Abstract
The association between tobacco smoke and acute myeloid leukemia (AML) is well established in adults but not in children. Individual-level data on parental cigarette smoking were obtained from 12 case-control studies from the Childhood Leukemia International Consortium (CLIC, 1974–2012), including 1,330 AML cases diagnosed at age <15 years and 13,169 controls. We conducted pooled analyses of CLIC studies, as well as meta-analyses of CLIC and non-CLIC studies. Overall, maternal smoking before, during, or after pregnancy was not associated with childhood AML; there was a suggestion, however, that smoking during pregnancy was associated with an increased risk in Hispanics (odds ratio = 2.08, 95% confidence interval (CI): 1.20, 3.61) but not in other ethnic groups. By contrast, the odds ratios for paternal lifetime smoking were 1.34 (95% CI: 1.11, 1.62) and 1.18 (95% CI: 0.92, 1.51) in pooled and meta-analyses, respectively. Overall, increased risks from 1.2- to 1.3-fold were observed for pre- and postnatal smoking (P < 0.05), with higher risks reported for heavy smokers. Associations with paternal smoking varied by histological type. Our analyses suggest an association between paternal smoking and childhood AML. The association with maternal smoking appears limited to Hispanic children, raising questions about ethnic differences in tobacco-related exposures and biological mechanisms, as well as study-specific biases.
Keywords: acute myeloid leukemia, childhood cancer, international collaboration, parental smoking
Acute lymphoblastic leukemia is the most common cancer in children 0–14 years of age, whereas acute myeloid leukemia (AML) represents 2%–5% of childhood cancers (1, 2). The incidence of childhood AML varies geographically, with higher rates in children from Asian and Hispanic descents (3). It is well established that exposure to tobacco smoke is a risk factor for AML in adults (4). Numerous studies demonstrate that myeloid cells can be damaged by tobacco smoke carcinogens, including benzene, formaldehyde, 1,3-butadiene, polycyclic aromatic hydrocarbons, and polonium (4–8). Although it has been hypothesized that tobacco smoke may affect both somatic cells and germ cells during critical periods of a child's development (7), the potential contribution of tobacco smoke to the development of AML in children has not been as well studied as it has in adults. Most studies of childhood AML report no associations with maternal smoking during pregnancy (9–22), although modest increased risks were observed in 2 small case-control studies (11, 12) and 2 registry-based Swedish cohort studies (23, 24). Studies on paternal smoking and childhood AML have yielded mixed results, some reporting elevated risks for lifetime smoking (18, 25), some for preconception smoking (17), and others no associations (9, 12, 26, 27).
Individual studies of childhood AML to date are based on small numbers because of the rarity of the disease and have included mostly Caucasian children. To overcome these shortcomings, we pooled data on maternal and paternal tobacco smoking from 12 case-control studies participating in the Childhood Leukemia International Consortium (CLIC) (28) and examined the associations of pre- and postnatal exposures to tobacco smoke with risk of childhood AML. Additionally, we conducted meta-analyses of CLIC and non-CLIC studies.
METHODS
Study population
Twelve case-control studies of childhood leukemia (age <15 years) conducted in 10 countries from 1974 to 2012 and participating in CLIC (28) contributed data to the pooled analyses (Web Table 1 available at http://aje.oxfordjournals.org/). This included 9 studies with published data: Brazil (limited to infants <24 months of age) (29), the United Kingdom (United Kingdom Childhood Cancer Study) (19), France (Adele; Epidemiologic Study on Childhood Cancer and Leukemia) (20, 21), Germany (German Childhood Cancer Registry) (22), Greece (Nationwide Registration for Childhood Hematological Malignancies) (30), Italy (Study on the Etiology of Childhood Lymphohematopoietic Malignancies) (25), and the United States (Northern California Childhood Leukemia Study (18) and linked registry data from Washington State (31)), as well as 3 studies with unpublished data at the time of this report from Costa Rica, Mexico (Mexican Interinstitutional Group for the Identification of the Causes of Childhood Leukemia), and New Zealand (New Zealand Childhood Cancer Study). Study characteristics are presented in Web Table 2 (28). Data for 1,330 newly diagnosed de novo AML cases and 13,169 controls were available for the pooled analyses.
Data collection and standardization
CLIC studies provided original data on self-reported maternal and paternal tobacco (cigarette) smoking for “ever/never smoking,” smoking during specific time windows (preconception, during pregnancy as a whole and/or by trimester, and after birth), and duration (Web Table 1). Data on maternal tobacco smoking during pregnancy were the most complete (12 studies), compared with other smoking-related data (6–9 studies) (Web Table 1). Information on parental smoking was collected from interviews with mothers and/or fathers (Northern California Childhood Leukemia Study, German Childhood Cancer Registry, Nationwide Registration for Childhood Hematological Malignancies, United Kingdom Childhood Cancer Study, Costa Rica, Study on the Etiology of Childhood Lymphohematopoietic Malignancies, the Mexican Interinstitutional Group for the Identification of the Causes of Childhood Leukemia), mothers only (Adele; Epidemiologic Study on Childhood Cancer and Leukemia, New Zealand Childhood Cancer Registry, Brazil), or via maternal self-report or medical/prenatal record review (United States-Washington State). We also obtained data on child's race/ethnicity, sex, age (at diagnosis for cases and corresponding age for matched controls or age at enrollment/interview), birth weight, parental ages at the time of the child's birth, maternal consumption of alcohol during pregnancy, home exposure to paints and solvents, and parental educational level (highest level achieved by mother or father at the time of the child's birth). The Washington State study provided imputed values for missing data on parental education, using methods previously described (32). All studies, except those in Brazil, Costa Rica, and Germany (German Childhood Cancer Registry), provided data on AML subtype according to the French-American-British (FAB) classification (Table 1). Four studies (United Kingdom Childhood Cancer Study, Adele, Epidemiologic Study on Childhood Cancer and Leukemia, and Northern California Childhood Leukemia Study) provided information on the presence of the mixed lineage leukemia gene (MLL) translocation (11q23) detected by fluorescence in situ hybridization.
Table 1.
Clinical and Sociodemographic Characteristics of Acute Myeloid Leukemia Cases and Controls, Childhood Leukemia International Consortium, 1974–2012a
| Variable | Controls(n = 13,169) |
Cases(n = 1,330) |
||
|---|---|---|---|---|
| No. | % | No. | % | |
| FAB subtype (cases only) | ||||
| M0 | 46 | 3 | ||
| M1 | 94 | 7 | ||
| M2 | 256 | 19 | ||
| M3 | 140 | 11 | ||
| M4 | 162 | 12 | ||
| M5 | 158 | 12 | ||
| M6 | 28 | 3 | ||
| M7 | 101 | 2 | ||
| Unspecified/missing | 346 | 26 | ||
| Child's age, yearsb | ||||
| <1 | 1,174 | 9 | 176 | 13 |
| 1–4 | 5,752 | 44 | 474 | 36 |
| 5–9 | 3,566 | 27 | 330 | 25 |
| 10–14 | 2,677 | 20 | 350 | 26 |
| Child's sex | ||||
| Male | 7,299 | 55 | 706 | 53 |
| Female | 5,870 | 45 | 624 | 47 |
| Parent's highest educational levelc | ||||
| None/primary | 2,611 | 20 | 284 | 22 |
| Secondary | 4,710 | 37 | 458 | 35 |
| Tertiary | 5,386 | 42 | 533 | 41 |
| Missing | 154 | 1 | 21 | 2 |
| Child's ethnicityd | ||||
| Non-Hispanic white | 10,363 | 79 | 860 | 65 |
| Hispanic | 1,711 | 13 | 372 | 28 |
| Non-Hispanic other | 1,018 | 8 | 90 | 7 |
| Missing | 77 | 1 | 8 | 1 |
| Mother's age at child's birth, years | ||||
| <25 | 3,688 | 28 | 414 | 31 |
| 25–29 | 4,555 | 35 | 448 | 34 |
| 30–34 | 3,319 | 25 | 305 | 23 |
| ≥35 | 1,575 | 12 | 158 | 12 |
| Missing | 32 | 0 | 5 | 0 |
| Father's age at child's birth, years | ||||
| <25 | 1,785 | 14 | 247 | 19 |
| 25–29 | 3,762 | 29 | 360 | 27 |
| 30–34 | 3,868 | 29 | 350 | 26 |
| ≥35 | 3,297 | 25 | 331 | 25 |
| Missing | 457 | 4 | 42 | 3 |
Abbreviations and designations: FAB, French-American-British; M0, acute myeloblastic leukemia, minimally differentiated; M1, acute myeloblastic leukemia, without maturation; M2, acute myeloblastic leukemia, with graulocytic maturation; M3, acute promyelocytic leukemia; M4, acute myelomonocytic leukemia; M5, acute monoblastic/monocytic leukemia; M6, acute erythroid leukemia; M7, acute megaloblastic leukemia.
a Percentages may not sum to 100 because of rounding.
b Child's age at diagnosis for cases and recruitment for controls.
c Does not include missing values for Washington State.
d Studies that did not provide ethnicity data were automatically assigned the most common ethnicity based on population-based data.
