine leukemia virus is directed towards sites of cell-cell contact. PLoS Biol. 2009;7:e1000163. doi: 10.1371/journal.pbio.1000163. • This study demonstrates polarized virus assembly at virological synapses.
- 13.Groot F, Welsch S, Sattentau QJ. Efficient HIV-1 transmission from macrophages to T cells across transient virological synapses. Blood. 2008;111:4660–4663. doi: 10.1182/blood-2007-12-130070. [DOI] [PubMed] [Google Scholar]
- 14.Gousset K, Ablan SD, Coren LV, Ono A, Soheilian F, Nagashima K, Ott DE, Freed EO. Real-time visualization of HIV-1 GAG trafficking in infected macrophages. PLoS Pathog. 2008;4:e1000015. doi: 10.1371/journal.ppat.1000015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sewald X, Ladinsky MS, Uchil PD, Beloor J, Pi R, Herrmann C, Motamedi N, Murooka TT, Brehm MA, Greiner DL, et al. Retroviruses use CD169-mediated trans-infection of permissive lymphocytes to establish infection. Science. 2015;350:563–567. doi: 10.1126/science.aab2749. •• This study demonstrates the key concept of contact-dependent trans-infection in vivo.
- 16.Koyuncu OO, Hogue IB, Enquist LW. Virus infections in the nervous system. Cell Host Microbe. 2013;13:379–393. doi: 10.1016/j.chom.2013.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Taylor MP, Enquist LW. Axonal spread of neuroinvasive viral infections. Trends Microbiol. 2015;23:283–288. doi: 10.1016/j.tim.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cabrera JR, Viejo-Borbolla A, Martinez-Martin N, Blanco S, Wandosell F, Alcami A. Secreted herpes simplex virus-2 glycoprotein G modifies NGF-TrkA signaling to attract free nerve endings to the site of infection. PLoS Pathog. 2015;11:e1004571. doi: 10.1371/journal.ppat.1004571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abaitua F, Zia FR, Hollinshead M, O'Hare P. Polarized cell migration during cell-to-cell transmission of herpes simplex virus in human skin keratinocytes. J Virol. 2013;87:7921–7932. doi: 10.1128/JVI.01172-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen P, Hubner W, Spinelli MA, Chen BK. Predominant mode of human immunodeficiency virus transfer between T cells is mediated by sustained Env-dependent neutralization-resistant virological synapses. J Virol. 2007;81:12582–12595. doi: 10.1128/JVI.00381-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Feldmann J, Schwartz O. HIV-1 Virological Synapse: Live Imaging of Transmission. Viruses. 2010;2:1666–1680. doi: 10.3390/v2081666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Phillips DM. The role of cell-to-cell transmission in HIV infection. AIDS. 1994;8:719–731. doi: 10.1097/00002030-199406000-00001. [DOI] [PubMed] [Google Scholar]
- 23.Koethe S, Avota E, Schneider-Schaulies S. Measles virus transmission from dendritic cells to T cells: formation of synapse-like interfaces concentrating viral and cellular components. J Virol. 2012;86:9773–9781. doi: 10.1128/JVI.00458-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aubert M, Yoon M, Sloan DD, Spear PG, Jerome KR. The virological synapse facilitates herpes simplex virus entry into T cells. J Virol. 2009;83:6171–6183. doi: 10.1128/JVI.02163-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rudnicka D, Feldmann J, Porrot F, Wietgrefe S, Guadagnini S, Prevost MC, Estaquier J, Haase AT, Sol-Foulon N, Schwartz O. Simultaneous cell-to-cell transmission of human immunodeficiency virus to multiple targets through polysynapses. J Virol. 2009;83:6234–6246. doi: 10.1128/JVI.00282-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dustin ML, Colman DR. Neural and immunological synaptic relations. Science. 2002;298:785–789. doi: 10.1126/science.1076386. [DOI] [PubMed] [Google Scholar]
- 27.Huppa JB, Davis MM. T-cell-antigen recognition and the immunological synapse. Nat Rev Immunol. 2003;3:973–983. doi: 10.1038/nri1245. [DOI] [PubMed] [Google Scholar]
