Abstract
Neovascularization is an understudied aspect of calcific aortic valve disease (CAVD). Within diseased valves, cells along the neovessels’ periphery stain for pericyte markers, but it is unclear whether valvular interstitial cells (VICs) can demonstrate a pericyte-like phenotype. This investigation examined the perivascular potential of VICs to regulate valve endothelial cell (VEC) organization and explored the role of Angiopoeitin1-Tie2 signaling in this process. Porcine VECs and VICs were fluorescently tracked and co-cultured in Matrigel over 7 days. VICs regulated early VEC network organization in a ROCK-dependent manner, then guided later VEC network contraction through chemoattraction. Unlike vascular control cells, the valve cell cultures ultimately formed invasive spheroids with 3D angiogenic-like sprouts. VECs co-cultured with VICs displayed significantly more invasion than VECs alone; with VICs generally leading and wrapping around VEC invasive sprouts. Lastly, Angiopoietin1-Tie2 signaling was found to regulate valve cell organization during VEC/VIC spheroid formation and invasion. VICs demonstrated pericyte-like behaviors toward VECs throughout sustained co-culture. The change from a vasculogenic network to an invasive sprouting spheroid suggests that both cell types undergo phenotypic changes during long-term culture in the model angiogenic environment. Valve cells organizing into spheroids and undergoing 3D invasion of Matrigel demonstrated several typical angiogenic-like phenotypes dependent on basal levels of Angiopoeitin1-Tie2 signaling and ROCK activation. These results suggest that the ectopic sustained angiogenic environment during the early stages of valve disease promotes organized activity by both VECs and VICs, contributing to neovessel formation and the progression of CAVD.
Keywords: Aortic Valve, Angiogenesis, CAVD, Angiopoietin, Valve Endothelial Cell, Valve Interstitial Cell
Introduction
Angiogenetic signaling plays an important role not only in valve formation and normal valve function, but also in the pathophysiology of calcific aortic valve disease (CAVD).12,29 Several studies have established the occurrence of neovascularization during CAVD.11,18,31 There has been little investigation, however, into how the cell-mediated mechanisms that underlie vascular angiogenesis play a role in the pathology of CAVD. During valve development and normal endothelial-to-mesenchymal transformation (EndMT), valve endothelial cells (VECs) undergo a physiological transdifferentiation that shares several characteristics with the process of vascular angiogenic root formation.36 Due to the increased abundance of factors important for angiogenic signaling within calcified valves, and the predisposition of VECs to undergo EndMT, we hypothesized that VECs cultured long-term in a model environment rich in pro-angiogenic signaling factors would maintain their endothelial phenotype while also gaining invasive mesenchymal characteristics, ultimately leading to an intermediate phenotype. The presence of alpha smooth muscle actin (aSMA) – the marker for activated valve interstitial cells (VICs) – was demonstrated surrounding neovessels in CAVD,21 and it is also reported that a subpopulation of VICs demonstrate the perictye marker NG235 and respond to pericyte-related signaling molecules.34 However, it has also been shown that VICs can be anti-angiogenic in basal conditions.38 Therefore, it is unclear whether myofibroblast-like VICs can serve as pericytes during neoangiogenesis.
Studying how VEC and VIC signaling guides their phenotypes is an important step toward understanding the cell-mediated pathobiology of CAVD. The Angiopoietin1 (Ang1)-Tie2 pathway is an important pathway in vascular angiogenesis influencing endothelial cell growth, migration, vessel maintenance and destabilization, and maturation,23 but the role of this pathway is understudied in the context of valve cell biology. There is some evidence for activation of this pathway during CAVD,7 but its significance in VEC/VIC interactions or CAVD histopathology has not been established. Prior studies of VECs and VICs have investigated the importance of endothelial nitric oxide synthase (eNOS) and Rho-associated protein kinase (ROCK) in VEC/VIC signaling behavior;9 the Ang-Tie2 pathway is upstream of both of these mechanisms. Ang-Tie2 is also important in NF-κB activation and leukocyte recruitment, which are speculated to have significant roles in CAVD.20 Moreover, previous co-culture investigations of VICs and VECs have demonstrated communication between these two cell types,4,9,32 but have not examined the pericyte-like potential of VICs to influence VEC angiogenic behavior.
The purpose of this study was to investigate how direct contact co-culture with VICs affected short-term and long-term (7 days) VEC network formation in a Matrigel model in comparison to a co-culture of vascular-mimetic cells, to quantify their spatial-temporal relationships, and to identify the role of Ang-Tie2 and its downstream signaling pathways in VEC/VIC dynamics. Clarification of these valve cell relationships would provide fundamental insights into this transformative stage of CAVD pathology.
Methods and Materials
Isolation, Purification, and Culture of Valve Cells and Vascular Cells
Valve cells were harvested from healthy 6-month-old adult porcine hearts from a local commercial abattoir (Fisher Ham and Meats, Spring, TX) or Animal Technologies (Tyler, TX). VECs and VICs were harvested and cultured from the aortic valves as previously described 1,32, and used between passages 2-4.
Immortalized mouse cardiac endothelial cells (MCECs) (CELLutions Biosystems Inc, Toronto, Canada) were cultured in the same manner as VECs.
10T1/2 fibroblast cells (American Type Culture Collection, Manassas, VA) were cultured in DMEM supplemented with 10% fetal bovine serum (Lonza) and 2 mM L-glutamine, 1000 U/mL penicillin, and 100 mg/L streptomycin (Sigma, St. Louis, MO).
PKH Co-culture Tubule-Like Structure Matrigel Assay
The Tubule-Like Structure (TLS) Matrigel Assay was used to investigate valve and vascular endothelial cell (EC)-PC dynamics in a model pro-angiogenic environment as previously described 3. In order to track relative EC-PC dynamics, cells were differentially stained with PKH26 (red) or PKH67 (green) fluorescent dyes (10 μM, Invitrogen) following the manufacturer's guidelines. The EC and PC cells were then seeded on phenol red-free Matrigel (BD Biosciences, San Jose, CA) in a 3 EC:1 PC ratio at 240,000 cells/ml in order to recreate culture conditions from previous vascular EC/PC matrigel studies.6 Intermittently throughout the culture period, live cell movement was tracked for 4-6 hours using a Nikon A1-Rsi confocal fluorescence microscope (Tokyo, Japan).
To investigate the role of ROCK signaling on VEC/VIC network organization, once seeded the co-cultures were treated with the small molecule ROCK inhibitor Y-27632 (Calbiochem, San Diego, CA)1.
Immunocytochemistry
Immunocytochemistry was performed as described previously1 to demonstrate the cells’ phenotypic characteristics (Table 1). In order to confirm that VECs retained their endothelial cell phenotype, co-cultures were stained with Dil-Ac-LDL (Invitrogen) at 10 μg/ml for 4 hours before washing with PBS.
Table 1.
Primary antibodies for immunocytochemistry.
| Antibody Target | Dilution | Function |
|---|---|---|
| CD31a | 1:100 | VEC Marker |
| Alpha smooth muscle actin (aSMA)a | 1:50 | VIC Marker |
| Delta-like ligand 4 (DLL4)a | 1:100 | Angiogenic Tip Cell Marker |
| Phosphorylated Akt1b | 1:100 | AKT activation Marker |
Abcam, Cambridge UK
Cell Signaling Technology, Danvers, MA
Automated Network Analysis
Networks were analyzed using the automated image analysis toolset Angiogenesis Analyzer in ImageJ (Bethesda, MA) on 10X phase contrast microscopy images from the middle of each well as used previously.1 The total network length and number of tubule like structures were analyzed, and the resultant average tubule length was calculated using equation (1):
| (1) |
Groups were statistically compared using one-way ANOVA with post-hoc Tukey tests.
