Abstract
Ageing decreases exercise performance and is frequently accompanied by reductions in cognitive performance. Deterioration in the physiological capacity to stand, locomote and exercise can manifest itself as falling over and represents a significant deterioration in sensorimotor control. In the elderly, falling leads to serious morbidity and mortality with major societal costs. Measurement of a suite of physiological capacities that are required for successful motor performance (including vision, muscle strength, proprioception and balance) has been used to produce a physiological profile assessment (PPA) which has been tracked over the age spectrum and in different diseases (e.g. multiple sclerosis, Parkinson's disease). As well as measures of specific physiological capacities, the PPA generates an overall ‘score’ which quantitatively measures an individual's cumulative risk of falling. The present review collates data from the PPA (and the physiological capacities it measures) as well as its use in strategies to reduce falls in the elderly and those with different diseases. We emphasise that (i) motor impairment arises via reductions in a wide range of sensorimotor abilities; (ii) the PPA approach not only gives a snapshot of the physiological capacity of an individual, but it also gives insight into the deficits among groups of individuals with particular diseases; and (iii) deficits in seemingly restricted and disparate physiological domains (e.g. vision, strength, cognition) are funnelled into impairments in tasks requiring upright balance. Motor impairments become more prevalent with ageing but careful physiological measurement and appropriate interventions offer a way to maximise health across the lifespan.
Abbreviations
- AMD
age‐related macular degeneration
- MS
multiple sclerosis
- PD
Parkinson's disease
- PPA
physiological profile assessment
Background
There is a growing health and economic burden associated with ageing combined with the high prevalence of diseases which impair the ability to perform essential tasks for daily living. The link between physiological performance and ageing is complex as it is affected so prominently by exercise and inactivity (Pollock et al. 2015). This provides an impetus for strategies in ageing to measure and improve function and to delay its deterioration (Seals et al. 2015). A range of diseases and age‐related health conditions cause physical disability that impose limitations upon daily activities and threaten autonomy (Barbotte et al. 2001). For example, Parkinson's disease is associated with motor slowing, poliomyelitis is associated with reduced muscle strength, age‐related macular degeneration is associated with vision loss, and diseases like dementia and depression are associated with reduced cognitive processing. Motor impairment is often the final common pathway that causes physical disability in a wide range of these diseases. Figure 1 shows a conceptual model for the control of movement which incorporates the central nervous system, muscular apparatus and the actual motor outcomes. It emphasises that afferent feedback arises continuously from muscles and the motor actions themselves which must be incorporated with feedback from centrally generated motor signals. Deficits at multiple levels within this schema reduce the likelihood that movements related to standing and locomotion are performed successfully.
Figure 1. A simple model underlying production of movements and maintenance of postures .
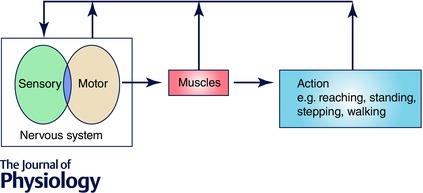
The two horizontal arrows from left to right show the integrated output from the central nervous system which drives muscle contractions and then functional actions. The vertical arrows emphasise the feedback resulting from the actions themselves, from the muscles, as well as sensorimotor signals generated at supraspinal levels.
Our research has used falls as a paradigm reflecting the risk that a fundamental motor output, namely reaching, standing, stepping and walking, is dysfunctional. Hence, falls can be viewed within the framework of what constitutes normal physiological performance. Falls and fall‐related injuries are leading causes of disease burden among people aged 65 years and over. Over one‐third of community‐dwelling older adults fall each year with about 15% of falls being injurious (O'Loughlin et al. 1993). Any fall, particularly in older people, is a significant threat to health, and when measured in individuals or in populations, the frequency or incidence of falling provides a robust marker of impaired physiological function. Therefore, not surprisingly, there has been much research on key risk factors for falls. The traditional approach is to identify medical conditions which predispose to falls (Tinetti & Kumar, 2010). Several studies have identified many medical conditions which contribute to falls risk, including chronic and degenerative diseases such as stroke (Tinetti et al. 1988; Campbell et al. 1989; Nevitt et al. 1989; Forster & Young, 1995; Jørgensen et al. 2002), Parkinson's disease (Ashburn et al. 2001; Schrag et al. 2002; Wood et al. 2002), arthritis (Sturnieks et al. 2004), foot problems (Menz & Lord, 2001), cognitive impairment (Tinetti et al. 1988; van Dijk et al. 1993; Shaw et al. 2003), diabetes (Richardson et al. 1992; Schwartz et al. 2000), cataracts (Ivers et al. 1998) and vestibular disorders (Herdman et al. 2000; Whitney et al. 2000). However, attribution of a degree of fall risk to a medical diagnosis is problematic because the severity of such conditions varies across individuals and comorbidity is common in older age. Furthermore, sensory and motor impairments associated with increased age, inactivity, medication use, or minor pathology are highly prevalent in older people without documented medical conditions.
We have proposed an ‘impairment profiling’ rather than a ‘disease‐based/medical’ approach to address this issue (Lord et al. 2003), placed within the context of the simple model that underlies the production of movements and maintenance of postures (Fig. 1). Termed the physiological profile assessment (PPA), it involves quantitative assessment of sensorimotor abilities critical for the control of balance. Function in each of these systems declines with age (Lord & Ward, 1994) and impairments in each system increase the risk of falling (Lord et al. 1991, 1992). Further, the sensorimotor impairment due to medical conditions, whether diagnosed or not, will usually manifest in one or more of the physiological tests. A marked deficit in any one system may be sufficient to predispose an older person to fall, but a combination of mild or moderate impairments across physiological systems is also likely to increase the risk of falling. Measurement of an individual's sensorimotor abilities identifies impairments in one or more physiological systems and determines cumulative fall risk.
