Abstract
Bloom syndrome (BSx) is a rare autosomal-recessive chromosome-instability disorder manifested by a constellation of clinical features including a significant predisposition to early onset of neoplasia. BSx cells display cytogenetic abnormalities, the pathognomonic feature being an increased rate of spontaneous sister chromatid exchanges (SCEs), 10- to 15-fold more frequent than SCEs seen in control cells. Identification of the primary biochemical defect in BSx and its relationship to SCE frequency and neoplasia have been complicated by reports that BSx cell lines exhibit defects in the structure and/or activity of a number of different enzymes. The rare occurrence of the disorder and lack of informative families have precluded mapping of the primary defect by standard linkage analysis. We have utilized BSx cells as recipients for microcell-mediated chromosome transfer to map a locus that renders complementation of the elevated SCE phenotype. Studies with the BSx cell line GM08505 demonstrated a stable frequency of SCEs 10-fold higher than control values, offering a phenotype suitable for complementation studies. Transfer of different independent human chromosomes from somatic cell hybrids into BSx cells permitted identification of a single chromosome that dramatically reduced the SCE frequency to a level near that seen in control cells. Detailed characterization revealed this complementing element to be human chromosome 15.
Full text
PDF

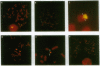
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bacchetti S., Graham F. L. Transfer of the gene for thymidine kinase to thymidine kinase-deficient human cells by purified herpes simplex viral DNA. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1590–1594. doi: 10.1073/pnas.74.4.1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom D. The syndrome of congenital telangiectatic erythema and stunted growth. J Pediatr. 1966 Jan;68(1):103–113. doi: 10.1016/s0022-3476(66)80426-2. [DOI] [PubMed] [Google Scholar]
- Chaganti R. S., Schonberg S., German J. A manyfold increase in sister chromatid exchanges in Bloom's syndrome lymphocytes. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4508–4512. doi: 10.1073/pnas.71.11.4508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan J. Y., Becker F. F., German J., Ray J. H. Altered DNA ligase I activity in Bloom's syndrome cells. Nature. 1987 Jan 22;325(6102):357–359. doi: 10.1038/325357a0. [DOI] [PubMed] [Google Scholar]
- Flejter W. L., McDaniel L. D., Johns D., Friedberg E. C., Schultz R. A. Correction of xeroderma pigmentosum complementation group D mutant cell phenotypes by chromosome and gene transfer: involvement of the human ERCC2 DNA repair gene. Proc Natl Acad Sci U S A. 1992 Jan 1;89(1):261–265. doi: 10.1073/pnas.89.1.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- German J., Bloom D., Passarge E. Bloom's syndrome. V. Surveillance for cancer in affected families. Clin Genet. 1977 Sep;12(3):162–168. doi: 10.1111/j.1399-0004.1977.tb00919.x. [DOI] [PubMed] [Google Scholar]
- German J., Passarge E. Bloom's syndrome. XII. Report from the Registry for 1987. Clin Genet. 1989 Jan;35(1):57–69. doi: 10.1111/j.1399-0004.1989.tb02905.x. [DOI] [PubMed] [Google Scholar]
- Goto K., Maeda S., Kano Y., Sugiyama T. Factors involved in differential Giemsa-staining of sister chromatids. Chromosoma. 1978 May 16;66(4):351–359. doi: 10.1007/BF00328535. [DOI] [PubMed] [Google Scholar]
- Heartlein M. W., Tsuji H., Latt S. A. 5-Bromodeoxyuridine-dependent increase in sister chromatid exchange formation in Bloom's syndrome is associated with reduction in topoisomerase II activity. Exp Cell Res. 1987 Mar;169(1):245–254. doi: 10.1016/0014-4827(87)90242-4. [DOI] [PubMed] [Google Scholar]
