Abstract
Acidocalcisomes are organelles rich in polyphosphate and cations and acidified by proton pumps. Although they have also been described in prokaryotes they have been better characterized in unicellular and multicellular eukaryotes. Eukaryotic acidocalcisomes belong to the group of lysosome-related organelles. They have a variety of functions, from the storage of cations and phosphorus to calcium signaling, autophagy, osmoregulation, blood coagulation, and inflammation. Acidocalcisomes of several unicellular eukaryotes possess a variety of transporters, channels and pumps implying a large energetic requirement for their maintenance and suggesting other important functions waiting to be discovered.
Graphical Abstract
Introduction
Since their first description in the protist parasites Trypanosoma brucei [1] and Trypanosoma cruzi [2], the etiologic agents of African and American trypanosomiasis, respectively, acidocalcisomes have been reported in bacteria [3,4], as well as in many unicellular and multicellular eukaryotes, including humans [5-7]. Two organelles in mammals, the platelet dense granules [8] and the mast cell granules [9], have characteristics in common to acidocalcisomes of protists. The biogenesis of these organelles in several protists [10,11] and mammalian cells [12] involves the function of the adaptor protein 3 (AP-3) complex, as is typical of lysosome-related organelles. Ablation of β3 and δ subunits of the AP-3 complex in T. brucei resulted in disappearance of acidocalcisomes with no alterations in trafficking of different markers to lysosomes [11].
Acidocalcisomes are characterized by their high electron-density when observed by electron microscopy (Fig. 1), and by their acidity and high calcium concentration. These characteristics are the reasons for their name and their grouping as one of the acidic calcium stores of the cell [13]. Another peculiarity common to acidocalcisomes of different species is their high content of phosphorus in the form of orthophosphate, pyrophosphate, and short- and long-chain polyphosphate [14]. Polyphosphate is a polymer of three to hundreds of orthophosphate monomers linked by high-energy phosphoanhydride bonds similar to those present in ATP [15].
Figure 1.
Morphological and elemental composition of acidocalcisomes. (a) Electron spectroscopic image of the monogenetic trypanosomatid Herpetomonas muscarum adhered to a formvar/carbon coated grid showing the electron-dense acidocalcisomes (white arrows). Bar = 2 μm. (b) Semi-quantitative analysis of acidocalcisomes by X-ray microanalysis indicating the presence of oxygen (O), magnesium (Mg), phosphorus (P), potassium (K), calcium (Ca), iron (Fe), and zinc (Zn). (c) Conventional transmission electron microscopy of procyclic stages of T. brucei showing the acidocalcisomes as “empty” vacuoles containing electron dense inclusions (black arrows). Bar = 1 μm. (d) Direct transmission electron microscopy of several Toxoplasma gondii tachyzoites showing electron-dense acidocalcisomes (black granules). Bar = 1 μm. (a) and (b) are from Ref. 25 with permission. © Elsevier. (c) is from Ref. 22 with permission. © American Association for Microbiology.
In protists the acidity of acidocalcisomes is maintained by a vacuolar H+-pyrophosphatase (V-H+-PPase), an enzyme also present in the vacuole of plants, and in some species acidocalcisomes have, like the plant vacuole, an additional proton pump, a vacuolar H+-ATPase (V-H+-ATPase). In multicellular organisms lacking a V-H+-PPase, the V-H+-ATPase maintains their acidity [5].
Recent reviews [5,16] have described the acidocalcisomes from prokaryotes. In this review we will limit our discussion to recent work on acidocalcisomes from eukaryotes.
Acidocalcisomes in protists
A large number of protists possess acidocalcisomes, among them trypanosomatids and Apicomplexan parasites, algae, slime molds, and fungi. We will discuss each of these organisms in the following sections.
Trypanosomatids
These have been the organisms in which acidocalcisomes were first described and that were studied in more detail. Early reports in T. brucei [1] and T. cruzi [2] described the presence of an energy-driven calcium transport mechanism (Ca2+-ATPase) in a compartment that is acidified by an ATP-dependent proton pump sensitive to bafilomycin A1 (V-H+-ATPase) and that was named the acidocalcisome. Evidence was also provided of the presence of a Na+/H+ and Ca2+/H+ exchangers in the membrane of this compartment in the procyclic stages (forms present in the insect vector) of T. brucei [17] and in promastigotes of Leishmania donovani [18], one of the agents of visceral leishmaniasis. These compartments were later [19] found to correspond to what were known as polyphosphate bodies, which are characterized by their high electron density when examined by electron microscopy (Fig. 1), and by the presence of phosphorus in the form of polyphosphate, and several cations like Ca2+, Mg2+, Na+, K+, and Zn2+, as detected by X-ray microanalysis [19,20]. These cations balance the negative charges of polyphosphate. The discovery of a V-H+-PPase in these organelles [21,22] provided an important marker for their subcellular identification and characterization in other trypanosomatids such as L. donovani [23], Phytomonas françai [24], and monogenetic trypanosomatids [25], as well as in other protists (see below). This period of morphological characterization was followed by the molecular identification of different transporters, pumps and channels present in the membrane of the organelles. Genes encoding the acidocalcisome Ca2+-ATPase (plasma membrane-type or PMCA) were reported in T. cruzi [26], and T. brucei [27]. The gene encoding the V-H+-PPase of acidocalcisomes was cloned from T. cruzi and functionally expressed in yeasts [28], and knocked down in T. brucei, showing its essential role for growth in the insect and mammalian forms of the parasite [29]. A water channel or aquaporin was found in acidocalcisomes of T. cruzi and shown to translocate to the contractile vacuole complex of these parasites in a cyclic AMP- and microtubule-dependent process during hyposmotic stress [30,31]. The identification of the nature of the abundant phosphorus content of acidocalcisomes as orthophosphate, pyrophosphate and short- and long-chain polyphosphate, was done first by 31P-NMR spectroscopy [32-34] and later by biochemical techniques [14]. The mechanism for synthesis and translocation of the polymer, was first discovered in the acidocalcisome-like organelle (vacuole) of yeasts [35,36] and later in trypanosomatids [37,38]. The mechanism involves the function of a vacuolar transporter chaperone (VTC) complex that in yeast consists of four proteins (Vtc1-4) that form heterotrimeric complexes. Two of these subunits (Vtc1 and Vtc4) were identified in trypanosomatids, where Vtc4 is the catalytic subunit. The complex is essential in both insect and mammalian stages of T. brucei [37]. A Ca2+ channel, the inositol 1,4,5-trisphosphate receptor (IP3R), was also localized to the acidocalcisomes of T. brucei and demonstrated to be essential for growth and infection [39]. Its presence suggests a role for acidocalcisomes in Ca2+ signaling.
