Abstract
Despite the common assumption that genetic generalized epilepsies are characterized by a macroscopically normal brain on magnetic resonance imaging, subtle structural brain alterations have been detected by advanced neuroimaging techniques in Childhood Absence Epilepsy syndrome. We applied quantitative structural MRI analysis to a group of adolescents and adults with Juvenile Absence Epilepsy (JAE) in order to investigate micro-structural brain changes using different brain measures. We examined grey matter volumes, cortical thickness, surface areas, and subcortical volumes in 24 patients with JAE compared to 24 healthy controls; whole-brain voxel-based morphometry (VBM) and Freesurfer analyses were used. When compared to healthy controls, patients revealed both grey matter volume and surface area reduction in bilateral frontal regions, anterior cingulate, and right mesial-temporal lobe. Correlation analysis with disease duration showed that longer disease was correlated with reduced surface area in right pre- and post-central gyrus. A possible effect of valproate treatment on brain structures was excluded. Our results indicate that subtle structural brain changes are detectable in JAE and are mainly located in anterior nodes of regions known to be crucial for awareness, attention and memory.
Keywords: Juvenile Absence Epilepsy, Voxel based morphometry, MRI, Cortical thickness, Surface area, Sodium valproate
Highlights
-
•
Subtle brain changes are detectable in Juvenile Absence Epilepsy.
-
•
Patients showed reduced gray matter and surface area in attentional and awareness networks.
-
•
Longer disease was correlated with reduced surface area in pre- and post-central regions.
-
•
Grey matter volume and surface analyses showed concordant findings.
1. Introduction
Childhood Absence Epilepsy (CAE) and Juvenile Absence Epilepsy (JAE) are two sub-syndromes of Genetic Generalized Epilepsy (GGE; formerly known as idiopathic generalized epilepsies) whose hallmark seizures are represented by absences (Fisher et al., 2005). CAE is a childhood epilepsy syndrome occurring in 10–17% of all childhood onset epilepsy, making it the most common paediatric epileptic syndrome. JAE usually begins between 10 and 17 years of age, but, at lower age limit, there is a great deal of overlap with CAE. By definition, GGE patients have no abnormalities on visual inspection of brain magnetic resonance imaging (MRI), but recently focal structural and functional abnormalities are emerging from studies using advanced MRI techniques. Volumetric studies using voxel-based morphometry (VBM) have shown both regional cortical grey matter (GM) and thalamic volumes alterations in GGE compared to controls: thalamic volume reduction was the most consistent finding across all the studies, while both increased and decreased cortical volumes were found (Seneviratne et al., 2014). Among all GGEs, only few studies have analysed structural MRI specifically in absence epilepsy patients separately from other sub-syndromes, and studies mainly focused to investigate populations with childhood onset absence seizures. Chan et al. (2006) compared CAE with controls and found areas of GM decrease in both thalami and in the subcallosal gyrus together with white matter reduction in extranuclear subcortical areas and basal forebrain (Chan et al., 2006). Pardoe et al. (2008), found a consistent reduction in thalami volume in CAE both within and across three different sites of MRI acquisition (Pardoe et al., 2008). Caplan et al. (2009) demonstrated that children with CAE have smaller grey matter volumes in orbito-frontal gyrus and in bilateral temporal lobe compared to children without epilepsy (Caplan et al., 2009). Finally, in a study analysing both CAE and JAE, an increased GM volume in superior mesio-frontal regions was reported in patients compared to controls (Betting et al., 2006). In conclusion, despite some variability in the location and direction of volumetric changes, there is an emerging body of evidence suggesting that subtle alterations in brain structure occur in absence epilepsy syndromes. This heterogeneity could be due to several factors, including mixed patients groups, different neuroimaging techniques, different sample size, and publication bias reporting only positive results. In addition, if we consider recent evidence suggesting that GGE syndromes are neurodevelopmental disorders with different patterns of prospective grey and white matter volume changes across childhood and adolescence (Hermann et al., 2006, Tosun et al., 2011a, Tosun et al., 2011b), one would expect distinct structural imaging patterns in CAE and JAE patients. Given the lack of specific structural neuroimaging studies on juvenile onset of Absence Epilepsy, we focused our study on JAE. We analysed cortical and subcortical brain changes in patients compared to healthy controls by means of different methods/measures: VBM, cortical thickness and surface, and subcortical volumes. We also correlated MRI abnormalities with the clinical features of epilepsy and we analysed the potential effect of drug treatment with sodium valproate on the brain structure (Pardoe et al., 2013).
2. Methods
2.1. Subjects
Twenty-four patients with JAE were recruited. Diagnosis of JAE was based on electroclinical criteria according to the International League Against Epilepsy (ILAE) classification (Berg et al., 2010). Demographic data and clinical information such as age of seizure onset, duration of epilepsy, antiepileptic drugs (AED) prescription and response to treatment, were collected. For group comparison, 24 volunteers were recruited to serve as healthy controls (HC). Healthy subjects had no history of neurological diseases or family history of epilepsy. All subjects, both patients and controls, had normal MRI at visual inspection. The human ethic committee of the University of Modena and Reggio Emilia approved this study and written informed consent was obtained from all the patients recruited and their parents if underage.
