Abstract
Background:
Bone marrow-derived mesenchymal stem cells (BMMSCs) transplantation has been considered as a promising milestone in liver fibrosis treatment. However, low amounts of homing are a major obstacle. We aimed to investigate the role of melatonin pretreatment in BMMSC homing into experimental liver fibrosis.
Methods:
BMMSCs were obtained, grown, propagated and preconditioned with 5 µM melatonin and analyzed for multipotency and immunophenotypic features at passage three. The cells were labelled with CM-Dil and infused into the rats received the i.p. injection of carbon tetrachloride (CCl4) for five weeks to induce liver fibrosis. Animals were divided into two groups: One group received BMMSCs, whereas the other group received melatonin-pretreated BMMSCs (MT-BMMSCs). After cell injection at 72 h, animals were sacrificed, and the liver tissues were assessed for further evaluations: fibrosis using Masson’s trichrome and hematoxylin and eosin staining and homing using fluorescent microscopy and flow cytometry.
Results:
BMMSCs and MT-BMMSCs expressed a high level of CD44 but low levels of CD11b, CD45 and CD34 (for all P≤0.05) and were able to differentiate into adipocytes and Schwann cells. CCl4 induction resulted in extensive collagen deposition, tissue disruption and fatty accumulation with no obvious difference between the two groups. There was a significant increase in homing of MT-BMMSCs in both florescent microscopy (P≤0.001) and flow cytometry (P≤0.01) assays, as compared with non-treated BMMSCs.
Conclusion:
This study indicates the improved homing potential of BMMSCs in pretreatment with melatonin. Therefore, this strategy may represent an applied approach for improving the stem cell therapy of liver fibrosis.
Keywords: Bone marrow, Mesenchymal stem cells, Melatonin
INTRODUCTION
In spite of great scientific breakthroughs toward treatment protocols, there are still a huge number of people suffer from incurable diseases around the world. Mesenchymal stem cells (MSCs) could be a promising source of therapy for patients to achieve their normal life[1]. Some standard criteria have been presented for MSCs, including plastic adherence, CD marker expression and multilineage differentiation. MSCs express CD44 at the high level and CD11b, CD34 and CD45 at the low levels; these cells have the capability to differentiate into adipocytes and Schwann cells[2,3]. MSCs constitute approximately 0.001% to 0.01% of all bone marrow cells[4].
Bone marrow-derived MSCs (BMMSCs) are an expandable population of stem cells with the capability of self-renewal[4] and are considered to have a potential in stem cell-based therapy[5]. Evidence has suggested the contribution of BMMSCs in regression of liver fibrosis induced by carbon tetrachloride (CCl4) as a well-known animal model[6]. The fact is that the use of MSCs for cell therapies depends on the capability of these cells to home into the damaged area[1]. Local injection of MSCs could be advantageous in certain aspects; however, there are special interests toward systemic infusion of MSCs with the possibility of minimal invasiveness. A predominant obstacle to the efficacious implementation of MSCs therapy is the inability to target these cells to organs of interests with high efficacy[7,8] because of oxidative stress, inflammation and hypoxia[9]. It might be presumed that increasing the number of injected cells could compensate the incidence of homing. However, this technique might not be helpful due to disturbing blood flow and demanding long-term cultivation, which in turn may change their MSCs properties and could be unsuitable for clinical usage[1]. Therefore, it is essential to pursue the strategy of ex vivo handling for better therapeutic outcome[9].
The pineal gland hormone melatonin (N-acetyl-5-methoxytryptamine) is known for its antioxidant activity[10] as well as MSCs survival[9] and motility[11]. Its preconditioning application in BMMSCs has been applied so far in targeting cerebral[12] and renal[9] ischemia and also myocardial infarction[13]. The purpose of the present study is to evaluate whether melatonin pretreatment could improve the mentioned significant barrier, namely the lack of homing to the efficacious implementation of MSC-based therapy in the fibrotic liver model.
MATERIALS AND METHODS
Cell isolation and culture
Under sterile conditions, the femurs and tibias of six-week male Sprague-Dawley rats were excised. The excision was carried out with special attention to remove all connective tissue attached to bones. The ends of the bones were cut, and bone marrow cells were obtained by carefully flashing DMEM (Gibco, USA) using a 23G syringe. The cells were then centrifuged (Hettich, Germany) at 224×g for 5 min, plated onto 25-cm2 culture flasks and cultured in an amplification medium, i.e. a low-glucose DMEM supplemented with 15% FBS (Gibco, USA) and 100 U/ml penicillin and 100 μg/ml streptomycin(Gibco, USA). Cells were incubated at 37°C in a humidified atmosphere of 5% CO2. Medium was replaced after 24 h and once every three days thereafter. The isolation of mesenchymal population was based on their capability to adhere to the culture plate[2]. At 90% confluence, the cells were recovered by digestion with 0.25% trypsin-EDTA (Gibco, USA) for 5 min and passaged to 25-cm2 flasks at 1:2 ratios and observed under a phase-contrast microscope (Olympus CKX41, Japan) for evaluation of morphologic features. For further experiments, passage-three cells were used.
