Abstract
Bacterial resistance to antibiotic therapy is on the rise and threatens to evolve into a worldwide emergency: alternative solutions to current therapies are urgently needed. Cationic amphipathic peptides are potent membrane-active agents that hold promise as the next-generation therapy for multidrug-resistant infections. The peptides’ behavior upon encountering the bacterial cell wall is crucial, and much effort has been dedicated to the investigation and optimization of this amphipathicity-driven interaction. In this study we examined the interaction of a novel series of nine-membered flexible cyclic AMPs with liposomes mimicking the characteristics of bacterial membranes. Employed techniques included circular dichroism and marker release assays, as well as microbiological experiments. Our analysis was aimed at correlating ring flexibility with their antimicrobial, hemolytic, and membrane activity. By doing so, we obtained useful insights to guide the optimization of cyclic antimicrobial peptides via modulation of their backbone flexibility without loss of activity.
Keywords: Cyclic peptides, antimicrobial peptides, flexible peptides, hemolytic activity, membrane activity
Bacterial resistance to antibiotic therapy is a pressing worldwide emergency, and the need for new antimicrobial drugs and strategies is higher than ever.1
Antimicrobial peptides (AMPs) are a fundamental component of the innate immune system of multicellular organisms2 but have also been isolated from many bacteria and fungi.3 The majority of them are amphipathic and bear a net positive charge. They interact with the negatively charged bacterial membrane, and after assuming an amphipathic secondary structure (e.g., an α-helix or β-hairpin), they (i) insert stably and eventually disrupt its integrity or (ii) are translocated to the cytoplasm and bind to other essential targets.4 AMPs are promising drug candidates for the treatment of multidrug-resistant (MDR) infections.5
Cyclic AMPs are an attractive scaffold for novel antimicrobial agents. Due to the entropic cost of assuming an ordinated conformation, constrained cyclic AMPs have been proposed to be better suited to membrane insertion and pore formation.6 Moreover, they are more resistant to proteases.7
Our recent work has been focused on combining the beneficial conformational bias of macrocycles with recent insights concerning the effect of flexibility on membrane insertion and phospholipidosis.8 Specifically, Wu and co-workers concluded that more constrained AMPs have the potential of producing a higher degree of phospholipidosis when inserted, but their rigidity hinders the insertion process itself.
We recently described how the introduction of lipophilic ω-amino acids increased the antimicrobial activity of a seven-membered cyclic AMP as effectively as lipidation.9 Single flexible residues can also prove extremely useful for the cyclization reaction, and based on this concept, we recently developed a novel class of macrocyclic peptidomimetics (macropeptoids).10
From our previous study it emerged that the heptamer bearing an 8-aminooctanoyl (8-Aoc) residue was the most active. Because 8-Aoc is approximately as long as an α-tripeptide, in this study we evaluated the antimicrobial and hemolytic activity of a novel series of nine-membered cyclic AMPs bearing different ω-amino acid residues. Biological data were compared with structural and functional information obtained by examining their interaction with liposomes. Ultimately, we were able to identify clear design guidelines for the dissociation of antimicrobial and hemolytic activity of cyclic membrane-active AMPs without loss of potency.
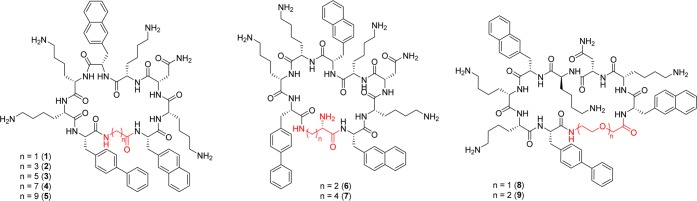
All compounds investigated in this study are displayed in Figure 1. The all-L macrocycle 1 was identified from a combinatorial library. Compounds 2–9 are based on the same scaffold and result from the replacement of Gly with a variety of flexible ω-amino and α,ω-diamino acids. Peptides were synthesized via standard Fmoc/tBu strategy starting from Fmoc-Asp-Odmb.11 Head-to-tail cyclization was performed on resin using DMTMM·BF4 as coupling reagent.12 Upon release from the Rink Amide Linker (RAM), the C-terminal Asp was converted to Asn, and the cyclic peptides were obtained as TFA-salts. Peptides were tested against an array of pathogens inspired to the ESKAPE panel:13E. coli (ATCC 25922), S. aureus (ATCC 33591, MRSA), K. pneumoniae (ATCC 700603, MDR), A. baumannii (ATCC BAA-1605, MDR), P. aeruginosa (ATCC 27853), E. faecium (ATCC 700221, VRE).
