Abstract
Background
Cognitive impairments contribute significantly to disease burden in young individuals presenting with major psychiatric disorders. The capacity to encode the consequences of one's actions may be of particular importance for real-world functioning due to its fundamental role in goal-directed behavior.
Methods
Here, we investigated a dimensional measure of causal awareness during a probabilistic learning task in 92 young individuals with an admixture of major mood and psychotic illnesses, at early and more established stages. Using automated gray matter segmentation of T1-weighted images, we estimated the volume and shapes of major subcortical structures and investigated their association with causal awareness.
Results
The low causal awareness (LCA) group (n = 35) reported increased social disability (p = .004) and reduced right pallidal size, specifically within the dorsolateral surfaces (p = .02), relative to the unimpaired high causal awareness (HCA) patients (n = 57). In early-stage illness, LCA had a smaller right thalamus (p = .002) relative to HCA. Exploratory investigations suggested that in developed psychotic syndromes, causal awareness was correlated with left hippocampal size (p = .006) whereas, in more persistent affective disorders, causal awareness was correlated with left amygdala size (p = .013), specifically within the anterior aspect.
Discussion
Low causal awareness occurs across diagnoses and stages of illness and is associated with poor functional outcomes. Our results suggest that there may be shared neural underpinnings of its dysfunction in the early course of mood and psychotic disorders, however in more established illness, there is greater neurobiological divergence in causal awareness correlates between diagnoses.
Keywords: Instrumental learning, Causal awareness, Volumetrics, Shape analysis, Pallidum, Youth
Highlights
-
•
Impaired awareness of causal relationships occurs trans-diagnostically.
-
•
Participants with low causal awareness have poorer functional outcomes.
-
•
Low causal awareness was associated with reduced right pallidal size
-
•
Low causal awareness was associated with a lateralized limbic-pallidal circuit.
-
•
Results suggest common neural dysfunction in early mood and psychotic disorders.
1. Introduction
Major psychiatric illnesses typically emerge between the ages of 15 and 30 (Kessler et al., 2005, Paus et al., 2008), which is a key period of social, academic and occupational development. Identifying factors that worsen the clinical and real-world functioning for these individuals is a crucial step towards early intervention strategies that enhance quality of life and reduce socio-economic burden.
Cognitive impairments have been shown to contribute significantly to functional disability across early stage bipolar disorder (Lee et al., 2014, Lin et al., 2011), schizophrenia (Allott et al., 2011, Lin et al., 2011) and depression (Bora et al., 2013, Lee et al., 2012). Importantly, traditional neuropsychological functions such as working memory and executive processes are reported to be better predictors of future functional outcomes than diagnostic category (Lee et al., 2013, Simonsen et al., 2010, Torres et al., 2010). The capacity to encode the consequences of one's actions is a fundamental capacity that draws upon these cognitive processes. It is critical for goal-directed behavior, allowing adaptation to changing environments so as to achieve desirable outcomes and avoid aversive ones (Balleine and Dickinson, 1998). Importantly, a reduced capacity for goal-directed behavior has also been associated with poor response to cognitive-behavior therapy (CBT) in patients with social anxiety disorder (Alvares et al., 2014). This highlights how causal knowledge may not only impact social and occupational outcomes by guiding optimal choices, but may also affect the efficacy of popular learning-based treatments for mental disorders.
Previous functional imaging studies in healthy subjects have demonstrated that a network including the medial prefrontal cortex (mPFC) and the anterior caudate nucleus (aCN) mediates learning of action-outcome contingencies (Liljeholm et al., 2011, Tanaka et al., 2008). Disruption within these regions, or within the subcortical feedback loops (e.g. pallidum, thalamus) connecting these regions, may contribute to causal awareness deficits. Indeed, we recently found that changes in the right external globus pallidus were associated with altered causal awareness in youth with depression (Griffiths et al., 2015).
Finding links between dimensional behavior and specific neural networks is a critical step towards an individualized understanding of compromised functioning, and may contribute to efforts in developing targeted treatment strategies (Insel, 2007).
During the early stages of mental illness, disorders commonly present with an admixture of clinical symptoms (e.g. anxiety, depression and general distress) and, as such, a reliable diagnosis is often difficult (Hermens et al., 2011). For this reason, it is particularly important to be able to identify behavioral or brain markers that are common across diagnoses, which may have greater utility in prognosis and predicting functional outcomes, especially during the early stages when illness characteristics are less entrenched (McGorry et al., 2006).
The relationship between causal awareness and brain structure with respect to stage of illness is unknown. In keeping with the Research Domain Criteria (RDoC; Insel et al., 2010), our overarching objective was to determine whether causal awareness is a functionally-relevant and biologically-specific endophenotype that cuts across traditional diagnostic boundaries and stage of illness. As such, the current study sought to determine whether causal awareness deficits are evident within early stages of psychiatric illness, as well as across more established major psychiatric disorders in adolescents and young adults. Secondly, we aimed to investigate whether there is a common association between poor causal awareness and differences in underlying cortical–basal ganglia structures, irrespective of stage of illness. This is important because differences in neural structure may occur between individuals in earlier phases of illness relative to those who have transitioned into more established disorders (Lagopoulos et al., 2012, Pantelis et al., 2003). As such, it is unclear if the relationship between causal awareness and specific structures may differ with early or later stage illness.
2. Methods
2.1. Demographics and clinical assessments
2.1.1. Participants
Ninety-two outpatients (aged 14–33 years) were recruited from specialized assessment and early intervention services for mental health problems in young people (headspace at the Brain and Mind Research Institute (Scott et al., 2012). Twenty demographically similar healthy controls were also recruited from the same geographical catchment area. Exclusion criteria for both clinical and control groups were history of neurological disease (e.g. head trauma, epilepsy), intellectual and/or developmental disability as reported to referring clinicians or research psychologists, and/or insufficient English language skills. A research psychologist screened controls for psychopathology via clinical interview. All patients continued to receive ‘treatment as usual’ with no interference to their prescribed course of treatment. All participants (and guardians if participants were < 16 years) gave written informed consent. The study was approved by the University of Sydney ethics committee and the investigation was carried out in accordance with the latest version of the Declaration of Helsinki.
