Abstract
Patients with Parkinson’s disease (PD) receiving L-3,4-dihydroxyphenylalanine (L-DOPA, the gold-standard treatment for this disease) frequently develop abnormal involuntary movements, termed L-DOPA-induced dyskinesias (LID). Glutamate overactivity is well documented in PD and LID. An approach to manage LID is to add to L-DOPA specific agents to reduce dyskinesias such as metabotropic glutamate receptor (mGlu receptor) drugs. This article reviews the contribution of mGlu type 5 (mGlu5) receptors in animal models of PD.
Several mGlu5 negative allosteric modulators acutely attenuate LID in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) monkeys and 6-hydroxydopamine(6-OHDA)-lesioned rats. Chronic administration of mGlu5 negative allosteric modulators to MPTP monkeys and 6-OHDA rats also attenuates LID while maintaining the anti-parkinsonian effect of L-DOPA.
Radioligand autoradiography shows an elevation of striatal mGlu5 receptors of dyskinetic L-DOPA-treated MPTP monkeys but not in those without LID. The brain molecular correlates of the long-term effect of mGlu5 negative allosteric modulators treatments with L-DOPA attenuating development of LID was shown to extend beyond mGlu5 receptors with normalization of glutamate activity in the basal ganglia of L-DOPA-induced changes of NMDA, AMPA, mGlu2/3 receptors and VGlut2 transporter.
In the basal ganglia, mGlu5 receptor negative allosteric modulators also normalize the L-DOPA-induced changes of dopamine D2 receptors, their associated signaling proteins (ERK1/2 and Akt/GSK3β) and neuropeptides (preproenkephalin, preprodynorphin) as well as the adenosine A2A receptors expression.
These results show in animal models of PD reduction of LID with mGlu5 negative allosteric modulation associated with normalization of glutamate, dopamine and adenosine receptors suggesting a functional link of these receptors in chronic treatment with L-DOPA.
Keywords: Adenosine receptor, dopamine receptor, glutamate receptor, L-DOPA, L-DOPA-induced dyskinesias, MPEP, MPTP monkey model, Parkinson’s disease
Introduction: Parkinson’s disease and L-DOPA-induced dyskinesias
Parkinson’s disease (PD) is the most common neurodegenerative movement disorder characterized by tremor, rigidity, bradykinesia and is likely to increase due to the aging populations [1]. PD involves principally the death of dopamine (DA) neurons in the substantia nigra pars compacta (SNc) but other neurotransmitters and neuro- modulators are also affected [2]. Treatment of motor symptoms of PD with the DA precursor, L-3,4-dihydroxyphenylalanine (L-DOPA), introduced 50 years ago still remains the gold standard for PD treatment [3]. However, various complications including motor fluctuations and abnormal involuntary movements, such as L-DOPA-induced dyskinesias (LID), limit the quality of life in PD patients and can be very difficult to manage [4]. LID increases with the duration of L-DOPA treatment and up to 95% of PD patients become afflicted after 15 years [5, 6]. Although the mechanisms of these involuntary movements are not well understood, they are paralleled by changes in various neurotransmitter systems and intracellular signaling pathways [7].
Glutamate receptors in Parkinson’s disease and L-DOPA-induced dyskinesias
Glutamate neurotransmission is reported to be increased in the basal ganglia in PD [7] and LID [8, 9]. Amantadine, a noncompetitive antagonist at N-methyl-D-aspartate (NMDA) ionotropic glutamate receptors and, to a lesser extent clozapine, are presently the only drug used in the clinic to reduce LID in some PD patients without worsening parkinsonian symptoms [10-14]. However, the antidyskinetic effect of amantadine may be transient [15] and high doses may not be tolerated in some PD patients because of cognitive impairment, thus limiting its use [15].
On the basis of these considerations, combined with the rich distribution and diverse physiological roles of metabotropic glutamate (mGlu) receptors within the basal ganglia, recent attention has been placed on these receptors as alternative targets to modulate glutamate hyperactivity in PD and LID [16]. Studies in animal models and PD patients indicate that antagonists of group I mGlu receptor, especially mGlu5 receptor, could be considered as a suitable therapeutic approach in PD and LID.
mGlu5 receptor specific binding was reported to be increased in the basal ganglia of parkinsonian monkeys with LID and in parkinsonian patients with motor complications [17-21].