Figure 1.
Structures of 1–9.
Backbone modifications have been shown to produce marked and strain-specific effects on the activity of linear AMPs.14 In a cyclic antimicrobial peptide they alter several parameters at once, including ring flexibility, ring size, lipophilic/hydrophilic ratio, and amphipathicity profile. For this reason 2–9 feature a selection of flexible linkers covering a variety of sizes and chemical compositions. For the scopes of this work, longer, unconstrained linker chains were considered as more flexible.
Previous work by Hodges and colleagues offers interesting parallelisms. The authors studied analogues of Gramicidin S with modified ring sizes based on the addition/deletion of α-amino acids.15 Initial studies on Gramicidin S analogues produced mixed results due to the periodicity of the β-sheet structure, while within a defined structural framework amphipathicity was found to be the key parameter.16 Later studies identified larger and more flexible 16-membered analogues showing more desirable biological activities.17 While the present study follows similar concepts, core differences are represented by the smaller size of the investigated macrocycles and by the use of ω-amino acids to alter ring size and flexibility. In agreement with previous literature concerning the influence of side-chains on peptide conformation,18 the difference between functionalized α- and linear ω-amino acids as ring extenders produced different results from those obtained by Hodges and co-workers (Table 1). Indeed, with a few exceptions, antimicrobial activity was maintained to similar levels throughout the 1–9 series. Gram-positive pathogens appeared overall more susceptible to the peptides and less affected by the studied backbone modifications. This is presumably related to the higher complexity characterizing the cell wall of Gram-negative bacteria. The activity against K. pneumoniae was negatively affected by long linkers (5) and/or hydrophilic linkers (6–9). MRSA and P. aeruginosa appeared more susceptible to medium-size lipophilic linkers. Cationic linkers abolished the activity against A. baumannii. The chemical characteristics of the linker appeared therefore to affect MIC values more than its length, with medium-long lipophilic linkers offering the strongest and broadest activity.
Table 1. Antimicrobial Activity (MIC in μM Concentrations), tR (RP-HPLC), and Hemolytic Activity of 1–9.
| no. | MRSA | E. coli | VRE | K. pneumoniae | P. aeruginosa | A. baumannii | HA,a EC50 | tR (min) |
|---|---|---|---|---|---|---|---|---|
| 1 | 8 | 32 | 4 | 32 | 32 | 32 | 45 | 17.2 |
| 2 | 16 | 32 | 4 | 32 | 32 | 32 | 155 | 17.0 |
| 3 | 8 | 16 | 2 | 8 | 16 | 8 | 18 | 17.6 |
| 4 | 4 | 16 | 2 | 16 | 16 | 16 | 12 | 18.0 |
| 5 | 16 | 16 | 8 | >64 | >64 | 16 | 5 | 18.8 |
| 6 | 16 | 64 | 2 | 64 | 32 | >64 | >300 | 15.9 |
| 7 | 16 | 32 | 2 | 64 | 16 | >64 | >300 | 15.9 |
| 8 | 16 | 32 | 4 | 64 | 32 | 32 | >300 | 16.9 |
| 9 | 16 | 64 | 2 | 64 | 32 | 16 | >300 | 17.2 |
Hemolytic activity, EC50 in μM concentrations.
Lytic activity toward red blood cells (RBC) was measured in order to evaluate the linker’s effect on the peptides’ affinity for the neutral/zwitterionic mammalian membranes. Compounds 1 and 3–5, bearing a lipophilic linker, resulted in high levels of hemolysis and EC50 values decreasing rapidly in concomitance with stronger hydrophobicity (Figure 2). Compound 2, while featuring a similar linker, exhibited an exceptional behavior and caused remarkably lower levels of hemolysis than its lipophilic congeners. Polar and cationic linkers (6–9) produced modest to undetectable levels of hemolysis. This is not surprising since hydrophobicity can be expected to be the main force driving the interaction of cationic peptides with the neutral/zwitterionic mammalian membranes. However, effective lipophilicity also depends on the spatial orientation (and therefore spatial concentration) of lipophilic residues, which in turn depends on the secondary structure assumed by the peptides. This parameter was investigated by circular dichroism in the presence of large unilamellar vesicles (LUVs) consisting of 1,2-dioleoyl-glycero-3-phosphoethanolamine (DOPE) and 1,2-dioleoyl-glycero-3-phosphoglycerol (DOPG) in a ratio of 3:1 (Figure 3). The presence of negatively charged PG lipids mimics the cell wall of bacteria.