The present review covers development of the profiling approach and then summarises the changes in physiological performance in a number of diseases including people with multiple sclerosis, stroke, cognitive impairment, depressed mood, macular degeneration, lower limb osteoarthritis and prior polio. The main aim is to assess and compare the physiological profiles across a number of seemingly ‘single’ diseases or disabilities. The value of this approach for assessing fall risk, tailoring a fall prevention programme and evaluating the effectiveness of interventions is also considered.
Components of the physiological profile assessment (PPA)
The physiological profile assessment (PPA) has two versions: a short and a comprehensive version, both of which comprise quantitative tests of vision, lower limb sensation, muscle strength, reaction time and balance, which are described in detail elsewhere (Lord et al. 2003). In order for the PPA to be practical in clinical settings, the tests are low‐tech, quick and easy to administer, and feasible for people with various levels of disability to undertake (i.e. have minimal floor or ceiling effects). Each measure has proven external validity with respect to falls and acceptable test–retest reliability.
For the comprehensive version, vision is assessed with tests of low and high contrast visual acuity, visual contrast sensitivity and depth perception (assessed using logMAR charts, the Melbourne Edge Test and a Howard–Dolman device, respectively). Lower limb sensation is assessed with a test of tactile sensitivity using a von Frey filament, and a test of proprioception measured using a lower limb‐matching task, with errors in degrees recorded using a protractor inscribed on a vertical clear acrylic sheet placed between the legs. Maximal voluntary knee extension, knee flexion and ankle dorsiflexion strength are measured isometrically in the dominant leg with participants seated. Simple reaction time is measured using a light as a stimulus and both a finger press and a foot press as the response. Balance is assessed with tests of postural sway (path length, measured using a sway meter recording displacements of the body at the pelvis with participants standing on the floor and a foam mat with eyes open and closed), and controlled leaning balance (maximal balance and coordinated stability tests) which assess the participant's ability to adjust body position in a steady and coordinated way when at or near the limits of their base of support.
The short version of the PPA includes the five non‐redundant items identified from discriminant function analyses (visual contrast sensitivity, lower limb proprioception, knee extension strength, finger press reaction time and sway on foam with eyes open) as significant independent discriminators between fallers and non‐fallers as identified in prospective studies in community‐dwelling older people (Lord et al. 1991, 1992, 2003; Lord & Dayhew, 2001). The two versions provide the same fall risk score based on discriminant function analysis (Lord et al. 2003). The five items from the short form and the leaning balance tasks are depicted in Fig. 2.
Figure 2. Depiction of the various tests of physiological functions used in the Physiological Profile Assessment (PPA) .
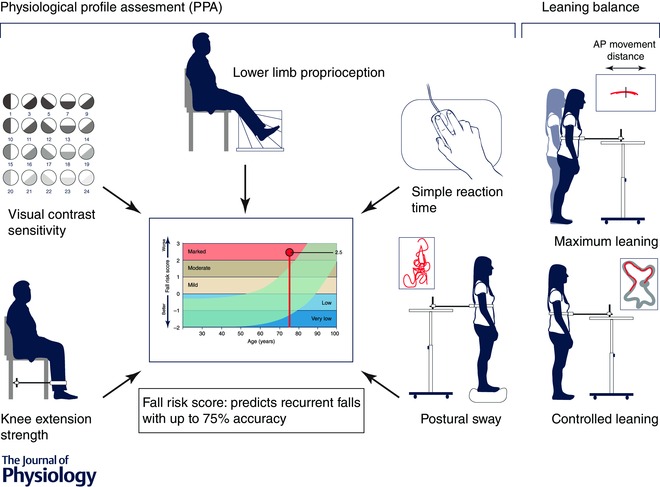
The diagram shows the key tests used in the short form of the PPA. Performance in these tests provides a global indication of performance and can be expressed as a risk score for falls (see text). This output of the PPA is shown in more detail in Fig. 5.
The final component of the PPA is a normative database compiled from assessments of over 4000 participants aged 20 years and older (www.neura.edu.au/fbrg) (Lord et al. 1991, 1992, 1994 a, 2003; Lord & Ward, 1994; Lord & Dayhew, 2001), in which the inclusion criteria were restricted to intact cognition and an ability to undertake the assessments. This resource allows an individual's PPA performance to be evaluated in relation to population norms for people aged 65 years and over (a representative group for many conditions in which motor impairments are manifest) to provide (a) an overall personalised estimate of fall risk and (b) a profile of individual test performances (Lord et al. 2003). As performance for each test is measured on a continuum, scores can be contrasted with the normative database and converted to standard (z‐scores) to provide a graphical representation of physiological ‘strengths’ and ‘weaknesses’.
Discriminative ability of the PPA for assessment of fall risk in older people
Several studies have evaluated the discriminative ability of the PPA tests with respect to falls in older people (Lord et al. 1991, 1992, 1994 a, 1996; Lord & Clark, 1996; Lord & Dayhew, 2001). In a 1‐year prospective study of 95 residents of an intermediate‐care hostel, aged 59–97 years, PPA measurements correctly classified participants into a multiple falls group (two or more falls) or a non‐multiple falls group (no falls or one fall) with an accuracy of 79% (Lord et al. 1991). This categorisation of fall status, i.e. multiple falls within a year, is more likely to indicate physiological impairments and chronic conditions than does a single fall (Nevitt et al. 1989; Lord et al. 1994 b; Ivers et al. 1998). In a subsequent study in a similar setting, the discrimination between faller and non‐faller groups increased to 86% with the addition of a validated assessment of cognitive functioning (Lord & Clark, 1996).