- 28.Vasiliver-Shamis G, Tuen M, Wu TW, Starr T, Cameron TO, Thomson R, Kaur G, Liu J, Visciano ML, Li H, et al. Human immunodeficiency virus type 1 envelope gp120 induces a stop signal and virological synapse formation in noninfected CD4+ T cells. J Virol. 2008;82:9445–9457. doi: 10.1128/JVI.00835-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vasiliver-Shamis G, Cho MW, Hioe CE, Dustin ML. Human immunodeficiency virus type-1 envelope gp120-induced partial T-cell receptor signaling creates an F-actin-depleted zone in the virological synapse. J Virol. 2009;83:11341–11355. doi: 10.1128/JVI.01440-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hioe CE, Tuen M, Vasiliver-Shamis G, Alvarez Y, Prins KC, Banerjee S, Nadas A, Cho MW, Dustin ML, Kachlany SC. HIV envelope gp120 activates LFA-1 on CD4 T-lymphocytes and increases cell susceptibility to LFA-1-targeting leukotoxin (LtxA) PLoS One. 2011;6:e23202. doi: 10.1371/journal.pone.0023202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sol-Foulon N, Sourisseau M, Porrot F, Thoulouze MI, Trouillet C, Nobile C, Blanchet F, di Bartolo V, Noraz N, Taylor N, et al. ZAP-70 kinase regulates HIV cell-to-cell spread and virological synapse formation. EMBO J. 2007;26:516–526. doi: 10.1038/sj.emboj.7601509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nejmeddine M, Negi VS, Mukherjee S, Tanaka Y, Orth K, Taylor GP, Bangham CR. HTLV-1-Tax and ICAM-1 act on T-cell signal pathways to polarize the microtubule-organizing center at the virological synapse. Blood. 2009;114:1016–1025. doi: 10.1182/blood-2008-03-136770. [DOI] [PubMed] [Google Scholar]
- 33.Li F, Jin J, Herrmann C, Mothes W. Basic residues in the matrix domain and multimerization target murine leukemia virus Gag to the virological synapse. J Virol. 2013;87:7113–7126. doi: 10.1128/JVI.03263-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jolly C, Mitar I, Sattentau QJ. Requirement for an intact T-cell actin and tubulin cytoskeleton for efficient assembly and spread of human immunodeficiency virus type 1. J Virol. 2007;81:5547–5560. doi: 10.1128/JVI.01469-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jolly C, Welsch S, Michor S, Sattentau QJ. The regulated secretory pathway in CD4(+) T cells contributes to human immunodeficiency virus type-1 cell-to-cell spread at the virological synapse. PLoS Pathog. 2011;7:e1002226. doi: 10.1371/journal.ppat.1002226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Groppelli E, Starling S, Jolly C. Contact-induced mitochondrial polarization supports HIV-1 virological synapse formation. J Virol. 2015;89:14–24. doi: 10.1128/JVI.02425-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Do T, Murphy G, Earl LA, Del Prete GQ, Grandinetti G, Li GH, Estes JD, Rao P, Trubey CM, Thomas J, et al. Three-dimensional imaging of HIV-1 virological synapses reveals membrane architectures involved in virus transmission. J Virol. 2014;88:10327–10339. doi: 10.1128/JVI.00788-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Martin N, Welsch S, Jolly C, Briggs JA, Vaux D, Sattentau QJ. Virological synapse-mediated spread of human immunodeficiency virus type 1 between T cells is sensitive to entry inhibition. Journal of virology. 2010;84:3516–3527. doi: 10.1128/JVI.02651-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Choudhuri K, Llodra J, Roth EW, Tsai J, Gordo S, Wucherpfennig KW, Kam LC, Stokes DL, Dustin ML. Polarized release of T-cell-receptor-enriched microvesicles at the immunological synapse. Nature. 2014;507:118–123. doi: 10.1038/nature12951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nikolic DS, Lehmann M, Felts R, Garcia E, Blanchet FP, Subramaniam S, Piguet V. HIV-1 activates Cdc42 and induces membrane extensions in immature dendritic cells to facilitate cell-to-cell virus propagation. Blood. 2011;118:4841–4852. doi: 10.1182/blood-2010-09-305417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Geijtenbeek TB, Kwon DS, Torensma R, van Vliet SJ, van Duijnhoven GC, Middel J, Cornelissen IL, Nottet HS, KewalRamani VN, Littman DR, et al. DC-SIGN, a dendritic cell-specific HIV-1-binding protein that enhances trans-infection of T cells. Cell. 2000;100:587–597. doi: 10.1016/s0092-8674(00)80694-7. [DOI] [PubMed] [Google Scholar]
- 42.Turville SG, Cameron PU, Handley A, Lin G, Pohlmann S, Doms RW, Cunningham AL. Diversity of receptors binding HIV on dendritic cell subsets. Nat Immunol. 2002;3:975–983. doi: 10.1038/ni841. [DOI] [PubMed] [Google Scholar]
- 43. Izquierdo-Useros N, Lorizate M, Contreras FX, Rodriguez-Plata MT, Glass B, Erkizia I, Prado JG, Casas J, Fabrias G, Krausslich HG, et al. Sialyllactose in viral membrane gangliosides is a novel molecular recognition pattern for mature dendritic cell capture of HIV-1. PLoS Biol. 2012;10:e1001315. doi: 10.1371/journal.pbio.1001315. • Together with Izquierdo-Useros et al. and Puryear et al. [44, 45, 46], these 4 studies identify the lectin CD169 on monocyte-derived dendritic cells to bind gangliosides within the retrovirus envelope for trans-infection.