Figure 2.
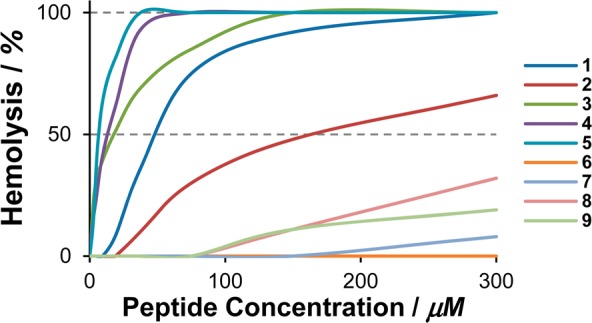
Concentration-dependent hemolytic activity of 1–9.
Figure 3.
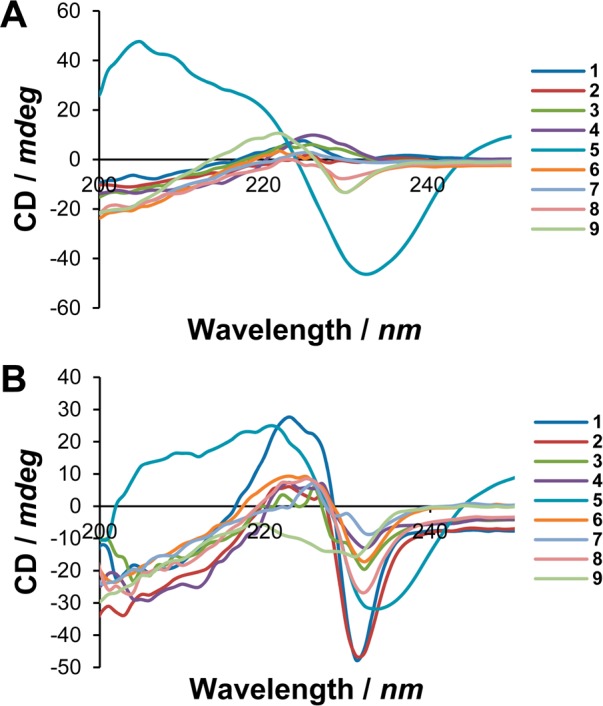
CD spectra of 1–9 acquired in NaHCO3 buffer (A) and after addition of DOPE/DOPG (3:1) LUVs (B).
Due to their high absorbance in the UV range, electronic transitions of the naphthyl and biphenyl groups are presumably responsible for the observable signals. However, it is accepted that signals generated by the side-chains of chiral aromatic amino acids are related to the backbone conformation.19 Moreover, being the most prominent lipophilic groups, such large aromatics can be expected to play a major role in membrane insertion and information about their orientation is highly valuable. In inorganic buffer, most peptides showed a disorganized structure (Figure 3A). However, a modest exciton split at approximately 225 nm was observed for 6, 9, and rather prominently for 5. In the presence of DOPE/DOPG liposomes, most peptides gave rise to the exciton split (Figure 3B). This spectral signature is often observed in peptides bearing multiple naphthyl groups.9,20 The most constrained 1 gave rise to a strong signal with low negative and high positive peaks; increasing ring flexibility produced an immediate effect on the positive maximum, but this was then kept constant throughout the series. On the contrary, the negative minimum kept increasing with a tendency to the zero line. Compound 9 was the only peptide not giving rise to a positive band in these experimental conditions.
