Abstract
A divergent selection experiment of Muscovy sires based on the residual feed intake (RFI) of their male mule progeny was initiated in 2009. Using electronic feeders, the aim of this study was to establish whether 3 generations of selection for RFI had an impact on feeding behavior traits and general behavior, and to examine its effect on liver and meat quality. Eighty mule ducks, issued from 8 Muscovy drakes per line with extreme RFI, were tested in a pen equipped with 4 electronic feeders. Feeding behaviors were recorded from 3 to 7 wk after hatching under ad libitum feeding conditions. Then animals were prepared for overfeeding with a 3-week period of restricted feeding, and overfed during 12 d before slaughter. The RFI was significantly lower in the low RFI line than in the high RFI line (−5.4 g/d, P = 0.0005) and daily feed intake was reduced both over the entire test period (−5 g/d, P = 0.049) and on a weekly basis (P = 0.006). Weekly and total feed conversion ratios were also significantly lower (−0.08, P = 0.03 and −0.06, P = 0.01, respectively). Low RFI ducks had more frequent meals, spent as much time eating as high RFI ducks, and their feeding rate was lower when analyzed at the wk level only. Additionally no significant correlation between feed efficiency and feeding behavior traits was evidenced, indicating only limited relationships between RFI and feeding patterns. Some differences in behavioral responses to stressors (open field test combined with a test measuring the response to human presence) suggested that a lower RFI is associated with less fearfulness. Selection for RFI had no effect on liver weight and quality and a slightly deleterious impact on meat quality (decreased drip loss and L*). Finally, low RFI animals had higher body weights after restricted feeding from wk 10 to wk 12 and after overfeeding than high RFI ducks. This suggests that selection for reduced RFI until 7 wk of age increases the feed efficiency up to slaughter.
Keywords: residual feed intake, feeding behavior, mule duck, carcass composition, general behavior
INTRODUCTION
Approximately 95% of the fatty liver produced in France comes from mule ducks, an infertile hybrid cross between female common ducks (Anas platyrhynchos) and Muscovy drakes (Cairina moschata). With a feed conversion ratio of more than 3.2 during growth, feed efficiency is a major concern for duck breeders. In 2009, the French National Institute for Agricultural Research (INRA) initiated a divergent selection experiment of Muscovy sires based on the residual feed intake (RFI) measured during the growing period of their male mule progeny. The RFI represents the fraction of feed intake that is not explained by maintenance and production requirements (Kennedy et al., 1993), typically the growth rate and body composition during growth. Selection for RFI was achieved by recording the feed intake of groups of ducks (Drouilhet et al., 2014). Some feeding behaviors, such as the feeding rate, have been considered for use as predictors of the overfeeding ability of the mule duck after growth. To test such hypotheses, the individual feed intake of group-housed ducks can now be measured accurately with electronic feeding systems (Basso et al., 2014). These devices record every feeding event, so, in addition to individual feed intakes, feeding behaviors also can be analyzed. Additionally, previous studies suggested that animals selected for high production levels, such as feed efficiency, eventually become less able to cope with stresses and challenges (Beilharz et al., 1993; Schülz et al., 2004). The aim of this study was to establish whether selection for RFI had an impact on feeding behavior traits and the ducks’ responses to a stressor. The impact on liver and meat quality, the major outcomes of this production system, also was examined. To achieve these goals, high and low RFI lines were compared for growth, feed intake, feeding and stress behaviors, and carcass traits after 3 generations of selection.
MATERIALS AND METHODS
Animals were bred in the INRA Duck experimental farm (UEPGF, Benquet, France), which has been approved for animal experimentation (B40-037-1).
Line Selection
The divergent lines were selected over 3 generations as described by Drouilhet et al. (2014). Briefly, about 48 Muscovy sires per line and generation were progeny tested at generations G0, G1, and G2 using their male offspring (300 mule ducks). Hybrid progeny were tested between 4 and 7 wk of age: Ducks were weighed at the beginning and at the end of the test (BW49d), and their ADG was computed for the test period. Individual total feed consumption over the test period was computed as the average consumption of small pens of 9 half-sibs. In vivo body lipid levels were estimated at the end of the test using TOBEC (Total Body Electrical Conductivity; EM-SCAN Inc., Springfield, IL) measurements (Cornuez et al., 2013). Individual RFI values during the test were computed as the residuals of a multiple phenotypic linear regression of individual daily feed consumption on the metabolic body weight at the end of the test (BW49d at the power 0.75) to account for maintenance requirements, and ADG and lipid levels to account for production requirements. Eight Muscovy drakes were then selected according to their estimated breeding value for RFI to produce the next generation of the tested line. The animals with the highest estimated breeding values for RFI were selected in the high-RFI line, and conversely the animals with the lowest estimated breeding values for RFI were selected in the low-RFI line. About 32 females were selected at random in each line to produce the next generation.
Animals
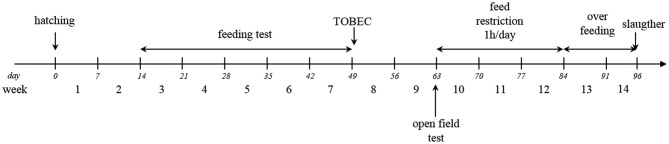
The 8 Muscovy sires selected in each line at generation G2 were mated with 37 Pekin females to produce 80 male mule ducks in one hatch (40 per line) — i.e., 5 progeny from 2 to 3 females per sire. The progeny were tested from the third wk of age (i.e., the beginning of feeding records) to slaughter (96 d of age). Testing wk were numbered from 3 to 14. Week 3 was defined from 14 to 20 d, wk 4 from 21 to 27 d, wk 5 from 28 to 34 d, wk 6 from 35 to 41 d, wk 7 from 42 to 48 d, wk 8 from 49 to 55 d, wk 9 from 56 to 62 d, wk 10 from 63 to 69 d, wk 11 from 70 to 76 d, wk 12 from 77 to 83 d, wk 13 from 84 to 90 d, and wk 14 from 91 to 96 days.
Housing
Immediately after hatching, the 80 mule ducks were placed in a single pen with additional infra-red light to adapt to the feeding system and environment. The pen measured 60 m2 and contained 2 bell drinkers and 4 automatic feeders. The bedding of straw shavings was renewed daily. The bell drinkers were located 3 meters away from the feeders. The structure of the feeders has already been described by Basso et al. (2014). Two modifications were later implemented to adapt the feeders to the behavior of different duck species (Muscovy, Pekin, and mule ducks). First, doors that close when a duck enters the feeder were added to restrict access to one duck at a time, replacing the original height limit bar placed at the entrance of the feeder. During the first 2 wk, ducks had free access to the electronic feeders, and an additional conventional feeder was available. The feeder doors were kept open during the first days after hatching. After a week, the doors closed when a duck entered the feeder. The second modification was an electronic scale placed under the access corridor to record the animal's weight at each visit. The body weight (BW) scale variations also were used to detect the presence of a duck in the feeder and to close the doors behind the animal.
At 2 wk of age, each bird was fitted with a small wing transponder bearing a unique identification code. Animals were identified when they entered and left a feeding station, using a radio frequency antenna system (Panel Reader V3; AGID, Dijon, France). Previous observations in the experimental facilities have suggested that the small, light wing transponder did not affect behavior of the duck in any way. Individual visits to feeders (n = 20,825) were recorded for the 80 mule ducks over a 5-week period from wk 3 to wk 7 (Figure 1). During the study, the maximum feeder occupancy observed was 58% of a 24-hour d, giving the ducks ample opportunity to feed. The feeding level was thus considered ad libitum. From hatching to wk 4 included, ducks received a commercial diet with 11.7 MJ/kg and 175 g digestible CP/kg of feed. From wk 5 to the end of wk 12, mule ducks received a commercial growing diet with 11.9 MJ/kg and 155 g digestible CP/kg of feed. From wk 10 to wk 12, ducks had access only to conventional feeders for one hr per d to prepare for the overfeeding phase (feed intake and feeding behavior were not recorded). They were then overfed over a 12-day period with 2 daily meals consisting of a soaked-corn mixture containing 62% DM with 26% grain and 36% flour (Figure 1). The overfeeding plan was the same for both lines. During the first 5 d after hatching, lights were 24L:0D, and they were subsequently changed to 16L:8D. The temperature of the building was set to approximately 23°C.