Quantification of Post-Network VIC Chemoattractant Behavior
In the Nikon AR automated tracking module, each cell could be identified uniquely due to the PKH fluorescent staining, cell size, and cell morphology. VECs and VICs could be distinguished by the different color PKH dyes.
VIC chemoattractant towards VECs was quantified during the period of network regression and spheroid formation. The null hypothesis was that the motion of each VEC was independent of the influence of nearby VICs, so that no net chemoattraction existed between the two cell types. For each VIC, a subset of VECs (VECsub) was identified as cells whose average Euclidian distance was less than 50 μm from the current VIC of interest (VICi). In order to quantify the movement of each VEC in the VECsub in relation to the VICi, their X/Y/time positions underwent a Lagrangian transformation in relation to the X/Y/time movements of the VICi. At each time point, for each set pairing of VICi and VECsub cells, the cosine similarity was calculated using equation (2):
| (2) |
where u represents the Lagrangian-corrected vector of the displacement that the currently paired VEC in the VECsub underwent during the current time step, v represents the vector if it was perfectly directed at the VICi which is by definition set to the new origin, and det(u,v) represented the determinant of (u,v). In this definition, an angle of 0 degrees represented a VEC that is perfectly converging towards the VICi at that time, and an angle of 180 degrees represented a cell moving perfectly away from the VICi. The cosine similarity of each pairing over time was averaged, and the average VECsub time-averaged cosine similarity was then calculated for each VICi. This metric quantified the directional tendency of the VECsub in relation to the VICi.
The radial distribution of these angles was compared with an even radial distribution using a Rayleigh's test for nonuniformity, with p<0.05 indicating a statistically different radial directionality. The average directionality of this migration of VECs toward VICs was calculated using equation (3):
| (3) |
where k represents the current VEC out of VECsub, n represents the number of cells in VECsub, t represents the current time step, and tf represents the final time step of the VECsub track. This metric represents the directional persistence of migration of VECsub in relation to the motion of the VICi with the maximal amount 1 representing a perfectly straight line of movement 26.
Finally, the Lagrangian-transformed Forward Migration Index (LaFMI) was calculated using equation (4) adapted from Martins et al. 17:
| (4) |
In this context, the LaFMI quantifies the percent of the total distance that the movement of a VECsub was directed towards the VICi, with the maximum value of 1 representing a VECsub moving perfectly towards the VICi at each step as the VICi moved. In this equation, k, t, u, t, tf, and n were defined as in equations (2) and (3), and Ɵ was defined as the cosine similarity between the u and v vectors as defined above at time t for VECk.
In order to benchmark the measured metrics to random independent motion, a Gaussian-based Brownian motion simulator was implemented to simulate the random movements of both the VECs and VICs from their starting positions, and each chemoattractant metric was calculated in parallel. A total of 732 cells from three independent cultures were tracked and used in the analysis. A t-test assuming unequal variances was used to compare the directionality and LaFMI distributions between the measured and random motions, with p<0.05 indicating a significant difference.
VEC/VIC Invasive Spheroid (VEVIS) Sprouting and Distribution Analysis
As the VECs and VICs co-cultured for 7 days on Matrigel formed spheroids and then formed angiogenetic sprouts into the Matrigel, additional methods were developed to quantify this behavior. To quantify the amount of Matrigel penetration by the VEVIS angiogenic sprouts, the spheroid core and outer perimeter of penetration were manually traced using Nikon AR software, and then the invasion ratio was calculated as the penetration perimeter divided by the core perimeter. Randomly chosen spheroids were selected in each of 9 independently-seeded VEC-only and VEC/VIC cultures after 7 days. Differences between VEC-only and VEC/VIC co-cultures were analyzed using a t-test assuming equal variance.
In order to quantify the distribution of VECs and VICs within the VEVISs, VEVISs with differentially PKH-tracked cells were imaged after 7 days of culture using confocal fluorescence microscopy (n=17 different VEVIS samples). Images were analyzed using a binary filter that assigned an X-Y position to each cell, and determined the area of that cell. Each VEVIS image was then divided into 4 concentric quartile rings with equal surface area, and each cell was allocated into one of the quartile rings based upon its Euclidean distance from the middle of each VEVIS. For each cell type (VIC or VEC), the total cell area per quartile ring was calculated and then normalized to the total cell area overall. The proportions of VICs and VECs in the quartile rings were compared using a two-way ANOVA with a Tukey's post hoc analysis comparing across cell type and quartile.
Investigating the Role of Angiopoietin1-Tie2 signaling on VEVIS Formation
To quantify the effect of Angiopoietin1 and its downstream effectors on VEC network formation, VECs were cultured alone in the TLS assay. Upon seeding, VECs were treated with Angiopoetin1 (Ang1) (R&D Systems, Minneapolis) at a final concentration of 14 μM, with Ang1 and the Tie2 Kinase Inhibitor (Calbiochem) at a final concentration of 3.5 μM, or with the Ang1 and the pAKT inhibitor LY 294002 (Cell Signaling Technology) at a final concentration of 14 μM. Treatment reagents were delivered in 2 μl of PBS or DMSO.
Since Angiopoetin1-Tie2 signaling is an important pathway in endothelial cell-pericyte communication, the effect of Angiopoietin1 and its downstream effectors were also quantified during VEVIS formation. VECs and VICs were co-cultured as described above for 7 days and treated with Angiopoetin1, the Tie2 Kinase Inhibitor, and the p-Akt inhibitor. Co-cultures were additionally treated with the Tie2 Kinase Inhibitor alone to investigate the role of basal Angiopoietin signaling. After 7 days, the sizes of at least 6 randomly selected spheroids from 6 independent wells from 2 independent experiments were quantified using ImageJ.
Quantification of Changes in Angiogenesis and Pericyte Related Markers with qRT-PCR
In order to investigate expression changes in angiogenesis and pericyte markersafter 7 days in culture on Matrigel, qRT-PCR was performed on valve cell mRNA before and after culture. mRNA was harvested using a Quick-RNA Micro Prep (Zymo Research). A PrimeScript™ 1st strand cDNA Synthesis Kit (Clontech) was then used to convert 100 ng of RNA to cDNA. qRT-PCR was then run at a 1:100 dilution of cDNA using the SYBR® Advantage® qPCR Premix for the targets in Table 2. Ubiquitin was used as the housekeeping gene in each run. Fold change was then calculated between cultures before and after 7 days in culture on the Matrigel. For VEC only cultures on Matrigel, mRNA harvested from the same sample of cells before seeding was used as their control. mRNA from a 3 to 1 mixture of VECs and VICs was used as the control for VEC and VIC co-cultures. ΔCt was averaged between runs of the same condition. This averaged ΔCt was then used to calculate the fold increase between the reference condition and the target by taking the ratio of (Efficiency of amplification)^avgΔCt for the target and the Ubiquitin reference. The error is represented by the square root of the average of the variances of the reference condition and the target.
Table 2.