2.2. MRI acquisition
Three-dimensional (3D) T1-weighted MRI images were acquired using a 3 Tesla Philips Intera MRI scanner (Best, The Netherlands). A SPGR pulse sequence (echo time (TE) = 4.6, repetition time (TR) = 9.9 ms) was used. One hundred seventy contiguous sagittal slices were acquired (voxel size = 1 × 1 × 1 mm) and the field of view was 240 mm with a matrix size of 256 × 256 × 170. A T2-weighted axial scan was also acquired to allow visual determination of vascular burden or tissue abnormalities.
2.3. Statistical analysis of demographical and clinical variables
Demographical and clinical characteristics of subjects were analysed using Stata11 software and parametric and non-parametric statistics were used as appropriate. Independent samples t-tests were used to compare age and years of education between patients and controls; chi-square was used to compare the two groups according to gender.
2.4. Voxel-based morphometry analysis
VBM was performed using VBM8 (http://dbm.neuro.uni-jena.de/vbm/) a toolbox of SPM8 (http://www.fil.ion.ucl.ac.uk/spm/); default settings were applied. Structural images were bias-corrected, tissue-classified, and normalized to standard template using high-dimensional DARTEL normalization. Grey-matter volume was calculated modulating the normalized segmented images with a non-linear only warping resulting in an analysis of relative differences in regional grey-matter volumes corrected for individual brain size. To check the quality of the segmentation and normalization procedures, the normalized, bias-corrected images were visually inspected and covariance between normalized images was calculated to check homogeneity of variance and to identify potential outliers. Finally the normalized, segmented, and modulated images were smoothed with a 8 mm FWHM isotropic Gaussian kernel. To identify GM differences between JAE and controls, we performed a t-test comparison between the two groups and a double statistical threshold was used (voxel-wise p < 0.001 and cluster size ≥ 686 voxels, as determined by AlphaSim with 10,000 Monte Carlo simulations) to obtain an overall alpha level of < 0.05 (see details on the procedure at http://afni.nimh.nih.gov/afni/doc/manual/AlphaSim) (Forman et al., 1995). In addition, a correlation analysis with GM volume and disease duration was performed in the patients' group and a statistical threshold of p < 0.001 with a cluster size ≥ 808 voxels was accepted. Finally, we performed a t-test comparison between patients taking valproate (either alone or in polytherapy, VPA +) versus patients not taking valproate or drugs-free (VPA −) at the time of MRI. In this model a double statistical threshold of p < 0.001 with a cluster size ≥ 785 voxels was used. In all analyses, age, gender, and education were entered as covariates of no interest in order to control results for the potential effect of these variables.
2.5. Cortical and subcortical Freesurfer analyses
Scans were analysed using standardized image toolbox (Freesurfer, version 5.0) (Fischl, 2012), quality assurance (outlier detection based on inter quartile of 1.5 standard deviations along with visual inspection of segmentations), and statistical methods. Briefly, the pipeline involves removal of non-brain tissue, automated Talaraich transformation, segmentation of white matter and grey matter, tessellation of grey/white matter boundary, automated correction of topology defects, surface deformation to form the grey/white matter boundary and grey/cerebrospinal fluid boundary, and parcellation of cerebral cortex. Cortical thickness estimates were calculated as the distance between the grey/white matter border and the pial surface at each vertex; surface area was derived by taking the sum of the area of the vertices in each parcellation. Labels were constructed and values were extracted based on automatic algorithm (Desikan et al., 2006, Fischl et al., 2004). According to previous literature (Jalbrzikowski et al., 2013), we combined caudal and rostral regions of the middle frontal cortex and anterior cingulate respectively to make a unique label; the same approach was used to combine pars orbitalis, pars triangularis, and pars opercularis to create the inferior frontal cortex label. In conclusion, we calculated cortical thickness and surface area measures from 30 regions for hemisphere. Subcortical volumes were calculated with FreeSurfer's automated procedure for volumetric measures. Each voxel in the normalized brain volume was assigned to one label using a probabilistic atlas obtained from a manually labeled training set (Fischl et al., 2002). The labels we used for the analysis were the putamen, caudate nucleus, globus pallidus, nucleus accumbens, thalamus, amygdala, hippocampus, and the ventricular system.
After visual inspection and quality control, one patient was removed from analyses because of poor segmentation. Statistical analyses were performed using SPSS software (IBM, Chicago, IL). All neuroanatomical measures were examined for normality using Shapiro-Wilk test and transformed appropriately if they violated assumptions of normality. To compare cortical and subcortical measures between patients and controls, we conducted a univariate ANCOVA with each neuroanatomical value as the dependent variable, group diagnosis as fixed factor, and age, gender, education, and intracranial volume as covariates. The same approach was uses to compare patients taking valproate (VPA +) versus patients not taking valproate (VPA −) at the time of MRI. False discovery rate (FDR) was used to correct for multiple comparisons and a threshold of q < 0.05 estimated using SPSS command, according to Bejamini and Hochberg methods (Bejamini and Hochberg, 1995), was considered statically significant. Finally, we performed a correlation analysis in the patient group between both cortical and subcortical neuroanatomical measures and disease duration calculated in years; for this analysis, we regressed out the effect of education, age, total brain volume, and gender and we considered significant results with a p < 0.05, corrected for multiple comparisons.