2.1.2. Clinical and neuropsychological assessment
All participants underwent clinical and neuropsychological assessment as previously described (Lee et al., 2015, Scott et al., 2012). Patients were seeking help primary for a unipolar depressive disorder (n = 50), bipolar disorder (n = 22), and/or a psychotic syndrome (first-episode psychosis, schizophrenia, schizoaffective disorder) (n = 19). Research psychologists rated clinical symptoms using the Brief Psychiatric Rating Scale (Overall and Gorham, 1962), Hamilton Depressive Rating Scale (Hamilton, 1967) and Young Mania Rating Scale (Young et al., 1978). Positive, negative, depressive, and manic symptom sub-scores of the BPRS were also calculated (Dingemans et al., 1995). Real-world functioning was indexed using the observer-rated Social and Occupational Functioning Assessment Scale (SOFAS; Goldman et al., 2012) and the patient-rated World Health Organization Disability Assessment Scale version 2 (WHODAS-II; Chwastiak and Von Korff, 2003). Premorbid intelligence (‘predicted IQ’) was estimated based on performance on the Wechsler Test of Adult Reading (WTAR; Wechsler, 2001).
2.1.3. Clinical staging
All patients were assigned an illness stage according to a clinical staging model (c.f. see Appendix 1 in Hickie et al., 2013 for details), according to consensus between experienced clinical researchers. Clinical staging involves a detailed description of where an individual exists on a continuum of disorder progression from stage 0 (an at-risk or latency stage) through to stage IV (late or end-stage disease) (Scott et al., 2013). Using this model, individuals may be classified as: stage 1a = ‘help-seeking’; stage 1b = ‘attenuated syndrome’; stage 2 = ‘discrete disorder’; stage 3 = ‘recurrent or persistent disorder’; and stage 4 = ‘severe, persistent and unremitting illness’. Stage 1b is assigned when the individual has developed sub-threshold symptoms of anxiety, depression, hypomania and/or psychotic phenomena. It encompasses the symptoms, disability and need for care that typically are below the thresholds required by current diagnostic systems (e.g. brief or recurrent hypomania, disrupted sleep-wake cycle, increased or decreased energy). Stage 2 is assigned when the individual displays a full-threshold/frank psychotic, manic and/or severe depressive episode, and stage 3 when these symptoms persist or are recurrent (cf. Hickie et al., 2013: see Appendix 1—template used by clinicians to reach staging decisions; and Appendix 2—a guide linking supporting standard assessments and clinical measures to stage in Hickie et al., 2013 for details). Importantly, stage does not necessarily correlate with duration of illness or age. Some Stage 1b individuals may never progress to stage 2, while others may transition almost immediately.
In order to determine whether stage of illness affected relationships between causal awareness and subcortical structure volumes, the patient sample was classified as being within either the early (1b) or later stages (2 or 3) of illness. These stages were selected as they account for the majority of the adolescent and young adult patients at headspace (Scott et al., 2012).
2.2. Causal awareness task
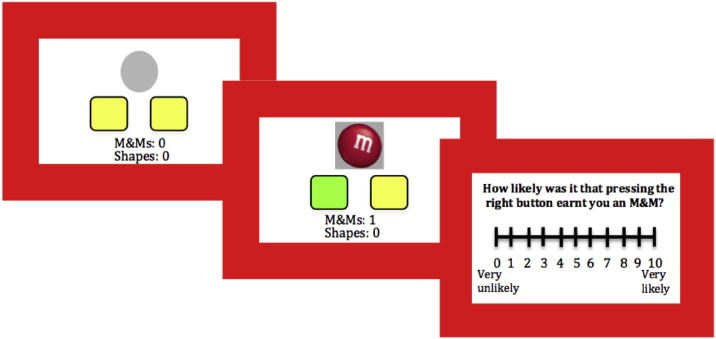
The instrumental causal awareness task involved participants choosing between two actions (AHIGH or ALOW) in order to earn food rewards (chocolate or crackers) (see Fig. 1). During each block, reward was always more contingent on one action (AHIGH) than the other action (ALOW). Across blocks, AHIGH switched location (left or right), and the reward ratio schedule varied (0.25, 0.125, 0.08). The schedule of ALOW always remained at 0.05, thus the relative difference in contingency between actions was altered, allowing us to assay sensitivity to these changes. Responding was self-paced during the 12 blocks, each 40-s in length. At the end of each block, participants were asked to judge, on a 10-point Likert scale, how likely it was that pressing each button earned them rewards on the previous trial (0—not at all likely, 10—extremely likely). The task began with a 0.25 contingency practice block, followed by ratings of pre-task hunger (0, not hungry at all, to 10, extremely hungry) and pleasantness of each food outcome (− 5, not at all pleasant, to + 5, extremely pleasant). Participants were made aware that they would be given the food rewards that they had earned at the end of the task.
Fig. 1.
Experimental task. Participants were able to choose between two buttons, to maximize reward. Unsuccessful button presses were signaled by a gray circle, while rewarded responses were signaled by a 1000 ms reward stimulus presentation, and the responsible button was highlighted green. After each block of trials, participants rated how causal each button was. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Choice performance and causal awareness were computed by averaging the proportion of optimal key choices and optimal key ratings (AHIGH/AHIGH + ALOW) from the three contingency conditions. Participants with a causal awareness metric 1SD or more below the healthy control group mean were classed as low causal awareness (LCA) with the remaining individuals classified as high causal awareness (HCA). Importantly, this division allowed us to specifically target our main group of interest, youth with psychiatric illness and low causal awareness, and to compare them to the most robust control group: youth with psychiatric illness with non-impaired causal awareness.
Statistical analyses were performed using IBM SPSS 22.0 (SPSS Inc., Chicago). Analyses of variance (ANOVAs) and Pearson's chi squared tests were used to examine group differences in demographics, clinical factors and contingency task performance. Assumptions of normality were assessed using Kolmogorov–Smirnov tests and equality of variance was assessed using Levene's test. For any significant group differences (LCA v HCA), Pearson correlations were conducted to further examine the association with causal awareness as a continuous variable. Alpha levels were set at 0.05 (2 tailed).
2.3. Image acquisition
Participants underwent structural MRI scanning using a 3-Tesla GE MR750 scanner at the Brain and Mind Research Institute, Camperdown, NSW Australia. Images were acquired using an optimized MP-RAGE 3D T1-weighted sequence to resolve anatomy at high resolution (0.9 mm isotropic resolution); TR = 726 ms; TE = 2784 ms; flip angle = 10°; coronal orientation; FOV 230 mm3; matrix of 256 × 256 × 196.
