Abstract
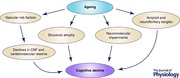
The rise in incidence of age‐related cognitive impairment is a global health concern. Ageing is associated with a number of changes in the brain that, collectively, contribute to the declines in cognitive function observed in older adults. Structurally, the ageing brain atrophies as white and grey matter volumes decrease. Oxidative stress and inflammation promote endothelial dysfunction thereby hampering cerebral perfusion and thus delivery of energy substrates and nutrients. Further, the development of amyloid plaques and neurofibrillary tangles contributes to neuronal loss. Of interest, there are substantial inter‐individual differences in the degree to which these physical and functional changes impact upon cognitive function as we grow older. This review describes how engaging in physical activity and cognitive activities and adhering to a Mediterranean style diet promote ‘brain health’. From a physiological perspective, we discuss the effects of these modifiable lifestyle behaviours on the brain, and how some recent human trials are beginning to show some promise as to the effectiveness of lifestyle behaviours in combating cognitive impairment. Moreover, we propose that these lifestyle behaviours, through numerous mechanisms, serve to increase brain, cerebrovascular and cognitive reserve, thereby preserving and enhancing cognitive function for longer.
Abbreviations
- AD
Alzheimer's disease
- BDNF
brain derived neutrophic factor
- CA
cognitive activity
- CBF
cerebral blood flow
- CVR
cerebrovascular resistance
- DHA
docosahexaenoic acid
- EPA
eicosapentaenoic acid
- MABP
mean arterial blood pressure
- NO
nitric oxide
- PA
physical activity
- PUFA
polyunsaturated fatty acid
- VCI
vascular cognitive impairment
Introduction
The ageing of the ‘baby boom’ population and the high costs of providing health care for cognitively impaired individuals make it imperative to develop interventions to delay and/or blunt the age‐related development of cognitive impairment. Indeed, without effective intervention or treatment, the prevalence of dementia worldwide could exceed 135 million individuals by the year 2050 (Prince et al. 2013). Declining cognitive function occurs with increasing age, but the inter‐individual trajectory of this decline varies widely and can be accounted for by a combination of genetic, physiological, environmental and behavioural factors, some of the latter of which are modifiable. Indeed, while the brain deteriorates and degrades with engagement in unhealthy behaviours, it can be enhanced by participation in healthy behaviours. As such, it has been shown that a sedentary lifestyle and ‘Western’ style dietary pattern characterised by high intake of red meat, saturated fat and refined carbohydrates increase the risk for cognitive impairment as well as a wide variety of chronic diseases. On the other hand, physical activity and following a Mediterranean style dietary pattern are two such lifestyle behaviours known to promote and support the brain's structural integrity and function, namely ‘brain health’.
The positive relationship between brain health and cognitive function in older adults can be described within the context of reserve. Broadly defined, reserve is the capacity of the brain to maintain function in the face of acute injury (e.g. head trauma and ischaemia) or degenerative damage (e.g. ageing), and can be divided into biological, cerebrovascular and cognitive reserve. Biological or brain reserve refers to the structural integrity of the brain and is typically assessed by measuring brain volumes (Stern, 2009). Cerebrovascular reserve is the capacity of the blood vessels of the brain to maintain blood flow in response to chemical, mechanical or neural stimuli (Davenport et al. 2012), whilst cognitive reserve relates to a capacity for compensatory brain function in order to maintain cognitive performance (Stern, 2009). The present review will discuss the effects of dietary and physical activity lifestyle behaviours on brain physiology and how they may contribute to increasing reserve and cognitive function. In addition, enhancing brain health and cognitive function via increased cognitive activity and its interaction with physical activity will also be discussed.
Physical activity
Studies in animals and humans show that physical exercise has direct effects on brain health by altering mechanisms of neuronal plasticity involved with learning and memory (Cotman & Berchtold, 2002; Cotman et al. 2007). Exercise may prevent age‐related deterioration of cognitive and brain function and reduce age‐related brain atrophy (Voss et al. 2013). For example, both prospective and retrospective observational studies have shown a lower incidence of cognitive impairment, depression and dementia in people who maintained regular physical activity (PA) (Lautenschlager et al. 2008; Sofi et al. 2011). Similarly, rodents given regular access to running wheels are less impaired than their sedentary counterparts on memory tests that involve the hippocampus (van Praag et al. 1999). In rodents, running also increases neurogenesis in the hippocampal dentate gyrus and induces angiogenesis in the cortex and other brain regions (Swain et al. 2003; Creer et al. 2010) thereby improving oxygen and glucose delivery. In humans, the majority of research examining the effects of PA and fitness on the brain has been conducted in older cognitively normal populations and has predominantly focused on the size and function of the hippocampus. The hippocampus is an important structure in the context of ageing as it plays a dominant role in declarative memory functions and faster rates of hippocampal atrophy are linked to more rapid conversion to Alzheimer's disease (AD). Consistent with animal research, higher cardiorespiratory fitness levels in cognitively normal adults were associated with better performance on a spatial memory task and greater volume of the hippocampus (Erickson et al. 2009). This effect remained significant even after controlling for variance associated with age, sex, education and intracranial volume. Interestingly, this effect has now been replicated in children (Chaddock et al. 2010), in adolescents (Herting & Nagel, 2012) and in adults with early stage dementia (Honea et al. 2009).
Prospective longitudinal studies have also found that engaging in greater amounts of PA is associated with elevated hippocampal and prefrontal cortex volume later in life. For example, in one study of 299 cognitively normal adults, a greater amount of self‐reported walking was associated with a greater grey matter volume 9 years later in several regions, including the hippocampus. Further, greater grey matter volume was predictive of a reduced risk of developing cognitive impairment 4 years later (Erickson et al. 2010). This and results from other studies (Rovio et al. 2010) suggest that participation in greater amounts of PA may have long‐term consequences for maintaining grey matter volume into late adulthood. To test the hypothesis that engaging in exercise could increase the size of the hippocampus, 120 inactive older adults were randomized to either 12 months of moderate intensity brisk walking or to a non‐aerobic stretching and toning control condition (Erickson et al. 2011). Both groups received site‐based exercise 3 times per week for 30–45 min. After 12 months of exercise, the brisk walking group showed a significant increase in the size of the hippocampus while the control group showed a slight decline. Further, changes in the size of the hippocampus were correlated with increases in fitness levels, improvements in spatial memory, and increased levels of brain derived neutrophic factor (BDNF) (also see Niemann et al. 2014; ten Brinke et al. 2015). This study suggests that engagement in regular amounts of moderate intensity activity is sufficient for increasing the size of the hippocampus.
Other studies have also linked exercise and fitness to the connectivity of the hippocampus and prefrontal cortex. For example, greater fitness and exercise were associated with an increase in grey matter volume in the prefrontal cortex (Colcombe et al. 2006; Weinstein et al. 2012) and greater grey matter volume statistically mediated the association between fitness and cognitive performance (Weinstein et al. 2012). Similarly, exercise and fitness have been linked to greater intrinsic brain connectivity between the hippocampus and prefrontal cortex as measured by both seed‐based and graph theory approaches (Burdette et al. 2010; Voss et al. 2010). Although other brain areas like the basal ganglia have been linked to fitness (Verstynen et al. 2012), the prefrontal cortex and hippocampus are regions most consistently observed across studies (Erickson et al. 2014).
In addition to enhancing the structural integrity of the brain, PA has also been shown to contribute to brain health via modulation of vascular function and inflammation. The pathology of vascular brain lesions develops in a similar manner to that of atherosclerosis, as increased inflammation and oxidative stress are evident in both conditions (Casserly & Topol, 2004). Recent translational investigations performed in high risk ageing patients and in corresponding animal models suggest a link between populations with high‐risk cardiovascular factors and neurodegenerative diseases. In the context of ageing, the brain is highly susceptible to reactive oxygen species (ROS)‐induced damage due to its high rate of oxidative metabolism and relatively low levels of antioxidant enzymes (Coyle & Puttfarcken, 1993). In addition, risk factors for atherosclerosis could be involved in the development of inflammatory conditions in the brain, and ultimately lead to ischaemic or haemorrhagic stroke (Dutta et al. 2012). It was indeed suggested that blood–brain barrier permeability (Hafezi‐Moghadam et al. 2007) and macrophage infiltration – two pathological features in atherosclerosis – which affect brain vessel function during the ageing process are specifically associated with a cholesterol‐rich diet (Casserly & Topol, 2004). On the other hand, regular physical training has been shown to reduce these risk factors by upregulating antioxidant enzymatic systems and anti‐inflammatory processes, which may slow down the usual increase in oxidative stress and inflammation during ageing and therefore potential neurovascular and neurodegenerative diseases (Garcia‐Mesa et al. 2015).