Data were reviewed by CLIC principal investigators and harmonized for pooling. Variables with heterogeneous definitions across studies were categorized for periods of smoking (Web Table 3), highest levels of parental education (none/primary, secondary, and tertiary), and ethnicity (Hispanic, non-Hispanic white/Caucasian/European, later referred to as non-Hispanic white, and non-Hispanic other) (Web Table 4). Studies from Germany, Italy, and Costa Rica did not collect data on subject-specific ethnicity; on the basis of population-based data, all subjects in Germany and Italy were considered non-Hispanic white, and all subjects in Costa Rica were considered Hispanic.
Statistical analyses
Study-specific and pooled odds ratios and 95% confidence intervals were estimated by using unconditional logistic regression models. Parents' and child's age and birth weight were treated as continuous variables. The amount of smoking was analyzed by using data as reported and categorical variables based on the observed frequency distribution among controls (nonsmokers, moderate smokers (1–15 cigarettes per day), and heavy smokers (≥16 cigarettes per day)). Models were adjusted for child's age, sex, and ethnicity, because these variables were used as matching factors in the individual studies. We also adjusted for parental educational level (a surrogate for socioeconomic status found to be a confounder and/or indicator of selection bias in individual studies) and study center. Other potential confounders (parental age at delivery, maternal alcohol intake, birth weight, and home exposure to paints and solvents) were not retained in the final additive models because their inclusion did not alter the odds ratio by more than 10%, and none were effect modifiers in the multiplicative models. In stratified analyses, P < 0.20 was used as a threshold for the statistical significance of heterogeneity between strata, using likelihood ratio techniques or Woolf's test. We conducted sensitivity analyses to assess the robustness of our results by systematically excluding 2 studies at a time (Web Figure 1).
To further assess study heterogeneity, we conducted meta-analyses for CLIC studies and non-CLIC studies. For CLIC studies, we computed the study-specific odds ratios using the original study design and corresponding unconditional or conditional regression models adjusted for study-specific confounders. A systematic search in PubMed, Embase, and Web of Science identified 24 studies of childhood AML and parental smoking, including 13 CLIC and 11 non-CLIC studies (details in Web Figure 2, based on Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines) (33). The following search terms were used: “(pediatric OR childhood) AND leukemia AND (tobacco OR smok*) AND (parent* OR maternal OR paternal)” in PubMed, Embase and Web of Science; reference lists of a published review paper (16) and a meta-analysis (30) were also screened. The search resulted in 268 papers, and 23 passed the 6 exclusion criteria (Web Figure 2). For those, the following information was extracted: author's name, publication year, country where the study was conducted, case-control design (individually or frequency matched), sample size (cases and controls), and any measure of association for parental smoking. The search and data abstraction were conducted by 1 investigator, and quality control was performed by an independent investigator. Funnel plots were generated and showed no indication of publication biases (data not shown). Meta odds ratios for yes/no smoking at various times were estimated for CLIC and non-CLIC studies and all studies combined using random and fixed effect models; the I2 statistics were used as a measure of heterogeneity (34).
RESULTS
Sociodemographic characteristics were similar for cases and controls with the exception of ethnicity, likely because of the inclusion of a large number of controls from Europe (Table 1). Overall, paternal tobacco smoking was least prevalent in the United States, and fathers smoked more than mothers in all countries (Tables 2–4). The φ correlation coefficients between maternal and paternal smoking ranged from 0.26 to 0.32 for various periods (data not shown). The φ correlation coefficients between periods were greater than 0.68 for mothers and 0.91 for fathers. Among smokers, 91% of fathers and 49% of mothers smoked from preconception to after birth, and 37% of mothers stopped smoking during pregnancy.
Table 2.
Pooled Risk of Childhood Acute Myeloid Leukemia Associated With Maternal Smoking Across Time Periods, Childhood Leukemia International Consortium, 1974–2012
| Window of Exposure | Total Controls, no. | Total Cases, no. | Exposed, % |
ORa | 95% CI | |
|---|---|---|---|---|---|---|
| Controls | Cases | |||||
| Ever-smoking | 5,929 | 563 | 42 | 42 | 1.01 | 0.84, 1.22 |
| Smoking during prenatal periodb | 12,631 | 1,266 | 29 | 26 | 0.93 | 0.81, 1.07 |
| Smoking during preconception periodb | 10,137 | 1,012 | 31 | 27 | 0.89 | 0.76, 1.04 |
| Smoking during pregnancyb | 12,621 | 1,266 | 19 | 15 | 0.95 | 0.81, 1.13 |
| Smoking after birth | 10,229 | 1,028 | 28 | 26 | 0.96 | 0.82, 1.12 |
Abbreviations: CI, confidence interval; OR, odds ratio.
a Odds ratios were adjusted for age, sex, ethnicity, highest parental education, and study center.
b Odds ratios were computed by using imputed values of education.
Table 4.
Pooled Risk of Childhood Acute Myeloid Leukemia Associated With Paternal Smoking Across Time Periods, Childhood Leukemia International Consortium, 1974–2012
| Window of Exposure | Total Controls, no. | Total Cases, no. | Exposed, % |
ORa | 95% CI | |
|---|---|---|---|---|---|---|
| Controls | Cases | |||||
| Ever-smoking | 5,538 | 528 | 53 | 60 | 1.34 | 1.11, 1.62 |
| Smoking during prenatal period | 11,080 | 1,094 | 45 | 51 | 1.24 | 1.08, 1.42 |
| Smoking during preconception period | 7,699 | 860 | 46 | 50 | 1.18 | 1.01, 1.38 |
| Smoking during pregnancy | 7,337 | 810 | 44 | 48 | 1.24 | 1.06, 1.46 |
| Smoking after birth | 11,199 | 1,035 | 42 | 50 | 1.27 | 1.11, 1.45 |
Abbreviations: CI, confidence interval; OR, odds ratio.
a Odds ratios were adjusted for age, sex, ethnicity, highest parental education, and study center.
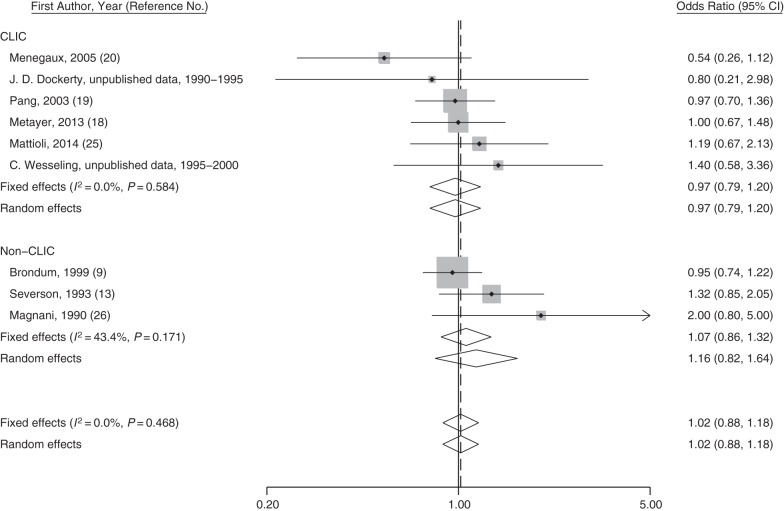
Overall, maternal smoking during any time period was not associated with increased risks of childhood AML in pooled analyses (Table 2) or in meta-analyses of CLIC and non CLIC studies (Figure 1 and Web Figures 3–5). However, in pooled analyses restricted to Hispanic children, maternal smoking during pregnancy was associated with a 2-fold increased risk of AML (95% CI: 1.20, 3.61), whereas no increased risks were observed for other ethnic groups (P for interaction < 0.01) (Table 3). Data on Hispanics were from studies in Mexico (257 cases and 520 controls; odds ratio (OR) = 1.68, 95% CI: 0.77, 3.66); Costa Rica (34 cases and 558 controls; OR = 2.42, 95% CI: 0.43, 13.62); California (64 cases and 517 controls; OR = 2.02, 95% CI: 0.84, 4.89); and Washington State (12 cases and 95 controls; OR = 6.30, 95% CI: 0.39, 101.1; data not shown). Analyses stratified by age, birth weight, and highest parental education led to inconclusive findings (Table 3).