Melatonin pretreatment of mesenchymal stem cells
To determine the effect of melatonin on BMMSCs, cells were subjected to a 24-h pretreatment with 5 µM melatonin (Sigma, USA). Melatonin-pretreated BMMSCs (MT-BMMSCs) were then washed three times with PBS (Sigma, USA) for complete removal of the hormone from cell suspension[9,12].
Bone marrow-derived mesenchymal stem cells multilineage differentiation potential
Adipogenic induction
For adipogenic differentiation, cells were incubated in an adipogenic medium containing 0.5 mM isobutylmethylxanthine, 0.5 µM dexamethasone, and 50 µM indomethacin (all from Sigma, USA) for 21 days with a medium exchange of every 3-4 days. Adipogenesis was measured by the accumulation of neutral lipids in vacuoles and stained with Oil Red O.
Transdifferentiation of Schwann cells
After sub-culturing at the concentration of 106 cells/cm2, BMMSCs and MT-BMMSCs were incubated in a serum-free DMEM containing 1 mM beta-mercaptoethanol (Sigma, USA) for 24 h. Then the culture media were replaced by DMEM containing 10% FBS and 35 ng/ml all-trans-retinoic acid (Sigma, USA) for three days. Finally, for further 10 days, the cells were placed in an inducer medium containing DMEM, 10% FBS and trophic factors, including 5 ng/ml platelet-derived growth factor (Peprotech, UK), 10 ng/ml beta fibroblast growth factor (Peprotech, UK), 5 µM forskolin (Calbiochem, Canada) and 200 ng/ml hergulin (R&D Systems, USA).
Immunostaining of cultured cells in Schwann cell transdifferentiation medium
Differentiated cells cultured on chamber slides (Lab-Tek, Denmark) were fixed in 4% (w/v) paraformaldehyde at 4°C for 20 min. The cells were incubated with primary antibody to S100 (rabbit polyclonal; 1:200; Dako, Denmark) at 4°C overnight. On the next day, the slides were incubated with fluorescein isothiocyanate(FITC)-conjugated secondary antibody (horse anti-mouse or goat anti-rabbit; 1:100; Vector Labs., USA) at room temperature for 2 h. Cell nuclei were labeled with Hoechst dye (Sigma, USA) at room temperature for 60 min, and the slides were examined under a fluorescent microscope (Olympus BX51TRF, Japan).
Characterization of bone marrow-derived mesenchymal stem cells
Cells, with or without melatonin preconditioning, were characterized by flow cytometric analysis of specific surface antigens. The cells were harvested with 0.25% Trypsin-EDTA, centrifuged at 112×g for 5 min, suspended in PBS and incubated in the dark with FITC-conjugated monoclonal antibodies against CD11b, CD45, CD34 and CD44 (all from eBioscience, CA, USA)— 1:100 dilution— at 4ºC for 30 min. The cells were then washed, resuspended in PBS and analyzed by flow cytometry (FACSCalibur, BDBiosciences, USA).
CM-Dil labeling of pretreated cells
BMMSCs and MT-BMMSCs were harvested by trypsinization and suspended in 1 ml PBS containing 4 µM fluorescent dye CM-Dil (C7000, Molecular Probes™, USA). Next, the cells were incubated in a humidified CO2 incubator (5% CO2/95% air) at 37°C for 15 min, followed by a centrifugation (at 112×g for 5 min) to remove unincorporated dye. The labelled cells were rinsed, resuspended in 1 ml PBS and kept in the dark before transplantation.
Animals
In total, 24 adult male Sprague-Dawley rats (Pasteur Institute of Iran, Tehran), weighing 200-220 g, were housed in groups of four in stainless steel cages. The animals were subjected to control conditions of constant humidity (55-65%), temperature (22°C) and illumination (12 h light-dark cycle), provided with unlimited access to food and water. The rats were allowed to acclimatize for at least one week before use. All animal experiments were carried out in accordance with the guidelines of Ethical Committee of Tehran University of Medical Sciences corresponding to the national and institutional guidelines for animal care and use.
Carbon tetrachloride-induced fibrotic rat model and cell transplantation
Induction of liver fibrosis was performed by i.p. administration of CCl4 (1 ml/kg body weight, Sigma, USA) dissolved in olive oil (Sigma, USA) at a ratio of 1:1 twice a week for 5 weeks. Twenty four hours after the 10th injection of CCl4, 1.5×106 BMMSCs (CM-Dil-labelled) were injected via tail vein. For examination of the homing rate and histological stainings, rats were randomly divided into two groups of eight animals: Group I received BMMSCs, while group II received MT-BMMSCs. Animals were killed 72 h after the cell injection. Moreover, eight intact rats were used as control for histological assessments.
Histological analysis
Liver specimens were collected, fixed in 10% neutral buffered formalin, embedded in paraffin, and cut into 5-µm sections. The slides were then heated in a vacuum oven at 60°C for 30 min and deparaffinized. The sections were stained with hematoxylin and eosin and Masson’s trichrome for structural and fibrosis detection and observed under a light microscope (Olympus CX31, Japan).