Chronic exercise training has been also shown to reduce most of the physiological processes involved in the pathogenesis of cardiovascular diseases including oxidative stress, vascular adhesion, impairment of nitric oxide metabolism (Szostak & Laurant, 2011) and inflammation (Lesniewski et al. 2011). Exercise is also connected with improvement in general metabolic conditions such as lipid dysfunction and insulin resistance. As shown by Pellegrin et al. (2009), the beneficial effects of exercise training on atherosclerosis have been established in ApoE−/− mice. ApoE−/− mice are known to spontaneously develop atherosclerotic lesions and this phenomenon is amplified when the animals are under fat diet (Meir & Leitersdorf, 2004). Since ApoE plays a central role in the brain response to injury and neurodegeneration, this model has also proven its relevance in fields related to neuroinflammation, Alzheimer's disease and dementia (Poirier, 2000).
In addition, the beneficial effects of exercise on oxidative stress and inflammation in the brain and aorta were also determined using a mouse model of atherosclerosis associated with ageing (Chirico et al. 2012). It was demonstrated that the occurrence of vascular brain and aortic damage (blood–brain barrier permeability, presence of micro‐haemorrhage and accumulation of macrophages measured by both MRI and histology) in an ageing model of atherosclerosis (60‐week‐old ApoE−/− mice under high fat–high cholesterol diet) was partially reversed by 12 weeks of exercise training. In parallel, exercise training decreased oxidative stress and inflammation directly in the brain and in the aorta. Interestingly, cultures of bone marrow‐derived macrophages of these mice also suggested that oxidative stress can modulate the macrophage pro‐ or anti‐inflammatory behaviour. Overall, this study suggests that regular PA may improve neurovascular health by reducing inflammation and oxidative stress.
In humans, the correlation between fitness, oxidative stress, nitric oxide production, mean arterial blood pressure (MABP) and cerebrovascular resistance (CVR) in a small cohort (n = 42) of postmenopausal women was examined (Pialoux et al. 2009). A greater level of oxidative stress was associated with higher MABP value, and CVR was reported. In contrast, lower levels of end‐products of nitric oxide metabolism were associated with higher MABP and CVR. Interestingly, postmenopausal women with higher fitness levels had higher antioxidant enzyme activity and lower levels of oxidative stress, which was associated with better cardio‐ and cerebrovascular outcomes (i.e. lower MABP and CVR). This study demonstrated that, after menopause, fitness level and regular PA mediate against oxidative stress by maintaining antioxidant enzyme efficiency and also that oxidative stress and NO production modulate CVR in this population.
The structural and physiological brain changes resulting from exercise might also translate to improved behavioural, emotional and cognitive performance. Consistent with this hypothesis, a meta‐analysis of 18 randomised exercise interventions found that exercise was an effective approach to improve cognitive function in older adults (Colcombe & Kramer, 2003). Similar meta‐analytic results have been reported showing smaller but significant effects across the adult lifespan (e.g. Smith et al. 2010), but other recent studies and meta‐analyses have reported negative findings on the consistency and robustness of the effects of PA on cognitive outcomes in older adults at risk for mobility disability (Sink et al. 2015) and the effects of exercise on cognitive function in older adults without known cognitive impairments (Young et al. 2015). Clearly there is an immediate need to better understand the effects of exercise on brain function and physiology with the brain's behavioural sequelae.
In summary, all available evidence suggests that participation in regular amounts of moderate intensity exercise may improve brain health throughout the lifespan and reduce the risk for neurological and psychiatric conditions. However, we still have a very poor understanding of how and if exercise influences cognition in humans. One proposal is that PA increases brain reserve. The aforementioned studies conducted by Erickson et al. (2014), which demonstrated simultaneous increases in hippocampal volume and performance on memory tasks following an exercise intervention, would certainly support the concept of increased brain reserve as a mediating factor in the relationship between PA and cognition in older adults (see Fig. 1). In addition, there is emerging evidence that habitual PA (Brown et al. 2010; Bailey et al. 2013; Guiney et al. 2015), aerobic fitness (i.e. maximal oxygen uptake) (Brown et al. 2010; J. N. Barnes et al. 2013), and aerobic exercise training (Vicente‐Campos et al. 2012) are associated with increases in cerebrovascular reserve (as measured by cerebrovascular reactivity to hypercapnia. However, not all studies report a beneficial effect of life‐long exercise training on age‐related changes in cerebral haemodynamics (Zhu et al. 2013). Further, higher cerebrovascular reactivity has been shown to be positively associated with executive function in young adults (Guiney et al. 2015) and overall cognitive functioning (including domains of executive function and processing speed) in older adults (Brown et al. 2010; Davenport et al. 2012). Moreover, as reported above, PA enhances vascular function by resolving endothelial dysfunction and reducing the oxidative stress and inflammation that may underpin this relationship. Davenport et al. (2012) also recently suggested that cerebrovascular reserve promotes neuro‐ and synaptogenesis via increased BDNF and NO production, which may serve to preserve or even improve cognitive function. In an attempt to progress our understanding of this complex relationship, the interaction between PA, cerebrovascular reserve and cognition is currently being investigated in a large prospective study (Tyndall et al. 2013).
Figure 1. Promoting brain health via a triad of healthy lifestyle behaviours .
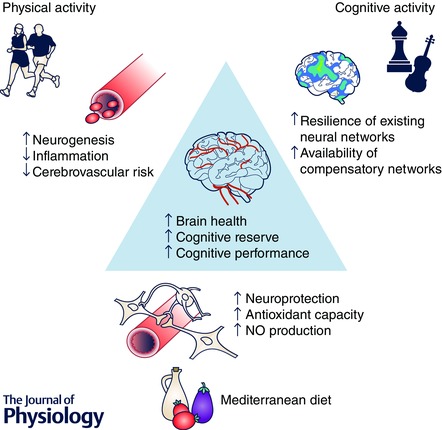
Proposed associations and potential underlying mechanisms between physical activity, cognitive activity and a Mediterranean‐style dietary pattern and increased physical and cognitive performance. The mechanisms underpinning the relationship between cognitive activity and cognitive function may be similar to those of physical activity and potentially include increased resting cerebral blood flow, synaptogenesis and neurogenesis. NO, nitric oxide.
Cognitive activity
Data from observational and longitudinal and cross sectional studies have overwhelmingly suggested that regular cognitive activity (CA) may convey benefits similar to those achieved by PA. Specifically, they have suggested that people who engage in more cognitively stimulating leisure (Crowe et al. 2003), social (Karp et al. 2006; Sorman et al. 2014) and job (Smart et al. 2014) activities are at a reduced risk for cognitive decline (Wilson et al. 2005; Hogan et al. 2012; Mitchell et al. 2012). CA has usually been measured by self‐reported frequency of participating in daily activities deemed to be cognitively, but not physically, stimulating, e.g. reading, playing games, crossword puzzles, etc. (Wilson et al. 2003; Eskes et al. 2010). Retrospective studies have examined the influence of leisure time activities on the risk of developing dementia. The Nun Study found that nuns whose diaries revealed greater time spent in reading, writing and other intellectual activities had a lower incidence of AD than their less academic counterparts (Iacono et al. 2009). Further, a retrospective study of the contributions of cognitive and physical activity on cognitive function in a sedentary group of post‐menopausal women also showed that greater frequency of different activities (not time) was also predictive of cognitive function (overall cognitive performance, attention and executive function), suggesting the importance of variety of stimulation (Eskes et al. 2010). Prospective studies also conclude that increased time spent reading, playing board games, and doing puzzles is associated with a reduction in dementia as diagnosed by neurological and neuropsychological exam, even when patients with possible preclinical dementia are excluded (Verghese et al. 2003).
Interventions aimed at increasing cognitive activities have shown consistently positive results in older adults engaging in structured cognitive training programmes, with a meta‐analysis reporting a moderate effect of cognitive training on subsequent performance (Willis et al. 2006; Papp et al. 2009). Overall, these trials have suggested that engaging in cognitively stimulating activities increases cognitive functioning – measured using standardised neuropsychological tests – and potentially decreases dementia risk. However, most cognitive training interventions are challenged to show generalisability across other cognitive domains or tasks (Papp et al. 2009), although some studies have reported long term benefits on untrained activity and cognitive domains (Ball et al. 2010; Wolinsky et al. 2010). The Cochrane review of cognitive training interventions in healthy older adults and those with mild cognitive impairment has concluded that cognitive training does have beneficial effects of cognitive performance, specific to the tasks that individuals were trained on (Martin et al. 2011). The greatest benefits were on immediate and delayed verbal memory performance; however no benefit was seen on cognitive performance when cognitive training was compared with an active control group.