Proteomic analyses of acidocalcisomes of trypanosomatids [40,41] identified several cation transporters, such as putative Zn2+ transporters, an orthologue to the vacuolar iron transporter (VIT) of plants and the Ca2+-sensitive cross-complementer 1 (CCC1) of the yeast vacuole, which are involved in iron and manganese sequestration, an orthologue to the phosphate-sodium symporter Pho91 of the yeast vacuole, involved in Na+ and phosphate release, and an orthologue to a polyamine transporter, suggesting roles in inorganic and organic cation uptake, and phosphorus release [41]. The presence of a V-H+-ATPase, a V-H+-PPase, Vtc1, and Vtc4, and the IP3R was also confirmed in these studies, as well as of an enzyme involved in pyrophosphate and polyphosphate metabolism, the vacuolar soluble pyrophosphatase (VSP), and an acid phosphatase [41].
More recent studies have been oriented towards the analysis of the functional role of acidocalcisomes. A role as a cation, phosphorus, and basic amino acid [42] store is evident. It was shown that in T. cruzi the polyphosphate content of the cells is significantly reduced after the lag phase of growth suggesting a requirement for phosphorus to resume growth [14]. As indicated above the presence of mechanisms for Ca2+ uptake (Ca2+-ATPase) and release (IP3R) suggest a role in Ca2+ signaling [6]. A role in autophagy in T. brucei was recently revealed [43]. Downregulation of components of the AP-3 complex, involved in acidocalcisome biogenesis, inhibits starvation-induced autophagosome formation [43]. In T. cruzi, several studies reported the role of acidocalcisomes in osmoregulation. Upon hyposmotic stress there is swelling and fusion of acidocalcisomes with the contractile vacuole complex and translocation of an aquaporin that favors contractile vacuole swelling and water expulsion [30,44]. Changes in acidocalcisome polyphosphate content accompany the changes in osmolarity [30]. Changes in acidocalcisome ions also occur in L. major submitted to hyposmotic stress [45]. The results suggest that hydrolysis of polyphosphate upon hyposmotic stress is followed by translocation of phosphorus and ions to the contractile vacuole enhancing their osmolarity and favoring water intake through the translocated aquaporin and then water elimination by the contractile vacuole to the extracellular medium [46,47]. A role in osmoregulation was also suggested by experiments that down-regulated [37] the expression of enzymes involved in the synthesis of acidocalcisome polyphosphate. On the other hand, overexpression of the acidocalcisome vacuolar soluble pyrophosphatase (VSP) resulted in decreased acidocalcisome polyphosphate levels and parasite persistence in tissues, suggesting a role for polyphosphate in infection [48]. Fig. 2 shows schemes of the pumps, channels, transporters, and enzymes known to the present in T. cruzi and T. brucei acidocalcisomes.
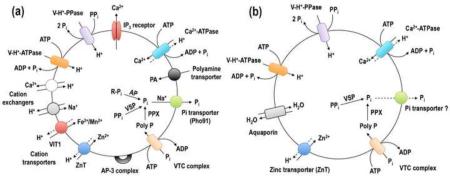
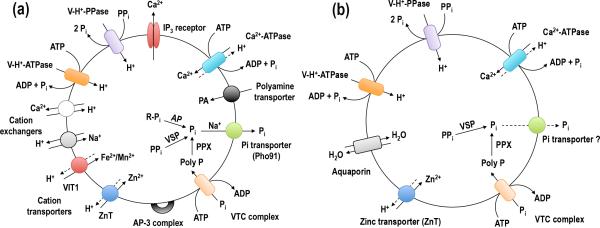
Figure 2.
Schematic representation of the acidocalcisomes of T. brucei and T. cruzi. (a) In the T. brucei acidocalcisomes, Ca2+ is taken up by a H+-countertransporting Ca2+-ATPase and released by the inositol 1,4,5,trisphosphate (IP3) receptor. H+ is pumped in electrogenically by either the V-H+-PPase or the multisubunit V-H+-ATPase. Ca2+/H+ and Na+/H+ exchangers could be used for Ca2+ release in exchange for Na+ uptake. A vacuolar iron transporter (VIT1) can be used for either Mn2+ or Fe2+ uptake and a Zn2+ transporter (ZnT) for Zn2+ uptake. There is also a polyamine (PA) transporter. A VTC with at least two subunits (Vtc1 and Vtc4) synthesizes polyphosphate using ATP and translocates it into the organelle. A Na+/Pi symporter (Pho91) releases Na+ and Pi from acidocalcisomes. Within acidocalcisomes there is a vacuolar soluble pyrophosphatase (VSP), an exopolyphosphatase (PPX) and an acid phosphatase (AP). Several adaptor protein 3 (AP-3) complex subunits also localize to the acidocalcisome. (b) In the acidocalcisomes of T. cruzi, there is also an aquaporin that allows water transport, but there are fewer channels, transporters, exchangers or enzymes identified thus far.