FSL (FMRIB Software Library) tools (www.fmrib.ox.ac.uk) were used in all analyses (version 5.0.1).
2.4. Structural imaging analysis
Two T1-weighted structural scans obtained from a single scanning session were averaged for each individual to increase signal-to-noise ratio. Non-brain material was removed (FSL BET; Smith, 2002), then T1-weighted images were transformed into standard space using a limited degrees-of-freedom non-linear model to ensure spatial alignment and images were corrected for non-uniformity.
2.4.1. Volumetrics
Volumetric data were obtained using FIRST (Patenaude et al., 2011) to automatically segment the following sub-cortical regions: bilateral amygdala, caudate nucleus, putamen, pallidum, thalamus and hippocampus. The caudate, pallidum and thalamus were selected due to evidence that the mPFC-caudate and feedback loops are involved in learning action-outcome contingencies (Griffiths et al., 2015, Liljeholm et al., 2011). The hippocampus may be important for mapping reward values to spatial locations (Izquierdo et al., 2006), while the nucleus accumbens and amygdala have important roles for assessing and utilizing current outcome value in choice situations (Griffiths et al., 2013).
Tissue-type segmentation carried out using FAST4 was used to calculate intracranial volumes (ICV), which were used to correct for differences in head size. These region of interest (ROI) volumes were then corrected for ICV variation so as to provide a common space for cross-sectional morphometric comparisons. Volumetric differences between LCA and HCA were assessed using independent-samples t-tests. For any significant group differences, associations between causal awareness and sub-cortical volume were further examined using Pearson correlations. Bonferroni corrections were applied to account for comparisons across multiple regions (.05/12 regions = .004).
2.4.2. Shape analysis
FIRST created a surface mesh for the subcortical structure in each subject, which was reconstructed in MNI space to normalize for inter-individual head size differences. Pose (rotation and translation) was removed by minimizing the sum of squares difference between the corresponding vertices of a subject's surface and the mean surface. Correlations between causal awareness and ROI shape were assessed within the whole patient group on a per-vertex basis using permutation-based GLM. Differences between groups were assessed on a per-vertex basis using F statistics. The directionality of significant F-tests was investigated using t-tests.
3. Results
3.1. Causal awareness and clinical associations
Thirty-five participants (38%) met criteria for low causal awareness (LCA), with the remaining individuals classed as high causal awareness (HCA) (n = 57, 62%). The LCA and HCA patient groups did not differ with regard to age, gender, years of education, predicted IQ, duration of illness, age of illness onset, medication-use, or any clinical symptoms on the BPRS or YMRS, p > .05. Controls (CON) (n = 20) however had significantly greater years of educational attainment than LCA, (F(2109) = 4.3, p = .019. LCA made significantly poorer causal judgments and fewer optimal choices than both HCA and CON (see Table 1). There were no significant differences in causal awareness or proportion of optimal choices between HCA and CON groups. Fifty-five patients were identified as being within the early stage (1b), while 33 were rated within the later stages of illness (2 or 3).
Table 1.
Demographic and clinical characteristics of participants. Means ± SD.
| LCA (n = 35) |
HCA (n = 57) |
CON (n = 20) |
F/X2 (p) | |
|---|---|---|---|---|
| Demographics | ||||
| Female N (%) | 25 (71%) | 19 (54%) | 11 (55%) | 2.57 (.28) |
| Age (years) | 21.4 ± 4.3 | 23.1 ± 4.9 | 23.9 ± 2.4 | 2.86 (.06) |
| Education | 12.3 ± 2.6 a | 13.2 ± 2.5 | 14.4 ± 2.4 a | 4.3 (.02)* |
| Predicted IQ | 105.0 ± 7.5 | 107.2 ± 6.4 | 106.4 ± 8.2 | 0.79 (.46) |
| Symptoms and history | ||||
| Age of onset (years) | 15.5 ± 3.2 | 15.7 ± 5.5 | 0.01 (.91) | |
| Duration of illness (years) | 6.3 ± 3.9 | 7.5 ± 4.2 | 1.43 (.24) | |
| WHO-DAS disability participating in society | 45.7 ± 17.7 | 35.3 ± 16.6 | 2.94 (.004) | |
| Medication | ||||
| N(%) of cases medicated | 27 (77.1%) | 43 (75.4%) | .035 (1.0) | |
| N(%) on antidepressants | 20 (57.1%) | 29 (50.9%) | .34 (.67) | |
| N(%) on mood stabilizers/anticonvulsants | 10 (28.5%) | 13 (22.8%) | .38 (.62) | |
| N(%) on antipsychotics | 14 (40.0%) | 19 (33.3%) | .41 (.66) | |
| N(%) on anxiolytics | 1 (2.8%) | 3 (5.2%) | .36 (1.0) | |
| Task performance | ||||
| Causal judgments | 0.56 ± 0.1 | 0.68 ± 0.1 | 0.67 ± 0.1 | 53.80 (< .001) |
| Optimal choices | 0.52 ± 0.1 | 0.59 ± 0.1 | 0.61 ± 0.1 | 7.59 (< .001) |
| Motivation measures | ||||
| Hunger (0–10) | 5.2 ± 1.8 | 5.9 ± 2.7 | 6.3 ± 1.6 | 1.60 (.21) |
| Food ratings (− 5: + 5) | 2.5 ± 1.7 | 2.1 ± 2.0 | 3.0 ± 1.4 | 1.78 (.18) |
| Press rate (per s) | 1.3 ± 0.4 | 1.4 ± 0.4 | 1.42 ± 0.4 | 0.78 (.47) |
HC, healthy controls; World Health Organisation Disability Assessment Scale. NB. Duration of illness indicates time since patient first experienced mental health problems, not time since diagnosis. *denotes < .05).
Of those with depression, 36% had low causal awareness, as did 36% of those with bipolar disorder, and 47% with a psychotic disorder. The proportion of individuals with low causal awareness did not differ statistically across diagnostic categories, X2(2) = .81, p = .67. Similarly, the proportion of individuals with low causal awareness did not differ across early (39%and later (36%stages of illness, X2(1) = .06, p = .82.