The PPA also has good predictive ability in community‐dwelling populations. In a 1‐year prospective study of 414 community‐dwelling women aged 65–99 years, PPA measurements correctly classified participants into a multiple falls group or a non‐multiple falls group with 75% accuracy (Lord et al. 1994 b). The largest study using the PPA was a cross‐sectional investigation of 1762 community‐dwelling people aged 60–100 years. Independent of age, participants with a history of falls exhibited reduced knee extension strength, poorer tactile sensitivity and greater sway than those without a history of falls (Lord & Dayhew, 2001). PPA measures also discriminate between older community‐dwelling people with and without a history of injurious falls (Lord et al. 1992).
Physiological profiling assessment (PPA) in clinical groups
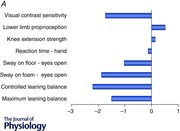
The PPA has been used in clinical groups with balance disorders and subsequent increased risk of falling, including people with multiple sclerosis (Hoang et al. 2014), stroke (Dean et al. 2012), cognitive impairment (Lorbach et al. 2007; Suttanon et al. 2012; Taylor et al. 2012), Parkinson's disease (Latt et al. 2009; Paul et al. 2014), depressed mood (Kvelde et al. 2015), macular degeneration (Szabo et al. 2008), diabetes (Lord et al. 1993), lower limb osteoarthritis (Sturnieks et al. 2004) and a history of poliomyelitis (Lord et al. 2002). Figure 3 shows the standard (z) score for tests of visual contrast sensitivity, lower limb proprioception, knee extension strength, hand reaction time, postural sway on a firm and compliant surface and maximal and coordinated leaning balance for several of these clinical groups as well as for normal young people. In each case, the young and clinical group z‐scores are based on the large normative database of the reference group of community‐dwelling people aged 65 years and over. The population profiles reveal large differences among the groups. As expected, the young normal group score well above the mean normative scores for the older population group in each test. Marked differences are evident for visual contrast sensitivity, knee extension strength, reaction time and the leaning balance tests: measures for which there are large age‐related declines (Lord & Ward, 1994).
Figure 3. z‐scores from the physiological profile assessment (PPA) for different groups .
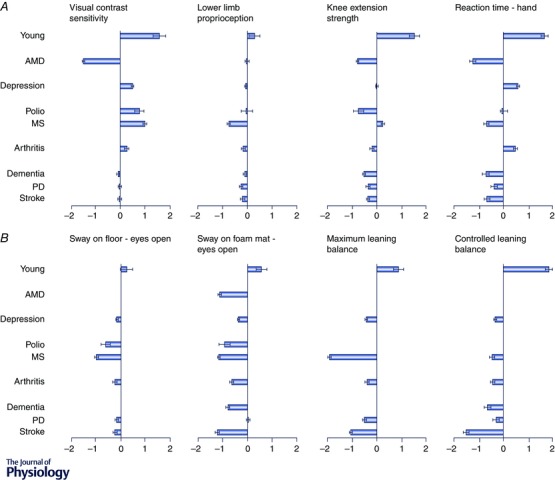
Data are from a young group and from patient groups in which the participants have age‐related macular degeneration (AMD), prior polio, multiple sclerosis (MS), osteoarthritis, depression, dementia, Parkinson's disease (PD) and stroke. A, data for key physiological abilities: detection of visual edges (edge‐control sensitivity), proprioception using a matching task for knee angle, maximal strength of knee extensors and reaction time using a hand response. B, data for sway on the floor with eyes open, sway on a foam rubber mat, maximum leaning balance and controlled leaning balance. Horizontal bars show the mean z‐score (compared to the reference group) with standard error of the mean. The reference group in each case comprises over 4000 community‐living people aged 65+ years in which the inclusion criteria were restricted to intact cognition and an ability to undertake the assessments (www.neura.edu.au/fbrg). Note some data were not collected in the dementia and macular degeneration groups.
The group with age‐related macular degeneration performed poorly in the visual contrast sensitivity test, documenting the large effect this condition has on visual functioning (Szabo et al. 2008). However, this group had impaired strength, reaction time and balance, particularly in the postural sway test with eyes open on the foam rubber mat. This latter measure relies strongly on visual input because proprioceptive input from the feet and ankles is reduced (Menz & Lord, 2001). This indicates that people with macular degeneration are at risk for falling, not only because of a reduced ability to perceive hazards in the environment but also because of reduced strength, slow reaction times and impaired balance.
Two of the clinical groups (i.e. those with prior polio and multiple sclerosis) were relatively young (both with a mean age of 51 years) in relation to the reference norms. The multiple sclerosis group performed relatively well in the tests of visual contrast sensitivity and knee extension strength. However, despite their younger age, this group performed below the mean reference values for proprioception and reaction time, and performed poorly in each of the balance tests. In the multivariate logistic regression analyses, both postural sway and coordinated stability were significant and independent predictors of multiple falls. This indicates that people with multiple sclerosis have both impairments to quiet standing (underpinned by reduced peripheral sensation and lower limb muscle strength) and controlled leaning balance control. The relatively poor performance in the reaction time test illustrated in Fig. 3 A as well as in tests of executive functioning (trail‐making test) and coordination (nine‐hole peg test) indicate people with multiple sclerosis suffer from central deficits in planning and movement control in addition to poor balance control (Hoang et al. 2014).
The group with prior polio performed poorly on the knee extension strength and postural sway tests, but had good vision and average proprioception and reaction time. This is consistent with the physiological concept that people with prior polio are a group with lower‐limb weakness, but in which other systems associated with balance are similar to those in the general community (Lord et al. 2002). Consistent with previous studies of older people (Lord & Ward, 1994; Lord & Menz, 2000), reduced muscle strength impairs standing balance as measured by postural sway, particularly when participants stand with eyes closed or on a compliant surface.