Angiogenesis and pericyte related gene expression PCR targets.
| Gene | Primer |
|---|---|
| Ubiquitin FWD | TGA CCA GCA GCG TCT GAT T |
| Ubiquitin REV | TCT TGT CGC AGT TGT ATT TCT GAG |
| NG2 FWD | CAT CTT GCC TCT GCT CTT CTA C |
| NG2 REV | TGT CCC TCC CTT CTC TTT CT |
| Periostin FWD | ACC TGG AGA TTG GAC CTT ATT T |
| Periostin REV | CTG CTG GGT AGA GGA GTT TAT C |
| Ang1 FWD | TTT CCA GAG AGG TTG GAA AGA A |
| Ang1 REV | GTA CTG CCT CTG ACT GGT TAT G |
| Ang2 FWD | CCG TCA ACT GCA TCA CTT AAA C |
| Ang2 REV | TTA TCT TCC CAC AGG CTT TCT C |
| VEGF FWD | TAT GCG GAT CAA ACC TCA CC |
| VEGF REV | CTT GCC TCG CTC TAT CTT TCT T |
| tie2 FWD | TGG AAC CTC GAA CAG AAT ATG AG |
| tie2 REV | GGA GGA GGA AGA CCG ATA GAA |
| FGFR2 FWD | AAC GAT TAC GGG TCC ATC AA |
| FGFR2 REV | CCG TTC TTT TCC ACG TGT TT |
Results
VIC-Driven Collapse of VEC Vasculogenic Networks is ROCK-Dependent
To test the role of VICs in VEC vascular networks, we grew VECs and VICs in direct co-culture in a 3:1 (VEC:VIC) ratio in the model pro-angiogenic environment of Matrigel. After 7 hours of co-culture, VECs cultured alone were organized (Figure 1A) as previously reported.1 PKH labeling of VECs (green) and VICs (red) in co-culture demonstrated that they organized together in well-defined networks with VICs scattered among the nodes and along the length of the tubule-like structures (Figure 1B). This arrangement was similar to previous reports of PKH-tracked vascular ECs and pericytes (PCs).6 Automated network analysis showed that co-culturing with VICs reduced total network length while increasing average tubule length between nodes compared to VECs cultured alone (Figure 1D).
Figure 1.

VICs altered VEC vasculogenic network formation in a ROCK-dependent manner at 7 h after seeding. (a) VECs cultured alone formed complex networks. (b) PKH-stained VECs (green) and VICs (red) formed complex vasculogenic networks when co-cultured. VICs were found connected to VEC tubules and in the nodes between tubules. (c) Treatment of co-cultures with ROCK inhibitor Y-27632 (3.5 μM) eliminated macro changes to network architectures, although localization of VICs along VEC tubules and nodes was still found. Scale bars: 100 μm in (a–c). (d) Quantification of changes to total network lengths and average tubule length in the co-cultures compared to VEC-only controls. Data shown as mean ± standard error across 15 independently seeded wells. The p-value for comparison between groups is displayed above the denoted pairs. Immunocytochemistry of (e) VEC/VIC co-cultures and (f) VEC/VIC co-cultures treated with Y-27632 stained for CD31 (green), actin (red), aSMA (yellow), and cell nuclei (blue). (e) Polymerized aSMA+ VICs (yellow arrow) were found wrapped around and in contact with several elongated CD31+/aSMA− VECs. (f) Treatment with the ROCK inhibitor did not affect VIC expression of aSMA, but it was present diffusely and not in polymerized stress fibers (green arrow). ROCK inhibition did not eliminate VIC-to-VEC connections, demonstrated by the multiple connections to VECs made by each VIC in panel F. Co-cultures treated with the ROCK inhibitor displayed notably fewer VICs covering VEC network vertexes. Scale bars: 50 μm in (e–f).
ROCK inhibition (3.5 μM Y-27632) largely prevented the VIC-mediated network formation, while maintaining multicellular clusters of VECs and VICs in direct contact (Figure 1C), and brought the total network length and average tubule length metrics closer to the scale of the VEC-only cultures (Figure 1D). Immunocytochemistry of the co-cultures demonstrated networks consisting of CD31+ (green) and aSMA− VECs with polymerized aSMA+ (yellow) VICs covering VEC tubules (Figure 1E, yellow arrow). Treatment with the ROCK inhibitor did not affect VIC expression of aSMA, but it was present more diffusely within VICs (green arrow) as opposed to presenting as stress fibers. ROCK inhibition did not ablate VIC-VEC connections, given the multiple VIC-to-VEC connections observed (Figure 1F). Co-cultures treated with the ROCK inhibitor displayed notably fewer VICs covering around VEC tubules.
VEC/VIC Long Term Co-cultures Form 3D Invasive Spheroids
As demonstrated by the MCEC/10T1/2 co-culture in Figure 2A and quantified in Supplemental Figure 1, network organization and stabilization is a major characteristic of EC/PC interactions in vitro. These results, in which the 10T1/2 cells stabilized the MCEC networks long-term, provided a benchmark of vascular EC/PC behavior for direct comparison with the effects of VIC co-culture on VECs in the same angiogenic assay. In contrast to the vascular controls, VEC/VIC co-cultures collapsed into spheroids at the network nodes by 24 hours in culture (Figure 2B). After 48 hours in culture, the VECs and VICs began to invade into the Matrigel. VICs were generally, though not exclusively, the first cells to migrate into the Matrigel in an angiogenic-like sprout. In these sprouts, the VIC would lead the way in a manner consistent with a tip cell, whereas the VECs acted more like the stalk cells of the new sprouts. The VEC/VIC spheroids continued to sprout into the gels, reaching a maximum invasion depth 5-7 days post-seeding. To demonstrate the dynamic VEC/VIC invasive spheroid (VEVIS) relationships, live cell imaging was performed using confocal fluorescence microscopy with an environmental chamber for periods of 4-6 hours; Supplemental Figures 2-8 show representative movies of significant time periods in the transition. After initial sprouting, VECs and VICs displayed a dynamic plasticity of roles in competing for the tip cell position, similar to how vascular ECs compete during angiogenic root formation.15 Similar to the MCEC-only cultures, the networks of VECs cultured alone slowly regressed over the first 72 hours of culture. By 7 days in culture, the VECs coalesced into spheroids and exhibited sprouts similar to VEVIS sprouts, but these sprouts tended to be fewer in number and smaller in size compared to the co-cultures (Supplemental Figure 13).
Figure 2.

VEC/VIC co-cultures form 3D invasive spheroids during sustained culture in a pro-angiogenic environment. (a) MCECs cultured alone form networks that regress after 48 h. MCEC/10T1/2 co-cultures display typical EC/PC dynamics by sustaining MCEC networks after 48 h of culture. Scale bars: 100 μm. (b) Representative images of PKH-stained VEC (green)/VIC (red) co-cultures over long-term culture. Within 24 h of seeding, VIC/VEC co-cultures formed EC/PC-like vasculogenic networks, spontaneously regressed, and reorganized into spheroid-like structures. After another 24 h, the VICs begin to invade into the Matrigel, generally acting as tip cells with the VECs acting as the stalk cells. VIC/VEC co-cultures continued to sprout radially for 5–7 days. In the 120 h panel, only the VECs (green) are highlighted to demonstrate their distribution throughout the VEVIS. Scale bars: 100 μm. Images were taken from 4 independent experiments. 0 and 7 h panels were taken from the same position in the same well, as were the 24 and 48 h panels.