Homing assay
Florescent microscopic study
After permeabilization with 0.2% Triton X-100 (Merck, Germany) and subsequent washing with PBS, deparaffinized sections of 5-μm thickness (three slides from each liver samples) were incubated with Hoechst dye under dark conditions at room temperature for 30 min to label the nuclei. The slides were visualized with an Olympus BX51 microscope (Olympus, Tokyo, Japan) equipped with an E.30 digital camera (Olympus, Tokyo, Japan). The average intensity (the average pixel value in the fluorescent stained cells) was measured in 10 high-power fields of each section with ImageQuant software (TotalLab Quant, UK) based on the area value, which is the number of pixels in the stained cells. Briefly, all the whole specific fluorescent areas of CM-Dil-positive cells were drawn in the software, the resulting table of data was exported to Excel, and all the fluorescent area values were sum up. Then, the number was divided by the area of the image and multiplied by 100.
Flow cytometry
The identification of homing efficiency was assayed by flow cytometry. For this purpose, rats were perfused with normal saline 72 h after transplantation, liver samples were finely smashed by forceps and placed in a tube containing collagenase IV (Invitrogen, USA). The resulting suspension was passed through 150-μm mesh, centrifuged (134×g, 5 min), exposed to 1 ml lysis buffer and eventually suspended in 1 ml PBS for further analysis of fluorescence intensity using flow cytometry. The samples were conducted in triplets and evaluated for red fluorescence CM-Dil at 570 nm. The percentage of the transplanted labeled cells homed to liver was determined as follows: %homing=[(A×B)/C] ×100%, where A is the percent of Dil cells analyzed by flow cytometry, B is the total number of cells transplanted, and C is the whole organ cellularity[14].
Statistical analysis
The results were expressed as the mean±SD. The significant differences between the groups were evaluated using independent samples t-test using SPSS16 computer software. P≤0.05 was considered to be statistically significant.
RESULTS
Bone marrow-derived mesenchymal stem cells morphologic feature
A single-step primary rat BMMSCs purification method was employed using adhesion to cell culture plastic[15]. Phase-contrast microscopy revealed that the adherent cells had a spindle-shaped fibroblastic morphology (Fig. 1A), which began to form colonies and became confluent (Fig. 1B).
Fig. 1.
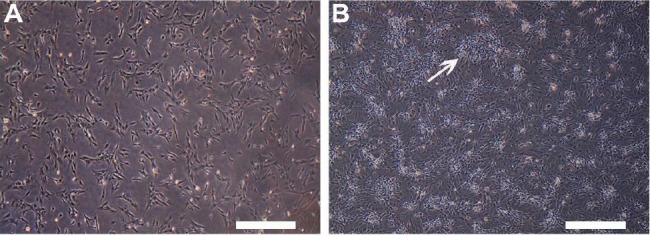
Morphologic features of bone marrow-derived mesenchymal stem cells (BMMSCs). A) Cultured BMMSCs showing spindle-shaped fibroblastic morphology; B) BMMSCs formed colonies (arrow) and became confluent (Phase-contrast microscope, scale bar = 100 µm).
Multilineage differentiation potential
Adipogenic differentiation
BMMSCs were cultured under adipogenic condition to examine whether the cells have the differentiation capability. After three weeks of exposure to adipogenic supplementation, Oil Red O-stained BMMSCs and MT-BMMSCs showed morphological changes from fibroblastic to spherical and the accumulation of red-stained intracellular fat deposits similar to differentiated adipocytes. However, there were no apparent differences between the two groups of the cells (Fig. 2).
Fig. 2.
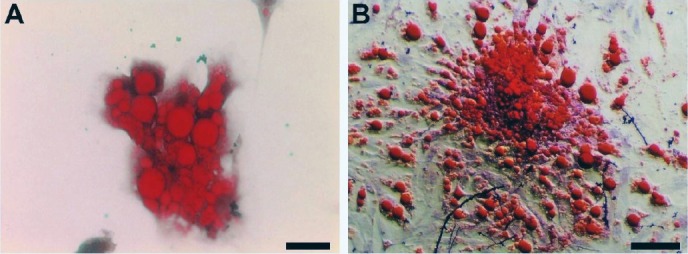
Adipogenic differentiation potential of bone marrow-derived mesenchymal stem cells (BMMSCs) stained with Oil Red O. A) By culturing in adipogenic medium, melatonin-pretreated BMMSCs exhibited a spherical shape and red-stained intracellular fat deposits; B) Non-treated BMMSCs represented the same feature as A (Light microscope, scale bar=50 µm).
Immunocytochemistry of Schwann cell trans-differentiation of bone marrow-derived mesenchymal stem cells
To evaluate the nature of Schwann cells, we detected the expression of Schwann cell marker S-100. Almost all the transdifferentiated BMMSCs and MT-BMMSCs were positive to the S-100 antibody, and after 14 days of induction, the expression of S-100 protein was demonstrated in the cytoplasm of Schwann cells (Fig. 3).
Fig. 3.