Cognitive interventions that focus on real‐world skills and lifestyle engagement have had greater success in improving functional outcomes in older adults. Participants in one study were assigned to receive a 14‐week intervention including social interaction and learning quilting, digital photography or both skills (Park et al. 2014). Participants were also expected to engage with the study community for a minimum of 15 hours per week. After participation in this intervention, episodic memory performance was improved in the participants who engaged in the cognitively demanding tasks. A follow‐up to this study found that engaging older adults in a similar intervention learning how to operate a tablet computer also improved memory and processing speed (Chan et al. 2015). Likewise, a structured community‐based volunteer intervention enhanced executive function and figural memory performance, and increased associated prefrontal cortex activation in an at‐risk population (Carlson et al. 2008; Carlson et al. 2009).
Combined cognitive and physical activity interventions
Increasing evidence indicates that the causes of neurodegeneration and ischaemic cell death are multi‐factorial (Iadecola & Anrather, 2011). Indeed, the brain responds to vascular insults by marshalling a wide array of self‐protective mechanisms (Iadecola & Anrather, 2011) that stands in marked contrast to the single‐target approach used to treat AD, stroke and other neurological disorders. Consequently, a series of studies (Langdon & Corbett, 2012; Langdon et al. 2013, 2014) that combined a PA intervention with a CA intervention were conducted in rodents to determine if this combined approach was more effective in enhancing cognitive function than either intervention alone. In the first of these studies rats were exposed to running wheels (PA) for 2 h per day, 5 days a week or to different versions of a Hebb–Williams maze (CA) for an identical time period or the combination of PA and CA. Results showed that only the combined group exhibited improved cognition as reflected by a reduction in the number of working memory errors and improved choice accuracy in an eight‐arm radial arm maze. Increasing the amount of PA from 2 h to 4 h did not enhance cognition. Indeed, only the combined PA–CA group displayed improved learning and memory (Langdon & Corbett, 2012). Subsequently, this ‘cognitive rehabilitation’ paradigm was used in an animal model of vascular cognitive impairment (VCI) to simulate human disease co‐morbidities, which consisted of chronic bilateral carotid artery occlusion of the carotid arteries in middle‐aged rats maintained on a diet high in fat and sugar (Langdon et al. 2013). In this study, VCI rats showed progressive impairment in spatial learning in the Morris water maze with repeated testing over 24 weeks following carotid artery occlusion. In VCI rats exposed to the PA–CA intervention, the cognitive impairments were significantly reduced. Interestingly, the untreated VCI rats had significant hypertrophy in the CA1 area of the hippocampus that was reduced (or prevented) by the PA–CA treatment. Whether the hippocampal CA1 hypertrophy represents a pathological state preceding cell death or instead an intrinsic repair response is not clear, but a similar form of CA1 hypertrophy was found in asymptomatic autopsy brains in the Nun study (Iacono et al. 2009). Finally, a note of caution must be expressed because the PA–CA intervention used to enhance cognitive performance in young, intact male rats (Langdon et al. 2013) and to blunt the cognitive impairments in a middle‐aged, male rat model of VCI did not provide cognitive benefit in a cohort of ovariectomised, middle‐aged female rats (Langdon et al. 2014). The reasons why the cognitive rehabilitation paradigm was effective in male but not female rats is unclear but it may relate to reduced levels of BDNF as a result of ageing and ovariectomy (Langdon et al. 2014). Thus a greater ‘dose’ of exercise may be required to achieve the same levels of BDNF and cognitive performance in female as in male rats, which is in agreement with the aforementioned findings of Pialoux et al. (2009) in post‐menopausal women.
In humans, a 16 week intervention including unsupervised physical activity, computer based brain training, or both found that older adults (60–85 years) who completed both training types had significantly higher long‐term verbal memory compared to the control group (Shah et al. 2014). Intervention studies that have included physical activity, cognition and emotional or cultural training have suggested that the combination of physical and cognitive training can improve both cognitive performance and functional outcomes in healthy older adults (Oswald et al. 2006; Pieramico et al. 2012), although this difference disappears when physical and cognitive activity interventions are compared to an active control group (Lautenschlager & Cox, 2013; D. E. Barnes et al. 2013), suggesting that the benefits of social interaction may also be contributing to the positive effects. Indeed, correlational and interventional studies have indicated positive effects of social interaction on cognitive function (e.g. Winocur et al. 1987; Krueger et al. 2009; Mortimer et al. 2012). To test if, and how, these three factors may interact to slow cognitive decline and reduce the risk of dementia there have been some multi‐modal interventions including physical activity and cognitive activity. One large longitudinal study has suggested that cognitive, social and physical activity equally contribute to reducing dementia risk in older adults. Individuals who had high engagement in all three areas were at a 37% reduced risk for developing dementia compared with individuals who had low engagement (Karp et al. 2006). A combined approach was also adopted in the Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (FINGER), which included a computer‐based cognitive intervention in 1200 individuals who were at risk of cognitive decline (Ngandu et al. 2015). Participants who completed the cognitive intervention, in addition to an exercise and diet intervention, had a modest increase in overall cognitive performance when compared to control participants who received general health advice only. Whilst this study has high external generalisability, it is impossible to disentangle the cognitive benefit associated with cognitive training from the diet and exercise interventions.
Overall these data suggest that engaging with stimulating cognitive activities also has a role in preventing cognitive decline, possibly by increasing cognitive reserve (see Fig. 1). In support of this, it has been demonstrated that CA is positively associated with resting cerebral blood flow (CBF) and increased neural efficiency, both of which correlate well with cognitive function in normal ageing and clinical impairment (Stern, 2009). Moreover, it appears that using a combination approach whereby exercise combined with CA is used to either aid stroke recovery or enhance cognitive function in healthy older adults appears to improve cognitive performance more than PA or CA alone.
Diet
Along with physical and cognitive activity, engaging with a healthy diet is a third modifiable lifestyle factor that has been linked to overall brain health and attenuated cognitive decline. One diet in particular, the Mediterranean Diet (MeDi), characterised by high intake of fruits, vegetables, cereals, fish, nuts and olive oil, has received particular attention in the literature. The benefits of adherence to this type of diet have been evidenced in both epidemiological studies and clinical trials and include reduced risk for developing cancer, metabolic syndrome and vascular disease as well as lower incidence of dementia and AD (Lourida et al. 2013). Results from the Prevención con Dieta Mediterránea (PREDIMED) study showed that risk of stroke – a major risk factor for cognitive impairment – was reduced by 46% during the 4.8 year follow‐up period (median follow‐up time) in participants who followed a Mediterranean style dietary pattern including 30 g of mixed nuts (7.5 g hazelnuts, 7.5 g almonds, 15 g walnuts). In addition, in a subsample of the participants that were tested for neuropsychological function, higher intakes of olive oil, coffee, walnuts and wine were associated with better global cognition and memory function, with walnuts in particular linked to better working memory function (Pribis & Shukitt‐Hale, 2014). The specific effects of some of the component parts of the MeDi such as polyunsaturated fatty acids (PUFAs) and polyphenols on indicators of brain health appear to underpin these positive findings.
Increased consumption of the omega‐3 PUFAs docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA), found predominantly in oily fish, is associated with attenuated cognitive decline and incidence of dementia or AD (Fotuhi et al. 2009). Recent MRI studies have indicated that higher circulating levels of omega‐3 PUFAs are associated with reduced white matter damage and grey matter atrophy, outcomes which were also shown to improve along with executive function following a 6 month dietary intervention (2.2 g day−1 EPA+DHA) in healthy 50–75 year olds (Witte et al. 2014). Underpinning these effects are the many actions of DHA and EPA in the brain. DHA in particular is uniquely accumulated in central nervous system tissue and its presence beneficially impacts upon plasma membrane function, synaptic transmission and cellular signalling (Yehuda et al. 1999). In vitro studies have also revealed a number of neuroprotective properties of DHA and EPA that may improve brain health including attenuation of inflammatory responses by either suppressing pro‐inflammatory pathways and upregulating pro‐resolving mediators such as neuroprotectin D1, or modulating mitochondrial function and reducing oxidative stress (Denis et al. 2015). There is also evidence from animal and human studies to suggest that dietary supplementation with omega‐3 PUFAs improves regional CBF, which may contribute to the preservation of both brain structure and function (Tsukada et al. 2000; Jackson et al. 2012). Adult hippocampal neurogenesis declines with age but appears to be stimulated with omega‐3 supplementation in aged rodents with concurrent improvements in cognitive function (Dyall et al. 2010; Cutuli et al. 2014). Supplementation studies in healthy adults have been less conclusive, however, with two recent meta‐analyses reporting that supplementation with omega‐3 PUFAs has a beneficial effect on episodic memory in samples characterised as suffering from mild memory complaints (Yurko‐Mauro et al. 2015), and a marginal effect of supplementation on working memory in samples that have low baseline omega‐3 PUFA status (Cooper et al. 2015). Therefore, despite compelling cross‐sectional data, these studies suggest either a cognitive or physiological deficit must be present before beneficial effects of relatively short‐term dietary interventions (6 months) are observed. Alternatively, it could be that greater consideration should be given to provision of both EPA and DHA in high quantities (Witte et al. 2014), which may explain the null effects on cognition of a recent 5 year omega‐3 PUFA supplementation study in older adults who were at risk for developing age‐related macular degeneration (Chew et al. 2015), a possibility that requires further exploration.