In the 6-hydroxydopamine (6-OHDA)-lesioned rat model, mGlu5 receptor negative allosteric modulators 2-methyl-6-(phenylethynyl)pyridine (MPEP), 3-[(2-methyl-1,3-thiazol-4-yl)ethynyl]pyridine (MTEP) and MRZ-8676 administered acutely inhibit LID [22-26]. The mGlu5 receptor negative allosteric modulators MPEP, MTEP, fenobam and AFQ056 (mavoglurant) were found to acutely reduce LID in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-lesioned monkeys [27-29]. In the light of these acute studies, it becomes important to test the long-term effects of mGlu5 receptor negative allosteric modulators behaviourally and their associated brain molecular changes.
Sub-chronic and chronic administrations of mGlu5 receptor negative allosteric modulators in MPTP monkeys and 6-OHDA rats reduce the development of L-DOPA induced motor complications (Table 1). In previous studies, we reported that development of LID over a month of treatment were lower by overall ∼70% with addition of MPEP to the L-DOPA treatment in de novo MPTP monkeys [17] and this was associated with a normalization of glutamate receptors [18] as shown for mGlu5, NMDA containing NR2B subunit, AMPA and mGlu2/3 receptors (Table 2). In 6-OHDA rats the vesicular glutamate transporter 2 (VGlut2) was also normalized by the addition of MPEP to the chronic treatment with L-DOPA [30].
Table 1. Behavioral effects of repeated mGlu5 negative allosteric modulator treatments in 6-OHDA-lesioned rats and MPTP monkeys.
|
Animal Model
and Treatment |
mGlu5 Receptor
Negative Allosteric Modulators(s) |
Regimen | Dose Tested | Main Behavioral Effect | Refs. |
|---|---|---|---|---|---|
| 6-OHDA-lesioned rat, Sub-chronic | MTEP | L-DOPA and MTEP administered simultaneous once daily for 7 days | 5 mg/kg | •Reduced already established abnormal involuntary movement scale (AIMS) up to the 7th day of co-treatment with L-DOPA, comparable to single acute administration of MTEP | [24] |
| MPEP | MPEP administered 5 min before L-DOPA once daily for 14 days |
1 mg/kg | •Reduced severity of limb dyskinesia and axial dystonia over the whole duration of L-DOPA treatment | [127] | |
| MRZ-8676 | L-DOPA and MRZ-8676 administered simultaneous once daily for 6 days | 75 mg/kg | •Reduced already established AIMS •No tolerance of antidyskinetic effect following repetitive treatment |
[22] | |
| MPEP | MPEP administered 10 min before L-DOPA twice daily for 10 days | 1.5 mg/kg | •Reduced LID | [30] | |
| 6-OHDA-lesioned rat, Chronic | MTEP | L-DOPA and MTEP administered simultaneous once daily for 21 days | 5 mg/kg | •Reduced AIMS compared to L-DOPA alone in drug naïve animals • Reduced already established AIMS |
[26] |
| MPEP | 30 min before L-DOPA challenge once daily during 21 days | 1.5 mg/kg | •MPEP virtually abolished AIMS | [25] | |
| MTEP | L-DOPA and MTEP administered simultaneous once daily for 21 days | 5 mg/kg | •Attenuated dyskinesias without adverse motor effects | [128] | |
| MPTP-lesioned monkey, Chronic | MPEP | de novo, MPEP administered 15 min prior to L-DOPA for one month | 10 mg/kg | •Decreased dyskinesias and maintained anti-parkinsonian effect •No decrease of duration of the L-DOPA antiparkinsonian effect |
[18] |
| Fenobam | L-DOPA and Fenobam administered simultaneous for 17 days | 10 mg/kg | •Attenuated development of peak-dose dyskinesias •No effect on antiparkinsonian activity of L-DOPA |
[132] |
Table 2. Biochemical effects of mGlu5 negative allosteric modulator treatments on glutamate neurotransmission in animal models of Parkinson’s disease.