VICs Guide VECs During Network Reorganization
Upon further investigation of these co-cultures, it became evident that a substantial number of the VICs was attracting and guiding the organization of VECs during the time period before spheroid formation (7-24 hours). Therefore, a dual color PKH fluorescent speckle tracking method was implemented to track movement of VECs in relation to nearby VICs (Figure 3A) to quantify three relative chemotaxis metrics. The average cosine similarity measurements of VEC movements showed a narrow radial normal distribution around 0° (±30°), indicating movement in the direction of VICs, unlike the simulated Brownian motion control, which had an even radial distribution (Figure 3B). The measured average directionality of VECs and their average Lagrangian Forward Migration Index (LaFMI) were significantly larger than in the simulated Brownian motion data sets, again indicating the movement of VECs in the direction of nearby VICs. Assuming that a LaFMI > 0.17 represents a lower threshold for a chemoattractant effect (as demonstrated in prior chemotaxis studies17), 46% of VICs demonstrated this central tendency, i.e., this effect on nearby VECs, whereas this chemoattractant proportion was only 0.14% in the simulated Brownian motion data set. An example of a VIC demonstrating a LaFMI > 0.17 (a chemoattractive effect on local VECs) is shown in Figure 3C and in Supplemental Figure 9.
Figure 3.
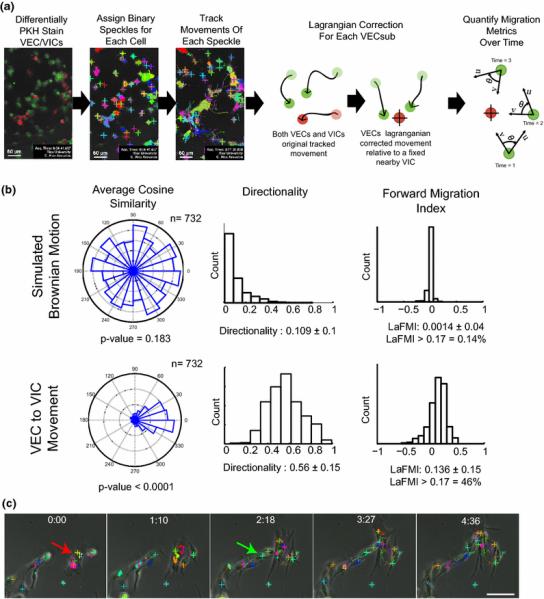
During organization into spheroids, tracking of VEC movement in relation to nearby VICs reveals a subpopulation of VICs that demonstrate chemoattraction. (a) Illustration of method to quantify chemotaxis metrics. Confocal microscopy images of differentially stained VECs and VICs were reconstructed into z-stacks at each time point. For each color channel, the PKH staining was assigned a binary threshold, and each cell was tracked by its resultant unique speckle pattern. For each VIC, all nearby VECs were grouped together and a Lagrangian transformation was performed on their movements relative to the VIC of interest. Lagrangian-corrected chemotaxis metrics were then calculated. (b) Resultant chemotaxis metrics for a Brownian motion simulation (negative control) and the measured VEC and VIC movements. Compared to the independent-motion negative control simulation, VECs demonstrated a pronounced movement toward VICs in terms of average cosine similarity, directionality, and forward migration index. As defined by a forward migration index >0.17, 46% of VICs had an overall positive chemotaxis effect on nearby VECs. (c) Representative time track of a VIC (red arrow) exhibiting positive chemoattraction on nearby VECs (green arrow) during network contraction and reorganization into spheroids.
VICs Promote VEC Invasion and Are Found Throughout VEVIS Sprouts
VECs cultured alone formed spheroids that displayed a limited amount of invasive sprouts after 7 days in culture. In contrast, VEVIS spheroids displayed a significantly larger invasion ratio (area of invasion/ area of core) by spreading more from the core spheroid into the Matrigel (Figure 4A). Quantification of the radial position of VECs and VICS revealed that a higher percentage of VICs were localized on the exterior of the spheroids (75% and 100% quartile rings) compared to VECs, whereas a higher percentage of VECs were found in the inner 25% quartile ring of the spheroid compared to VICs (Figure C). The PKH-labeled VICs were observed throughout the VEVIS. As demonstrated by the red arrows in Figure 4, VICs were frequently found wrapped around early 2D networks (Figure 4E), on the leading edge of invading sprouts (Figure 4F, yellow arrow), and wrapped around 3D invasive sprouts (Figure 4G, red arrow). Interestingly, VICs often displayed direct cell-to-cell contact to the leading edge of 3D invading VECs, as shown by Figure 4H. As shown by the Z-stack reconstructed confocal microscopy images (Figure 4H and 4I), VEVIS sprouts formed complex geometries spreading in 3D. The continued dynamic plasticity of the sprout edge after 7 days of co-culture is shown in Supplemental Figure 10.
Figure 4.

VICs display a pericyte-like phenotype promoting and stabilizing VEC invasive angiogenic root formation throughout VEVIS. (a) Representative image of quantification of sprouting ratio after 7 days. Scale bar: 100 μm. (b) Sprouting was significantly greater in VEC/VIC spheroids than in VEC-only spheroids. (c) Representative image of the segmentation of a VEVIS into four quartile rings of equal surface area. Scale bar: 100 μm. Both VECs and VICs were found throughout the VEVIS. (d) A significant relationship was found between cell type and position by two-way ANOVA (p < 0.05). The inner quartile ring contained a significantly larger percentage of VECs than VICs, whereas a larger percentage of VICs tended to be located in the third quartile ring. Throughout the VEC/VIC Matrigel co-culture, VICs were found in diverse locations that included (e) wrapping around VEC tubules at 7 h (red arrow); (f) leading (yellow arrow) VEC angiogenic stalk-like cell (white arrow) sprouting from spheroids after 24 h and co-localized with VEC spheroid nodes; (g) wrapping (red arrow) around and stabilizing formed VEC invasive stalks (white arrow); and (h) occupying the leading edge (yellow arrow) of VEC sprouts (white arrow) after 5 days in co-culture. Direct VIC-to-VEC contacts could also be found on invasive sprouts. (i) 3D reconstruction of panel H demonstrating the 3D nature of VEVIS sprouts. Each arrow in panel I points to the same cell as in panel H. Scale bars represent 25 μm in C and 50 μm in (d–f).
VEVIS Sprouts Display Characteristics of Vascular Angiogenesis Sprouts
The VEVIS sprouts demonstrated several of the characteristics found in vascular sprouts, including DLL4 polarization, activated AKT, organized actin, 3D invasion, branching, lamellipodia at the tips, and multicellular tube formation (Figure 5). VECs maintained the ability to uptake acetylated LDL (Figure 5A). Most cells in VEVISs displayed low levels of unpolarized aSMA (blue arrows, Figure 5B and Supplemental Figure 11) as opposed to the polarized aSMA found in VICs but not in CD31+ VECs during the network formation at earlier time points (Figure 1C). VECs demonstrated continued expression of CD31 after 7 days of co-culture (magenta arrows in Figure 5C and in Supplemental Figure 12). Interestingly, both CD31− (green arrows) and CD31+ cells could be found on the tips of VEVIS sprouts (Figure 5D). These tip cells displayed direct cell-cell contact with CD31+ stalk cells emerging from the core VEVIS (magenta arrows in Figure 5D). VEVIS sprouts displayed DLL4+ polarization from tip to stalk, i.e., the expression of DLL4 was strongest at the leading cell and decreased toward the origin of the sprout (red arrows for tip and white arrows for origin in Figure 5E and Supplemental Figure 13). Interestingly, phosphorylated AKT+ cells were found prevalently on the tips of sprouts (yellow arrows in Figure 5F). The linear and tube-like organization of actin structures along VEVIS sprouts is recognizable in the higher magnification images (white arrows in Figure 5E and F). Similar to DLL4, VEVIS displayed β-Catenin polarity down the angiogenic like-sprouts (Figure 5G). In addition, all cells in the VEVIS were pMLC+ (Figure 5H).