The group with osteoarthritis was drawn from a cohort of 764 older people in whom the presence of medical conditions was determined by self‐report (Sachdev et al. 2010). As over half of the participants (n = 399, 52.2%) reported osteoarthritis, the group findings overlap considerably with the normative reference data. This reflects why in our example this group does not appear to have notable proprioceptive or strength impairments compared with previously reported studies in people with lower limb osteoarthritis (Sturnieks et al. 2004). However, the postural sway, maximal balance range and coordinated stability scores were below values for community‐dwelling people as a whole. This is consistent with results from a study that compared 283 older people with lower limb arthritis with 401 age‐matched control participants, in which those with self‐reported arthritis had significantly more falls and fall injuries than their peers who did not report this condition (Sturnieks et al. 2004).
The group with depressive symptoms was also drawn from the Sydney Memory and Ageing cohort (Sachdev et al. 2010). This group of 126 people had similar vision, proprioception, strength and reaction time, but below average postural sway, maximal leaning balance range and controlled leaning balance when compared with the normative values for older community‐dwelling people. Younger depressed people also have slower reaction times compared to controls (Caligiuri & Ellwanger, 2000). Much research indicates that depression in older age is not an isolated psychological phenomenon and is linked with muscle weakness, impaired balance and slower gait (Kvelde et al. 2013).
While the PPA was devised for physically and cognitively intact older people, it has been feasible to assess fall risk in clinical groups of people with cognitive impairment (Lorbach et al. 2007; Suttanon et al. 2012; Taylor et al. 2012), Parkinson's disease (Latt et al. 2009; Paul et al. 2014) and stroke (Dean et al. 2012). The profile for the cognitively impaired group indicates poor performances in the tests of reaction time, knee extension strength, postural sway and controlled leaning balance while being comparable in vision and proprioception compared to normative values for community‐dwelling people. This profile indicates substantial motor impairments which add to concomitant cognitive, psychological and medication‐related risk factors (Taylor et al. 2014). People with stroke and Parkinson's disease have very high falling rates (Dean et al. 2012; Taylor et al. 2014). Their profiles indicate poor performances across a range of tests (knee extension strength, reaction time, postural sway and the maximal leaning balance range and controlled leaning balance tests).
The above profiles have been presented to show group data for clinical groups in which balance disorders and a high risk of falls are common. However, as indicated in the ‘Background’ (above), attribution of a degree of fall risk to a specific medical diagnosis can be problematic because the severity of such conditions varies considerably among individuals. This is illustrated with two profiles of individuals with stroke drawn from a large community‐based trial (Dean et al. 2012) (Fig. 4). The heterogeneity of this condition is readily apparent. The profile depicted in Fig. 4 A shows above‐average performance for vision, proprioception, knee extension strength, reaction time and maximal leaning balance but average performance (i.e. one standard deviation below average) for only one balance measure. In contrast, the profile depicted in Fig. 4 B shows performances for knee extension strength are more than one standard deviation below average, and reaction time, sway and controlled leaning balance are more than two standard deviations below average. Such divergent profiles provide valuable information for the design of targeted exercise and personalised interventions (Buford & Pahor, 2012).
Figure 4. Contrasting data from two individuals with stroke for the components of the physiological profile assessment (PPA) .
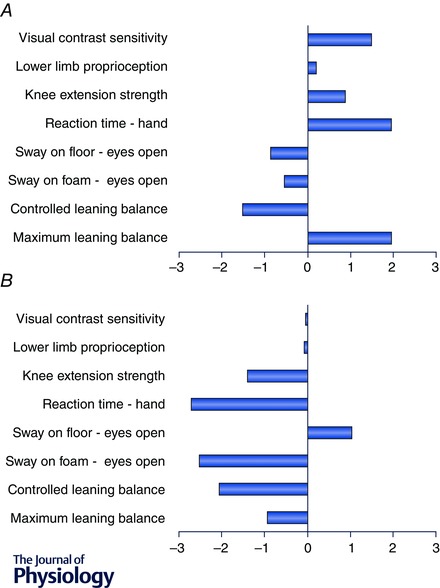
The x‐axis depicts the z‐score for the components. The knee extension strength score is the average of the two lower limbs.
Figure 5 shows the overall fall risk scores derived from the PPA for the clinical populations in relation to age, with the fall risk categorised from ‘very low’ to ‘very marked’. Ageing is associated with a predictable temporal pattern of functional decline. The clinical groups all have high scores, with the younger prior polio and multiple sclerosis groups scoring well above levels found in their peers of the same age without these conditions.
Figure 5. Relationship between the risk of falls derived from the physiological profile assessments and age .
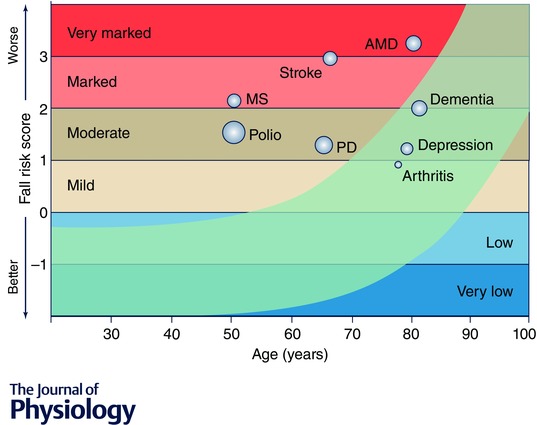
The light blue curved band shows the normal range across the lifespan – top border indicating 75th percentile and bottom border 10th percentile. Superimposed on this established relationship are group scores for a number of diseases and disabilities including polio, multiple sclerosis (MS), stroke, Parkinson's disease (PD), age‐related macular degeneration (AMD), dementia, depression and arthritis. The middle of the bubble points indicate the group mean scores, and the bubble size indicates the group standard deviations.