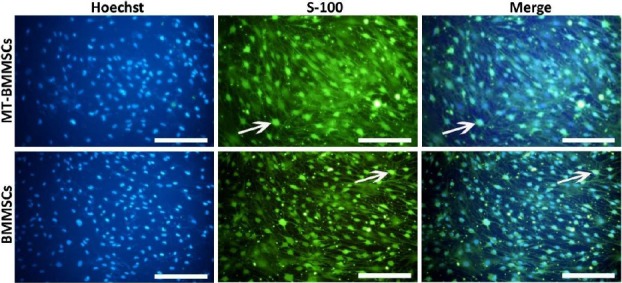
Immunocytochemistry of Schwann cell marker, S-100, in bone marrow-derived mesenchymal stem cells (BMMSCs) after 14 days of placing in transdifferentiation medium. Subsequent to the induction, differentiated melatonin-pretreated BMMSCs and BMMSCs became positive for S-100 antibody (arrows). Hoechst was used for highlighting nuclei (blue). (Fluorescent microscope, scale bar=100 µm).
Immunophenotypic characterization of bone marrow -derived mesenchymal stem cells
Concerning cell-surface antigens, flow cytometry analysis of both BMMSCs and MT-BMMSCs revealed an upward trend forCD44 expression with95.6±1.3% and 85.21±0.82%, whereas a downward for CD11b, 45, and 34 with 0.45±0.43% and 0.51±0.16%, 0.7±0.74% and 0.64±0.28%, and 1.47±0.51% and 1.71±0.17 (for all P≤0.05), respectively (Fig. 4).
Fig. 4.
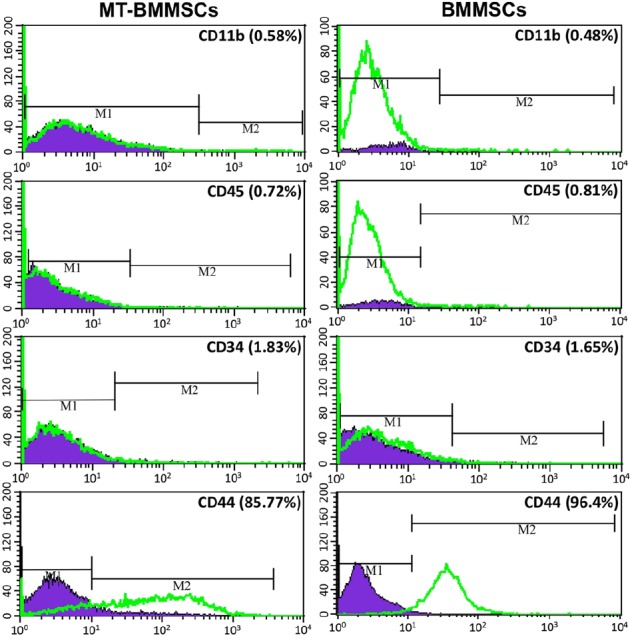
Immunophenotypic characterization of bone marrow-derived mesenchymal stem cells (BMMSCs). Flow cytometry analysis confirmed that both melatonin-pretreated BMMSCs (MT-BMMSCs) and BMMSCs expressed the low levels of CD11b, CD45 and CD34, but high level of CD44. CD44 had a fractionally higher expression in BMMSCs. M1, Isotype control; M2, CD markers expression level of BMMSCs
Carbon tetrachloride administration to induce fibrosis
Rats subjected to CCl4 revealed the average weight loss of 40-60 g, as compared with normal animals. There was also a small fraction, around 5%, of mortality rates subsequent to CCl4 intoxication (data not shown). The evaluation of liver fibrosis was performed by two histological methods: hematoxylin and eosin and Masson’s trichrome staining. The histology of hepatic lobule and hepatocytes was normal, and slight collagen deposition was identified in the peripheral blood vessels and portal area in control group. In both MT-BMMSCs and BMMSCs infusion of CCl4-injected rats, the swelling of hepatocytes was observed; meanwhile, significant vacuolar degeneration was noticed in hepatic fatty tissues. Widespread necrosis and ballooning degeneration were seen in the majority of hepatocytes. Infiltration of inflammatory cells was found in the portal area and interval of fibers. Significant fibroplasia was observed in the collagen fibers located in the parenchyma of the two groups (Fig. 5).
Fig. 5.