Apicomplexan parasites
The presence of acidocalcisomes in Toxoplasma gondii, the agent of toxoplasmosis, was reported soon after their discovery in trypanosomatids [49]. Since then several Apicomplexan parasites like Plasmodium falciparum [50], agent of malaria, Eimeria spp., agents of coccidiosis [51], and Garnia gonadati, a parasite of an Amazonian reptile [52], were found to have acidocalcisomes. A Ca2+-ATPase [53,54] and Na+/H+ and Ca+/H+ exchangers [55] were found in acidocalcisomes of the tachyzoite forms of T. gondii, together with a V-H+-PPase, although this last enzyme also localizes in other compartments, like the plant-like vacuole [56]. Two subunits of the VTC complex were found in T. gondii, Vtc2 and Vtc4, although Vtc2 does not appear to co-localize with the V-H+-PPase [57]. Both the acidocalcisome Ca2+-ATPase [54] and the V-H+-PPase [58] are essential for growth and virulence of T. gondii.
Algae
Chlamydomonas reinhardtii has typical electron-dense acidocalcisomes rich in polyphosphate and cations (Ca2+, Mg2+, Zn2+), which are acidified by both a V-H+-PPase and a V-H+-ATPase [59]. Acidocalcisomes are also in close association with the contractile vacuole complex of these organisms [59]. They were recently found to contain copper, and suggested to have a role in preventing mismetallation during zinc deficiency [60]. Copper was also found in acidocalcisome-like organelles of Euglena gracilis [61]. C. reinhardtii acidocalcisomes also possess an orthologue of Vtc1, and mutants deficient in this protein have less acidocalcisomes and polyphosphate [62]. A Vtc4 orthologue is also present in the genome of this alga. A proteomic analysis of acidocalcisomes (polyphosphate vacuoles) of the red alga Cyanidioschyzon merolae detected evidence of several pumps and transporters in common with those of acidocalcisomes of T. brucei, such as V-H+-PPase, V-H+-ATPase, Zn2+ transporter, acid phosphatase, Vtc1 and VIT, as well as of other proteins (putative metallopeptidase, prenylated Rab receptor, ABC transporter and o-methyltransferase) identified by epitope tagging and specific antibodies [63].
Slime molds and fungi
The slime mold Dictyostellium discoideun possesses mass-dense granules rich in polyphosphate and cations (Ca2+, Mg2+) with similar characteristics to the acidocalcisomes of trypanosomatids and algae [64]. Antibodies against a Ca2+-ATPase (PAT1) and a V-H+-ATPase were found to co-localize in acidocalcisomes with antibodies against the plant V-H+-PPase and with pyrophosphatase activity [64]. However, despite the detection of membrane-bound pyrophosphatase activity [65], a gene orthologue to V-H+-PPases has not been reported in the genome of the slime mold. As occurs with C. reinhardtii and T. cruzi, D. discoideum acidocalcisomes are also in close contact with the contractile vacuole complex of the organism [64]. Two polyphosphate kinases were identified in D. discoideum and their vacuolar (potentially acidocalcisome) localization was proposed [66,67].
The yeast vacuole has been considered an acidocalcisome-like organelle [36] since it possesses large amounts of polyphosphate and cations, and pumps and transporters such as V-H+-ATPase, Ca2+-ATPase (PMC1), Ca2+/H+ exchanger (CAX), VTC complex (Vtc1p-4p), transporters for Zn2+ (ZnT), Mn2+ and Fe2+ (CCC1), polyamines, basic amino acids, and phosphorus (Pho91) [68]. However, the yeast vacuole possesses hydrolytic enzymes and do not possess a V-H+-PPase, although this could be the result of their divergent evolution. Recent studies have shown that the polyphosphate metabolism in yeast is connected to that of highly phosphorylated inositol species and that mutants unable to produce inositol pyrophosphates have undetectable levels of polyphosphate, suggesting the presence of another potential pathway for their synthesis [69]. Vacuoles containing transporters similar to those in yeast acidocalcisome-like vacuoles are also found in other fungi [70,71].
Insect, echinoderm and bird eggs
Acidocalcisomes have been found in the yolk of insect [72], echinoderms [73], and bird [74] eggs. They are electron-dense and rich in short chain polyphosphate (chains of less than 100 orthophosphate residues) and cations. In chicken eggs acidocalcisomes are within larger acidic vacuoles forming compound organelles [74]. Acidification is through a V-H+-ATPase in sea urchin and chicken eggs [73,74]. Although a V-H+-PPase activity was detected in insect eggs [75] there is no genomic information of the presence of orthologues to the V-H+-PPases present in acidocalcisomes of insect species.
Mammalian cells
Several lysosome-related organelles of mammalian cells have similarities with acidocalcisomes. The best studied are the platelet dense granules [8] and the mast cell granules [9]. Human platelet dense granules are electron-dense, possess polyphosphate of about 80 orthophosphate monomers, and cations (Ca2+, K+), and are also known to contain pyrophosphate, ATP, ADP and serotonin. Their acidification is through a V-H+-ATPase [8]. Polyphosphate is also present in mast cell granules (RBL-2H3 cells) and human basophils although their size is smaller (~60 phosphate units). In mast cells polyphosphate is present in serotonin-containing granules but it does not co-localize with histamine-containing granules. These granules are also acidic, and presumably contain a V-H+-ATPase [9]. Polyphosphate release from human dense granules is associated with promotion of blood clotting, and anti-fibrinolysis [76], and has a pro-inflammatory role [77]. Interestingly, human platelets appear to depend on the inositol pyrophosphate pathway for the synthesis of polyphosphate [78].