Use of the PPA in interventions
The tests of strength, speed and balance in the PPA have been used to assess the effectiveness of exercise in older people (Sherrington et al. 2008) and clinical groups with balance disorders, including people with multiple sclerosis (Hoang et al. 2015), stroke (Dean et al. 2012), Parkinson's disease (Allen et al. 2010) and cognitive impairment (Wesson et al. 2013). The inclusion of these measures in clinical trials helps in elucidating the mechanisms by which interventions may prevent falls. For example in older people, the evidence shows that exercise programmes with moderate‐ to high‐intensity balance training are the most effective exercise interventions to diminish the incidence of falls (Sherrington et al. 2008).
The PPA fall risk score has also been used as an outcome measure in randomised controlled trials in studies of older people (Liu‐Ambrose et al. 2004; Lord et al. 2005) and people with stroke (Allen et al. 2010) and cognitive impairment (Wesson et al. 2013). The first of these compared the effectiveness of group resistance and agility training programmes in reducing fall risk in 98 community‐dwelling older women with low bone mass (Liu‐Ambrose et al. 2004). At the end of the 25 week trial, PPA fall risk scores were reduced by 57% and 48% in the resistance and agility training groups, respectively, but by only 20% in a control group that undertook stretching exercises. In both the resistance and agility groups, the reduction in fall risk was mediated primarily by improved postural stability, with sway reduced by 31% and 29%, respectively.
A second randomised controlled trial examined whether tailored interventions identified by the PPA could reduce fall risk by maximising vision, muscle strength, coordination and balance in 620 community‐dwelling people aged 75 years and over (Lord et al. 2005). The participants underwent the PPA at baseline and at the mid‐point of the trial. At retest, the participants randomised to the exercise intervention showed significant improvements in knee flexion strength and sit‐to‐stand times compared with the controls. Similarly, participants randomised to the visual intervention (referral to an eye‐care specialist) showed significant improvements in visual acuity and contrast sensitivity. Overall, PPA fall risk scores decreased significantly in the intervention group.
The studies involving exercise interventions for people with stroke and cognitive impairment have been of a more pilot nature and have not demonstrated significantly improved PPA scores or reduced fall rates (Allen et al. 2010; Wesson et al. 2013). These findings agree with those of other recent studies and may indicate that exercise alone is not an effective fall prevention strategy in high‐risk populations. For example, exercise programmes have failed to prevent falls in older people recently discharged from hospital (Sherrington et al. 2014) or older people with documented frailty (Fairhall et al. 2014). This area requires further investigation but it seems that multifactorial interventions may be needed to prevent falls in high‐risk people with these more complex conditions.
Conclusions and implications
As implied by our simple model (Fig. 1), the deterioration in physical performance with ageing is much more than simply the well‐described reduction in muscle function (Conley et al. 2000). The above findings illustrate the use of a physiological profiling approach not only to identify sensory and motor impairments in older people at risk of falls but also to document reduced physiological performance in people with a range of disorders such as multiple sclerosis, stroke, cognitive impairment, depressed mood, macular degeneration, lower limb osteoarthritis and a history of polio. The group profiles presented in Fig. 3 show condition‐specific impairments and the fall risk graph (Fig. 5) reveals the extent to which these groups are at an elevated risk of falls.
Concomitant impairments in physiological, psychological and cognitive function are common across diseases and in old age. It is expected that multiple impairments across this triad add cumulatively to disability and to limitations in physical activity (Herman et al. 2010; Martin et al. 2013). For example, higher levels of emotional distress (i.e. depression, anxiety) can reduce autonomy through restricting activities of daily living and consequent loss in physical function (Van Haastregt et al. 2008). Furthermore, a large body of literature has described the role of higher cognitive processing in relation to standing and locomotion (Woollacott & Shumway‐Cook, 2002). Therefore, while the physiological domains within the profiles are able to distinguish fallers from non‐fallers between disease groups as shown in Fig. 3, related research has shown that disease‐specific factors — such as executive functioning and freezing of gait in people with Parkinson's disease (Latt et al. 2009; Paul et al. 2014) or impaired cognition in people following stroke (Liu‐Ambrose et al. 2007), depressive symptoms in people with cognitive impairment (Taylor et al. 2012), and reduced fine motor control in people with multiple sclerosis (Hoang et al. 2014) — provide added ability to predict an adverse outcome such as falls. The PPA measures include cognitive processing speed by means of simple hand reaction time, which was affected in Parkinson's disease, stroke, multiple sclerosis and dementia, as shown in Fig. 3 A. While it is likely that general slowing in processing of information is the key cause of delayed motor responses (Salthouse, 1996), other cognitive functions (e.g. executive functioning and attention) have recently been investigated in more detail in relation to balance control and walking (Yogev‐Seligmann et al. 2008). Impaired executive functioning reduces the ability of an individual to attend to relevant sensory information required for maintaining balance when walking (Yogev‐Seligmann et al. 2008). The effect of attentional limits on balance and gait is especially apparent during dual‐tasks when sensorimotor and cognitive tasks are performed concurrently (e.g. walking when talking) or when walking in complex environments (e.g. walking in crowded areas) (Woollacott & Shumway‐Cook, 2002).
Our collated findings from across the age and disease range emphasise that functionally relevant motor impairments, such as an increased risk of falling, arise from many different deficits in sensorimotor capacities. A deficit in one capacity may be sufficient to increase the risk (e.g. poor leg muscle strength in prior polio), but commonly several physiological deficits are combined. Furthermore, deficits in a restricted physiological domain (e.g. vision, strength, cognition) can be ‘amplified’ such that they impair more complex physiological tasks such as standing, balancing and walking. The motor impairment of impaired balance (with its concomitant risk of recurrent falling) is a final common outcome in ageing and a range of highly prevalent disorders.
Finally, the profiling approach has clinical implications. If used on an individual basis, it gives a measurement of physiological vigor (conversely viewed as frailty) and fall risk, and it allows subsequent tailoring of personalised interventions to maximise sensorimotor and balance function to enhance mobility. When viewed from the perspective of disease groups, the profiling approach can provide practical insight into the physiology of tasks such as standing and how this task is commonly affected in different diseases. With the challenges of population ageing being felt across most nations, increased emphasis must be placed on strategies to measure important functional abilities and then to intervene to improve them and slow their decline.