- Henning K. A., Schultz R. A., Sekhon G. S., Friedberg E. C. Gene complementing xeroderma pigmentosum group A cells maps to distal human chromosome 9q. Somat Cell Mol Genet. 1990 Jul;16(4):395–400. doi: 10.1007/BF01232467. [DOI] [PubMed] [Google Scholar]
- Kuhn E. M., Therman E. Cytogenetics of Bloom's syndrome. Cancer Genet Cytogenet. 1986 May;22(1):1–18. doi: 10.1016/0165-4608(86)90132-9. [DOI] [PubMed] [Google Scholar]
- Kyoizumi S., Nakamura N., Takebe H., Tatsumi K., German J., Akiyama M. Frequency of variant erythrocytes at the glycophorin-A locus in two Bloom's syndrome patients. Mutat Res. 1989 Oct;214(2):215–222. doi: 10.1016/0027-5107(89)90166-8. [DOI] [PubMed] [Google Scholar]
- Lambert C., Schultz R. A., Smith M., Wagner-McPherson C., McDaniel L. D., Donlon T., Stanbridge E. J., Friedberg E. C. Functional complementation of ataxia-telangiectasia group D (AT-D) cells by microcell-mediated chromosome transfer and mapping of the AT-D locus to the region 11q22-23. Proc Natl Acad Sci U S A. 1991 Jul 1;88(13):5907–5911. doi: 10.1073/pnas.88.13.5907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langlois R. G., Bigbee W. L., Jensen R. H., German J. Evidence for increased in vivo mutation and somatic recombination in Bloom's syndrome. Proc Natl Acad Sci U S A. 1989 Jan;86(2):670–674. doi: 10.1073/pnas.86.2.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledbetter S. A., Garcia-Heras J., Ledbetter D. H. "PCR-karyotype" of human chromosomes in somatic cell hybrids. Genomics. 1990 Dec;8(4):614–622. doi: 10.1016/0888-7543(90)90247-r. [DOI] [PubMed] [Google Scholar]
- Ledbetter S. A., Nelson D. L., Warren S. T., Ledbetter D. H. Rapid isolation of DNA probes within specific chromosome regions by interspersed repetitive sequence polymerase chain reaction. Genomics. 1990 Mar;6(3):475–481. doi: 10.1016/0888-7543(90)90477-c. [DOI] [PubMed] [Google Scholar]
- Lichter P., Cremer T., Borden J., Manuelidis L., Ward D. C. Delineation of individual human chromosomes in metaphase and interphase cells by in situ suppression hybridization using recombinant DNA libraries. Hum Genet. 1988 Nov;80(3):224–234. doi: 10.1007/BF01790090. [DOI] [PubMed] [Google Scholar]
- Lichter P., Ledbetter S. A., Ledbetter D. H., Ward D. C. Fluorescence in situ hybridization with Alu and L1 polymerase chain reaction probes for rapid characterization of human chromosomes in hybrid cell lines. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6634–6638. doi: 10.1073/pnas.87.17.6634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maas R. L., Jepeal L. I., Elfering S. L., Holcombe R. F., Morton C. C., Eddy R. L., Byers M. G., Shows T. B., Leder P. A human gene homologous to the formin gene residing at the murine limb deformity locus: chromosomal location and RFLPs. Am J Hum Genet. 1991 Apr;48(4):687–695. [PMC free article] [PubMed] [Google Scholar]
- Matsubara Y., Ito M., Glassberg R., Satyabhama S., Ikeda Y., Tanaka K. Nucleotide sequence of messenger RNA encoding human isovaleryl-coenzyme A dehydrogenase and its expression in isovaleric acidemia fibroblasts. J Clin Invest. 1990 Apr;85(4):1058–1064. doi: 10.1172/JCI114536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mezzina M., Nardelli J., Nocentini S., Remault G., Sarasin A. DNA ligase activity in human cell lines from normal donors and Bloom's syndrome patients. Nucleic Acids Res. 1989 Apr 25;17(8):3091–3106. doi: 10.1093/nar/17.8.3091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicotera T. M., Notaro J., Notaro S., Schumer J., Sandberg A. A. Elevated superoxide dismutase in Bloom's syndrome: a genetic condition of oxidative stress. Cancer Res. 1989 Oct 1;49(19):5239–5243. [PubMed] [Google Scholar]
