Abstract
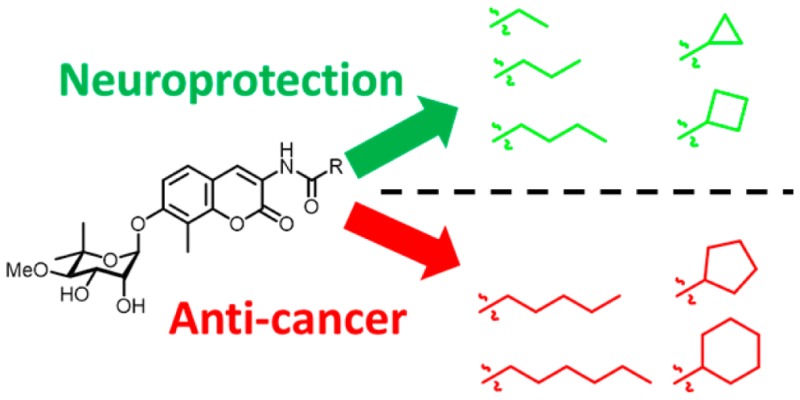
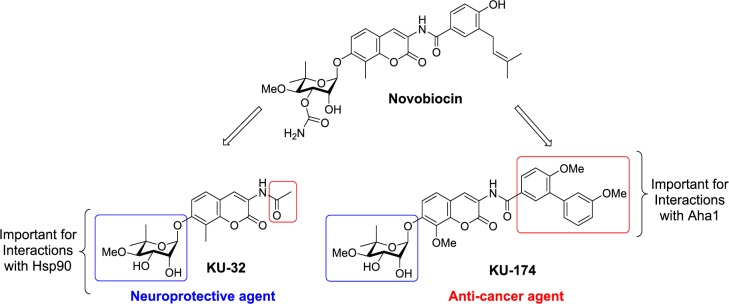
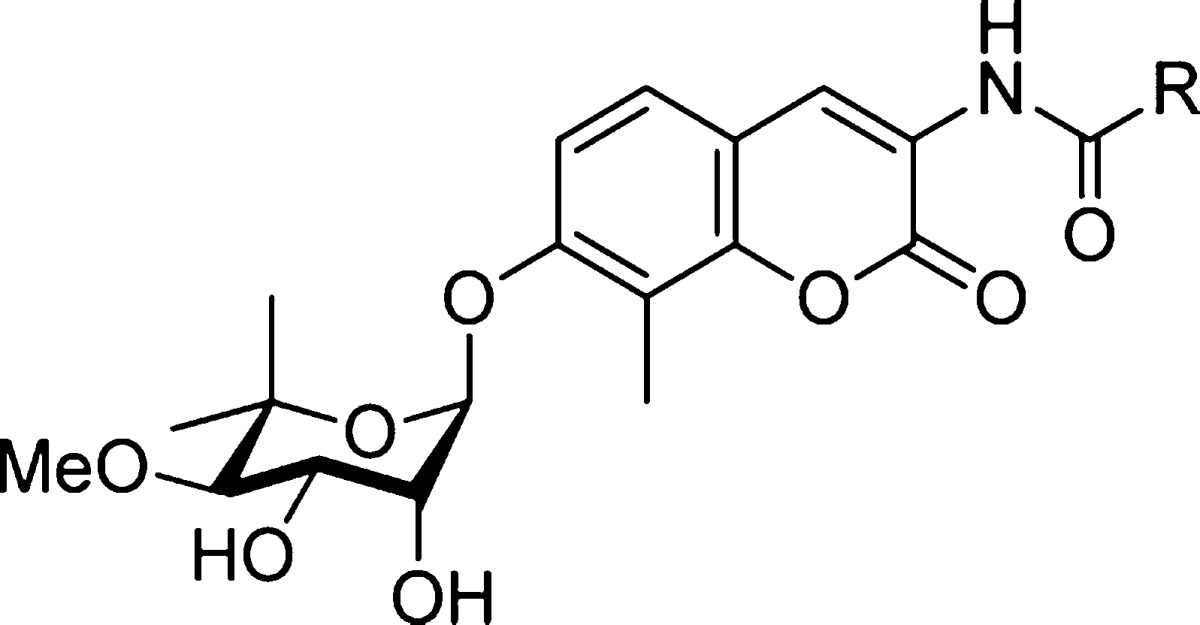
Novobiocin is a natural product that binds the Hsp90 C-terminus and manifests Hsp90 inhibitory activity. Structural investigations on novobiocin led to the development of both anti-cancer and neuroprotective agents. The varied pharmacological activity manifested by these novobiocin analogs prompted the investigation of structure–function studies to identify these contradictory effects, which revealed that modifications to the amide side chain produce either anti-cancer or neuroprotective activity. Compounds that exhibit neuroprotective activity contain a short alkyl or cycloalkyl amide side chain. In contrast, anti-cancer agents contain five or more carbons, disrupt interactions between Hsp90α and Aha1, and induce the degradation of Hsp90-dependent client proteins.
Keywords: Hsp90, Hsp90α, Aha1, anti-cancer, neuroprotection, novobiocin, structure−activity relationship
Introduction
Hsp90 represents a highly sought after target for the treatment of cancer, as substrates dependent upon Hsp90 are associated with all ten hallmarks of cancer.1−3 Hsp90 is overexpressed in cancer to fold oncogenic substrates in the presence of various cochaperones. While inhibition of Hsp90 can induce the degradation of oncogenic clients via the ubiquitin-proteasome pathway, it also induces the pro-survival heat shock response, which leads to concomitant induction of the heat shock proteins, including Hsp27, Hsp40, Hsp70, and Hsp90, and often results in cytostatic activity.4 Although difficult to overcome for the treatment of cancer, increased chaperone levels are beneficial for the treatment of neurodegenerative disorders that result from the accumulation of aggregated or misfolded proteins, such as Alzheimer’s and Huntington’s disease.5 Thus, Hsp90 is considered a target for both cancer and neurodegeneration.6−9 Therefore, methods to segregate these activities represent a novel paradigm for which Hsp90 modulators can be developed to treat cancer or neurodegeneration.