3. Results
3.1. Demographical and clinical characteristics
JAE patients (n = 24) had a mean age of 26.33 years (± 11.9; range 16–52); 19 were female. Mean age of onset was 13.8 years (range between 10 and 24 years of age); mean duration of epilepsy was 12.9 years. Control subjects (n = 24) had a mean age of 30.6 years (± 5.4; range 19–38); 14 were female. Patients and controls only differed in years of education (p = 0.000), with controls showing higher level of education (17.2 years ± 1.38) compared to patients (11.38 years ± 3.24); no statistical significant difference was found in age (p = 0.11) and gender (p = 0.21).
Table 1 reports demographical and clinical characteristics of each patient.
Table 1.
Demographical and clinical characteristic of patients. Gender: F, female; M, male. Seizure types: A, absence; TC, tonico-clonic; M, myoclonic. Anti-epileptic drugs (AED): PB, phenobarbital; VPA, sodium valproate; LEV, levetiracetam; TPM, topiramate; CBZ, carbamazepine; LTG, lamotrigine; CLB, clobazam.
| Pt. ID | Age at MRI (years) | Gender | Seizures onset (years) | Disease duration (years) | Past seizures types | AED | Seizure-free > 1-year |
|---|---|---|---|---|---|---|---|
| 1 | 26 | F | 12 | 14 | A | PB | Yes |
| 2 | 21 | M | 15 | 6 | A; TC | VPA | Yes |
| 3 | 22 | F | 15 | 7 | A; TC; M | LEV | Yes |
| 4 | 16 | F | 10 | 6 | A | VPA | Yes |
| 5 | 16 | M | 12 | 4 | A; TC | Drug-naive | Yes |
| 6 | 52 | F | 16 | 36 | A; TC | VPA | No |
| 7 | 19 | F | 12 | 7 | A; TC | VPA | Yes |
| 8 | 23 | F | 16 | 7 | A; TC | VPA | Yes |
| 9 | 20 | F | 12 | 8 | A; TC | VPA | Yes |
| 10 | 20 | F | 13 | 7 | A | VPA | Undetermined |
| 11 | 18 | M | 16 | 2 | A; TC | VPA | Yes |
| 12 | 45 | F | 10 | 35 | A | LEV | Yes |
| 13 | 19 | F | 12 | 7 | A; TC | VPA | Yes |
| 14 | 28 | M | 18 | 10 | A; TC | PB;TPM | No |
| 15 | 31 | F | 14 | 17 | A | Drug-free | Undetermined |
| 16 | 48 | F | 13 | 35 | A | LEV | No |
| 17 | 37 | F | 13 | 24 | A; TC | CBZ; PB | No |
| 18 | 19 | F | 10 | 9 | A | VPA; LTG; CLB | No |
| 19 | 16 | F | 14 | 2 | A | VPA | Yes |
| 20 | 21 | F | 11 | 10 | A; TC | VPA | No |
| 21 | 17 | M | 17 | 0 | A; TC | Drug-naive | Yes |
| 22 | 41 | F | 12 | 29 | A; TC | VPA;TPM | Yes |
| 23 | 45 | F | 16 | 29 | A; TC | Drug-free | Yes |
| 24 | 24 | F | 24 | 0 | A; TC | LEV; CLB | No |
Fifteen patients (62%) were seizure-free during the 12 months before MRI. 7 patients (29%) reported persistence of absence seizures. For two patients seizures outcomes could not be established. As far as AED prescription, 13 patients were taking sodium valproate at the time of the study (11 in monotherapy; two in combination therapy) with a mean daily dose of 870 ± 376 mg (mean plasma level of 59,8 ± 18,6 μg/mL; ‘therapeutic’ range 40–100 μg/mL). Eleven patients were not taking sodium valproate (VPA − group; four were in monotherapy; three in combination therapy; four patients did not take any drug).
3.2. VBM results
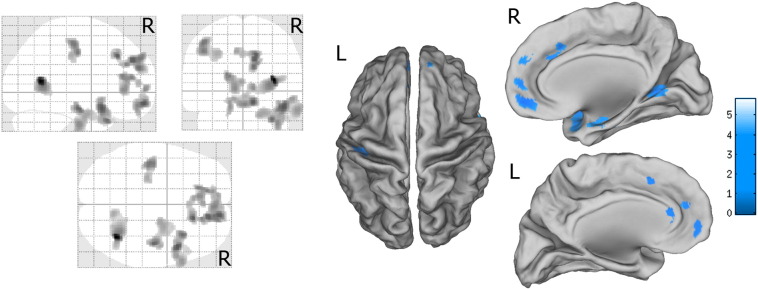
Group comparison analysis between patients and controls showed that patients had reduced GM volume in several cortical regions compared to controls, including right mesial-temporal lobe, right superior temporal gyrus, bilateral medial orbitofrontal cortex, right calcarine cortex and lingual gyrus, left anterior cingulate, bilateral superior frontal gyrus, and left post-central gyrus (Table 2, Fig. 1). No regions of increased GM volume were detected in patients compared to controls. Correlation analysis with disease duration after controlling for age, gender, and education did not show significant results. The direct comparison between VPA + and VPA − patients did not show significant differences for increased or decreased GM.
Table 2.
