Abstract
Neurotransmitter and receptor systems are involved in different neurological and neuropsychological disorders such as Parkinson's disease, depression, Alzheimer’s disease and epilepsy. Recent advances in studies of signal transduction pathways or interacting proteins of neurotransmitter receptor systems suggest that different receptor systems may share the common signal transduction pathways or interacting proteins which may be better therapeutic targets for development of drugs to effectively control brain diseases. In this paper, we reviewed metabotropic glutamate receptors (mGluRs) and their related signal transduction pathways or interacting proteins in status epilepticus and temporal lobe epilepsy, and proposed some novel therapeutical drug targets for controlling epilepsy and epileptogenesis.
Keywords: Calmodulin, drug target, epileptogenesis, homer, interacting proteins, metabotropic glutamate receptors (mGluRs), protein kinase C (PKC), signal transduction pathways
INTRODUCTION
The three groups of metabotropic glutamate receptors (mGluRs) are G-protein coupled receptors (GPCR) similar to calcium-sensing receptors and γ-aminobutyric acid B (GABAB) receptors [1]. The significant work in the 80s [2-4] led to the discovery of metabotropic glutamate receptor 1 (mGluR1), one of group I mGluRs in 1991 and it was the first of the eight mGluR subtypes identified [5, 6]. Group I mGluRs regulate neuronal excitability through modulation of ionotropic glutamate receptors (iGluRs), activity at postsynaptic density. Activation of group II (mGluR2 and 3) and group III receptors (mGluR4, 6, 7 and 8) reduces the concentration of cyclic adenosine monophosphate (cAMP) by inhibiting the activity of adenylyl cyclase (AC). Group II and III mGluRs are located presynaptically to regulate the release of glutamate or other neurotransmitters [7, 8]. Activation of group I mGluRs positively affects postsynaptic neuronal excitability, may induce specific long lasting synaptic and cellular plasticity including long term potentiation (LTP) and depression (LTD) [9-11]. Over activation of group I mGluRs may therefore initiate epileptogenesis [11-14]. Group II and III mGluRs are located presynaptically and activation these mGluRs may suppress the excitatory transmission in a glutamatergic synapse [15-18]. Theses receptors are negatively coupled with G-protein linked AC and voltage gated K+ and Ca2+ channels leading to a lower calcium concentration in presynaptic nerve ending which limits the release of glutamate [19, 20]. MGluRs have been considered as promising drug targets in the treatment of epileptogensis as Group I mGluRs antagonists and group II and III mGluRs agonists are both anticonvulsive and neuroprotective [8, 17, 21]. The regulation of mGluRs plays a vital role in functional modulation of synapses and neuronal networks within central nervous system (CNS) [1, 8]. By binding to their interacting proteins, mGluRs dynamically interact with pre- or postsynaptic enzymes, ion channels and other proteins to assemble into various macromolecular signal complexes. The coupling between mGluRs and their interacting protein may result in abnormal signal transduction and consequently cause neurodegenerative diseases including temporal lobe epilepsy [8]. Understanding the distribution and function of mGluRs and their interacting proteins in the brain is fundamental to understand the mechanisms of epilepto- genesis and develop new anti-epileptogenic drugs. While rational designs of treatment by targeting on one particular neurotransmitter and receptor system including glutamate, acetylcholine (ACh), dopamine, GABAB, serotonin have improved the suffering of these patients in past half a century, these treatments are all symptomatic with limited therapeutic effects but some obvious side effects, as targeting on one neurotransmitter and receptor system may result in the imbalance of other systems.
MGLURS & INTERACTING PROTEINS
MGluRs possess a large extracellular N-terminus containing about 560 amino acids that forms the binding pocket for glutamate and their subtype selective agonists. Their intracellular C-termini which are modified extensively in length and phosphorylation level have various binding affinities for intracellular interacting proteins [22]. By binding with different cytosolic proteins, these C-termini can regulate the surface expression level, ligand binding affinity and the interaction with G-protein [1, 23]. Based on similarity in amino acid sequence, neuropharmacology of agonist and antagonist and the signal transduction pathways to which they couple, mGluRs are classified into three groups. Group I mGluRs (mGluR1 and 5) promote the activity of phospholipase C (PLC) and increase the intracellular concentrations of diacylglycerol (DAG) and inositol triphosphate (IP3). They may also increase the concentration of cAMP by activating AC [24]. In addition to signaling proteins, mGluRs also interact with other three groups of proteins, i.e., cytoskeleton, membrane and scaffold proteins. Signaling proteins include kinases [25, 26], phosphatases [27, 28] and those directly involved in signal cascades such as calmodulin, calcineurin inhibitor and norbin [29, 30]. The cytoskeleton or its associated proteins play a vital role in trafficking and anchoring mGluRs at specialized location of synapses [31]. In the membranes, mGluRs form homodimer or heterodimer between mGluRs subtypes or with other functional membrane proteins [32]. These oligomerized GPCRs increase the diversity of mGluR associated signal transduction pathways which may be different from the individual mGluR subtypes. Scaffold proteins serve as platform for mGluRs and down-stream proteins involved in mGluR associated signal transduction pathways [33-35]. They tether together to form macromolecular signaling complexes at synaptic terminals. Furthermore, scaffold proteins also regulate mGluR mediated signal transduction cascades by changing their molecular composition in these signaling complexes [36, 37]. Abnormal protein-protein interactions between mGluRs and their interacting proteins, such as Homer, have been involved in Fragile X mental retardation, schizophrenia, anxiety, attention deficit, neuroplasticity associated with acute and chronic action of drugs of abuse such as cocaine and alcohol and the reduction of neuronal excitability, especially during epileptic seizure and inflammatory pain [38].
MGLURS IN EPILEPTOGENESIS
Epileptogenesis refers to the process whereby the brain becomes epileptic due to inborn brain malformations, acquired structural brain lesions, alterations in neuronal signaling, and defects in maturation and plasticity of neuronal networks [39]. Both animal and human experimental studies have clearly shown that glutamate is released just before and during spontaneous seizures. The time course of this in vivo glutamate release from epileptic foci correlates well with the time course of the epileptic hyperactivity, as it begins immediately before seizure onset, indicating a causal relationship between glutamate release and seizure onset [40]. This causal relationship is further confirmed by in vitro model of glutamate injury-induced epileptogenesis [41, 42]. In vitro study also suggests that stimulation of group I mGluRs elicits epileptogenesis [42], which is supported by our in vivo animal experimental study showing that group I mGluR antagonist 2-methyl-6-(phenylethynyl)- pyridine (MPEP) prevents status epilepticus and subsequent neuronal loss and epileptogenesis [8, 40, 43].
At molecular levels, the involvement of mGluRs in epileptogenesis has also been well documented. Up-regulation of mGluR1 mRNA and protein occurs in the hippocampus of different animal models of epilepsy, suggesting that it may be involved in the neuronal hyperexcitability, loss, and subsequent epileptogenesis at acute stages after status epilepticus or kindling [12, 13, 44]. Down-regulation of mGluR5 mRNA in the hippocampus suggests that mGluR1 and mGluR5 may contribute to epileptogenesis differentially [13]. Increases in the expression of functional mGluR1 in the supraoptic nucleus may contribute to the development of the long-lasting plastic changes [45]. In the pilocarpine model of epilepsy, up-regulation of mGluR2 and 3 in the stratum lacunosum moleculare [46], mGluR4 in the granular layer [47], mGluR7 mRNA [48] and mGluR8 in the molecular layer of the dentate gyrus [49] 24 h after status epilepticus may indicate a compensatory mechanism to reduce excitoneurotoxicity and epileptogenesis. However, down-regulation of group II and group III mGluRs at chronic stages of animal models of epilepsy may indicate a reduced inhibitory effect or negative feedback which may be related to epileptogenesis [18, 49-52]. Animal experimental results therefore suggest that increased group I mGluRs and reduced group II and III mGluRs in the hippocampus may be involved in chronic epileptogenesis [11, 17]. It is supported by data from patients with temporal lobe epilepsy [44, 46, 53-57]. Decreased group I mGluR or increased group II and III mGluRs in previous studies may be due to the use of different animal models and experimental protocols [53, 58]. Consistent increase in expression of group I mGluRs mRNA and protein at acute stages of seizures in the animal models [12, 59] and patients [44, 54, 60], suggests that group I mGluRs may be therapeutic drug targets to control seizures and prevent epileptogenesis. This is supported by neuropharmacological studies showing anticonvulsive and neuroprotective effects of antagonists of group I mGluRs [17, 40]. However, a significant down-regulation of the expression levels of group II and III mGluRs suggests that targeting on group II and III mGluRs may not be so effective to control the occurrence of epilepsy at chronic stages [21, 46, 54].
MGLUR INTERACTING PROTEINS IN EPILEPTO- GENESIS
Homer proteins are scaffolds connecting mGluRs and other ligands to form a macrocomplex via the N-terminal Ena/VASP homology domain 1 [33, 61]. The long Homer isoforms use C-terminal coiled coil domain for dimerization [61, 62]. Homer 1 and 2 but not Homer 3 physically hold group I mGluRs, PLCβ and insitol-1,4,5- trisphosphate (IP3) receptors in a signaling complex which is involved in intracellular calcium signaling [61, 63]. The short Homer isoform 1a (H1a) lacks the dimerization domain and thus inhibits the formation of signaling complex by uncoupling Homer scaffolds [62]. “In neocortex pyramidal cells, activation of mGluR by Homer-1a induces IP3 which causes inositol-induced calcium release and a consequent potassium channel opening, thus hyperpolarizing the intracellularly Homer1a protein injected neurons” [64]. It has been reported that H1a expression is immediately up-regulated in the acute stage of kindling and pilocarpine induced animal model of epilepsy. H1a may therefore act as an anticonvulsant [37, 65]. H1a also plays a role in certain forms of homeostatic scaling which may lead to changes in synaptic function in epileptogenesis [66]. Furthermore, H1a modulates endocannabinoid (eCB) mediated synaptic plasticity in cultured hippocampal neurons following a seizure activity [36]. “eCBs are produced in the postsynaptic neuron upon strong depolarization and / or activation of mGluRs and act on presynaptic cannabinoid receptor-1 (CB1) to inhibit the release of neurotransmitter” [67]. They serve as an on-demand neuroprotective system. “However, the induced epileptiform activity by a group I mGluR agonist, dihydroxyphenylglycine (DHPG), was significantly reduced by CB1 receptor antagonists, SR 141716 or AM 251” [68]. Increased H1a expression following an epileptic stimulus subsequently uncouples mGluR from the signaling complex and affects mGluR-mediated eCB production [36]. Current data suggest that during the early stage of epileptogenesis, overexpression of H1a can counteract hyperexcitability and thus H1a may be a molecular target to prevent epilepto- genesis [36, 37].
In mGluRs related signal transduction systems, PLCβ4 has been identified as one down-stream protein for mGluR1 in the mouse cerebellum [63, 69, 70] and for mGluR5 in the suprachiasmatic nucleus [71]. MGluR5-PLCβ1 signal transduction plays an important role in the coordinated development of the neocortex [72] and in the aged striatum [73]. In the hippocampus, group I mGluR elicited epileptiform discharges through PLCβ1 signaling [74]. Cuellar et al. [75] indicated that “l-cysteine sulfinic acid (CSA), an agonist of phospholipase D (PLD)-coupled mGluRs, mediated its effect by PLD-driven activation of protein kinase C (PKC), which may desensitize PLC-linked group I mGluRs and thereby prevent group I mGluR-induced epileptogenesis”. “It suggested that CSA-mediated suppression of group I mGluR-induced epileptogenesis was PKC dependent” [75]. Our previous studies indicated that PKCβ1, PKCβ2, and PKCγ might be involved in mGluR1α -related excitoneurotoxicity and epileptogenesis [76], and mGluR5-PLCβ4- PKCβ2/PKCγ pathways was involved in the hyperexcitability of hippocampal CA1 pyramidal neurons leading to the loss of these neurons [77]. Our findings are in agreement with in vitro study in HEK cells that mGluR5-stimulated oscillatory activation of PKCγ [78] and PKCβ2 [79] is mediated by PLCβ4 and Ca2+. In vitro study also indicated that “PKC-induced prolongation of epileptiform bursts was dependent on changes specific to mGluR5 and not mediated simply by a generalized increase in transmitter release” [80]. In pilocarpine model, mGluR5 may regulates the PKCζ activation in the hippocampal interneurons in epilepsy [77]. The increased PKCε may support the “epsilon theory” of epileptogenesis [81]. Alteration of the expression of cyclic-AMP dependent protein kinase (cPKA) subtypes cPKAβ and cPKAγ in mouse hippocampus [25] suggests that hippocampal PKC and cPKA isoforms play different roles in neuronal hyperexcitability and epileptogenesis, and may be targets for development of anti-convulsive and anti-epilepto- genic drugs [26].