Additional information
Competing interests
The PPA (NeuRA FallScreen) is commercially available through Neuroscience Research Australia.
Funding
Our work is supported by the National Health and Medical Research Council.
Biographies
Stephen Lord is a Senior Principal Research Scientist at Neuroscience Research Australia. He has published over 350 papers in the areas of balance, gait and falls in older people and is acknowledged as a leading international researcher in his field. His research follows two main themes: the identification of physiological risk factors for falls and the development and evaluation of fall prevention strategies.
Kim Delbaere is a Senior Research Scientist at Neuroscience Research Australia. Her research has contributed to the understanding of physical, psychological and cognitive factors causing falls. Her multidisciplinary approach incorporates elements from physiotherapy, psychology, brain imaging and software engineering towards preventing falls and promoting healthy ageing.
Simon Gandevia is a Senior Principal Research Fellow of the NHMRC. He works at Neuroscience Research Australia and is one of its founders. His work focuses on human sensory and motor control particularly in areas of motor performance and fatigue, respiratory muscles, proprioception and passive muscle behaviour. A current programme of work aims to examine motor impairments in healthy ageing and disease.

This review was presented at the symposium “From TOP to TOE: What falls reveal about physiological ageing and degeneration”, which took place at Ageing and Degeneration: A Physiological Perspective in Edinburgh, UK, 10–11 April 2015.
References
- Allen NE, Canning CG, Sherrington C, Lord SR, Latt MD, Close JCT, O'Rourke SD, Murray SM & Fung VSC (2010). The effects of an exercise program on fall risk factors in people with Parkinson's disease: A randomized controlled trial. Mov Disord 25, 1217–1225. [DOI] [PubMed] [Google Scholar]
- Ashburn A, Stack E, Pickering RM & Ward CR (2001). A community‐dwelling sample of people with Parkinson's disease: characteristics of fallers and non‐fallers. Age Ageing 30, 47–52. [DOI] [PubMed] [Google Scholar]
- Barbotte E, Guillemin F & Chau N; Lorhandicap Group (2001). Prevalence of impairments, disabilities, handicaps and quality of life in the general population: a review of recent literature. Bull World Health Organ 79, 1047–1055. [PMC free article] [PubMed] [Google Scholar]
- Buford TW & Pahor M (2012). Making preventive medicine more personalized: implications for exercise‐related research. Prev Med 55, 34–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caligiuri MP & Ellwanger J (2000). Motor and cognitive aspects of motor retardation in depression. J Affect Disord 57, 83–93. [DOI] [PubMed] [Google Scholar]
- Campbell AJ, Borrie MJ & Spears GF (1989). Risk factors for falls in a community‐based prospective study of people 70 years and older. J Gerontol 44, M112–117. [DOI] [PubMed] [Google Scholar]
- Conley KE, Esselman PC, Jubrias SA, Cress ME, Inglin B, Mogadam C & Schoene RB (2000). Ageing, muscle properties and maximal O2 uptake rate in humans. J Physiol 526, 211–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean CM, Rissel C, Sherrington C, Sharkey M, Cumming RG, Lord SR, Barker RN, Kirkham C & O'Rourke S (2012). Exercise to enhance mobility and prevent falls after stroke: the community stroke club randomized trial. Neurorehabil Neural Repair 26, 1046–1057. [DOI] [PubMed] [Google Scholar]
- Fairhall N, Sherrington C, Lord SR, Kurrle SE, Langron C, Lockwood K, Monaghan N, Aggar C & Cameron ID (2014). Effect of a multifactorial, interdisciplinary intervention on risk factors for falls and fall rate in frail older people: a randomised controlled trial. Age Ageing 43, 616–622. [DOI] [PubMed] [Google Scholar]
- Forster A & Young J (1995). Incidence and consequences of falls due to stroke: a systematic inquiry [see comments]. BMJ 311, 83–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herdman SJ, Blatt P, Schubert MC & Tusa RJ (2000). Falls in patients with vestibular deficits. Am J Otology 21, 847–851. [PubMed] [Google Scholar]
- Herman T, Mirelman A, Giladi N, Schweiger A & Hausdorff JM (2010). Executive control deficits as a prodrome to falls in healthy older adults: A prospective study linking thinking, walking, and falling. J Gerontol A Biol Sci Med Sci 65, 1086–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoang P, Schoene D, Gandevia S, Smith S & Lord SR (2015). Effects of a home‐based step training programme on balance, stepping, cognition and functional performance in people with multiple sclerosis – a randomized controlled trial. Mult Scler; DOI: 10.1177/1352458515579442. [DOI] [PubMed] [Google Scholar]
- Hoang PD, Cameron MH, Gandevia SC & Lord SR (2014). Neuropsychological, balance, and mobility risk factors for falls in people with multiple sclerosis: a prospective cohort study. Arch Phys Med Rehabil 95, 480–486. [DOI] [PubMed] [Google Scholar]
- Ivers RQ, Cumming RG, Mitchell P & Attebo K (1998). Visual impairment and falls in older adults: the Blue Mountains Eye Study. J Am Geriatr Soc 46, 58–64. [DOI] [PubMed] [Google Scholar]
- Jørgensen L, Engstad T & Jacobsen BK (2002). Higher incidence of falls in long‐term stroke survivors than in population controls: Depressive symptoms predict falls after stroke. Stroke 33, 542–547. [DOI] [PubMed] [Google Scholar]