Conclusions
The common properties of acidocalcisomes of different eukaryotes are their high electron-density, their acidic nature, and their high calcium and polyphosphate content. Acidocalcisome acidification is by a V-H+-PPase, by a V-H+-ATPase, or by both. Ca2+ uptake is, at least in protists, by a Ca2+-ATPase, while polyphosphate synthesis and translocation in several protists is by a VTC complex, and by unknown mechanisms in mammalian cells, possibly involving the inositol pyrophosphate pathway. The best-studied acidocalcisomes are those of trypanosomatids, in which several functions have been demonstrated or suggested such as their roles in autophagy, osmoregulation, calcium signaling, and phosphorus and cation storage. The discovery of the presence of polyphosphate in human platelets and mast cells and that this polymer can be released when cells are stimulated led to the discovery of the potent pro-coagulant, anti-fibrinolytic, and pro-inflammatory actions of polyphosphate, suggesting that this role could also be important for the pathogenesis of bacterial and parasitic infections since microorganisms are also rich in polyphosphate. Future studies will need to identify the enzymes involved in polyphosphate synthesis in mammalian cells, and demonstrate the role of acidocalcisomes in Ca2+ signaling. Other major challenges for the future are the determination of the structural organization of the acidocalcisome matrix and the mechanisms involved in their replication and fusion to other organelles, like the contractile vacuole complex, and its contact with the mitochondria, which could be very important for Ca2+ signaling.
Acknowledgements
Research in the authors’ laboratory is funded by the United States National Institutes of Health [grant numbers AI-077538 and AI-108222]. RD is a Barbara and Sanford Orkin/Georgia Research Alliance Eminent Scholar.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
Paper of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1••.Vercesi AE, Moreno SN, Docampo R. Ca2+/H+ exchange in acidic vacuoles of Trypanosoma brucei. Biochem J. 1994;304:227–233. doi: 10.1042/bj3040227. [This was the first report of a vacuolar compartment in trypanosomes acidified by a V-H+− ATPase and possessing a Ca2+ ATPase activity. It was named the acidocalcisome.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Docampo R, Scott DA, Vercesi AE, Moreno SN. Intracellular Ca2+ storage in acidocalcisomes of Trypanosoma cruzi. Biochem J. 1995;310:1005–1012. doi: 10.1042/bj3101005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3••.Seufferheld M, Vieira MC, Ruiz FA, Rodrigues CO, Moreno SN, Docampo R. Identification of organelles in bacteria similar to acidocalcisomes of unicellular eukaryotes. J Biol Chem. 2003;278:29971–29978. doi: 10.1074/jbc.M304548200. [This work and the following one (4••)reported the presence of acidocalcisomes in bacteria. The organelles are electron-dense, possess polyphosphate and cations, and a membrane bilayer where a V-H+-PPase localizes. They are also able to take up acidophilic dyes.] [DOI] [PubMed] [Google Scholar]
- 4••.Seufferheld M, Lea CR, Vieira M, Oldfield E, Docampo R. The H+-pyrophosphatase of Rhodospirillum rubrum is predominantly located in polyphosphate-rich acidocalcisomes. J Biol Chem. 2004;279:51193–51202. doi: 10.1074/jbc.M406099200. [See annotation to Ref. [3••].] [DOI] [PubMed] [Google Scholar]
- 5.Docampo R, Moreno SN. Acidocalcisomes. Cell Calcium. 2011;50:113–119. doi: 10.1016/j.ceca.2011.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Docampo R, Huang G. Calcium signaling in trypanosomatid parasites. Cell Calcium. 2015;57:194–202. doi: 10.1016/j.ceca.2014.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Docampo R, de Souza W, Miranda K, Rohloff P, Moreno SN. Acidocalcisomes - conserved from bacteria to man. Nat Rev. Microbiol. 2005;3:251–261. doi: 10.1038/nrmicro1097. [DOI] [PubMed] [Google Scholar]
- 8••.Ruiz FA, Lea CR, Oldfield E, Docampo R. Human platelet dense granules contain polyphosphate and are similar to acidocalcisomes of bacteria and unicellular eukaryotes. J Biol Chem. 2004;279:44250–44257. doi: 10.1074/jbc.M406261200. [This paper reported the presence of polyphosphate in human platelet dense granule and its release upon stimulation. The results suggest conservation of the organelles from bacteria to humans.] [DOI] [PubMed] [Google Scholar]
- 9.Moreno-Sanchez D, Hernandez-Ruiz L, Ruiz FA, Docampo R. Polyphosphate is a novel pro-inflammatory regulator of mast cells and is located in acidocalcisomes. J Biol Chem. 2012;287:28435–28444. doi: 10.1074/jbc.M112.385823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Besteiro S, Tonn D, Tetley L, Coombs GH, Mottram JC. The AP3 adaptor is involved in the transport of membrane proteins to acidocalcisomes of Leishmania. J Cell Sci. 2008;121:561–570. doi: 10.1242/jcs.022574. [DOI] [PubMed] [Google Scholar]