3.2. Causal awareness and real-world functioning
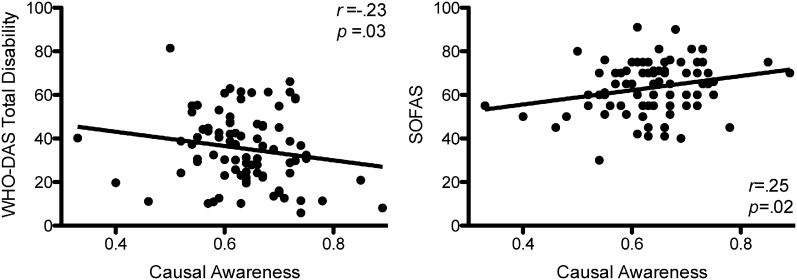
To investigate the functional associations of causal awareness, we compared LCA and HCA on the SOFAS and WHODAS-II. LCA reported higher levels of disability ‘participating in society’ relative to the HCA, t(79) = 2.94, p = .004. Using causal awareness as a continuous measure, reductions in causal awareness were significantly associated with increased self-reported total disability (r = −.26, p = .04) and reduced clinician-rated social and occupational functioning (r = .26, p = .033) (Fig. 2).
Fig. 2.
Causal awareness, disability, and social and occupational functioning. Patients with greater causal awareness tended to have reduced levels of disability and higher levels of social and occupational functioning.
3.3. Sub-cortical differences in participants with low causal awareness
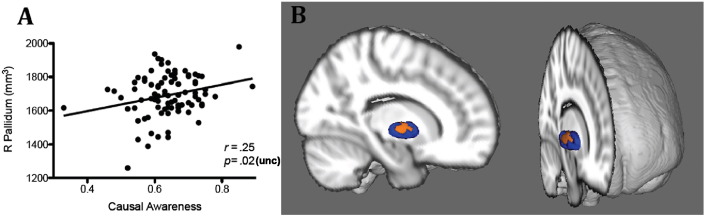
T1-weighted images were not obtained from 4 patients due to scanning artifacts or missing data. LCA had smaller mean right pallidum size than HCA, t(86) = 0.2, p = .038, which was supported by a positive association between causal awareness and right pallidal size in the whole patient group, r = .25, p = .02, though these results did not survive stringent Bonferroni correction (p = .004). There were no other significant differences in any other subcortical region (Table 2). Shape analysis determined that the correlation between causal awareness and right pallidal size was specifically associated with an inward movement of vertices on the central region of the right pallidum, on the lateral aspect (p = 0.02) (Fig. 3). This is situated within the globus pallidus externa (GPe). This finding was replicated using our causal awareness classification groupings, ruling out an effect due to covariance in symptomology or other matched factors.
Table 2.
ICV-corrected volumetric data of ROIs; mean ± SEM (mm3).
| LCA | HCA | CON | ||
|---|---|---|---|---|
| Left hemisphere | Caudate | 3770 ± 58 | 3831 ± 69 | 3822 ± 69 |
| Putamen | 4911 ± 73 | 4918 ± 58 | 4922 ± 59 | |
| Pallidum | 1676 ± 19 | 1676 ± 21 | 1725 ± 71 | |
| Thalamus | 8069 ± 73 | 8190 ± 71 | 8391 ± 119 | |
| Amygdala | 1208 ± 36 | 1245 ± 25 | 1289 ± 43 | |
| Hippocampus | 3611 ± 67 | 3684 ± 68 | 3750 ± 89 | |
| Right hemisphere | Caudate | 3924 ± 70 | 4022 ± 82 | 4035 ± 100 |
| Putamen | 4820 ± 70 | 4939 ± 53 | 4950 ± 54 | |
| Pallidum | 1656 ± 25 | 1725 ± 27 | 1716 ± 27 | |
| Thalamus | 7848 ± 84 | 8031 ± 68 | 8173 ± 99 | |
| Amygdala | 1253 ± 33 | 1316 ± 24 | 1229 ± 44 | |
| Hippocampus | 3789 ± 78 | 3833 ± 65 | 3828 ± 91 |
Comparison of ICV corrected volumes showed larger mean right pallidal size in HCA relative to LCA. This did not survive correction for multiple comparisons. N.B. Control sub-cortical volumes are included to provide a normative reference point.
Fig. 3.
Causal awareness and subcortical volume and shape in youth with psychiatric illnesses. A. Positive correlation between contingency awareness and volume of the right pallidum B. Vertex-wise shape analysis. Average shape of the right pallidum in all patients (blue), with orange regions representing surfaces where individuals with LCA had an inward location of vertices relative to HCA. Image shows varying rotations of a 3D MNI template brain, with the right hemisphere cut away to reveal the 3D mesh of the pallidum. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.4. Sub-cortical associations with causal awareness according to stage of illness
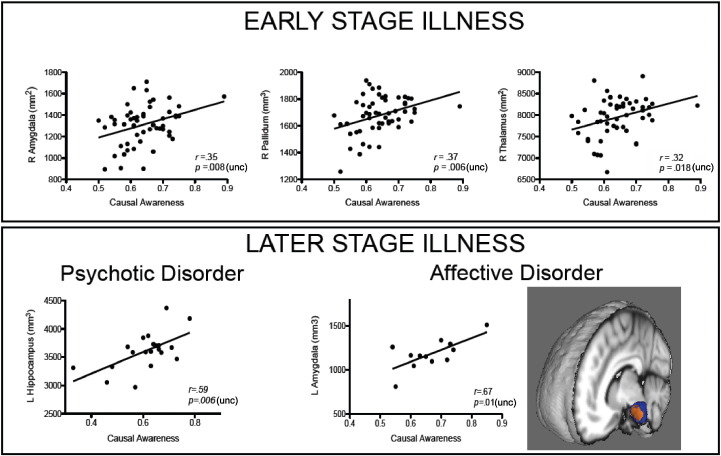
For those within an earlier stage of illness (1b), HCA and LCA groups remained matched on all demographic and clinical variables.LCA had a smaller right amygdala, t(53) = − 2.0, p = .047, right pallidum, t(30.8) = − 2.2, p = .04, and right thalamus, t(53) = − 3.2, p = .002, relative to HCA (note that only the right thalamic size group difference survives Bonferroni multiple comparisons correction (p = .004)). This was supported by positive correlations between causal awareness and volumes within a right lateralized circuit containing the pallidum (r = .37, p = .006), thalamus (r = .32, p = .018), and the amygdala (r = .35, p = .008) (Fig. 4). For those rated in a later stage (i.e. 2 or 3) of illness however, no subcortical structures size were correlated with causal awareness. As an exploratory investigation, we split the later stage group into affective (depression and bipolar disorder without psychosis) versus psychosis (schizophrenia, first episode psychosis, schizoaffective disorder, bipolar disorder with psychosis) groups. In the later stage psychosis group (n = 21), there was a positive relationship between causal awareness and left hippocampal size, r = .59, p = .006, while in the affective group (n = 12), there was a positive correlation between causal awareness and left amygdala size, r = .67, p = .013. Shape analysis revealed that this correlation was specific to the anterior surface of the left amygdala (Fig. 4).