Polyphenols are phytochemical micronutrients that are ubiquitous in the diet and are predominantly found in fruits and vegetables, as well as coffee, tea, soy, red wine and chocolate. Despite their diversity, many of the beneficial physiological effects resulting from their consumption are common to many polyphenolic compounds and include promoting vascular function, reducing inflammation, combating oxidative stress and enhancing neuroprotection, all of which contribute to the maintenance of brain health during ageing. For example, the effects of cocoa flavan‐3‐ols (flavanols) have been well characterised in the literature; at the epidemiological level their consumption is associated with lower blood pressure and increased peripheral blood flow and inversely related to incidence of cardiovascular disease in late adulthood (Haskell & Watson, 2013). Studies in young adults have demonstrated improved cognitive function during demanding tasks following an acute dose of 520 mg cocoa flavanols (Scholey et al. 2010), and in older adults following an 8 week dietary intervention with both high (993 mg day−1) and intermediate (520 mg day−1) doses (Mastroiacovo et al. 2015), although null effects have also been reported (e.g. Pase et al. 2013). These effects may be attributable to a number of mechanisms including improved insulin sensitivity (Mastroiacovo et al. 2015), increased CBF (Haskell‐Ramsay et al. 2015) and improved functioning of the dentate gyrus (Brickman et al. 2014).
The phytoalexin resveratrol found in the skin of red grapes and hence red wine also shows potential for preserving brain health. In vivo studies have revealed an impressive list of effects of this polyphenol, which has been shown to possess anti‐inflammatory, antiviral and antioxidant properties, to protect against the development of cancer and cardiovascular disease, to improve insulin sensitivity and even to increase longevity (Kennedy & Wightman, 2011). In addition, resveratrol offers neuroprotection by increasing CBF and perfusion and attenuating amyloid‐β‐plaque formation in a transgenic mouse model of AD (Karuppagounder et al. 2009; Kennedy et al. 2010). Whilst increased CBF following an acute dose of resveratrol (250 mg) in healthy young adults has been repeatedly observed (Kennedy et al. 2010; Wightman et al. 2014), to date no effect on cognition in humans has been reported. This may be due in part to the cognitive testing paradigms that have been employed, or the bioavailability of the parent compound, or it may simply be that the cognitive effects of resveratrol may only be observed in populations where cognitive function is already impaired. To date, however, studies assessing the effect of resveratrol on cognition in older adults with cognitive impairment have yet to be conducted.
Berry fruits are another example of food high in polyphenols shown to contribute towards brain health and preserve cognitive function in ageing. Impressively, a large epidemiological study concluded that high consumption of berry fruits such as blueberries, strawberries and blackberries – rich in anthocyanin polyphenols – actually delayed cognitive ageing by 2.5 years (Devore et al. 2012). The effects of blueberries have been most widely studied, the anthocyanin compounds of which have been shown to cross the blood–brain barrier. In the brains of aged rodents, blueberry supplementation appears to reduce nuclear transcription factor κΒ, a marker of oxidative stress and inflammation; enhance activation of the cAMP response element binding protein, which is pivotal for maintaining neuronal plasticity; and increase BDNF, which supports neuronal survival and neurogenesis (Pribis & Shukitt‐Hale, 2014). Further, these changes in the brain correlate well with memory performance in these supplemented animals. The study of the effects of anthocyanins in humans is limited to a couple of small scale studies; however, the results of these show promise for improving learning, and episodic and spatial memory in older adults with mild cognitive impairment (Pribis & Shukitt‐Hale, 2014).
Other, less well‐researched dietary components are also showing promise for promoting brain health. Of these, there has been rapidly growing interest in dietary nitrate, found in high concentrations in red beetroot as well as lettuce and spinach. Dietary nitrate is reduced to nitric oxide in vivo, a cellular signalling molecule that promotes endothelial function, a depletion of which is observed in ageing and may contribute to cerebral hypoperfusion commonly associated with cognitive decline (Lidder & Webb, 2013). Beetroot, either in juice or supplement form, has been shown to attenuate oxidative stress and inflammation in vitro and in rodent models, and positive effects on endothelial function in humans have also been observed (Clifford et al. 2015). Interestingly, two recent intervention studies have shown differing effects of acute beetroot juice consumption on CBF in the prefrontal cortex in older and younger adults with increased perfusion in this area being observed in older adults at rest (Presley et al. 2011), whilst reductions in local CBF during completion of cognitive tasks was observed in younger adults (Thompson et al. 2014). In the case of the latter, concurrent improvement on one of the cognitive tasks was observed, which the authors suggest as potentially indicative of a positive effect of dietary nitrate on neural efficiency. Whether the observed increased cerebral perfusion in older adults is associated with similar improvements in cognitive performance remains to be seen. Therefore, larger, longer intervention studies are required, with a specific focus on the effect of beetroot in older populations.
All the available evidence suggests that following a Mediterranean style diet is beneficial for preserving brain health and hence function during ageing. The individual effects of some of the active components of this diet with regard to brain function are the topic of intense current investigation. Although not consistently translated into observable effects in humans, in vitro and in vivo work indicates that each of these has numerous positive actions that serve to enhance cerebral circulation and offer neuroprotection, which may work to increase both brain and cerebrovascular reserve and thus maintain cognitive function (see Fig. 1). It must be said, however, that the effectiveness of a dietary strategy to combat decline, improve current functioning or even as an adjunctive treatment to pharmacological approaches in combating neurodegenerative diseases associated with ageing is still greatly under‐researched. Further, if these dietary components are indeed effective in attenuating brain ageing, whether there is a criterial period of intervention remains to be addressed. Lastly, whilst the specific action and effectiveness of these dietary components is of academic interest – especially given that the dosages provided in the studies discussed here are not always achievable by dietary means alone – the diet as a whole, which includes other fats, proteins and micronutrients, must be considered and the possibility of interactions and synergies explored. The type of long‐term large‐scale studies that are required to adequately illuminate these issues are resource intensive and thus uncommon in the literature. In this way, the in vitro and animal studies will continue to complement more practicable human trials in order to advance our understanding of the effects of diet on the ageing brain.
Conclusions
The evidence described here suggests that the older adult brain retains its capacity for plasticity and that a triad of healthy behaviours may be key to taking advantage of this fundamental neural property. We propose that reserve is built up through engaging with physical and cognitive activities and adhering to a diet rich in healthy fats and plant phytochemicals so that the clinical manifestations of ageing and neurodegenerative disease are kept at bay for longer. Adopting a brain health‐promoting lifestyle may even improve current cognition function as well as attenuate decline in addition to preventing the development of other age‐related diseases, although this is not universally reported. Interestingly, particularly promising effects on cognitive performance are found when physical and cognitive activities are combined; a three‐pronged approach including a MeDi intervention has yet to be developed and may prove to be even more beneficial. Therefore, the design of future studies should allow for a comparison of the effects of singular and combined approaches.
Finally, there are still many unanswered questions. The majority of human research in this area has focused on the effects of these lifestyle interventions in healthy older adults; their effectiveness as a potential adjunctive treatment to traditional therapies for cognitive impairment is currently not established, although animal models continue to show promise. In addition, it is also unknown whether there exists a window of opportunity during which implementing these behaviours yields the best results in terms of functional outcome and at which point the downward trajectory of cognitive function can be influenced no further. Moreover, other, less well understood modifiable lifestyle factors such as sleep may also promote brain health in similar ways as described here, which is a current emerging area of interest. In 2013, the G8 Dementia Summit Declaration revealed the worldwide annual cost of dementia associated care as US$604 billion. Moreover, the cost of care is expected to increase as the prevalence of dementia increases beyond the current capacity of many health care systems across the globe. Therefore, cost‐effective approaches to combating cognitive impairment are imperative and additional research is needed to determine effective and sustainable ways of increasing these promising healthy behaviours at the community level.
Additional information
Competing interests
None declared.