- 11.Huang G, Fang J, Sant'Anna C, Li ZH, Wellems DL, Rohloff P, Docampo R. Adaptor protein-3 (AP-3) complex mediates the biogenesis of acidocalcisomes and is essential for growth and virulence of Trypanosoma brucei. J Biol Chem. 2011;286:36619–36630. doi: 10.1074/jbc.M111.284661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Theos AC, Tenza D, Martina JA, Hurbain I, Peden AA, Sviderskaya EV, Stewart A, Robinson MS, Bennett DC, Cutler DF, et al. Functions of adaptor protein (AP)-3 and AP-1 in tyrosinase sorting from endosomes to melanosomes. Mol Biol Cell. 2005;16:5356–5372. doi: 10.1091/mbc.E05-07-0626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Patel S, Docampo R. Acidic calcium stores open for business: expanding the potential for intracellular Ca2+ signaling. Trends Cell Biol. 2010;20:277–286. doi: 10.1016/j.tcb.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ruiz FA, Rodrigues CO, Docampo R. Rapid changes in polyphosphate content within acidocalcisomes in response to cell growth, differentiation, and environmental stress in Trypanosoma cruzi. J Biol Chem. 2001;276:26114–26121. doi: 10.1074/jbc.M102402200. [DOI] [PubMed] [Google Scholar]
- 15.Kornberg A. Inorganic polyphosphate: toward making a forgotten polymer unforgettable. J Bacteriol. 1995;177:491–496. doi: 10.1128/jb.177.3.491-496.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shively JM CG, Heinhorst S, Fuerst JA, Bryant DA, Maupin-Furlow JA, Schüler D, Pfeifer F, Docampo R, Dahl C, Preiss J, Steinbüchel A, Federici BA. Elsevier Reference Modular in Biomedical Sciences (Third Edition) 404-424. Elsevier; 2014. Intracellular structures of prokaryotes: inclusions, compartments and assemblages. Reference module in biomedical sciences. [Google Scholar]
- 17.Vercesi AE, Docampo R. Sodium-proton exchange stimulates Ca2+ release from acidocalcisomes of Trypanosoma brucei. Biochem J. 1996;315:265–270. doi: 10.1042/bj3150265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vercesi AE, Rodrigues CO, Catisti R, Docampo R. Presence of a Na+/H+ exchanger in acidocalcisomes of Leishmania donovani and their alkalization by anti-leishmanial drugs. FEBS Lett. 2000;473:203–206. doi: 10.1016/s0014-5793(00)01531-3. [DOI] [PubMed] [Google Scholar]
- 19.Scott DA, Docampo R, Dvorak JA, Shi S, Leapman RD. In situ compositional analysis of acidocalcisomes in Trypanosoma cruzi. J Biol Chem. 1997;272:28020–28029. doi: 10.1074/jbc.272.44.28020. [This work identifies the electron-dense granules (polyphosphate bodies) as the acidocalcisomes.] [DOI] [PubMed] [Google Scholar]
- 20.Dvorak JA, Engel JC, Leapman RD, Swyt CR, Pella PA. Trypanosoma cruzi: elemental composition heterogeneity of cloned stocks. Mol Biochem Parasitol. 1988;31:19–26. doi: 10.1016/0166-6851(88)90141-7. [DOI] [PubMed] [Google Scholar]
- 21.Scott DA, de Souza W, Benchimol M, Zhong L, Lu HG, Moreno SN, Docampo R. Presence of a plant-like proton-pumping pyrophosphatase in acidocalcisomes of Trypanosoma cruzi. J Biol Chem. 1998;273:22151–22158. doi: 10.1074/jbc.273.34.22151. [DOI] [PubMed] [Google Scholar]
- 22.Rodrigues CO, Scott DA, Docampo R. Characterization of a vacuolar pyrophosphatase in Trypanosoma brucei and its localization to acidocalcisomes. Mol Cell Biol. 1999;19:7712–7723. doi: 10.1128/mcb.19.11.7712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rodrigues CO, Scott DA, Docampo R. Presence of a vacuolar H+-pyrophosphatase in promastigotes of Leishmania donovani and its localization to a different compartment from the vacuolar H+-ATPase. Biochem J. 1999;340:759–766. [PMC free article] [PubMed] [Google Scholar]
- 24.Miranda K, Rodrigues CO, Hentchel J, Vercesi A, Plattner H, de Souza W, Docampo R. Acidocalcisomes of Phytomonas francai possess distinct morphological characteristics and contain iron. Microsc Microanal. 2004;10:647–655. doi: 10.1017/S1431927604040887. [DOI] [PubMed] [Google Scholar]
- 25.Miranda K, Docampo R, Grillo O, de Souza W. Acidocalcisomes of trypanosomatids have species-specific elemental composition. Protist. 2004;155:395–405. doi: 10.1078/1434461042650361. [DOI] [PubMed] [Google Scholar]
- 26.Lu HG, Zhong L, de Souza W, Benchimol M, Moreno S, Docampo R. Ca2+ content and expression of an acidocalcisomal calcium pump are elevated in intracellular forms of Trypanosoma cruzi. Mol Cell Biol. 1998;18:2309–2323. doi: 10.1128/mcb.18.4.2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Luo S, Rohloff P, Cox J, Uyemura SA, Docampo R. Trypanosoma brucei plasma membrane-type Ca2+-ATPase 1 (TbPMC1) and 2 (TbPMC2) genes encode functional Ca2+-ATPases localized to the acidocalcisomes and plasma membrane, and essential for Ca2+ homeostasis and growth. J Biol Chem. 2004;279:14427–14439. doi: 10.1074/jbc.M309978200. [DOI] [PubMed] [Google Scholar]