KU-32 (Figure 1) is a neuroprotective novobiocin derivative that binds the Hsp90 C-terminal dimerization domain10−12 and induces a robust heat shock response without concomitant client protein degradation. KU-32 manifests efficacy in attenuating the death of cortical neurons induced by β-amyloid peptides12 and can improve multiple physiological indices of diabetic peripheral neuropathy.9,13−15 The efficacy exhibited by KU-32 to protect against glucotoxicity correlates directly with an increase in mitochondrial bioenergetics.9 KU-32 does not induce the degradation of Hsp90-dependent client proteins such as Akt and Raf until much higher concentrations than those needed to induce the heat shock response and promote neuroprotection.13,14
Figure 1.
Structures of novobiocin based Hsp90 C-terminal inhibitors.
In contrast to the acetamide side chain on KU-32, KU-174 is a novobiocin derivative that contains an aryl amide side chain and exhibits potent anti-cancer activity by inducing the degradation of Hsp90-dependent client proteins without concomitant induction of the heat shock response.16 Unfortunately, the mechanisms for distinguishing between these opposing activities remain unclear. Since Hsp90 forms a complex with several proteins to assist in the protein folding process, interactions between Hsp90 and its cochaperones were investigated, which ultimately revealed the subtle nuances manifested by these two distinct classes of novobiocin analogs.
Activator of Hsp90 ATPase Activity (Aha1) is a cochaperone that binds to and facilitates the ATPase activity of Hsp90, which is required during the protein folding process.17−23 We have shown previously that a novobiocin-derived, Hsp90 C-terminal inhibitor could disrupt the Hsp90α/Aha1 complex.22 Those studies indicated that the noviose sugar was responsible for binding Hsp90 while the benzamide side chain present in KU-174 (Figure 1) interacted with Aha1, and, when combined, manifested anti-cancer activity.22 In contrast, replacement of the benzamide with an acetamide chain, as in the case of KU-32, did not disrupt the Hsp90α/Aha1 complex and, consequently, did not exhibit anti-cancer activity.22
In an effort to systematically investigate the differences manifested by the alkyl and aryl containing amide side chains, structure–function studies were investigated to identify the point of divergence at which a neuroprotective agent is transformed into an anti-cancer agent.
Results and Discussion
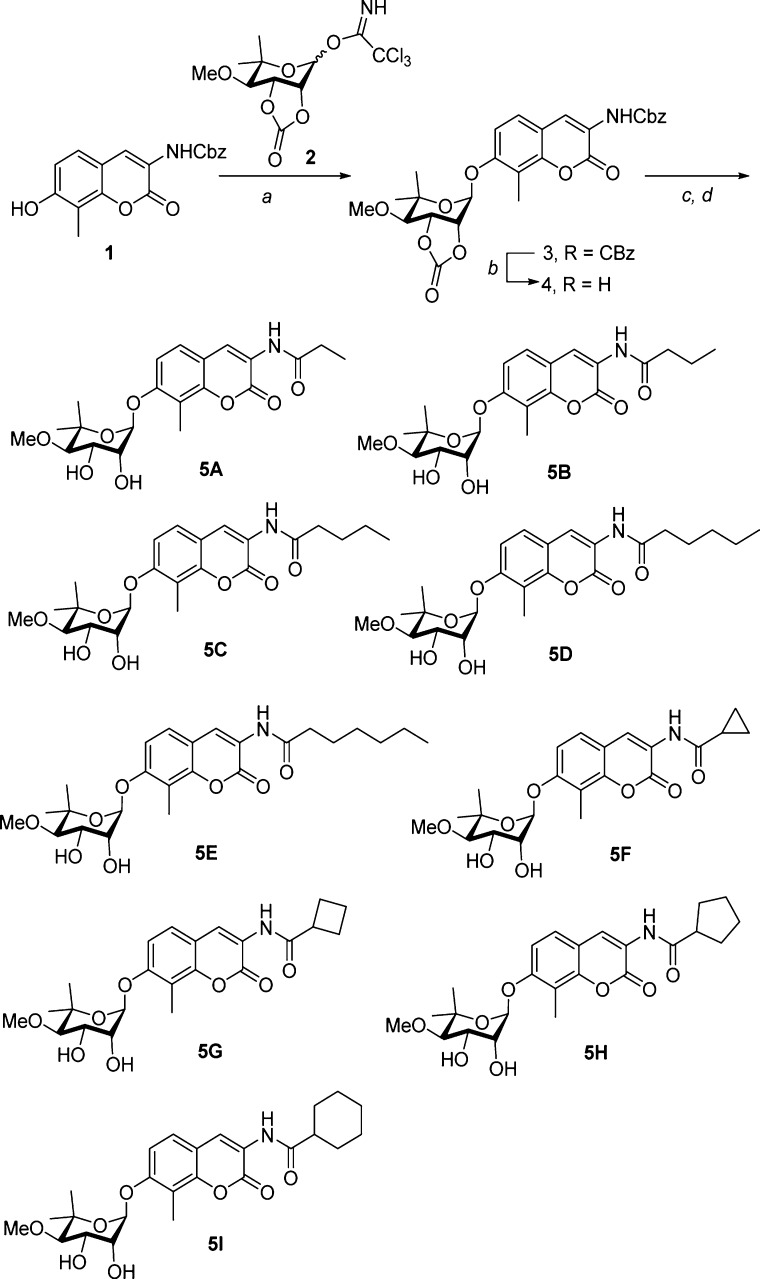
Analogs containing increasingly larger alkyl and cycloalkyl groups on the amide side chain were pursued (Scheme 1) to identify the point at which KU-32 is transformed from a neuroprotective agent into an anti-cancer agent. As shown in Scheme 1, synthesis of these analogs began by the noviosylation of phenol 1(24) with activated noviose25−29 (2) in the presence of catalytic boron trifluoride etherate to give 3 in good yield. Hydrogenolysis of the resulting benzyl carbonate furnished aniline 4, which underwent amide coupling with acids containing sequentially larger alkyl substituents, followed by solvolysis of the carbonate to afford the desired diols, 5A–I, in moderate to good yields.