A highly structured exciton split, measurable in terms of distance between minimum and maximum, can be considered as an indication of conformational stability. In this assumption, in the presence of anionic LUVs 1 and 5 appeared to be the most structured of the series. However, 5 showed signs of a clearly defined structure also in inorganic buffer. The behavior of 1 can be rationalized in terms of its higher constraint since increasing flexibility intuitively leads to more conformational variability. When interacting with anionic LUVs, a constrained macrocycle is expected to adopt the lowest-energy amphipathic conformation, deviating from which is energetically costly due to the very nature of structural constraints. Therefore, it does not surprise that more flexible peptides gave rise to weaker CD signals. However, it is clear that the behavior of 5 diverged considerably from its flexible congeners. We have observed this same behavior in our previous study, also in concomitance with the introduction of 10-aminodecanoic acid.9 We hypothesize that the high hydrophobicity of this linker, as well as its length, drives the peptide to fold (i.e., hydrophobic collapse21) in order to minimize the hydrophobic areas in contact with water. In spite of its flexibility, these effects result in a peptide with a definite conformational bias. In this respect, it appears that a linker length of ten atoms is the maximum that a small macrocyclic peptide can tolerate without major conformational rearrangements. Based on CD spectra and previous literature,22 the lower hemolytic activity of 2 can find explanation in a favorable combination of destabilized amphipathic conformation paired with a negligible difference in lipophilicity (see tR in Table 1). Peptides 6 and 7 feature chiral linkers potentially capable of imparting a different conformation. Interestingly, they either (a) do not do so in a discernible manner from achiral linkers and/or (b) do not produce remarkable changes in antimicrobial activity. Peptide 9 also behaved quite exceptionally, as this peptide appeared more structured in buffer than in the presence of LUVs.
Overall, biological activity seems to be better correlated to the lipophilic/cationic properties of the linker in a classic amphipathicity perspective. Structural effects, where present, seem predominant only when the difference in amphipathicity is small (e.g., peptides 1 and 2).
Peptide-induced leakage of sulforhodamine B from LUVs was determined for all peptides at the biologically relevant 16, 32, and 64 μM concentrations (Figure 4). When entrapped inside vesicles at high concentration, the fluorescence of sulforhodamine B is self-quenched.23 Release and following dilution restore the fluorescence emission properties. The dye has been trapped into LUVs consisting of 1,2-dioleoyl-glycero-3-phosphocholine (DOPC) and 2-oleoyl-1-palmitoyl-glycero-3-phosphoglycerole (POPG) in a 3:1 ratio. To avoid leakage due to vesicle instability, PE-lipids have been avoided24 and palmitoyl has replaced one oleyl chain to achieve tighter lipid packing.
Figure 4.
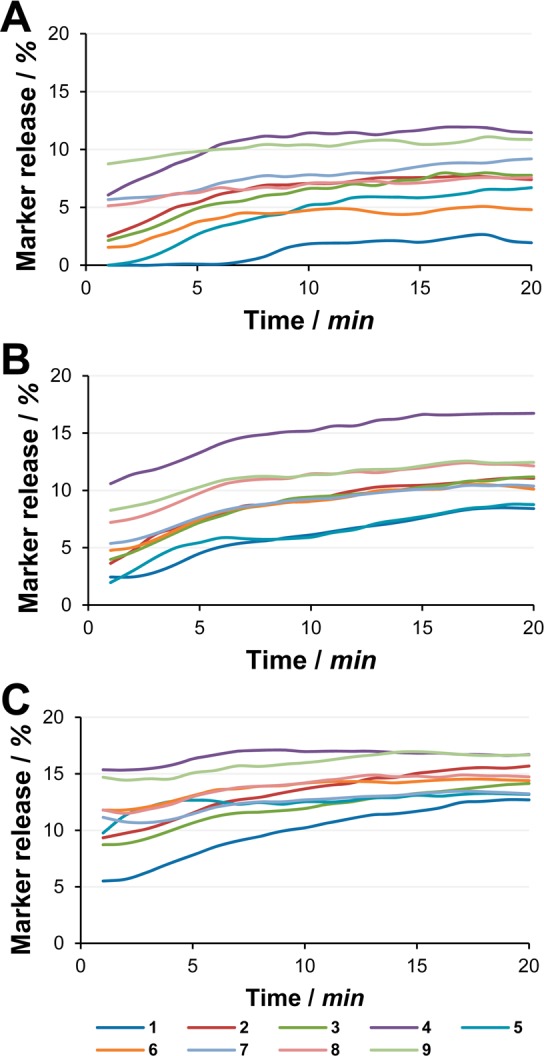
Time-dependent leakage of sulforhodamine B from DOPC/POPG LUVs determined at peptide concentrations of 16 (A), 32 (B), and 64 μM (C).