- 28.Hill JE, Scott DA, Luo S, Docampo R. Cloning and functional expression of a gene encoding a vacuolar-type proton-translocating pyrophosphatase from Trypanosoma cruzi. Biochem J. 2000;351:281–288. doi: 10.1042/0264-6021:3510281. [First description of a vacuolar proton pyrophosphate in a species other than bacteria or plants.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lemercier G, Dutoya S, Luo S, Ruiz FA, Rodrigues CO, Baltz T, Docampo R, Bakalara N. A vacuolar-type H+-pyrophosphatase governs maintenance of functional acidocalcisomes and growth of the insect and mammalian forms of Trypanosoma brucei. J Biol Chem. 2002;277:37369–37376. doi: 10.1074/jbc.M204744200. [DOI] [PubMed] [Google Scholar]
- 30.Rohloff P, Montalvetti A, Docampo R. Acidocalcisomes and the contractile vacuole complex are involved in osmoregulation in Trypanosoma cruzi. J Biol Chem. 2004;279:52270–52281. doi: 10.1074/jbc.M410372200. [DOI] [PubMed] [Google Scholar]
- 31.Montalvetti A, Rohloff P, Docampo R. A functional aquaporin co-localizes with the vacuolar proton pyrophosphatase to acidocalcisomes and the contractile vacuole complex of Trypanosoma cruzi. J Biol Chem. 2004;279:38673–38682. doi: 10.1074/jbc.M406304200. [DOI] [PubMed] [Google Scholar]
- 32.Urbina JA, Moreno B, Vierkotter S, Oldfield E, Payares G, Sanoja C, Bailey BN, Yan W, Scott DA, Moreno SN, et al. Trypanosoma cruzi contains major pyrophosphate stores, and its growth in vitro and in vivo is blocked by pyrophosphate analogs. J Biol Chem. 1999;274:33609–33615. doi: 10.1074/jbc.274.47.33609. [DOI] [PubMed] [Google Scholar]
- 33.Moreno B, Urbina JA, Oldfield E, Bailey BN, Rodrigues CO, Docampo R. 31P NMR spectroscopy of Trypanosoma brucei, Trypanosoma cruzi, and Leishmania major. Evidence for high levels of condensed inorganic phosphates. J Biol Chem. 2000;275:28356–28362. doi: 10.1074/jbc.M003893200. [DOI] [PubMed] [Google Scholar]
- 34.Moreno B, Rodrigues CO, Bailey BN, Urbina JA, Moreno SN, Docampo R, Oldfield E. Magic-angle spinning 31P NMR spectroscopy of condensed phosphates in parasitic protozoa: visualizing the invisible. FEBS Lett. 2002;523:207–212. doi: 10.1016/s0014-5793(02)02977-0. [DOI] [PubMed] [Google Scholar]
- 35••.Hothorn M, Neumann H, Lenherr ED, Wehner M, Rybin V, Hassa PO, Uttenweiler A, Reinhardt M, Schmidt A, Seiler J, et al. Catalytic core of a membrane-associated eukaryotic polyphosphate polymerase. Science. 2009;324:513–516. doi: 10.1126/science.1168120. [This study identified the VTC complex as a polyphosphate polymerase in yeast. The first enzyme described in eukaryotes that is involved in the synthesis of polyphosphate.] [DOI] [PubMed] [Google Scholar]
- 36.Gerasimaite R, Sharma S, Desfougeres Y, Schmidt A, Mayer A. Coupled synthesis and translocation restrains polyphosphate to acidocalcisome-like vacuoles and prevents its toxicity. J Cell Sci. 2014;127:5093–5104. doi: 10.1242/jcs.159772. [DOI] [PubMed] [Google Scholar]
- 37.Lander N, Ulrich PN, Docampo R. Trypanosoma brucei vacuolar transporter chaperone 4 (TbVtc4) is an acidocalcisome polyphosphate kinase required for in vivo infection. J Biol Chem. 2013;288:34205–34216. doi: 10.1074/jbc.M113.518993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ulrich PN, Lander N, Kurup SP, Reiss L, Brewer J, Soares Medeiros LC, Miranda K, Docampo R. The acidocalcisome vacuolar transporter chaperone 4 catalyzes the synthesis of polyphosphate in insect-stages of Trypanosoma brucei and T. cruzi. J Eukaryot Microbiol. 2014;61:155–165. doi: 10.1111/jeu.12093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huang G, Bartlett PJ, Thomas AP, Moreno SN, Docampo R. Acidocalcisomes of Trypanosoma brucei have an inositol 1,4,5-trisphosphate receptor that is required for growth and infectivity. Proc Natl Acad Sci U S A. 2013;110:1887–1892. doi: 10.1073/pnas.1216955110. [An inositol 1,4,5-trisphosphate receptor is found to localize to the acidocalcisomes of T. brucei.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ferella M, Nilsson D, Darban H, Rodrigues C, Bontempi EJ, Docampo R, Andersson B. Proteomics in Trypanosoma cruzi--localization of novel proteins to various organelles. Proteomics. 2008;8:2735–2749. doi: 10.1002/pmic.200700940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huang G, Ulrich PN, Storey M, Johnson D, Tischer J, Tovar JA, Moreno SN, Orlando R, Docampo R. Proteomic analysis of the acidocalcisome, an organelle conserved from bacteria to human cells. PLoS Pathog. 2014;10:e1004555. doi: 10.1371/journal.ppat.1004555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rohloff P, Rodrigues CO, Docampo R. Regulatory volume decrease in Trypanosoma cruzi involves amino acid efflux and changes in intracellular calcium. Mol Biochem Parasitol. 2003;126:219–230. doi: 10.1016/s0166-6851(02)00277-3. [DOI] [PubMed] [Google Scholar]