- 44. Izquierdo-Useros N, Lorizate M, Puertas MC, Rodriguez-Plata MT, Zangger N, Erikson E, Pino M, Erkizia I, Glass B, Clotet B, et al. Siglec-1 is a novel dendritic cell receptor that mediates HIV-1 trans-infection through recognition of viral membrane gangliosides. PLoS Biol. 2012;10:e1001448. doi: 10.1371/journal.pbio.1001448. • See annotation to Ref. [43].
- 45. Puryear WB, Yu X, Ramirez NP, Reinhard BM, Gummuluru S. HIV-1 incorporation of host-cell-derived glycosphingolipid GM3 allows for capture by mature dendritic cells. Proc Natl Acad Sci U S A. 2012;109:7475–7480. doi: 10.1073/pnas.1201104109. • See annotation to Ref. [43].
- 46. Puryear WB, Akiyama H, Geer SD, Ramirez NP, Yu X, Reinhard BM, Gummuluru S. Interferon-inducible mechanism of dendritic cell-mediated HIV-1 dissemination is dependent on Siglec-1/CD169. PLoS Pathog. 2013;9:e1003291. doi: 10.1371/journal.ppat.1003291. • See annotation to Ref. [43].
- 47.Akiyama H, Ramirez NG, Gudheti MV, Gummuluru S. CD169-mediated trafficking of HIV to plasma membrane invaginations in dendritic cells attenuates efficacy of anti-gp120 broadly neutralizing antibodies. PLoS Pathog. 2015;11:e1004751. doi: 10.1371/journal.ppat.1004751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rempel H, Calosing C, Sun B, Pulliam L. Sialoadhesin expressed on IFN-induced monocytes binds HIV-1 and enhances infectivity. PLoS One. 2008;3:e1967. doi: 10.1371/journal.pone.0001967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Erikson E, Wratil PR, Frank M, Ambiel I, Pahnke K, Pino M, Azadi P, Izquierdo-Useros N, Martinez-Picado J, Meier C, et al. Mouse Siglec-1 Mediates trans-Infection of Surface-bound Murine Leukemia Virus in a Sialic Acid N-Acyl Side Chain-dependent Manner. J Biol Chem. 2015;290:27345–27359. doi: 10.1074/jbc.M115.681338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pino M, Erkizia I, Benet S, Erikson E, Fernandez-Figueras MT, Guerrero D, Dalmau J, Ouchi D, Rausell A, Ciuffi A, et al. HIV-1 immune activation induces Siglec-1 expression and enhances viral trans-infection in blood and tissue myeloid cells. Retrovirology. 2015;12:37. doi: 10.1186/s12977-015-0160-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yu HJ, Reuter MA, McDonald D. HIV traffics through a specialized, surface-accessible intracellular compartment during trans-infection of T cells by mature dendritic cells. PLoS Pathog. 2008;4:e1000134. doi: 10.1371/journal.ppat.1000134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang JH, Kwas C, Wu L. Intercellular adhesion molecule 1 (ICAM-1), but not ICAM-2 and -3, is important for dendritic cell-mediated human immunodeficiency virus type 1 transmission. J Virol. 2009;83:4195–4204. doi: 10.1128/JVI.00006-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang JH, Wells C, Wu L. Macropinocytosis and cytoskeleton contribute to dendritic cell-mediated HIV-1 transmission to CD4+ T cells. Virology. 2008;381:143–154. doi: 10.1016/j.virol.2008.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Aggarwal A, Iemma TL, Shih I, Newsome TP, McAllery S, Cunningham AL, Turville SG. Mobilization of HIV spread by diaphanous 2 dependent filopodia in infected dendritic cells. PLoS pathogens. 2012;8:e1002762. doi: 10.1371/journal.ppat.1002762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Menager MM, Littman DR. Actin dynamics regulates dendritic cell-mediated transfer of HIV-1 to T cells. Cell. 2016;164:695–709. doi: 10.1016/j.cell.2015.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Felts RL, Narayan K, Estes JD, Shi D, Trubey CM, Fu J, Hartnell LM, Ruthel GT, Schneider DK, Nagashima K, et al. 3D visualization of HIV transfer at the virological synapse between dendritic cells and T cells. Proc Natl Acad Sci U S A. 2010;107:13336–13341. doi: 10.1073/pnas.1003040107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Joseph SB, Arrildt KT, Sturdevant CB, Swanstrom R. HIV-1 target cells in the CNS. J Neurovirol. 