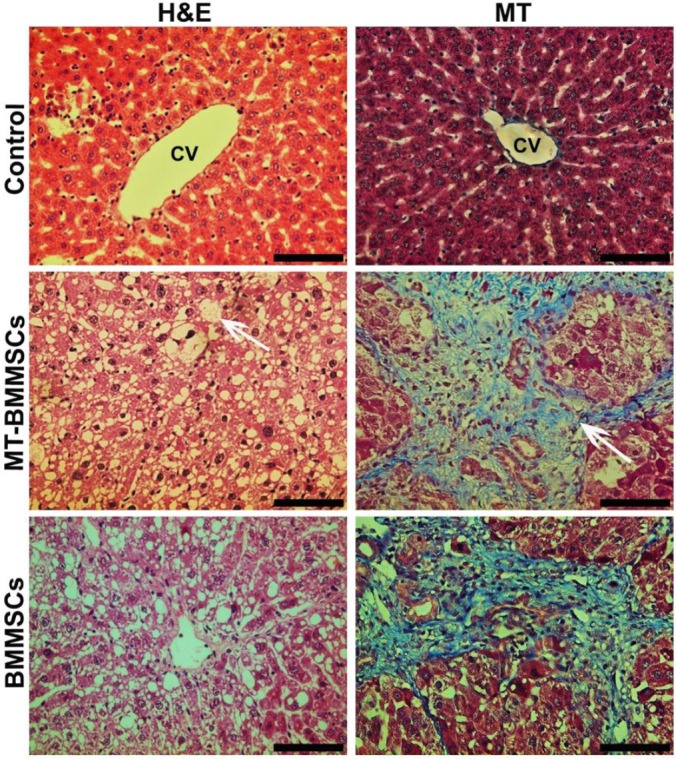
Liver sections of carbon tetrachloride-treated rats stained with hematoxylin and eosin (H&M) and Masson’s trichrome (MT). H&M staining showed tissue disruption and also high numbers of macro (arrow)- and micro-vesicular fatty change in hepatocytes of melatonin-pretreated BMMSCs (MT-BMMSCs)- and BMMSCs-infused animals. MT revealed extensive collagen deposition (arrow) in both groups. CV, central vein (Light microscope, scale bar=50 µm).
Homing assay
Fluorescent microscopic study
For in vivo cell tracking, BMMSCs, with or without melatonin incubation, were labelled with CM-Dil, and then transplanted into CCl4-injured rats. Following melatonin pretreatment, labelled BMMSCs displayed much high numbers of fluorescence. On the other hand, the number of detectable CM-Dil-labelled cells in non-incubated cells was markedly smaller than incubated cells (Fig. 6). For quantifying the number of homed cells, ImageQuant software was used. The proportion for MT-BMMSCs-injected animals rose by almost 2-fold at 0.84±0.07, as compared with BMMSCs with 0.47±0.03 (P≤0.001) (Fig. 7A).
Fig. 6.
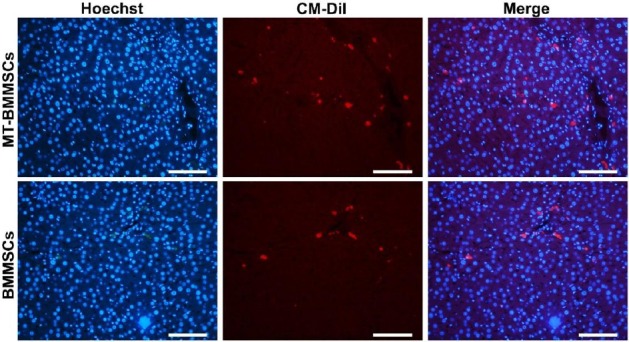
In vivo homing of CM-Dil-labelled bone marrow-derived mesenchymal stem cells (BMMSCs) with or without melatonin preconditioning toward tetrachloride-injured liver. Using CM-Dil (red), to track the fate of the transplanted BMMSCs, the high numbers of red stained cells were identified in melatonin-pretreated BMMSCs (MT-BMMSCs) group, as compared with not-treated BMMSCs. Nuclei were labeled with Hoechst (fluorescent microscope, scale bar=100 µm).
Fig. 7.
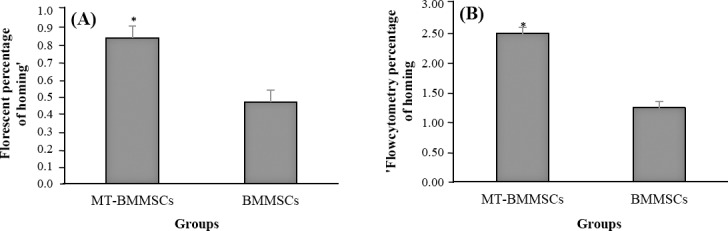
Quantifying the percentage of CM-Dil-labelled bone marrow-derived mesenchymal stem cells (BMMSCs) homing into tetrachloride-treated liver. Values are the means±SD of eight different animals. A) Florescent micrograph quantification was determined by counting the labelled-BMMSCs, which have homed to the liver by using imageQuant software. Melatonin-pretreated BMMSCs (MT-BMMSCs) showed a significant higher rate of homing (*P≤0.001); B) The total number of BMMSCs homed to the liver through the mentioned formula, based on flow cytometry data, was also significantly revealed the higher number for MT-BMMSCs (*P≤0.01).
Flow cytometry
The evaluation of CM-Dil-labelled BMMSCs homing was performed via flow cytometry. In comparison to not-exposed BMMSCs (Fig. 8A), BMMSCs exposed to CM-Dil showed a high percentage of labeling (Fig. 8B). Seventy two hours after transplantation of melatonin-pretreated cells, 1 ml homogenized liver sample represented 20.54±0.4% CM-Dil-labelled cells (Fig. 8C), which was approximately 2-fold as opposed to untreated cells with 10.24±0.2% (Fig. 8D). Determination of the formula number of homed cells from the total 1.5×106 injected cells was carried out based on the earlier mentioned. As shown in Figure 7B, the mean of 2.5±0.2% from the whole injection was belonged to the pretreated cells. In contrast, the number for untreated cells was 1.25±0.1%. The discrepancy between the two groups was significant (P≤0.01).