Figure 1.
Experimental design. Week 3 was defined from 14 to 20 d, wk 4 from 21 to 27 d, wk 5 from 28 to 34 d, wk 6 from 35 to 41 d, wk 7 from 42 to 48 d, wk 8 from 49 to 55 d, wk 9 from 56 to 62 d, wk 10 from 63 to 69 d, wk 11 from 70 to 76 d, wk 12 from 77 to 83 d, wk 13 from 84 to 90 d and wk 14 from 91 to 96 days.
Automatic Feeder Records
Visits to the feeders were set to start when the BW scale recorded a BW over 300 g and ended when it dropped below 300 g. BW scales measured weights every 0.5 s. A second set of scales was connected to each feeder (Balea POD 3 kg/0.5 g; Balea S.A., Saint Mathieu de Tréviers, France) and recorded the weight of the feed in the tray at the beginning and end of each visit. Beginning and end of visit times were recorded to the nearest second, feed weight was recorded to the nearest 0.5 g, and animal weight to the nearest one g. The amount of feed consumed during a visit was calculated as the difference between the feed weights at the beginning and at the end of the visit. Hence, the feed intake for each visit, the beginning and end of visit dates and times, and the identification code of the feeder were assigned to individual birds through electronic identification. The duration of the visit was calculated as the difference between the end and beginning times of the visit. The duration of the interval between subsequent visits of the same bird was calculated from these records, independently from the feeder in which the bird ate.
Any visit that could not be assigned to a specific duck was removed from the data set before analysis. Such visits represented less than 1.4% of the visits, during which less than 2.7% of feed consumption occurred.
Trait Records
Growth and Feeding Traits.
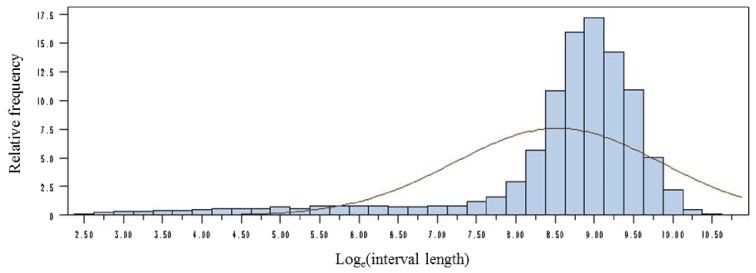
Duck BW was recorded individually at each visit from the beginning of wk 3 to the end of wk 7 via the electronic feeders. Individual average BW (ABW) was calculated as the average of the BW measurements over one day. Body lipid levels were estimated at the end of wk 7 (49 d of age) for each mule duck using TOBEC measurements (Cornuez et al., 2013) (Figure 1). In addition, individual BW was recorded at the beginning of wk 13 before overfeeding (BW84d) and before slaughter after 11 h of fasting (BW96d). First, a meal criterion, i.e., the duration of non-feeding intervals between 2 biologically defined individual meals, was estimated to group visits to the feeders into meals, as proposed by Howie et al. (2009). Two visits were considered to be part of separate meals if the interval between them was greater than the meal criterion. In our data set, the meal criterion could be estimated as 2208s (Figure 2), but only a very small proportion of small interval durations was observed (11% of 20,552 intervals). Consequently, based on this criterion, meals consisted on average of 1.10 visits. As compared to previous studies (Howie et al., 2010), and in particular Basso et al. (2014), who used similar feeders (but without doors closing behind the animals) and the same algorithm to compute the meal criterion, this distribution suggested that the visits to the feeders after their modification (addition of doors) are actually consistent with biological meals. Therefore, meals were considered as the recorded visits in our study.
Figure 2.
Frequency distribution of loge-transformed intervals between visits to feeders (n = 20,825 interval lengths).
The raw data set contained 5 wk of records. Traits were first computed on a daily basis to obtain 35 daily measurements for each trait and each duck: daily feed intakes, daily feeding times, daily feeding rates (ratio between the daily feed intake and daily feeding time), daily interval between meals and number of meals per day. In a second step, the different traits were computed per week and over the feeding test period. For example, daily feed intake values were computed per wk and over the feeding test period to obtain the weekly feed intake (5 measures per duck) and total feed intake (one measure per duck). Similarly, the ADG and feed conversion ratio (FCR) were computed weekly and over the entire test period. The RFI for the feeding test period was computed as the residual of a multiple phenotypic linear regression of the daily feed intake on the ADG during the feeding test, lipid level at the end of the test, and the metabolic BW at the end of the test, as given in the following equation (R2 = 0.70):
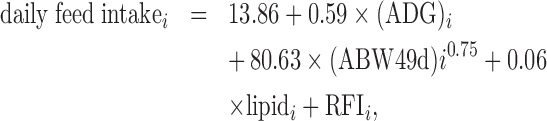 |
where the daily feed intake, ADG, and RFI are in grams per d, ABW49d and lipid are measured in grams, and i is the animal. To summarize, our data set was composed of 35 daily/5 weekly/one over the feeding test period measures for each of the following traits: daily feed intake, daily feeding time, daily feeding rate, number of meals per d, and daily interval between meals. For ADG and FCR, we computed 5 weekly/one over the feeding test period values. Each duck had one value of RFI for the feeding test period.
Behavioral Tests During Growth.
An open field (OF) test combined with a test measuring the response to human presence was performed to evaluate the response to a stressor of the 2 lines. Birds were tested individually, in the morning, on 3 successive d between age 62 d and 64 d (Figure 1, approximately one-third of the birds were tested each d), in an OF measuring 3.0 × 3.0 m2 subdivided into 9 equal zones of one m2 each. Birds were transported individually to the testing place in an opaque transport box (20 cm width, 50 cm length and 30 cm height). The box containing the tested duck was transported to a building different from the raising unit (duration of transport approx. one min), placed on the ground in a corridor for 10 min to rest, and then carried through a room to the OF area. Ducks entered the OF through a trapdoor in zone 1. Latency to emergence did not exceed 222 seconds. Next, behavior was video recorded during 5 min with a webcam (Trust Widescreen HD 720P; Trust International BV, Dordrecht, The Netherlands). Behavior traits recorded were the number of visited zones, vocalizations, body shaking, wing flapping, and long breaths. To measure the bird's response to the presence of a human (same person for all birds), a person entered the OF through a door located in zone 3 and then remained still in zone 2 for one min. The latency to the first immobilization of the duck, the number of visited zones, long breaths, and beak openings were later visually assessed from the video. Vocalizations were counted during the test.
Carcass Characteristics and Meat Quality.
Male mule ducks were slaughtered at the experimental farm at the end of the overfeeding period (age 96 d) after approx. 11 h of fasting. They were electrically stunned, bled, plucked, and the whole carcasses entered the chilling room (4°C) approximately 20 min postmortem on D0 (the slaughter d). On D1, carcasses were weighed (carcass weight) providing the cold bled-plucked carcass weight, dissected, and the following weights were recorded: abdominal fat, leg, liver, “magret” (Pectoralis major of overfed waterfowl) muscle, and magret skin (including subcutaneous fat). The cooking losses of the fatty livers (mainly lipid losses, the so called “melting rate”) were measured at D1 on a sample of 60 g of fatty liver under sterilization conditions (50 min at 105°C) and expressed as a percentage of the initial weight. Meat quality traits were recorded for the magret muscle. First, drip loss was evaluated. At D1, the magret muscles were weighed and stored for 5 d at 4°C in a polystyrene tray covered with a standard plastic wrap (air permeable). They were weighed again after 48 h and 5 d storage (on D3 and D6, respectively). Drip loss, which is the difference of magret muscle weight compared to D1, was expressed as a percentage of the weight on D1 (Honikel, 1998). Color was measured twice with a chromameter (CR 300 Minolta; Minolta Corporation, Ramsey, NJ) on a fresh cut (perpendicular to the main axis) of the magret muscle, and on the internal surface of the muscle using the trichromatic CIE Lab coordinates system (L*, a*, b*). The color was assessed on D1, D3, and D6.