Scheme 1. Synthesis of KU-32 Analogs.
Reagents and conditions: (a) BF3·Et2O, DCM, rt, 2 h, 70%; (b) 10% Pd/C, H2, EtOAc, rt, 4 h, ∼100%; (c) R-COOH, EDCl·HCl, DMAP, pyridine, DCM, rt, 12 h, 50–70%; (d) 2% Et3N/MeOH, rt, 12 h, 40–70%.
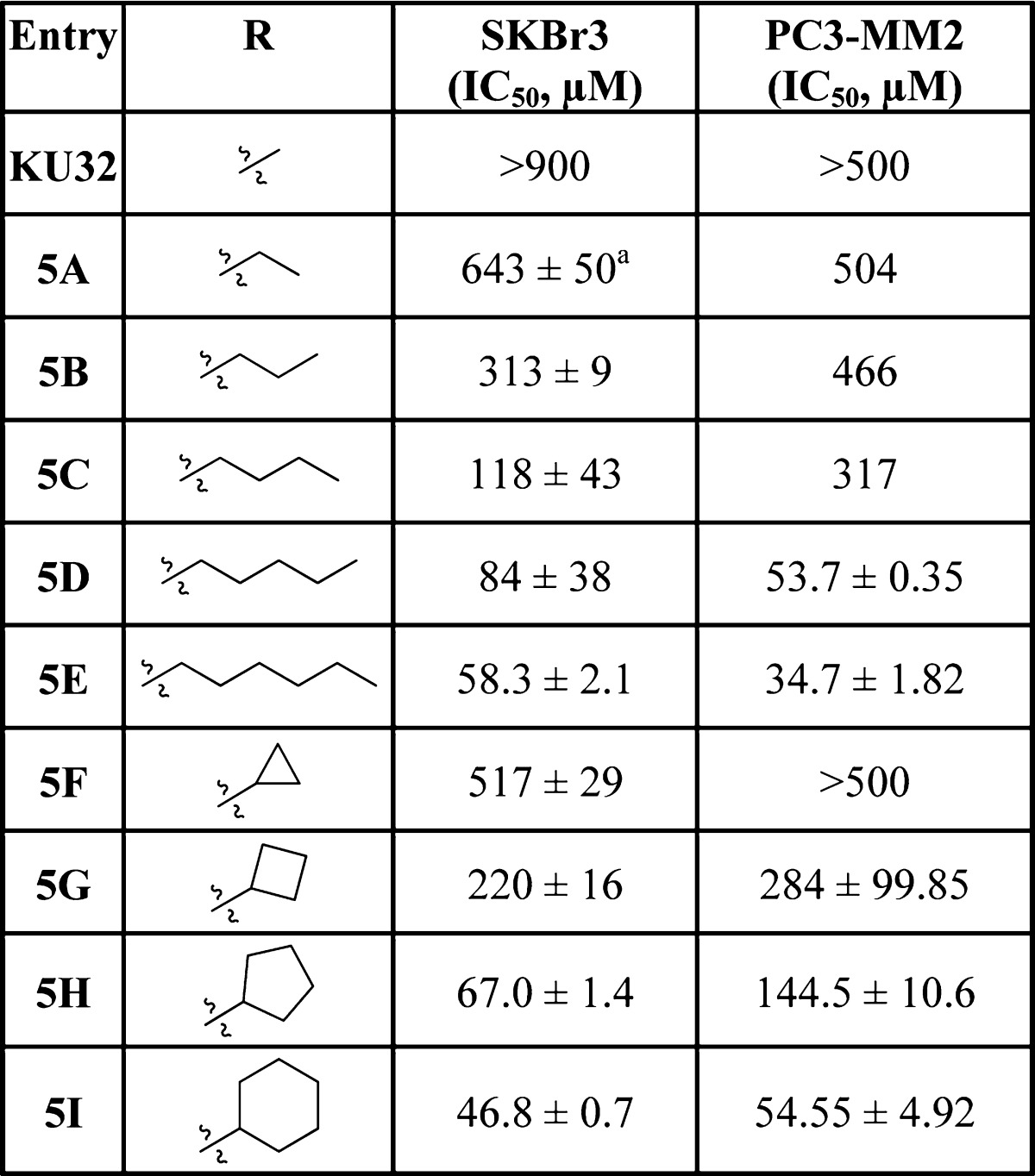
The anti-proliferative activity manifested by these compounds was evaluated against the highly metastatic, Her2 overexpressing SkBr3 breast and the androgen-independent PC3-MM2 prostate cancer cell lines. Increasing the alkyl chain length or the inclusion of a cycloalkyl ring onto the amide side chain resulted in a size-dependent increase in anti-proliferative activity as shown in Table 1, which was dependent upon chain length (R2 = 0.8732) or bulk (R2 = 0.8626) (Figure 2).
Table 1. Anti-proliferative Activity of KU-32 Analogsa.
Values represent mean ± standard deviation for at least two separate experiments performed in triplicate.
Figure 2.