The surface expression level of mGluRs is controlled by interacting proteins such as calmodulin (CaM), seven in absentia homolog (Siah)-1A and norbin. CaM, a calcium binding protein in brain, regulates the synaptic plasticity by interacting with numerous GPCRs [82-84], NMDA receptors and voltage-gated calcium channels [85]. CaM binds to group I and III mGluRs in a Ca2+-dependent manner. Activation of mGluR5 triggers PKC mediated phosphorylation of serine 901 (S901) on its C terminus, which consequently disrupts the binding of CaM to mGluR5 [86], results in an increased mGluR5 endocytosis and decreased surface expression. In contrast, CaM binding prevents phosphorylation of mGluR5 C-terminus by PKC [87]. Furthermore, E3 ligase seven in absentia homolog (Siah)-1A competes with CaM for binding to mGluR5 and degrades mGluR1 and mGluR5 [29, 88]. It has been shown that “the phosphorylation of mGluR5 by PKC disassociates CaM from its C terminus and enhances the binding of Siah-1A leading to a decreased surface expression of mGluR5” [29]. Like mGluR5, “activation of mGluR7 also leads to decreased surface expression of CaM” [29]. However, “CaM affects mGluR7 trafficking by competing with protein interacting with C kinase 1 (PICK1) binding and leading to dephosphorylation of the major PKC phosphorylation site on the mGluR7 C terminus and increase of mGluR7 surface expression” [29, 89, 90]. Furthermore, the CaM competes with the G-Protein βγ-subunit and displaces pre-bound G-Protein βγ-subunit from mGluR7a which may affect the glutamate release in presynaptic terminals [91]. Norbin also competes with CaM for mGluR5 binding which is regulated by PKC phosphorylation of S901 on mGluR5 C-terminus [92]. In knock-in mice lacking the PDZ-ligand motif of mGluR7a, the interaction between mGluR7a and PICK1 is disrupted and then no down-regulatory effects on spontaneous excitatory currents were observed from the group III mGluR agonist L-AP4 [93]. This suggests that “PICK1 binding to the C-terminal region of mGluR7a is crucial for protein kinase C-mediated inhibition of glutamate release” [93].
In hippocampal pyramidal cells, the endogenous activation of mGluRs is dependent on the rate of glutamate reuptake mainly through the excitatory amino-acid transporters (EAAT). The postsynaptic mGluRs located around the synaptic centre are tonically activated after using EAAT inhibitor, TBOA [94]. The mRNA and protein levels of EAAT3 were significantly increased in pyramidal cells of the hippocampus after pilocarpine-induced status epilepticus [95]. This up-regulation of EAAT should work as a neuroprotective mechanism during and after seizure.
AGONISTS AND ANTAGONISTS OF MGLURS IN THE CONTROL OF EPILEPSY AND EPILEPTO- GENESIS
The anticonvulsive and anti-epileptogenic effect of various agonists and antagonists of mGluRs has been reviewed by different research groups [8, 96, 97]. In brief, agonists of group I mGluRs such as (S)-3,5-dihydro- xyphenylglycine (DHPG), (1S,3R)-1-aminocyclopentane dicarboxylic acid (APDC) and other group I mGluR agonists enhances neuronal excitability, induce seizures and neuronal injury [11, 43, 98]. Whereas group I mGluR antagonists such as MPEP [40], [(2-methyl-1,3-thiazol-4-yl)ethynyl] pyridine (MTEP) [99], SIB1893 [100], 1-aminoindan-1,5-dicarboxylic acid (AIDA) [17], BAY36-7620 [101], LY367385 [102], LY357366 [103], LY339764, LY367335, LY367366 and LY339840 [104] have potent anticonvulsant activity in animal models of epilepsy [105, 106]. The mGluR2 agonists such as (S)-4-carboxy-3-hydroxyphenylglycine (C3HPG) [107], (2R,4R)-APDC [15] and (2S,2'R,3'R)-2-(2',3'-dicarboxycyclopropyl)glycine (DCG-IV) [16, 108] reduce audiogenic seizures in DBA/2 mice and genetically epilepsy prone rats and enhance the generalised seizure threshold in kindled rats. DCG-IV significantly depresses medial perforant path-evoked responses in epileptic tissue from pilocarpine-treated rats more than control which may be due to a significant increase of mGluR2 [18]. In addition, group II mGluRs agonists, LY379268 and LY389795 reduce spike and wave discharge (SWD) duration of absence seizures in lh/lh mice [109]. LY379268 reduces both behavioral correlates and power in EEG bandwidths in pilocarpine model [21]. Activation of group III mGluRs by their agonists such as L-(+)-2-amino-4- phosphonobutyric acid (L-AP4) [17], L-serine-O-phosphate (L-SOP) [110], (R,S)-4-phosphonophenylglycine (PPG) [111] and (1S,3R,4S) -1- aminocyclopentane- 1,2,4-tricarboxylic acid (ACPT-1) [112] produces anticonvulsant effects. Moreover, “the selective agonist for mGluR8, (S)-3,4-dicarboxyphenylglycine (DCPG) reduces DL-homocysteic acid induced seizures in immature rats and suppresses generalized clonic-tonic seizures” [97, 113]. Intracerebroventricular administration of group III mGluR antagonist (RS)-a-cyclopropyl-4-phosphonophenylglycine (CPPG) reduces PTZ-induced seizures. However, L-AP4 does not significantly affect the kindling of seizures [113], suggesting that the dose of L-AP4 may significantly affect its anticonvulsant effects. At lower doses of 10 and 20 nmol/site, i.c.v., it produces anticonvulsant effect, at high doses of 50 and 100 nmol/site, i.c.v., it has no anticonvulsive effects [81, 114], whereas at very high doses such as 300 nmol/site, i.c.v., it induces convulsions [115]. In pilocarpine model, mGluR5 antagonist MPEP shows significant anticonvulsive and neuroprotective. Its combination with NMDA receptor antagonist and GABA receptor agonist is more effective than MPEP alone [40], suggesting that mGluR5 antagonists may be promising candidate anti-convulsive, neuroprotective or anti-epileptogenic drugs.
MGLUR INTERACTING PROTEINS AS THERAPEUTIC TARGETS IN THE CONTROL OF EPILEPSY AND EPILEPTOGENESIS
As scaffold proteins, Homers connect various intra- cellular and membrane proteins to form signaling complexes which play vital roles in neuronal activity (Fig. 1). “Down-regulation of Homer1b/c could attenuate group I mGluR dependent Ca2+ signaling through regulating endoplasmic reticulum Ca2+ release and then protect neurons from glutamate excitotoxicity after injury” [116, 117]. Homer1b/c promotes “neuronal apoptosis via the Bax/Bcl-2 dependent pathway during neuroinflammation in CNS, and inhibition of Homer1b/c expression may provide a novel neuroprotective strategy against the inflammation-related neuronal apoptosis” [118]. Up-regulation of postsynaptic Homer1a could protect against neuronal injury by reducing the level of phosphorylated extracellular signal-regulated kinase (ERK) and then disrupting mGluR-ERK signaling [119, 120]. “It can reduce mGluR5 coupling to postsynaptic effectors without relying on large changes in the subcellular distribution of the receptor” [121]. “Homer protein-metabotropic glutamate receptor binding also regulates endocannabinoid signaling and affects hyperexcitability in a mouse model of fragile X syndrome” [122]. The expression of Homer1a has been shown to be upregulated selectively and rapidly by neural stimulation [37, 65]. “NMDA receptor agonists and brain-derived neurotrophic factor (BDNF) could upregulate homer1a mRNA via the mitogen-activated protein kinase (MAPK) cascade in cultured cerebellar granule cells” [123]. Whereas its antagonists “reduced Homer1b and PSD-95 expression in cortical and striatal regions” [124]. It was also reported that the MAPK/ERK cascade played an important role in vascular endothelial growth factor (VEGF)-stimulated induction of Homer1a mRNA [125]. The short Homer1a lacks the dimerization domain comparing with long Homers and thus inhibits the formation of the signaling complexes by uncoupling Homer scaffolds [62]. Therefore, uncoupling Homer scaffolds by blocking the dimerization domain of long Homer isoforms may prevent neuronal hyperexcitability in epilepsy (Fig. 1). It suggests that Homers may be promising therapeutic drug targets to control seizures and neuronal loss, and prevent epileptogenesis.
Fig. (1).
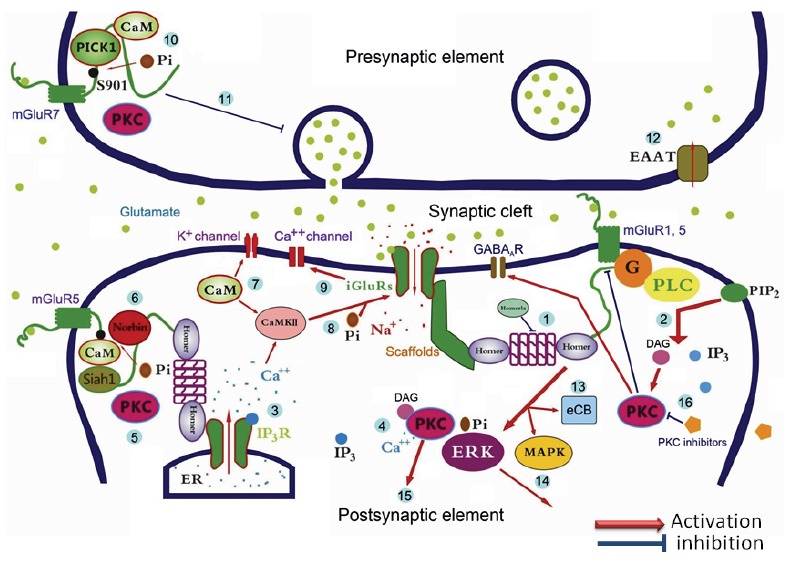
Interactions among mGluRs and the interacting proteins in the pre- and post-synaptic membrane and subsequent epileptogenesis. Glutamate released from the pre-synaptic membrane bind and open ligand-gated ionotropic glutamate receptors (iGluRs) allowing Na+ and/or Ca2+ influx and/or K+ efflux at the central area of postsynaptic membrane. Over release of glutamate activates metabotropic glutamate receptors (mGluRs) at the peripheral of post-synaptic membrane, induces the following cascades of reactions: 1) the formation of signaling complexes by dimerization of the long form Homers which then enhances the activity of iGluRs; 2) phospholipase C (PLC) cleaves the phospholipid phosphatidylinositol 4,5-bisphosphate (PIP2) leading to an increase in diacylglycerol (DAG) and inositol triphosphate (IP3); 3) IP3 activates IP3 receptors which induces Ca2+ efflux from endoplasmic reticulum (ER); 4) Protein kinase C (PKC) activation by the increased concentration of Ca2+ and DAG; 5) PKC phosphorylation of the S901 of mGluR5 to reduce its postsynaptic membrane expressing level; 6) calmodulin (CaM) binding which prevents phosphorylation of the mGluRs by PKC in a Ca2+-dependent manner, while the Seven in absentia homolog (Siah)-1 and norbin compete with CaM to enhance the possibility of PKC phosphorylation; 7) dissociation of CaM and released Ca2+ open K+ channels on postsynaptic membrane to induce a neuroprotective hyperpolarization; 8-9) phosphorylation of NMDAR by the activated CaMKII and then opening of the NMDAR-dependent Ca2+ channel; 10) binding of glutamate with presynaptic mGluR7 to enhance the possibility of PKC phosphorylation with the help of the protein interacting with C kinase 1 (PICK1) which compete with CaM; 11) PKC phosphorylation of the S901 of mGluR7 to down-regulate the release of presynaptic neurotransmitters; 12) uptaking glutamate back to presynaptic nerve ending by excitatory amino-acid transporters (EAAT) to reduce excitatory response on postsynaptic membrane; 13-14) up-regulation of endocannabinoid (eCB) and activation of mitogen-activated protein kinase (MAPK) and extracellular signal-regulated kinase (ERK) by PKC phosphorylation; 15) fine-tuning PKC activities may induce synergic action of various neurotransmitters and receptors system, for instance, 16) PKC inhibition induces GABAA receptor hypersensitivity, reduces mGluR5 related epileptiform bursts, and therefore, PKC may be an ideal therapeutic target to prevent epileptogenesis.