Fig. 4.
Associations between causal awareness and subcortical structure sizes in the early and later stages of illness. Top panel. Within our group of sub-syndromal helping-seeking participants, causal awareness was positively correlated with the size of right amygdala, pallidum and thalamus. Bottom panel. Within later stage psychosis, causal awareness was positively correlated with left hippocampal size, while in later stage affective disorders, causal awareness was positively correlated with left amygdala size. On the right, the average shape of the left amygdala in all later stage affective disorder individuals is depicted in blue, with orange regions representing surfaces where individuals with lower causal awareness had an inward location of vertices. Image shows a 3D MNI template brain, with the left hemisphere cut away to reveal the 3D mesh of the amygdala. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
4. Discussion
4.1. Reduced volume within right external pallidum in participants with low causal awareness
Cognitive deficits and structural changes within the cortical–basal ganglia network are shared illness characteristics across major mood and psychotic disorders. Here, we sought to compare adolescents and young adults with psychiatric illnesses on the basis of awareness of the causal efficacy of their actions. Despite equivalence on all demographic and clinical measures, two key differences emerged in the low causal awareness group relative to patients unimpaired in this capacity: the LCA group reported increased social disability and exhibited reduced right pallidal size. Specifically, LCA had inverted vertices on the dorsolateral pallidal surfaces relative to HCA. In early stage psychiatric illness, LCA exhibited reduced size of the right thalamus relative to HCA. Exploratory investigations suggest that for those with later stage psychosis, causal awareness was positively correlated with left hippocampal size, whereas in later stage affective disorders, causal awareness was positively correlated with left amygdala size, specifically with the anterior aspect. In summary, although deficits in causal awareness were found to occur to a similar extent across the early and later stages of illness, there were differences in the underlying neurobiological correlates.
4.2. Reduced causal awareness as a trans-diagnostic impairment
As discussed recently by Robbins et al. (2012), there is an urgent need to identify neurocognitive endophenotypes that can be applied trans-diagnostically. Our results show that almost 40% of young individuals with psychiatric disorders experience a common difficulty in recognizing the consequences of their actions, irrespective of diagnosis, illness severity or stage of illness. Although not statistically significant, a larger proportion of individuals with psychosis demonstrated low causal awareness relative to those with depression or bipolar disorder. This is consistent with previous reports of general cognitive deficits often being greater in psychotic cohorts compared with the affective disorders (Hellvin et al., 2012, Reppermund et al., 2009, Simonsen et al., 2010). While this measure identifies individuals with a similar behavioral deficit across diagnosis and stage of illness, its neural correlates are not consistent, likely ruling it out as an endophenotype with a shared genetic mechanism.
Importantly though, the early identification of problems with encoding the causal consequences of one's actions may have relevance for guiding clinical treatment decisions. Reduced causal awareness was associated with increased social and occupational dysfunction, suggesting that this may represent a useful cognitive marker for identifying individuals who require additional social support. In addition, reduced causal awareness may impact on the efficacy of specific treatments. Individuals with social anxiety and a reduced capacity for goal-directed behavior experienced poorer response to CBT (Alvares et al., 2014). For these individuals steps may need to be taken to target the updating of belief systems, or alternate therapies may need to be considered. The relationship between altered causal awareness and response to cognitive therapies is clinically important and requires further investigation.
We failed to find any significant association between causal awareness and clinical symptoms such as depression, psychosis, or anxiety. This supports previous findings that clinical symptomology and cognition may to some extent lie on separate dimensions (i.e. Bora et al., 2013, Lee et al., 2012). Although psychosis, mania, depression and anxiety have been shown to introduce state-based cognitive impairments (Millan et al., 2012), when acute clinical symptoms abate, cognitive deficits often persist (Bora et al., 2013). Importantly however, as reduced causal awareness was associated with poorer real-world functioning, this could potentially predispose future depressive episodes and progression to more severe psychopathology (Lin et al., 2013, Martinez-Aran et al., 2004).
4.3. Neural correlates of causal awareness
Tracking of action-outcome contingencies has previously been shown to be modulated by neural circuits including the mPFC and caudate nuclei (Liljeholm et al., 2011, Tanaka et al., 2008)—regions that are connected by wider basal ganglia loops. Our results indicated that those with low causal awareness had reduced right pallidal volumes, specifically in the central region of the pallidum (anterior–posterior axis) on the lateral aspects. This replicates our recent finding that volumetric reduction within the indirect pathway involving the right dorsolateral pallidum is associated with reduced awareness of the causal efficacy of goal-directed actions in young depressed individuals (Griffiths et al., 2015), and extends this finding by showing that it is not specific to depression.
Targeting adolescents and young adults enabled us to examine individuals in the earlier, often less differentiated phases of mental illness when diagnosis is less reliable (Bromet et al., 2011). Despite low rates of conversion from an ultra high-risk (UHR) state to an established disorder (Simon et al., 2011), UHR groups exhibit discernible differences from controls in both gray matter and white matter morphology (Lagopoulos et al., 2013, Lagopoulos et al., 2012). Further, in this early stage, affective and psychotic disorders share significantly overlapping neurobiological changes relative to controls. Within early stage individuals in the current study, the low causal awareness group had reduced right thalamic size relative to the high causal awareness group. The mediodorsal thalamus is an important relay structure between the striatum and prefrontal cortex (Parnaudeau et al., 2013), therefore dysfunction here may also disrupt other prefrontal-dependent cognitive behavior. Although the findings did not survive Bonferrroni correction, volumes of the right pallidum and amygdala were also reduced in the low causal awareness group. Causal awareness was positively correlated with structural size in this extended right lateralized circuit, including pallidum, amygdala and thalamus, suggesting that volume reductions within this circuit may be associated with generalized cognitive impairment. Later stage illness may however exhibit greater neurobiological divergence between diagnoses, and pathophysiology more typical of established, older cohorts. Within later stage psychosis, causal awareness was correlated with left hippocampal size, and in affective disorders, with left amygdala size, specifically on the anterior aspects. Hippocampal pathology in schizophrenia has been well established across a range of imaging modalities and post-mortem studies (Adriano et al., 2012, Heckers, 2001) and the hippocampal-striatum circuit has previously been found to be important for learning to map spatial locations onto reward values (Izquierdo et al., 2006, Rossato et al., 2006). Alternatively, retrospective causal ratings may rely upon context-dependent episodic memory, which the left hippocampus is thought to modulate (Burgess et al., 2002). Depression has been often been associated with volumetric abnormality in the amygdala (Hamilton et al., 2008). Interestingly, exploratory investigations showed that causal awareness was correlated specifically with surface vertices on the anterior aspects of the structure, which may correspond to the basolateral amygdala (BLA). This region is a key part of evaluative learning circuitry, and plays a fundamental role in linking value information with the sensory features of reward or reward-related cues (Balleine et al., 2003). Impairments in this region might critically impact value based learning as well as causal awareness.