2015;21:276–289. doi: 10.1007/s13365-014-0287-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Peters PJ, Bhattacharya J, Hibbitts S, Dittmar MT, Simmons G, Bell J, Simmonds P, Clapham PR. Biological analysis of human immunodeficiency virus type 1 R5 envelopes amplified from brain and lymph node tissues of AIDS patients with neuropathology reveals two distinct tropism phenotypes and identifies envelopes in the brain that confer an enhanced tropism and fusigenicity for macrophages. J Virol. 2004;78:6915–6926. doi: 10.1128/JVI.78.13.6915-6926.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Thomas ER, Dunfee RL, Stanton J, Bogdan D, Taylor J, Kunstman K, Bell JE, Wolinsky SM, Gabuzda D. Macrophage entry mediated by HIV Envs from brain and lymphoid tissues is determined by the capacity to use low CD4 levels and overall efficiency of fusion. Virology. 2007;360:105–119. doi: 10.1016/j.virol.2006.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Baxter AE, Russell RA, Duncan CJ, Moore MD, Willberg CB, Pablos JL, Finzi A, Kaufmann DE, Ochsenbauer C, Kappes JC, Groot F, Sattentau QJ. Macrophage infection via selective capture of HIV-1-infected CD4+ T cells. Cell Host Microbe. 2014;16:711–721. doi: 10.1016/j.chom.2014.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sattentau QJ, Stevenson M. Macrophages and HIV-1: An Unhealthy Constellation. Cell Host Microbe. 2016;19:304–310. doi: 10.1016/j.chom.2016.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bird SW, Kirkegaard K. Escape of non-enveloped virus from intact cells. Virology. 2015;479–480:444–449. doi: 10.1016/j.virol.2015.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jackson WT, Giddings TH, Jr, Taylor MP, Mulinyawe S, Rabinovitch M, Kopito RR, Kirkegaard K. Subversion of cellular autophagosomal machinery by RNA viruses. PLoS biology. 2005;3:e156. doi: 10.1371/journal.pbio.0030156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Feng Z, Hensley L, McKnight KL, Hu F, Madden V, Ping L, Jeong SH, Walker C, Lanford RE, Lemon SM. A pathogenic picornavirus acquires an envelope by hijacking cellular membranes. Nature. 2013;496:367–371. doi: 10.1038/nature12029. • The study shows that eHAV is a distinct version of the virus that carries a pseudo-membrane, has a distinct capsid protein and is sensitive to solvent-treatment.
- 65.Takahashi M, Tanaka T, Takahashi H, Hoshino Y, Nagashima S, Jirintai, Mizuo H, Yazaki Y, Takagi T, Azuma M, et al. Hepatitis E Virus (HEV) strains in serum samples can replicate efficiently in cultured cells despite the coexistence of HEV antibodies: characterization of HEV virions in blood circulation. J Clin Microbiol. 2010;48:1112–1125. doi: 10.1128/JCM.02002-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Bird SW, Maynard ND, Covert MW, Kirkegaard K. Nonlytic viral spread enhanced by autophagy components. Proc Natl Acad Sci U S A. 2014;111:13081–13086. doi: 10.1073/pnas.1401437111. • This study describes how induction of autophagy by chemical compounds can increase non-lytic release of poliovirus.
- 67.Clayson ET, Brando LV, Compans RW. Release of simian virus 40 virions from epithelial cells is polarized and occurs without cell lysis. J Virol. 1989;63:2278–2288. doi: 10.1128/jvi.63.5.2278-2288.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tucker SP, Thornton CL, Wimmer E, Compans RW. Vectorial release of poliovirus from polarized human intestinal epithelial cells. J Virol. 1993;67:4274–4282. doi: 10.1128/jvi.67.7.4274-4282.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chen YH, Du W, Hagemeijer MC, Takvorian PM, Pau C, Cali A, Brantner CA, Stempinski ES, Connelly PS, Ma HC, et al. Phosphatidylserine vesicles enable efficient en bloc transmission of enteroviruses. Cell. 2015;160:619–630. doi: 10.1016/j.cell.2015.