Figure 1.
Study-specific odds ratios and fixed- and random-effects meta-analysis odds ratios for maternal ever-smoking and risk of acute myeloid leukemia in selected Childhood Leukemia International Consortium (CLIC) and non-CLIC studies between 1990–2014. Odds ratios for CLIC studies were generated by using original data. The P value for heterogeneity between groups is 0.552. The vertical solid line represents the reference for “no association,” and the dashed line represents the risk estimate from the fixed-effect meta-analysis. CI, confidence interval.
Table 3.
Risk of Acute Myeloid Leukemia Associated With Maternal Smoking During Pregnancy, by Selected Characteristics, Childhood Leukemia International Consortium, 1974–2012
| Variable | Total Controls, no. | Total Cases, no. | Exposed, % |
ORa,b | 95% CI | P Value for Interactionc | |
|---|---|---|---|---|---|---|---|
| Controls | Cases | ||||||
| Child's ethnicity | <0.01 | ||||||
| Non-Hispanic white | 7,732 | 644 | 23 | 19 | 0.82 | 0.66, 1.01 | |
| Hispanic | 1,130 | 332 | 5 | 7 | 2.08 | 1.20, 3.61 | |
| Non-Hispanic other | 1,013 | 89 | 13 | 16 | 1.08 | 0.56, 2.07 | |
| Highest parental education | 0.98 | ||||||
| None/primary | 2,605 | 284 | 27 | 23 | 0.85 | 0.58, 1.24 | |
| Secondary | 4,851 | 464 | 23 | 17 | 0.93 | 0.69, 1.24 | |
| Tertiary | 5,493 | 553 | 12 | 10 | 0.99 | 0.71, 1.39 | |
| Child's birth weight, g | 0.53 | ||||||
| Low birth weight (<2,500) | 1,039 | 108 | 25 | 16 | 0.58 | 0.29, 1.17 | |
| Normal birth weight (2,500–4,000) | 10,831 | 1,091 | 19 | 15 | 0.95 | 0.77, 1.17 | |
| High birth weight (>4,000) | 1,219 | 121 | 12 | 16 | 1.20 | 0.65, 2.19 | |
| Child's age, years | 0.93 | ||||||
| Age <1 | 1,169 | 175 | 17 | 17 | 1.08 | 0.66, 1.78 | |
| Age 1–4 | 5,725 | 472 | 20 | 15 | 0.97 | 0.72, 1.32 | |
| Age 5–9 | 3,542 | 327 | 19 | 17 | 0.83 | 0.55, 1.25 | |
| Age 10–14 | 2,653 | 346 | 18 | 14 | 0.83 | 0.57, 1.20 | |
Abbreviations: CI, confidence interval; OR, odds ratio.
a Odds ratios were adjusted for age, sex, ethnicity, highest parental education, and study center, except when it was the variable of interest.
b Odds ratios were computed by using imputed values of education.
c P value for interaction derived from the Woolf test.
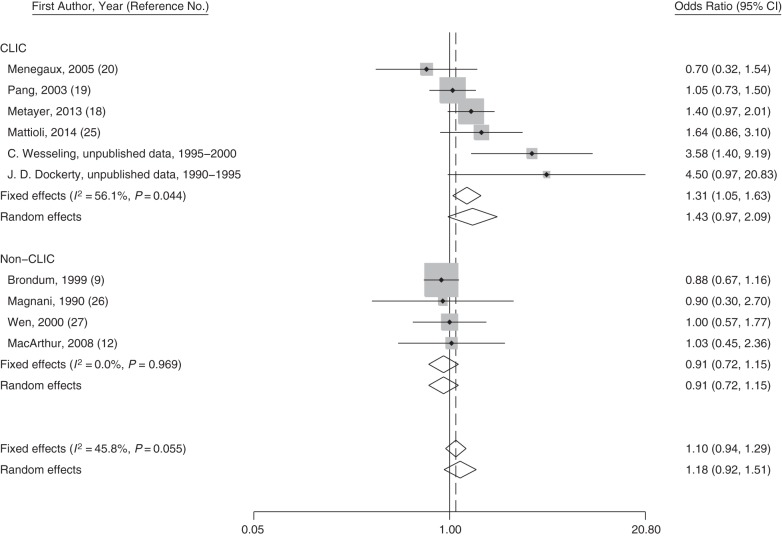
Paternal smoking during any period was associated with increased risks of childhood AML with pooled odds ratios ranging from 1.18 to 1.34 (Table 4). Similar results were observed when analyses were restricted to households with nonsmoking mothers (∼25%) and for analyses of concurrent paternal and maternal ever-smoking versus nonsmoking parents (data not shown). Sensitivity analyses indicated that the mean odds ratio for childhood AML associated with paternal ever-smoking remained >1 for all 15 possible combinations of studies (Web Figure 1). The magnitude of the association with paternal ever-smoking was lower among non-Hispanic whites, compared with other ethnicities (P for interaction = 0.08) (Table 5). Despite heterogeneity among CLIC studies, the fixed- and random-effects meta odds ratios were similar to the pooled odds ratios, ranging from 1.2 to 1.4 depending on the time period (Figure 2 and Web Figures 6–8). For non-CLIC studies, the study-specific odds ratios for paternal smoking were below or close to 1 for all periods, resulting in overall meta odds ratios of smaller magnitude, yet above 1 (Figure 2 and Web Figures 6–8).
Table 5.
Risk of Acute Myeloid Leukemia Associated With Paternal Ever-Smoking, by Selected Characteristics, Childhood Leukemia International Consortium, 1974–2012
| Variable | Total Controls, no. | Total Cases, no. | Exposed, % |
ORa | 95% CI | P Value for Interactionb | |
|---|---|---|---|---|---|---|---|
| Controls | Cases | ||||||
| Child's ethnicity | 0.08 | ||||||
| Non-Hispanic white | 3,951 | 307 | 54 | 58 | 1.10 | 0.87, 1.41 | |
| Hispanic | 506 | 61 | 42 | 52 | 1.45 | 0.84, 2.50 | |
| Non-Hispanic other | 475 | 47 | 47 | 64 | 1.81 | 0.94, 3.49 | |
| Highest parental education | 0.26 | ||||||
| None/primary | 815 | 116 | 66 | 78 | 1.71 | 0.87, 3.39 | |
| Secondary | 2,242 | 215 | 58 | 60 | 1.09 | 0.79, 1.50 | |
| Tertiary | 2,487 | 197 | 44 | 50 | 1.25 | 0.92, 1.70 | |
| Child's birth weight, g | 0.96 | ||||||
| Low birth weight (<2,500) | 479 | 41 | 56 | 71 | 1.39 | 0.58, 3.32 | |
| Normal birth weight (2,500–4,000) | 4,482 | 426 | 53 | 61 | 1.21 | 0.96, 1.53 | |
| High birth weight (>4,000) | 599 | 64 | 49 | 55 | 1.32 | 0.76, 2.29 | |
| Child's age, years | 0.74 | ||||||
| Age <1 | 247 | 56 | 52 | 50 | 1.04 | 0.55, 1.96 | |
| Age 1–4 | 2,574 | 187 | 49 | 58 | 1.36 | 0.96, 1.94 | |
| Age 5–9 | 1,583 | 141 | 55 | 66 | 1.35 | 0.86, 2.12 | |
| Age 10–14 | 1,156 | 147 | 58 | 63 | 1.11 | 0.74, 1.65 | |
Abbreviations: CI, confidence interval; OR, odds ratio.
a Odds ratios were adjusted for age, sex, ethnicity, highest parental education level, and study center, except when it was the variable of interest.
b P value for interaction was derived from the −2 log likelihood estimate from multivariate analyses.