Fig. 8.
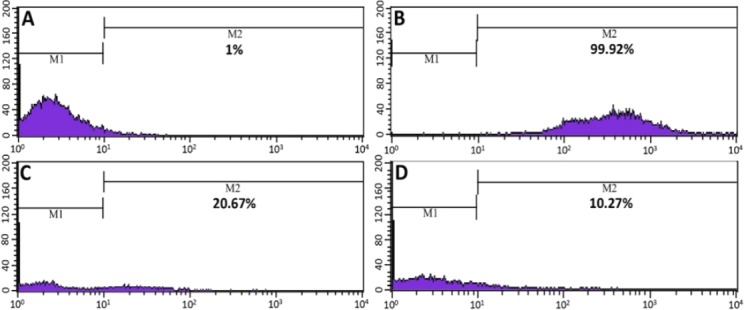
Flow cytometric assessment of bone marrow-derived mesenchymal stem cells (BMMSCs) homing. A and B showing non-injected BMMSCs: A) Unlabeled cells; B) CM-Dil-labelled cells; C) Melatonin-pretreated BMMSCs; D) Untreated cells. C and D show the percentage of red-stained CM-Dil-labelled injected BMMSCs in 1 ml homogenized liver sample. M1, Isotype control; M2, Labelled BMMSCs
DISCUSSION
In the present investigation, the effect of melatonin preconditioning on homing capability of BMMSCs into CCl4-induced hepatic fibrosis has been surveyed. Our findings disclose the high purity of BMMSCs in multipotency and immunophenotypic features with no conspicuous change between BMMSCs and MT-BMMSCs, except for CD44 that had slightly higher expression in non-treated cells. This high purity is beneficial for using BMMSCs in therapeutic approaches[1]. Similarly, the results of the present study pointed out no remarkable difference in the extent of fibrosis as well as in liver architecture among the two groups, which may relate to the short-time period of cell exposure.
As a part of host repair and defense, homing reveals the integration and functional efficiency of exogenously injected stem cells in target organ[1], Which exerts through signals of chemokines, growth factors and adhesion molecules[16]. Chemokine stromal-derived factor-1 (SDF-1) and its receptor, G-protein-coupled C-X-C chemokine receptor type 4 (CXCR4), play a pivotal role in MSCs homing[17] so that SDF-1 has a key regulatory role in stem/progenitor cell trafficking[18]. SDF-1/CXCR4 axis expressed constitutively in liver, and the targeting MSC migration to the site of injury exert through interaction in this axis. Furthermore, in response to tissue damage (i.e. toxic agent exposure), the level of SDF-1 expression will be increased in that organ[17]. Thus, it can be assumed that CCl4 intoxication promotes SDF-1 expression in liver for allowing the BMMSCs homing to some extent.
Here, we verified the enhancement of homing in transplanted BMMSCs under pretreatment with melatonin. To illustrate this, for better BMMSCs homing into liver, transforming growth factor beta 1 (TGF-β1) signaling inhibition and restoration of the matrix metalloproteinase (MMP)/tissue inhibitor of MMP balance are of importance[19]. It has been claimed that both SDF-1/CXCR4 interaction and MMP-9 seem to be essential for stem cell homing potential; the latter degrades extracellular matrix components[20] and activates TGF-β[21]. Consistently, in the case of cerebral ischemia, elevation in the level of MMP-9 via glycogen synthase kinase-3β suppression caused an increase in CXCR4 expression for subsequent mediating BMMSCs migration[1]. Besides, in the liver fibrosis model, infused bone marrow-derived cells migrate to the injured liver through the expression of MMP-9[22]. On the contrary, melatonin administration in pharmacologic dosage has a negative effect on MMP-9 by its inhibition through suppressing nuclear factor-κB. Nuclear factor-κB pathway induces MMP-9 expression[23] and also inhibits TGF-β via down-regulating TGF-β/Smad signaling[24]. However, the incubation of MT-BMMSCs to the culture fibroblasts was followed by the increased expression of melatonin receptor 1-MMP-9 and decreased tissue inhibitor of MMP-2[13]. MMP-9 secretion is induced by protein kinase Cζ[25], which in turn is known to increase nuclear factor-κB activity[26] and reinforce CXCR4 expression[25]. Therefore, we assume that melatonin could have opposite functions in pharmacologic and precondition-ing dosage. It might also be an indirect relation between melatonin preconditioning and the mentioned axis, although it highly demands further studies. In addition, the receptor-mediated effects of melatonin on MSCs exert through the enhancement of survival[9] or the stimulation of engraftment[11]. For the latter function, melatonin receptor 2 is involved whereby infused cells are targeted to the injured site[11]. This is not the solely contribution of this receptor, as it also acts in cell differentiation[27]. Overall, considering this point that both melatonin receptors are expressed in BMMSCs[9], melatonin receptors/matrix enzymes interaction might be a reason for higher homing of the pretreated group, and probably in a dose-dependent manner one function of melatonin will be superior by the other.