PKC isoforms are involved in different neurotransmitter receptor signal transduction pathways and play important roles in neuronal hyperexcitability and epileptogenesis [26, 76, 80]. Induced transient expression of protein kinase C beta1(PKCβ1), beta2 (PKCβ2), and gamma (PKCγ) in hippocampal interneurons may result in the excitoneuro- toxicity, leading to the death of interneurons, over excitation of pyramidal neurons and epileptogenesis. This activation is Group I mGluR dependent [76]. The long term increase in PKCγ has been strongly suggested to associate with epilepsy [26, 126]. PKC isoforms are also important downstream proteins and play important roles in nicotinic [127], muscarinic [128] cholinergic receptors, gamma-aminobutyric acid type A (GABAA) receptor [129], serotonin (5-HT) receptor [130], NMDA receptor [131], AMPA receptor [132] regulated neuronal activities. Many inhibitors of different PKC isoforms were unraveled as potential drugs for the cure and prevention of various PKC elevation disorders such as diabetic complications, cancer, Alzheimer’s disease, autoimmune diseases and cardiovascular diseases [133]. “Thymeleatoxin, an activator for PKCα, PKCβ1, and PKCγ, up-regulated basolateral Na,K,2Cl-cotransporter (NKCC) activity during 6 h hypoxia/aglycemia treatment” [134]. The activation of PKC was also applied as therapeutic strategy for treating Alzheimer’s disease because it directly decreased the formation of amyloid β [135]. It was reported that “the SUMOylation of PKC isoforms prevented them from activation and the synaptic SUMOylation levels were dynamically regulated by neuronal activity” [136]. “Sentrin-specific peptidase 1 (SENP1) mediated deSUMOylation of PKC” [136] should be a potential strategic drug target for promoting PKC’s effects. The inhibitors of other kinase may also be potential candidate drugs for controlling acute and chronic neurodegenerative diseases [137]. We therefore proposed that by fine tuning activity of PKC isoforms at downstream of different neurotransmitter receptor signal transduction common pathways, neuronal activity or degenerative process may be more effectively controlled than targeting on one or two neurotransmitter receptor(s) (Fig. 1). This approach may prevent epileptogenesis.
PKC has a well-established role in governing GABA receptor surface expression and receptor turnover. PKC activity may block α1β2γ2 receptor recycling to the cell surface [138]. Its inhibition induces activation of GABAA receptor, suggesting an existence of negative feedback relationship between PKC and GABAA receptor activation [139]. The antiepileptic effect of valproate has been related to PKCε inhibition induced GABAA receptor hypersensitivity in nucleus reticularis thalami [81]. On the other hand, PKC-induced prolongation of epileptiform bursts is dependent on changes specific to mGluR5, and not mediated simply by a generalized increase in transmitter release [80]. This study suggests that inhibition of PKC may not only induce GABAA receptor hypersensitivity, but also reduce mGluR5 related epileptiform bursts, and therefore, it may be an ideal therapeutic target to prevent epileptogenesis. However, it has to emphasize that due to numerous functionally different PKC isoforms, activation of each PKC isoform may produce different effects. In addition, the timing and precise location of the PKC activation may also affect therapeutic effect. Therefore, further study is still needed to find candidate drugs to activate PKC in a targeted and isoform-specific manner in order to effectively prevent epileptogenesis.
The involvement of mGluRs interacting protein CaM in epilepsy and epileptogenesis has been well documented. “Calmodulin-mediated processes play important roles in the development of altered neuronal excitability and in some forms of seizure disorders” [140]. “Decreased calmodulin-NMDAR1 co-assembly contributes to hyperexcitability in dysplastic cortical neurons and in focal seizure onsets” [141]. Inhibition of calcium and calmodulin-dependent kinase II (CaMKII) activity occurred in the rat status epilepticus model, which involved NMDA receptor activation [142], as pretreatment with MK-801 blocked inhibition in CaM kinase II activity and the development of epilepsy [143]. In in vitro model, “Ca2+ influxes through L-type voltage-dependent- and NMDA receptor-dependent-Ca2+ channels contribute to the development of a kindling-like state which was also mediated by CaMKII-dependent mechanisms” [144, 145]. In vivo study also demonstrated that “the loss of CaMKII observed with multiple pathological states in the central nervous system, including epilepsy, brain trauma, and ischemia, likely exacerbated programmed cell death by sensitizing vulnerable neuronal populations to excitotoxic glutamate signaling and inducing an excitotoxic insult itself” [146]. Overexpression of CaM increased K+ efflux through CaM-dependent voltage-gated K+ channels [147]. The dysfunction of Ca2+/CaM-dependent protein kinase II (CaMKII) may result in various neuropsychiatric disorders such as epilepsy through maladaptations in glutamate signaling and neuroplasticity [148]. By targeting CaMKII, microRNA 219 (miR-219) has been shown to negatively regulate the function of NMDA receptors and then protect against seizure in kainic acid induced mouse model of epilepsy [149]. Phenytoin, carbamazepine, and the benzodiazepines have been reported to reduce the pre-synaptic glutamate release by inhibiting Ca2+/CaM-dependent phosphorylation of membrane proteins [140]. CaM binding with mGluR5 promoted by Ca2+ competitively occupied the inactive CaMKII binding site in mGluR5 [150]. The dissociated CaMKII was then activated and phosphorylated adjacent NMDA receptor. As mentioned above, the phosphorylation of mGluR5 by active PKC could also be inhibited by binding with CaM in a Ca2+ dependent manner. Recent studies suggest that mGluR interacting protein Siah1 [29], Norbin [92] and PICK1 [151] which work as competitive inhibitors of CaM may be potential therapeutic anti-epileptic and/or anti-epileptogenic targets. It is therefore reasonable to believe that targeting on CaM may produce significant antiepileptic and/ or antiepileptogenic effect.
CONCLUSION
Accumulated data suggest that agonists or antagonists of mGluRs, especially group I mGluR antagonists are promising candidate drugs to control seizures and subsequent neurodegeneration, i.e., to prevent epileptogenesis. The interacting or down-stream proteins of mGluRs such as Homers, PKCs and CaM may be more promising therapeutic targets due to the fact that these proteins are shared by different neurotransmitter receptor signal transduction pathways, i.e, serve as common signal transduction molecules (Fig. 1). For instance, inhibition of PKC may not only reduce mGluR5- related neuronal hyperactivity, but also induce GABAA receptor hypersensitivity. Therefore, PKC inhibitor may be more effective in controlling epilepto- genesis than single mGluR5 antagonist or GABAA receptor agonist. By careful selection of isoforms of PKCs as therapeutic target, we may effectively prevent epileptogenesis and meanwhile significantly reduce side–effect caused by targeting on either excitatory or inhibitory neurotransmitter system, as the latter may result in imbalanced inhibition or excitation of the epileptic brain activity. Nevertheless, the variation of the expression of mGluRs in the pathological brain, complicated interactions of mGluRs and their interacting proteins, doses of mGluR agonists or antagonists, stages of the epileptogenesis may significantly affect therapeutic effects of mGluR agonists or antagonists, and inhibitors or promoters of mGluR interacting proteins. Comprehensive evaluation of patient’s pathological brain, careful designing therapeutic approaches and fine-tuning the doses of candidate drugs may therefore be needed to effectively control epileptogenesis.
ACKNOWLEDGEMENTS
This work was supported by grants from the Sciences Foundation of the Hubei Provincial Department of Education (Q20141301) to Dr. F. Qian, Singapore NMRC grants (No: NMRC/0960/2005) and Singhealth Research Foundation (Nos: SHF/FG217P/2005 and SHF/FG382P/2007) to Dr. F.R. Tang, Singapore National Research Foundation to Singapore Nuclear Research and Safety initiative, National University of Singapore, Singapore.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
REFERENCES
- 1.Enz R. Metabotropic glutamate receptors and interacting proteins: evolving drug targets. Curr. Drug Targets. 2012;13(1):145–156. doi: 10.2174/138945012798868452. [http://dx.doi.org/10.2174/138945012798868452]. [PMID: 21777188]. [DOI] [PubMed] [Google Scholar]
- 2.Sladeczek F., Pin J.P., Récasens M., Bockaert J., Weiss S. Glutamate stimulates inositol phosphate formation in striatal neurones. Nature. 1985;317(6039):717–719. doi: 10.1038/317717a0. [http://dx.doi.org/ 10.1038/317717a0]. [PMID: 2865680]. [DOI] [PubMed] [Google Scholar]
- 3.Cotman C.W., Flatman J.A., Ganong A.H., Perkins M.N. Effects of excitatory amino acid antagonists on evoked and spontaneous excitatory potentials in guinea-pig hippocampus. J. Physiol. 1986;378:403–415. doi: 10.1113/jphysiol.1986.sp016227. [http://dx.doi.org/10.1113/jphysiol.1986.sp016227]. [PMID: 3795109]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nicoletti F., Wroblewski J.T., Novelli A., Alho H., Guidotti A., Costa E. The activation of inositol phospholipid metabolism as a signal-transducing system for excitatory amino acids in primary cultures of cerebellar granule cells. J. Neurosci. 1986;6(7):1905–1911. doi: 10.1523/JNEUROSCI.06-07-01905.1986. [PMID: 3016212]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Masu M., Tanabe Y., Tsuchida K., Shigemoto R., Nakanishi S. Sequence and expression of a metabotropic glutamate receptor. Nature. 1991;349(6312):760–765. doi: 10.1038/349760a0. [http://dx.doi.org/10.1038/ 349760a0]. [PMID: 1847995]. [DOI] [PubMed] [Google Scholar]
- 6.Houamed K.M., Kuijper J.L., Gilbert T.L., Haldeman B.A., O’Hara P.J., Mulvihill E.R., Almers W., Hagen F.S. Cloning, expression, and gene structure of a G protein-coupled glutamate receptor from rat brain. Science. 1991;252(5010):1318–1321. doi: 10.1126/science.1656524. [http://dx.doi.org/10.1126/science.1656524]. [PMID: 1656524]. [DOI] [PubMed] [Google Scholar]
- 7.Conn P.J., Pin J.P. Pharmacology and functions of metabotropic glutamate receptors. Annu. Rev. Pharmacol. Toxicol. 1997;37:205–237. doi: 10.1146/annurev.pharmtox.37.1.205. [http://dx.doi.org/10.1146/annurev.pharmtox.37.1.205]. [PMID: 9131252]. [DOI] [PubMed] [Google Scholar]
- 8.Tang F.R., Bradford H.F., Ling E.A. Metabotropic glutamate receptors in the control of neuronal activity and as targets for development of anti-epileptogenic drugs. Curr. Med. Chem. 2009;16(17):2189–2204. doi: 10.2174/092986709788612710. [http://dx.doi.org/10.2174/092986709788612710]. [PMID: 19519386]. [DOI] [PubMed] [Google Scholar]