Figure 5.

VEVIS sprouts display characteristics of vascular angiogenesis sprouts after 7 days in co-culture. (a) VECs maintained endothelial phenotype as demonstrated by acetylated LDL uptake (red). Scale bar: 100 μm. (b) Most cells throughout the VEVIS, including tip and stalk cells, stained positive for diffusive non-polymerized aSMA (green; indicated by blue arrow). Counterstained with phalloidin (red) and DAPI (blue). Scale bar: 200 μm. (c) VECs maintained CD31 (green) expression in VEVIS core and sprouts (magenta arrow). Counterstained with phalloidin (red) and DAPI (blue). Scale bar: 200 μm. (d) CD31− VIC (green arrow) displayed direct cell-to-cell contact (white arrow) and organized actin on the tip of a stalk of CD31+ (green) VECs (magenta arrow). Counterstained with phalloidin (red) and DAPI (blue). Scale bar: 25 μm. (e) DLL4 (green) expression is polarized from tip of the invasive root to the core spheroid (red arrow). In addition, linear and tube-like organized actin structures were recognizable along VEVIS sprouts (white arrow). Cells are counterstained with phalloidin (red) and DAPI (blue). Scale bar: 25 μm. (f) pAKT + (green) expression is localized on the tip cells of VEVIS sprouts (yellow arrow). Counterstained with phalloidin (red) and DAPI (blue). Scale bar: 100 μm. (g) VEVIS demonstrate β-Catenin (red) polarity down the angiogenic-like root. Counterstained with phalloidin (green) and DAPI (blue). Scale bar: 25 μm. (h) pMLC (red) was present in most cells with no appreciable pattern. Counterstained with phalloidin (green) and DAPI (blue). Scale bar: 200 μm.
Tie2-Angiopoietin Signaling Is Important in Valve Cell Organization
VEC-only cultures treated with Ang1 displayed no significant change in total network size after 7 hours (93±11% of untreated control, Figure 6A-B). In contrast, treatment with Tie2 Inhibitor or pAKT inhibitor in addition to Ang1 resulted in significantly smaller networks relative to untreated controls at 7 hours (12±7% and 50±9% respectively). After 3 days of culture, VECs treated with Ang1 demonstrated significantly larger total network size compared to controls (218±3%), indicating the networks of Ang1-treated VECs were more stable than those of untreated VECs. This network size effect was significantly abated with the addition of the Tie2 kinase inhibitor (62±4%) and the pAKT inhibitor (86±3%).
Figure 6.

Angiopoietin-Tie2 signaling regulates organization of VIC-VEC co-cultures. (a) Representative images of the effects of Ang1 and its downstream inhibitors on VEC network formation over 3 days. Scale bars: 100 μm. (b) Quantification of the effects of Ang1 and its downstream inhibitors on VEC network formation over time. Results are presented as mean ± standard error for 12 independently seeded wells. p < 0.05 between groups. (c) Representative images of the effects of Ang1 and its downstream inhibitors on VEVIS formation after 7 days in culture. Scale bars: 100 μm. Quantification of the effects of Ang1 and its downstream inhibitors on VEC/VIC co-culture after 7 days. Results are presented as mean ± standard error of the VEVIS of 12 independently seeded wells normalized to the (d) total spheroid size and (e) the invasion ratio of the control VEVIS.
Treatment with Ang1 was insufficient to prevent VIC-mediated contraction into the VEVIS structures during 7 day cultures, as there was no significant difference in size between the VEVIS formed from untreated VEC/VIC co-cultures and the Ang1-treated group (91±7% of control). Treatment with the Tie2 inhibitor, either alone or in addition to Ang, significantly reduced VEVIS size (4±1% and 11±1.9%, respectively) compared to control VEVIS. Treatment with the pAKT inhibitor in combination with Ang1 significantly reduced VEVIS formation (20±7%) compared to the control.
Long Term Co-Culture in the TLS Assay ± VICs Significantly Increases the Expression of Angiogenesis and Pericyte-Related Genes
As shown by Figure 7, VECs and VICs demonstrated elevated levels of several angiogenesis- and pericyte-related markers after long term culture in the Matrigel model. After VECs were cultured alone in the Matrigel model, they showed significantly greater expression of FGFR2 and Tie2 compared to VECs cultured on the control condition of tissue culture plastic. Angiopoetin1 expression was undetectable in control or Matrigel-cultured VECs. No change in Periostin expression was detected between control and Matrigel-cultured VECs. When VECs and VICs were co-cultured for 7 days in the Matrigel model, there was significantly greater expression of the angiogenesis- and pericyte-related markers FGFR2, Angiopoetin1, Tie2, NG2, and Periostin compared to a control 3:1 mixture of VECs and VICs mixed together prior to co-culture.
Figure 7.

VECs and VICs demonstrate several increases in angiogenesis and pericyte-related markers after long term culture in the Matrigel model. (a) Significant increases in FGFR2 and Tie2 were measured when comparing VECs cultured in the Matrigel model after 7 days to Day 0 control VECs. Angiopoetin1 did not prime in isolated culture or after 7 days in the Matrigel model. No significant change in periostin was measured. (b) Significant increases in the angiogenesis and pericyte-related markers of FGFR2, Angiopoetin1, Tie2, NG2, and Periostin (Perio) were measured after 7 days in co-culture in the Matrigel model when compared to a control 3:1 mixture of VECs and VICs before co-culture. ND = not detected.
Discussion
During CAVD, the valve tissue undergoes an important change from an anti-angiogenic environment to a pro-angiogenic environment.2,12,29 This change is demonstrated by the presence of neovasculature in the normally avascular adult aortic valve.31 These neovessels show characteristics of vascular microvessels, such as a CD31+ endothelial cell lining with aSMA+ pericytes wrapped circumferentially.31 Although it would be ideal to confirm the valvular origin of these cells in vivo, it is compelling to investigate whether VECs and VICs have the potential to be the source of the endothelial cells and pericytes in these microvessels in vitro first, but there has been no previous study on whether VEC/VIC co-cultures could form 3D angiogenetic structures.