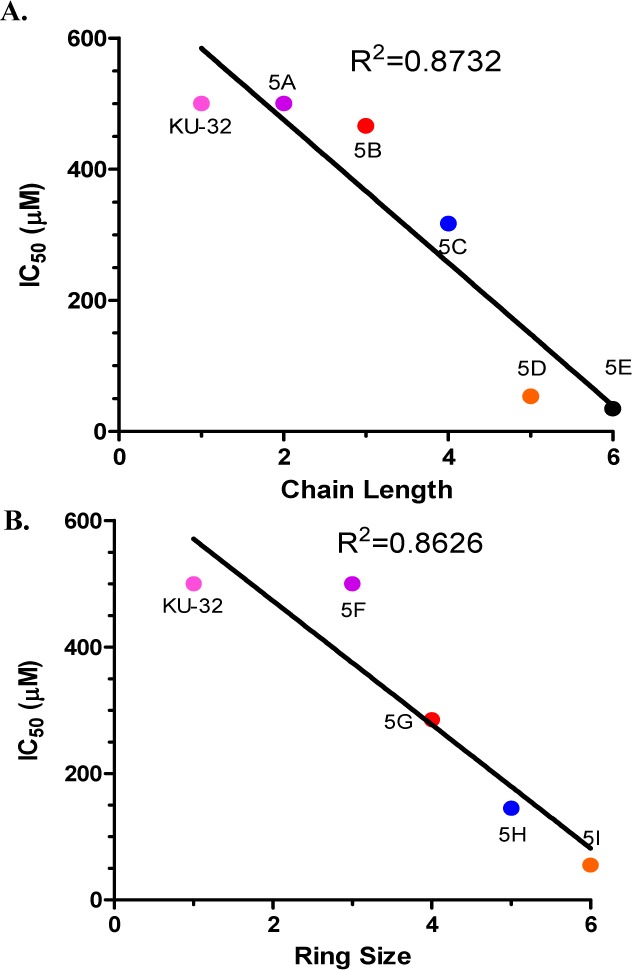
IC50 (μM) values of PC3-MM2 cells were plotted against the chain length (A) or the ring size (B).
Since rematuration of firefly luciferase is dependent upon the Hsp90 protein folding machinery, the refolding of denatured firefly luciferase was assessed to confirm whether these analogs retained the ability to inhibit Hsp90.10,29−31 As expected, KU-32 did not inhibit the ability of Hsp90 to refold luciferase, whereas compounds containing longer chains (5D and 5E) or carbocycles (5H and 5I) inhibited the rematuration of firefly luciferase (Figure 3). In exciting contrast, analogs with shorter chains (KU-32, 5A-5C) or small carbocycles (KU-32, 5F-5G) stimulated the rematuration of firefly luciferase. In fact, there was a clear point of divergence for the inhibition of luciferase refolding. These data suggest that the anti-proliferative activities manifested by these compounds (Table 1) result from modulation of the Hsp90 protein folding machinery (Figure 3).
Figure 3.
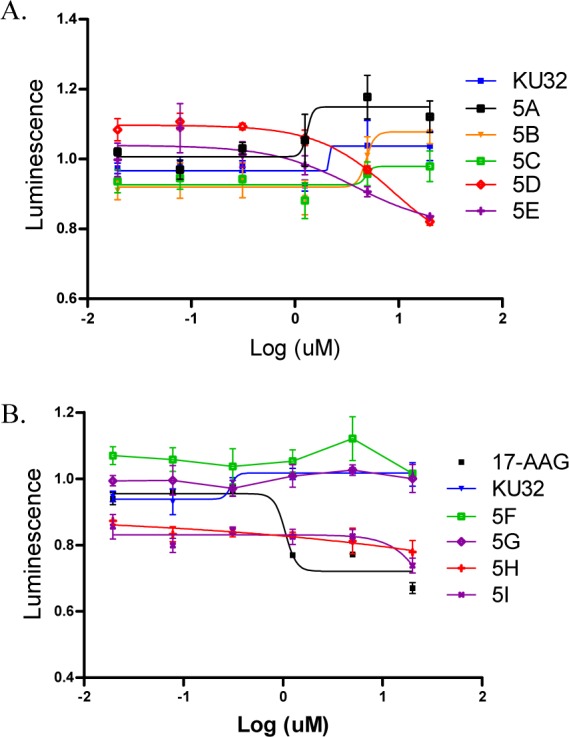
KU-32 analogs inhibit Hsp90-dependent luciferase refolding. Luciferase activity fold change compared to DMSO (1.0-fold) after incubation of heat-denatured luciferase with (A) KU-32, 5A, 5B, 5C, 5D, 5E, or (B) KU-32, 5F, 5G, 5H, or 5I. 17-AAG was used as a positive control. The concentrations of KU-32 and analogs used during this assay ranged from 0.02 to 20 μM.
Increased chaperone activity can produce neuroprotection that can be readily assessed through measurement of mitochondrial function using extracellular flux technology. Previous studies revealed that KU-32 improved mitochondrial bioenergetics and could reverse sensory neuropathy in vivo.9,12 Since the preliminary studies demonstrated that a systematic increase in chain length or the larger cycloalkyl rings resulted in anti-proliferative activity, analogs with larger side chains were expected to reduce the maximal respiratory capacity in the neuronal cells. As shown in Figure 4, the inclusion of propyl to heptyl alkyl chains led to a progressive decrease in maximal respiratory capacity, compared to KU-32. Similarly, substitution of propyl to hexyl carbocycles also produced a decline in maximal respiration. Thus, the decrease in maximal respiratory capacity correlated directly with the anti-proliferative activity manifested by these analogs (Table 1).
Figure 4.