- 9.Lüscher C., Huber K.M. Group 1 mGluR-dependent synaptic long-term depression: mechanisms and implications for circuitry and disease. Neuron. 2010;65(4):445–459. doi: 10.1016/j.neuron.2010.01.016. [http://dx.doi.org/10. 1016/j.neuron.2010.01.016]. [PMID: 20188650]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anwyl R. Metabotropic glutamate receptor-dependent long-term potentiation. Neuropharmacology. 2009;56(4):735–740. doi: 10.1016/j.neuropharm.2009.01.002. [http:// dx.doi.org/10.1016/j.neuropharm.2009.01.002]. [PMID: 19705571]. [DOI] [PubMed] [Google Scholar]
- 11.Pan Y.Z., Rutecki P.A. Enhanced excitatory synaptic network activity following transient group I metabotropic glutamate activation. Neuroscience. 2014;275:22–32. doi: 10.1016/j.neuroscience.2014.05.062. [http://dx.doi.org/10.1016/ j.neuroscience.2014.05.062]. [PMID: 24928353]. [DOI] [PubMed] [Google Scholar]
- 12.Tang F.R., Lee W.L., Yang J., Sim M.K., Ling E.A. Expression of metabotropic glutamate receptor 1alpha in the hippocampus of rat pilocarpine model of status epilepticus. Epilepsy Res. 2001;46(2):179–189. doi: 10.1016/s0920-1211(01)00276-5. [http://dx.doi.org/10.1016/S0920-1211(01)00276-5]. [PMID: 11463519]. [DOI] [PubMed] [Google Scholar]
- 13.Akbar M.T., Rattray M., Powell J.F., Meldrum B.S. Altered expression of group I metabotropic glutamate receptors in the hippocampus of amygdala-kindled rats. Brain Res. Mol. Brain Res. 1996;43(1-2):105–116. doi: 10.1016/s0169-328x(96)00162-3. [http://dx.doi.org/10.1016/S0169-328X(96)00162-3]. [PMID: 9037524]. [DOI] [PubMed] [Google Scholar]
- 14.Bianchi R., Wong R.K., Merlin L.R. Glutamate Receptors in Epilepsy: Group I mGluR-Mediated Epileptogenesis. In: Noebels J.L., Avoli M., Rogawski M.A., Olsen R.W., Delgado-Escueta A.V., editors. Jasper's Basic Mechanisms of the Epilepsies. Bethesda, MD: 2012. [http://dx.doi.org/10.1093/med/9780199746545.003.0011] [PubMed] [Google Scholar]
- 15.Attwell P.J., Koumentaki A., Abdul-Ghani A.S., Croucher M.J., Bradford H.F. Specific group II metabotropic glutamate receptor activation inhibits the development of kindled epilepsy in rats. Brain Res. 1998;787(2):286–291. doi: 10.1016/s0006-8993(97)01500-x. [http://dx.doi.org/10.1016/ S0006-8993(97)01500-X]. [PMID: 9518652]. [DOI] [PubMed] [Google Scholar]
- 16.Attwell P.J., Singh Kent N., Jane D.E., Croucher M.J., Bradford H.F. Anticonvulsant and glutamate release-inhibiting properties of the highly potent metabotropic glutamate receptor agonist (2S,2’R, 3’R)-2-(2′,3′-dicarboxycyclopropyl)glycine (DCG-IV). Brain Res. 1998;805(1-2):138–143. doi: 10.1016/s0006-8993(98)00698-2. [http://dx.doi.org/10.1016/S0006-8993(98)00698-2]. [PMID: 9733953]. [DOI] [PubMed] [Google Scholar]
- 17.Watanabe Y., Kaida Y., Fukuhara S., Takechi K., Uehara T., Kamei C. Participation of metabotropic glutamate receptors in pentetrazol-induced kindled seizure. Epilepsia. 2011;52(1):140–150. doi: 10.1111/j.1528-1167.2010.02764.x. [http://dx.doi.org/10.1111/j.1528-1167.2010.02764.x]. [PMID: 21054350]. [DOI] [PubMed] [Google Scholar]
- 18.Rohde J., Kirschstein T., Wilkars W., Müller L., Tokay T., Porath K., Bender R.A., Köhling R. Upregulation of presynaptic mGluR2, but not mGluR3 in the epileptic medial perforant path. Neuropharmacology. 2012;62(4):1867–1873. doi: 10.1016/j.neuropharm.2011.12.012. [http://dx.doi.org/ 10.1016/j.neuropharm.2011.12.012]. [PMID: 22202905]. [DOI] [PubMed] [Google Scholar]
- 19.Abdul-Ghani A.S., Attwell P.J., Singh Kent N., Bradford H.F., Croucher M.J., Jane D.E. Anti-epileptogenic and anticonvulsant activity of L-2-amino-4-phosphonobutyrate, a presynaptic glutamate receptor agonist. Brain Res. 1997;755(2):202–212. doi: 10.1016/s0006-8993(97)00098-x. [http://dx.doi. org/10.1016/S0006-8993(97)00098-X]. [PMID: 9175888]. [DOI] [PubMed] [Google Scholar]
- 20.Pacheco Otalora L.F., Skinner F., Oliveira M.S., Farrell B., Arshadmansab M.F., Pandari T., Garcia I., Robles L., Rosas G., Mello C.F., Ermolinsky B.S., Garrido-Sanabria E.R. Chronic deficit in the expression of voltage-gated potassium channel Kv3.4 subunit in the hippocampus of pilocarpine-treated epileptic rats. Brain Res. 2011;1368:308–316. doi: 10.1016/j.brainres.2010.10.047. [http://dx.doi.org/10.1016/ j.brainres.2010.10.047]. [PMID: 20971086]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Caulder E.H., Riegle M.A., Godwin D.W. Activation of group 2 metabotropic glutamate receptors reduces behavioral and electro- graphic correlates of pilocarpine induced status epilepticus. Epilepsy Res. 2014;108(2):171–181. doi: 10.1016/j.eplepsyres.2013.10.009. [http://dx.doi.org/10.1016/j.eplepsyres. 2013.10.009]. [PMID: 24305700]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mao L.M., Liu X.Y., Zhang G.C., Chu X.P., Fibuch E.E., Wang L.S., Liu Z., Wang J.Q. Phosphorylation of group I metabotropic glutamate receptors (mGluR1/5) in vitro and in vivo. Neuropharmacology. 2008;55(4):403–408. doi: 10.1016/j.neuropharm.2008.05.034. [http://dx.doi.org/ 10.1016/j.neuropharm.2008.05.034]. [PMID: 18585398]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Enz R. The trick of the tail: protein-protein interactions of metabotropic glutamate receptors. BioEssays. 2007;29(1):60–73. doi: 10.1002/bies.20518. [http://dx.doi.org/10.1002/bies.20518]. [PMID: 17187376]. [DOI] [PubMed] [Google Scholar]
- 24.Aramori I., Nakanishi S. Signal transduction and pharmacological characteristics of a metabotropic glutamate receptor, mGluR1, in transfected CHO cells. Neuron. 1992;8(4):757–765. doi: 10.1016/0896-6273(92)90096-v. [http://dx.doi. org/10.1016/0896-6273(92)90096-V]. [PMID: 1314623]. [DOI] [PubMed] [Google Scholar]
- 25.Liu J.X., Tang Y.C., Liu Y., Tang F.R. Status epilepticus alters hippocampal PKAbeta and PKAgamma expression in mice. Seizure. 2010;19(7):414–420. doi: 10.1016/j.seizure.2010.06.008. [http://dx.doi.org/10.1016/j.seizure.2010.06. 008]. [PMID: 20630779]. [DOI] [PubMed] [Google Scholar]
- 26.Liu J.X., Liu Y., Tang F.R. Pilocarpine-induced status epilepticus alters hippocampal PKC expression in mice. Acta Neurobiol. Exp. (Warsz.) 2011;71(2):220–232. doi: 10.55782/ane-2011-1842. [PMID: 21731076]. [DOI] [PubMed] [Google Scholar]
- 27.Croci C., Sticht H., Brandstätter J.H., Enz R. Group I meta- botropic glutamate receptors bind to protein phosphatase 1C. Mapping and modeling of interacting sequences. J. Biol. Chem. 2003;278(50):50682–50690. doi: 10.1074/jbc.M305764200. [http://dx.doi.org/10.1074/jbc. M305764200]. [PMID: 14519764]. [DOI] [PubMed] [Google Scholar]
- 28.Flajolet M., Rakhilin S., Wang H., Starkova N., Nuangchamnong N., Nairn A.C., Greengard P. Protein phosphatase 2C binds selectively to and dephosphorylates metabotropic glutamate receptor 3. Proc. Natl. Acad. Sci. USA. 2003;100(26):16006–16011. doi: 10.1073/pnas.2136600100. [http://dx.doi.org/10.1073/pnas. 2136600100]. [PMID: 14663150]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ko S.J., Isozaki K., Kim I., Lee J.H., Cho H.J., Sohn S.Y., Oh S.R., Park S., Kim D.G., Kim C.H., Roche K.W. PKC phosphorylation regulates mGluR5 trafficking by enhancing binding of Siah-1A. J. Neurosci. 2012;32(46):16391–16401. doi: 10.1523/JNEUROSCI.1964-12.2012. [http://dx.doi.org/10.1523/JNEUROSCI.1964-12.2012]. [PMID: 23152621]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ferreira L.T., Dale L.B., Ribeiro F.M., Babwah A.V., Pampillo M., Ferguson S.S. Calcineurin inhibitor protein (CAIN) attenuates Group I metabotropic glutamate receptor endocytosis and signaling. J. Biol. Chem. 2009;284(42):28986–28994. doi: 10.1074/jbc.M109.050872. [http://dx.doi.org/10. 1074/jbc.M109.050872]. [PMID: 19717561]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ciruela F., Canela L., Burgueño J., Soriguera A., Cabello N., Canela E.I., Casadó V., Cortés A., Mallol J., Woods A.S., Ferré S., Lluis C., Franco R. Heptaspanning membrane receptors and cytoskeletal/scaffolding proteins: focus on adenosine, dopamine, and metabotropic glutamate receptor function. J. Mol. Neurosci. 2005;26(2-3):277–292. doi: 10.1385/JMN:26:2-3:277. [http://dx.doi.org/10.1385/JMN:26:2-3:277]. [PMID: 16012201]. [DOI] [PubMed] [Google Scholar]
- 32.Doumazane E., Scholler P., Zwier J.M., Trinquet E., Rondard P., Pin J.P. A new approach to analyze cell surface protein complexes reveals specific heterodimeric metabotropic glutamate receptors. FASEB J. 2011;25(1):66–77. doi: 10.1096/fj.10-163147. [http://dx.doi.org/10. 1096/fj.10-163147]. [PMID: 20826542]. [DOI] [PubMed] [Google Scholar]
- 33.Paquet M., Ribeiro F.M., Guadagno J., Esseltine J.L., Ferguson S.S., Cregan S.P. Role of metabotropic glutamate receptor 5 signaling and homer in oxygen glucose deprivation-mediated astrocyte apoptosis. Mol. Brain. 2013;6:9. doi: 10.1186/1756-6606-6-9. [http://dx.doi.org/ 10.1186/1756-6606-6-9]. [PMID: 23406666]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Renner M., Specht C.G., Triller A. Molecular dynamics of postsynaptic receptors and scaffold proteins. Curr. Opin. Neurobiol. 2008;18(5):532–540. doi: 10.1016/j.conb.2008.09.009. [http://dx.doi.org/10.1016/j.conb.2008.09. 009]. [PMID: 18832033]. [DOI] [PubMed] [Google Scholar]
- 35.Schütt J., Falley K., Richter D., Kreienkamp H.J., Kindler S. Fragile X mental retardation protein regulates the levels of scaffold proteins and glutamate receptors in postsynaptic densities. J. Biol. Chem. 2009;284(38):25479–25487. doi: 10.1074/jbc.M109.042663. [http://dx.doi.org/10.1074/ jbc.M109.042663]. [PMID: 19640847]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li Y., Krogh K.A., Thayer S.A. Epileptic stimulus increases Homer 1a expression to modulate endocannabinoid signaling in cultured hippocampal neurons. Neuropharmacology. 2012;63(6):1140–1149. doi: 10.1016/j.neuropharm.2012.07.014. [http://dx.doi.org/10.1016/j.neuropharm.2012.07.014]. [PMID: 22814532]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cavarsan C.F., Tescarollo F., Tesone-Coelho C., Morais R.L., Motta F.L., Blanco M.M., Mello L.E. Pilocarpine-induced status epilepticus increases Homer1a and changes mGluR5 expression. Epilepsy Res. 2012;101(3):253–260. doi: 10.1016/j.eplepsyres.2012.04.011. [http://dx.doi.org/10.1016/ j.eplepsyres.2012.04.011]. [PMID: 22591751]. [DOI] [PubMed] [Google Scholar]
- 38.Bockaert J., Perroy J., Bécamel C., Marin P., Fagni L. GPCR interacting proteins (GIPs) in the nervous system: Roles in physiology and pathologies. Annu. Rev. Pharmacol. Toxicol. 2010;50:89–109. doi: 10.1146/annurev.pharmtox.010909.105705. [http://dx.doi.org/10.1146/annurev.pharmtox.010909. 105705]. [PMID: 20055699]. [DOI] [PubMed] [Google Scholar]