Figure 2.
Study-specific odds ratios and fixed- and random-effects meta-analysis odds ratios for paternal ever-smoking and risk of acute myeloid leukemia in Childhood Leukemia International Consortium (CLIC) and non-CLIC studies between 1990 and 2014. Odds ratios for CLIC studies were generated by using original data. The P value for heterogeneity between groups is 0.026. The solid line represents the reference for “no association,” and the dashed line represents the risk estimate from the fixed-effect meta-analysis. CI, confidence interval.
Among non-Hispanic white controls, 36% and 17% of smoking mothers smoked 16 cigarettes or more per day before conception and during pregnancy, respectively, suggesting that heavy smoking among smokers was not rare. However, fewer Hispanic mothers reported smoking 16 cigarettes or more per day before conception (7%) and during pregnancy (3%). Overall, the odds ratios did not increase with increasing amount of maternal smoking (Table 6), except for Hispanic mothers during pregnancy (for an increase of 5 cigarettes per day, OR = 1.81, 95% CI: 1.11, 2.96). The highest level of paternal smoking (≥16 cigarettes per day) in all time periods was associated with the greatest risk of AML (Table 6); however, dose-response relationships were statistically significant only for the prenatal periods. The mean duration of paternal smoking in the prenatal period was similar between cases and controls (16.2 and 17.2 years, respectively; P for t test = 0.21), and the odds ratio for every year of smoking was 1.01 (95% CI: 0.98, 1.03). About 80% of fathers continued to smoke after the child's birth, and the mean duration of smoking did not vary between cases and controls (P for t test = 0.60).
Table 6.
Risk of Acute Myeloid Leukemia Associated With Number of Cigarettes Smoked per Day by the Mother and Father, by Period of Exposure, Childhood Leukemia International Consortium, 1974–2012
| Window of Exposure and Smoking Status | No. of Studies | Controls, no. | Cases, no. | ORa | 95% CI |
|---|---|---|---|---|---|
| Mother | |||||
| Prenatal | 11 | ||||
| Nonsmokers | 6,037 | 512 | 1.00 | Referent | |
| Moderate smokersb | 2,074 | 130 | 0.82 | 0.66, 1.00 | |
| Heavy smokersc | 4,321 | 404 | 0.74 | 0.50, 1.11 | |
| Per 5 cigarettes/dayd | 12,432 | 1,046 | 0.99 | 0.98, 1.00 | |
| Preconception | 6 | ||||
| Nonsmokers | 5,569 | 461 | 1.00 | Referent | |
| Moderate smokersb | 1,576 | 99 | 0.82 | 0.65, 1.04 | |
| Heavy smokersc | 854 | 54 | 0.83 | 0.61, 1.13 | |
| Per 5 cigarettes/dayd | 7,999 | 614 | 0.95 | 0.89, 1.01 | |
| Pregnancy | 9 | ||||
| Nonsmokers | 8,448 | 723 | 1.00 | Referent | |
| Moderate smokersb | 1,812 | 139 | 0.96 | 0.79, 1.17 | |
| Heavy smokersc | 383 | 23 | 0.68 | 0.44, 1.07 | |
| Per 5 cigarettes/dayd | 10,643 | 885 | 0.96 | 0.89, 1.03 | |
| Postnatal | 4 | ||||
| Nonsmokers | 3,544 | 301 | 1.00 | Referent | |
| Moderate smokersb | 570 | 41 | 0.87 | 0.61, 1.23 | |
| Heavy smokersc | 206 | 10 | 0.54 | 0.28, 1.04 | |
| Per 5 cigarettes/dayd | 4,320 | 352 | 0.92 | 0.82, 1.04 | |
| Father | |||||
| Prenatal | 7 | ||||
| Nonsmokers | 5,296 | 375 | 1.00 | Referent | |
| Moderate smokersb | 1,726 | 141 | 1.18 | 0.96, 1.45 | |
| Heavy smokersc | 1,949 | 174 | 1.31 | 1.08, 1.60 | |
| Per 5 cigarettes/dayd | 8,971 | 690 | 1.06 | 1.02, 1.10 | |
| Preconception | 5 | ||||
| Nonsmokers | 3,252 | 254 | 1.00 | Referent | |
| Moderate smokersb | 1,113 | 85 | 1.04 | 0.79, 1.35 | |
| Heavy smokersc | 1,217 | 117 | 1.37 | 1.07, 1.77 | |
| Per 5 cigarettes/dayd | 5,582 | 456 | 1.06 | 1.02, 1.11 | |
| Pregnancy | 3 | ||||
| Nonsmokers | 2,283 | 149 | 1.00 | Referent | |
| Moderate smokersb | 884 | 58 | 1.03 | 0.74, 1.44 | |
| Heavy smokersc | 997 | 89 | 1.41 | 1.06, 1.89 | |
| Per 5 cigarettes/dayd | 4,164 | 296 | 1.06 | 1.01, 1.12 | |
| Postnatal | 3 | ||||
| Nonsmokers | 2,546 | 139 | 1.00 | Referent | |
| Moderate smokersb | 779 | 43 | 0.93 | 0.65, 1.33 | |
| Heavy smokersc | 937 | 68 | 1.27 | 0.93, 1.73 | |
| Per 5 cigarettes/dayd | 4,262 | 250 | 1.03 | 0.97, 1.10 | |
Abbreviations: CI, confidence interval; OR, odds ratio.
a Odds ratios were adjusted for age, sex, ethnicity, highest parental education (using imputed values for mothers when needed), and study center.
b Moderate smokers are defined as smoking 1–15 cigarettes per day.
c Heavy smokers are defined as smoking 16 or more cigarettes per day.
d Odds ratios were computed with the continuous variable provided, divided by 5.
Although modestly increased odds ratios were observed for some AML subtypes, only the association between paternal ever-smoking and acute myelomonocytic leukemia (designated M4 in the French-American-British (FAB) classification) was statistically significant (OR = 1.87, 95% CI: 1.08, 3.25) (Table 7). Maternal ever-smoking was also associated with an increased risk of M4 (OR = 1.80, 95% CI: 1.09, 2.97), but this association weakened after adjustment for paternal smoking (OR = 1.49, 95% CI: 0.87, 2.53). There were no statistically significant increased risks of AML with MLL fusion in relation to paternal or maternal ever-smoking.
Table 7.
Risk of Acute Myeloid Leukemia FAB Subtype and MLL Rearrangement Status in Relation to Paternal Ever-Smoking, Childhood Leukemia International Consortium, 1974–2012
| FAB Subtype | Total Cases, no. | Exposed Cases, % | ORa | 95% CI |
|---|---|---|---|---|
| M0 | 23 | 65 | 1.33 | 0.54, 3.24 |
| M1 | 43 | 51 | 0.83 | 0.45, 1.55 |
| M2 | 116 | 62 | 1.35 | 0.91, 2.01 |
| M3 | 45 | 62 | 1.55 | 0.83, 2.91 |
| M4 | 60 | 65 | 1.87 | 1.08, 3.25 |
| M5 | 80 | 48 | 0.76 | 0.48, 1.21 |
| M6 | 10 | 40 | 0.59 | 0.16, 2.20 |
| M7 | 42 | 55 | 1.25 | 0.66, 2.36 |
| MLL rearrangement | ||||
| Negative | 338 | 57 | 1.19 | 0.95, 1.50 |
| Positive | 41 | 51 | 1.08 | 0.57, 2.03 |
Abbreviations and designations: CI, confidence interval; FAB, French-American-British (classification); M0, acute myeloblastic leukemia, minimally differentiated; M1, acute myeloblastic leukemia, without maturation; M2, acute myeloblastic leukemia, with graulocytic maturation; M3, acute promyelocytic leukemia; M4, acute myelomonocytic leukemia; M5, acute monoblastic/monocytic leukemia; M6, acute erythroid leukemia; M7, acute megaloblastic leukemia; MLL, mixed lineage leukemia gene; OR, odds ratio.
a Odds ratios were adjusted for age, sex, ethnicity, highest parental education, and study center.