Meat (magret muscle) and fatty liver samples were taken on D1, immediately frozen at −20°C, and stored until analysis of water and lipid contents. Water content was determined by oven drying at 105°C for 24 h to constant weight (JOCE, 1971). Total lipids were extracted and measured gravimetric analysis as described by Folch et al. (1957). The results were expressed as a percentage of the raw tissue (fresh matter [FM]).
Data Quality Check
Before analysis, the fixed effects to be accounted for were tested using various linear models depending on the traits (see below). The 2 × 2 interactions between the fixed effects of these linear models were systematically tested and were never found to be significant (P > 0.05). They were excluded from later analyses. Three animals were removed from the data set: one died during the experiment and the others systematically had outlier values for the weekly feed intake.
Growth and Feeding Traits.
Non-normally distributed data were transformed with a logarithmic function — all the weekly traits and the average interval between meals(t).
For weekly based traits, the fixed effects to be accounted for were tested using a mixed linear model (MIXED procedure, SAS, Inst. Inc., Cary, NC, 2008) as follows:
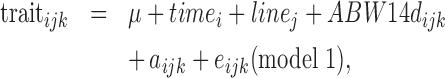 |
with linej the line (2 levels), timei the d (35 levels), or wk (5 levels) of the record, aijk the animal repeated across d or wk (random), ABW14d, the covariable ABW measured at 14 d of age, i.e., at the beginning of the feeding test period, and eijk the residual (random).
For the entire test period, the fixed effects to be accounted for were tested using a linear model (GLM procedure, SAS, Inst. Inc., Cary, NC, 2008) as follows:
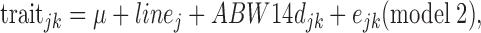 |
with linej the line (2 levels), ABW14d the covariable of ABW measured at 14 d of age, and ejk the residual.
Behavioral Tests During Growth.
Except for the number of long breaths, performances were analyzed using a linear model (GLM procedure, SAS, Inst. Inc., Cary, NC, 2008) as follows:
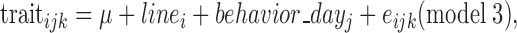 |
with linei the line (2 levels), behavior_dayj the d of test (3 levels), and eijk the residual. As many animals did not exhibit long breaths (68% during the OF test and 50% during the test measuring the response to the presence of a human), this trait was analyzed as a binary trait (presence or absence of long breaths, binomial distribution) (GENMOD procedure, SAS, Inst. Inc., Cary, NC, 2008) as follows:
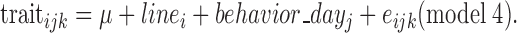 |
The effect of the duck position at the beginning of the test measuring the response to the presence of a human was not found to be significant; it was therefore not retained in the model.
Carcass Characteristics and Meat Quality.
The fixed effects to be accounted for were tested using a linear model (GLM procedure (model 5), MIXED procedure (model 6), SAS, Inst. Inc., Cary, NC, 2008) as follows:
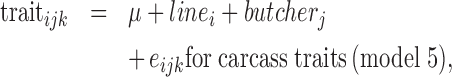 |
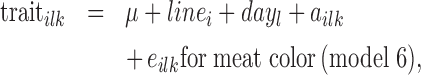 |
with linei the line (2 levels), butcherj the person who cut the carcass (10 levels), dayl the d of measure (3 levels, D1, D3, and D6), ailk the animal repeated across d (random), and eijk or eilk the residual. The ADBW14d was not significant for these traits.
Data Analysis
For traits measured on a weekly basis, the least square means (LSmeans) of the weekly effects on feeding behaviors and line effects were compared for each trait using model 1. For other traits measured over the entire test, line effects were compared using models 2 to 6, as applicable, using a Student t test (SAS, Inst. Inc., Cary, NC, 2008). The phenotypic correlations between RFI, FCR, liver weight, and melting rate, and the feeding behavior traits and carcass traits were estimated for all traits recorded during the test period.
RESULTS
Descriptive Statistics
Table 1 shows the descriptive statistics of the traits measured in the 2 lines. The average RFI was null by construction with a SD of 6.9 g/d. During the test period, the average FCR was 2.54 (SD = 0.12), and ADG was 76.6 g/d (SD = 5.3 g/d). The average liver weight of mule ducks was 526 g (SD = 109 g).
Table 1.
Descriptive statistics of performance, feeding behavior, carcass and meat quality traits, and behavioral recordings for all the mule ducks.
| N | Mean | SD | |
|---|---|---|---|
| Performance traits over the feeding test period | |||
| Residual feed intake (g/d) | 77 | 0.0 | 6.9 |
| Lipid quantity (g) | 77 | 423 | 72 |
| ABW49d (g) | 77 | 3335 | 210 |
| BW84d (g) | 77 | 4352 | 306 |
| BW96d (g) | 77 | 6001 | 361 |
| Feed intake during overfeeding (g) | 77 | 9498 | 145 |
| Total average daily gain (g/d) | 77 | 76.6 | 5.3 |
| Total feed conversion ratio | 77 | 2.54 | 0.12 |
| Feeding behavior traits over the feeding test period | |||
| Daily feed intake(t) (g) | 77 | 194 | 13 |
| Meal feed intake(t) (g) | 77 | 28.7 | 6.0 |
| Meal duration(t) (s) | 77 | 118 | 29 |
| Daily feeding time(t) (min) | 77 | 14.3 | 2.5 |
| Daily feeding rate(t) (g/min) | 77 | 15.1 | 2.6 |
| Number of meals per day(t) | 77 | 7.4 | 1.2 |
| Daily interval between meals(t) (min) | 77 | 135 | 28 |
| Carcass traits | |||
| Carcass weight (g) | 75 | 5071 | 316 |
| Liver weight (g) | 75 | 526 | 109 |
| Leg weight (g) | 74 | 478 | 44 |
| Abdominal fat (g) | 75 | 159 | 22 |
| Muscle magret weight (g) | 74 | 296 | 24 |
| Skin magret weight (g) | 75 | 166 | 26 |
| Quality traits | |||
| Fatty liver | |||
| Lipid content (% FM2) | 75 | 57.0 | 4.0 |
| Dry matter (% FM) | 75 | 67.4 | 3.2 |
| Melting rate (%) | 75 | 32 | 13 |
| Magret | |||
| lipid content (% FM) | 75 | 6.16 | 0.80 |
| Dry matter (% FM) | 75 | 27.78 | 0.69 |
| Drip loss D1-D3 (%) | 75 | 0.78 | 0.33 |
| Drip loss D3-D6 (%) | 75 | 0.46 | 0.20 |
| Drip loss D1-D6 (%) | 75 | 1.24 | 0.40 |
| Open field | |||
| Number of zones visited | 72 | 22 | 11 |
| Number of vocalizations | 72 | 103 | 53 |
| Number of long breaths | 72 | 0.32 | 0.47 |
| Number of body shaking | 72 | 2.11 | 0.93 |
| Number of wing flapping | 72 | 1.49 | 0.98 |
| Response to the presence of human | |||
| Latency to the first immobilization (s) | 72 | 6.0 | 2.9 |
| Number of zones visited | 72 | 4.9 | 2.2 |
| Number of long breaths | 72 | 0.50 | 0.50 |
| Number of vocalizations | 72 | 45 | 19 |
| Number of beak opening | 72 | 3.9 | 3.4 |
ABW49d: Average body weight at 49 d; BW84d and BW96d: body weight at 84 d and 96 d, respectively. FM: fresh matter. Behavioral traits recorded during the open field test lasting 5 min and the response to the presence of human test lasting one min.