- 39.Bozzi Y., Casarosa S., Caleo M. Epilepsy as a neuro- developmental disorder. Front. Psychiatry. 2012;3:19. doi: 10.3389/fpsyt.2012.00019. [http:// dx.doi.org/10.3389/fpsyt.2012.00019]. [PMID: 22457654]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tang F.R., Chen P.M., Tang Y.C., Tsai M.C., Lee W.L. Two-methyl-6-phenylethynyl-pyridine (MPEP), a metabotropic glutamate receptor 5 antagonist, with low doses of MK801 and diazepam: a novel approach for controlling status epilepticus. Neuropharmacology. 2007;53(7):821–831. doi: 10.1016/j.neuropharm.2007.08.012. [http://dx.doi.org/10.1016/ j.neuropharm.2007.08.012]. [PMID: 17904168]. [DOI] [PubMed] [Google Scholar]
- 41.DeLorenzo R.J., Sun D.A., Blair R.E., Sombati S. An in vitro model of stroke-induced epilepsy: elucidation of the roles of glutamate and calcium in the induction and maintenance of stroke-induced epileptogenesis. Int. Rev. Neurobiol. 2007;81:59–84. doi: 10.1016/S0074-7742(06)81005-6. [http://dx.doi.org/10.1016/S0074-7742(06)81005-6]. [PMID: 17433918]. [DOI] [PubMed] [Google Scholar]
- 42.Zhao W., Chuang S.C., Young S.R., Bianchi R., Wong R.K. Extracellular glutamate exposure facilitates group I mGluR-mediated epileptogenesis in the hippocampus. J. Neurosci. 2015;35(1):308–315. doi: 10.1523/JNEUROSCI.1944-14.2015. [http://dx.doi.org/10.1523/JNEUROSCI.1944-14.2015]. [PMID: 25568123]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tang F.R. Agonists and antagonists of metabotropic glutamate receptors: anticonvulsants and antiepileptogenic agents? Curr. Neuropharmacol. 2005;3(4):299–307. doi: 10.2174/157015905774322525. [http://dx.doi.org/10.2174/ 157015905774322525]. [PMID: 18369399]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Blümcke I., Becker A.J., Klein C., Scheiwe C., Lie A.A., Beck H., Waha A., Friedl M.G., Kuhn R., Emson P., Elger C., Wiestler O.D. Temporal lobe epilepsy associated up-regulation of metabotropic glutamate receptors: correlated changes in mGluR1 mRNA and protein expression in experimental animals and human patients. J. Neuropathol. Exp. Neurol. 2000;59(1):1–10. doi: 10.1093/jnen/59.1.1. [http://dx. doi.org/10.1093/jnen/59.1.1]. [PMID: 10744030]. [DOI] [PubMed] [Google Scholar]
- 45.Al-Ghoul W.M., Meeker R.B., Greenwood R.S. Kindled seizures increase metabotropic glutamate receptor expression and function in the rat supraoptic nucleus. J. Neurosci. Res. 1998;54(3):412–423. doi: 10.1002/(SICI)1097-4547(19981101)54:3<412::AID-JNR12>3.0.CO;2-E. [http://dx.doi.org/10.1002/(SICI)1097-4547(19981101) 54:3<412:AID-JNR12>3.0.CO;2-E]. [PMID: 9819146]. [DOI] [PubMed] [Google Scholar]
- 46.Tang F.R., Chia S.C., Chen P.M., Gao H., Lee W.L., Yeo T.S., Burgunder J.M., Probst A., Sim M.K., Ling E.A. Metabotropic glutamate receptor 2/3 in the hippocampus of patients with mesial temporal lobe epilepsy, and of rats and mice after pilocarpine-induced status epilepticus. Epilepsy Res. 2004;59(2-3):167–180. doi: 10.1016/j.eplepsyres.2004.04.002. [http://dx.doi.org/10.1016/j.eplepsyres.2004.04.002]. [PMID: 15246118]. [DOI] [PubMed] [Google Scholar]
- 47.Chen J., Larionov S., Pitsch J., Hoerold N., Ullmann C., Elger C.E., Schramm J., Becker A.J. Expression analysis of metabotropic glutamate receptors I and III in mouse strains with different susceptibility to experimental temporal lobe epilepsy. Neurosci. Lett. 2005;375(3):192–197. doi: 10.1016/j.neulet.2004.11.008. [http://dx.doi.org/10.1016/ j.neulet.2004.11.008]. [PMID: 15694259]. [DOI] [PubMed] [Google Scholar]
- 48.Guilfoyle D.N., Gerum S., Vadasz C. In vivo Proton NMR spectroscopy of genetic mouse models BALB/cJ and C57BL/6By: variation in hippocampal glutamate level and the metabotropic glutamate receptor, subtype 7 (Grm7) gene. J. Mol. Neurosci. 2014;53(1):135–141. doi: 10.1007/s12031-013-0211-5. [http://dx.doi.org/10.1007/s12031-013-0211-5]. [PMID: 24390354]. [DOI] [PubMed] [Google Scholar]
- 49.Tang F.R., Lee W.L., Yang J., Sim M.K., Ling E.A. Metabotropic glutamate receptor 8 in the rat hippocampus after pilocarpine induced status epilepticus. Neurosci. Lett. 2001;300(3):137–140. doi: 10.1016/s0304-3940(01)01579-8. [http://dx.doi.org/10.1016/S0304-3940(01)01579-8]. [PMID: 11226630]. [DOI] [PubMed] [Google Scholar]
- 50.Bough K.J., Mott D.D., Dingledine R.J. Medial perforant path inhibition mediated by mGluR7 is reduced after status epilepticus. J. Neurophysiol. 2004;92(3):1549–1557. doi: 10.1152/jn.00315.2004. [http://dx.doi.org/ 10.1152/jn.00315.2004]. [PMID: 15152022]. [DOI] [PubMed] [Google Scholar]
- 51.Ermolinsky B., Pacheco Otalora L.F., Arshadmansab M.F., Zarei M.M., Garrido-Sanabria E.R. Differential changes in mGlu2 and mGlu3 gene expression following pilocarpine-induced status epilepticus: a comparative real-time PCR analysis. Brain Res. 2008;1226:173–180. doi: 10.1016/j.brainres.2008.05.073. [http://dx.doi.org/10.1016/j.brainres.2008. 05.073]. [PMID: 18585369]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jiang F.L., Tang Y.C., Chia S.C., Jay T.M., Tang F.R. Anticonvulsive effect of a selective mGluR8 agonist (S)-3,4-dicarboxyphenylglycine (S-3,4-DCPG) in the mouse pilocarpine model of status epilepticus. Epilepsia. 2007;48(4):783–792. doi: 10.1111/j.1528-1167.2007.01000.x. [http://dx.doi.org/10.1111/j.1528-1167.2007.01000.x]. [PMID: 17430409]. [DOI] [PubMed] [Google Scholar]
- 53.Das A., Wallace G.C., IV, Holmes C., McDowell M.L., Smith J.A., Marshall J.D., Bonilha L., Edwards J.C., Glazier S.S., Ray S.K., Banik N.L. Hippocampal tissue of patients with refractory temporal lobe epilepsy is associated with astrocyte activation, inflammation, and altered expression of channels and receptors. Neuroscience. 2012;220:237–246. doi: 10.1016/j.neuroscience.2012.06.002. [http://dx.doi.org/10.1016/ j.neuroscience.2012.06.002]. [PMID: 22698689]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kandratavicius L., Rosa-Neto P., Monteiro M.R., Guiot M.C., Assirati J.A., Jr, Carlotti C.G., Jr, Kobayashi E., Leite J.P. Distinct increased metabotropic glutamate receptor type 5 (mGluR5) in temporal lobe epilepsy with and without hippocampal sclerosis. Hippocampus. 2013;23(12):1212–1230. doi: 10.1002/hipo.22160. [http://dx.doi. org/10.1002/hipo.22160]. [PMID: 23804486]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lie A.A., Becker A., Behle K., Beck H., Malitschek B., Conn P.J., Kuhn R., Nitsch R., Plaschke M., Schramm J., Elger C.E., Wiestler O.D., Blümcke I. Up-regulation of the metabotropic glutamate receptor mGluR4 in hippocampal neurons with reduced seizure vulnerability. Ann. Neurol. 2000;47(1):26–35. [http://dx. doi.org/10.1002/1531-8249(200001)47:1<26:AID-ANA7>3.0.CO;2-P]. [PMID: 10632098]. [PubMed] [Google Scholar]
- 56.Notenboom R.G., Hampson D.R., Jansen G.H., van Rijen P.C., van Veelen C.W., van Nieuwenhuizen O., de Graan P.N. Up-regulation of hippocampal metabotropic glutamate receptor 5 in temporal lobe epilepsy patients. Brain. 2006;129(Pt 1):96–107. doi: 10.1093/brain/awh673. [http://dx.doi.org/10.1093/brain/awh673]. [PMID: 16311265]. [DOI] [PubMed] [Google Scholar]
- 57.Tang F.R., Lee W.L. Expression of the group II and III metabotropic glutamate receptors in the hippocampus of patients with mesial temporal lobe epilepsy. J. Neurocytol. 2001;30(2):137–143. doi: 10.1023/a:1011939223872. [http://dx.doi.org/10.1023/A:1011939223872]. [PMID: 11577252]. [DOI] [PubMed] [Google Scholar]
- 58.Grigorenko E., Glazier S., Bell W., Tytell M., Nosel E., Pons T., Deadwyler S.A. Changes in glutamate receptor subunit composition in hippocampus and cortex in patients with refractory epilepsy. J. Neurol. Sci. 1997;153(1):35–45. doi: 10.1016/s0022-510x(97)00180-9. [http://dx.doi.org/10. 1016/S0022-510X(97)00180-9]. [PMID: 9455976]. [DOI] [PubMed] [Google Scholar]
- 59.Camón L., Vives P., de Vera N., Martínez E. Seizures and neuronal damage induced in the rat by activation of group I metabotropic glutamate receptors with their selective agonist 3,5-dihydroxyphenylglycine. J. Neurosci. Res. 1998;51(3):339–348. doi: 10.1002/(SICI)1097-4547(19980201)51:3<339::AID-JNR7>3.0.CO;2-H. [http://dx.doi.org/10.1002/(SICI)1097-4547(19980201)51:3<339: AID-JNR7>3.0.CO;2-H]. [PMID: 9486769]. [DOI] [PubMed] [Google Scholar]
- 60.Aronica E., Gorter J.A., Jansen G.H., van Veelen C.W., van Rijen P.C., Ramkema M., Troost D. Expression and cell distribution of group I and group II metabotropic glutamate receptor subtypes in taylor-type focal cortical dysplasia. Epilepsia. 2003;44(6):785–795. doi: 10.1046/j.1528-1157.2003.54802.x. [http://dx.doi.org/10.1046/j.1528-1157.2003. 54802.x]. [PMID: 12790891]. [DOI] [PubMed] [Google Scholar]
- 61.Worley P.F., Zeng W., Huang G., Kim J.Y., Shin D.M., Kim M.S., Yuan J.P., Kiselyov K., Muallem S. Homer proteins in Ca2+ signaling by excitable and non-excitable cells. Cell Calcium. 2007;42(4-5):363–371. doi: 10.1016/j.ceca.2007.05.007. [http://dx.doi.org/10.1016/j.ceca.2007. 05.007]. [PMID: 17618683]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hayashi M.K., Tang C., Verpelli C., Narayanan R., Stearns M.H., Xu R.M., Li H., Sala C., Hayashi Y. The postsynaptic density proteins Homer and Shank form a polymeric network structure. Cell. 2009;137(1):159–171. doi: 10.1016/j.cell.2009.01.050. [http://dx.doi.org/10.1016/ j.cell.2009.01.050]. [PMID: 19345194]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nakamura M., Sato K., Fukaya M., Araishi K., Aiba A., Kano M., Watanabe M. Signaling complex formation of phospholipase Cbeta4 with metabotropic glutamate receptor type 1alpha and 1,4,5-trisphosphate receptor at the perisynapse and endoplasmic reticulum in the mouse brain. Eur. J. Neurosci. 2004;20(11):2929–2944. doi: 10.1111/j.1460-9568.2004.03768.x. [http://dx.doi.org/10.1111/j.1460-9568.2004.03768.x]. [PMID: 15579147]. [DOI] [PubMed] [Google Scholar]
- 64.Sakagami Y., Yamamoto K., Sugiura S., Inokuchi K., Hayashi T., Kato N. Essential roles of Homer-1a in homeostatic regulation of pyramidal cell excitability: a possible link to clinical benefits of electroconvulsive shock. Eur. J. Neurosci. 2005;21(12):3229–3239. doi: 10.1111/j.1460-9568.2005.04165.x. [http://dx.doi.org/10.1111/j.1460-9568.2005.04165.x]. [PMID: 16026461]. [DOI] [PubMed] [Google Scholar]
- 65.Potschka H., Krupp E., Ebert U., Gümbel C., Leichtlein C., Lorch B., Pickert A., Kramps S., Young K., Grüne U., Keller A., Welschof M., Vogt G., Xiao B., Worley P.F., Löscher W., Hiemisch H. Kindling-induced overexpression of Homer 1A and its functional implications for epileptogenesis. Eur. J. Neurosci. 2002;16(11):2157–2165. doi: 10.1046/j.1460-9568.2002.02265.x. [http://dx.doi.org/10.1046/j.1460-9568. 2002.02265.x]. [PMID: 12473083]. [DOI] [PubMed] [Google Scholar]