VBM group comparison results: areas of decreased volume in patients compared to controls. Only regions surviving to the statistical threshold (voxel-wise p < 0.001 and cluster size ≥ 686 voxels, as determined by AlphaSim with 10,000 Monte Carlo simulations) are showed. L, left; R, right; MNI, Montreal Neurological Institute.
| Region |
Cluster size |
t |
MNI coordinates |
||
|---|---|---|---|---|---|
| x | y | z | |||
| R, Hippocampus | 1173 | 4,42 | 40 | − 12 | − 19 |
| R, Parahippocampus | 20 | − 9 | 25 | ||
| R, Amygdala | 25 | 3 | − 27 | ||
| R, Superior temporal gyrus | 1215 | 4,77 | 45 | 12 | − 16 |
| R, Frontal medial orbital cortex | 1030 | 4,73 | 7 | 54 | − 11 |
| L, Frontal medial orbital cortex | − 5 | 56 | − 11 | ||
| R, Calcarine cortex | 1176 | 4,33 | 25 | − 56 | 6 |
| R, Lingual gyrus (BA19–30) | 8 | − 58 | 6 | ||
| L, Anterior cingulate (BA32) | 743 | 3,96 | − 3 | 36 | 8 |
| R, Frontal superior gyrus (BA6–9) | 928 | 4,67 | 9 | 26 | 41 |
| L, Frontal superior gyrus (BA6–9) | − 3 | 25 | 44 | ||
| L, Post-central gyrus (BA3) | 731 | 4,43 | − 43 | − 19 | 52 |
Fig. 1.
VBM group comparison results. In blue are depicted areas of decreased volume in patients compared to controls; only regions surviving to the statistical threshold (voxel-wise p < 0.001 and cluster size ≥ 686 voxels) are showed. The morphometric results are displayed onto the normalized SPM-glass brain (left images) and warped to the PALS-B12 atlas in Caret (Caret, http://brainvis.wustl.edu/wiki/index.php/Caret:About; (Van Essen, 2005)) (mesial and dorsal view) for right (R) and left (L) hemisphere (right images). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.3. Freesurfer results
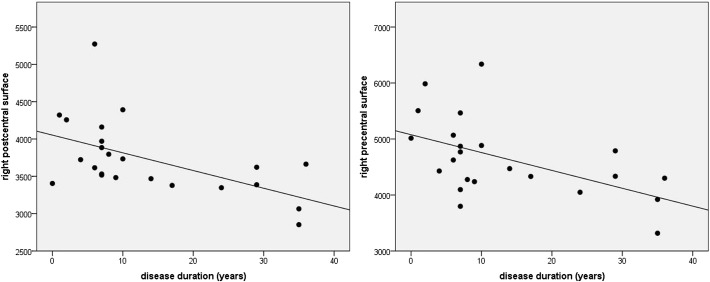
After correcting for multiple comparisons, patients had significant reduced surface area in right middle frontal, right peri-calcarine, bilateral superior frontal cortices, as well as in bilateral insula and anterior cingulate. In contrast, after correcting for multiple comparisons, no differences in cortical thickness emerged between the two groups, although a trend towards significance (p = 0.06) was found in anterior cingulate and isthmus cingulate cortex (decreased cortical thickness in patients compared to controls). Subcortical analyses confirmed the volume reduction in right hippocampus and amygdala in patients versus controls. In Table 3 we reported significant results for cortical and subcortical group comparison analyses. The direct comparison between VPA + and VPA − patients did not show significant differences for the three measures. Finally, the correlation analysis with disease duration showed a statistically significant negative correlation in right pre-central (r = − 0.52) and post-central gyrus (r = − 0.64) surface measures, meaning that longer disease was associated with decrease surface area in these areas (Fig. 2). The potential effect of age on this correlation analysis was controlled by adjusting for this variable with partial correlation. No correlation emerged for cortical thickness and subcortical regions volumes.
Table 3.
Group comparisons significant results for neuroanatomical cortical and subcortical measures extracted with Freesurfer.
| Hemisphere | F | p | Group comparison | |
|---|---|---|---|---|
| Surface areas | ||||
| Middle frontal | R | 13,78 | 0,001 | CTR > PT |
| Superior frontal | L | 9,75 | 0,004 | CTR > PT |
| R | 13,64 | 0,001 | CTR > PT | |
| Insula | L | 15,62 | 0,000 | CTR > PT |
| R | 17,76 | 0,000 | CTR > PT | |
| Anterior cingulate | L | 12,38 | 0,001 | CTR > PT |
| R | 17,62 | 0,000 | CTR > PT | |
| Pericalcarine | R | 9,27 | 0,004 | CTR > PT |
| Subcortical areas | ||||
| Hippocampus | R | 8,04 | 0,007 | CTR > PT |
| Amygdala | R | 5,13 | 0,003 | CTR > PT |
Fig. 2.
Correlation scatter plot between disease duration and surface measures. In each graph, y axis represents surface area measures as resulted from Freesurfer analysis, x axis represents disease duration in years.
4. Discussion
In this study, we investigated morphological and structural brain alterations in JAE patients and we found focal cortical and subcortical areas reduction in several brain regions in patients compared to controls. Conversely, no brain area showed increased values for any measure compared to controls. With regard to the population studied, the clinical outcomes of our JAE population are in line with previous studies on the prognosis of juvenile onset absence epilepsy (Bartolomei et al., 1997, Trinka et al., 2004, Wolf and Inoue, 1984), reporting percentages of seizure-free patients ranging from 60 to 85%. Our population is therefore representative of the syndrome of JAE, and does not feature a group of patients with a more ‘severe’ disease respect with the usual clinical practice.