Author contributions
All authors have approved the final version of the manuscript and agree to be accountable for all aspects of the work. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Funding
M.J.P. holds the Brenda Strafford Foundation Chair in Alzheimer Research. His research program is supported by funding from the Canadian Institutes of Health Research (CIHR), the Heart and Stroke Foundation of Canada (HSFC), the Natural Sciences and Engineering Research Council of Canada (NSERC), the Alzheimer Society of Canada, the Hotchkiss Brain Institute, and Alberta Innovates Health Solutions (AIHS). V.P. is funded by the Institut Universitaire de France. Research support to D.C. from the CIHR, the HSFC and NSERC is gratefully acknowledged. L.D. holds an AIHS Postdoctoral Fellowship. K.E.’s research is funded by National Institutes for Health (NIH) (NIH R01 DK095172, NIH P30 MH90333), and also by the Alzheimer's Association (NPSASA‐14‐321093). G.E. was supported by an Alberta Heritage Foundation for Medical Research Visiting Scientist Award, a HSFC Visiting Scholar Award and the CIHR.
Acknowledgements
The authors would like to thank Rich Rawling for his graphic design contributions to Fig. 1.
Biographies
Philippa A. Jackson completed her PhD at Northumbria University (UK) in 2010; for it she investigated the effects of supplemental omega‐3 polyunsaturated fatty acids on cognition in healthy young adults. She has since then continued at Northumbria University as a Research Fellow focusing on the effects of dietary interventions for promoting cognitive function and cerebral blood flow throughout the lifespan.
![]()
Marc J. Poulin completed a PhD in exercise physiology at the University of Western Ontario (Canada). He then completed a DPhil and postdoctoral fellowship in respiratory and cerebrovascular physiology at Oxford with Peter Robbins as supervisor and mentor. He was recruited to the University of Calgary (Canada) in 2000 and he is now Professor and The Brenda Strafford Foundation Chair in Alzheimer Research. His research focuses on healthy brain ageing. Vincent Pialoux completed a PhD in exercise physiology at the University Blaise Pascal (Clermont‐Ferrand, France). He was then postdoctoral fellow in respiratory and cerebrovascular physiology at the University of Calgary with Marc Poulin as supervisor. He was recruited to the University of Lyon (France) in 2009 as assistant Professor and he is now Professor and holds an Institut Universitaire de France Chair. His research focusses on the role of oxidative stress on vascular and cerebrovascular diseases and the effects of physical activity on this pathological mechanism. Kirk Erickson completed a PhD in cognitive psychology in 2005 at the University of Illinois at Urbana‐Champaign under the mentorship of Dr. Arthur Kramer. He then went on to a postdoctoral fellowship at the Beckman Institute for Advanced Science and Technology at the University of Illinois. He was recruited to the University of Pittsburgh in 2008 and is now an Associate Professor of Psychology, Medicine, and is appointed in the Center for the Neural Basis of Cognition and the Center for Neuroscience at the University of Pittsburgh. His research focuses on healthy brain aging with an emphasis on the role of physical activity on brain health. Dale Corbett completed a PhD in behavioural neuroscience at Concordia University and post‐doctoral studies at McGill. He was a faculty member at Harvard and Memorial University of Newfoundland prior to his recruitment to the University of Ottawa as Professor and Scientific Director & CEO of the Heart and Stroke Foundation Canadian Partnership for Stroke Recovery. His research focuses on recovery of function following stroke. Gail A. Eskes completed a PhD in Psychology at the University of California, Berkeley, USA and a postdoctoral fellowship focused on memory and aging at the Rotman Research Institute, Toronto, Canada. She is currently a clinical neuropsychologist and Professor at Dalhousie University, Halifax, Canada and her research focuses on developing evidence‐based rehabilitation tools for cognitive deficits due to ageing or brain disorders such as Parkinsons disease or stroke. Lauren L. Drogos completed a PhD in psychology at the University of Illinois at Chicago, under the mentorship of Dr. Pauline M. Maki, investigating the effects of vasomotor symptoms on autonomic nervous system activity in midlife women. She is currently working as a postdoctoral fellow at the University of Calgary with Dr. Marc J. Poulin, investigating the effects of exercise on cognitive performance in healthy older adults. Her specific focus is on identifying potential predictors of cognitive decline, such as sleep disturbances or neuroendocrine changes.
This review was presented at the symposium ‘Ageing, exercise and brain health’, which took place at The Physiological Society's Ageing and Degeneration: A Physiological Perspective meeting in Edinburgh, UK between 10–11 April 2015.
References
- Bailey DM, Marley CJ, Brugniaux JV, Hodson D, New KJ, Ogoh S & Ainslie PN (2013). Elevated aerobic fitness sustained throughout the adult lifespan is associated with improved cerebral hemodynamics. Stroke 44, 3235–3238. [DOI] [PubMed] [Google Scholar]
- Ball K, Edwards JD, Ross LA & McGwin G Jr (2010). Cognitive training decreases motor vehicle collision involvement of older drivers. J Am Geriatr Soc 58, 2107–2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes DE, Santos‐Modesitt W, Poelke G, Kramer AF, Castro C, Middleton LE & Yaffe K (2013). The Mental Activity and eXercise (MAX) trial: a randomized controlled trial to enhance cognitive function in older adults. JAMA Intern Med 173, 797–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes JN, Taylor JL, Kluck BN, Johnson CP & Joyner MJ (2013). Cerebrovascular reactivity is associated with maximal aerobic capacity in healthy older adults. J Appl Physiol (1985) 114, 1383–1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickman AM, Khan UA, Provenzano FA, Yeung LK, Suzuki W, Schroeter H, Wall M, Sloan RP & Small SA (2014). Enhancing dentate gyrus function with dietary flavanols improves cognition in older adults. Nat Neurosci 17, 1798–1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AD, McMorris CA, Longman RS, Leigh R, Hill MD, Friedenreich CM & Poulin MJ (2010). Effects of cardiorespiratory fitness and cerebral blood flow on cognitive outcomes in older women. Neurobiol Aging 31, 2047–2057. [DOI] [PubMed] [Google Scholar]
- Burdette JH, Laurienti PJ, Espeland MA, Morgan A, Telesford Q, Vechlekar CD, Hayasaka S, Jennings JM, Katula JA, Kraft RA & Rejeski WJ (2010). Using network science to evaluate exercise‐associated brain changes in older adults. Front Aging Neurosci 2, 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson MC, Erickson KI, Kramer AF, Voss MW, Bolea N, Mielke M, McGill S, Rebok GW, Seeman T & Fried LP (2009). Evidence for neurocognitive plasticity in at‐risk older adults: the experience corps program. J Gerontol A Biol Sci Med Sci 64, 1275–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson MC, Saczynski JS, Rebok GW, Seeman T, Glass TA, McGill S, Tielsch J, Frick KD, Hill J & Fried LP (2008). Exploring the effects of an “everyday” activity program on executive function and memory in older adults: Experience Corps. Gerontologist 48, 793–801. [DOI] [PubMed] [Google Scholar]
- Casserly I & Topol E (2004). Convergence of atherosclerosis and Alzheimer's disease: inflammation, cholesterol, and misfolded proteins. Lancet 363, 1139–1146. [DOI] [PubMed] [Google Scholar]
- Chaddock L, Erickson KI, Prakash RS, Kim JS, Voss MW, Vanpatter M, Pontifex MB, Raine LB, Konkel A, Hillman CH, Cohen NJ & Kramer AF (2010). A neuroimaging investigation of the association between aerobic fitness, hippocampal volume, and memory performance in preadolescent children. Brain Res 1358, 172–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan MY, Haber S, Drew LM & Park DC (2015). Training older adults to use tablet computers: does it enhance cognitive function? Gerontologist 56, 475–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chew EY, Clemons TE, Agron E, Launer LJ, Grodstein F, Bernstein PS & Age‐Related Eye Disease Study 2 (AREDS2) Research Group (2015). Effect of omega‐3 fatty acids, lutein/zeaxanthin, or other nutrient supplementation on cognitive function: The AREDS2 randomized clinical trial. JAMA 314, 791–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirico EN, Patsouris D, Geloen A, Rieusset J, Abidi R, Canet‐Soulas E & Pialoux V (2012). The role of exercise on oxidative stress and inflammation in the aging brain. FASEB J 26, 1138.18. [Google Scholar]