- 43.Li FJ, He CY. Acidocalcisome is required for autophagy in Trypanosoma brucei. Autophagy. 2014;10:1978–1988. doi: 10.4161/auto.36183. [The study identifies a role for acidocalcisomes in starvation-induced authophagy.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Niyogi S, Jimenez V, Girard-Dias W, de Souza W, Miranda K, Docampo R. Rab32 is essential for maintaining functional acidocalcisomes, and for growth and infectivity of Trypanosoma cruzi. J Cell Sci. 2015;128:2363–2373. doi: 10.1242/jcs.169466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.LeFurgey A, Ingram P, Blum JJ. Elemental composition of polyphosphate-containing vacuoles and cytoplasm of Leishmania major. Mol Biochem Parasitol. 1990;40:77–86. doi: 10.1016/0166-6851(90)90081-v. [DOI] [PubMed] [Google Scholar]
- 46.Docampo R, Jimenez V, Lander N, Li ZH, Niyogi S. New insights into roles of acidocalcisomes and contractile vacuole complex in osmoregulation in protists. Int Rev Cell Mol Biol. 2013;305:69–113. doi: 10.1016/B978-0-12-407695-2.00002-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Docampo R, Jimenez V, King-Keller S, Li ZH, Moreno SN. The role of acidocalcisomes in the stress response of Trypanosoma cruzi. Adv Parasitol. 2011;75:307–324. doi: 10.1016/B978-0-12-385863-4.00014-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Galizzi M, Bustamante JM, Fang J, Miranda K, Soares Medeiros LC, Tarleton RL, Docampo R. Evidence for the role of vacuolar soluble pyrophosphatase and inorganic polyphosphate in Trypanosoma cruzi persistence. Mol Microbiol. 2013;90:699–715. doi: 10.1111/mmi.12392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moreno SN, Zhong L. Acidocalcisomes in Toxoplasma gondii tachyzoites. Biochem J. 1996;313:655–659. doi: 10.1042/bj3130655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ruiz FA, Luo S, Moreno SN, Docampo R. Polyphosphate content and fine structure of acidocalcisomes of Plasmodium falciparum. Microsc Microanal. 2004;10:563–567. doi: 10.1017/S1431927604040875. [DOI] [PubMed] [Google Scholar]
- 51.Soares Medeiros LC, Gomes F, Maciel LR, Seabra SH, Docampo R, Moreno S, Plattner H, Hentschel J, Kawazoe U, Barrabin H, et al. Volutin granules of Eimeria parasites are acidic compartments and have physiological and structural characteristics similar to acidocalcisomes. J Eukaryot Microbiol. 2011;58:416–423. doi: 10.1111/j.1550-7408.2011.00565.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Diniz JA, Silva EO, Lainson R, de Souza W. The fine structure of Garnia gonadati and its association with the host cell. Parasitol Res. 2000;86:971–977. doi: 10.1007/pl00008528. [DOI] [PubMed] [Google Scholar]
- 53.Luo S, Ruiz FA, Moreno SN. The acidocalcisome Ca2+-ATPase (TgA1) of Toxoplasma gondii is required for polyphosphate storage, intracellular calcium homeostasis and virulence. Mol Microbiol. 2005;55:1034–1045. doi: 10.1111/j.1365-2958.2004.04464.x. [DOI] [PubMed] [Google Scholar]
- 54.Luo S, Vieira M, Graves J, Zhong L, Moreno SN. A plasma membrane-type Ca2+-ATPase co-localizes with a vacuolar H+-pyrophosphatase to acidocalcisomes of Toxoplasma gondii. EMBO J. 2001;20:55–64. doi: 10.1093/emboj/20.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rohloff P, Miranda K, Rodrigues JC, Fang J, Galizzi M, Plattner H, Hentschel J, Moreno SN. Calcium uptake and proton transport by acidocalcisomes of Toxoplasma gondii. PLoS One. 2011;6:e18390. doi: 10.1371/journal.pone.0018390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Miranda K, Pace DA, Cintron R, Rodrigues JC, Fang J, Smith A, Rohloff P, Coelho E, de Haas F, de Souza W, et al. Characterization of a novel organelle in Toxoplasma gondii with similar composition and function to the plant vacuole. Mol Microbiol. 2010;76:1358–1375. doi: 10.1111/j.1365-2958.2010.07165.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rooney PJ, Ayong L, Tobin CM, Moreno SN, Knoll LJ. TgVTC2 is involved in polyphosphate accumulation in Toxoplasma gondii. Mol Biochem Parasitol. 2011;176:121–126. doi: 10.1016/j.molbiopara.2010.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu J, Pace D, Dou Z, King TP, Guidot D, Li ZH, Carruthers VB, Moreno SN. A vacuolar-H+− pyrophosphatase (TgVP1) is required for microneme secretion, host cell invasion, and extracellular survival of Toxoplasma gondii. Mol Microbiol. 2014;93:698–712. doi: 10.1111/mmi.12685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ruiz FA, Marchesini N, Seufferheld M, Govindjee, Docampo R. The polyphosphate bodies of Chlamydomonas reinhardtii possess a proton-pumping pyrophosphatase and are similar to acidocalcisomes. J Biol Chem. 2001;276:46196–46203. doi: 10.1074/jbc.M105268200. [DOI] [PubMed] [Google Scholar]
- 60.Hong-Hermesdorf A, Miethke M, Gallaher SD, Kropat J, Dodani SC, Chan J, Barupala D, Domaille DW, Shirasaki DI, Loo JA, et al. Subcellular metal imaging identifies dynamic sites of Cu accumulation in Chlamydomonas. Nat Chem Biol. 2014;10:1034–1042. doi: 10.1038/nchembio.1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Einicker-Lamas M, Mezian GA, Fernandes TB, Silva FL, Guerra F, Miranda K, Attias M, Oliveira MM. Euglena gracilis as a model for the study of Cu2+ and Zn2+ toxicity and accumulation in eukaryotic cells. Environ Pollut. 2002;120:779–786. doi: 10.1016/s0269-7491(02)00170-7. [DOI] [PubMed] [Google Scholar]