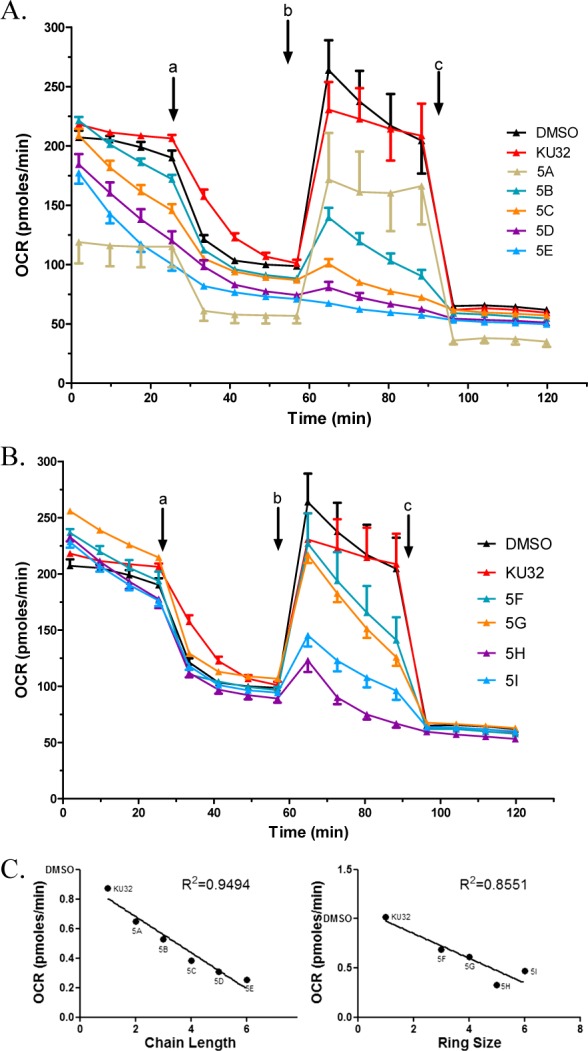
KU-32 analogs worsen mitochondrial bioenergetics in the 50B11 neuronal cell line. (A and B) 50B11 cells were treated for 24 h with KU-32 or its analogs (5 μM each) for mitochondrial bioenergetic analysis. The basal oxygen consumption rate (OCR) was measured prior to the addition of (a) oligomycin, to assess ATP-coupled respiration; (b) FCCP, to measure uncoupled respiration; and (c) rotenone + antimycin A, to assess nonmitochondrial respiration. (C) OCR values at 88.3 min were plotted and compared to the DMSO value.
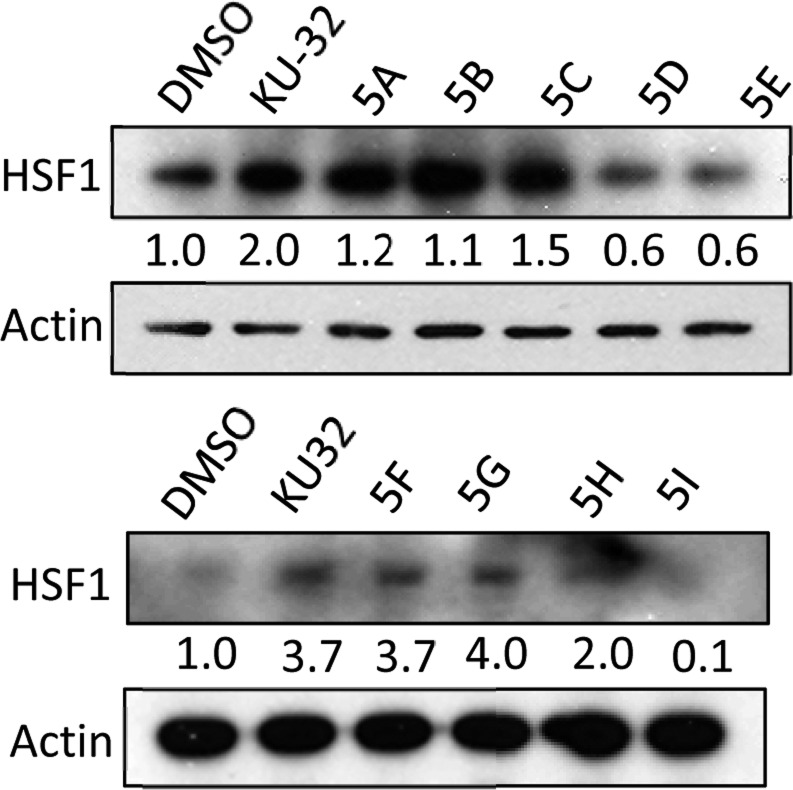
Heat Shock Factor 1 (HSF1) is the transcription factor that serves to regulate the heat shock response, and is required to manifest neuroprotective activity.32 Hsp90 binds HSF1 to form a stable complex, which prevents induction of the heat shock response under normal conditions. However, upon exposure to high temperature or Hsp90 inhibitors, this complex can disassemble and induce the heat shock response, which is necessary to refold proteins that become denatured at high temperature. Neuroprotective agents are sought that activate HSF1 and refold protein aggregates that occur in many neurodegenerative disorders.32 Therefore, the levels of HSF1 were evaluated in PC3-MM2 cells treated with KU-32 and its analogs. The results clearly show that treatment with KU-32 activated HSF1 2–3.7-fold (Figure 5). However, as the size of the alkyl or cycloalkyl substitution increased, the levels of HSF1 decreased (Figure 5), once again suggesting that smaller groups (KU-32, 5A-5C, 5F-5H) induce the pro-survival response, whereas larger groups (5D, 5E, 5I) prevent induction of the heat shock response.
Figure 5.
Expression of HSF1 upon treatment with the KU-32 analogs. PC3-MM2 cells were treated with KU-32 or its analogs (10 μM each) for 24 h, and the levels of HSF1 were measured compared to the levels of β-actin.