Considering the observed cortical alterations, these involved extensively brain regions known to participate in specific brain networks (i.e. Default Mode Network and Fronto-Parietal Network) devoted to attention, goal-oriented cognition, and adaptive control processes (Cole et al., 2014, Spreng et al., 2010, Uddin, 2015). In particular, we found alterations located predominantly in the anterior nodes of these networks (the orbito-frontal cortex, the anterior cingulate and insular cortex). This finding suggests that a disruption of attention and awareness circuits is a core feature in absences epilepsies (Danielson et al., 2011, Killory et al., 2011, Kim et al., 2014, Luo et al., 2011). In particular, the orbito-frontal cortex was hypothesized to be important in absence seizures generation (Holmes, 2004, Holmes et al., 2004) and abnormal GM volume reduction in this area was previously found in CAE compared to children without epilepsy (Caplan et al., 2009).
Interestingly, and quite unexpectedly, we found group differences in surface area, but no differences emerged for cortical thickness (except by lowering the statistical threshold). Several studies have shown different trajectories in cortical thickness and surface area changes across childhood and adulthood (Aleman-Gomez et al., 2013, Raznahan et al., 2011). Moreover, studies on psychiatric disorder like autism have shown increased cortical thinning but comparable surface area growth rates in adolescents with autism respect with controls (Wallace et al., 2015). This evidence suggests that cortical surface and cortical thickness measures might reflect independent neuropathological mechanisms. Recent findings indicate that cortical thickness and surface area may be driven by different genetic and neurobiological mechanisms involving different progenitor cells (Panizzon et al., 2009, Pontious et al., 2008, Winkler et al., 2010). Therefore, if we consider that JAE is a neurodevelopmental disorder and that cortical thickness and surface may be the expression of different corticogenesis pathways, it is not surprising to observe predominantly or selective alterations in one of the two cortical measures (Jalbrzikowski et al., 2013).
With regards to the alterations observed at subcortical level our study did not reveal a significant thalamic atrophy in adults with JAE. As a matter of fact, the thalamus volume reduction seen across the majority of studies on absence epilepsy was detected mainly in CAE subjects (Chan et al., 2006, Pardoe et al., 2008), whereas the only other study that included also JAE subjects did not find thalamic changes in whole-brain analysis (Betting et al., 2006). Several factors, beyond a possible syndrome-specific effect, could contribute to this difference. Possible explanations relate to the different age at fMRI, but also to different imaging analyses techniques. Moreover, not all previous studies considered potentially confounding covariates (i.e age, gender) in the analyses, therefore we cannot rule out a possible effect of these variables on reported results.
Interestingly, we observed a consistent mesial-temporal lobe volume reduction both with VBM and Freesurfer analyses. This finding is in accordance with recent studies showing hippocampal damage and spatial memory deficits in animal models of absence epilepsy (Arcaro et al., 2016, Jafarian et al., 2015, Marques-Carneiro et al., 2016). However, human data on memory impairment in absence epilepsy are still conflicting (Verrotti et al., 2015) and specific studies focusing on cognitive-brain structure correlation should be performed to better analyse this issue.
Considering the impact of disease duration on brain structures we observed a negative correlation between ‘years of epilepsy’ and both pre- and post-central gyrus surface area, even after adjusting the analysis for age with partial correlation. This means that it is not ‘age’ per se that leads to sensorymotor area reduction rather the interaction of epilepsy with disease duration. The impact of disease duration on sensorymotor cortex has also been reported in children with CAE (Tosun et al., 2011b), in adults with GGE with tonic-clonic seizures only (Bernhardt et al., 2009), as well as in adult temporal lobe epilepsy patients with and without mesial temporal sclerosis (Bernhardt et al., 2008, Mueller et al., 2009). This finding may not be specific to absence epilepsy. However, it is important to highlight that this correlation in our population is relevant because we can reasonably exclude that this result is the expression/consequence of repeated tonic-clonic seizures. Indeed, in our JAE population the burden of generalized convulsion was small. In particular, 15 out of 24 patients did not experience any seizure in the 12-month before MRI and considering the remaining 9 patients only three of them reported 1 to 3 tonic-clonic convulsions in the previous year. Notably, the involvement of the pericentral cortex (and in particular of the somatosensory cortex) was observed both by VBM and Freesurfer group comparisons and by the correlation analysis. This finding corroborates evidence from animal and human studies for a role of this brain region in absence generation and maintenance (Blumenfeld, 2005, Polack et al., 2009, Tosun et al., 2011b).
Finally, an important secondary finding of our study concerns the potential effects of sodium valproate on brain structure. This widely used AED has been demonstrated to be associated with rare cases of reversible brain atrophy (Guerrini et al., 1998) and more recently to parietal cortical thinning and whole brain volume reduction in different epilepsy syndromes (Pardoe et al., 2013). Indeed, in our population, morphometry changes were not driven by potential effects of valproate treatment given the lack of difference in comparison between VPA + and VPA − patients. However, considering our study and the one of Pardoe et al. (2013), several possible factors can explain the different findings. Importantly, beyond different analysis methodology, our patients' group differs for two important variables: first, the mean valproate dose exposure in our patients was about the half respect with the one reported in the study of Pardoe et al. (2013); second, our cohort shows a striking preponderance of female patients, while the study of Pardoe was performed on male patients only. Given these differences in patients' populations, it is conceivable if it is the dose of valproate exposure and/or the subject gender that can make a difference on the effects of valproate on brain structures. It will be important to design future studies to address properly the effects on valproate treatment (as well as of other drugs) on brain structure, possibly with longitudinal pre/post treatment design.
