Highlights
-
•
We report the safe removal of a migrated intragastric balloon.
-
•
We advise multiple preoperative follow-ups to prevent complications.
-
•
Adherence to manufacturers’ and doctors’ advice to avoid complications are key.
-
•
Most intra-gastric balloons should be removed after 6 months.
-
•
Quick diagnosis of bowel obstruction needs adequate history and investigations.
Keywords: Case report, Intragastric balloon, Bariatric, Weight loss, Obesity, Complication
Abstract
Introduction
Intragastric balloons are a non-surgical treatment method for obesity.
Presentation of case
We report a 28 year old Kuwaiti women presented with vomiting and epigastric pain two years following insertion of a intra gastric balloon. The women was diagnosed as a case of intestinal obstruction secondary to intragastric balloon migration.
Discussion
Most intragastric balloons, should be removed in 6 months, to prevent complications. In our case, failure to follow up and remove the intragastric balloons in time led to bowel obstruction secondary to balloon migration.
Conclusion
We report a rare complication of the BioEnterics intragastric balloon. Adherence to manufacturer and physician recommendations may prevent serious complications of intra gastric balloon insertion.
1. Introduction
Obesity is one of the leading global health problems in the world today. It is considered a major risk factor for many serious diseases. To treat this risk factor, doctors have developed invasive and noninvasive therapeutic methods. One of these methods is the intragastric balloons, which are known by some studies to be efficient, safe, and easy [1].
These balloons are placed endoscopically in the stomach then inflated with either air or saline and methylene blue. After the procedure, patient is discharged within a few hours and is followed up in outpatient clinic for the next 6 months. After 6 months, the balloon is removed using the same method.
We report a case of subacute bowel obstruction caused by a deflated BioEnterics intragastric balloon, two years after its original insertion.
2. Presentation of case
A 28-year-old Kuwaiti female, with a history of two cesarean sections, was brought to emergency department with one day history of severe epigastric colicky pain of a few minutes duration accompanied by anorexia, nausea, and repeated episodes of vomiting. She also added that she has passed flatus, but not motion during the last day.
The patient had history of a saline-filled BioEnteric intra-gastric balloon insertion two years ago. However, since the insertion she voluntarily admitted that she has not been to any of her outpatient appointments and does not want the balloon to be removed due to the weight loss results she had. Her initial body mass index (BMI) was 36.2. On admission, it showed that she had lost 18 kg of her weight, reaching a BMI of 29.2.
The patient was mildly dehydrated and in pain. On abdominal examination, the abdomen was distended and she had severe tenderness on soft palpation of the epigastric region, without any guarding or rebound tenderness. In addition, her bowel sounds were audible. Abdominal ultrasound showed a partially filled balloon in the ileocecal region Urgent esophagogastroduodenoscopy was done which showed no balloon in the stomach, which confirms balloon migration.
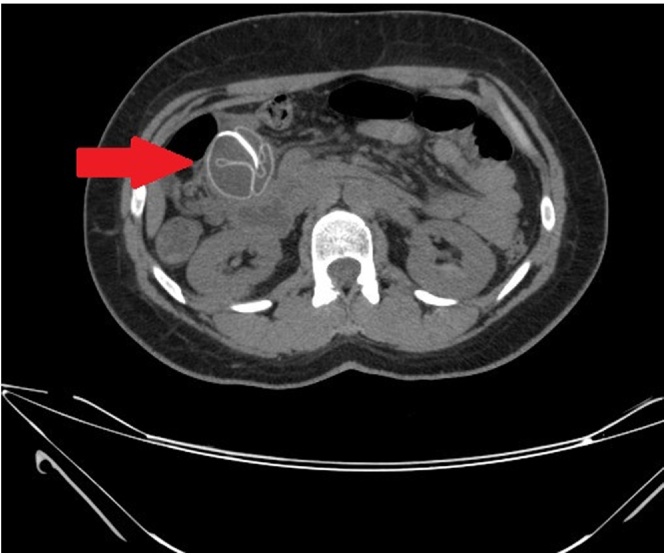
The patient was kept on nil per oral and on intravenous fluids. After a negative pregnancy test, computed tomography of the abdomen was performed and showed a metallic head seen within the small bowel loop in the right side of the abdomen (Fig. 1). However, the definite site could not be clearly assessed as the bowel loops were collapsed and crowded with the ileocecal junction not clearly identified. The patient was diagnosed as migrated BioEnterics intragastric balloon causing a subacute bowel obstruction.
Fig. 1.
Computed tomography of the small intestine showed a folded structure (arrow) with metallic head seen within the small bowel loop in the right side of the abdomen.