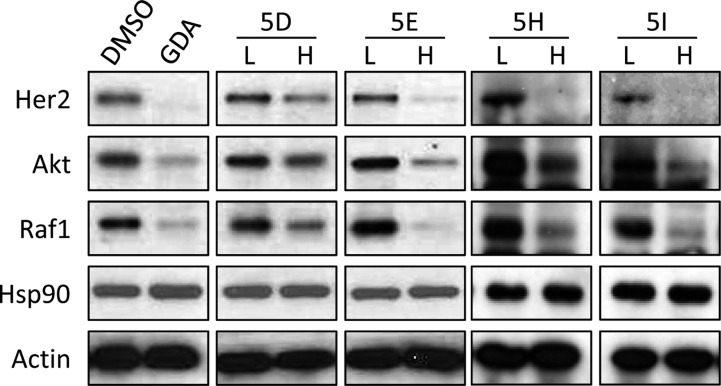
Compounds containing larger alkyl or cycloalkyl substitutions, 5D, 5E, 5H, and 5I, did not induce the heat shock response (Figure 5) but did exhibit decreased mitochondrial respiration (Figure 4), increased anti-proliferative activity (Table 1), and Hsp90 inhibitory activity (Figure 3). Consequently, these compounds were further evaluated to determine whether they could induce the degradation of known Hsp90-dependent client proteins. As shown in Figure 6, the degradation of Hsp90-dependent client proteins such as Her2, Akt, and Raf-1 occurred in the presence of 5D, 5E, 5H, and 5I without induction of Hsp90 levels, which is a hallmark of Hsp90 C-terminal anti-cancer agents.26,33−35 Compounds 5A–5C, 5F, and 5G were unable to induce Hsp90-client protein degradation at 100 μM.
Figure 6.
Cytotoxic KU-32 analogs down-regulate the expression of Hsp90 client proteins. PC3-MM2 cells were treated with the cytotoxic analogs of KU-32 (5D, 5E, 5H, and 5I) with the concentrations of (H) 3 × IC50 and (L) 0.5 × IC50 for 24 h, and the levels of known Hsp90 client proteins were measured. Actin was used as the loading control.
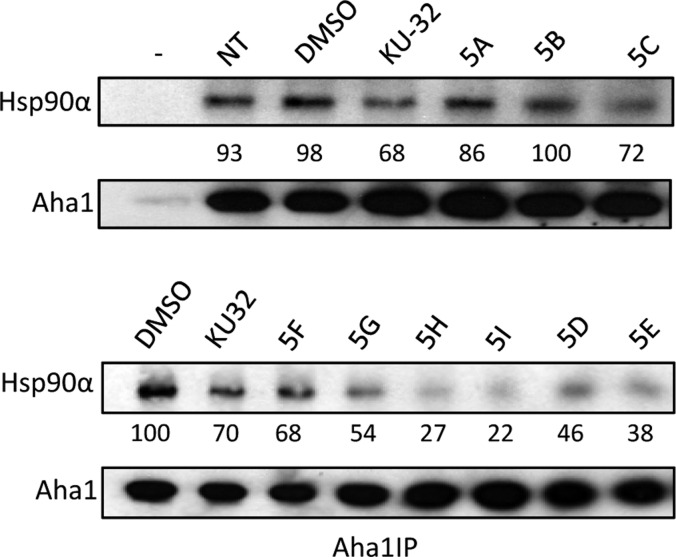
Since an increase in luciferase maturation was observed in Figure 3 with shorter amide side chains, it was hypothesized that Aha1 remained bound to Hsp90 under these conditions, whereas, in the presence of larger side chains, Aha1 association was disrupted. Therefore, Hsp90α/Aha1 interactions were investigated in PC3-MM2 cells treated with compounds 5A–I for 24 h. Aha1 was immunoprecipitated and the percent of Hsp90α remained bound to Aha1 was determined. As expected, Hsp90α/Aha1 disruption occurred more readily for analogs with large alkyl and cycloalkyl amide side chains (Figure 7). The percent of Hsp90α that remained bound to Aha1 was plotted against the IC50 values obtained from Table 1, and this once again demonstrated a correlation between Hsp90α/Aha1 disruption based on chain length or ring size, as well as anti-proliferative activity. Collectively, these data suggest that larger side chains disrupt Hsp90α/Aha1 interactions to result in anti-proliferative activity, whereas smaller side chains promote protein folding and induction of the heat shock response to manifest neuroprotective activity (Figure 7).
Figure 7.
KU-32 analogs disrupt the Hsp90α/Aha1 complex. (A–B) PC3-MM2 cells were treated with nothing (NT), DMSO (0.1%), KU-32 (100 μM), 5A (100 μM), 5B (100 μM), 5C (100 μM), 5D (50 μM), 5E (35 μM), or 5F (100 μM), 5G (100 μM), 5H (100 μM), 5I (50 μM) for 24 h. Aha1 was immunoprecipitated, and Hsp90α and Aha1 were analyzed by Western blotting. Aha1 bound Hsp90α was quantified using ImageJ software and expressed as percent bound compared to the cells treated with 0.1% DMSO (control).
Conclusion
In summary, it was shown that KU-32 analogs containing an alkyl or cycloalkyl group with increased carbon atoms manifested a linear inhibition of Hsp90-dependent refolding of thermally denatured luciferase, disruption of Hsp90α/Aha1 interactions, and increased anti-proliferative activity, while simultaneously decreasing mitochondrial bioenergetics. In fact, the results demonstrate that a shorter carbon chain or small ring retains the activities needed for neuroprotective activity (Figures 4 and 5), while an amide side chain containing at least five carbons exhibits anti-cancer activity by inhibiting Hsp90 function. These results suggest that a length of five carbons on the amide side chain is sufficient for the disruption of Hsp90α/Aha1 interactions and defines a point of divergence that transforms a neuroprotective agent into an anti-cancer agent.
Acknowledgments
This work was supported by National Institutes of Health grants [CA109265] to BSJB, [DK095911] to RTD, and [NS075311] to BSJB and RTD.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsmedchemlett.6b00224.
Experimental details (PDF)
Author Contributions
# SG and YL contributed equally.
The authors declare no competing financial interest.