Line Effects on Growth and Feeding Traits
The line effect was tested on the feeding behavior and performance traits, for both traits recorded over the whole test period (Table 2) and traits measured weekly (Table 3). Residual feed intake was significantly lower in the low-RFI line than in the high-RFI line (−5.4 g/d, P = 0.0005). Lipid levels, ADG(t) (Table 2) and ADG(w) (Table 3), were not found to differ significantly between the lines (P > 0.74). The BW at the beginning (ABW14d) and at the end of the feeding test (ABW49d) were not significantly different between the lines, but the live BW at 84 d of age (before the overfeeding period) and at 96 d of age (after the overfeeding period) were significantly higher in the low-RFI line (+151 g (3.4%) and +280 g (4.6%), respectively, P < 0.03) than in the high-RFI line (Table 2).
Table 2.
LSmeans of the line effect for feeding behavior and performance traits measured weekly.
| P | High-RFI | Low-RFI | |||
|---|---|---|---|---|---|
| Trait (w) | Line | LSmeans | SE | LSmeans | SE |
| Daily feed intake (w) (g/d) | ** | 193.4a | 1.0 | 188.4b | 1.0 |
| Meal feed intake (w) (g) | ** | 30.2a | 1.0 | 27.8b | 1.0 |
| Meal duration (w) (s) | ns | 106.8 | 1.0 | 104.9 | 1.0 |
| Daily feeding time (w) (min) | ns | 12.32 | 0.02 | 12.76 | 0.02 |
| Daily feeding rate (w) (g/min) | ** | 17.0a | 1.0 | 15.9b | 1.0 |
| Average daily gain (w) (g/d) | ns | 76.4 | 0.7 | 76.6 | 0.7 |
| Feed conversion ratio (w) | * | 2.68a | 0.03 | 2.60b | 0.03 |
| Number of meals per day (w) | * | 6.9a | 1.0 | 7.3b | 1.0 |
| Daily interval between meals (w) (min) | * | 144.14a | 1.0 | 135.08b | 1.0 |
N = 78. Least square means (LSmeans) and SE of the line effect were estimated from linear models accounting for the following fixed effects: week, line, and the ABW14d covariable. The weekly effect was significant for all the traits studied (P < 0.0001). The ABW14d was significant for all traits studied except daily feeding time (w) and average daily gain (w). The interaction between week and line was tested but was never significant.
In a row, values with different superscripts (a, b) were found to be significantly different (P < 0.05) for the line effect.
*: P < 0.05; **: P < 0.01; ***: P < 0.001
Table 3.
LSmeans of the line effect for traits computed over the feeding test period (from wk 3 to wk 7).
| P | High-RFI line | Low-RFI line | |||
|---|---|---|---|---|---|
| Trait (t) | Line | LSmeans | SE | LSmeans | SE |
| Residual feed intake (g/d) | *** | 2.8a | 1.0 | −2.6b | 1.0 |
| Lipid quantity (g) | ns | 421 | 11 | 426 | 11 |
| ABW49d (g) | ns | 3336 | 30 | 3334 | 30 |
| BW84d (g) | * | 4280a | 48 | 4431b | 50 |
| BW96d (g) | *** | 5866a | 54 | 6146b | 56 |
| Feed intake during overfeeding (g) | ns | 9508 | 23 | 9487 | 24 |
| Total average daily gain (g/d) | ns | 76.5 | 0.9 | 76.6 | 0.9 |
| Total feed conversion ratio | * | 2.57a | 0.02 | 2.51b | 0.02 |
| Daily feed intake(t) (g) | * | 197a | 2 | 192b | 2 |
| Meal feed intake(t) (g) | ns | 29.4 | 0.9 | 28.1 | 0.9 |
| Meal duration(t) (s) | ns | 117 | 5 | 18 | 5 |
| Daily feeding time(t) (min) | ns | 14.0 | 0.4 | 14.6 | 0.4 |
| Daily feeding rate(t) (g/min) | ns | 15.6 | 0.4 | 14.6 | 0.4 |
| Number of meals per day(t) | ns | 7.2 | 1.0 | 7.5 | 1.0 |
| Daily interval between meals(t) (min) | ns | 137 | 4 | 133 | 4 |
N = 77. Least square means (LSmeans) and SE of the line effect were estimated from linear models accounting for the line effect and the ABW14d covariable (average body weight at 14 d). ABW14d was significant for all traits studied except residual feed intake, BW84d, BW96d, total average daily gain, meal duration and daily feeding time. ABW49d: average body weight at 49 d; BW84d and BW96d: body weight at 84 d and 96 d, respectively.
In a row, values with different superscripts (a, b) were found to be significantly different (P < 0.05) for the line effect.
*: P < 0.05; **: P < 0.01; ***: P < 0.001
This resulted in a higher ADG during the restriction period (+7.5 g/d, P = 0.02, results not shown) and the overfeeding period in the low-RFI line (+10.2 g/d, P = 0.004, results not shown). The overfeeding plan being the same, the feed consumed during the overfeeding period did not differ between lines, so the FCR during the overfeeding phase was lower in the low-RFI line (−0.54, P = 0.02, results not shown).
The LSmeans for DFI(t) (Table 2) were significantly lower in the low-RFI line than in the high-RFI line (P < 0.05). Over the entire feeding test, the average daily feeding time was similar between lines (P > 0.13) but the LSmeans for the average daily feeding rate tended to be different between lines (P = 0.07). The LSmeans for total FCR were significantly lower in the low-RFI line than in the high-RFI line (−0.06, P = 0.01). The LSmeans for the daily interval between meals(t) and the number of meals per day(t) were similar between lines (P > 0.42). When compared wk by wk, similar results were found (Table 3) except for the LSmeans for the number of meals per d(w) of the high-RFI line, which were significantly lower than for the low-RFI line (−0.4, P = 0.03) and the LSmeans for the daily interval between meals(w), which were shorter in the low-RFI line than in the high-RFI line (−9.0 min, P = 0.01).
Changes in Feeding Behavior and Performance Traits Over Time
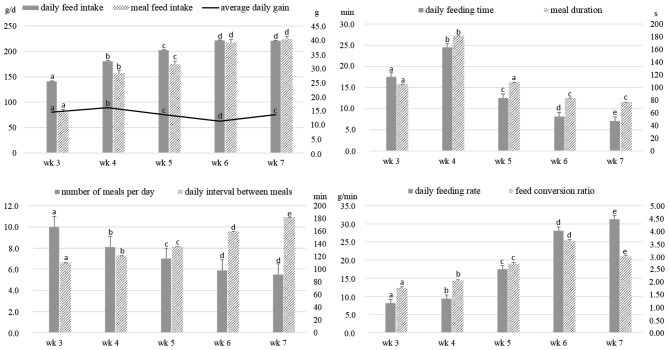
The LSmeans of the weekly effect for production traits and feeding behavior traits are presented in Figure 3. The weekly effect was significant for all feeding behavior and performance traits (P < 0.0001). The LSmeans for the DFI(w) increased from 141.3 g during wk 3 to 221.0 g during wk 7 (P < 0.0001). The ADG(w) remained relatively constant across the test, approximately 76 g/d, even if a slight decrease was observed during wk 6. The LSmeans for the average number of meals per d (w) decreased from 10.0 during wk 3 to 5.5 during wk 7 (P < 0.0001), and the LSmeans for the average meal duration(w) increased from wk 3 (105.3 s/meal) to wk 4 (181.3 s/meal), and then decreased to 77.2 s/meal (P < 0.0001 between wk 3 and wk 7). As a consequence, the ducks ate more in a shorter time as they got older, and the LSmeans for their average daily feeding rate(w) increased from 8.2 g/min during wk 3 to 31.3 g/min during wk 7 (P < 0.0001). As ADG(w) was relatively constant and DFI(w) increased with age, FCR(w) increased from 1.77 during wk 3 to 3.01 during wk 7 (P < 0.0001).