| Animal Model |
mGlu5 Receptor
Negative Allosteric Modulator(s) |
Dose(s) Tested and Treatment Regimen | Main Biochemical Effects | Refs. |
|---|---|---|---|---|
| MPTP-lesioned monkey | MPEP | 10 mg/kg, once daily for one month (de novo), 15 min prior to L-DOPA |
•Prevented the increase by L-DOPA of striatal mGlu5 receptors density measured with [3H]ABP688 specific binding •Maintained at control levels striatal binding affinity of [3H]ABP688 to mGlu5 receptors •Maintained at control levels striatal mGlu5 receptor mRNA levels •Prevented the increase by L-DOPA of [3H]Ro-25-6981 specific binding to NMDA receptors containing NR1/NR2B subunits in the caudate nucleus and putamen •Prevented the increase by L-DOPA of [3H]Ro-48-8587 specific binding to AMPA receptors in the caudate nucleus and putamen •Prevented the decrease by L-DOPA of [3H]LY341495 specific binding to mGlu2/3 receptor in the caudate nucleus and putamen |
[18] |
| 6-OHDA-lesioned rat | MPEP | MPEP (1.5 mg/kg) administered 10 min before L-DOPA twice daily for 10 days |
•MPEP with L-DOPA decreased the levels of VGlut2 in the striatum ipsilateral to the lesion | [30] |
mGlu5 receptor negative allosteric modulators, mavoglurant and ADX-48621 (dipraglurant), were shown to reduce LID in parkinsonian patients and were well tolerated without worsening motor symptoms [31-33]. However, recent studies with mavoglurant did not show reduced LID in PD patients [34, 35].
Dopamine receptors in Parkinson’s disease and L-DOPA-induced dyskinesias
The mechanisms underlying the development of LID remain unknown, but evidence suggests that LID is the result of maladaptive plasticity at striatal synapses [7, 36] and in an altered activity of dopaminergic neurotransmission in the basal ganglia [37]. DA binds to five different subtypes of G protein-coupled DA receptors divided in two classes, the D1 (D1 and D5) and the D2 class (D2, D3 and D4) of receptors [38]. D1 receptors were shown to be expressed in neurons containing substance P and dynorphin, projecting to the substantia nigra pars reticulata (SNr) and to the internal globus pallidus (GPi), which constitute the direct striatal output pathway [39]. D2 receptors are predominantly localized in neurons expressing enkephalin, projecting to the external globus pallidus (GPe), constituting the indirect pathway [39]. D2 receptors are found on postsynaptic and pre-synaptic nigrostriatal dopaminergic terminals, of the substantia nigra neurons and of presynaptic corticostriatal terminals where they can inhibit striatal glutamate release [40].
Denervation-induced supersensitivity of D1 and D2 receptors was initially recognized as a plausible mechanism of LID [41, 42]. Numerous studies measured the density of D1 and D2 receptors in the brain of human and animal models, but no general consensus emerged; the wide methodological discrepancies, time to sacrifice, post-mortem delays, etc, may account for this lack of consensus. Post-mortem studies have shown that striatal DA receptors particularly the D2 subtype were increased in PD patients [42, 43] or unchanged [44, 45], while both D1 and D2 receptor subtypes were increased in MPTP monkeys [46-48]. Administration of L-DOPA was shown to reverse these increases in PD patients [42, 43] and primates in many studies [47-49]. No general consensus also emerged for DA receptor mRNA. Hence, D1 mRNA levels are reported to remain unchanged [50-52] or to be reduced after MPTP lesion in monkeys [51-53]. This decrease was corrected with L-DOPA [52, 53]. D2 receptors mRNA levels were reported to be increased concomitantly with its corresponding protein levels in striatum with MPTP [51-54] and returned to control levels when L-DOPA was administered [52]. These reports support that LID are more complex than hypersensitivity due to a simple increase in the density of striatal DA receptors and its mRNA.
Numerous interactions between mGlu5 receptor and D1, D2, NMDA, A2A adenosine receptors suggest that these receptors may function together as closely associated signaling partners in the development of LID [55, 56].
A chronic treatment with MPEP in MPTP monkeys treated with L-DOPA that prevented the development of LID was shown to normalize changes produced by L-DOPA on D2 receptor and its mRNA, preproenkephalin (PPE) mRNA, preprodynorphin (PPD) mRNA, phosphorylated extracellular signal-regulated kinase 1 and 2 (ERK1/2) and phosphorylated Akt/GSK3β signaling proteins, but not D1 and its mRNA (Table 3). Similar findings for PPE and PPD mRNA levels in 6-OHDA rats were observed with chronic MPEP or MTEP treatment with L-DOPA associated with the prevention of development of abnormal involuntary movement scale (AIMS) (Table 3). MTEP was also shown to normalize phosphorylated ERK1/2 and phospho-mitogen- and stress-activated protein kinase-1 (MSK-1) in L-DOPA treated 6-OHDA rats (Table 3). While both MPEP and MTEP have off-target activities these are at higher doses [57-61] and at the doses shown in Table 3 the results reported are more likely due to mGlu5 receptor negative allosteric modulator activity.