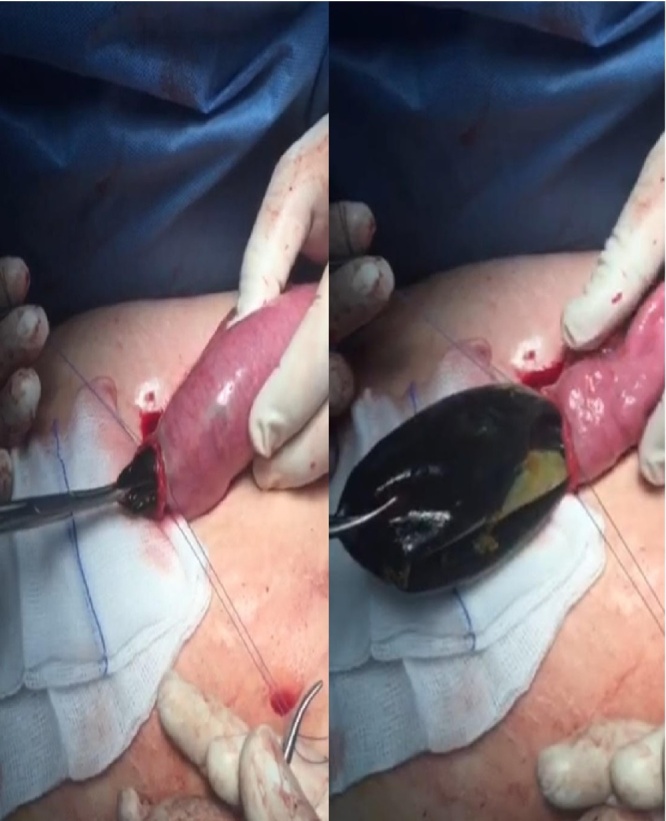
Laparoscopic approach was used and the bowel loop containing the migrated balloon was identified and brought out through the umbilical wound. Longitudinal enterotomy was performed, and the balloon was completely deflated and extracted from the bowel (Fig. 2). On the first postoperative day, the patient was started on an oral diet.
Fig. 2.
Operative image of the longitudinal enterotomy, showing the balloon deflated and being extracted from the small bowel.
Postoperative recovery was uneventful. She was discharged on the 3rd post-operative day. Two weeks later, the patient was reassessed in the outpatient clinic where she was completely asymptomatic with normal daily function.
3. Discussion
Obesity is a worldwide epidemic. In 2010, the World Health Organization reported that 43 million children were estimated to be overweight and obese [2]. Different management methods are available to these patients, ranging from lifestyle and dietary modifications, bariatric procedures, and intra-gastric balloons. Intra-gastric balloons have been in practice since the 1980s when it was first introduced [3]. They are a temporary non-operative method in losing weight by filling the stomach and increasing the sense of satiety and reduce oral intake. Due the easy insertion, modification and retrieval, it was a worthy option for doctors and patients.
Throughout the years, a number of complications have been reported with the use of fluid-filled BIB including esophagitis, gastric erosions or ulcerations, gastric perforation, gastric obstruction, balloon rupture, intestinal obstruction [1], [4]. BioEnterics intragastric balloons are still widely used to treat morbid obesity in Kuwait, even after their questionable long-term efficacy and significant rate of complications [5], [6].
The general recommendation for most intra-gastric balloons, is that it should be removed in 6 months, to reduce complications. In our case, lack of follow-up and removal lead to the deflation migration of the BIB. This migration lead to the bowel obstruction.
4. Conclusion
In conclusion, we report the safe removal of a migrated BioEnterics intragastric balloon.We advise, regular follow up and adherence to manufacturer and physician recommendations, which are important tools in preventing serious complications of intra gastric balloon insertion.
Consent
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Conflict of interest statement
None. The authors declare that they have no competing interests and did not receive any funding.
The work has been reported in line with the CARE criteria.
Funding
The authors declare that they have no competing interests and did not receive any funding.
Ethical approval
NA.
Author contribution
AJA and AA, contributed equally by examining the patient, following up the patient, writing up the manuscript, and reviewing the literature. NMA and MAA were the surgeons assigned to the case. All authors read and approve the final manuscript.
Guarantor
NMA is the Guarantor and head of our surgical unit in Adan Hospital, Kuwait.
Acknowledgement
We acknowledge all the great efforts and contributions of the Unit D Surgical Team of Adan Hospital, Kuwait including our Consultant Dr. Hamid Labib.
References
- 1.Genco A., Bruni T., Doldi S.B. BioEnterics intragastric balloon: the Italian experience with 2,515 patients. Obes. Surg. 2005;15(8):1161–1164. doi: 10.1381/0960892055002202. [DOI] [PubMed] [Google Scholar]
- 2.De onis M., Blössner M., Borghi E. Global prevalence and trends of overweight and obesity among preschool children. Am. J. Clin. Nutr. 2010;92(5):1257–1264. doi: 10.3945/ajcn.2010.29786. [DOI] [PubMed] [Google Scholar]
- 3.Nieben O.G., Harboe H. Intragastric balloon as an artificial bezoar for treatment of obesity. Lancet. 1982;1(8265):198–199. doi: 10.1016/s0140-6736(82)90762-0. [DOI] [PubMed] [Google Scholar]
- 4.Al-momen A., El-mogy I. Intragastric balloon for obesity: a retrospective evaluation of tolerance and efficacy. Obes. Surg. 2005;15(1):101–105. doi: 10.1381/0960892052993558. [DOI] [PubMed] [Google Scholar]
- 5.Dumonceau J.M. Evidence-based review of the BioEnterics intragastric balloon for weight loss. Obes. Surg. 2008;18(12):1611–1617. doi: 10.1007/s11695-008-9593-9. [DOI] [PubMed] [Google Scholar]
- 6.Imaz I., Martínez-cervell C., García-alvarez E.E., Sendra-gutiérrez J.M. Safety and effectiveness of the intragastric balloon for obesity: a meta-analysis. Obes. Surg. 2008;18(7):841–846. doi: 10.1007/s11695-007-9331-8. [DOI] [PubMed] [Google Scholar]