DISCUSSION
The current study, based on individual data from 12 case-control studies in Europe, the Americas, and Oceania, is the largest to date to investigate the role of parental smoking in the etiology of childhood AML. Our pooled analyses support the association between paternal smoking and childhood AML; however, the high correlation between pre- and postnatal paternal smoking limited our ability to identify specific windows of exposure. Overall, no associations were detected between maternal smoking and childhood AML. Meta-analyses including both CLIC and non-CLIC case-control studies (9–15, 35–39) led to similar findings for maternal smoking, although the magnitude of the associations was decreased for paternal smoking.
There was a suggestion that the increased risk of AML associated with maternal smoking during pregnancy was observed in Hispanic children. The findings for Hispanics were based on studies in Costa Rica and Mexico, as well as 2 US studies in California and Washington states that accounted for 42% and 11% of Hispanics, respectively (including 83%–88% from Mexican origin on the basis of census data from 2011 (40, 41) and self-report in the California study). This is the first study to examine the risk of tobacco smoking and childhood AML in Hispanics, an ethnic group that has experienced a rising incidence of childhood leukemia (3), as well as rising prevalence of smoking among women (42–44). Our findings may be due to population heterogeneity, study-specific biases, or chance. Alternatively, differences by ethnic group may be explained by variation in types of tobacco used (black or blond) (45–49) and, consequently, differences in concentrations of carcinogens, such as N-nitroso compounds. Also, genes involved in transport and metabolism of tobacco-related chemicals have been found to differ in non-Hispanics versus Hispanics (50, 51).
Similar to our findings, those of previous studies have suggested a positive dose-response relationship with paternal smoking, although based on unstable estimates. A study from China reported a 2.3-fold (95% CI: 0.4, 14.8) increase in risk of childhood AML with preconception paternal smoking of 5 or more pack-years, whereas no associations were seen for lesser amounts (35). In a US study of 88 infants with AML (15), higher risk of childhood leukemia was observed among fathers’ smoking more than 20 cigarettes per day (OR = 1.29, 95% CI: 0.44, 3.74). A study conducted in the United Kingdom reported a positive dose-response relationship with lifetime paternal smoking and childhood AML, using death as a surrogate for leukemia diagnosis (n = 190 from 1971 to 1976) (39), although a later study did not (n = 145 from 1977 to 1981) (37). A larger case-control study conducted in the United States failed to support an association with paternal ever-smoking or during any time period, and no evidence of a dose-response relationship was found (n = 517 from 1989 to 1993) (9). Duration of smoking was not associated with an increased risk of childhood AML in our study. Brondum et al. (9) reported a decreased risk with increasing smoking duration (P for trend = 0.06), whereas no other childhood AML studies have published data.
Few fathers changed their smoking habits over time, limiting our ability to assess the independent contribution of pre- versus. postnatal exposure to tobacco. Under the assumption that maternal smoking before birth (for non-Hispanic children) and after birth does not induce AML in the index child, it is possible that preconception paternal smoking contributes to AML risk, either solely, or in combination with postnatal smoking. Such associations have also been reported for risk of childhood acute lymphoblastic leukemia (18, 30, 52). These epidemiologic observations are supported by laboratory studies showing that tobacco smoke is a germ-cell mutagen in rodents, and likely in humans (53), and can induce germ cell epigenetic changes (54). A child's exposure to secondhand smoking, however, may also contribute to the development of AML (18). The CLIC study from Italy reported an increased risk of AML associated with preconception paternal smoking only among mothers less than 30 years of age at delivery (25), but we did not reproduce this finding in our pooled analyses (data not shown).
We observed increased risks of M4 in children of smoking fathers and, possibly, smoking mothers. Nonstatistically significant odds ratios were also observed between paternal smoking and acute myeloblastic leukemia, minimally differentiated (FAB subtype M0), acute myeloblastic leukemia, with granulocytic maturation (FAB subtype M2), and acute promyelocytic leukemia (FAB subtype M3). However, the interpretation of our subtype analyses is limited by small sample sizes. Another case-control study also reported associations of larger magnitude between childhood M4 leukemia and maternal ever-smoking (n = 37 cases) (13). Studies conducted in adults have reported a higher frequency of M2, M4, acute monoblastic/monocytic leukemia (FAB subtype M5), and acute erythroid leukemia (FAB subtype M6) among smokers, whereas acute myeloblastic leukemia without maturation (FAB subtype M1) and M3 were more frequent in nonsmokers (16, 39, 55). Treatment-related M2 frequently occurs in patients treated with alkylating agents and radiation, and FAB subtypes M3, M4, and M5 occur after exposure to topoisomerase II inhibitors (56, 57), suggesting that some metabolic pathways may be shared between tobacco- and treatment-induced AML. We observed no difference in risk for children classified as MLL positive and MLL negative. The MLL fusion is more frequent among infants with leukemia (58), and a study of 88 infant AMLs reported no association with parental smoking (15). In contrast, a recent case-case study found that maternal smoking during pregnancy resulted in a 3-fold increased risk of AML with MLL rearrangement (n = 57) compared with MLL negative (n = 59) (59). The occurrence of AML with MLL fusion proteins may be further modulated by inherited genetic susceptibility factors (60, 61).
Our analyses have some limitations. Selection bias may occur in case-control studies if parents' participation is dependent on the exposure of interest, here smoking habit, and is correlated with sociodemographic factors or race/ethnicity. The distribution of educational levels varied among participating CLIC studies (i.e., lower education in cases vs. controls in 4 studies, lower education in controls vs. cases in 1 study, and no difference between cases and controls in 7 studies). As a result, it is difficult to assess the magnitude of selection bias and the influence on the overall pooled risk estimates. However, the observation of both positive and negative findings in our analyses argues against strong selection bias. There is a concern, especially among pregnant women, that recall of smoking history may be inaccurate (62). Studies have reported a lack of concordance between self-reported smoking during pregnancy and cotinine blood level in mothers and newborns (63, 64). Also, compared with nonpregnant women, pregnant women are more likely to underreport smoking (63) or exhibit unstable smoking habits (65). In most studies, mothers provided information for paternal smoking, and it has been shown that spouses of cases and controls usually report paternal smoking accurately (18, 66, 67). We compared smoking prevalence in CLIC studies with population-based data, and for CLIC studies conducted in the United Kingdom, Greece, Italy, Mexico, and Brazil, the prevalence of smoking for mothers and fathers (among controls) was higher by approximately 6%–8%, compared with population-based data (68–72) that were available for the corresponding study periods and the corresponding age groups; the population-based data for Germany, France, Costa Rica, and California were available after the corresponding CLIC study period and suggested the same trend (73–76). In contrast, the prevalence of smoking among controls in the birth registry-based study conducted in Washington State was lower than the prevalence reported in the general population (77), and it is possible that mothers underreported smoking at the interviews conducted by hospital staff soon after the child's birth. Overall, this may indicate that subjects participating in the CLIC case-control studies overreported smoking habits or represent a selected group not representative of the general population. However, our data indicated that maternal smoking was correlated with low birth weight in both cases and controls (data not shown), consistent with the well-established observation that maternal smoking induces intrauterine growth retardation (78). This observation indirectly suggests that recall bias may not fully explain the negative findings for maternal smoking (among non-Hispanics). Alternatively, maternal smoking during pregnancy is associated with stillbirth and miscarriage (79), which could represent a competing outcome preventing our evaluation of childhood leukemia risk.