Supplementary Material
References
- Peterson L. B.; Blagg B. S. To fold or not to fold: modulation and consequences of Hsp90 inhibition. Future Med. Chem. 2009, 1, 267. 10.4155/fmc.09.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg G.; Khandelwal A.; Blagg B. S. Anticancer Inhibitors of Hsp90 Function: Beyond the Usual Suspects. Adv. Cancer Res. 2016, 129, 51. 10.1016/bs.acr.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neckers L. Hsp90 inhibitors as novel cancer chemotherapeutic agents. Trends Mol. Med. 2002, 8, S55. 10.1016/S1471-4914(02)02316-X. [DOI] [PubMed] [Google Scholar]
- Luo W.; Sun W.; Taldone T.; Rodina A.; Chiosis G. Heat shock protein 90 in neurodegenerative diseases. Mol. Neurodegener. 2010, 5, 24. 10.1186/1750-1326-5-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyttenbach A. Role of heat shock proteins during polyglutamine neurodegeneration: mechanisms and hypothesis. J. Mol. Neurosci. 2004, 23, 69. 10.1385/JMN:23:1-2:069. [DOI] [PubMed] [Google Scholar]
- Kim Y. S.; Alarcon S. V.; Lee S.; Lee M. J.; Giaccone G.; Neckers L.; Trepel J. B. Update on Hsp90 inhibitors in clinical trial. Curr. Top. Med. Chem. 2009, 9, 1479. 10.2174/156802609789895728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt G. E.; Blagg B. S. Alternate strategies of Hsp90 modulation for the treatment of cancer and other diseases. Curr. Top. Med. Chem. 2009, 9, 1447. 10.2174/156802609789895683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y.; Ansar S.; Michaelis M. L.; Blagg B. S. Neuroprotective activity and evaluation of Hsp90 inhibitors in an immortalized neuronal cell line. Bioorg. Med. Chem. 2009, 17, 1709. 10.1016/j.bmc.2008.12.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J.; Farmer K. L.; Pan P.; Urban M. J.; Zhao H.; Blagg B. S.; Dobrowsky R. T. J Heat shock protein 70 is necessary to improve mitochondrial bioenergetics and reverse diabetic sensory neuropathy following KU-32 therapy. J. Pharmacol. Exp. Ther. 2014, 348, 281. 10.1124/jpet.113.210435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matts R. L.; Brandt G. E.; Lu Y.; Dixit A.; Mollapour M.; Wang S.; Donnelly A. C.; Neckers L.; Verkhivker G.; Blagg B. S. A systematic protocol for the characterization of Hsp90 modulators. Bioorg. Med. Chem. 2011, 19, 684. 10.1016/j.bmc.2010.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matts R. L.; Dixit A.; Peterson L. B.; Sun L.; Voruganti S.; Kalyanaraman P.; Hartson S. D.; Verkhivker G. M.; Blagg B. S. Elucidation of the Hsp90 C-Terminal Inhibitor Binding Site. ACS Chem. Biol. 2011, 6, 800. 10.1021/cb200052x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansar S.; Burlison J. A.; Hadden M. K.; Yu X. M.; Desino K. E.; Bean J.; Neckers L.; Audus K. L.; Michaelis M. L.; Blagg B. S. A non-toxic Hsp90 inhibitor protects neurons from Abeta-induced toxicity. Bioorg. Med. Chem. Lett. 2007, 17, 1984. 10.1016/j.bmcl.2007.01.017. [DOI] [PubMed] [Google Scholar]
- Urban M. J.; Li C.; Yu C.; Lu Y.; Krise J. M.; McIntosh M. P.; Rajewski R. A.; Blagg B. S.; Dobrowsky R. T. Inhibiting heat-shock protein 90 reverses sensory hypoalgesia in diabetic mice. ASN Neuro 2010, 2, e00040. 10.1042/AN20100015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L.; Zhao H.; Blagg B. S.; Dobrowsky R. T. C-terminal heat shock protein 90 inhibitor decreases hyperglycemia-induced oxidative stress and improves mitochondrial bioenergetics in sensory neurons. J. Proteome Res. 2012, 11, 2581. 10.1021/pr300056m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J.; Pan P.; Anyika M. A.; Blagg B. S. J.; Dobrowsky R. T. Modulating molecular chaperones improves mitochondrial bioenergetics and decreases the inflammatory transcriptome in diabetic sensory neurons. ACS Chem. Neurosci. 2015, 6, 1637. 10.1021/acschemneuro.5b00165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskew J. D.; Sadikot T.; Morales P.; Duren A.; Dunwiddie I.; Swink M.; Zhang X.; Hembruff S.; Donnelly A.; Rajewski R. A.; Blagg B. S.; Manjarrez J. R.; Matts R. L.; Holzbeierlein J. M.; Vielhauer G. A. Development and characterization of a novel C-terminal inhibitor of Hsp90 in androgen dependent and independent prostate cancer cells. BMC Cancer 2011, 11, 468. 10.1186/1471-2407-11-468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotz G. P.; Lin H.; Harst A.; Obermann W. M. Aha1 binds to the middle domain of Hsp90, contributes to client protein activation, and stimulates the ATPase activity of the molecular chaperone. J. Biol. Chem. 2003, 278, 17228. 10.1074/jbc.M212761200. [DOI] [PubMed] [Google Scholar]