- Petrini J. H., Huwiler K. G., Weaver D. T. A wild-type DNA ligase I gene is expressed in Bloom's syndrome cells. Proc Natl Acad Sci U S A. 1991 Sep 1;88(17):7615–7619. doi: 10.1073/pnas.88.17.7615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxon P. J., Schultz R. A., Stanbridge E. J., Friedberg E. C. Human chromosome 15 confers partial complementation of phenotypes to xeroderma pigmentosum group F cells. Am J Hum Genet. 1989 Apr;44(4):474–485. [PMC free article] [PubMed] [Google Scholar]
- Schultz R. A., Saxon P. J., Glover T. W., Friedberg E. C. Microcell-mediated transfer of a single human chromosome complements xeroderma pigmentosum group A fibroblasts. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4176–4179. doi: 10.1073/pnas.84.12.4176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seal G., Brech K., Karp S. J., Cool B. L., Sirover M. A. Immunological lesions in human uracil DNA glycosylase: association with Bloom syndrome. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2339–2343. doi: 10.1073/pnas.85.7.2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiraishi Y., Taguchi T., Ozawa M., Bamezai R. Different mutations responsible for the elevated sister-chromatid exchange frequencies in Bloom syndrome and X-irradiated B-lymphoblastoid cell lines originating from acute leukemia. Mutat Res. 1989 Apr;211(2):273–278. doi: 10.1016/0027-5107(89)90010-9. [DOI] [PubMed] [Google Scholar]
- Tanaka K., Miura N., Satokata I., Miyamoto I., Yoshida M. C., Satoh Y., Kondo S., Yasui A., Okayama H., Okada Y. Analysis of a human DNA excision repair gene involved in group A xeroderma pigmentosum and containing a zinc-finger domain. Nature. 1990 Nov 1;348(6296):73–76. doi: 10.1038/348073a0. [DOI] [PubMed] [Google Scholar]
- Tasset D. M., Hartz J. A., Kao F. T. Isolation and analysis of DNA markers specific to human chromosome 15. Am J Hum Genet. 1988 Jun;42(6):854–866. [PMC free article] [PubMed] [Google Scholar]
- Trent J. M., Stanbridge E. J., McBride H. L., Meese E. U., Casey G., Araujo D. E., Witkowski C. M., Nagle R. B. Tumorigenicity in human melanoma cell lines controlled by introduction of human chromosome 6. Science. 1990 Feb 2;247(4942):568–571. doi: 10.1126/science.2300817. [DOI] [PubMed] [Google Scholar]
- Vilpo J. A., Vilpo L. M. Normal uracil-DNA glycosylase activity in Bloom's syndrome cells. Mutat Res. 1989 Jan;210(1):59–62. doi: 10.1016/0027-5107(89)90044-4. [DOI] [PubMed] [Google Scholar]
- Warren S. T., Schultz R. A., Chang C. C., Wade M. H., Trosko J. E. Elevated spontaneous mutation rate in Bloom syndrome fibroblasts. Proc Natl Acad Sci U S A. 1981 May;78(5):3133–3137. doi: 10.1073/pnas.78.5.3133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman B. E., Saxon P. J., Pasquale S. R., Jones G. R., Geiser A. G., Stanbridge E. J. Introduction of a normal human chromosome 11 into a Wilms' tumor cell line controls its tumorigenic expression. Science. 1987 Apr 10;236(4798):175–180. doi: 10.1126/science.3031816. [DOI] [PubMed] [Google Scholar]
- Weksberg R., Smith C., Anson-Cartwright L., Maloney K. Bloom syndrome: a single complementation group defines patients of diverse ethnic origin. Am J Hum Genet. 1988 Jun;42(6):816–824. [PMC free article] [PubMed] [Google Scholar]
- Willis A. E., Lindahl T. DNA ligase I deficiency in Bloom's syndrome. Nature. 1987 Jan 22;325(6102):355–357. doi: 10.1038/325355a0. [DOI] [PubMed] [Google Scholar]
- Willis A. E., Weksberg R., Tomlinson S., Lindahl T. Structural alterations of DNA ligase I in Bloom syndrome. Proc Natl Acad Sci U S A. 1987 Nov;84(22):8016–8020. doi: 10.1073/pnas.84.22.8016. [DOI] [PMC free article] [PubMed] [Google Scholar]