4.1. Conclusion
Our study shows the presence of focal structural brain changes in JAE syndrome detectable with advanced automated techniques; these changes are mainly located in the medial frontal cortex, anterior cingulate, and mesial-temporal lobe, partially overlapping the anterior nodes of attentional and awareness networks. The fact that quite overlapping brain pattern alterations were detected with different neuroimaging approaches gave a reasonable confidence that subtle alterations in these areas may be related to JAE syndrome. Larger and possibly longitudinal studies will be required to confirm these findings and to better specify the link between structural abnormalities and clinical, neuropsychological, and electrophysiological data in absence epilepsy syndromes.
Study funding
No funding.
Disclosures
S. Meletti received Research grant support from the Italian Ministry of Health (NET-2013-02355313-3), from the non-profit organization CarisMo Foundation (N.A.010@FCRMO RINT@MELFONINFO); has received personal compensation as scientific advisory board member for UCB and EISAI. A.E. Vaudano received Research Grant support from the Italian Ministry of Health, Emilia-Romagna Region (N. PRUA1GR-2013-00000120).
References
- Aleman-Gomez Y., Janssen J., Schnack H., Balaban E., Pina-Camacho L., Alfaro-Almagro F., Castro-Fornieles J., Otero S., Baeza I., Moreno D., Bargallo N., Parellada M., Arango C., Desco M. The human cerebral cortex flattens during adolescence. J. Neurosci. 2013;33(38):15004–15010. doi: 10.1523/JNEUROSCI.1459-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arcaro J., Ma J., Chu L., Kuo M., Mirsattari S.M., Stan Leung L. The hippocampus participates in a pharmacological rat model of absence seizures. Epilepsy Res. 2016;120:79–90. doi: 10.1016/j.eplepsyres.2015.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartolomei F., Roger J., Bureau M., Genton P., Dravet C., Viallat D., Gastaut J.L. Prognostic factors for childhood and juvenile absence epilepsies. Eur. Neurol. 1997;37(3):169–175. doi: 10.1159/000117429. [DOI] [PubMed] [Google Scholar]
- Bejamini Y., Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. 1995;57:289–300. [Google Scholar]
- Berg A.T., Berkovic S.F., Brodie M.J., Buchhalter J., Cross J.H., van Emde Boas W., Engel J., French J., Glauser T.A., Mathern G.W., Moshe S.L., Nordli D., Plouin P., Scheffer I.E. Revised terminology and concepts for organization of seizures and epilepsies: report of the ILAE commission on classification and terminology, 2005–2009. Epilepsia. 2010;51(4):676–685. doi: 10.1111/j.1528-1167.2010.02522.x. [DOI] [PubMed] [Google Scholar]
- Bernhardt B.C., Worsley K.J., Besson P., Concha L., Lerch J.P., Evans A.C., Bernasconi N. Mapping limbic network organization in temporal lobe epilepsy using morphometric correlations: insights on the relation between mesiotemporal connectivity and cortical atrophy. NeuroImage. 2008;42(2):515–524. doi: 10.1016/j.neuroimage.2008.04.261. [DOI] [PubMed] [Google Scholar]
- Bernhardt B.C., Rozen D.A., Worsley K.J., Evans A.C., Bernasconi N., Bernasconi A. Thalamo-cortical network pathology in idiopathic generalized epilepsy: insights from MRI-based morphometric correlation analysis. NeuroImage. 2009;46(2):373–381. doi: 10.1016/j.neuroimage.2009.01.055. [DOI] [PubMed] [Google Scholar]
- Betting L.E., Mory S.B., Li L.M., Lopes-Cendes I., Guerreiro M.M., Guerreiro C.A., Cendes F. Voxel-based morphometry in patients with idiopathic generalized epilepsies. NeuroImage. 2006;32(2):498–502. doi: 10.1016/j.neuroimage.2006.04.174. [DOI] [PubMed] [Google Scholar]
- Blumenfeld H. Cellular and network mechanisms of spike-wave seizures. Epilepsia. 2005;46(Suppl. 9):21–33. doi: 10.1111/j.1528-1167.2005.00311.x. [DOI] [PubMed] [Google Scholar]
- Caplan R., Levitt J., Siddarth P., Wu K.N., Gurbani S., Sankar R., Shields W.D. Frontal and temporal volumes in childhood absence epilepsy. Epilepsia. 2009;50(11):2466–2472. doi: 10.1111/j.1528-1167.2009.02198.x. [DOI] [PubMed] [Google Scholar]
- Chan C.H., Briellmann R.S., Pell G.S., Scheffer I.E., Abbott D.F., Jackson G.D. Thalamic atrophy in childhood absence epilepsy. Epilepsia. 2006;47(2):399–405. doi: 10.1111/j.1528-1167.2006.00435.x. [DOI] [PubMed] [Google Scholar]