This study was designed to quantify the extent to which VECs and VICs demonstrate characteristics representative of dynamic endothelial-pericyte interactions within a long-term Matrigel co-culture model that introduces the cells to a pro-angiogenic microenvironment. Directly comparing these aspects of the behavior of valve cells to vascular-mimetic endothelial cells and pericyte precursors elucidated several typical and atypical vasculogenic/angiogenic behaviors of the valve cells. The main findings were that the VICs do act like pericytes in stabilizing early VEC vasculogenic network formation, but later drive network collapse in a manner involving chemoattraction of VECs. In time, the co-cultured VICs and VECs form a spheroid, from which the VICs lead invasive angiogenic sprouting. Lastly, it was shown that these processes were regulated by ROCK and the Ang-Tie2 pathway. It should be noted that there are caveats associated with the use of this Matrigel model. It is unclear the effect of stiffness-mediated VIC aSMA contractility on the differences between the early time point identified phenotypes between the VICs and the 10T1/2 cells. Although the cells used in this study were not of human origin, our preliminary work with human-derived VECs and VICs in the same Matrigel model (data now shown) indicates that human cells form the VEVIS structure similar to the porcine-derived valve cells. Additionally, the question of whether the VEVIS invasive sprouts are indicative of functional in vivo angiogenesis complete with lumen formation and blood carrying capacity remains unclear. Future studies should include an in vitro angiogenesis assay that incorporates flow to better demonstrate an EC lumen22 and an in vivo model to test VIC ability to support vessel formation19. Nonetheless, this work expands our understanding of valve cell-cell organization and communication with implications for valve homeostasis, heart valve tissue engineering, and the pathology of CAVD.
This study was centered on the ability of VICs to act as pericytes. During vascular angiogenesis, pericyte-endothelial signaling plays a very important role in the organization and stabilization of neovascularization. Pericytes guide vessel formation through paracrine and direct contact signaling mechanisms, several of which have been investigated in regards to valve cells.8,9,25,30,37 Despite the evidence for a subpopulation of cells in calcified valves that have pericyte-like characteristics,35 VIC-endothelial cell studies to date have yet to induce quantifiable pericyte-mimicking behavior.
In this study, VICs demonstrated characteristics of pericyte phenotypes throughout sustained co-culture with VECs in the Matrigel model. During the initial network formation, co-culture with VICs led to a quantifiably smaller VEC network size, which was reversed by inhibition of ROCK. ROCK inhibition prevented aSMA polymerization while maintaining VEC-VIC direct cell-cell contacts (Figure 1E compared to 1F), similar to how pericytes have been previously described in vitro.6 This effect of ROCK inhibition on network size of VIC-VEC co-cultures is consistent with previous reports that ROCK inhibition increases vasculogenic network size in VEC-only cultures.1 Given that ROCK also regulates contraction of collagen gels by VICs,10 this data suggests that early VIC-mediated VEC network organization is ROCK dependent. Furthermore, there is recent evidence that VICs can regulate the process of endothelial to mesenchyme transformation in VECs14,28; a process that is also ROCK-dependent27. The relationship between VEC transformation and invasion into the valve leaflets and the angiogenesis found in CAVD is a compelling area for future study.
By tracking and quantifying cell motion to determine chemotaxis metrics, this study demonstrated that a large percentage of VICs (46%) strongly demonstrated a chemoattractant phenotype, attracting VECs. This behavior was demonstrated during VEC/VIC self-organization into spheroids by 24 hours after seeding in Matrigel. In contrast, co-culturing of the vascular cells (MCECs and 10T1/2 cells) led to sustained networks over time. Although not all VICs showed this chemoattractive behavior (LaFMI>0.17), it is unclear if VICs with a LaFMI<0.17 were unable to attract local VECs due to another VIC nearby with a stronger chemoattraction. It is currently not fully understood whether dysfunction of VECs, VICs, or both leads to initiation of CAVD.9 These results indicate that VICs cultured in Matrigel develop a pro-angiogenic and chemoattractant phenotype. These VICs are then capable of driving VECs from their physiologically quiescent phenotype toward a more pro-angiogenic and invasive phenotype. These results suggest that under the proper stimuli VICs have the ability to organize VECs into pathological structures and, thus, the capacity to disrupt valve homeostasis.
Tracking of differentially-stained valve cell types in co-culture revealed several pericyte-like behaviors of VICs towards VECs. After our first observations of the spontaneous formation of VEVIS structures, we hypothesized that VECs would be the first cell type to invade the gel from the spheroids, and implemented PKH tracking to monitor cell behavior during this culture period. Unexpectedly, PKH tracking revealed that VICs tended to be the first cell to leave the spheroid and invade the Matrigel, were found preferentially on the exterior of the VEVIS compared to the VECs, and facilitated greater invasion by VECs. These results reveal that the significant differences between the localization and arrangement pattern of VECs and VICs was not random, and these interactions were likely driven by a combination of cell-cell and cell-matrix signaling. These results were also distinct from the MCEC/10T1/2 co-cultures, which did not form spheroids. In the future, it will be important to elucidate further the key molecular regulators in valve cells that are responsible for their atypical translation of these angiogenetic signals compared to the vascular controls.
The invading VEVIS sprouts were similar in some ways to classic angiogenetic sprouts, but also demonstrated unique characteristics. The VICs on the leading edges of the sprouts were CD31− and aSMA+, but the VICs maintained cell-cell adhesions with following CD31+ VECs. As shown by Figure 7, VECs demonstrated a pronounced elevation in periostin expression during the culture period, but this elevation was only found in co-culture. This result suggests an association between the greater degree of cell invasion of Matrigel in the VEVIS condition and expression of this ECM protein, which has been strongly linked to neo-angiogenesis in aortic valve sclerosis.11 Interestingly, the VEVIS sprouts demonstrated DLL4 and β-catenin polarity from the tip to stalk of sprouts, thus exhibiting evidence of cell-cell contact-mediated DLL4 as well as β-catenin-linked24 regulation of angiogenic-like sprout initiation. Further studies are required to investigate if manipulation of NOTCH or DLL4 expression in valve cells affects VEC/VIC tip cell dynamics. In vascular angiogenesis, DLL4 to NOTCH signaling plays an important role in cell-cell contact-mediated initiation and tip-stalk cell organization in neovessel formation.13 NOTCH is the expected ligand for the DLL4 receptor, and there is a reported role for NOTCH signaling in VEC-VEC signaling, VIC-induced calcification, and valvulogenesis.5,30 However, it is unclear if the same downstream pathways previously related to calcification and NOTCH are at play in this model. Further studies are needed to examine whether sustained angiogenic signaling leads towards valve calcification through angiogenetic-related cell-cell contact NOTCH inhibition.
Since there is growing evidence for significant involvement of nitrous oxide (NO) signaling in VEC-VIC interactions,9,32 this study examined the role of the Ang1-Tie2 pathway (an upstream regulator of NO via eNOS and AKT) in valve cell signaling and organization into networks and VEVISs. Stimulation with Ang1, the ligand for Tie2 receptor, had no significant effect on early network formation in VIC-VEC co-cultures, but inhibition of direct and downstream signaling via inhibitors for Tie2 and AKT markedly reduced network formation. After 3 days in culture Ang1 stimulation significantly slowed network collapse and, correspondingly, inhibitors for Tie2 or AKT prevented this slowing. Long-term ROCK inhibition had a similar effect as Ang1 stimulation on VIC-VEC network stabilization (data not shown), which is not surprising given that ROCK inhibition is a downstream effect of Tie2 activation.16 Ultimately, this network stabilizing effect of Ang1 could not prevent the VIC-mediated network collapse and eventual formation of VEVIS. Tie2 inhibition, however, completely ablated VEVIS formation, suggesting that basal levels of Ang-Tie2 signaling are significant contributors to VEC/VIC signaling during VEVIS formation. Although treatment with the AKT inhibitor failed to prevent VEVIS formation, the resulting VEVIS were much smaller, suggesting a role for basal levels of AKT activation in VEC/VIC communication. Collectively, these results suggest that Angiopoietin signaling, via the Tie2 and AKT pathways, mediates VIC-VEC organization, as well as evolution towards invasive angiogenic behavior. This finding is significant due to the role of Ang-Tie2 signaling in initial vascular destabilization,33 and therefore suggests a role for this pathway in initial VEC dysfunction during the early pathology of CAVD. Further studies are required to confirm the role and source of Tie2 signaling activity and its effects on VEC monolayer homeostasis during CAVD. It would be particularly useful to employ siRNAs to knock down key signaling components (i.e., NOTCH, Tie2, or Ang1) in VECs or VICs independent of one another to determine how each factor drives the different cell types towards their angiogenic-like organization.