Although alcohol consumption during pregnancy has been associated with a 1.5-fold increased risk of childhood AML (17, 80), there was no evidence of confounding (or effect modification) with alcohol use in our analyses. No other factors affected the observed associations, but residual confounding from unmeasured factors is possible. In previous CLIC pooled analyses on prenatal folate and vitamin supplementation, we observed that adjustment for study site accounted for confounding by measured and unmeasured study characteristics, even when the heterogeneity between studies is large (81). Meta-analyses indicated some heterogeneity for paternal smoking, and to a lesser extent for maternal smoking. Nevertheless, exclusion of studies resulted in relatively stable estimates, indicating that results were not largely driven by specific studies. Finally, it is possible that multiple comparisons may have led to false positive findings. Despite limitations, the strengths of the CLIC pooled analyses are the inclusion of published and unpublished original data, large sample size for a rare disease like childhood AML, inclusion of Hispanic children, and the ability to conduct subgroup and dose-response analyses, as well as adjustment for confounders.
In conclusion, we observed an association between paternal smoking and childhood AML. Findings for maternal smoking remained mostly null, except possibly for Hispanic children exposed in utero. Until associations with maternal smoking are further elucidated, and given the many health risks associated with smoking, it is important to educate families and health professionals on preventing fathers, mothers, and children from being exposed to tobacco smoke before and after the child's birth.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: School of Public Health, University of California, Berkeley, Berkeley, California (Catherine Metayer, Kathryn McCauley, Steve Selvin, Alice Y. Kang); Department of Hygiene, Epidemiology, and Medical Statistics, National and Kapostridian University of Athens Medical School, Athens, Greece (Eleni Petridou, Maria Moschovi); Clinical Epidemiology, Pediatric Hospital, Centro Medico Nacional “Siglo XXI,” Instituto Mexicano del Seguro Social, Mexico City, Mexico (Juan Manuel Mejía Aranguré, Janet Flores-Lujano, the Mexican Interinstitutional Group for the Identification of the Causes of Childhood Leukemia Group); Department of Health Services, University of York, Heslington, York, United Kingdom (Eve Roman, Tracy Lightfoot); International Agency for Research on Cancer Section of Environment and Radiation, Lyon, France (Joachim Schüz); Dipartimento di Medicina Traslazionale, Universitá del Piemonte Orientale, SCDU Epidemiologia del Tumori, Novara, Italy (Corrado Magnani); Central American Institute for Studies on Toxic Substances (IRET), Universidad Nacional, Heredia, Costa Rica (Ana Maria Mora, Catharina Wesseling); Public Health Sciences Division, Fred Hutchinson Cancer Research Center, Seattle, Washington (Beth A. Mueller, David R. Doody); Department of Epidemiology, School of Public Health, University of Washington, Seattle, Washington (Beth A. Mueller); Pediatric Hematology-Oncology Program, Instituto Nacional de Cancer, Rio de Janeiro, Brazil (Maria S. Pombo de Oliveira); Dean's Department and Department of Preventative and Social Medicine, Dunedin School of Medicine, University of Otago, Dunedin, New Zealand (John D. Dockerty); Pediatric Hematology Oncology Unit, Second Department of Pediatrics, Aristotle University School of Medicine, AHEPA General Hospital, Thessaloniki, Greece (Emmanouel Hatzipantelis); INSERM U1153, Epidemiology and Biostatistics Sourbonne Paris Cité Center (CRESS), Epidemiology of Childhood and Adolescent Cancers Team (EPICEA), Paris-Descartes University, Villejuif, France (Jacqueline Clavel, Jérémie Rudant, Laurent Orsi); German Childhood Cancer Registry at the Institute for Medical Biostatistics, Epidemiology, and Informatics, University Medical Centre, Johnanes Gutenberg University, Mainz, Germany (Peter Kaatsch); Occupational and Environmental Epidemiology Unit, ISPO-Cancer Prevention and Research Institute, Ponte Nuovo, Florence, Italy (Lucia Miligi); and Department of Medical and Surgical Sciences, University of Bologna, Bologna, Italy (Stefano Mattioli).
This work was supported by the National Institute of Environmental Health Sciences (P01 ES018172) and the US Environmental Protection Agency (RD83451101), as part of the Center for Integrative Research on Childhood Leukemia and the Environment (CIRCLE).
We would like to thank our late colleague, Dr. Patricia Buffler (Chair of CLIC, 2007–2013, and Principal Investigator of CIRCLE, 2009–2013), as well as Somdat Mahabir (US National Cancer Institute) and Denis Henshaw and Katie Martin (Children with Cancer, United Kingdom) for the scientific and administrative support to CLIC. Additional acknowledgments for CLIC studies are provided in the Web Appendix.
Members of the Mexican Interinstitutional Group for the Identification of the Causes of Childhood Leukemia (MIGICCL) include Juan Carlos Nuñez-Enriquez, Elva Jiménez-Hernández, and Rogelio Paredes-Aguilera.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Environmental Health Sciences or the US Environmental Protection Agency.
Conflict of interest: none declared.
Contributor Information
Collaborators: the MIGICCL Group, Catherine Metayer, Kathryn McCauley, Steve Selvin, Alice Y. Kang, Eleni Petridou, Maria Moschovi, Juan Manuel Mejía Aranguré, Janet Flores-Lujano, Eve Roman, Tracy Lightfoot, Joachim Schüz, Corrado Magnani, Ana Maria Mora, Catharina Wesseling, Beth A. Mueller, David R. Doody, Maria S. Pombo de Oliveira, John D. Dockerty, Emmanouel Hatzipantelis, Jacqueline Clavel, Jérémie Rudant, Laurent Orsi, Peter Kaatsch, Lucia Miligi, and Stefano Mattioli
REFERENCES
- 1.Ward E, DeSantis C, Robbins A et al. . Childhood and adolescent cancer statistics, 2014. CA Cancer J Clin. 2014;642:83–103. [DOI] [PubMed] [Google Scholar]
- 2.Haematological Malignancy Research Network (HMRN). Incidence. York, United Kingdom: Epidemiology and Cancer Statistics Group; 2015. https://www.hmrn.org/statistics/incidence. Accessed November 30, 2015. [Google Scholar]
- 3.Linet MS, Brown LM, Mbulaiteye SM et al. . International long-term trends and recent patterns in the incidence of leukemias and lymphomas among children and adolescents ages 0–19 years. Int J Cancer. 2016;1388:1862–1874. [DOI] [PubMed] [Google Scholar]
- 4.Cogliano VJ, Baan R, Straif K et al. . Preventable exposures associated with human cancers. J Natl Cancer Inst. 2011;10324:1827–1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.International Agency for Research on Cancer. Overall Evaluation of Carcinogenicity: An Updating of IARC Monographs. Vol. 1–42. Lyon, Fance: International Agency for Research on Cancer; 1987. [Google Scholar]
- 6.Goldstein BD. Benzene as a cause of lymphoproliferative disorders. Chem Biol Interact. 2010;184(1-2):147–150. [DOI] [PubMed] [Google Scholar]
- 7.Smith MT. Advances in understanding benzene health effects and susceptibility. Annu Rev Public Health. 2010;31:133–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zagà V, Lygidakis C, Chaouachi K et al. . Polonium and lung cancer. J Oncol. 2011;2011:860103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brondum J, Shu XO, Steinbuch M et al. . Parental cigarette smoking and the risk of acute leukemia in children. Cancer. 1999;856:1380–1388. [PubMed] [Google Scholar]