Figure 3.
Evolution of feeding behavior and performance traits during the 5 wk of the feeding test period. Least square means (LSmeans) and SE of the weekly effect was estimated from linear models accounting for the following fixed effects: week, line, and the ABW14d covariable. The interaction between week and line was tested but was never significant. For each trait, values with different superscripts (a, b, c, d, e) were found to be significantly different (P < 0.05) for the weekly effect. The difference between values with the same superscript was not statistically significant. Week 3 was defined from 14 to 20 d, wk 4 from 21 to 27 d, wk 5 from 28 to 34 d, wk 6 from 35 to 41 d, wk 7 from 42 to 48 d, wk 8 from 49 to 55 d, wk 9 from 56 to 62 d, wk 10 from 63 to 69 d, wk 11 from 70 to 76 d, wk 12 from 77 to 83 d, wk 13 from 84 to 90 d and wk 14 from 91 to 96 days.
Line Effects on Carcass Traits
Line effects on carcass traits are presented in Table 4. The carcass weight (cold bled-plucked carcass) was significantly higher in the low-RFI line than in the high-RFI line (+220 g (4.2%), P = 0.002). Line differences were found for leg and magret muscle weights, the low-RFI animals having higher cut weights (+34 g and +18 g, respectively, P < 0.001). A significant line effect on abdominal fat (+17 g for the low-RFI line, P = 0.0006) also was found. The latter differences between the lines were no longer observed when expressed as a percentage of the carcass weight.
Table 4.
LSmeans of the line effect for carcass and quality traits.
| P | High-RFI | Low-RFI | |||
|---|---|---|---|---|---|
| Line | LSmeans | SE | LSmeans | SE | |
| Carcass traits1 | |||||
| Carcass weight (g) | ** | 4965a | 48 | 5185b | 50 |
| Liver weight (g) | ns | 514 | 17 | 538 | 18 |
| Leg weight (g) | ** | 462a | 7 | 496b | 7 |
| abdominal fat (g) | *** | 153a | 3 | 170b | 4 |
| Muscle magret weight (g) | *** | 288a | 7 | 306b | 7 |
| Skin magret weight (g) | ns | 168 | 4 | 166 | 4 |
| Quality trait | |||||
| fatty liver | |||||
| Lipid content (% FM1) | ns | 57.3 | 0.6 | 56.6 | 0.7 |
| Dry matter (% FM) | ns | 67.9 | 0.5 | 66.9 | 0.5 |
| Melting rate (%) | ns | 35 | 2 | 29 | 2 |
| magret | |||||
| Lipid content (% FM) | ns | 6.28 | 0.13 | 6.02 | 0.13 |
| Dry matter (% FM) | ns | 27.9 | 0.11 | 27.7 | 0.12 |
| Drip loss D1-D3 (%) | * | 0.69a | 0.05 | 0.88b | 0.05 |
| Drip loss D3-D6 (%) | ns | 0.46 | 0.03 | 0.46 | 0.03 |
| Drip loss D1-D6 (%) | * | 1.15a | 0.06 | 1.35b | 0.06 |
| Magret color/cut2 | |||||
| L* | ** | 41.93a | 0.19 | 42.72b | 0.20 |
| a* | ** | 23.37a | 0.07 | 23.05b | 0.07 |
| b* | ns | 8.70 | 0.08 | 8.70 | 0.08 |
| Magret color/surface2 | |||||
| L* | ns | 46.30 | 0.19 | 46.76 | 0.19 |
| a* | ns | 19.28 | 0.11 | 19.06 | 0.11 |
| b* | ns | 10.01 | 0.12 | 10.08 | 0.13 |
Concerning carcass traits, least square means (LSmeans) and SE of the line effect were estimated from linear models accounting for the following fixed effects: operator and line. Concerning the color parameters, LSmeans and SE of the line effect were estimated from linear models accounting for the following fixed effects: line and day. The d effect was significant for all color parameters (P ≤ 0.0008). The interaction between line and day was tested but was never significant.
In a row, values with different superscripts (a, b) were found to be significantly different (P < 0.05) for the line effect.
1FM: fresh matter.
2L* = lightness; a* = redness; b* = yellowness. *: P < 0.05; **: P < 0.01; ***: P < 0.001.
Line Effects on Quality Traits and Chemical Composition
Few line differences were significant for meat and fatty liver traits (Table 4). No line effect was found for the liver characteristics. The drip loss between D1 and D3 was significantly higher in the low-RFI line (+0.19%, P = 0.01) compared to the high-RFI line. This difference also was observed for drip loss after 5 d of storage, but no significant difference was observed between D3 and D6 (P = 0.99). Concerning color measurements, the parameters L* and a* were significantly different between lines for freshly cut meat only, with the meat of the low-RFI line being lighter (+0.79, P = 0.006) and less red (−0.32, P = 0.001) compared with the high-RFI line.
Phenotypic Correlations with Feed Efficiency Traits
Residual feed intake and FCR(t) were highly phenotypically correlated (rp = 0.74, P < 0.0001) (Table 5). Not surprisingly, both RFI and the FCR(t) were phenotypically correlated to DFI(t) (P < 0.01). A negative phenotypic correlation was found between ADG(t) and total FCR (−0.42, P = 0.0002), but none between ADG(t) and RFI. Feed efficiency traits were not phenotypically correlated with feeding behavior traits (P > 0.14), nor with feed intake during overfeeding. Moderate and negative phenotypic correlations between RFI and BW at slaughter and leg and magret weight were found, and similar correlations were observed with FCR(t). No significant phenotypic correlations were found with liver traits (weight or quality), nor between liver traits and feeding behavior traits. The liver melting rate was highly correlated with the liver weight (0.75, P < 0.0001) and negatively correlated with the abdominal fat (−0.28, P < 0.02). The average daily feeding rate(t) was correlated with the average number of meals per d (t) (−0.37, P = 0.0009), the average meal feed intake(t) (0.33, P = 0.003), the average meal duration(t) (−0.38, P = 0.0006), and the average interval between meals(t) (0.39, P = 0.0005) (data not shown).
Table 5.
Phenotypic correlations (rp) with the feed efficiency traits, RFI, and total feed conversion ratio.
| RFI | FCR(t) | Liver weight | Melting rate | |||||
|---|---|---|---|---|---|---|---|---|
| rp | P | rp | P | rp | P | rp | P | |
| Growth traits | ||||||||
| BW84d (g) | −0.36 | ** | −0.45 | *** | ns | ns | ||
| BW96d (g) | −0.49 | *** | −0.48 | *** | 0.26 | * | ns | |
| Feed intake during overfeeding | ns | ns | ns | ns | ||||
| Total average daily gain | ns | −0.42 | *** | ns | ns | |||
| Total feed conversion ratio | 0.74 | *** | ns | ns | ||||
| Daily feeding rate(t) | Ns | ns | ns | ns | ||||
| Daily feed intake(t) | 0.54 | *** | 0.29 | * | ns | ns | ||
| Meal feed intake(t) | Ns | ns | ns | ns | ||||
| Number of meals per day(t) | Ns | ns | ns | ns | ||||
| Meal duration(t) | Ns | ns | ns | ns | ||||
| Daily interval between visits(t) | Ns | ns | ns | ns | ||||
| Carcass traits | ||||||||
| Carcass weight | −0.45 | *** | −0.46 | *** | 0.30 | * | ns | |
| Liver weight | Ns | ns | 0.75 | *** | ||||
| Melting rate | Ns | ns | 0.75 | *** | ||||
| Leg weight | −0.37 | ** | −0.39 | *** | ns | ns | ||
| Abdominal fat | Ns | ns | ns | −0.28 | * | |||
| Muscle magret weight | −0.28 | * | −0.31 | ** | ns | ns | ||
| Skin magret weight | Ns | ns | ns | ns | ||||
P are P-values for the test of H0: rp = 0. (t) : traits were computed over the entire test period. RFI: residual feed intake, BW84d and BW96d: body weight at 84 d and 96 d, respectively.