In conclusion, angiogenesis has long been associated with valvulogenesis and the pathology of CAVD. This study sought to identify the phenotypic changes of valve cells cultured long term in a pro-angiogenic model to provide further insight to the role of angiogenic signaling in VEC/VIC dynamics. Tracking of the cells revealed that many VICs demonstrate chemoattractant behavior towards VECs and showed a novel invasive spheroid phenotype of these valve cells compared to their vascular counterparts. Lastly, this study identified a new set of angiogenic regulators, the Ang-Tie2 family, affecting valve cell communication and organization. This study contributes to the growing understanding of valve cell biology and how the atypical translation of angiogenetic signaling in valve cells could translate to the pathology of CAVD through enabling neovascular invasion of the ECM through coordinated valve cell behavior and subsequently through increased inflammatory infiltrates and blood flow to the normally avascular aortic valve.
Supplementary Material
Acknowledgments
The authors thank Jennifer Connell, PhD, for editorial assistance and Cindy Farach-Carson, PhD, for use of her microscopy equipment.
Funding Sources
Funding was provided by AHA 11IRG5550052 and an NSF Graduate Research Fellowship to C.A.A.
Abbreviations
- AKT
RAC-alpha serine/threonine-protein kinase
- Ang1
Angiopoetin1
- CAVD
Calcific aortic valve disease
- DLL4
Delta like ligand four
- EndMT
Endothelial to mesenchymal transformation
- LaFMI
Lagrangian corrected forward migration index
- MCEC
Mouse cardiac endothelial cells
- NO
Nitrous oxide
- ROCK
Rho-associated protein kinase
- VEC
Valve endothelial cell
- VIC
Valvular interstitial cell
Footnotes
Conflicts of Interest
The authors have nothing to disclose.
References
- 1.Arevalos CA, Walborn AT, Rupert AA, Berg JM, Godfrey EL, V Nguyen JM, Grande-Allen KJ. Regulation of valve endothelial cell vasculogenic network architectures with ROCK and Rac inhibitors. Microvasc. Res. 2015;98C:108–118. doi: 10.1016/j.mvr.2015.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Armstrong EJ, Bischoff J. Heart valve development: endothelial cell signaling and differentiation. Circ. Res. 2004;95:459–70. doi: 10.1161/01.RES.0000141146.95728.da. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arnaoutova I, Kleinman HK. In vitro angiogenesis: endothelial cell tube formation on gelled basement membrane extract. Nat. Protoc. 2010;5:628–35. doi: 10.1038/nprot.2010.6. [DOI] [PubMed] [Google Scholar]
- 4.Balaoing LR, Post AD, Liu H, Minn KT, Grande-Allen KJ. Age-related changes in aortic valve hemostatic protein regulation. Arterioscler. Thromb. Vasc. Biol. 2014;34:72–80. doi: 10.1161/ATVBAHA.113.301936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bosse K, Hans CP, Zhao N, Koenig SN, Huang N, Guggilam A, LaHaye S, Tao G, Lucchesi PA, Lincoln J, Lilly B, Garg V. Endothelial nitric oxide signaling regulates Notch1 in aortic valve disease. J. Mol. Cell. Cardiol. 2013;60:27–35. doi: 10.1016/j.yjmcc.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bryan BA, D’Amore PA. Pericyte isolation and use in endothelial/pericyte coculture models. Methods Enzymol. 2008;443:315–31. doi: 10.1016/S0076-6879(08)02016-8. [DOI] [PubMed] [Google Scholar]
- 7.Chalajour F, Treede H, Ebrahimnejad A, Lauke H, Reichenspurner H, Ergun S. Angiogenic activation of valvular endothelial cells in aortic valve stenosis. Exp. Cell Res. 2004;298:455–64. doi: 10.1016/j.yexcr.2004.04.034. [DOI] [PubMed] [Google Scholar]
- 8.Fernández-Pisonero I, López J, Onecha E, Dueñas AI, Maeso P, Crespo MS, Román JAS, García-Rodríguez C. Synergy between sphingosine 1-phosphate and lipopolysaccharide signaling promotes an inflammatory, angiogenic and osteogenic response in human aortic valve interstitial cells. PLoS One. 2014;9:e109081. doi: 10.1371/journal.pone.0109081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gould ST, Matherly EE, Smith JN, Heistad DD, Anseth KS. The role of valvular endothelial cell paracrine signaling and matrix elasticity on valvular interstitial cell activation. Biomaterials. 2014;35:3596–606. doi: 10.1016/j.biomaterials.2014.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gu X, Masters KS. Role of the Rho pathway in regulating valvular interstitial cell phenotype and nodule formation. Am. J. Physiol. Heart Circ. Physiol. 2011;300:H448–58. doi: 10.1152/ajpheart.01178.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hakuno D, Kimura N, Yoshioka M. Periostin advances atherosclerotic and rheumatic cardiac valve degeneration by inducing angiogenesis and MMP production in humans and rodents. J. Clin. Invest. 2010;120 doi: 10.1172/JCI40973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hakuno D, Kimura N, Yoshioka M, Fukuda K. Molecular mechanisms underlying the onset of degenerative aortic valve disease. J. Mol. Med. (Berl) 2009;87:17–24. doi: 10.1007/s00109-008-0400-9. [DOI] [PubMed] [Google Scholar]
- 13.Hellstrom M, Phng L-K, Hofmann JJ, Wallgard E, Coultas L, Lindblom P, Alva J, Nilsson A-K, Karlsson L, Gaiano N, Yoon K, Rossant J, Iruela-Arispe ML, Kalen M, Gerhardt H, Betsholtz C. Dll4 signalling through Notch1 regulates formation of tip cells during angiogenesis. Nature. 2007;445:776–780. doi: 10.1038/nature05571. [DOI] [PubMed] [Google Scholar]
- 14.Hjortnaes J, Shapero K, Goettsch C, Hutcheson JD, Keegan J, Kluin J, Mayer JE, Bischoff J, Aikawa E. Valvular interstitial cells suppress calcification of valvular endothelial cells. Atherosclerosis. 2015;242:251–260. doi: 10.1016/j.atherosclerosis.2015.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jakobsson L, Franco CA, Bentley K, Collins RT, Ponsioen B, Aspalter IM, Rosewell I, Busse M, Thurston G, Medvinsky A, Schulte-Merker S, Gerhardt H. Endothelial cells dynamically compete for the tip cell position during angiogenic sprouting. Nat. Cell Biol. 2010;12:943–53. doi: 10.1038/ncb2103. [DOI] [PubMed] [Google Scholar]