Table 3. Biochemical effects of mGlu5 negative allosteric modulator treatments on dopamine receptors, their associated signaling proteins and neuropeptides in animal models of Parkinson’s disease.
| Animal Model |
mGlu5 Receptor
Negative Allosteric Modulator(s) |
Dose(s) Tested and Treatment Regimen | Main Biochemical Effects | Refs. |
|---|---|---|---|---|
| MPTP-lesioned monkey | MPEP | 10 mg/kg, once daily for one month (de novo), 15 min prior to L-DOPA |
•[3H]SCH23390 specific binding to D1 DA receptors remained low in the striatum and globus pallidus of MPTP, MPTP+L-DOPA, and L-DOPA+MPEP-treated monkeys while no changes were observed compared to control in the levels of D1 receptor mRNA. •[3H]raclopride specific binding to D2 DA receptor and its corresponding mRNA levels in the striatum were elevated in L-DOPA+MPEP-treated monkeys compared to L-DOPA alone and was comparable to MPTP-treated monkeys •Prevented the increase by L-DOPA of striatal preproenkephalin and preprodynorphin mRNA levels •Prevented the increase by L-DOPA of striatal phosphorylated ERK1 and ERK2 •Prevented the increase by L-DOPA of striatal phosphorylated forms of Akt (Ser473) and GSK3b (Ser9) |
[109] |
| 6-OHDA-lesioned rat | MTEP | L-DOPA and MTEP (0.25, 1.25 and 6.25 mg/kg) administered simultaneous (acute) | •Prevented the increase by L-DOPA of striatal prodynorphin mRNA with MTEP 1.25 and 6.25 mg/kg •MTEP combined with L-DOPA did not modify up-regulation of striatal preproenkephalin mRNA-induced by DA denervation •MTEP alone did not modify striatal prodynorphin and preproenkephalin mRNA expression compared to vehicle-treated 6-OHDA rats. |
[26] |
| 6-OHDA-lesioned rat | MTEP | L-DOPA and MTEP (5 mg/kg) administered simultaneous once daily for 21 days |
•Up-regulation of striatal prodynorphin on the lesion side of L-DOPA+MTEP-treated rats less pronounced that with L-DOPA alone treatment •MTEP alone reversed the lesion-induced down-regulation of striatal prodynorphin •MTEP with L-DOPA partially blocked the additional up-regulation of striatal preproenkephalin induced by L-DOPA alone •MTEP alone did not reverse the lesion-induced up-regulation of striatal preproenkephalin |
[26] |
| 6-OHDA-lesioned rat | MPEP | MPEP (1 mg/kg) administered once daily 5 min before L-DOPA for 14 days |
•MPEP reduced the increases in preprodynorphin mRNA levels in striatonigral neurons ipsilateral to the lesion | [127] |
| 6-OHDA-lesioned rat | MPEP | MPEP (1.5 mg/kg) 30 min before L-DOPA challenge once daily for 21 days | •MPEP combined with L-DOPA reduced dramatically the increased striatal expression of FosB/Delta FosB induced by L-DOPA | [25] |
| 6-OHDA-lesioned rat | MTEP | L-DOPA and MTEP (1.25 and 6. 25 mg/kg, acute; 5 mg/kg once daily for 21 days) administered simultaneous |
•Acute administration of MTEP with L-DOPA reduced striatal L-DOPA-induced phospho-ERK1/2 and phospho-MSK-1 expression at the two doses tested •Chronic administration of MTEP with L-DOPA blocked the up-regulation of striatal prodynorphin mRNA induced by L-DOPA |
[128] |
Adenosine receptors in Parkinson’s disease and L-DOPA-induced dyskinesias
Adenosine, a purinergic messenger, plays a crucial role in many physiological processes and is released by many cells including neurons and glia [62, 63]. In the basal ganglia, adenosine interacts closely with DA and is involved in the function of striatal GABAergic striatopallidal neurons projecting from the caudate nucleus and the putamen, mainly to the GPe [64-69]. The implication of adenosine to regulate the excessive glutamate neurotransmission observed in PD and LID is also demonstrated [37, 70, 71]. Hence, adenosine has received increasing attention because of its interaction with DA and glutamate receptors; this could have major implications for the development of new pharmacological targets for the treatment of PD and LID.