*: P < 0.05; **: P < 0.01; ***: P < 0.001.
Open Field Test Combined with a Test Measuring the Response to Human Presence
Table 6 presents the descriptive statistics for the behavioral tests. In both the OF and response to human presence tests, the ducks were active (4.4 to 4.9 zones visited per min) and vocalized (21 to 45 vocalizations per min). During the OF test, respectively, 68% and 15% of the ducks did not exhibit long breaths or body shaking. During the test measuring the response to human presence, 50% and 18% of the ducks did not show long breaths or beak opening, respectively. Among the different traits measured during the OF test, only the number of vocalizations significantly differed between lines (+27 vocalizations in the low-RFI line compared with the high-RFI line, P = 0.03). In the test measuring the response to human presence, mobility traits did not differ significantly between lines (P > 0.05), although the number of vocalizations (−9 in the low-RFI line compared with the high-RFI line, P = 0.04) and the number of beak openings (+2.5 in the low-RFI line compared with the high-RFI line, P = 0.001) did differ between lines.
Table 6.
P-values and LSmeans of the line effect of behavioral recordings (N = 72).
| Line effect | LSmeans | ||
|---|---|---|---|
| Open field | P | Low-RFI line | High-RFI line |
| Number of zones visited | ns | 19 ± 2 | 24 ± 2 |
| Number of vocalizations | * | 117 ± 9a | 90 ± 9b |
| Number of long breaths | ns | 0.2 ± 0.1 | 0.4 ± 0.1 |
| Number of body shaking | ns | 1.4 ± 0.2 | 1.6 ± 0.2 |
| Number of wing flapping | ns | 2.2 ± 0.2 | 2.0 ± 0.2 |
| Response to the presence of human | |||
| Latency to the first immobilization (s) | ns | 6.2 ± 0.4 | 5.8 ± 0.4 |
| Number of zones visited | ns | 4.7 ± 0.4 | 5.2 ± 0.4 |
| Number of long breaths | ns | 0.4 ± 0.1 | 0.6 ± 0.1 |
| Number of vocalizations | * | 41 ± 3a | 50 ± 3b |
| Number of beak opening | ** | 5.1 ± 0.5a | 2.6 ± 0.5b |
Behavioral traits recorded during the open field test lasting 5 min and the response to the presence of human test lasting one min. Least square means (LSmeans) ± SE were estimated.
In a row, values with different superscripts (a, b) were found to be significantly different (P < 0.05) for the line effect.
*: P < 0.05; **: P < 0.01; ***: P < 0.001
DISCUSSION
Electronic Feeder Development
The automatic feeder without doors, as used by Basso et al. (2014), was appropriate to study the feeding behaviors of mule ducks, but preliminary studies with Muscovy ducks showed an undesired increase of simultaneous visits to the feeders (i.e., multiple ducks in the feeder at the same time). To propose a reliable device suitable for different species of ducks and their hybrid, the automatic feeders were equipped with doors that closed behind the animals. The use of doors affected the feeding behavior of ducks, so visits were consistent with full meals in this different configuration. Indeed, previous studies (Basso et al., 2014; Howie et al., 2010) found 2 patterns of feeding behaviors (short-term and long-term) whereas we essentially evidenced long-term feeding behaviors, with a very small proportion of short term feeding patterns (Figure 2). Consequently and conversely to Howie et al. (2010), no within-meal drinking events were observed in our experiment (data not shown). Despite these changes, our estimates of the average daily feed intake(w) were similar to those reported by Basso et al. (2014) between wk 3 and 7 in a test performed with 19 mule ducks (10 males and 9 females) with one automatic feeder without doors. The growth traits of mule ducks issued from the 2 divergent lines have already been described by Drouilhet et al. (2014) from wk 4 to wk 7. The total ADG and BW at the end of the test were similar in both studies. To conclude, the modifications made to feeder structure led to a significant change by structuring the visits into full meals; however, the growth and feed intake of ducks did not seem to be altered.
RFI Line Effect on Feeding Behavior Traits
Apart from the daily feed intake, no line differences were significant for feeding traits at the feeding test level. When compared wk by wk, the low-RFI ducks also had a lower average daily feed intake(w) than the high-RFI ducks, which translated into significant feeding behavior differences between lines. Ducks from the low-RFI line had more frequent meals and spent as much time eating as high-RFI ducks, so their feeding rate was lower. The power of our experimental plan was probably insufficient to significantly detect such modest line differences at the feeding test level. In any case, the selection process does not seem to have changed to a large extent the mechanisms underlying the control of feeding behavior. The feeding behaviors reported in the literature for poultry and mammals selected on feed efficiency are quite different and depend on the species. In the third generation of a divergent selection experiment on RFI in laying hens, Braastad and Katle (1989) found that low RFI animals spent less time feed pecking than high-RFI hens. In pigs, after 4 and 7 generations of selection for low RFI, different studies found that low-RFI pigs had a lower average daily feed intake, spent less time in the feeder per d, and ate significantly faster than control or high-RFI pigs (Meunier-Salaün et al., 2014; Young et al., 2011). In Angus bulls, low-RFI animals consumed less feed, with similar feeding rates to high-RFI animals (Golden et al., 2008; Lancaster et al., 2009). These results in ruminants are consistent with our results in ducks. Consistently, RFI was not phenotypically correlated with the average daily feeding rate(t), or any feeding behavior trait in our study. In pigs, Young et al. (2011) also did not evidence a significant correlation between the feeding rate and occupation time per d, despite line differences after selection on RFI. Other studies reported only high and positive correlations between RFI and occupation time per d (in pigs, De Haer et al., 1993; in beef cattle, Nkrumah et al., 2007; in boars, Von Felde et al., 1996). In beef cattle, depending on the study and the breed, the phenotypic correlations reported between RFI and feeding rate varied to a great extent, ranging from lowly positive (r = 0.14, Robinson and Oddy, 2004) or null (Durunna et al., 2011; Golden et al., 2008; Lancaster et al., 2009) to moderately negative (r = −0.31 for Angus bulls and r = −0.51 for Hereford bulls, Kayser and Hill, 2013). It therefore seems that the relationships between feed efficiency and feeding behavior traits are population and feed dependent. The only difference we observed between the divergently selected duck lines was a trend in terms of attendance to watering places, with a lesser attendance in the low-RFI line (data not shown). However, attendance cannot be correlated with the quantity of water consumed and, as ducks like paddling, it was difficult to distinguish actual water consumption on the video records. Nevertheless, in laying hens, Bordas and Minvielle (1997) also found that the low-RFI line had lower a water intake (−51%) and Renaudeau et al. (2013) found similar trends in pigs.