- Clifford T, Howatson G, West DJ & Stevenson EJ (2015). The potential benefits of red beetroot supplementation in health and disease. Nutrients 7, 2801–2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colcombe S & Kramer AF (2003). Fitness effects on the cognitive function of older adults: A meta‐analytic study. Psychol Sci 14, 125–130. [DOI] [PubMed] [Google Scholar]
- Colcombe SJ, Erickson KI, Scalf PE, Kim JS, Prakash R, McAuley E, Elavsky S, Marquez DX, Hu L & Kramer AF (2006). Aerobic exercise training increases brain volume in aging humans. J Gerontol A Biol Sci Med Sci 61, 1166–1170. [DOI] [PubMed] [Google Scholar]
- Cooper RE, Tye C, Kuntsi J, Vassos E & Asherson P (2015). Omega‐3 polyunsaturated fatty acid supplementation and cognition: A systematic review and meta‐analysis. J Psychopharmacol 29, 753–763. [DOI] [PubMed] [Google Scholar]
- Cotman CW & Berchtold NC (2002). Exercise: A behavioral intervention to enhance brain health and plasticity. Trends Neurosci 25, 295–302. [DOI] [PubMed] [Google Scholar]
- Cotman CW, Berchtold NC & Christie LA (2007). Exercise builds brain health: key roles of growth factor cascades and inflammation. Trends Neurosci 30, 464–472. [DOI] [PubMed] [Google Scholar]
- Coyle JT & Puttfarcken P (1993). Oxidative stress, glutamate, and neurodegenerative disorders. Science 262, 689–695. [DOI] [PubMed] [Google Scholar]
- Creer DJ, Romberg C, Saksida LM, van Praag H & Bussey TJ (2010). Running enhances spatial pattern separation in mice. Proc Natl Acad Sci USA 107, 2367–2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowe M, Andel R, Pedersen NL, Johansson B & Gatz M (2003). Does participation in leisure activities lead to reduced risk of Alzheimer's disease? A prospective study of Swedish twins. J Gerontol B Psychol Sci Soc Sci 58, P249–255. [DOI] [PubMed] [Google Scholar]
- Cutuli D, De Bartolo P, Caporali P, Laricchiuta D, Foti F, Ronci M, Rossi C, Neri C, Spalletta G, Caltagirone C, Farioli‐Vecchioli S & Petrosini L (2014). n‐3 polyunsaturated fatty acids supplementation enhances hippocampal functionality in aged mice. Front Aging Neurosci 6, 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davenport MH, Hogan DB, Eskes GA, Longman RS & Poulin MJ (2012). Cerebrovascular reserve: the link between fitness and cognitive function? Exerc Sport Sci Rev 40, 153–158. [DOI] [PubMed] [Google Scholar]
- Denis I, Potier B, Heberden C & Vancassel S (2015). Omega‐3 polyunsaturated fatty acids and brain aging. Curr Opin Clin Nutr Metab Care 18, 139–146. [DOI] [PubMed] [Google Scholar]
- Devore EE, Kang JH, Breteler MM & Grodstein F (2012). Dietary intakes of berries and flavonoids in relation to cognitive decline. Ann Neurol 72, 135–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta P, Courties G, Wei Y, Leuschner F, Gorbatov R, Robbins CS, Iwamoto Y, Thompson B, Carlson AL, Heidt T, Majmudar MD, Lasitschka F, Etzrodt M, Waterman P, Waring MT, Chicoine AT, van der Laan AM, Niessen HW, Piek JJ, Rubin BB, Butany J, Stone JR, Katus HA, Murphy SA, Morrow DA, Sabatine MS, Vinegoni C, Moskowitz MA, Pittet MJ, Libby P, Lin CP, Swirski FK, Weissleder R & Nahrendorf M (2012). Myocardial infarction accelerates atherosclerosis. Nature 487, 325–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyall SC, Michael GJ & Michael‐Titus AT (2010). Omega‐3 fatty acids reverse age‐related decreases in nuclear receptors and increase neurogenesis in old rats. J Neurosci Res 88, 2091–2102. [DOI] [PubMed] [Google Scholar]
- Erickson KI, Leckie RL & Weinstein AM (2014). Physical activity, fitness, and gray matter volume. Neurobiol Aging 35 Suppl 2, S20–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson KI, Prakash RS, Voss MW, Chaddock L, Hu L, Morris KS, White SM, Wojcicki TR, McAuley E & Kramer AF (2009). Aerobic fitness is associated with hippocampal volume in elderly humans. Hippocampus 19, 1030–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson KI, Raji CA, Lopez OL, Becker JT, Rosano C, Newman AB, Gach HM, Thompson PM, Ho AJ & Kuller LH (2010). Physical activity predicts gray matter volume in late adulthood: the Cardiovascular Health Study. Neurology 75, 1415–1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson KI, Voss MW, Prakash RS, Basak C, Szabo A, Chaddock L, Kim JS, Heo S, Alves H, White SM, Wojcicki TR, Mailey E, Vieira VJ, Martin SA, Pence BD, Woods JA, McAuley E & Kramer AF (2011). Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci USA 108, 3017–3022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskes GA, Longman S, Brown AD, McMorris CA, Langdon KD, Hogan DB & Poulin M (2010). Contribution of physical fitness, cerebrovascular reserve and cognitive stimulation to cognitive function in post‐menopausal women. Front Aging Neurosci 2, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fotuhi M, Mohassel P & Yaffe K (2009). Fish consumption, long‐chain omega‐3 fatty acids and risk of cognitive decline or Alzheimer disease: a complex association. Nat Clin Pract Neurol 5, 140–152. [DOI] [PubMed] [Google Scholar]
- Garcia‐Mesa Y, Colie S, Corpas R, Cristofol R, Comellas F, Nebreda AR, Gimenez‐Llort L & Sanfeliu C (2015). Oxidative stress is a central target for physical exercise neuroprotection against pathological brain aging. J Gerontol A Biol Sci Med Sci 71, 40–49. [DOI] [PubMed] [Google Scholar]
- Guiney H, Lucas SJ, Cotter JD & Machado L (2015). Evidence cerebral blood‐flow regulation mediates exercise‐cognition links in healthy young adults. Neuropsychology 29, 1–9. [DOI] [PubMed] [Google Scholar]
- Hafezi‐Moghadam A, Thomas KL & Wagner DD (2007). ApoE deficiency leads to a progressive age‐dependent blood‐brain barrier leakage. Am J Physiol Cell Physiol 292, C1256–1262. [DOI] [PubMed] [Google Scholar]
- Haskell‐Ramsay CF, Jackson PA, Forster JS, Laverick B & Sutherland, K (2015). Acute consumption of a cocoa flavanol‐enriched drink increases cerebral haemoglobin levels at rest and during cognitive performance in healthy adults In 9th World Congress on Polyphenols Applications. International Society of Antioxidants, Malta. [Google Scholar]
- Haskell CF & Watson AW (2013). Cocoa, blood flow and the brain In Bioactives in Fruit: Health Properties and Functional Foods, ed. Skinner M. & Hunter D, pp. 367–388. Wiley‐Blackwell. [Google Scholar]
- Herting MM & Nagel BJ (2012). Aerobic fitness relates to learning on a virtual Morris Water Task and hippocampal volume in adolescents. Behav Brain Res 233, 517–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan MJ, Staff RT, Bunting BP, Deary IJ & Whalley LJ (2012). Openness to experience and activity engagement facilitate the maintenance of verbal ability in older adults. Psychol Aging 27, 849–854. [DOI] [PubMed] [Google Scholar]
- Honea RA, Thomas GP, Harsha A, Anderson HS, Donnelly JE, Brooks WM & Burns JM (2009). Cardiorespiratory fitness and preserved medial temporal lobe volume in Alzheimer disease. Alzheimer Dis Assoc Disord 23, 188–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacono D, Markesbery WR, Gross M, Pletnikova O, Rudow G, Zandi P & Troncoso JC (2009). The Nun study: clinically silent AD, neuronal hypertrophy, and linguistic skills in early life. Neurology 73, 665–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iadecola C & Anrather J (2011). Stroke research at a crossroad: asking the brain for directions. Nat Neurosci 14, 1363–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson PA, Reay JL, Scholey AB & Kennedy DO (2012). DHA‐rich fish oil modulates the cerebral hemodynamic response to cognitive tasks in healthy young adults. Biol Psychol 89, 183–190. [DOI] [PubMed] [Google Scholar]
- Karp A, Paillard‐Borg S, Wang HX, Silverstein M, Winblad B & Fratiglioni L (2006). Mental, physical and social components in leisure activities equally contribute to decrease dementia risk. Dement Geriatr Cogn Disord 21, 65–73. [DOI] [PubMed] [Google Scholar]