With respect to total marker leakage most peptides stably occupied a middle tier, with limited differences between them, as likewise observed for antimicrobial activity. However, the most constrained 1 always occupied the bottom tier, revealing that a flexible linker conferred higher membrane activity to all peptides, disregarding the size and chemical characteristics of the linker itself. Compound 4 always occupied the top tier, while the similarly sized but more hydrophilic 9 occupied either the middle or top tier depending on concentration. Overall, increasing peptide concentration was found to affect the extent of marker release, but in particular, it affected the initial outset. Flexible peptides reached their plateau faster than the more constrained 1, whose release kinetics were in turn the most dependent on concentration.
The bactericidal activity of 1 and the less hemolytic but more membrane active 2 and 6 against S. aureus was assessed in a time-course experiment (Figure 5). Notably, 2 proved a faster bactericidal than 1 and caused a 3-Log reduction in cell count within 30 min. The related, but more hydrophilic 6 maintained the bactericidal activity, but the same reduction was achieved in 3.5 h. This discrepancy with model membrane assays is likely a consequence of a filter effect operated by the cell wall. However, at t = 24 h (not shown) no colonies were detected in any of the samples.
Figure 5.

Time-kill kinetics of selected peptides at 32 μM concentration against S. aureus ATCC 29213.
Based on the presented data, it appears that “stretching” the ring within its tolerable maximum grants cyclic membrane-active AMPs an advantage in the early stage of membrane insertion. These observations agree with the conclusion by Wu and co-workers.8 In addition, the destabilization of the amphipathic secondary structure will decrease the peptides’ affinity for mammalian membranes, whenever total lipophilicity is not substantially increased. Antimicrobial activity appeared relatively insensitive to conformational modifications, probably due to the fact that the necessary amphipathic conformation is induced and supported by electrostatic attraction.25 The kinetics of bacterial killing might however be hampered after major increases in hydrophilicity.
From a drug design perspective, simple and effective strategies for the chemical optimization of biologically relevant structural parameters are extremely useful. In conclusion, we found that membrane-active macrocyclic AMPs are very tolerant to the insertion of single flexible residues (consisting of 10 atoms or less) both structurally and functionally. From a medicinal chemistry perspective, this tolerance can be leveraged to modulate other desirable characteristics without loss of activity. Overall, replacing Gly in 1 with a γ-aminobutyryl in 2 produced the rather ideal result of potentiating membrane and bactericidal activity while also reducing affinity for RBC. The presented strategies and insights should find application in the future design and optimization of cyclic membrane-active AMPs.
Acknowledgments
The authors wish to thank Jytte M. Andersen and Birgitte Simonsen for excellent technical support. The members of the Marie Curie ITN “TRAIN-ASAP” (www.train-asap.eu) are kindly acknowledged for their valuable scientific input.
Glossary
ABBREVIATIONS
- 8-Aoc
8-aminooctanoic acid
- AMP
antimicrobial peptide
- DMTMM·BF4
4-(4,6-dimethoxy-1,3,5-triazin-2-yl)-4-methylmorpholinium tetrafluoroborate
- DOPE
1,2-dioleoyl-glycero-3-phosphoethanolamine
- DOPC
1,2-dioleoyl-glycero-3-phosphocholine
- DOPG
1,2-dioleoyl-glycero-3-phosphoglycerole
- LUV
large unilamellar vesicle
- MDR
multidrug resistant
- MIC
minimum inhibitory concentration
- MRSA
methicillin-resistant S. aureus
- PE
phosphoethanolamine
- POPG
2-oleoyl-1-palmitoyl-glycero-3-phosphoglycerole
- VRE
vancomycin-resistant E. faecium
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsmedchemlett.5b00400.
Experimental details, thermogravimetric analysis graphs, HPLC chromatograms, MALDI-ToF-MS spectra, magnified CD spectra, and standard deviations for marker release (PDF)
Author Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
This study has been performed as part of the TRAIN-ASAP project and funded by the Marie Curie Actions under the Seventh Framework Programme for Research and Technological Development of the EU (Grant Agreement N° 289285).