- Mollapour M.; Bourboulia D.; Beebe K.; Woodford M. R.; Polier S.; Hoang A.; Chelluri R.; Li Y.; Guo A.; Lee M. J.; Fotooh-Abadi E.; Khan S.; Prince T.; Miyajima N.; Yoshida S.; Tsutsumi S.; Xu W.; Panaretou B.; Stetler-Stevenson W. G.; Bratslavsky G.; Trepel J. B.; Prodromou C.; Neckers L. Asymmetric Hsp90 N domain SUMOylation recruits Aha1 and ATP-competitive inhibitors. Mol. Cell 2014, 53, 317. 10.1016/j.molcel.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panaretou B.; Siligardi G.; Meyer P.; Maloney A.; Sullivan J. K.; Singh S.; Millson S. H.; Clarke P. A.; Naaby-Hansen S.; Stein R.; Cramer R.; Mollapour M.; Workman P.; Piper P. W.; Pearl L. H.; Prodromou C. Activation of the ATPase activity of hsp90 by the stress-regulated cochaperone aha1. Mol. Cell 2002, 10, 1307. 10.1016/S1097-2765(02)00785-2. [DOI] [PubMed] [Google Scholar]
- Sun L.; Hartson S. D.; Matts R. L. Identification of proteins associated with Aha1 in HeLa cells by quantitative proteomics. Biochim. Biophys. Acta, Proteins Proteomics 2015, 1854, 365. 10.1016/j.bbapap.2015.01.002. [DOI] [PubMed] [Google Scholar]
- Synoradzki K.; Bieganowski P. Middle domain of human Hsp90 isoforms differentially binds Aha1 in human cells and alters Hsp90 activity in yeast. Biochim. Biophys. Acta, Mol. Cell Res. 2015, 1853, 445. 10.1016/j.bbamcr.2014.11.026. [DOI] [PubMed] [Google Scholar]
- Ghosh S.; Shinogle H. E.; Garg G.; Vielhauer G. A.; Holzbeierlein J. M.; Dobrowsky R. T.; Blagg B. S. Hsp90 C-terminal Inhibitors Exhibit Anti-migratory Activity by Disrupting the Hsp90alpha/Aha1 Complex in PC3-MM2 Cells. ACS Chem. Biol. 2014, 10, 577. 10.1021/cb5008713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L.; Prince T.; Manjarrez J. R.; Scroggins B. T.; Matts R. L. Characterization of the interaction of Aha1 with components of the Hsp90 chaperone machine and client proteins. Biochim. Biophys. Acta, Mol. Cell Res. 2012, 1823, 1092. 10.1016/j.bbamcr.2012.03.014. [DOI] [PubMed] [Google Scholar]
- Burlison J. A.; Neckers L.; Smith A. B.; Maxwell A.; Blagg B. S. J. Novobiocin: Redesigning a DNA Gyrase Inhibitor for Selective Inhibition of Hsp90. J. Am. Chem. Soc. 2006, 128, 15529. 10.1021/ja065793p. [DOI] [PubMed] [Google Scholar]
- Zhao H.; Donnelly A. C.; Kusuma B. R.; Brandt G. E.; Brown D.; Rajewski R. A.; Vielhauer G.; Holzbeierlein J.; Cohen M. S.; Blagg B. S. Engineering an antibiotic to fight cancer: optimization of the novobiocin scaffold to produce anti-proliferative agents. J. Med. Chem. 2011, 54, 3839. 10.1021/jm200148p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly A. C.; Mays J. R.; Burlison J. A.; Nelson J. T.; Vielhauer G.; Holzbeierlein J.; Blagg B. S. The design, synthesis, and evaluation of coumarin ring derivatives of the novobiocin scaffold that exhibit antiproliferative activity. J. Org. Chem. 2008, 73, 8901. 10.1021/jo801312r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H.; Anyika M.; Girgis A.; Blagg B. S. Novologues containing a benzamide side chain manifest anti-proliferative activity against two breast cancer cell lines. Bioorg. Med. Chem. Lett. 2014, 24, 3633. 10.1016/j.bmcl.2014.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusuma B. R.; Khandelwal A.; Gu W.; Brown D.; Liu W.; Vielhauer G.; Holzbeierlein J.; Blagg B. S. Synthesis and biological evaluation of coumarin replacements of novobiocin as Hsp90 inhibitors. Bioorg. Med. Chem. 2014, 22, 1441. 10.1016/j.bmc.2013.12.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galam L.; Hadden M. K.; Ma Z.; Ye Q. Z.; Yun B. G.; Blagg B. S.; Matts R. L. High-throughput assay for the identification of Hsp90 inhibitors based on Hsp90-dependent refolding of firefly luciferase. Bioorg. Med. Chem. 2007, 15, 1939. 10.1016/j.bmc.2007.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davenport J.; Balch M.; Galam L.; Girgis A.; Hall J.; Blagg B. S.; Matts R. L. High-throughput screen of natural product libraries for hsp90 inhibitors. Biology 2014, 3, 101. 10.3390/biology3010101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avila C.; Hadden M. K.; Ma Z.; Kornilayev B. A.; Ye Q. Z.; Blagg B. S. High-throughput screening for Hsp90 ATPase inhibitors. Bioorg. Med. Chem. Lett. 2006, 16, 3005. 10.1016/j.bmcl.2006.02.063. [DOI] [PubMed] [Google Scholar]
- Neef D. W.; Jaeger A. M.; Thiele D. Heat shock transcription factor 1 as a therapeutic target in neurodegenerative diseases. Nat. Rev. Drug Discovery 2011, 10, 930. 10.1038/nrd3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H.; Garg G.; Zhao J.; Moroni E.; Girgis A.; Franco L. S.; Singh S.; Colombo G.; Blagg B. S. Design, synthesis and biological evaluation of biphenylamide derivatives as Hsp90 C-terminal inhibitors. Eur. J. Med. Chem. 2015, 89, 442. 10.1016/j.ejmech.2014.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg G.; Zhao H.; Blagg B. S. Design, synthesis, and biological evaluation of ring-constrained novobiocin analogues as hsp90 C-terminal inhibitors. ACS Med. Chem. Lett. 2015, 6, 204. 10.1021/ml5004475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samadi A. K.; Zhang X.; Mukerji R.; Donnelly A. C.; Blagg B. S.; Cohen M. S. A novel C-terminal HSP90 inhibitor KU135 induces apoptosis and cell cycle arrest in melanoma cells. Cancer Lett. 2011, 312, 158. 10.1016/j.canlet.2011.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.