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Taylor MP, Burgon TB, Kirkegaard K, Jackson WT. Role of microtubules in extracellular release of poliovirus. J Virol. 2009;83:6599–6609. doi: 10.1128/JVI.01819-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wirblich C, Bhattacharya B, Roy P. Nonstructural protein 3 of bluetongue virus assists virus release by recruiting ESCRT-I protein Tsg101. J Virol. 2006;80:460–473. doi: 10.1128/JVI.80.1.460-473.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dimitrov DS, Willey RL, Sato H, Chang LJ, Blumenthal R, Martin MA. Quantitation of human immunodeficiency virus type 1 infection kinetics. J Virol. 1993;67:2182–2190. doi: 10.1128/jvi.67.4.2182-2190.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Carr JM, Hocking H, Li P, Burrell CJ. Rapid and efficient cell-to-cell transmission of human immunodeficiency virus infection from monocyte-derived macrophages to peripheral blood lymphocytes. Virology. 1999;265:319–329. doi: 10.1006/viro.1999.0047. [DOI] [PubMed] [Google Scholar]
- 74.Zhong P, Agosto LM, Ilinskaya A, Dorjbal B, Truong R, Derse D, Uchil PD, Heidecker G, Mothes W. Cell-to-cell transmission can overcome multiple donor and target cell barriers imposed on cell-free HIV. PLoS One. 2013;8:e53138. doi: 10.1371/journal.pone.0053138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Richardson MW, Carroll RG, Stremlau M, Korokhov N, Humeau LM, Silvestri G, Sodroski J, Riley JL. Mode of transmission affects the sensitivity of human immunodeficiency virus type 1 to restriction by rhesus TRIM5alpha. Journal of virology. 2008;82:11117–11128. doi: 10.1128/JVI.01046-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jolly C, Booth NJ, Neil SJ. Cell-cell spread of human immunodeficiency virus type 1 overcomes tetherin/BST-2-mediated restriction in T cells. Journal of virology. 2010;84:12185–12199. doi: 10.1128/JVI.01447-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhong P, Agosto LM, Munro JB, Mothes W. Cell-to-cell transmission of viruses. Current opinion in virology. 2013;3:44–50. doi: 10.1016/j.coviro.2012.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Malbec M, Porrot F, Rua R, Horwitz J, Klein F, Halper-Stromberg A, Scheid JF, Eden C, Mouquet H, Nussenzweig MC, et al. Broadly neutralizing antibodies that inhibit HIV-1 cell to cell transmission. J Exp Med. 2013;210:2813–2821. doi: 10.1084/jem.20131244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Abela IA, Berlinger L, Schanz M, Reynell L, Gunthard HF, Rusert P, Trkola A. Cell-cell transmission enables HIV-1 to evade inhibition by potent CD4bs directed antibodies. PLoS Pathog. 2012;8:e1002634. doi: 10.1371/journal.ppat.1002634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Duncan CJ, Williams JP, Schiffner T, Gartner K, Ochsenbauer C, Kappes J, Russell RA, Frater J, Sattentau QJ. High-multiplicity HIV-1 infection and neutralizing antibody evasion mediated by the macrophage-T cell virological synapse. J Virol. 2014;88:2025–2034. doi: 10.1128/JVI.03245-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Reh L, Magnus C, Schanz M, Weber J, Uhr T, Rusert P, Trkola A. Capacity of Broadly Neutralizing Antibodies to Inhibit HIV-1 Cell-Cell Transmission Is Strain- and Epitope-Dependent. PLoS Pathog. 2015;11:e1004966. doi: 10.1371/journal.ppat.1004966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Russell RA, Martin N, Mitar I, Jones E, Sattentau QJ. Multiple proviral integration events after virological synapse-mediated HIV-1 spread. Virology. 2013;443:143–149. doi: 10.1016/j.virol.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 83. Del Portillo A, Tripodi J, Najfeld V, Wodarz D, Levy DN, Chen BK. Multiploid inheritance of HIV-1 during cell-to-cell infection. J Virol. 2011;85:7169–7176. doi: 10.1128/JVI.00231-11. • First experimental demonstration of the high multiplicity of infection during HIV-1 cell-to-cell transmission.