Collectively, the data of the current study suggest that melatonin could be considered as a preconditioning agent for promoting homing potential of BMMSCs. Therefore, this strategy may represent a novel and beneficial applied approach for improving targeted stem cell therapy of liver fibrosis.
ACKNOWLEDGEMENTS
This study was supported by the Tehran University of Medical Sciences and Health Services, Tehran, Iran (grant number 7733-30-03-87). We thank Stuart Cullen, the manager of ImageQuant software, for his valuable technical support regarding data quantification.
Footnotes
CONFLICT OF INTEREST. None declared.
REFERENCE
- 1.Naderi-Meshkin H, Bahrami AR, Bidkhori HR, Mirahmadi M, Ahmadiankia N. Strategies to improve homing of mesenchymal stem cells for greater efficacy in stem cell therapy. Cell biologyinternational. 2015;39(1):23–34. doi: 10.1002/cbin.10378. [DOI] [PubMed] [Google Scholar]
- 2.Ladak A, Olson J, Tredget E, Gordon T. Differentiation of mesenchymal stem cells to support peripheral nerve regeneration in a rat model. Experimental neurology. 2011;28(2):242–252. doi: 10.1016/j.expneurol.2011.01.013. [DOI] [PubMed] [Google Scholar]
- 3.Pokrywczynska M, Lewandowska MA, Krzyzanowska S, Jundzill A, Rasmus M, Warda K, Gagat M, Deptula A, Helmin-Basa A, Holysz M, Nowacki M, Buchholz L, Bodnar M, Marszalek A, Grzanka A, Jozwicki W, Michalkiewicz J, Drewa T. Transdifferentiation of bone marrow mesenchymal stem cells into the islet-like cells:the role of extracellular matrix proteins. Archivun immunologiae et therapiae experimentalis. 2015;63(5):377–384. doi: 10.1007/s00005-015-0340-3. [DOI] [PubMed] [Google Scholar]
- 4.Wu Y, Zhao RC. The role of chemokines in mesenchymal stem cell homing to myocardium. Stem cell reviews and reports. 2012;8(1):243–250. doi: 10.1007/s12015-011-9293-z. [DOI] [PubMed] [Google Scholar]
- 5.Darabi S, Tiraihi T, Delshad A, Sadeghizadeh M. A new multistep induction protocol for the transdifferentiation of bone marrow stromal stem cells into GABAergic neuron-like cells. Iranian biomedical journal. 2013;17(1):8–14. doi: 10.6091/IBJ.1112.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Higashiyama R, Inagaki Y, Hong YY, Kushida M, Nakao S, Niioka M, Watanabe T, Okano H, Matsuzaki Y, Shiota G, Okazaki I. Bone marrow–derived cells express matrix metalloproteinases and contribute to regression of liver fibrosis in mice. Hepatology. 2007;45(1):213–222. doi: 10.1002/hep.21477. [DOI] [PubMed] [Google Scholar]
- 7.Karp JM, Leng Teo GS. Mesenchymal stem cell homing:the devil is in the details. Cell stem cell. 2009;4(3):206–216. doi: 10.1016/j.stem.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 8.Cai H, Zhang Z, Yang GY. Preconditioned stem cells:a promising strategy for cell-based ischemic stroke therapy. Current drug targets. 2014;15(8):771–779. doi: 10.2174/1389450115666140623120010. [DOI] [PubMed] [Google Scholar]
- 9.Mias C, Trouche E, Seguelas MH, Calcagno F, Dignat-George F, Sabatier F, Piercecchi-Marti MD, Daniel L, Bianchi P, Calise D, Bourin P, Parini A, Cussac D. Ex vivo pretreatment with melatonin improves survival, proangiogenic/mitogenic activity, and efficiency of mesenchymal stem cells injected into ischemic kidney. Stem cells. 2008;6(7):1749–1757. doi: 10.1634/stemcells.2007-1000. [DOI] [PubMed] [Google Scholar]
- 10.Mehraein F, Talebi R, Jameie B, Joghataie MT, Madjd Z. Neuroprotective effect of exogenous melatonin on dopaminergic neurons of the substantia nigra in ovariectomized rats. Iranian biomedical journal. 2011;15(1-2):44–50. [PMC free article] [PubMed] [Google Scholar]
- 11.Lee SJ, Jung YH, Oh SY, Yun SP, Han HJ. Melatonin enhances the human mesenchymal stem cells motility via melatonin receptor 2 coupling with Gαq in skin wound healing. Journal of pineal research. 2014;57(4):393–407. doi: 10.1111/jpi.12179. [DOI] [PubMed] [Google Scholar]
- 12.Tang Y, Cai B, Yuan F, He X, Lin X, Wang J, Wang Y, Yang GY. Melatonin pretreatment improves the survival and function of transplanted mesenchymal stem cells after focal cerebral ischemia. Stem cell transplant. 2014;3(10):1279–1291. doi: 10.3727/096368913x667510. [DOI] [PubMed] [Google Scholar]