The authors declare no competing financial interest.
Supplementary Material
References
- Antimicrobial Resistance: Global Report on Surveillance; World Health Organisation (WHO), 2014. [Google Scholar]
- Zasloff M. Antimicrobial peptides of multicellular organisms. Nature 2002, 415 (6870), 389–95. 10.1038/415389a. [DOI] [PubMed] [Google Scholar]
- Liskamp R. M.; Rijkers D. T.; Kruijtzer J. A.; Kemmink J. Peptides and proteins as a continuing exciting source of inspiration for peptidomimetics. ChemBioChem 2011, 12 (11), 1626–53. 10.1002/cbic.201000717. [DOI] [PubMed] [Google Scholar]
- Brogden K. A. Antimicrobial peptides: Pore formers or metabolic inhibitors in bacteria?. Nat. Rev. Microbiol. 2005, 3 (3), 238–250. 10.1038/nrmicro1098. [DOI] [PubMed] [Google Scholar]
- Mercer D. K.; O’Neil D. A. Peptides as the next generation of anti-infectives. Future Med. Chem. 2013, 5 (3), 315–337. 10.4155/fmc.12.213. [DOI] [PubMed] [Google Scholar]
- Mika J. T.; Moiset G.; Cirac A. D.; Feliu L.; Bardají E.; Planas M.; Sengupta D.; Marrink S. J.; Poolman B. Structural basis for the enhanced activity of cyclic antimicrobial peptides: The case of BPC194. Biochim. Biophys. Acta, Biomembr. 2011, 1808 (9), 2197–2205. 10.1016/j.bbamem.2011.05.001. [DOI] [PubMed] [Google Scholar]
- Tyndall J. D.; Nall T.; Fairlie D. P. Proteases universally recognize beta strands in their active sites. Chem. Rev. 2005, 105 (3), 973–99. 10.1021/cr040669e. [DOI] [PubMed] [Google Scholar]
- Liu L.; Fang Y.; Wu J. Flexibility is a mechanical determinant of antimicrobial activity for amphipathic cationic α-helical antimicrobial peptides. Biochim. Biophys. Acta, Biomembr. 2013, 1828 (11), 2479–2486. 10.1016/j.bbamem.2013.06.017. [DOI] [PubMed] [Google Scholar]
- Oddo A.; Nyberg N. T.; Frimodt-Moller N.; Thulstrup P. W.; Hansen P. R. The effect of glycine replacement with flexible omega-amino acids on the antimicrobial and haemolytic activity of an amphipathic cyclic heptapeptide. Eur. J. Med. Chem. 2015, 102, 574–581. 10.1016/j.ejmech.2015.08.028. [DOI] [PubMed] [Google Scholar]
- Oddo A.; Münzker L.; Hansen P. R. Peptide Macrocycles Featuring a Backbone Secondary Amine: A Convenient Strategy for the Synthesis of Lipidated Cyclic and Bicyclic Peptides on Solid Support. Org. Lett. 2015, 17 (10), 2502–2505. 10.1021/acs.orglett.5b01026. [DOI] [PubMed] [Google Scholar]
- Mcmurray J. S. Solid-Phase Synthesis of a Cyclic Peptide Using Fmoc Chemistry. Tetrahedron Lett. 1991, 32 (52), 7679–7682. 10.1016/0040-4039(91)80563-L. [DOI] [Google Scholar]
- Kaminski Z. J.; Kolesinska B.; Kolesinska J.; Sabatino G.; Chelli M.; Rovero P.; Blaszczyk M.; Glowka M. L.; Papini A. M. N-triazinylammonium tetrafluoroborates. A new generation of efficient coupling reagents useful for peptide synthesis. J. Am. Chem. Soc. 2005, 127 (48), 16912–16920. 10.1021/ja054260y. [DOI] [PubMed] [Google Scholar]
- Boucher H. W.; Talbot G. H.; Bradley J. S.; Edwards J. E.; Gilbert D.; Rice L. B.; Scheld M.; Spellberg B.; Bartlett J. Bad Bugs, No Drugs: No ESKAPE! An Update from the Infectious Diseases Society of America. Clin. Infect. Dis. 2009, 48 (1), 1–12. 10.1086/595011. [DOI] [PubMed] [Google Scholar]