- 84.Agosto LM, Zhong P, Munro J, Mothes W. Highly active antiretroviral therapies are effective against HIV-1 cell-to-cell transmission. PLoS Pathog. 2014;10:e1003982. doi: 10.1371/journal.ppat.1003982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sigal A, Kim JT, Balazs AB, Dekel E, Mayo A, Milo R, Baltimore D. Cell-to-cell spread of HIV permits ongoing replication despite antiretroviral therapy. Nature. 2011;477:95–98. doi: 10.1038/nature10347. [DOI] [PubMed] [Google Scholar]
- 86.Doitsh G, Cavrois M, Lassen KG, Zepeda O, Yang Z, Santiago ML, Hebbeler AM, Greene WC. Abortive HIV infection mediates CD4 T cell depletion and inflammation in human lymphoid tissue. Cell. 2010;143:789–801. doi: 10.1016/j.cell.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Doitsh G, Galloway NL, Geng X, Yang Z, Monroe KM, Zepeda O, Hunt PW, Hatano H, Sowinski S, Munoz-Arias I, et al. Cell death by pyroptosis drives CD4 T-cell depletion in HIV-1 infection. Nature. 2014;505:509–514. doi: 10.1038/nature12940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cooper A, Garcia M, Petrovas C, Yamamoto T, Koup RA, Nabel GJ. HIV-1 causes CD4 cell death through DNA-dependent protein kinase during viral integration. Nature. 2013;498:376–379. doi: 10.1038/nature12274. [DOI] [PubMed] [Google Scholar]
- 89.Galloway NL, Doitsh G, Monroe KM, Yang Z, Munoz-Arias I, Levy DN, Greene WC. Cell-to-Cell Transmission of HIV-1 Is Required to Trigger Pyroptotic Death of Lymphoid-Tissue-Derived CD4 T Cells. Cell Rep. 2015;12:1555–1563. doi: 10.1016/j.celrep.2015.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Swartz MA. The physiology of the lymphatic system. Adv Drug Deliv Rev. 2001;50:3–20. doi: 10.1016/s0169-409x(01)00150-8. [DOI] [PubMed] [Google Scholar]
- 91.Iannacone M, Moseman EA, Tonti E, Bosurgi L, Junt T, Henrickson SE, Whelan SP, Guidotti LG, von Andrian UH. Subcapsular sinus macrophages prevent CNS invasion on peripheral infection with a neurotropic virus. Nature. 2010;465:1079–1083. doi: 10.1038/nature09118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Farrell HE, Davis-Poynter N, Bruce K, Lawler C, Dolken L, Mach M, Stevenson PG. Lymph Node Macrophages Restrict Murine Cytomegalovirus Dissemination. J Virol. 2015;89:7147–7158. doi: 10.1128/JVI.00480-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Prestwood TR, May MM, Plummer EM, Morar MM, Yauch LE, Shresta S. Trafficking and replication patterns reveal splenic macrophages as major targets of dengue virus in mice. J Virol. 2012;86:12138–12147. doi: 10.1128/JVI.00375-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Winkelmann ER, Widman DG, Xia J, Johnson AJ, van Rooijen N, Mason PW, Bourne N, Milligan GN. Subcapsular sinus macrophages limit dissemination of West Nile virus particles after inoculation but are not essential for the development of West Nile virus-specific T cell responses. Virology. 2014;450–451:278–289. doi: 10.1016/j.virol.2013.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hickman HD, Takeda K, Skon CN, Murray FR, Hensley SE, Loomis J, Barber GN, Bennink JR, Yewdell JW. Direct priming of antiviral CD8+ T cells in the peripheral interfollicular region of lymph nodes. Nat Immunol. 2008;9:155–165. doi: 10.1038/ni1557. [DOI] [PubMed] [Google Scholar]
- 96.Frederico B, Chao B, May JS, Belz GT, Stevenson PG. A murid gamma-herpesviruses exploits normal splenic immune communication routes for systemic spread. Cell host & microbe. 2014;15:457–470. doi: 10.1016/j.chom.2014.03.010. [DOI] [PubMed] [Google Scholar]
- 97.Frederico B, Chao B, Lawler C, May JS, Stevenson PG. Subcapsular sinus macrophages limit acute gammaherpesvirus dissemination. J Gen Virol. 2015;96:2314–2327. doi: 10.1099/vir.0.000140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Murooka TT, Deruaz M, Marangoni F, Vrbanac VD, Seung E, von Andrian UH, Tager AM, Luster AD, Mempel TR. HIV-infected T cells are migratory vehicles for viral dissemination. Nature. 2012;490:283–287. doi: 10.1038/nature11398. •• Together with Daley-Bauer et al. [99], this study documents that viruses use migrating cells as vehicles for systemic spread in vivo.
- 99. Daley-Bauer LP, Roback LJ, Wynn GM, Mocarski ES. Cytomegalovirus hijacks CX3CR1(hi) patrolling monocytes as immune-privileged vehicles for dissemination in mice. Cell Host Microbe. 2014;15:351–362. doi: 10.1016/j.chom.2014.02.002. •• See annotation to Ref. [98].