4.4. Limitations and future directions
As for many studies using heterogeneous clinical groups, we should be cautious in interpreting gray matter volumes in a cohort treated with various medication classes. For instance, it is well documented that lithium increases gray matter volumes (Moore et al., 2000) while atypical antipsychotics are associated with thalamic enlargement (Dazzan et al., 2005). It is possible that medications for specific symptoms (e.g. antipsychotic or antidepressant) may contribute to the differentiation in neural correlates of causal awareness in later stage psychotic and affective disorders. Longitudinal data is necessary however for making more substantiated claims about illness progression. This would also allow for a better understanding of the causal nature of the association between causal awareness and real-world functioning and an examination of modulatory effects on treatment outcomes. Due to the relatively small sample size, our exploratory findings need to also be replicated in larger samples before firm conclusions can be drawn. Future work may benefit from utilizing a battery of behavioral tests designed to dissociate deficits in specific aspects of goal-directed behavior more broadly (see Balleine and O'Doherty, 2010). This may assist with identifying individual-specific deficits which may lead to targeted pharmacological treatment.
4.5. Summary and conclusion
Gauging the consequences of one's actions relies upon a broad neural network that integrates capacities such as learning, memory and executive functioning. Given this complexity, aiming to determine underlying pathology from a single behavioral metric may appear beyond current methodology. Nevertheless, poor causal awareness appears to be a cross-diagnostic impairment that was associated with social functioning. Early identification of problems such as these may provide impetus for targeted cognitive interventions, to ultimately reduce disease burden or delay the development of full threshold disorders. On balance, causal awareness is a functionally relevant dimensional capacity, however further research is necessary to clarify the underlying individual-specific neural correlates.
Financial disclosures
IBH has led projects for health professionals and the community supported by governmental, community agency and drug industry partners (Wyeth, Eli Lily, Servier, Pfizer and AstraZeneca) for the identification and management of depression and anxiety. He has served on advisory boards convened by the drug industry in relation to specific antidepressants, including nefazodone, duloxetine and desvenlafaxine, and has participated in a multicenter clinical trial of agomelatine effects on sleep architecture in depression. He has participated in Servier-sponsored educational programs related to circadian-based therapies. DFH has previously received honoraria for educational seminars from Janssen-Cilag. The remaining authors declare no conflict of interest.
Acknowledgments
The preparation of this manuscript was supported by a grant from the Australian Research Council (ARC FL0992409) to Bernard W. Balleine.
References
- Adriano F., Caltagirone C., Spalletta G. Hippocampal volume reduction in first-episode and chronic schizophrenia: a review and meta-analysis. Neuroscientist. 2012;18:180–200. doi: 10.1177/1073858410395147. [DOI] [PubMed] [Google Scholar]
- Allott K., Liu P., Proffitt T.M., Killackey E. Cognition at illness onset as a predictor of later functional outcome in early psychosis: systematic review and methodological critique. Schizophr. Res. 2011;125(2):221–235. doi: 10.1016/j.schres.2010.11.001. [DOI] [PubMed] [Google Scholar]
- Alvares G.A., Balleine B.W., Guastella A.J. Impairments in goal-directed actions predict treatment response to cognitive-behavioral therapy in social anxiety disorder. PLoS One. 2014;9(4) doi: 10.1371/journal.pone.0094778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balleine B.W., Dickinson A.D. Goal-directed instrumental action: contingency and incentive learning and their cortical substrates. Neuropharmacology. 1998;37(4):407–419. doi: 10.1016/s0028-3908(98)00033-1. [DOI] [PubMed] [Google Scholar]
- Balleine B.W., O'Doherty J.P. Human and rodent homologies in action control: corticostriatal determinants of goal-directed and habitual action. Neuropsychopharmacology. 2010;35(1):48–69. doi: 10.1038/npp.2009.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balleine B.W., Killcross S., Dickinson A.D. The effect of lesions of the basolateral amygdala on instrumental conditioning. J. Neurosci. 2003;23(2):666–675. doi: 10.1523/JNEUROSCI.23-02-00666.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bora E., Harrison B.J., Yücel M., Pantelis C. Cognitive impairment in euthymic major depressive disorder: a meta-analysis. Psychol. Med. 2013;43(10):2017–2026. doi: 10.1017/S0033291712002085. [DOI] [PubMed] [Google Scholar]
- Bromet E.J., Kotov R., Fochtmann L.J., Carlson G.A., Tanenberg-Karant M., Ruggero C., Chang S.-W. Diagnostic shifts during the decade following first admission for psychosis. Am. J. Psychiatry. 2011;168(11):1186–1194. doi: 10.1176/appi.ajp.2011.11010048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess N., Maguire E.A., O'Keefe J. The human hippocampus and spatial and episodic memory. Neuron. 2002;35(4):625–641. doi: 10.1016/s0896-6273(02)00830-9. [DOI] [PubMed] [Google Scholar]
- Chwastiak L.A., Von Korff M. Disability in depression and back pain: evaluation of the World Health Organization Disability Assessment Schedule (WHO DAS II) in a primary care setting. J. Clin. Epidemiol. 2003;56(6):507–514. doi: 10.1016/s0895-4356(03)00051-9. [DOI] [PubMed] [Google Scholar]