Adenosine binds to four classes of specific G-protein-coupled receptor subtypes named A1, A2A, A2B and A3 [72]. Adenosine receptor subtypes A1 and A2A are mainly localized in the basal ganglia, more precisely in the striatum, whereas A2B and A3 receptors are widely distributed in the brain [73, 74].
In the basal ganglia, A1 receptors are mainly present in the striatonigral GABAergic and corticostriatal glutamatergic neurons [75]. The stimulation of A1 receptors generally leads to inhibition of neurotransmitter release, such as the inhibition of glutamate release at corticostriatal terminals in the striatum [62, 76].
The A2A receptor is co-expressed at postsynaptic sites of the same medium spiny neurons as those bearing DA D2 receptors [77, 78] and projecting to the GPe of the indirect pathway. The restricted and specific distribution of A2A receptors to the indirect pathway of the basal ganglia provide specificity that could lead to a reduced incidence of adverse effects. Presynaptic and glial A2A receptors are also localized in the caudate nucleus and the putamen [73]. A2A receptors are found on presynaptic glutamatergic corticostriatal terminals and these receptors can modulate positively the glutamatergic cortical input by stimulating glutamate release [73].
Adenosine A2A antagonists can reduce the excessive striatopallidal and subthalamic neuronal activity and bring a new target in PD therapy [37, 65, 67, 79]. Several behavioural analyses have shown the potential efficacy of adenosine receptor modulators in the treatment of PD and LID, such as A2A receptor antagonists [79-83]. The A2A receptor antagonists Preladenant and KW-6002 (istradefylline), are reported to extend the duration of the antiparkinsonian effect of L-DOPA and enhance the parkinsonian activity of low doses of DA agonists; this is without worsening dyskinesias showing the DOPA-sparing activity of A2A receptor antagonists [81, 84-87]; reviewed in: [88-91]. Moreover a recent paper showed that the A2A receptor antagonist istradefylline did not increase LID of MPTP-treated marmosets in a chronic treatment and at days 21 and 28 it slightly but significantly reduced dyskinesias [92].
Presynaptically, A2A receptors can colocalize with A1 receptors in corticostriatal afferents, where they act together to modulate and regulate glutamate release [93]. Experiments using isolated striatal nerve terminal preparations have shown that most of the striatal glutamatergic terminals contain both A1 and A2A receptors [93]. In response to variation in adenosine concentrations, functional studies in striatal glutamatergic terminals have shown that the A1–A2A adenosine receptor heteromer provides a “switch mechanism” that can produce opposite effects on glutamate release [93-95].
Functional interactions between DA and adenosine receptors are supported by the anatomic localization of these receptors in striatal projection neurons [67]. A1 receptors are mainly co-expressed with D1 DA receptors on striatal neurons that project to the GPi and the substantia nigra [56]. D1 and adenosine A1 receptors are known to form functionally interacting complexes, the heteromer D1-A1, in cortical neurons and basal ganglia [96, 97].
Using co-immunoprecipitation and colocalization, as well as bioluminescence resonance energy transfer (BRET) and fluorescence resonance energy transfer (FRET) techniques, D2 and adenosine A2A receptors were shown to form functional homo- and hetero-oligomers [96, 98, 99]. Moreover, in cell lines cotransfected with plasmids containing D2 and A2A receptors and in primary culture of striatal neurons, long-term administration of A2A or D2 agonists induces an internalization and desensitization of the D2-A2A complex [98, 99], whereas D2 antagonists trigger an increase in D2 and A2A immunoreactivity [100]. Also, in SH-SY5Y neuroblastoma cells cotransfected with the D2 receptor, A2A antagonists enhance striatal D2 receptor signaling and block increased A2A receptor signaling [101]. Interestingly, a recent report in rats and monkeys has shown that D2-A2A-cannabinoid (CB)1 receptor heteromers are present in the striatum of intact and hemiparkinsonian 6-OHDA-lesioned rat as well as in intact and MPTP parkinsonian monkeys [102, 103] and that this heteromer expression is altered in lesioned striatum. Also, acute or chronic L-DOPA treatment induced disruption of D2-A2A-CB1 heteromers [102, 103]. These authors suggested that LID could be induced, at least in part, by an imbalance in the indirect pathway due to absence of cross-talk between D2-A2A-CB1 receptor heteromers functional components [102, 103].