- Kvelde T, Lord SR, Close JCT, Reppermund S, Kochan NA, Sachdev P, Brodaty H & Delbaere K (2015). Depressive symptoms increase fall risk in older people, independent of antidepressant use, and reduced executive and physical functioning. Arch Gerontol Geriatr 60, 190–195. [DOI] [PubMed] [Google Scholar]
- Kvelde T, McVeigh C, Toson B, Greenaway M, Lord SR, Delbaere K & Close JCT (2013). Depressive symptomatology as a risk factor for falls in older people: Systematic review and meta‐analysis. J Am Geriatr Soc 61, 694–706. [DOI] [PubMed] [Google Scholar]
- Latt MD, Lord SR, Morris JGL & Fung VSC (2009). Clinical and physiological assessments for elucidating falls risk in Parkinson's disease. Mov Disord 24, 1280–1289. [DOI] [PubMed] [Google Scholar]
- Liu‐Ambrose T, Khan KM, Eng JJ, Lord SR & McKay HA (2004). Balance confidence improves with resistance or agility training: Increase is not correlated with objective changes in fall risk and physical abilities. Gerontology 50, 373–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu‐Ambrose T, Pang MYC & Eng JJ (2007). Executive function is independently associated with performances of balance and mobility in community‐dwelling older adults after mild stroke: Implications for falls prevention. Cerebrovasc Dis 23, 203–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorbach ER, Webster KE, Menz HB, Wittwer JE & Merory JR (2007). Physiological falls risk assessment in older people with Alzheimer's disease. Dement Geriatr Cogn Disord 24, 260–265. [DOI] [PubMed] [Google Scholar]
- Lord SR, Allen GM, Williams P & Gandevia SC (2002). Risk of falling: Predictors based on reduced strength in persons previously affected by polio. Arch Phys Med Rehabil 83, 757–763. [DOI] [PubMed] [Google Scholar]
- Lord SR, Caplan GA, Colagiuri R, Colagiuri S & Ward JA (1993). Sensori‐motor function in older persons with diabetes. Diabet Med 10, 614–618. [DOI] [PubMed] [Google Scholar]
- Lord SR & Clark RD (1996). Simple physiological and clinical tests for the accurate prediction of falling in older people. Gerontology 42, 199–203. [DOI] [PubMed] [Google Scholar]
- Lord SR, Clark RD & Webster IW (1991). Physiological factors associated with falls in an elderly population. J Am Geriatr Soc 39, 1194–1200. [DOI] [PubMed] [Google Scholar]
- Lord SR & Dayhew J (2001). Visual risk factors for falls in older people. J Am Geriatr Soc 49, 508–515. [DOI] [PubMed] [Google Scholar]
- Lord SR, Lloyd DG, Nirui M, Raymond J, Williams P & Stewart RA (1996). The effect of exercise on gait patterns in older women: a randomized controlled trial. J Gerontol A Biol Sci Med Sci 51, M64–70. [DOI] [PubMed] [Google Scholar]
- Lord SR, McLean D & Stathers G (1992). Physiological factors associated with injurious falls in older people living in the community. Gerontology 38, 338–346. [DOI] [PubMed] [Google Scholar]
- Lord SR & Menz HB (2000). Visual contributions to postural stability in older adults. Gerontology 46, 306–310. [DOI] [PubMed] [Google Scholar]
- Lord SR, Menz HB & Tiedemann A (2003). A physiological profile approach to falls risk assessment and prevention. Phys Ther 83, 237–252. [PubMed] [Google Scholar]
- Lord SR, Sambrook PN, Gilbert C, Kelly PJ, Nguyen T, Webster IW & Eisman JA (1994. a). Postural stability, falls and fractures in the elderly: results from the Dubbo Osteoporosis Epidemiology Study. Med J Aust 160, 684–691. [PubMed] [Google Scholar]
- Lord SR, Tiedemann A, Chapman K, Munro B, Murray SM & Sherrington C (2005). The effect of an individualized fall prevention program on fall risk and falls in older people: A randomized, controlled trial. J Am Geriatr Soc 53, 1296–1304. [DOI] [PubMed] [Google Scholar]
- Lord SR & Ward JA (1994). Age‐associated differences in sensori‐motor function and balance in community dwelling women. Age Ageing 23, 452–460. [PubMed] [Google Scholar]
- Lord SR, Ward JA, Williams P & Anstey K (1994. b). Physiological factors associated with falls in older community‐dwelling women. J Am Geriatr Soc 42, 1110–1117. [DOI] [PubMed] [Google Scholar]
- Martin KL, Blizzard L, Srikanth VK, Wood A, Thomson R, Sanders LM & Callisaya ML (2013). Cognitive function modifies the effect of physiological function on the risk of multiple falls – a population‐based study. J Gerontol A Biol Sci Med Sci 68, 1091–1097. [DOI] [PubMed] [Google Scholar]
- Menz HB & Lord SR (2001). The contribution of foot problems to mobility impairment and falls in community‐dwelling older people. J Am Geriatr Soc 49, 1651–1656. [PubMed] [Google Scholar]
- Nevitt MC, Cummings SR, Kidd S & Black D (1989). Risk factors for recurrent nonsyncopal falls. A prospective study. JAMA 261, 2663–2668. [PubMed] [Google Scholar]
- O'Loughlin JL, Robitaille Y, Boivin JF & Suissa S (1993). Incidence of and risk factors for falls and injurious falls among the community‐dwelling elderly. Am J Epidemiol 137, 342–354. [DOI] [PubMed] [Google Scholar]
- Paul SS, Sherrington C, Canning CG, Fung VSC, Close JCT & Lord SR (2014). The relative contribution of physical and cognitive fall risk factors in people with Parkinson's disease: A large prospective cohort study. Neurorehabil Neural Repair 28, 282–290. [DOI] [PubMed] [Google Scholar]