- 66.Hu J.H., Park J.M., Park S., Xiao B., Dehoff M.H., Kim S., Hayashi T., Schwarz M.K., Huganir R.L., Seeburg P.H., Linden D.J., Worley P.F. Homeostatic scaling requires group I mGluR activation mediated by Homer1a. Neuron. 2010;68(6):1128–1142. doi: 10.1016/j.neuron.2010.11.008. [http://dx.doi.org/10.1016/j.neuron.2010.11.008]. [PMID: 21172614]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Maejima T., Hashimoto K., Yoshida T., Aiba A., Kano M. Presynaptic inhibition caused by retrograde signal from metabotropic glutamate to cannabinoid receptors. Neuron. 2001;31(3):463–475. doi: 10.1016/s0896-6273(01)00375-0. [http://dx.doi.org/10.1016/S0896-6273(01)00375-0]. [PMID: 11516402]. [DOI] [PubMed] [Google Scholar]
- 68.Karr L., Pan Y.Z., Rutecki P.A. CB1 receptor antagonism impairs the induction of epileptiform activity by group I metabotropic glutamate receptor activation. Epilepsia. 2010;51(Suppl. 3):121–125. doi: 10.1111/j.1528-1167.2010.02625.x. [http://dx.doi.org/10.1111/j.1528-1167. 2010.02625.x]. [PMID: 20618416]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Maejima T., Oka S., Hashimotodani Y., Ohno-Shosaku T., Aiba A., Wu D., Waku K., Sugiura T., Kano M. Synaptically driven endocannabinoid release requires Ca2+-assisted metabotropic glutamate receptor subtype 1 to phospholipase Cbeta4 signaling cascade in the cerebellum. J. Neurosci. 2005;25(29):6826–6835. doi: 10.1523/JNEUROSCI.0945-05.2005. [http://dx.doi.org/10.1523/JNEUROSCI.0945-05.2005]. [PMID: 16033892]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kim D., Jun K.S., Lee S.B., Kang N.G., Min D.S., Kim Y.H., Ryu S.H., Suh P.G., Shin H.S. Phospholipase C isozymes selectively couple to specific neurotransmitter receptors. Nature. 1997;389(6648):290–293. doi: 10.1038/38508. [http://dx.doi.org/10.1038/38508]. [PMID: 9305844]. [DOI] [PubMed] [Google Scholar]
- 71.Park D., Lee S., Jun K., Hong Y.M., Kim D.Y., Kim Y.I., Shin H.S. Translation of clock rhythmicity into neural firing in suprachiasmatic nucleus requires mGluR-PLCbeta4 signaling. Nat. Neurosci. 2003;6(4):337–338. doi: 10.1038/nn1033. [http://dx.doi.org/10.1038/nn1033]. [PMID: 12640460]. [DOI] [PubMed] [Google Scholar]
- 72.Hannan A.J., Blakemore C., Katsnelson A., Vitalis T., Huber K.M., Bear M., Roder J., Kim D., Shin H.S., Kind P.C. PLC-beta1, activated via mGluRs, mediates activity-dependent differentiation in cerebral cortex. Nat. Neurosci. 2001;4(3):282–288. doi: 10.1038/85132. [http://dx.doi.org/10.1038/85132]. [PMID: 11224545]. [DOI] [PubMed] [Google Scholar]
- 73.Domenici M.R., Pintor A., Potenza R.L., Gaudi S., Grò M.C., Passarelli F., Reggio R., Galluzzo M., Massotti M., Popoli P. Metabotropic glutamate receptor 5 (mGluR5)-mediated phosphoinositide hydrolysis and NMDA-potentiating effects are blunted in the striatum of aged rats: a possible additional mechanism in striatal senescence. Eur. J. Neurosci. 2003;17(10):2047–2055. doi: 10.1046/j.1460-9568.2003.02649.x. [http://dx.doi.org/10.1046/j.1460-9568.2003.02649.x]. [PMID: 12786971]. [DOI] [PubMed] [Google Scholar]
- 74.Chuang S.C., Bianchi R., Kim D., Shin H.S., Wong R.K. Group I metabotropic glutamate receptors elicit epileptiform discharges in the hippocampus through PLCbeta1 signaling. J. Neurosci. 2001;21(16):6387–6394. doi: 10.1523/JNEUROSCI.21-16-06387.2001. [PMID: 11487662]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cuellar J.C., Griffith E.L., Merlin L.R. Contrasting roles of protein kinase C in induction versus suppression of group I mGluR-mediated epileptogenesis in vitro. J. Neurophysiol. 2005;94(5):3643–3647. doi: 10.1152/jn.00548.2005. [http://dx.doi.org/10.1152/jn.00548.2005]. [PMID: 16049142]. [DOI] [PubMed] [Google Scholar]
- 76.Tang F.R., Lee W.L., Gao H., Chen Y., Loh Y.T., Chia S.C. Expression of different isoforms of protein kinase C in the rat hippocampus after pilocarpine-induced status epilepticus with special reference to CA1 area and the dentate gyrus. Hippocampus. 2004;14(1):87–98. doi: 10.1002/hipo.10156. [http://dx.doi.org/10.1002/hipo.10156]. [PMID: 15058486]. [DOI] [PubMed] [Google Scholar]
- 77.Liu J.X., Tang Y.C., Liu Y., Tang F.R. mGluR5-PLCbeta4-PKCbeta2/PKCgamma pathways in hippocampal CA1 pyramidal neurons in pilocarpine model of status epilepticus in mGluR5+/+ mice. Epilepsy Res. 2008;82(2-3):111–123. doi: 10.1016/j.eplepsyres.2008.07.014. [http://dx.doi.org/ 10.1016/j.eplepsyres.2008.07.014]. [PMID: 18774262]. [DOI] [PubMed] [Google Scholar]
- 78.Uchino M., Sakai N., Kashiwagi K., Shirai Y., Shinohara Y., Hirose K., Iino M., Yamamura T., Saito N. Isoform-specific phosphorylation of metabotropic glutamate receptor 5 by protein kinase C (PKC) blocks Ca2+ oscillation and oscillatory trans- location of Ca2+-dependent PKC. J. Biol. Chem. 2004;279(3):2254–2261. doi: 10.1074/jbc.M309894200. [http://dx.doi.org/10.1074/jbc.M309894200]. [PMID: 14561742]. [DOI] [PubMed] [Google Scholar]
- 79.Dale L.B., Babwah A.V., Bhattacharya M., Kelvin D.J., Ferguson S.S. Spatial-temporal patterning of metabotropic glutamate receptor-mediated inositol 1,4,5-triphosphate, calcium, and protein kinase C oscillations: protein kinase C-dependent receptor phosphorylation is not required. J. Biol. Chem. 2001;276(38):35900–35908. doi: 10.1074/jbc.M103847200. [http://dx.doi.org/10.1074/jbc.M103847200]. [PMID: 11461909]. [DOI] [PubMed] [Google Scholar]
- 80.Fuortes M.G., Faria L.C., Merlin L.R. Impact of protein kinase C activation on epileptiform activity in the hippocampal slice. Epilepsy Res. 2008;82(1):38–45. doi: 10.1016/j.eplepsyres.2008.07.002. [http://dx.doi.org/10.1016/j. eplepsyres.2008.07.002]. [PMID: 18715754]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Toth M. The epsilon theory: a novel synthesis of the underlying molecular and electrophysiological mechanisms of primary generalized epilepsy and the possible mechanism of action of valproate. Med. Hypotheses. 2005;64(2):267–272. doi: 10.1016/j.mehy.2004.07.019. [http://dx.doi. org/10.1016/j.mehy.2004.07.019]. [PMID: 15607553]. [DOI] [PubMed] [Google Scholar]
- 82.Turner J.H., Raymond J.R. Interaction of calmodulin with the serotonin 5-hydroxytryptamine2A receptor. A putative regulator of G protein coupling and receptor phosphorylation by protein kinase C. J. Biol. Chem. 2005;280(35):30741–30750. doi: 10.1074/jbc.M501696200. [http://dx.doi.org/ 10.1074/jbc.M501696200]. [PMID: 15970592]. [DOI] [PubMed] [Google Scholar]
- 83.Turner J.H., Garnovskaya M.N., Coaxum S.D., Vlasova T.M., Yakutovich M., Lefler D.M., Raymond J.R. Ca2+-calmodulin and janus kinase 2 are required for activation of sodium-proton exchange by the Gi-coupled 5-hydroxytryptamine 1a receptor. J. Pharmacol. Exp. Ther. 2007;320(1):314–322. doi: 10.1124/jpet.106.112581. [http://dx.doi.org/ 10.1124/jpet.106.112581]. [PMID: 17050776]. [DOI] [PubMed] [Google Scholar]
- 84.Navarro G., Aymerich M.S., Marcellino D., Cortés A., Casadó V., Mallol J., Canela E.I., Agnati L., Woods A.S., Fuxe K., Lluís C., Lanciego J.L., Ferré S., Franco R. Interactions between calmodulin, adenosine A2A, and dopamine D2 receptors. J. Biol. Chem. 2009;284(41):28058–28068. doi: 10.1074/jbc.M109.034231. [http://dx.doi.org/10.1074/ jbc.M109.034231]. [PMID: 19632986]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Xia Z., Storm D.R. The role of calmodulin as a signal integrator for synaptic plasticity. Nat. Rev. Neurosci. 2005;6(4):267–276. doi: 10.1038/nrn1647. [http://dx.doi.org/10.1038/nrn1647]. [PMID: 15803158]. [DOI] [PubMed] [Google Scholar]
- 86.Lee J.H., Lee J., Choi K.Y., Hepp R., Lee J.Y., Lim M.K., Chatani-Hinze M., Roche P.A., Kim D.G., Ahn Y.S., Kim C.H., Roche K.W. Calmodulin dynamically regulates the trafficking of the metabotropic glutamate receptor mGluR5. Proc. Natl. Acad. Sci. USA. 2008;105(34):12575–12580. doi: 10.1073/pnas.0712033105. [http://dx.doi.org/10.1073/ pnas.0712033105]. [PMID: 18715999]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Minakami R., Jinnai N., Sugiyama H. Phosphorylation and calmodulin binding of the metabotropic glutamate receptor subtype 5 (mGluR5) are antagonistic in vitro. J. Biol. Chem. 1997;272(32):20291–20298. doi: 10.1074/jbc.272.32.20291. [http://dx.doi.org/10.1074/jbc.272.32. 20291]. [PMID: 9242710]. [DOI] [PubMed] [Google Scholar]
- 88.Ishikawa K., Nash S.R., Nishimune A., Neki A., Kaneko S., Nakanishi S. Competitive interaction of seven in absentia homolog-1A and Ca2+/calmodulin with the cytoplasmic tail of group 1 metabotropic glutamate receptors. Genes Cells. 1999;4(7):381–390. doi: 10.1046/j.1365-2443.1999.00269.x. [http://dx.doi.org/10.1046/j.1365-2443.1999.00269.x]. [PMID: 10469171]. [DOI] [PubMed] [Google Scholar]
- 89.Suh Y.H., Pelkey K.A., Lavezzari G., Roche P.A., Huganir R.L., McBain C.J., Roche K.W. Corequirement of PICK1 binding and PKC phosphorylation for stable surface expression of the metabotropic glutamate receptor mGluR7. Neuron. 2008;58(5):736–748. doi: 10.1016/j.neuron.2008.03.028. [http://dx.doi.org/10.1016/j.neuron.2008.03.028]. [PMID: 18549785]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Nakajima Y., Yamamoto T., Nakayama T., Nakanishi S. A relationship between protein kinase C phosphorylation and calmodulin binding to the metabotropic glutamate receptor subtype 7. J. Biol. Chem. 1999;274(39):27573–27577. doi: 10.1074/jbc.274.39.27573. [http://dx.doi.org/ 10.1074/jbc.274.39.27573]. [PMID: 10488094]. [DOI] [PubMed] [Google Scholar]
- 91.O’Connor V., El Far O., Bofill-Cardona E., Nanoff C., Freissmuth M., Karschin A., Airas J.M., Betz H., Boehm S. Calmodulin dependence of presynaptic metabotropic glutamate receptor signaling. Science. 1999;286(5442):1180–1184. doi: 10.1126/science.286.5442.1180. [http:// dx.doi.org/10.1126/science.286.5442.1180]. [PMID: 10550060]. [DOI] [PubMed] [Google Scholar]
- 92.Wang H., Westin L., Nong Y., Birnbaum S., Bendor J., Brismar H., Nestler E., Aperia A., Flajolet M., Greengard P. Norbin is an endogenous regulator of metabotropic glutamate receptor 5 signaling. Science. 2009;326(5959):1554–1557. doi: 10.1126/science.1178496. [http://dx.doi.org/10.1126/science.1178496]. [PMID: 20007903]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhang C.S., Bertaso F., Eulenburg V., Lerner-Natoli M., Herin G.A., Bauer L., Bockaert J., Fagni L., Betz H., Scheschonka A. Knock-in mice lacking the PDZ-ligand motif of mGluR7a show impaired PKC-dependent autoinhibition of glutamate release, spatial working memory deficits, and increased susceptibility to pentylenetetrazol. J. Neurosci. 2008;28(34):8604–8614. doi: 10.1523/JNEUROSCI.0628-08.2008. [http:// dx.doi.org/10.1523/JNEUROSCI.0628-08.2008]. [PMID: 18716219]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Molinari F., Cattani A.A., Mdzomba J.B., Aniksztejn L. Glutamate transporters control metabotropic glutamate receptors activation to prevent the genesis of paroxysmal burst in the developing hippocampus. Neuroscience. 2012;207:25–36. doi: 10.1016/j.neuroscience.2012.01.036. [http:// dx.doi.org/10.1016/j.neuroscience.2012.01.036]. [PMID: 22326967]. [DOI] [PubMed] [Google Scholar]