RFI Line Effect on Production Traits
As a result of the selection, RFI and FCR were improved in the low-RFI line during the growing phase, with no impact on the end BW. Additionally, the higher BW observed at 84 d in the low-RFI line at the end of the restriction phase prior to overfeeding suggests that low-RFI animals used the restricted amount of feed more efficiently, as previously observed in pigs (Nguyen et al., 2004) and rabbits (Drouilhet et al., 2015; Gilbert et al., 2015). However actual feed intake during restriction could not be recorded to validate this assumption, but a lower (better) feed conversion for low-RFI ducks during the overfeeding period can be hypothesized, so selection during the growing period generally improved the feed efficiency from wk 3 to slaughter. Moreover, the correlations with carcass, meat, and liver quality were globally favorable. The higher weight of low-RFI ducks at slaughter was partly due to higher cut weights (leg and magret) and abdominal fat weight. The lack of difference in liver and muscle lipid content could be due to overfeeding: Lipid synthesis is emphasized during this period and might mask differences between lines. Despite reduced spontaneous feed intake during growth, selection for low RFI had no effect on liver weight and melting rate, as already suggested in an earlier stage of the experiment (Drouilhet et al., 2014). It can be hypothesized that better feed efficiency compensated the impact of reduced spontaneous feed intake, leading to an unexpected favorable impact on the preparation to overfeeding. Liver weight and melting rate also showed no significant correlations with feeding behaviors during growth, so a higher feeding rate might not be indicative of better overfeeding capacity. However, low-RFI ducks exhibited a higher drip loss and a lighter-colored magret (L*). Similarly in pigs, a low-RFI line was shown to have a higher drip loss and a lighter meat color than a high-RFI line (Faure et al., 2013), but contrary results were found by Colpoys et al. (2015) in another selection experiment. In both experiments selection for low RFI was linked to an increased glycolytic energy metabolism in the muscle (Le Naou et al., 2012). The lipids synthesized from the starch of maize during this period were exported and mainly stored as abdominal fat. If we consider that the maximum liver storage capacity was not reached for an average liver weight of 526 g, then genetic selection could influence the destination of the lipids synthetized by the liver with increased storage as abdominal fat. It would be interesting to explore the molecular basis that determines lipid destination and storage in both lines.
RFI Line Effect on Behavioral Responses
The possibility of decreased behavioral reactivity due to selection for feed efficiency has been investigated by comparing divergent lines in several species (Canario et al., 2013). Indeed, such selection might reduce the buffer capacity of the animal in its reaction to stress (Rauw, 2007). Mule ducks are known to have a high sensitivity to stress, high social motivation, and a high level of fear of humans (Arnaud et al., 2010; Guéméné et al., 2006; Marie-Etancelin et al., 2008). Our results indicate that selection for lower RFI increases vocal expression during novelty investigation and lowers vocal reactivity to a human. The latency of emergence from the box into the open unfamiliar area, a good measurement of emotional reactivity (Arnaud et al., 2008), was not found to differ between lines. Genetic associations between RFI and global activity have been reported in hens (Braastad and Katle, 1989; Luiting and Urff, 1991), cattle (Herd et al., 2004; Richardson et al., 1999), and pigs (Meunier-Salaün et al., 2014): Animals with lower RFI are less active. In the present study, the level of locomotor activity (here the number of zones crossed), indicative of the motivation to re-establish contact with the group, was similar in both lines. On the contrary, beak opening in the presence of a human was more frequent in low-RFI birds as compared to high-RFI birds; however its interpretation is ambiguous. Globally, the few behavioral differences observed between the divergent lines in the present study reveal that better feed efficiency is associated with lower fearfulness, which is desirable. To conclude, selection for reduced RFI should result only in slight changes in mule duck behavior, and no indication of increased responses to a stressor was evidenced. This should be correlated with the responses to handling during overfeeding, to provide a criterion for selecting ducks with better capability to cope with the novelty of overfeeding.
In conclusion, after 3 generations the divergent genetic selection for RFI did not change the mechanisms underlying the control of feeding behaviors to a significant extent, nor the general behavior of the animals. Feed efficiency traits such as RFI and FCR were not phenotypically linked to any feeding behavior traits, and no deleterious impact on duck reactivity to novelty could be evidenced in our study. Selection had no effect on liver weight and quality, and these traits were not correlated with feeding behaviors. The better feed efficiency was associated with higher abdominal fat, and higher weights of leg and magret, but unfavorable impacts on meat quality were suggested.
The ADG during the feeding test period was similar in both lines. However, the body weight measured at 12 wk and after the overfeeding period differed between the lines, and the contrast was even stronger at slaughter, suggesting that ducks selected for lower RFI under ad libitum feeding conditions are also more efficient when fed a fixed amount compared with ducks selected for high RFI. The study suggests that a lower RFI during growth is related to better feed efficiency up to slaughter. Further studies focusing on the restriction and overfeeding periods will be necessary to better understand this difference and its impact on duck fat metabolism.
Acknowledgments
The authors are grateful to the staff of the waterfowl experimental farm for taking care of the animals.
REFERENCES
- Arnaud I., Gardin E., Sauvage E., Bernadet M. D., Couty M., Guy G., Guéméné D. Behavioral and adrenal responses to various stressors in mule ducks from different commercial genetic selection schemes and their respective parental genotypes. Poult. Sci. 2010;89:1097–1109. doi: 10.3382/ps.2009-00553. [DOI] [PubMed] [Google Scholar]
- Arnaud I., Mignon-Grasteau S., Larzul C., Guy G., Faure J.-M., Guéméné D. Behavioural and physiological fear responses in ducks: genetic cross effects. Animal. 2008;2:1518–1525. doi: 10.1017/S1751731108002784. [DOI] [PubMed] [Google Scholar]
- Basso B., Lagüe M., Guy G., Ricard E., Marie-Etancelin C. Detailed analysis of the individual feeding behavior of male and female mule ducks. J. Anim. Sci. 2014;92:1639–1646. doi: 10.2527/jas.2013-7110. [DOI] [PubMed] [Google Scholar]
- Beilharz R. G., Luxford B. G., Wilkinson J. L. Quantitative genetics and evolution: Is our understanding of genetics sufficient to explain evolution? J. Anim. Breed. Genet. 1993;110:161–170. doi: 10.1111/j.1439-0388.1993.tb00728.x. [DOI] [PubMed] [Google Scholar]
- Bordas A., Minvielle F. Réponse à la chaleur de poules pondeuses issues de lignées sélectionnées pour une faible (R−) ou forte (R+) consommation alimentaire résiduelle. Genet. Sel. Evol. 1997;29:279–290. [Google Scholar]
- Braastad B. O., Katle J. Behavioral differences between laying hen populations selected for high and low efficiency of food utilization. Br. Poult. Sci. 1989;30:533–544. doi: 10.1080/00071668908417177. [DOI] [PubMed] [Google Scholar]
- Canario L., Mignon-Grasteau S., Dupont-Nivet M., Phocas F. Genetics of behavioural adaptation of livestock to farming conditions. Animal. 2013;7:357–377. doi: 10.1017/S1751731112001978. [DOI] [PubMed] [Google Scholar]