- 100.Randolph GJ, Angeli V, Swartz MA. Dendritic-cell trafficking to lymph nodes through lymphatic vessels. Nat Rev Immunol. 2005;5:617–628. doi: 10.1038/nri1670. [DOI] [PubMed] [Google Scholar]
- 101.Villablanca EJ, Mora JR. A two-step model for Langerhans cell migration to skin-draining LN. Eur J Immunol. 2008;38:2975–2980. doi: 10.1002/eji.200838919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cunningham AL, Abendroth A, Jones C, Nasr N, Turville S. Viruses and Langerhans cells. Immunol Cell Biol. 2010;88:416–423. doi: 10.1038/icb.2010.42. [DOI] [PubMed] [Google Scholar]
- 103.Roozendaal R, Mempel TR, Pitcher LA, Gonzalez SF, Verschoor A, Mebius RE, von Andrian UH, Carroll MC. Conduits mediate transport of low-molecular-weight antigen to lymph node follicles. Immunity. 2009;30:264–276. doi: 10.1016/j.immuni.2008.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gretz JE, Norbury CC, Anderson AO, Proudfoot AE, Shaw S. Lymph-borne chemokines and other low molecular weight molecules reach high endothelial venules via specialized conduits while a functional barrier limits access to the lymphocyte microenvironments in lymph node cortex. J Exp Med. 2000;192:1425–1440. doi: 10.1084/jem.192.10.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sixt M, Kanazawa N, Selg M, Samson T, Roos G, Reinhardt DP, Pabst R, Lutz MB, Sorokin L. The conduit system transports soluble antigens from the afferent lymph to resident dendritic cells in the T cell area of the lymph node. Immunity. 2005;22:19–29. doi: 10.1016/j.immuni.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 106.Gray EE, Cyster JG. Lymph node macrophages. J Innate Immun. 2012;4:424–436. doi: 10.1159/000337007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chang JE, Turley SJ. Stromal infrastructure of the lymph node and coordination of immunity. Trends Immunol. 2015;36:30–39. doi: 10.1016/j.it.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 108.Kuka M, Iannacone M. The role of lymph node sinus macrophages in host defense. Ann N Y Acad Sci. 2014;1319:38–46. doi: 10.1111/nyas.12387. [DOI] [PubMed] [Google Scholar]
- 109.Kraal G, Mebius R. New insights into the cell biology of the marginal zone of the spleen. Int Rev Cytol. 2006;250:175–215. doi: 10.1016/S0074-7696(06)50005-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Saunderson SC, Dunn AC, Crocker PR, McLellan AD. CD169 mediates the capture of exosomes in spleen and lymph node. Blood. 2014;123:208–216. doi: 10.1182/blood-2013-03-489732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Chan R, Uchil PD, Jin J, Shui G, Ott DE, Mothes W, Wenk MR. Retroviruses human immunodeficiency virus and murine leukemia virus are enriched in phosphoinositides. Journal of virology. 2008;82:11228–11238. doi: 10.1128/JVI.00981-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bennett AE, Narayan K, Shi D, Hartnell LM, Gousset K, He H, Lowekamp BC, Yoo TS, Bliss D, Freed EO, et al. Ion-abrasion scanning electron microscopy reveals surface-connected tubular conduits in HIV-infected macrophages. PLoS Pathog. 2009;5:e1000591. doi: 10.1371/journal.ppat.1000591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Deneka M, Pelchen-Matthews A, Byland R, Ruiz-Mateos E, Marsh M. In macrophages, HIV-1 assembles into an intracellular plasma membrane domain containing the tetraspanins CD81, CD9, and CD53. J Cell Biol. 2007;177:329–341. doi: 10.1083/jcb.200609050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Izquierdo-Useros N, Naranjo-Gomez M, Archer J, Hatch SC, Erkizia I, Blanco J, Borras FE, Puertas MC, Connor JH, Fernandez-Figueras MT, et al. Capture and transfer of HIV-1 particles by mature dendritic cells converges with the exosome-dissemination pathway. Blood. 2009;113:2732–2741. doi: 10.1182/blood-2008-05-158642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sewald X, Gonzalez DG, Haberman AM, Mothes W. In vivo imaging of virological synapses. Nat Commun. 2012;3:1320. doi: 10.1038/ncomms2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Haase AT, Henry K, Zupancic M, Sedgewick G, Faust RA, Melroe H, Cavert W, Gebhard K, Staskus K, Zhang ZQ, et al. Quantitative image analysis of HIV-1 infection in lymphoid tissue. Science. 1996;274:985–989. doi: 10.1126/science.274.5289.985. [DOI] [PubMed] [Google Scholar]
- 117.Ho J, Moir S, Kulik L, Malaspina A, Donoghue ET, Miller NJ, Wang W, Chun TW, Fauci AS, Holers VM. Role for CD21 in the establishment of an extracellular HIV reservoir in lymphoid tissues. J Immunol. 2007;178:6968–6974. doi: 10.4049/jimmunol.178.11.6968. [DOI] [PubMed] [Google Scholar]