- 95.Ross J.R., Ramakrishnan H., Porter B.E., Robinson M.B. Group I mGluR-regulated translation of the neuronal glutamate transporter, excitatory amino acid carrier 1. J. Neurochem. 2011;117(5):812–823. doi: 10.1111/j.1471-4159.2011.07233.x. [http://dx.doi.org/10.1111/j.1471-4159.2011.07233.x]. [PMID: 21371038]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gregory K.J., Noetzel M.J., Niswender C.M. Pharmacology of metabotropic glutamate receptor allosteric modulators: structural basis and therapeutic potential for CNS disorders. Prog. Mol. Biol. Transl. Sci. 2013;115:61–121. doi: 10.1016/B978-0-12-394587-7.00002-6. [http://dx.doi.org/10.1016/B978-0-12-394587-7.00002-6]. [PMID: 23415092]. [DOI] [PubMed] [Google Scholar]
- 97.Hovelsø N., Sotty F., Montezinho L.P., Pinheiro P.S. [DOI] [PMC free article] [PubMed]; Herrik K.F., Mørk A. Therapeutic potential of metabotropic glutamate receptor modulators. Curr. Neuropharmacol. 2012;10(1):12–48. doi: 10.2174/157015912799362805. [http://dx.doi.org/10.2174/157015912799362805]. [PMID: 22942876]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bianchi R., Chuang S.C., Zhao W., Young S.R., Wong R.K. Cellular plasticity for group I mGluR-mediated epileptogenesis. J. Neurosci. 2009;29(11):3497–3507. doi: 10.1523/JNEUROSCI.5447-08.2009. [http://dx.doi.org/10.1523/ JNEUROSCI.5447-08.2009]. [PMID: 19295155]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lojková-Janecková D., Ng J., Mares P. Antagonists of group I metabotropic glutamate receptors and cortical afterdischarges in immature rats. Epilepsia. 2009;50(9):2123–2129. doi: 10.1111/j.1528-1167.2009.02091.x. [http://dx.doi. org/10.1111/j.1528-1167.2009.02091.x]. [PMID: 19486355]. [DOI] [PubMed] [Google Scholar]
- 100.Chapman A.G., Nanan K., Williams M., Meldrum B.S. Anticonvulsant activity of two metabotropic glutamate group I antagonists selective for the mGlu5 receptor: 2-methyl-6-(phenylethynyl)-pyridine (MPEP), and (E)-6-methyl-2-styryl-pyridine (SIB 1893). Neuropharmacology. 2000;39(9):1567–1574. doi: 10.1016/s0028-3908(99)00242-7. [http://dx.doi.org/10.1016/S0028-3908(99)00242-7]. [PMID: 10854901]. [DOI] [PubMed] [Google Scholar]
- 101.De Vry J., Horváth E., Schreiber R. Neuroprotective and behavioral effects of the selective metabotropic glutamate mGlu(1) receptor antagonist BAY 36-7620. Eur. J. Pharmacol. 2001;428(2):203–214. doi: 10.1016/s0014-2999(01)01296-1. [http://dx.doi.org/10.1016/S0014-2999(01)01296-1]. [PMID: 11675037]. [DOI] [PubMed] [Google Scholar]
- 102.Chapman A.G., Yip P.K., Yap J.S., Quinn L.P., Tang E., Harris J.R., Meldrum B.S. Anticonvulsant actions of LY 367385 ((+)-2-methyl-4-carboxyphenylglycine) and AIDA ((RS)-1-aminoindan-1,5-dicarboxylic acid). Eur. J. Pharmacol. 1999;368(1):17–24. doi: 10.1016/s0014-2999(99)00014-x. [http://dx.doi.org/10.1016/S0014-2999(99)00014-X]. [PMID: 10096765]. [DOI] [PubMed] [Google Scholar]
- 103.Bruno V., Battaglia G., Kingston A., O’Neill M.J., Catania M.V., Di Grezia R., Nicoletti F. Neuroprotective activity of the potent and selective mGlu1a metabotropic glutamate receptor antagonist, (+)-2-methyl-4 carboxyphenylglycine (LY367385): comparison with LY357366, a broader spectrum antagonist with equal affinity for mGlu1a and mGlu5 receptors. Neuropharmacology. 1999;38(2):199–207. doi: 10.1016/s0028-3908(98)00159-2. [http://dx.doi.org/10.1016/ S0028-3908(98)00159-2]. [PMID: 10218860]. [DOI] [PubMed] [Google Scholar]
- 104.Kingston A.E., Griffey K., Johnson M.P., Chamberlain M.J., Kelly G., Tomlinson R., Wright R.A., Johnson B.G., Schoepp D.D., Harris J.R., Clark B.P., Baker R.S., Tizzano J.T. Inhibition of group I metabotropic glutamate receptor responses in vivo in rats by a new generation of carboxyphenylglycine-like amino acid antagonists. Neurosci. Lett. 2002;330(2):127–130. doi: 10.1016/s0304-3940(02)00751-6. [http://dx.doi. org/10.1016/S0304-3940(02)00751-6]. [PMID: 12231428]. [DOI] [PubMed] [Google Scholar]
- 105.Nagaraja R.Y., Grecksch G., Reymann K.G., Schroeder H., Becker A. Group I metabotropic glutamate receptors interfere in different ways with pentylenetetrazole seizures, kindling, and kindling-related learning deficits. Naunyn Schmiedebergs Arch. Pharmacol. 2004;370(1):26–34. doi: 10.1007/s00210-004-0942-5. [http://dx.doi.org/10.1007/ s00210-004-0942-5]. [PMID: 15241581]. [DOI] [PubMed] [Google Scholar]
- 106.Renaud J., Emond M., Meilleur S., Psarropoulou C., Carmant L. AIDA, a class I metabotropic glutamate-receptor antagonist limits kainate-induced hippocampal dysfunction. Epilepsia. 2002;43(11):1306–1317. doi: 10.1046/j.1528-1157.2002.10402.x. [http://dx.doi.org/10.1046/j.1528-1157.2002.10402.x]. [PMID: 12423379]. [DOI] [PubMed] [Google Scholar]
- 107.Thomsen C., Klitgaard H., Sheardown M., Jackson H.C., Eskesen K., Jacobsen P., Treppendahl S., Suzdak P.D. (S)-4-carboxy-3-hydroxyphenylglycine, an antagonist of metabotropic glutamate receptor (mGluR) 1a and an agonist of mGluR2, protects against audiogenic seizures in DBA/2 mice. J. Neurochem. 1994;62(6):2492–2495. doi: 10.1046/j.1471-4159.1994.62062492.x. [http://dx.doi.org/10.1046/j.1471-4159.1994. 62062492.x]. [PMID: 8189254]. [DOI] [PubMed] [Google Scholar]
- 108.Miyamoto M., Ishida M., Shinozaki H. Anticonvulsive and neuroprotective actions of a potent agonist (DCG-IV) for group II metabotropic glutamate receptors against intraventricular kainate in the rat. Neuroscience. 1997;77(1):131–140. doi: 10.1016/s0306-4522(96)00442-3. [http://dx.doi.org/10. 1016/S0306-4522(96)00442-3]. [PMID: 9044381]. [DOI] [PubMed] [Google Scholar]
- 109.Moldrich R.X., Jeffrey M., Talebi A., Beart P.M., Chapman A.G., Meldrum B.S. Anti-epileptic activity of group II meta- botropic glutamate receptor agonists (--)-2-oxa-4-aminobicyclo [3.1.0]hexane-4,6-dicarboxylate (LY379268) and (--)-2-thia-4-aminobicyclo[3.1.0]hexane-4,6-dicarboxylate (LY389795). Neuropharmacology. 2001;41(1):8–18. doi: 10.1016/s0028-3908(01)00044-2. [http://dx.doi.org/10.1016/ S0028-3908(01)00044-2]. [PMID: 11445181]. [DOI] [PubMed] [Google Scholar]
- 110.Yip P.K., Meldrum B.S., Rattray M. Elevated levels of group-III metabotropic glutamate receptors in the inferior colliculus of genetically epilepsy-prone rats following intracollicular administration of L-serine-O-phosphate. J. Neurochem. 2001;78(1):13–23. doi: 10.1046/j.1471-4159.2001.00418.x. [http:// dx.doi.org/10.1046/j.1471-4159.2001.00418.x]. [PMID: 11432969]. [DOI] [PubMed] [Google Scholar]
- 111.Chapman A.G., Nanan K., Yip P., Meldrum B.S. Anticonvulsant activity of a metabotropic glutamate receptor 8 preferential agonist, (R,S)-4-phosphonophenylglycine. Eur. J. Pharmacol. 1999;383(1):23–27. doi: 10.1016/s0014-2999(99)00615-9. [http://dx.doi.org/10.1016/S0014-2999(99)00615-9]. [PMID: 10556677]. [DOI] [PubMed] [Google Scholar]
- 112.Chapman A.G., Talebi A., Yip P.K., Meldrum B.S. Anticonvulsant activity of a mGlu(4alpha) receptor selective agonist, (1S,3R,4S)-1-aminocyclopentane-1,2,4-tricarboxylic acid. Eur. J. Pharmacol. 2001;424(2):107–113. doi: 10.1016/s0014-2999(01)01013-5. [http://dx.doi.org/10. 1016/S0014-2999(01)01013-5]. [PMID: 11476756]. [DOI] [PubMed] [Google Scholar]
- 113.Folbergrová J., Druga R., Haugvicová R., Mares P., Otáhal J. Anticonvulsant and neuroprotective effect of (S)-3,4-dicarbo- xyphenylglycine against seizures induced in immature rats by homocysteic acid. Neuropharmacology. 2008;54(4):665–675. doi: 10.1016/j.neuropharm.2007.11.015. [http:// dx.doi.org/10.1016/j.neuropharm.2007.11.015]. [PMID: 18191956]. [DOI] [PubMed] [Google Scholar]
- 114.Maciejak P., Szyndler J., Turzyńska D., Sobolewska A., Taracha E., Skórzewska A., Lehner M., Bidziński A., Hamed A., Wisłowska-Stanek A., Płaźnik A. The effects of group III mGluR ligands on pentylenetetrazol-induced kindling of seizures and hippocampal amino acids concentration. Brain Res. 2009;1282:20–27. doi: 10.1016/j.brainres.2009.05.049. [http://dx.doi.org/10.1016/j.brainres.2009.05.049]. [PMID: 19481536]. [DOI] [PubMed] [Google Scholar]
- 115.Thomsen C., Dalby N.O. Roles of metabotropic glutamate receptor subtypes in modulation of pentylenetetrazole-induced seizure activity in mice. Neuropharmacology. 1998;37(12):1465–1473. doi: 10.1016/s0028-3908(98)00138-5. [http://dx.doi.org/10.1016/S0028-3908(98)00138-5]. [PMID: 9886669]. [DOI] [PubMed] [Google Scholar]
- 116.Fei F., Rao W., Zhang L., Chen B.G., Li J., Fei Z., Chen Z. Downregulation of Homer1b/c improves neuronal survival after traumatic neuronal injury. Neuroscience. 2014;267:187–194. doi: 10.1016/j.neuroscience.2014.02.037. [http://dx.doi.org/10.1016/j.neuroscience.2014.02.037]. [PMID: 24607348]. [DOI] [PubMed] [Google Scholar]
- 117.Lv M.M., Cheng Y.C., Xiao Z.B., Sun M.Y., Ren P.C., Sun X.D. Down-regulation of Homer1b/c attenuates group I metabotropic glutamate receptors dependent Ca2+ signaling through regulating endoplasmic reticulum Ca2+ release in PC12 cells. Biochem. Biophys. Res. Commun. 2014;450(4):1568–1574. doi: 10.1016/j.bbrc.2014.07.044. [http://dx.doi.org/10.1016/j.bbrc.2014.07.044]. [PMID: 25026550]. [DOI] [PubMed] [Google Scholar]
- 118.Cui Z., Zhou L., Liu C., Zhu G., Wu X., Yan Y., Xia X., Ben Z., Song Y., Zhou Y., Zhang H., Zhang D. The role of Homer1b/c in neuronal apoptosis following LPS-induced neuroinflammation. Neurochem. Res. 2015;40(1):204–215. doi: 10.1007/s11064-014-1460-6. [http://dx.doi.org/10.1007/s11064-014-1460-6]. [PMID: 25503822]. [DOI] [PubMed] [Google Scholar]
- 119.Luo P., Chen T., Zhao Y., Zhang L., Yang Y., Liu W., Li S., Rao W., Dai S., Yang J., Fei Z. Postsynaptic scaffold protein Homer 1a protects against traumatic brain injury via regulating group I metabotropic glutamate receptors. Cell Death Dis. 2014;5:e1174. doi: 10.1038/cddis.2014.116. [http://dx.doi.org/10.1038/cddis.2014.116]. [PMID: 24722299]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Fei F., Li J., Rao W., Liu W., Chen X., Su N., Wang Y., Fei Z. Upregulation of Homer1a Promoted Retinal Ganglion Cell Survival After Retinal Ischemia and Reperfusion via Interacting with Erk Pathway. Cell. Mol. Neurobiol. 2015;35(7):1039–1048. doi: 10.1007/s10571-015-0198-2. [http://dx.doi.org/10.1007/s10571-015-0198-2]. [PMID: 25924704]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kammermeier P.J., Worley P.F. Homer 1a uncouples metabotropic glutamate receptor 5 from postsynaptic effectors. Proc. Natl. Acad. Sci. USA. 2007;104(14):6055–6060. doi: 10.1073/pnas.0608991104. [http://dx. doi.org/10.1073/pnas.0608991104]. [PMID: 17389377]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Tang A.H., Alger B.E. Homer protein-metabotropic glutamate receptor binding regulates endocannabinoid signaling and affects hyperexcitability in a mouse model of fragile X syndrome. J. Neurosci. 2015;35(9):3938–3945. doi: 10.1523/JNEUROSCI.4499-14.2015. [http://dx.doi.org/10.1523/ JNEUROSCI.4499-14.2015]. [PMID: 25740522]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sato M., Suzuki K., Nakanishi S. NMDA receptor stimulation and brain-derived neurotrophic factor upregulate homer 1a mRNA via the mitogen-activated protein kinase cascade in cultured cerebellar granule cells. J. Neurosci. 2001;21(11):3797–3805. doi: 10.1523/JNEUROSCI.21-11-03797.2001. [PMID: 11356868]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.de Bartolomeis A., Sarappa C., Buonaguro E.F., Marmo F., Eramo A., Tomasetti C., Iasevoli F. Different effects of the NMDA receptor antagonists ketamine, MK-801, and memantine on postsynaptic density transcripts and their topography: role of Homer signaling, and implications for novel antipsychotic and pro-cognitive targets in psychosis. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2013;46:1–12. doi: 10.1016/j.pnpbp.2013.06.010. [http://dx.doi.org/10.1016/ j.pnpbp.2013.06.010]. [PMID: 23800465]. [DOI] [PubMed] [Google Scholar]