- Jahnsen R. D.; Frimodt-Møller N.; Franzyk H. Antimicrobial Activity of Peptidomimetics against Multidrug-Resistant Escherichia coli: A Comparative Study of Different Backbones. J. Med. Chem. 2012, 55 (16), 7253–7261. 10.1021/jm300820a. [DOI] [PubMed] [Google Scholar]
- Kondejewski L. H.; Farmer S. W.; Wishart D. S.; Kay C. M.; Hancock R. E. W.; Hodges R. S. Modulation of Structure and Antibacterial and Hemolytic Activity by Ring Size in Cyclic Gramicidin S Analogs. J. Biol. Chem. 1996, 271 (41), 25261–25268. 10.1074/jbc.271.41.25261. [DOI] [PubMed] [Google Scholar]
- Kondejewski L. H.; Lee D. L.; Jelokhani-Niaraki M.; Farmer S. W.; Hancock R. E. W.; Hodges R. S. Optimization of Microbial Specificity in Cyclic Peptides by Modulation of Hydrophobicity within a Defined Structural Framework. J. Biol. Chem. 2002, 277 (1), 67–74. 10.1074/jbc.M107825200. [DOI] [PubMed] [Google Scholar]
- Jelokhani-Niaraki M.; Kondejewski L. H.; Wheaton L. C.; Hodges R. S. Effect of Ring Size on Conformation and Biological Activity of Cyclic Cationic Antimicrobial Peptides. J. Med. Chem. 2009, 52 (7), 2090–2097. 10.1021/jm801648n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sak K.; Karelson M.; Järv J. Modeling of the Amino Acid Side Chain Effects on Peptide Conformation. Bioorg. Chem. 1999, 27 (6), 434–442. 10.1006/bioo.1999.1150. [DOI] [Google Scholar]
- Kelly S. M.; Price N. C. The Use of Circular Dichroism in the Investigation of Protein Structure and Function. Curr. Protein Pept. Sci. 2000, 1 (4), 349–384. 10.2174/1389203003381315. [DOI] [PubMed] [Google Scholar]
- Wu J.-M.; Wei S.-Y.; Chen H.-L.; Weng K.-Y.; Cheng H.-T.; Cheng J.-W. Solution structure of a novel D-naphthylalanine substituted peptide with potential antibacterial and antifungal activities. Biopolymers 2007, 88 (5), 738–745. 10.1002/bip.20736. [DOI] [PubMed] [Google Scholar]
- Zhou R.; Huang X.; Margulis C. J.; Berne B. J. Hydrophobic collapse in multidomain protein folding. Science 2004, 305 (5690), 1605–9. 10.1126/science.1101176. [DOI] [PubMed] [Google Scholar]
- Zelezetsky I.; Tossi A. Alpha-helical antimicrobial peptides—Using a sequence template to guide structure–activity relationship studies. Biochim. Biophys. Acta, Biomembr. 2006, 1758 (9), 1436–1449. 10.1016/j.bbamem.2006.03.021. [DOI] [PubMed] [Google Scholar]
- Faudry E.; Perdu C.; Attree I.. Pore formation by T3SS translocators: liposome leakage assay. In Bacterial Cell Surfaces: Methods and Protocols; Delcour A. H., Ed.; Springer Science & Business Media: New York, 2013; Vol. 966, pp 173–85. [DOI] [PubMed] [Google Scholar]
- Epand R. F.; Schmitt M. A.; Gellman S. H.; Epand R. M. Role of membrane lipids in the mechanism of bacterial species selective toxicity by two α/β-antimicrobial peptides. Biochim. Biophys. Acta, Biomembr. 2006, 1758 (9), 1343–1350. 10.1016/j.bbamem.2006.01.018. [DOI] [PubMed] [Google Scholar]
- Oren Z.; Ramesh J.; Avrahami D.; Suryaprakash N.; Shai Y.; Jelinek R. Structures and mode of membrane interaction of a short alpha helical lytic peptide and its diastereomer determined by NMR, FTIR, and fluorescence spectroscopy. Eur. J. Biochem. 2002, 269 (16), 3869–80. 10.1046/j.1432-1033.2002.03080.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.