- Colpoys J. D., Abell C. E., Gabler N. K., Keating A. F., Millman S. T., Siegford J. M., Young J. M., Johnson A. K. Feed efficiency effects on barrow and gilt behavioral reactivity to novel stimuli tests. J Anim Sci. 2015;93:1267–1275. doi: 10.2527/jas.2014-8660. [DOI] [PubMed] [Google Scholar]
- Cornuez A., Bannelier C., Gouraud P., Lamothe L., Manse H., Basso B., Marie-Etancelin C. 10èmes Journées de la Recherche Avicole et Palmipèdes à Foie Gras. La Rochelle: 2013. Développement de la méthode TOBEC pour prédire l'état d'engraissement du canard mulard in vivo; pp. 577–580. [Google Scholar]
- De Haer L. C. M., Luiting P., De Vries A. G. Relations among individual (residual) feed intake, growth performance and feed intake pattern of growing pigs in group housing. Livest. Prod. Sci. 1993;36:233–253. [Google Scholar]
- Drouilhet L., Achard C. S., Zemb O., Molette C., Gidenne T., Larzul C., Ruesche J., Tircazes A., Segura M., Bouchez T., Theau-Clement M., Joly T., Balmisse E., Garreau H., Gilbert H. Direct and correlated responses to selection in two lines of rabbits selected for feed efficiency under ad libitum and restricted feeding: I. Production traits and gut microbiota characteristics. J. Anim. Sci. 2015 doi: 10.2527/jas.2015-9402. doi:10.2527/jas2015-9402. [DOI] [PubMed] [Google Scholar]
- Drouilhet L., Basso B., Bernadet M. D., Cornuez A., David I., Bodin L., Gilbert H., Marie-Etancelin C. Improving residual feed intake of mule progeny of Muscovy duck: Genetic parameters and responses to selection with emphasis on carcass composition and fatty liver quality. J. Anim. Sci. 2014;92:4287–4296. doi: 10.2527/jas.2014-8064. [DOI] [PubMed] [Google Scholar]
- Durunna O. N., Wang Z., Basarab J. A., Okine E. K., Moore S. S. Phenotypic and genetic relationships among feeding behavior traits, feed intake, and residual feed intake in steers fed grower and finishers diets. J. Anim. Sci. 2011;89:3401–3409. doi: 10.2527/jas.2011-3867. [DOI] [PubMed] [Google Scholar]
- Faure J., Lefaucheur L., Bonhomme N., Ecolan P., Meteau K., Coustard S. M., Kouba M., Gilbert H., Lebret B. Consequences of divergent selection for residual feed intake in pigs on muscle energy metabolism and meat quality. Meat Sci. 2013;93:37–45. doi: 10.1016/j.meatsci.2012.07.006. [DOI] [PubMed] [Google Scholar]
- Folch J., Lees M., Sloane Stanley G. H. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- Gilbert H., Drouilhet L., Ruesche J., Tircazes A., Theau-Clement M., Joly T., Balmisse E., Garreau H. Selection for Feed Efficiency: Direct and Correlated Responses on Production Traits in Two Rabbit Lines Selected Under Ad Libitum and Restricted Feeding. Des Moines, USA: ASAS Midwest Meeting; 2015. [Google Scholar]
- Golden J. W., Kerley M. S., Kolath W. H. The relationship of feeding behavior to residual feed intake in crossbred Angus steers fed traditional and no-roughage diets. J. Anim. Sci. 2008;86:180–186. doi: 10.2527/jas.2005-569. [DOI] [PubMed] [Google Scholar]
- Guéméné D., Bernadet M. D., Fournel E., Val-Laillet D., Bouy S., Arnaud I., Gardin E., Larzul C., Grasteau S., Guy G., Faure J. M. Symposium COA/INRA Scientific Cooperation in Agriculture. Tainan, Taiwan. INRA, Paris, France: 2006. Nervousness or fearfulness and social behaviour in male mule ducks: An update review. [Google Scholar]
- Herd R. M., Oddy V. H., Richardson E. C. Biological basis for variation in residual feed intake in beef cattle. 1. Review of potential mechanisms. Aust. J. Exp. Agric. 2004;44:423–430. [Google Scholar]
- Honikel K. O. Reference methods for the assessment of physical characteristics of meat. Meat Sci. 1998;49:447–457. doi: 10.1016/s0309-1740(98)00034-5. [DOI] [PubMed] [Google Scholar]
- Howie J. A., Tolkamp B. J., Avendaño S., Kyriazakis I. A novel flexible method to split feeding behaviour into bouts. Appl. Anim. Behav. Sci. 2009;116:101–109. [Google Scholar]
- Howie J. A., Tolkamp B. J., Bley T., Kyriazakis I. Short-term feeding behaviour has a similar structure in broilers, turkeys and ducks. Br. Poult. Sci. 2010;51:714–724. doi: 10.1080/00071668.2010.528749. [DOI] [PubMed] [Google Scholar]
- JOCE Dosage de l'humidité. Journal Officiel des Communautés Européennes L279/8. 1971 [Google Scholar]
- Kayser W., Hill R. A. Relationship between feed intake, feeding behaviors, performance and ultrasound carcass measurements in growing purebred Angus and Hereford bulls. J. Anim. Sci. 2013;91:5492–5499. doi: 10.2527/jas.2013-6611. [DOI] [PubMed] [Google Scholar]
- Kennedy B. W., van der Werf J. H., Meuwissen T. H. Genetic and statistical properties of residual feed intake. J. Anim. Sci. 1993;71:3239–3250. doi: 10.2527/1993.71123239x. [DOI] [PubMed] [Google Scholar]
- Lancaster P. A., Carstens G. E., Ribeiro F. R. B., Tedeschi L. O., Crews Jr D. H. Characterization of feed efficiency traits and relationships with feeding behavior and ultrasound carcass traits in growing bulls. J. Anim. Sci. 2009;87:1528–1539. doi: 10.2527/jas.2008-1352. [DOI] [PubMed] [Google Scholar]
- Le Naou T., Le Floc'h N., Louveau I., Gilbert H., Gondret F. Metabolic changes and tissue responses to selection on residual feed intake in growing pigs. J. Anim. Sci. 2012;90:4771–4780. doi: 10.2527/jas.2012-5226. [DOI] [PubMed] [Google Scholar]
- Luiting P., Urff E. M. Optimization of a model to estimate residual feed consumption in the laying hen. Livest. Prod. Sci. 1991;27:321–338. [Google Scholar]
- Marie-Etancelin C., Chapuis H., Brun J. M., Larzul C., Mialon-Richard M. M., Rouvier R. Genetics and selection of mule ducks in France: a review. Worlds Poult. Sci. J. 2008;64:187–208. [Google Scholar]
- Meunier-Salaün M.-C., Guérin C., Billon Y., Sellier P., Noblet J., Gilbert H. Divergent selection for residual feed intake in group-housed growing pigs: Characteristics of physical and behavioural activity according to line and sex. Animal. 2014;8:1898–1906. doi: 10.1017/S1751731114001839. [DOI] [PubMed] [Google Scholar]
- Nguyen N. H., McPhee C. P., Wade C. M. Genetic selection strategies for efficient lean growth in pigs. Pig News Inf. 2004;25:149N-163 N. [Google Scholar]
- Nkrumah J. D., Basarab J. A., Wang Z., Li C., Price M. A., Okine E. K., Crews D. H., Jr., Moore S. S. Genetic and phenotypic relationships of feed intake and measures of efficiency with growth and carcass merit of beef cattle. J. Anim. Sci. 2007;85:2711–2720. doi: 10.2527/jas.2006-767. [DOI] [PubMed] [Google Scholar]
- Rauw W. M. Conference of the Association for the Advancement of Animal Breeding and Genetics. Armidale, NSW, Australia: 2007. Physiological consequences of selection for increased performance; pp. 240–247. [Google Scholar]
- Reneaudeau D., Frances G., Dubois S., Gilbert H., Noblet J. Effect of thermal heat stress on energy utilization in two lines of pigs divergently selected for residual feed intake. J. Anim. Sci. 2013;91:1162–1175. doi: 10.2527/jas.2012-5689. [DOI] [PubMed] [Google Scholar]
- Richardson E. C., Kilgour R. J., Archer J. A., Herd R. M. Pedometers measure differences in activity in bulls selected for high or low net feed efficiency. Australian Society for the Study of Animal Behaviour. 1999:16. [Google Scholar]
- Robinson D. L., Oddy V. H. Genetic parameters for feed efficiency, fatness, muscle area and feeding behaviour of feedlot finished beef cattle. Livest. Prod. Sci. 2004;90:255–270. [Google Scholar]
- Schülz K. E., Kerje S., Jacobsson L., Forkman B., Carlbörg O., Andersson L., Jensen J. Major growth QTLs in fowl are related to fearful behavior: Possible genetic links between fear responses and production traits in a Red Junglefowl x White Leghorn intercross. Behav. Genet. 2004;34:121–130. doi: 10.1023/B:BEGE.0000009481.98336.fc. [DOI] [PubMed] [Google Scholar]
- Von Felde A., Roehe R., Looft H., Kalm E. Genetic association between feed intake and feed intake behaviour at different stages of growth of group-housed boars. Livest. Prod. Sci. 1996;47:11–22. [Google Scholar]
- Young J. M., Cai W., Dekkers J. C. M. Effect of selection for residual feed intake on feeding behavior and daily feed intake patterns in Yorkshire swine. J. Anim. Sci. 2011;89:639–647. doi: 10.2527/jas.2010-2892. [DOI] [PubMed] [Google Scholar]