- Pollock RD, Carter S, Velloso CP, Duggal NA, Lord JM, Lazarus NR & Harridge SD (2015). An investigation into the relationship between age and physiological function in highly active older adults. J Physiol 593, 657–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson JK, Ching C & Hurvitz EA (1992). The relationship between electromyographically documented peripheral neuropathy and falls. J Am Geriatr Soc 40, 1008–1012. [DOI] [PubMed] [Google Scholar]
- Sachdev PS, Brodaty H, Reppermund S, Kochan NA, Trollor JN, Draper B, Slavin MJ, Crawford J, Kang K, Broe GA, Mather KA & Lux O (2010). The Sydney Memory and Ageing Study (MAS): Methodology and baseline medical and neuropsychiatric characteristics of an elderly epidemiological non‐demented cohort of Australians aged 70–90 years. Int Psychogeriatr 22, 1248–1264. [DOI] [PubMed] [Google Scholar]
- Salthouse TA (1996). The processing‐speed theory of adult age differences in cognition. Psychol Rev 103, 403–428. [DOI] [PubMed] [Google Scholar]
- Schrag A, Ben‐Shlomo Y & Quinn N (2002). How common are complications of Parkinson's disease? J Neurol 249, 419–423. [DOI] [PubMed] [Google Scholar]
- Schwartz A, Nevitt M, Sellmeyer D, Resnick H, Ensrud K, Schreiner P, Margolis K, Hillier T, Gregg E, Cauley J, Black D & Cummings S (2000). Older women with diabetes have a higher risk of falls: A prospective study. Diabetes 49, A193. [DOI] [PubMed] [Google Scholar]
- Seals DR, Justice JN & LaRocca TJ (2015). Physiological geroscience: targeting function to increase healthspan and achieve optimal longevity. J Physiol 594, 2001–2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw FE, Bond J, Richardson DA, Dawson P, Steen IN, McKeith IG & Kenny RA (2003). Multifactorial intervention after a fall in older people with cognitive impairment and dementia presenting to the accident and emergency department: randomised controlled trial. BMJ 326, 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherrington C, Lord SR, Vogler CM, Close JCT, Howard K, Dean CM, Heller GZ, Clemson L, O'Rourke SD, Ramsay E, Barraclough E, Herbert RD & Cumming RG (2014). A post‐hospital home exercise program improved mobility but increased falls in older people: a randomised controlled trial. PLoS One 9, e104412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherrington C, Whitney JC, Lord SR, Herbert RD, Cumming RG & Close JCT (2008). Effective exercise for the prevention of falls: a systematic review and meta‐analysis. J Am Geriatr Soc 56, 2234–2243. [DOI] [PubMed] [Google Scholar]
- Sturnieks DL, Tiedemann A, Chapman K, Munro B, Murray SM & Lord SR (2004). Physiological risk factors for falls in older people with lower limb arthritis. J Rheumatol 31, 2272–2279. [PubMed] [Google Scholar]
- Suttanon P, Hill KD, Said CM, LoGiudice D, Lautenschlager NT & Dodd KJ (2012). Balance and mobility dysfunction and falls risk in older people with mild to moderate Alzheimer disease. Am J Phys Med Rehabil 91, 12–23. [DOI] [PubMed] [Google Scholar]
- Szabo SM, Janssen PA, Khan K, Potter MJ & Lord SR (2008). Older women with age‐related macular degeneration have a greater risk of falls: a physiological profile assessment study. J Am Geriatr Soc 56, 800–807. [DOI] [PubMed] [Google Scholar]
- Taylor ME, Delbaere K, Lord SR, Mikolaizak AS, Brodaty H & Close JCT (2014). Neuropsychological, physical, and functional mobility measures associated with falls in cognitively impaired older adults. J Gerontol A Biol Sci Med Sci 69, 987–995. [DOI] [PubMed] [Google Scholar]
- Taylor ME, Lord SR, Delbaere K, Mikolaizak AS & Close JCT (2012). Physiological fall risk factors in cognitively impaired older people: a one‐year prospective study. Dement Geriatr Cogn Disord 34, 181–189. [DOI] [PubMed] [Google Scholar]
- Tinetti ME & Kumar C (2010). The patient who falls: ‘It's always a trade‐off’. JAMA 303, 258–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinetti ME, Speechley M & Ginter S (1988). Risk factors for falls among elderly persons living in the community. N Engl J Med 319, 1701–1707. [DOI] [PubMed] [Google Scholar]
- van Dijk PT, Meulenberg OG, van de Sande HJ & Habbema JD (1993). Falls in dementia patients. Gerontologist 33, 200–204. [DOI] [PubMed] [Google Scholar]
- van Haastregt JCM, Zijlstra GAR, van Rossum E, van Eijk JTM & Kempen GIJM (2008). Feelings of anxiety and symptoms of depression in community‐living older persons who avoid activity for fear of falling. Am J Geriatr Psychiatry 16, 186–193. [DOI] [PubMed] [Google Scholar]
- Wesson J, Clemson L, Brodaty H, Lord S, Taylor M, Gitlin L & Close J (2013). A feasibility study and pilot randomised trial of a tailored prevention program to reduce falls in older people with mild dementia. BMC Geriatr 13, 89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitney S, Hudak MT & Marchetti GF (2000). The dynamic gait index relates to self reported fall history in individuals with vestibular dysfunction. J Vestib Res 10, 99–105. [PubMed] [Google Scholar]
- Wood BH, Bilclough JA, Bowron A & Walker RW (2002). Incidence and prediction of falls in Parkinson's disease: a prospective multidisciplinary study. J Neurol Neurosurg Psychiatry 72, 721–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woollacott M & Shumway‐Cook A (2002). Attention and the control of posture and gait: A review of an emerging area of research. Gait Posture 16, 1–14. [DOI] [PubMed] [Google Scholar]
- Yogev‐Seligmann G, Hausdorff JM & Giladi N (2008). The role of executive function and attention in gait. Mov Disord 23, 329–342. [DOI] [PMC free article] [PubMed] [Google Scholar]