- Cole M.W., Repovs G., Anticevic A. The frontoparietal control system: a central role in mental health. Neuroscientist. 2014;20(6):652–664. doi: 10.1177/1073858414525995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielson N.B., Guo J.N., Blumenfeld H. The default mode network and altered consciousness in epilepsy. Behav. Neurol. 2011;24(1):55–65. doi: 10.3233/BEN-2011-0310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desikan R.S., Segonne F., Fischl B., Quinn B.T., Dickerson B.C., Blacker D., Buckner R.L., Dale A.M., Maguire R.P., Hyman B.T., Albert M.S., Killiany R.J. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage. 2006;31(3):968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Fischl B. FreeSurfer. NeuroImage. 2012;62(2):774–781. doi: 10.1016/j.neuroimage.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B., Salat D.H., Busa E., Albert M., Dieterich M., Haselgrove C., van der Kouwe A., Killiany R., Kennedy D., Klaveness S., Montillo A., Makris N., Rosen B., Dale A.M. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33(3):341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Fischl B., van der Kouwe A., Destrieux C., Halgren E., Segonne F., Salat D.H., Busa E., Seidman L.J., Goldstein J., Kennedy D., Caviness V., Makris N., Rosen B., Dale A.M. Automatically parcellating the human cerebral cortex. Cereb. Cortex. 2004;14(1):11–22. doi: 10.1093/cercor/bhg087. [DOI] [PubMed] [Google Scholar]
- Fisher R.S., van Emde Boas W., Blume W., Elger C., Genton P., Lee P., Engel J., Jr. Epileptic seizures and epilepsy: definitions proposed by the International League Against Epilepsy (ILAE) and the International Bureau for Epilepsy (IBE) Epilepsia. 2005;46(4):470–472. doi: 10.1111/j.0013-9580.2005.66104.x. [DOI] [PubMed] [Google Scholar]
- Forman S.D., Cohen J.D., Fitzgerald M., Eddy W.F., Mintun M.A., Noll D.C. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magn. Reson. Med. 1995;33(5):636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Guerrini R., Belmonte A., Canapicchi R., Casalini C., Perucca E. Reversible pseudoatrophy of the brain and mental deterioration associated with valproate treatment. Epilepsia. 1998;39(1):27–32. doi: 10.1111/j.1528-1157.1998.tb01270.x. [DOI] [PubMed] [Google Scholar]
- Hermann B., Jones J., Sheth R., Dow C., Koehn M., Seidenberg M. Children with new-onset epilepsy: neuropsychological status and brain structure. Brain. 2006;129(Pt 10):2609–2619. doi: 10.1093/brain/awl196. [DOI] [PubMed] [Google Scholar]
- Holmes G.L. Models for generalized seizures. Suppl. Clin. Neurophysiol. 2004;57:415–424. doi: 10.1016/s1567-424x(09)70379-4. [DOI] [PubMed] [Google Scholar]
- Holmes M.D., Brown M., Tucker D.M. Are “generalized” seizures truly generalized? Evidence of localized mesial frontal and frontopolar discharges in absence. Epilepsia. 2004;45(12):1568–1579. doi: 10.1111/j.0013-9580.2004.23204.x. [DOI] [PubMed] [Google Scholar]
- Jafarian M., Karimzadeh F., Alipour F., Attari F., Lotfinia A.A., Speckmann E.J., Zarrindast M.R., Gorji A. Cognitive impairments and neuronal injury in different brain regions of a genetic rat model of absence epilepsy. Neuroscience. 2015;298:161–170. doi: 10.1016/j.neuroscience.2015.04.033. [DOI] [PubMed] [Google Scholar]
- Jalbrzikowski M., Jonas R., Senturk D., Patel A., Chow C., Green M.F., Bearden C.E. Structural abnormalities in cortical volume, thickness, and surface area in 22q11.2 microdeletion syndrome: relationship with psychotic symptoms. Neuroimage Clin. 2013;3:405–415. doi: 10.1016/j.nicl.2013.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killory B.D., Bai X., Negishi M., Vega C., Spann M.N., Vestal M., Guo J., Berman R., Danielson N., Trejo J., Shisler D., Novotny E.J., Jr., Constable R.T., Blumenfeld H. Impaired attention and network connectivity in childhood absence epilepsy. NeuroImage. 2011;56(4):2209–2217. doi: 10.1016/j.neuroimage.2011.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.B., Suh S.I., Seo W.K., Oh K., Koh S.B., Kim J.H. Altered thalamocortical functional connectivity in idiopathic generalized epilepsy. Epilepsia. 2014;55(4):592–600. doi: 10.1111/epi.12580. [DOI] [PubMed] [Google Scholar]
- Luo C., Li Q., Lai Y., Xia Y., Qin Y., Liao W., Li S., Zhou D., Yao D., Gong Q. Altered functional connectivity in default mode network in absence epilepsy: a resting-state fMRI study. Hum. Brain Mapp. 2011;32(3):438–449. doi: 10.1002/hbm.21034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques-Carneiro J.E., Faure J.B., Barbelivien A., Nehlig A., Cassel J.C. Subtle alterations in memory systems and normal visual attention in the GAERS model of absence epilepsy. Neuroscience. 2016;316:389–401. doi: 10.1016/j.neuroscience.2015.12.048. [DOI] [PubMed] [Google Scholar]