- 10.van Duijn CM, van Steensel-Moll HA, Coebergh JW et al. . Risk factors for childhood acute non-lymphocytic leukemia: an association with maternal alcohol consumption during pregnancy? Cancer Epidemiol Biomarkers Prev. 1994;36:457–460. [PubMed] [Google Scholar]
- 11.Cnattingius S, Zack M, Ekbom A et al. . Prenatal and neonatal risk factors for childhood myeloid leukemia. Cancer Epidemiol Biomarkers Prev. 1995;45:441–445. [PubMed] [Google Scholar]
- 12.MacArthur AC, McBride ML, Spinelli JJ et al. . Risk of childhood leukemia associated with parental smoking and alcohol consumption prior to conception and during pregnancy: the cross-Canada childhood leukemia study. Cancer Causes Control. 2008;193:283–295. [DOI] [PubMed] [Google Scholar]
- 13.Severson RK, Buckley JD, Woods WG et al. . Cigarette smoking and alcohol consumption by parents of children with acute myeloid leukemia: an analysis within morphological subgroups—a report from the Children's Cancer Group. Cancer Epidemiol Biomarkers Prev. 1993;25:433–439. [PubMed] [Google Scholar]
- 14.Slater ME, Linabery AM, Blair CK et al. . Maternal prenatal cigarette, alcohol and illicit drug use and risk of infant leukaemia: a report from the Children's Oncology Group. Paediatr Perinat Epidemiol. 2011;256:559–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shu XO, Ross JA, Pendergrass TW et al. . Parental alcohol consumption, cigarette smoking, and risk of infant leukemia: A Children's Cancer Group study. J Natl Cancer Inst. 1996;881:24–31. [DOI] [PubMed] [Google Scholar]
- 16.Chang JS. Parental smoking and childhood leukemia. In: Verma M, ed. Cancer Epidemiology. New York, NY: Humana Press; 2009:103–137. [DOI] [PubMed] [Google Scholar]
- 17.Orsi L, Rudant J, Ajrouche R et al. . Parental smoking, maternal alcohol, coffee and tea consumption during pregnancy, and childhood acute leukemia: the ESTELLE study. Cancer Causes Control. 2015;267:1003–1017. [DOI] [PubMed] [Google Scholar]
- 18.Metayer C, Zhang L, Wiemels JL et al. . Tobacco smoke exposure and the risk of childhood acute lymphoblastic and myeloid leukemias by cytogenetic subtype. Cancer Epidemiol Biomarkers Prev. 2013;229:1600–1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pang D, McNally R, Birch JM. Parental smoking and childhood cancer: results from the United Kingdom Childhood Cancer Study. Br J Cancer. 2003;883:373–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Menegaux F, Steffen C, Bellec S et al. . Maternal coffee and alcohol consumption during pregnancy, parental smoking and risk of childhood acute leukaemia. Cancer Detect Prev. 2005;296:487–493. [DOI] [PubMed] [Google Scholar]
- 21.Rudant J, Menegaux F, Leverger G et al. . Childhood hematopoietic malignancies and parental use of tobacco and alcohol: the ESCALE study (SFCE). Cancer Causes Control. 2008;1910:1277–1290. [DOI] [PubMed] [Google Scholar]
- 22.Schüz J, Kaatsch P, Kaletsch U et al. . Association of childhood cancer with factors related to pregnancy and birth. Int J Epidemiol. 1999;284:631–639. [DOI] [PubMed] [Google Scholar]
- 23.Mucci LA, Granath F, Cnattingius S. Maternal smoking and childhood leukemia and lymphoma risk among 1,440,542 Swedish children. Cancer Epidemiol Biomarkers Prev. 2004;139:1528–1533. [PubMed] [Google Scholar]
- 24.Pershagen G, Ericson A, Otterblad-Olausson P. Maternal smoking in pregnancy: Does it increase the risk of childhood cancer? Int J Epidemiol. 1992;211:1–5. [DOI] [PubMed] [Google Scholar]
- 25.Mattioli S, Farioli A, Legittimo P et al. . Tobacco smoke and risk of childhood acute non-lymphocytic leukemia: findings from the SETIL study. PLoS One. 2014;911:e111028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Magnani C, Pastore G, Luzzatto L et al. . Parental occupation and other environmental factors in the etiology of leukemias and non-Hodgkin's lymphomas in childhood: a case-control study. Tumori. 1990;765:413–419. [DOI] [PubMed] [Google Scholar]
- 27.Wen WQ, Shu XO, Steinbuch M et al. . Paternal military service and risk for childhood leukemia in offspring. Am J Epidemiol. 2000;1513:231–240. [DOI] [PubMed] [Google Scholar]
- 28.Metayer C, Milne E, Clavel J et al. . The Childhood Leukemia International Consortium. Cancer Epidemiol. 2013;373:336–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ferreira JD, Couto AC, Pombo-de-Oliveira MS et al. . Pregnancy, maternal tobacco smoking, and early age leukemia in Brazil. Front Oncol. 2012;2:151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Klimentopoulou A, Antonopoulos CN, Papadopoulou C et al. . Maternal smoking during pregnancy and risk for childhood leukemia: a nationwide case-control study in Greece and meta-analysis. Pediatr Blood Cancer. 2012;583:344–351. [DOI] [PubMed] [Google Scholar]
- 31.Podvin D, Kuehn CM, Mueller BA et al. . Maternal and birth characteristics in relation to childhood leukaemia. Paediatr Perinat Epidemiol. 2006;204:312–322. [DOI] [PubMed] [Google Scholar]
- 32.White IR, Royston P, Wood AM. Multiple imputation using chained equations: issues and guidance for practice. Stat Med. 2011;304:377–399. [DOI] [PubMed] [Google Scholar]
- 33.Moher D, Liberati A, Tetzlaff J et al. . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;85:336–341. [DOI] [PubMed] [Google Scholar]
- 34.Higgins JP, Thompson SG, Deeks JJ et al. . Measuring inconsistency in meta-analyses. BMJ . 2003;3277414:557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ji BT, Shu XO, Linet MS et al. . Paternal cigarette smoking and the risk of childhood cancer among offspring of nonsmoking mothers. J Natl Cancer Inst. 1997;893:238–244. [DOI] [PubMed] [Google Scholar]
- 36.John EM, Savitz DA, Sandler DP. Prenatal exposure to parents’ smoking and childhood cancer. Am J Epidemiol. 1991;1332:123–132. [DOI] [PubMed] [Google Scholar]
- 37.Sorahan T, Lancashire R, Prior P et al. . Childhood cancer and parental use of alcohol and tobacco. Ann Epidemiol. 1995;55:354–359. [DOI] [PubMed] [Google Scholar]
- 38.Sorahan T, Lancashire RJ, Hultén MA et al. . Childhood cancer and parental use of tobacco: deaths from 1953 to 1955. Br J Cancer. 1997;751:134–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sorahan T, Prior P, Lancashire RJ et al. . Childhood cancer and parental use of tobacco: deaths from 1971 to 1976. Br J Cancer. 1997;7611:1525–1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pew Research Center. Demographic profile of Hispanics in Washington, 2011. Washington, DC: Pew Research Center; 2011. http://www.pewhispanic.org/states/state/wa/. Accessed April 9, 2015. [Google Scholar]
- 41.Pew Research Center. Demographic profile of Hispanics in California, 2011. Washington, DC: Pew Research Center; 2011. http://www.pewhispanic.org/states/state/ca/. Accessed April 9, 2015. [Google Scholar]
- 42.Bethel JW, Schenker MB. Acculturation and smoking patterns among Hispanics: a review. Am J Prev Med. 2005;292:143–148. [DOI] [PubMed] [Google Scholar]
- 43.Franco-Marina F, Lazcano-Ponce E. Adult smoking trends in Mexico between 1988 and 2008 [in Spanish] Salud Publica Mex. 2010;52(suppl 2):S108–S119. [PubMed] [Google Scholar]
- 44.Kondo KK, Rossi JS, Schwartz SJ et al. . Acculturation and cigarette smoking in Hispanic women: a meta-analysis. J Ethn Subst Abuse. 2016;151:46–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Benhamou E, Benhamou S. Black (air-cured) and blond (flue-cured) tobacco and cancer risk. VI. Lung cancer. Eur J Cancer. 1993;29A12:1778–1780. [DOI] [PubMed] [Google Scholar]
- 46.Benhamou S, Sancho-Garnier H. Black and blond tobacco and cancer: a review [abstract]. Presented at the International Symposium on Cofactor Interactions and Cancer Prevention, Nice, France, March 17–19, 1993. [Google Scholar]