- 118.Smith BA, Gartner S, Liu Y, Perelson AS, Stilianakis NI, Keele BF, Kerkering TM, Ferreira-Gonzalez A, Szakal AK, Tew JG, et al. Persistence of infectious HIV on follicular dendritic cells. J Immunol. 2001;166:690–696. doi: 10.4049/jimmunol.166.1.690. [DOI] [PubMed] [Google Scholar]
- 119.Banki Z, Kacani L, Rusert P, Pruenster M, Wilflingseder D, Falkensammer B, Stellbrink HJ, van Lunzen J, Trkola A, Dierich MP, et al. Complement dependent trapping of infectious HIV in human lymphoid tissues. AIDS. 2005;19:481–486. doi: 10.1097/01.aids.0000162336.20439.8d. [DOI] [PubMed] [Google Scholar]
- 120.Banki Z, Wilflingseder D, Ammann CG, Pruenster M, Mullauer B, Hollander K, Meyer M, Sprinzl GM, van Lunzen J, Stellbrink HJ, et al. Factor I-mediated processing of complement fragments on HIV immune complexes targets HIV to CR2-expressing B cells and facilitates B cell-mediated transmission of opsonized HIV to T cells. J Immunol. 2006;177:3469–3476. doi: 10.4049/jimmunol.177.5.3469. [DOI] [PubMed] [Google Scholar]
- 121.Ebenbichler CF, Thielens NM, Vornhagen R, Marschang P, Arlaud GJ, Dierich MP. Human immunodeficiency virus type 1 activates the classical pathway of complement by direct C1 binding through specific sites in the transmembrane glycoprotein gp41. J Exp Med. 1991;174:1417–1424. doi: 10.1084/jem.174.6.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Joling P, Bakker LJ, Van Strijp JA, Meerloo T, de Graaf L, Dekker ME, Goudsmit J, Verhoef J, Schuurman HJ. Binding of human immunodeficiency virus type-1 to follicular dendritic cells in vitro is complement dependent. J Immunol. 1993;150:1065–1073. [PubMed] [Google Scholar]
- 123. Moir S, Malaspina A, Li Y, Chun TW, Lowe T, Adelsberger J, Baseler M, Ehler LA, Liu S, Davey RT, Jr, et al. B cells of HIV-1-infected patients bind virions through CD21-complement interactions and transmit infectious virus to activated T cells. J Exp Med. 2000;192:637–646. doi: 10.1084/jem.192.5.637. • See annotation to Ref. [124].
- 124. Heesters BA, Lindqvist M, Vagefi PA, Scully EP, Schildberg FA, Altfeld M, Walker BD, Kaufmann DE, Carroll MC. Follicular Dendritic Cells Retain Infectious HIV in Cycling Endosomes. PLoS Pathog. 2015;11:e1005285. doi: 10.1371/journal.ppat.1005285. • Together with Moir et al. [123], this study demonstrates that isolated follicular dendritic cells and B cells can store HIV for CD21-dependent trans-infection of T cells.
- 125.Kacani L, Prodinger WM, Sprinzl GM, Schwendinger MG, Spruth M, Stoiber H, Dopper S, Steinhuber S, Steindl F, Dierich MP. Detachment of human immunodeficiency virus type 1 from germinal centers by blocking complement receptor type 2. J Virol. 2000;74:7997–8002. doi: 10.1128/jvi.74.17.7997-8002.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Phan TG, Grigorova I, Okada T, Cyster JG. Subcapsular encounter and complement-dependent transport of immune complexes by lymph node B cells. Nat Immunol. 2007;8:992–1000. doi: 10.1038/ni1494. [DOI] [PubMed] [Google Scholar]
- 127.Carrasco YR, Batista FD. B cells acquire particulate antigen in a macrophage-rich area at the boundary between the follicle and the subcapsular sinus of the lymph node. Immunity. 2007;27:160–171. doi: 10.1016/j.immuni.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 128.Park C, Arthos J, Cicala C, Kehrl JH. The HIV-1 envelope protein gp120 is captured and displayed for B cell recognition by SIGN-R1(+) lymph node macrophages. eLife. 2015;4 doi: 10.7554/eLife.06467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Junt T, Moseman EA, Iannacone M, Massberg S, Lang PA, Boes M, Fink K, Henrickson SE, Shayakhmetov DM, Di Paolo NC, et al. Subcapsular sinus macrophages in lymph nodes clear lymph-borne viruses and present them to antiviral B cells. Nature. 2007;450:110–114. doi: 10.1038/nature06287. [DOI] [PubMed] [Google Scholar]
- 130.Heesters BA, Chatterjee P, Kim YA, Gonzalez SF, Kuligowski MP, Kirchhausen T, Carroll MC. Endocytosis and recycling of immune complexes by follicular dendritic cells enhances B cell antigen binding and activation. Immunity. 2013;38:1164–1175. doi: 10.1016/j.immuni.2013.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Keele BF, Tazi L, Gartner S, Liu Y, Burgon TB, Estes JD, Thacker TC, Crandall KA, McArthur JC, Burton GF. Characterization of the follicular dendritic cell reservoir of human immunodeficiency virus type 1. J Virol. 2008;82:5548–5561. doi: 10.1128/JVI.00124-08. [DOI] [PMC free article] [PubMed] [Google Scholar]