- Dazzan P., Morgan K.D., Orr K., Hutchinson G., Chitnis X., Suckling J. Different effects of typical and atypical antipsychotics on grey matter in first episode psychosis: the AESOP study. Neuropsychopharmacology. 2005;30(4):765–774. doi: 10.1038/sj.npp.1300603. [DOI] [PubMed] [Google Scholar]
- Dingemans P.M., Linszen D.H., Lenior M.E., Smeets R.M. Component structure of the expanded Brief Psychiatric Rating Scale (BPRS-E) Psychopharmacology. 1995;122(3):263–267. doi: 10.1007/BF02246547. [DOI] [PubMed] [Google Scholar]
- Goldman H., Skodol A.E., Lave T. 2012. Revising Axis V for DSM-IV: a Review of Measures of Social Functioning. [DOI] [PubMed] [Google Scholar]
- Griffiths K.R., Morris R.W., Balleine B.W. Translational studies of goal-directed action as a framework for classifying deficits across psychiatric disorders. Front. Syst. Neurosci. 2013;8:101. doi: 10.3389/fnsys.2014.00101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths K.R., Lagopoulos J., Hermens D.F., Hickie I.B., Balleine B.W. Right external globus pallidus changes are associated with altered causal awareness in youth with depression. Transl. Psychiatry. 2015;5(10):e653. doi: 10.1038/tp.2015.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton M. Development of a rating scale for primary depressive illness. Br. J. Soc. Clin. Psycho. 1967;6(4):278–296. doi: 10.1111/j.2044-8260.1967.tb00530.x. [DOI] [PubMed] [Google Scholar]
- Hamilton J.P., Siemer M., Gotlib I.H. Amygdala volume in major depressive disorder: a meta-analysis of magnetic resonance imaging studies. Mol. Psychiatry. 2008;13(11):993–1000. doi: 10.1038/mp.2008.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckers S. Neuroimaging studies of the hippocampus in schizophrenia. Hippocampus. 2001;11(5):520–528. doi: 10.1002/hipo.1068. [DOI] [PubMed] [Google Scholar]
- Hellvin T., Sundet K., Simonsen C., Aminoff S.R., Lagerberg T.V., Andreassen O.A., Melle I. Neurocognitive functioning in patients recently diagnosed with bipolar disorder. Bipolar Disord. 2012;14(3):227–238. doi: 10.1111/j.1399-5618.2012.01004.x. [DOI] [PubMed] [Google Scholar]
- Hermens D.F., Redoblado Hodge M.A., Naismith S.L., Kaur M., Scott E.M., Hickie I.B. Neuropsychological clustering highlights cognitive differences in young people presenting with depressive symptoms. J. Int. Neuropsychol. Soc. 2011;17(2):267–276. doi: 10.1017/S1355617710001566. [DOI] [PubMed] [Google Scholar]
- Hickie I.B., Scott E.M., Hermens D.F., Naismith S.L., Guastella A.J., Kaur M. Applying clinical staging to young people who present for mental health care. Early Interv. Psychiatry. 2013;7(1):31–43. doi: 10.1111/j.1751-7893.2012.00366.x. [DOI] [PubMed] [Google Scholar]
- Insel T.R. The arrival of preemptive psychiatry. Early Interv. Psychiatry. 2007;1(1):5–6. doi: 10.1111/j.1751-7893.2007.00017.x. [DOI] [PubMed] [Google Scholar]
- Insel T., Cuthbert B., Garvey M., Heinssen R., Pine D.S., Quinn K. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. Am. J. Psychiatry. 2010;167(7):748–751. doi: 10.1176/appi.ajp.2010.09091379. [DOI] [PubMed] [Google Scholar]
- Izquierdo I., Bevilaqua L.R., Rossato J.I., Bonini J.S., Da Silva W.C., Medina J.H., Cammarota M. The connection between the hippocampal and the striatal memory systems of the brain: a review of recent findings. Neurotox. Res. 2006;10(2):113–121. doi: 10.1007/BF03033240. [DOI] [PubMed] [Google Scholar]
- Kessler R.C., Berglund P., Demler O., Jin R., Merikangas K.R., Walters E.E. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch. Gen. Psychiatry. 2005;62(6):593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- Lagopoulos J., Hermens D.F., Naismith S.L., Scott E.M., Hickie I.B. Frontal lobe changes occur early in the course of affective disorders in young people. BMC Psychiatry. 2012;12(1):4. doi: 10.1186/1471-244X-12-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagopoulos J., Hermens D.F., Hatton S.N., Tobias-Webb J., Griffiths K., Naismith S.L. Microstructural white matter changes in the corpus callosum of young people with bipolar disorder: a diffusion tensor imaging study. PLoS One. 2013;8(3):e59108. doi: 10.1371/journal.pone.0059108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee R.S.C., Hermens D.F., Porter M.A., Redoblado Hodge M.A. A meta-analysis of cognitive deficits in first-episode major depressive disorder. J. Affect. Disord. 2012;140(2):113–124. doi: 10.1016/j.jad.2011.10.023. [DOI] [PubMed] [Google Scholar]
- Lee R.S.C., Hermens D.F., Redoblado Hodge M.A., Naismith S.L., Porter M.A., Kaur M. Neuropsychological and socio-occupational functioning in young psychiatric outpatients: a longitudinal investigation. PLoS One. 2013;8(3):e58176. doi: 10.1371/journal.pone.0058176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee R.S.C., Hermens D.F., Scott J., Redoblado Hodge M.A., Naismith S.L., Lagopoulos J. A meta-analysis of neuropsychological functioning in first-episode bipolar disorders. J. Psychiatr. Res. 2014;57:1–11. doi: 10.1016/j.jpsychires.2014.06.019. [DOI] [PubMed] [Google Scholar]
- Lee R.S.C., Hermens D.F., Naismith S.L., Lagopoulos J., Jones A., Scott J. Neuropsychological and functional outcomes in recent-onsetmajor depression, bipolar disorder and schizophrenia-spectrumdisorders: a longitudinal cohort study. 2015;5(4):1–10. doi: 10.1038/tp.2015.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liljeholm M., Tricomi E.M., O'Doherty J.P., Balleine B.W. Neural correlates of instrumental contingency learning: differential effects of action-reward conjunction and disjunction. J. Neurosci. 2011;31(7):2474–2480. doi: 10.1523/JNEUROSCI.3354-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin A., Wood S.J., Nelson B., Brewer W.J., Spiliotacopoulos D., Bruxner A. Neurocognitive predictors of functional outcome two to 13 years after identification as ultra-high risk for psychosis. Schizophr. Res. 2011;132(1):1–7. doi: 10.1016/j.schres.2011.06.014. [DOI] [PubMed] [Google Scholar]