- Karuppagounder SS, Pinto JT, Xu H, Chen HL, Beal MF & Gibson GE (2009). Dietary supplementation with resveratrol reduces plaque pathology in a transgenic model of Alzheimer's disease. Neurochem Int 54, 111–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy DO & Wightman EL (2011). Herbal extracts and phytochemicals: plant secondary metabolites and the enhancement of human brain function. Adv Nutr 2, 32–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy DO, Wightman EL, Reay JL, Lietz G, Okello EJ, Wilde A & Haskell CF (2010). Effects of resveratrol on cerebral blood flow variables and cognitive performance in humans: a double‐blind, placebo‐controlled, crossover investigation. Am J Clin Nutr 91, 1590–1597. [DOI] [PubMed] [Google Scholar]
- Krueger KR, Wilson RS, Kamenetsky JM, Barnes LL, Bienias JL & Bennett DA (2009). Social engagement and cognitive function in old age. Exp Aging Res 35, 45–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langdon KD & Corbett D (2012). Improved working memory following novel combinations of physical and cognitive activity. Neurorehabil Neural Repair 26, 523–532. [DOI] [PubMed] [Google Scholar]
- Langdon KD, Granter‐Button S, Harley CW, Moody‐Corbett F, Peeling J & Corbett D (2013). Cognitive rehabilitation reduces cognitive impairment and normalizes hippocampal CA1 architecture in a rat model of vascular dementia. J Cereb Blood Flow Metab 33, 872–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langdon KD, Granter‐Button S, Harley CW, Moody‐Corbett F, Peeling J & Corbett D (2014). A cognitive rehabilitation paradigm effective in male rats lacks efficacy in female rats. J Cereb Blood Flow Metab 34, 1673–1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lautenschlager NT & Cox KL (2013). Can participation in mental and physical activity protect cognition in old age?: Comment on “The Mental Activity and eXercise (MAX) trial: a randomized controlled trial to enhance cognitive function in older adults”. JAMA Intern Med 173, 805–806. [DOI] [PubMed] [Google Scholar]
- Lautenschlager NT, Cox KL, Flicker L, Foster JK, van Bockxmeer FM, Xiao J, Greenop KR & Almeida OP (2008). Effect of physical activity on cognitive function in older adults at risk for Alzheimer disease: a randomized trial. JAMA 300, 1027–1037. [DOI] [PubMed] [Google Scholar]
- Lesniewski LA, Durrant JR, Connell ML, Henson GD, Black AD, Donato AJ & Seals DR (2011). Aerobic exercise reverses arterial inflammation with aging in mice. Am J Physiol Heart Circ Physiol 301, H1025–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lidder S & Webb AJ (2013). Vascular effects of dietary nitrate (as found in green leafy vegetables and beetroot) via the nitrate‐nitrite‐nitric oxide pathway. Br J Clin Pharmacol 75, 677–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lourida I, Soni M, Thompson‐Coon J, Purandare N, Lang IA, Ukoumunne OC & Llewellyn DJ (2013). Mediterranean diet, cognitive function, and dementia: a systematic review. Epidemiology 24, 479–489. [DOI] [PubMed] [Google Scholar]
- Martin M, Clare L, Altgassen AM, Cameron MH & Zehnder F (2011). Cognition‐based interventions for healthy older people and people with mild cognitive impairment. Cochrane Database Syst Rev, CD006220. [DOI] [PubMed] [Google Scholar]
- Mastroiacovo D, Kwik‐Uribe C, Grassi D, Necozione S, Raffaele A, Pistacchio L, Righetti R, Bocale R, Lechiara MC, Marini C, Ferri C & Desideri G (2015). Cocoa flavanol consumption improves cognitive function, blood pressure control, and metabolic profile in elderly subjects: the Cocoa, Cognition, and Aging (CoCoA) Study–a randomized controlled trial. Am J Clin Nutr 101, 538–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meir KS & Leitersdorf E (2004). Atherosclerosis in the apolipoprotein E‐deficient mouse – A decade of progress. Arterioscler Thromb Vasc Biol 24, 1006–1014. [DOI] [PubMed] [Google Scholar]
- Mitchell MB, Cimino CR, Benitez A, Brown CL, Gibbons LE, Kennison RF, Shirk SD, Atri A, Robitaille A, Macdonald SW, Lindwall M, Zelinski EM, Willis SL, Schaie KW, Johansson B, Dixon RA, Mungas DM, Hofer SM & Piccinin AM (2012). Cognitively stimulating activities: effects on cognition across four studies with up to 21 years of longitudinal data. J Aging Res 2012, 461592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortimer JA, Ding D, Borenstein AR, DeCarli C, Guo QH, Wu YG, Zhao QH & Chu SG (2012). Changes in brain volume and cognition in a randomized trial of exercise and social interaction in a community‐based sample of non‐demented Chinese elders. J Alzheimers Dis 30, 757–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngandu T, Lehtisalo J, Solomon A, Levalahti E, Ahtiluoto S, Antikainen R, Backman L, Hanninen T, Jula A, Laatikainen T, Lindstrom J, Mangialasche F, Paajanen T, Pajala S, Peltonen M, Rauramaa R, Stigsdotter‐Neely A, Strandberg T, Tuomilehto J, Soininen H & Kivipelto M (2015). A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at‐risk elderly people (FINGER): a randomised controlled trial. Lancet 385, 2255–2263. [DOI] [PubMed] [Google Scholar]
- Niemann C, Godde B & Voelcker‐Rehage C (2014). Not only cardiovascular, but also coordinative exercise increases hippocampal volume in older adults. Front Aging Neurosci 6, 170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oswald W, Gunzelmann T, Rupprecht R & Hagen B (2006). Differential effects of single versus combined cognitive and physical training with older adults: the SimA study in a 5‐year perspective. Eur J Ageing 3, 179–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papp KV, Walsh SJ & Snyder PJ (2009). Immediate and delayed effects of cognitive interventions in healthy elderly: a review of current literature and future directions. Alzheimers Dement 5, 50–60. [DOI] [PubMed] [Google Scholar]
- Park DC, Lodi‐Smith J, Drew L, Haber S, Hebrank A, Bischof GN & Aamodt W (2014). The impact of sustained engagement on cognitive function in older adults: the Synapse Project. Psychol Sci 25, 103–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pase MP, Scholey AB, Pipingas A, Kras M, Nolidin K, Gibbs A, Wesnes K & Stough C (2013). Cocoa polyphenols enhance positive mood states but not cognitive performance: a randomized, placebo‐controlled trial. J Psychopharmacol 27, 451–458. [DOI] [PubMed] [Google Scholar]
- Pellegrin M, Miguet‐Alfonsi C, Bouzourene K, Aubert JF, Deckert V, Berthelot A, Mazzolai L & Laurant P (2009). Long‐term exercise stabilizes atherosclerotic plaque in ApoE knockout mice. Med Sci Sports Exerc 41, 2128–2135. [DOI] [PubMed] [Google Scholar]
- Pialoux V, Brown AD, Leigh R, Friedenreich CM & Poulin MJ (2009). Effect of cardiorespiratory fitness on vascular regulation and oxidative stress in postmenopausal women. Hypertension 54, 1014–1020. [DOI] [PubMed] [Google Scholar]
- Pieramico V, Esposito R, Sensi F, Cilli F, Mantini D, Mattei PA, Frazzini V, Ciavardelli D, Gatta V, Ferretti A, Romani GL & Sensi SL (2012). Combination training in aging individuals modifies functional connectivity and cognition, and is potentially affected by dopamine‐related genes. PLoS One 7, e43901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poirier J (2000). Apolipoprotein E and Alzheimer's disease – A role in amyloid catabolism Ann NY Acad Sci 924, 81–90. [DOI] [PubMed] [Google Scholar]
- Presley TD, Morgan AR, Bechtold E, Clodfelter W, Dove RW, Jennings JM, Kraft RA, King SB, Laurienti PJ, Rejeski WJ, Burdette JH, Kim‐Shapiro DB & Miller GD (2011). Acute effect of a high nitrate diet on brain perfusion in older adults. Nitric Oxide 24, 34–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pribis P & Shukitt‐Hale B (2014). Cognition: the new frontier for nuts and berries. Am J Clin Nutr 100 Suppl 1, 347S–352S. [DOI] [PubMed] [Google Scholar]
- Prince M, Bryce R, Albanese E, Wimo A, Ribeiro W & Ferri CP (2013). The global prevalence of dementia: a systematic review and metaanalysis. Alzheimers Dement 9, 63–75 e62. [DOI] [PubMed] [Google Scholar]
- Rovio S, Spulber G, Nieminen LJ, Niskanen E, Winblad B, Tuomilehto J, Nissinen A, Soininen H & Kivipelto M (2010). The effect of midlife physical activity on structural brain changes in the elderly. Neurobiol Aging 31, 1927–1936. [DOI] [PubMed] [Google Scholar]