- 13.Mias C, Lairez O, Trouche E, Roncalli J, Calise D, Seguelas MH, Ordener C, Piercecchi-Marti MD, Auge N, Salvayre AN, Bourin P, Parini A, Cussac D. Mesenchymal stem cells promote matrix metalloproteinase secretion by cardiac fibroblasts and reduce cardiac ventricular fibrosis after myocardial infarction. Stem cells. 2009;7(11):2734–2743. doi: 10.1002/stem.169. [DOI] [PubMed] [Google Scholar]
- 14.Szilvassy SJ, Meyerrose TE, Ragland PL, Grimes B. Differential homing and engraftment properties of hematopoietic progenitor cells from murine bone marrow, mobilized peripheral blood, and fetal liver. Blood. 2001;98(7):2108–2115. doi: 10.1182/blood.v98.7.2108. [DOI] [PubMed] [Google Scholar]
- 15.Li Y, Wei J, Lu J, Wu X, Teng G. Labeling and tracing of bone marrow mesenchymal stem cells for tendon-to-bone tunnel healing. Knee surgery, sports traumatology, arthroscopy. 2011;19:2153–2158. doi: 10.1007/s00167-011-1506-0. [DOI] [PubMed] [Google Scholar]
- 16.Li Q, Yu P, Wang W, Zhang P, Yang H, Li S, Zhang L. Gene expression profiles of various cytokines in mesenchymal stem cells derived from umbilical cord tissue and bone marrow following infection with human cytomegalovirus. Cellular and molecular biology letters. 2014;19(1):140–157. doi: 10.2478/s11658-014-0187-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hao NB, Li CZ, Lü MH, Tang B, Wang SM, Wu YY, Liang GP, Yang SM. SDF-1/CXCR4 axis promotes MSCs to repair liver injury partially through trans-differentiation and fusion with hepatocytes. Stem cells international. 2015 doi: 10.1155/2015/960387. doi:10.1155/2015/960387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kucia M, Jankowski K, Reca R, Wysoczynski M, Bandura L, Allendorf DJ, Zhang J, Ratajczak J, Ratajczak MZ. CXCR4–SDF-1 signalling, locomotion, chemotaxis and adhesion. Journal of molecular histology. 2004;35(3):233–245. doi: 10.1023/b:hijo.0000032355.66152.b8. [DOI] [PubMed] [Google Scholar]
- 19.Usunier B, Benderitter M, Tamarat R, Chapel A. Management of fibrosis:the mesenchymal stromal cells breakthrough. Stem cells international. 2014 doi: 10.1155/2014/340257. doi:10.1155/2014/340257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tsai LK, Wang Z, Munasinghe J, Leng Y, Leeds P, Chuang DM. Mesenchymal stem cells primed with valproate and lithium robustly migrate to infarcted regions and facilitate recovery in a stroke model. Stroke. 2011;42(10):2932–2939. doi: 10.1161/STROKEAHA.110.612788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mishra A, Paul S, Swarnakar S. Downregulation of matrix metalloproteinase-9 by melatonin during prevention of alcohol-induced liver injury in mice. Biochimie. 2011;93(5):854–866. doi: 10.1016/j.biochi.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 22.Sakaida I, Terai S, Yamamoto N, Aoyama K, Ishikawa T, Nishina H, Okita K. Transplantation of bone marrow cells reduces CCl4-induced liver fibrosis in mice. Hepatology. 2004;40(6):1304–1311. doi: 10.1002/hep.20452. [DOI] [PubMed] [Google Scholar]
- 23.Qin W, Lu W, Li H, Yuan X, Li B, Zhang Q, Xiu R. Melatonin inhibits IL1β-induced MMP9 expression and activity in human umbilical vein endothelial cells by suppressing NF-κB activation. Journal of endocrinology. 2012;214(2):145–153. doi: 10.1530/JOE-12-0147. [DOI] [PubMed] [Google Scholar]
- 24.Crespo I, San-Miguel B, Fernández A, de Urbina JO, González-Gallego J, Tuñón MJ. Melatonin limits the expression of profibrogenic genes and ameliorates the progression of hepatic fibrosis in mice. Translational research. 2015;165(2):346–357. doi: 10.1016/j.trsl.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 25.Zöller M. CD44, Hyaluronan, the hematopoietic stem cell, and leukemia-initiating cells. Frontiers in immunology. 2015;6:235. doi: 10.3389/fimmu.2015.00235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shravah J, Wang B, Pavlovic M, Kumar U, Chen DD, Luo H, Ansley DM. Propofol mediates signal transducer and activator of transcription 3 activation and crosstalk with phosphoinositide 3-kinase/AKT. The jak/stat signaling pathway. 2014;12:e29554. doi: 10.4161/jkst.29554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zaminy A, Ragerdi Kashani I, Barbarestani M, Hedayatpour A, Mahmoudi R, Farzaneh Nejad A. Osteogenic differentiation of rat mesenchymal stem cells from adipose tissue in comparison with bone marrow mesenchymal stem cells:melatonin as a differentiation factor. Iranian biomedical journal. 2008;12(3):133–141. [PubMed] [Google Scholar]