- 62.Aksoy M, Pootakham W, Grossman AR. Critical function of a Chlamydomonas reinhardtii putative polyphosphate polymerase subunit during nutrient deprivation. Plant Cell. 2014;26:4214–4229. doi: 10.1105/tpc.114.129270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yagisawa F, Nishida K, Yoshida M, Ohnuma M, Shimada T, Fujiwara T, Yoshida Y, Misumi O, Kuroiwa H, Kuroiwa T. Identification of novel proteins in isolated polyphosphate vacuoles in the primitive red alga Cyanidioschyzon merolae. Plant J. 2009;60:882–893. doi: 10.1111/j.1365-313X.2009.04008.x. [DOI] [PubMed] [Google Scholar]
- 64.Marchesini N, Ruiz FA, Vieira M, Docampo R. Acidocalcisomes are functionally linked to the contractile vacuole of Dictyostelium discoideum. J Biol Chem. 2002;277:8146–8153. doi: 10.1074/jbc.M111130200. [DOI] [PubMed] [Google Scholar]
- 65.MacDonald JI, Weeks G. Evidence for a membrane-bound pyrophosphatase in Dictyostelium discoideum. FEBS Lett. 1988;238:9–12. doi: 10.1016/0014-5793(88)80214-x. [DOI] [PubMed] [Google Scholar]
- 66.Zhang H, Gomez-Garcia MR, Shi X, Rao NN, Kornberg A. Polyphosphate kinase 1, a conserved bacterial enzyme, in a eukaryote, Dictyostelium discoideum, with a role in cytokinesis. Proc Natl Acad Sci U S A. 2007;104:16486–16491. doi: 10.1073/pnas.0706847104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gomez-Garcia MR, Kornberg A. Formation of an actin-like filament concurrent with the enzymatic synthesis of inorganic polyphosphate. Proc Natl Acad Sci U S A. 2004;101:15876–15880. doi: 10.1073/pnas.0406923101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Docampo R. The origin and evolution of the acidocalcisome and its interactions with other organelles. Mol Biochem Parasitol. 2015 doi: 10.1016/j.molbiopara.2015.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lonetti A, Szijgyarto Z, Bosch D, Loss O, Azevedo C, Saiardi A. Identification of an evolutionarily conserved family of inorganic polyphosphate endopolyphosphatases. J Biol Chem. 2011;286:31966–31974. doi: 10.1074/jbc.M111.266320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bowman BJ, Draskovic M, Freitag M, Bowman EJ. Structure and distribution of organelles and cellular location of calcium transporters in Neurospora crassa. Eukaryot Cell. 2009;8:1845–1855. doi: 10.1128/EC.00174-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Milani G, Schereiber AZ, Vercesi AE. Ca2+ transport into an intracellular acidic compartment of Candida parapsilosis. FEBS Lett. 2001;500:80–84. doi: 10.1016/s0014-5793(01)02585-6. [DOI] [PubMed] [Google Scholar]
- 72.Ramos I, Gomes F, Koeller CM, Saito K, Heise N, Masuda H, Docampo R, de Souza W, Machado EA, Miranda K. Acidocalcisomes as calcium- and polyphosphate-storage compartments during embryogenesis of the insect Rhodnius prolixus Stahl. PLoS One. 2011;6:e27276. doi: 10.1371/journal.pone.0027276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ramos IB, Miranda K, Pace DA, Verbist KC, Lin FY, Zhang Y, Oldfield E, Machado EA, De Souza W, Docampo R. Calcium- and polyphosphate-containing acidic granules of sea urchin eggs are similar to acidocalcisomes, but are not the targets for NAADP. Biochem J. 2010;429:485–495. doi: 10.1042/BJ20091956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ramos IB, Miranda K, Ulrich P, Ingram P, LeFurgey A, Machado EA, de Souza W, Docampo R. Calcium- and polyphosphate-containing acidocalcisomes in chicken egg yolk. Biol Cell. 2010;102:421–434. doi: 10.1042/BC20100011. [DOI] [PubMed] [Google Scholar]
- 75.Motta LS, Ramos IB, Gomes FM, de Souza W, Champagne DE, Santiago MF, Docampo R, Miranda K, Machado EA. Proton-pyrophosphatase and polyphosphate in acidocalcisome-like vesicles from oocytes and eggs of Periplaneta americana. Insect Biochem Mol Biol. 2009;39:198–206. doi: 10.1016/j.ibmb.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 76••.Smith SA, Mutch NJ, Baskar D, Rohloff P, Docampo R, Morrissey JH. Polyphosphate modulates blood coagulation and fibrinolysis. Proc Natl Acad Sci U S A. 2006;103:903–908. doi: 10.1073/pnas.0507195103. [The study describes the potent pro-coagulant and anti-fibrinolytic action of polyphosphate.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Muller F, Mutch NJ, Schenk WA, Smith SA, Esterl L, Spronk HM, Schmidbauer S, Gahl WA, Morrissey JH, Renne T. Platelet polyphosphates are proinflammatory and procoagulant mediators in vivo. Cell. 2009;139:1143–1156. doi: 10.1016/j.cell.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ghosh S, Shukla D, Suman K, Lakshmi BJ, Manorama R, Kumar S, Bhandari R. Inositol hexakisphosphate kinase 1 maintains hemostasis in mice by regulating platelet polyphosphate levels. Blood. 2013;122:1478–1486. doi: 10.1182/blood-2013-01-481549. [DOI] [PubMed] [Google Scholar]