- Lin A., Yung A.R., Nelson B., Brewer W.J., Riley R., Simmons M. Neurocognitive predictors of transition to psychosis: medium-to long-term findings from a sample at ultra-high risk for psychosis. Psychol. Med. 2013;43(11):2349–2360. doi: 10.1017/S0033291713000123. [DOI] [PubMed] [Google Scholar]
- Martinez-Aran A., Vieta E., Colom F., Torrent C., Sánchez Moreno J., Reinares M. Cognitive impairment in euthymic bipolar patients: implications for clinical and functional outcome. Bipolar Disord. 2004;6(3):224–232. doi: 10.1111/j.1399-5618.2004.00111.x. [DOI] [PubMed] [Google Scholar]
- McGorry P.D., Hickie I.B., Yung A.R., Pantelis C., Jackson H.J. Clinical staging of psychiatric disorders: a heuristic framework for choosing earlier, safer and more effective interventions. Aust. N. Z. J. Psychiatry. 2006;40(8):616–622. doi: 10.1080/j.1440-1614.2006.01860.x. [DOI] [PubMed] [Google Scholar]
- Millan M.J., Agid Y., Brüne M., Bullmore E.T., Carter C.S., Clayton N.S. Cognitive dysfunction in psychiatric disorders: characteristics, causes and the quest for improved therapy. Nat. Rev. Drug Discov. 2012;11(2):141–168. doi: 10.1038/nrd3628. [DOI] [PubMed] [Google Scholar]
- Moore G.J., Bebchuk J.M., Wilds I.B., Chen G., Menji H.K. Lithium-induced increase in human brain grey matter. Lancet. 2000;356(9237):1241–1242. doi: 10.1016/s0140-6736(00)02793-8. [DOI] [PubMed] [Google Scholar]
- Overall J.E., Gorham D.R. The brief psychiatric rating scale. Psychol. Rep. 1962;10(3):799–812. [Google Scholar]
- Pantelis C., Velakoulis D., McGorry P.D., Wood S.J., Suckling J., Phillips L.J. Neuroanatomical abnormalities before and after onset of psychosis: a cross-sectional and longitudinal MRI comparison. Lancet. 2003;361(9354):281–288. doi: 10.1016/S0140-6736(03)12323-9. [DOI] [PubMed] [Google Scholar]
- Parnaudeau S., O'Neill P.K., Bolkan S.S., Ward R.D., Abbas A.I., Roth B.L.…Kellendonk C. Inhibition of mediodorsal thalamus disrupts thalamofrontal connectivity and cognition. Neuron. 2013;77(6):1151–1162. doi: 10.1016/j.neuron.2013.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patenaude B., Smith S.M., Kennedy D.N., Jenkinson M. A Bayesian model of shape and appearance for subcortical brain segmentation. NeuroImage. 2011;56(3):907–922. doi: 10.1016/j.neuroimage.2011.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus T., Keshavan M., Giedd J.N. Why do many psychiatric disorders emerge during adolescence? Nat. Rev. Neurosci. 2008;9(12):947–957. doi: 10.1038/nrn2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reppermund S., Ising M., Lucae S., Zihl J. Cognitive impairment in unipolar depression is persistent and non-specific: further evidence for the final common pathway disorder hypothesis. Psychol. Med. 2009;39(04):603–614. doi: 10.1017/S003329170800411X. [DOI] [PubMed] [Google Scholar]
- Robbins T.W., Gillan C.M., Smith D.G., Wit S., Ersche K.D. Neurocognitive endophenotypes of impulsivity and compulsivity: towards dimensional psychiatry. Trends Cogn. Sci. 2012;16(1):81–91. doi: 10.1016/j.tics.2011.11.009. [DOI] [PubMed] [Google Scholar]
- Rossato J.I., Bevilaqua L.R., Medina J.H., Izquierdo I., Cammarota M. Retrieval induces hippocampal-dependent reconsolidation of spatial memory. Learn. Mem. 2006;13(4):431–440. doi: 10.1101/lm.315206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott E.M., Hermens D.F., Glozier N., Naismith S.L., Guastella A.J., Hickie I.B. Targeted primary care-based mental health services for young Australians. Med. J. Aust. 2012;196(2):136–140. doi: 10.5694/mja11.10481. [DOI] [PubMed] [Google Scholar]
- Scott J., Leboyer M., Hickie I., Berk M., Kapczinski F., Frank E.…McGorry P. Clinical staging in psychiatry: a cross-cutting model of diagnosis with heuristic and practical value. Br. J. Psychiatry. 2013;202(4):243–245. doi: 10.1192/bjp.bp.112.110858. [DOI] [PubMed] [Google Scholar]
- Simon A.E., Velthorst E., Nieman D.H., Linszen D., Umbricht D., de Haan L. Ultra high-risk state for psychosis and non-transition: a systematic review. Schizophr. Res. 2011;132(1):8–17. doi: 10.1016/j.schres.2011.07.002. [DOI] [PubMed] [Google Scholar]
- Simonsen C., Sundet K., Vaskinn A., Birkenaes A.B., Engh J.A., Faerden A. Neurocognitive dysfunction in bipolar and schizophrenia spectrum disorders depends on history of psychosis rather than diagnostic group. Schizophr. Bull. 2010;37(1):73–83. doi: 10.1093/schbul/sbp034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S.M. Fast robust automated brain extraction. Hum. Brain Mapp. 2002;17(3):143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka S.C., Balleine B.W., O'Doherty J.P. Calculating consequences: brain systems that encode the causal effects of actions. J. Neurosci. 2008;28(26):6750–6755. doi: 10.1523/JNEUROSCI.1808-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres I.J., DeFreitas C.M., DeFreitas V.G., Bond D.J., Kunz M., Honer W.G. Relationship between cognitive functioning and 6-month clinical and functional outcome in patients with first manic episode bipolar I disorder. Psychol. Med. 2010;41(05):971–982. doi: 10.1017/S0033291710001613. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Psychological Corporation; New York, NY, USA: 2001. Wechsler Test of Adult Reading. [Google Scholar]
- Young R.C., Biggs J.T., Ziegler V.E., Meyer D.A. A rating scale for mania: reliability, validity and sensitivity. Br. J. Psychiatry. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]