- 47.De Stefani E, Barrios E, Fierro L. Black (air-cured) and blond (flue-cured) tobacco and cancer risk. III. Oesophageal cancer. Eur J Cancer. 1993;29A5:763–766. [DOI] [PubMed] [Google Scholar]
- 48.Malaveille C, Vineis P, Estéve J et al. . Levels of mutagens in the urine of smokers of black and blond tobacco correlate with their risk of bladder cancer. Carcinogenesis. 1989;103:577–586. [DOI] [PubMed] [Google Scholar]
- 49.Momas I, Daures JP, Festy B et al. . Bladder cancer and black tobacco cigarette smoking. Some results from a French case-control study. Eur J Epidemiol. 1994;105:599–604. [DOI] [PubMed] [Google Scholar]
- 50.Chokkalingam AP, Metayer C, Scelo GA et al. . Variation in xenobiotic transport and metabolism genes, household chemical exposures, and risk of childhood acute lymphoblastic leukemia. Cancer Causes Control. 2012;238:1367–1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Searles Nielsen S, McKean-Cowdin R, Farin FM et al. . Childhood brain tumors, residential insecticide exposure, and pesticide metabolism genes. Environ Health Perspect. 2010;1181:144–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu R, Zhang L, McHale CM et al. . Paternal smoking and risk of childhood acute lymphoblastic leukemia: systematic review and meta-analysis. J Oncol. 2011;2011:854584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Demarini DM. Declaring the existence of human germ-cell mutagens. Environ Mol Mutagen. 2012;533:166–172. [DOI] [PubMed] [Google Scholar]
- 54.Marczylo EL, Amoako AA, Konje JC et al. . Smoking induces differential miRNA expression in human spermatozoa: a potential transgenerational epigenetic concern? Epigenetics. 2012;75:432–439. [DOI] [PubMed] [Google Scholar]
- 55.Chelghoum Y, Danaïla C, Belhabri A et al. . Influence of cigarette smoking on the presentation and course of acute myeloid leukemia. Ann Oncol. 2002;1310:1621–1627. [DOI] [PubMed] [Google Scholar]
- 56.Barnard DR, Woods WG. Treatment-related myelodysplastic syndrome/acute myeloid leukemia in survivors of childhood cancer—an update. Leuk Lymphoma. 2005;465:651–663. [DOI] [PubMed] [Google Scholar]
- 57.Seedhouse C, Russell N. Advances in the understanding of susceptibility to treatment-related acute myeloid leukaemia. Br J Haematol. 2007;1376:513–529. [DOI] [PubMed] [Google Scholar]
- 58.Chowdhury T, Brady HJ. Insights from clinical studies into the role of the MLL gene in infant and childhood leukemia. Blood Cells Mol Dis. 2008;402:192–199. [DOI] [PubMed] [Google Scholar]
- 59.Andrade FG, Furtado-Silva JM, Gonçalves BA et al. . RAS mutations in early age leukaemia modulated by NQO1 rs1800566 (C609T) are associated with second-hand smoking exposures. BMC Cancer. 2014;14:133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Smith MT, Wang Y, Skibola CF et al. . Low NAD(P)H:quinone oxidoreductase activity is associated with increased risk of leukemia with MLL translocations in infants and children. Blood. 2002;10013:4590–4593. [DOI] [PubMed] [Google Scholar]
- 61.Wiemels JL, Pagnamenta A, Taylor GM et al. . United Kingdom Childhood Cancer Study Investigators. A lack of a functional NAD(P)H:quinone oxidoreductase allele is selectively associated with pediatric leukemias that have MLL fusions. Cancer Res. 1999;5916:4095–4099. [PubMed] [Google Scholar]
- 62.Pickett KE, Kasza K, Biesecker G et al. . Women who remember, women who do not: a methodological study of maternal recall of smoking in pregnancy. Nicotine Tob Res. 2009;1110:1166–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dietz PM, Homa D, England LJ et al. . Estimates of nondisclosure of cigarette smoking among pregnant and nonpregnant women of reproductive age in the United States. Am J Epidemiol. 2011;1733:355–359. [DOI] [PubMed] [Google Scholar]
- 64.Spector LG, Murphy SE, Wickham KM et al. . Prenatal tobacco exposure and cotinine in newborn dried blood spots. Pediatrics. 2014;1336:e1632–e1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pickett KE, Rathouz PJ, Kasza K et al. . Self-reported smoking, cotinine levels, and patterns of smoking in pregnancy. Paediatr Perinat Epidemiol. 2005;195:368–376. [DOI] [PubMed] [Google Scholar]
- 66.Herrmann N. Retrospective information from questionnaires. I. Comparability of primary respondents and their next-of-kin. Am J Epidemiol. 1985;1216:937–947. [DOI] [PubMed] [Google Scholar]
- 67.Prochazka M, Hall P, Granath F et al. . Validation of smoking history in cancer patients. Acta Oncol. 2008;476:1004–1008. [DOI] [PubMed] [Google Scholar]
- 68.World Health Organization. WHO report on the global tobacco epidemic. In: Enforcing Bans on Tobacco Advertising, Promotion, and Sponsorship. Geneva, Switzerland: World Health Organization; 2013. [Google Scholar]
- 69.Walker A, Maher J, Coulthard M et al. . Living in Britain: Results from the 2000/01 General Household Survey. Norwich, United Kingdom: Office for National Statistics; 2001. [Google Scholar]
- 70.Kokkevi A, Loukadakis M, Plagianakou S et al. . Sharp increase in illicit drug use in Greece: trends from a general population survey on licit and illicit drug use. Eur Addict Res. 2000;61:42–49. [DOI] [PubMed] [Google Scholar]
- 71.Gallus S, Colombo P, Scarpino V et al. . Smoking in Italy, 2002. Tumori. 2002;886:453–456. [DOI] [PubMed] [Google Scholar]
- 72.Tapia R, Medina-Mora M, Cravioto P et al. . National Addictions Survey 1998. Mexico City, Mexico: Mexican Health Ministry; 2000. [Google Scholar]
- 73.Kraus L, Augustin R. Population survey on the consumption of psychoactive substances in the German adult population 2000. In: European Country Profiles on Tobacco Control, 2001. Copenhagen, Denmark: World Health Organization Regional Office for Europe; 2001. [Google Scholar]
- 74.INSEE. Enquetes permanentes sur les conditions de vie, 2000—indicateurs sociaux (in French). In: European Country Profiles on Tobacco Control, 2001. Copenhagen, Denmark: World Health Organization Regional Office for Europe; 2000. [Google Scholar]
- 75.California Tobacco Control Program. Adult smoking prevalence. Sacramento, California: Department of Public Health; 2010. https://www.cdph.ca.gov/programs/tobacco/Documents/Archived%20Files/CTCPAdultSmoking_10.pdf. Accessed July 12, 2016. [Google Scholar]
- 76.Bejarano J, Ugalde F. Current smoking in the past month. In: Estudio Nacional sobre consumo de drogas 2000–2001 (in Spanish). San Jose, CA: IAFA; 2003. [Google Scholar]
- 77.Office on Smoking and Health. State highlights. Washington, DC: Centers for Disease Control and Prevention; 2010. http://www.cdc.gov/tobacco/data_statistics/state_data/state_highlights/2010/states/washington/index.htm. Accessed December 19, 2014. [Google Scholar]
- 78.Topinka J, Milcova A, Libalova H et al. . Biomarkers of exposure to tobacco smoke and environmental pollutants in mothers and their transplacental transfer to the foetus. Part I. Bulky DNA adducts. Mutat Res. 2009;669(1-2):13–19. [DOI] [PubMed] [Google Scholar]
- 79.Mei-Dan E, Walfisch A, Weisz B et al. . The unborn smoker: association between smoking during pregnancy and adverse perinatal outcomes. J Perinat Med. 2015;435:553–558. [DOI] [PubMed] [Google Scholar]
- 80.Latino-Martel P, Chan DS, Druesne-Pecollo N et al. . Maternal alcohol consumption during pregnancy and risk of childhood leukemia: systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev. 2010;195:1238–1260. [DOI] [PubMed] [Google Scholar]
- 81.Metayer C, Milne E, Dockerty JD et al. . Maternal supplementation with folic acid and other vitamins and risk of leukemia in offspring: a Childhood Leukemia International Consortium study. Epidemiology. 2014;256:811–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.