- 125.Wang Y., Fei Z., Ma Y.H., Liu W.B., Zhu J., Zhang C., Lin W., Qu Y. VEGF upregulates Homer 1a gene expression via the mitogen-activated protein kinase cascade in cultured cortex neurons. Neurosci. Lett. 2012;515(1):44–49. doi: 10.1016/j.neulet.2012.03.016. [http://dx.doi.org/ 10.1016/j.neulet.2012.03.016]. [PMID: 22445886]. [DOI] [PubMed] [Google Scholar]
- 126.Beldhuis H.J., De Ruiter A.J., Maes F.W., Suzuki T., Bohus B. Long-term increase in protein kinase C-gamma and muscarinic acetylcholine receptor expression in the cerebral cortex of amygdala-kindled rats--a quantitative immunocytochemical study. Neuroscience. 1993;55(4):965–973. doi: 10.1016/0306-4522(93)90311-3. [http://dx.doi.org/10.1016/ 0306-4522(93)90311-3]. [PMID: 8232906]. [DOI] [PubMed] [Google Scholar]
- 127.Ohta K., Miyamoto H., Yaguchi T., Nagai K., Yamamoto S., Nomura T., Tanaka A., Nishizaki T. Stearic acid facilitates hippocampal neurotransmission by enhancing nicotinic ACh receptor responses via a PKC pathway. Brain Res. Mol. Brain Res. 2003;119(1):83–89. doi: 10.1016/j.molbrainres.2003.08.017. [http://dx.doi.org/10.1016/j.molbrainres.2003. 08.017]. [PMID: 14597232]. [DOI] [PubMed] [Google Scholar]
- 128.Fisher A. M1 muscarinic agonists target major hallmarks of Alzheimer’s disease--an update. Curr. Alzheimer Res. 2007;4(5):577–580. doi: 10.2174/156720507783018163. [http://dx.doi.org/10.2174/156720507783018163]. [PMID: 18220527]. [DOI] [PubMed] [Google Scholar]
- 129.Porcher C., Hatchett C., Longbottom R.E., McAinch K., Sihra T.S., Moss S.J., Thomson A.M., Jovanovic J.N. Positive feedback regulation between gamma-aminobutyric acid type A (GABA(A)) receptor signaling and brain-derived neurotrophic factor (BDNF) release in developing neurons. J. Biol. Chem. 2011;286(24):21667–21677. doi: 10.1074/jbc.M110.201582. [http://dx.doi.org/10.1074/jbc.M110.201582]. [PMID: 21474450]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Coelho W.S., Sola-Penna M. Serotonin regulates 6-phosphofructo-1-kinase activity in a PLC-PKC-CaMK II- and Janus kinase-dependent signaling pathway. Mol. Cell. Biochem. 2013;372(1-2):211–220. doi: 10.1007/s11010-012-1462-0. [http://dx.doi.org/10.1007/s11010-012-1462-0]. [PMID: 23010892]. [DOI] [PubMed] [Google Scholar]
- 131.Cavaliere F., Benito-Muñoz M., Panicker M., Matute C. NMDA modulates oligodendrocyte differentiation of subventricular zone cells through PKC activation. Front. Cell. Neurosci. 2013;7:261. doi: 10.3389/fncel.2013.00261. [http://dx.doi.org/10.3389/fncel.2013.00261]. [PMID: 24391542]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ren S.Q., Yan J.Z., Zhang X.Y., Bu Y.F., Pan W.W., Yao W., Tian T., Lu W. PKCλ is critical in AMPA receptor phosphorylation and synaptic incorporation during LTP. EMBO J. 2013;32(10):1365–1380. doi: 10.1038/emboj.2013.60. [http://dx.doi.org/10.1038/emboj.2013.60]. [PMID: 23511975]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Sobhia M.E., Grewal B.K., Paul M.L., Patel J., Kaur A., Haokip T., Kokkula A. Protein kinase C inhibitors: a patent review (2010 - present). Expert Opin. Ther. Pat. 2013;23(11):1451–1468. doi: 10.1517/13543776.2013.812073. [http://dx.doi.org/10.1517/13543776.2013.812073]. [PMID: 23795866]. [DOI] [PubMed] [Google Scholar]
- 134.Yang T., Roder K.E., Bhat G.J., Thekkumkara T.J., Abbruscato T.J. Protein kinase C family members as a target for regulation of blood-brain barrier Na,K,2Cl-cotransporter during in vitro stroke conditions and nicotine exposure. Pharm. Res. 2006;23(2):291–302. doi: 10.1007/s11095-005-9143-2. [http://dx.doi.org/10.1007/s11095-005-9143-2]. [PMID: 16450214]. [DOI] [PubMed] [Google Scholar]
- 135.Mach U.R., Lewin N.E., Blumberg P.M., Kozikowski A.P. Synthesis and pharmacological evaluation of 8- and 9-substituted benzolactam-v8 derivatives as potent ligands for protein kinase C, a therapeutic target for Alzheimer’s disease. ChemMedChem. 2006;1(3):307–314. doi: 10.1002/cmdc.200500068. [http://dx.doi.org/10.1002/cmdc.200500068]. [PMID: 16892365]. [DOI] [PubMed] [Google Scholar]
- 136.Sun H., Lu L., Zuo Y., Wang Y., Jiao Y., Zeng W.Z., Huang C., Zhu M.X., Zamponi G.W., Zhou T., Xu T.L., Cheng J., Li Y. Kainate receptor activation induces glycine receptor endocytosis through PKC deSUMOylation. Nat. Commun. 2014;5:4980. doi: 10.1038/ncomms5980. [http://dx.doi.org/10.1038/ncomms5980]. [PMID: 25236484]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Cuny G.D. Kinase inhibitors as potential therapeutics for acute and chronic neurodegenerative conditions. Curr. Pharm. Des. 2009;15(34):3919–3939. doi: 10.2174/138161209789649330. [http://dx.doi.org/10.2174/138161209789649330]. [PMID: 19751204]. [DOI] [PubMed] [Google Scholar]
- 138.Connolly C.N., Kittler J.T., Thomas P., Uren J.M., Brandon N.J., Smart T.G., Moss S.J. Cell surface stability of gamma-aminobutyric acid type A receptors. Dependence on protein kinase C activity and subunit composition. J. Biol. Chem. 1999;274(51):36565–36572. doi: 10.1074/jbc.274.51.36565. [http://dx.doi.org/10.1074/jbc.274.51.36565]. [PMID: 10593956]. [DOI] [PubMed] [Google Scholar]
- 139.Meier J., Akyeli J., Kirischuk S., Grantyn R. GABA(A) receptor activity and PKC control inhibitory synaptogenesis in CNS tissue slices. Mol. Cell. Neurosci. 2003;23(4):600–613. doi: 10.1016/s1044-7431(03)00079-4. [http://dx.doi. org/10.1016/S1044-7431(03)00079-4]. [PMID: 12932440]. [DOI] [PubMed] [Google Scholar]
- 140.DeLorenzo R.J. Calmodulin systems in neuronal excitability: a molecular approach to epilepsy. Ann. Neurol. 1984;16(Suppl.):S104–S114. doi: 10.1002/ana.410160716. [http://dx.doi.org/10.1002/ana.410160716]. [PMID: 6150680]. [DOI] [PubMed] [Google Scholar]
- 141.Mikuni N., Nishiyama K., Babb T.L., Ying Z., Najm I., Okamoto T., Lüders H.O., Wylie C. Decreased calmodulin-NR1 co-assembly as a mechanism for focal epilepsy in cortical dysplasia. Neuroreport. 1999;10(7):1609–1612. doi: 10.1097/00001756-199905140-00040. [http://dx.doi.org/10.1097/ 00001756-199905140-00040]. [PMID: 10380990]. [DOI] [PubMed] [Google Scholar]
- 142.Kochan L.D., Churn S.B., Omojokun O., Rice A., DeLorenzo R.J. Status epilepticus results in an N-methyl-D-aspartate receptor-dependent inhibition of Ca2+/calmodulin-dependent kinase II activity in the rat. Neuroscience. 2000;95(3):735–743. doi: 10.1016/s0306-4522(99)00462-5. [http://dx.doi.org/ 10.1016/S0306-4522(99)00462-5]. [PMID: 10670440]. [DOI] [PubMed] [Google Scholar]
- 143.Churn S.B., Kochan L.D., DeLorenzo R.J. Chronic inhibition of Ca(2+)/calmodulin kinase II activity in the pilocarpine model of epilepsy. Brain Res. 2000;875(1-2):66–77. doi: 10.1016/s0006-8993(00)02623-8. [http://dx.doi.org/10. 1016/S0006-8993(00)02623-8]. [PMID: 10967300]. [DOI] [PubMed] [Google Scholar]
- 144.Semyanov A., Godukhin O. Epileptiform activity and EPSP-spike potentiation induced in rat hippocampal CA1 slices by repeated high-K(+): involvement of ionotropic glutamate receptors and Ca(2+)/calmodulin-dependent protein kinase II. Neuropharmacology. 2001;40(2):203–211. doi: 10.1016/s0028-3908(00)00147-7. [http://dx.doi.org/10.1016/S0028-3908(00) 00147-7]. [PMID: 11114399]. [DOI] [PubMed] [Google Scholar]
- 145.Blair R.E., Sombati S., Churn S.B., Delorenzo R.J. Epileptogenesis causes an N-methyl-d-aspartate receptor/Ca2+-dependent decrease in Ca2+/calmodulin-dependent protein kinase II activity in a hippocampal neuronal culture model of spontaneous recurrent epileptiform discharges. Eur. J. Pharmacol. 2008;588(1):64–71. doi: 10.1016/j.ejphar.2008.04.021. [http://dx.doi.org/10.1016/j.ejphar.2008.04.021]. [PMID: 18495112]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Ashpole N.M., Song W., Brustovetsky T., Engleman E.A., Brustovetsky N., Cummins T.R., Hudmon A. Calcium/ calmodulin-dependent protein kinase II (CaMKII) inhibition induces neurotoxicity via dysregulation of glutamate/calcium signaling and hyperexcitability. J. Biol. Chem. 2012;287(11):8495–8506. doi: 10.1074/jbc.M111.323915. [http://dx.doi.org/10.1074/jbc.M111.323915]. [PMID: 22253441]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ambrosino P., Alaimo A., Bartollino S., Manocchio L., De Maria M., Mosca I., Gomis-Perez C., Alberdi A., Scambia G., Lesca G., Villarroel A., Taglialatela M., Soldovieri M.V. Epilepsy-causing mutations in Kv7.2 C-terminus affect binding and functional modulation by calmodulin. Biochim. Biophys. Acta. 2015;1852(9):1856–1866. doi: 10.1016/j.bbadis.2015.06.012. [http://dx.doi.org/10.1016/j.bbadis. 2015.06.012]. [PMID: 26073431]. [DOI] [PubMed] [Google Scholar]
- 148.Robison A.J. Emerging role of CaMKII in neuropsychiatric disease. Trends Neurosci. 2014;37(11):653–662. doi: 10.1016/j.tins.2014.07.001. [http://dx. doi.org/10.1016/j.tins.2014.07.001]. [PMID: 25087161]. [DOI] [PubMed] [Google Scholar]
- 149.Zheng H., Tang R., Yao Y., Ji Z., Cao Y., Liu Z., Peng F., Wang W., Can D., Xing H., Bu G., Xu H., Zhang Y.W., Zheng W. MiR-219 Protects Against Seizure in the Kainic Acid Model of Epilepsy. Mol. Neurobiol. 2016;53(1):1–7. doi: 10.1007/s12035-014-8981-5. [http://dx.doi.org/10. 1007/s12035-014-8981-5]. [PMID: 25394384]. [DOI] [PubMed] [Google Scholar]
- 150.Jin D.Z., Guo M.L., Xue B., Mao L.M., Wang J.Q. Differential regulation of CaMKIIα interactions with mGluR5 and NMDA receptors by Ca(2+) in neurons. J. Neurochem. 2013;127(5):620–631. doi: 10.1111/jnc.12434. [http://dx.doi.org/10.1111/jnc.12434]. [PMID: 24032403]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Suh Y.H., Park J.Y., Park S., Jou I., Roche P.A., Roche K.W. Regulation of metabotropic glutamate receptor 7 (mGluR7) internalization and surface expression by Ser/Thr protein phosphatase 1. J. Biol. Chem. 2013;288(24):17544–17551. doi: 10.1074/jbc.M112.439513. [http://dx.doi.org/10.1074/jbc.M112.439513]. [PMID: 23612982]. [DOI] [PMC free article] [PubMed] [Google Scholar]