- 16.Lukasz A, Kümpers P, David S. Role of angiopoietin/tie2 in critical illness: promising biomarker, disease mediator, and therapeutic target? Scientifica (Cairo) 2012;2012:160174. doi: 10.6064/2012/160174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martins M, Warren S, Kimberley C, Margineanu A, Peschard P, McCarthy A, Yeo M, Marshall CJ, Dunsby C, French PMW, Katan M. Activity of PLCε contributes to chemotaxis of fibroblasts towards PDGF. J. Cell Sci. 2012;125:5758–69. doi: 10.1242/jcs.110007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mazzone A, Epistolato MC, De Caterina R, Storti S, Vittorini S, Sbrana S, Gianetti J, Bevilacqua S, Glauber M, Biagini A, Tanganelli P. Neoangiogenesis, T-lymphocyte infiltration, and heat shock protein-60 are biological hallmarks of an immunomediated inflammatory process in end-stage calcified aortic valve stenosis. J. Am. Coll. Cardiol. 2004;43:1670–6. doi: 10.1016/j.jacc.2003.12.041. [DOI] [PubMed] [Google Scholar]
- 19.Melero-Martin JM, Khan ZA, Picard A, Wu X, Paruchuri S, Bischoff J. In vivo vasculogenic potential of human blood-derived endothelial progenitor cells. Blood. 2007;109:4761–8. doi: 10.1182/blood-2006-12-062471. [DOI] [PubMed] [Google Scholar]
- 20.van Meurs M, Kümpers P, Ligtenberg JJM, Meertens JHJM, Molema G, Zijlstra JG. Bench-to-bedside review: Angiopoietin signalling in critical illness - a future target? Crit. Care. 2009;13:207. doi: 10.1186/cc7153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mohler ER, Gannon F, Reynolds C, Zimmerman R, Keane MG, Kaplan FS. Bone formation and inflammation in cardiac valves. Circulation. 2001;103:1522–8. doi: 10.1161/01.cir.103.11.1522. [DOI] [PubMed] [Google Scholar]
- 22.Nguyen D-HT, Stapleton SC, Yang MT, Cha SS, Choi CK, Galie PA, Chen CS. Biomimetic model to reconstitute angiogenic sprouting morphogenesis in vitro. Proc. Natl. Acad. Sci. U. S. A. 2013;110:6712–7. doi: 10.1073/pnas.1221526110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Otrock ZKZ, Mahfouz R. a R., Makarem J. a, Shamseddine AI. Understanding the biology of angiogenesis: review of the most important molecular mechanisms. Blood Cells, Mol. 2007;39:212–20. doi: 10.1016/j.bcmd.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 24.Oubaha M, Lin MI, Margaron Y, Filion D, Price EN, Zon LI, Côté J-F, Gratton J-P. Formation of a PKCζ/β-catenin complex in endothelial cells promotes angiopoietin-1-induced collective directional migration and angiogenic sprouting. Blood. 2012;120:3371–81. doi: 10.1182/blood-2012-03-419721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paranya G, Vineberg S, Dvorin EL, Kaushal S, Roth SJ, Rabkin E, Schoen FJ, Bischoff J. Aortic valve endothelial cells undergo transforming growth factor-beta-mediated and non-transforming growth factor-beta-mediated transdifferentiation in vitro. Am. J. Pathol. 2001;159:1335–43. doi: 10.1016/s0002-9440(10)62520-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Petrie RJ, Doyle AD, Yamada KM. Random versus directionally persistent cell migration. Nat. Rev. Mol. Cell Biol. 2009;10:538–49. doi: 10.1038/nrm2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sakabe M, Ikeda K, Nakatani K, Kawada N, Imanaka-Yoshida K, Yoshida T, Yamagishi T, Nakajima Y. Rho kinases regulate endothelial invasion and migration during valvuloseptal endocardial cushion tissue formation. Dev. Dyn. 2006;235:94–104. doi: 10.1002/dvdy.20648. [DOI] [PubMed] [Google Scholar]
- 28.Shapero K, Wylie-Sears J, Levine RA, Mayer JE, Bischoff J. Reciprocal interactions between mitral valve endothelial and interstitial cells reduce endothelial-to-mesenchymal transition and myofibroblastic activation. J. Mol. Cell. Cardiol. 2015;80:175–85. doi: 10.1016/j.yjmcc.2015.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shworak NW. Angiogenic modulators in valve development and disease: does valvular disease recapitulate developmental signaling pathways? Curr. Opin. Cardiol. 2004;19:140–6. doi: 10.1097/00001573-200403000-00013. [DOI] [PubMed] [Google Scholar]
- 30.Stankunas K, Ma GK, Kuhnert FJ, Kuo CJ, Chang C-P. VEGF signaling has distinct spatiotemporal roles during heart valve development. Dev. Biol. 2010;347:325–36. doi: 10.1016/j.ydbio.2010.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Syväranta S, Helske S, Laine M, Lappalainen J, Kupari M, Mäyränpää MI, Lindstedt K. a, Kovanen PT. Vascular endothelial growth factor-secreting mast cells and myofibroblasts: a novel self-perpetuating angiogenic pathway in aortic valve stenosis. Arterioscler. Thromb. Vasc. Biol. 2010;30:1220–7. doi: 10.1161/ATVBAHA.109.198267. [DOI] [PubMed] [Google Scholar]
- 32.Tseng H, Balaoing LR, Grigoryan B, Raphael RM, Killian TC, Souza GR, Grande-Allen KJ. A three-dimensional co-culture model of the aortic valve using magnetic levitation. Acta Biomater. 2014;10:173–82. doi: 10.1016/j.actbio.2013.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ucuzian AA, Gassman AA, East AT, Greisler HP. Molecular mediators of angiogenesis. J. Burn Care Res. 2010;31:158–75. doi: 10.1097/BCR.0b013e3181c7ed82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Walker GA, Masters KS, Shah DN, Anseth KS, Leinwand LA. Valvular myofibroblast activation by transforming growth factor-beta: implications for pathological extracellular matrix remodeling in heart valve disease. Circ. Res. 2004;95:253–60. doi: 10.1161/01.RES.0000136520.07995.aa. [DOI] [PubMed] [Google Scholar]
- 35.Wang H, Sridhar B, Leinwand LA, Anseth KS. Characterization of cell subpopulations expressing progenitor cell markers in porcine cardiac valves. PLoS One. 2013;8:e69667. doi: 10.1371/journal.pone.0069667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Welch-Reardon KM, Wu N, Hughes CCW. A Role for Partial Endothelial-Mesenchymal Transitions in Angiogenesis? Arterioscler. Thromb. Vasc. Biol. 2014;35:303–8. doi: 10.1161/ATVBAHA.114.303220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Witt W, Jannasch A, Burkhard D, Christ T, Ravens U, Brunssen C, Leuner A, Morawietz H, Matschke K, Waldow T. Sphingosine-1-phosphate induces contraction of valvular interstitial cells from porcine aortic valves. Cardiovasc. Res. 2012;93:490–7. doi: 10.1093/cvr/cvs002. [DOI] [PubMed] [Google Scholar]
- 38.Yoshioka M, Yuasa S, Matsumura K, Kimura K, Shiomi T, Kimura N, Shukunami C, Okada Y, Mukai M, Shin H, Yozu R, Sata M, Ogawa S, Hiraki Y, Fukuda K. Chondromodulin-I maintains cardiac valvular function by preventing angiogenesis. Nat. Med. 2006;12:1151–9. doi: 10.1038/nm1476. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