- Mueller S.G., Laxer K.D., Barakos J., Cheong I., Garcia P., Weiner M.W. Widespread neocortical abnormalities in temporal lobe epilepsy with and without mesial sclerosis. NeuroImage. 2009;46(2):353–359. doi: 10.1016/j.neuroimage.2009.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panizzon M.S., Fennema-Notestine C., Eyler L.T., Jernigan T.L., Prom-Wormley E., Neale M., Jacobson K., Lyons M.J., Grant M.D., Franz C.E., Xian H., Tsuang M., Fischl B., Seidman L., Dale A., Kremen W.S. Distinct genetic influences on cortical surface area and cortical thickness. Cereb. Cortex. 2009;19(11):2728–2735. doi: 10.1093/cercor/bhp026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardoe H., Pell G.S., Abbott D.F., Berg A.T., Jackson G.D. Multi-site voxel-based morphometry: methods and a feasibility demonstration with childhood absence epilepsy. NeuroImage. 2008;42(2):611–616. doi: 10.1016/j.neuroimage.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardoe H.R., Berg A.T., Jackson G.D. Sodium valproate use is associated with reduced parietal lobe thickness and brain volume. Neurology. 2013;80(20):1895–1900. doi: 10.1212/WNL.0b013e318292a2e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polack P.O., Mahon S., Chavez M., Charpier S. Inactivation of the somatosensory cortex prevents paroxysmal oscillations in cortical and related thalamic neurons in a genetic model of absence epilepsy. Cereb. Cortex. 2009;19(9):2078–2091. doi: 10.1093/cercor/bhn237. [DOI] [PubMed] [Google Scholar]
- Pontious A., Kowalczyk T., Englund C., Hevner R.F. Role of intermediate progenitor cells in cerebral cortex development. Dev. Neurosci. 2008;30(1–3):24–32. doi: 10.1159/000109848. [DOI] [PubMed] [Google Scholar]
- Raznahan A., Shaw P., Lalonde F., Stockman M., Wallace G.L., Greenstein D., Clasen L., Gogtay N., Giedd J.N. How does your cortex grow? J. Neurosci. 2011;31(19):7174–7177. doi: 10.1523/JNEUROSCI.0054-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seneviratne U., Cook M., D'Souza W. Focal abnormalities in idiopathic generalized epilepsy: a critical review of the literature. Epilepsia. 2014;55(8):1157–1169. doi: 10.1111/epi.12688. [DOI] [PubMed] [Google Scholar]
- Spreng R.N., Stevens W.D., Chamberlain J.P., Gilmore A.W., Schacter D.L. Default network activity, coupled with the frontoparietal control network, supports goal-directed cognition. NeuroImage. 2010;53(1):303–317. doi: 10.1016/j.neuroimage.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosun D., Dabbs K., Caplan R., Siddarth P., Toga A., Seidenberg M., Hermann B. Deformation-based morphometry of prospective neurodevelopmental changes in new onset paediatric epilepsy. Brain. 2011;134(Pt 4):1003–1014. doi: 10.1093/brain/awr027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosun D., Siddarth P., Toga A.W., Hermann B., Caplan R. Effects of childhood absence epilepsy on associations between regional cortical morphometry and aging and cognitive abilities. Hum. Brain Mapp. 2011;32(4):580–591. doi: 10.1002/hbm.21045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinka E., Baumgartner S., Unterberger I., Unterrainer J., Luef G., Haberlandt E., Bauer G. Long-term prognosis for childhood and juvenile absence epilepsy. J. Neurol. 2004;251(10):1235–1241. doi: 10.1007/s00415-004-0521-1. [DOI] [PubMed] [Google Scholar]
- Uddin L.Q. Salience processing and insular cortical function and dysfunction. Nat. Rev. Neurosci. 2015;16(1):55–61. doi: 10.1038/nrn3857. [DOI] [PubMed] [Google Scholar]
- Van Essen D.C. A Population-average, Landmark- and Surface-based (PALS) atlas of human cerebral cortex. NeuroImage. 2005;28(3):635–662. doi: 10.1016/j.neuroimage.2005.06.058. [DOI] [PubMed] [Google Scholar]
- Verrotti A., Matricardi S., Rinaldi V.E., Prezioso G., Coppola G. Neuropsychological impairment in childhood absence epilepsy: review of the literature. J. Neurol. Sci. 2015;359(1–2):59–66. doi: 10.1016/j.jns.2015.10.035. [DOI] [PubMed] [Google Scholar]
- Wallace G.L., Eisenberg I.W., Robustelli B., Dankner N., Kenworthy L., Giedd J.N., Martin A. Longitudinal cortical development during adolescence and young adulthood in autism spectrum disorder: increased cortical thinning but comparable surface area changes. J. Am. Acad. Child Adolesc. Psychiatry. 2015;54(6):464–469. doi: 10.1016/j.jaac.2015.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler A.M., Kochunov P., Blangero J., Almasy L., Zilles K., Fox P.T., Duggirala R., Glahn D.C. Cortical thickness or grey matter volume? The importance of selecting the phenotype for imaging genetics studies. NeuroImage. 2010;53(3):1135–1146. doi: 10.1016/j.neuroimage.2009.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf P., Inoue Y. Therapeutic response of absence seizures in patients of an epilepsy clinic for adolescents and adults. J. Neurol. 1984;231(4):225–229. doi: 10.1007/BF00313944. [DOI] [PubMed] [Google Scholar]