- Scholey AB, French SJ, Morris PJ, Kennedy DO, Milne AL & Haskell CF (2010). Consumption of cocoa flavanols results in acute improvements in mood and cognitive performance during sustained mental effort. J Psychopharmacol 24, 1505–1514. [DOI] [PubMed] [Google Scholar]
- Shah T, Verdile G, Sohrabi H, Campbell A, Putland E, Cheetham C, Dhaliwal S, Weinborn M, Maruff P, Darby D & Martins RN (2014). A combination of physical activity and computerized brain training improves verbal memory and increases cerebral glucose metabolism in the elderly. Transl Psychiatry 4, e487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sink KM, Espeland MA, Castro CM, Church T, Cohen R, Dodson JA, Guralnik J, Hendrie HC, Jennings J, Katula J, Lopez OL, McDermott MM, Pahor M, Reid KF, Rushing J, Verghese J, Rapp S & Williamson JD (2015). Effect of a 24‐month physical activity intervention vs health education on cognitive outcomes in sedentary older adults: The LIFE randomized trial. JAMA 314, 781–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart EL, Gow AJ & Deary IJ (2014). Occupational complexity and lifetime cognitive abilities. Neurology 83, 2285–2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PJ, Blumenthal JA, Hoffman BM, Cooper H, Strauman TA, Welsh‐Bohmer K, Browndyke JN & Sherwood A (2010). Aerobic exercise and neurocognitive performance: a meta‐analytic review of randomized controlled trials. Psychosom Med 72, 239–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofi F, Valecchi D, Bacci D, Abbate R, Gensini GF, Casini A & Macchi C (2011). Physical activity and risk of cognitive decline: a meta‐analysis of prospective studies. J Intern Med 269, 107–117. [DOI] [PubMed] [Google Scholar]
- Sorman DE, Sundstrom A, Ronnlund M, Adolfsson R & Nilsson LG (2014). Leisure activity in old age and risk of dementia: a 15‐year prospective study. J Gerontol B Psychol Sci Soc Sci 69, 493–501. [DOI] [PubMed] [Google Scholar]
- Stern Y (2009). Cognitive reserve. Neuropsychologia 47, 2015–2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain RA, Harris AB, Wiener EC, Dutka MV, Morris HD, Theien BE, Konda S, Engberg K, Lauterbur PC & Greenough WT (2003). Prolonged exercise induces angiogenesis and increases cerebral blood volume in primary motor cortex of the rat. Neuroscience 117, 1037–1046. [DOI] [PubMed] [Google Scholar]
- Szostak J & Laurant P (2011). The forgotten face of regular physical exercise: a ‘natural’ anti‐atherogenic activity. Clin Sci (Lond) 121, 91–106. [DOI] [PubMed] [Google Scholar]
- ten Brinke LF, Bolandzadeh N, Nagamatsu LS, Hsu CL, Davis JC, Miran‐Khan K & Liu‐Ambrose T (2015). Aerobic exercise increases hippocampal volume in older women with probable mild cognitive impairment: a 6‐month randomised controlled trial. Br J Sports Med 49, 248–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson KG, Turner L, Prichard J, Dodd F, Kennedy DO, Haskell C, Blackwell JR & Jones AM (2014). Influence of dietary nitrate supplementation on physiological and cognitive responses to incremental cycle exercise. Respir Physiol Neuro 193, 11–20. [DOI] [PubMed] [Google Scholar]
- Tsukada H, Kakiuchi T, Fukumoto D, Nishiyama S & Koga K (2000). Docosahexaenoic acid (DHA) improves the age‐related impairment of the coupling mechanism between neuronal activation and functional cerebral blood flow response: a PET study in conscious monkeys. Brain Res 862, 180–186. [DOI] [PubMed] [Google Scholar]
- Tyndall AV, Davenport MH, Wilson BJ, Burek GM, Arsenault‐Lapierre G, Haley E, Eskes GA, Friedenreich CM, Hill MD, Hogan DB, Longman RS, Anderson TJ, Leigh R, Smith EE & Poulin MJ (2013). The brain‐in‐motion study: effect of a 6‐month aerobic exercise intervention on cerebrovascular regulation and cognitive function in older adults. BMC Geriatr 13, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H, Kempermann G & Gage FH (1999). Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neurosci 2, 266–270. [DOI] [PubMed] [Google Scholar]
- Verghese J, Lipton RB, Katz MJ, Hall CB, Derby CA, Kuslansky G, Ambrose AF, Sliwinski M & Buschke H (2003). Leisure activities and the risk of dementia in the elderly. N Engl J Med 348, 2508–2516. [DOI] [PubMed] [Google Scholar]
- Verstynen TD, Lynch B, Miller DL, Voss MW, Prakash RS, Chaddock L, Basak C, Szabo A, Olson EA, Wojcicki TR, Fanning J, Gothe NP, McAuley E, Kramer AF & Erickson KI (2012). Caudate nucleus volume mediates the link between cardiorespiratory fitness and cognitive flexibility in older adults. J Aging Res 2012, 939285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicente‐Campos D, Mora J, Castro‐Pinero J, Gonzalez‐Montesinos JL, Conde‐Caveda J & Chicharro JL (2012). Impact of a physical activity program on cerebral vasoreactivity in sedentary elderly people. J Sports Med Phys Fitness 52, 537–544. [PubMed] [Google Scholar]
- Voss MW, Prakash RS, Erickson KI, Basak C, Chaddock L, Kim JS, Alves H, Heo S, Szabo AN, White SM, Wojcicki TR, Mailey EL, Gothe N, Olson EA, McAuley E & Kramer AF (2010). Plasticity of brain networks in a randomized intervention trial of exercise training in older adults. Front Aging Neurosci 2, 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss MW, Vivar C, Kramer AF & van Praag H (2013). Bridging animal and human models of exercise‐induced brain plasticity. Trends Cogn Sci 17, 525–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein AM, Voss MW, Prakash RS, Chaddock L, Szabo A, White SM, Wojcicki TR, Mailey E, McAuley E, Kramer AF & Erickson KI (2012). The association between aerobic fitness and executive function is mediated by prefrontal cortex volume. Brain Behav Immun 26, 811–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wightman EL, Reay JL, Haskell CF, Williamson G, Dew TP & Kennedy DO (2014). Effects of resveratrol alone or in combination with piperine on cerebral blood flow parameters and cognitive performance in human subjects: a randomised, double‐blind, placebo‐controlled, cross‐over investigation. Br J Nutr 112, 203–213. [DOI] [PubMed] [Google Scholar]
- Willis SL, Tennstedt SL, Marsiske M, Ball K, Elias J, Koepke KM, Morris JN, Rebok GW, Unverzagt FW, Stoddard AM, Wright E & Group AS (2006). Long‐term effects of cognitive training on everyday functional outcomes in older adults. JAMA 296, 2805–2814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RS, Barnes LL & Bennett DA (2003). Assessment of lifetime participation in cognitively stimulating activities. J Clin Exp Neuropsychol 25, 634–642. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Barnes LL, Krueger KR, Hoganson G, Bienias JL & Bennett DA (2005). Early and late life cognitive activity and cognitive systems in old age. J Int Neuropsychol Soc 11, 400–407. [PubMed] [Google Scholar]
- Winocur G, Moscovitch M & Freedman J (1987). An investigation of cognitive function in relation to psychosocial variables in institutionalized old people. Can J Psychol 41, 257–269. [DOI] [PubMed] [Google Scholar]
- Witte AV, Kerti L, Hermannstadter HM, Fiebach JB, Schreiber SJ, Schuchardt JP, Hahn A & Floel A (2014). Long‐chain omega‐3 fatty acids improve brain function and structure in older adults. Cereb Cortex 24, 3059–3068. [DOI] [PubMed] [Google Scholar]
- Wolinsky FD, Mahncke H, Vander Weg MW, Martin R, Unverzagt FW, Ball KK, Jones RN & Tennstedt SL (2010). Speed of processing training protects self‐rated health in older adults: enduring effects observed in the multi‐site ACTIVE randomized controlled trial. Int Psychogeriatr 22, 470–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehuda S, Rabinovitz S & Mostofsky DI (1999). Essential fatty acids are mediators of brain biochemistry and cognitive functions. J Neurosci Res 56, 565–570. [DOI] [PubMed] [Google Scholar]
- Young J, Angevaren M, Rusted J & Tabet N (2015). Aerobic exercise to improve cognitive function in older people without known cognitive impairment. Cochrane Database Syst Rev 4, 145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurko‐Mauro K, Alexander DD & Van Elswyk ME (2015). Docosahexaenoic acid and adult memory: a systematic review and meta‐analysis. PLoS One 10, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu YS, Tarumi T, Tseng BY, Palmer DM, Levine BD & Zhang R (2013). Cerebral vasomotor reactivity during hypo‐ and hypercapnia in sedentary elderly and Masters athletes. J Cereb Blood Flow Metab 33, 1190–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]