In addition to adenosine A2A and DA D2 receptors, mGlu5 receptors are also known to interact and colocalize postsynaptically in the striatopallidal GABAergic efferent neurons [16, 71, 104]. Experiments using optical sectioning techniques found that A2A and mGlu5 receptors are also co-localized in rat striatal cultures [105]. In addition, double labelling electron microscopy colocalization experiments in the putamen of monkey have shown a substantial degree of A2A and mGlu5 receptor colocalization mainly in postsynaptic elements [104]. In fact, 60-70% of A2A receptors immuno- reactive dendrites or spines in the monkey putamen co-express mGlu5 receptors [104]. Accordingly, A2A receptors activation can increase the phosphorylation of DARPP-32 at Thr-34 via an ERK pathway and the induction of c-fos expression is also increased in striatopallidal neurons when A2A and mGlu5 receptors are co-activated [71, 106]. An important increase in adenosine release was observed when glutamatergic neurotransmission becomes overactive [71, 107]. Functional interaction of A2A and mGlu5 receptors have also been demonstrated at the behavioural level, a synergistic effect on the induction of motor activity being observed following acute combined treatment with a low doses of mGlu5 negative allosteric modulator, MPEP, and A2A antagonist KW-6002 [108]. Hence, the mGlu5 receptor-mediated effect in the striatum was abolished by the blockade of A2A receptors [70]. This colocalization provides a structural framework for the existence of multiple functional interactions of A2A, D2 and mGlu5 receptors [16]. A better understanding of the functional interactions that exist between these receptor heteromers in normal and lesioned striatum as well as following acute or chronic L-DOPA treatment foreshadow a potential therapy targeting mGlu5-D2-A2A receptor heteromers in PD patients.
As presented in the previous section of this review, in de novo MPTP monkeys, development of LID was shown to be lower with addition of MPEP to the L-DOPA treatment [17] and to be associated with a normalization of glutamate [18] and DA receptors [18, 109]. This antidyskinetic activity of MPEP could also extend to A2A receptors. Therefore, we investigated A2A receptors in the brain of these de novo MPTP monkeys treated with L-DOPA and MPEP where motor behaviour [17], glutamate [18] and DA receptors [109] were previously reported. A2A receptors were measured by in situ hybridization of their mRNA levels using oligonucleotides probes corresponding to bases 593–637 and 714–757 of human A2A receptors cDNA [110, 111] under conditions we previously described for MPTP monkeys [112] and human post-mortem brains [80].
Fig. 1 shows representative A2A receptor mRNA levels in brain slices of control and MPTP monkeys treated with vehicle, L-DOPA, and L-DOPA+ MPEP. High expression of this receptor in control monkeys is observed in the caudate nucleus and putamen but not in the GPi and GPe as we previously reported [112].
Fig. (1).
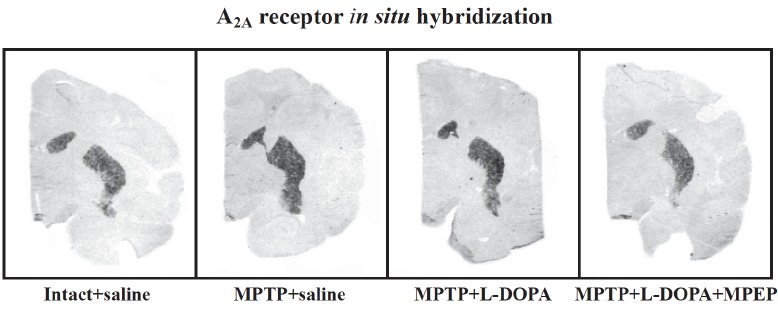
Representative autoradiograms of coronal brain sections showing A2A receptor mRNA labeling measured by in situ hybridization in the caudate nucleus and putamen of an intact monkey (intact + saline), saline-treated MPTP monkey (MPTP + saline), a monkey chronically treated with L-DOPA (MPTP + L-DOPA) that developed dyskinesias, and a monkey chronically treated with L-DOPA and MPEP (MPTP + L-DOPA + MPEP) that developed significantly less dyskinesias.
In the posterior caudate nucleus analyzed (Fig. 2), no significant effect of lesion and treatment was measured on A2A receptor mRNA levels (one-way analysis of variance (ANOVA) followed by post-hoc pairwise comparisons with Fisher’s least significant difference test, (Fisher’s test) F3,13 = 2.026, p = 0.1600) consistent with our previous findings in another group of MPTP monkeys [112]. By contrast, significant changes of A2A receptor mRNA levels were measured in the lateral (ANOVA and Fisher’s test, F3,13 = 6.140 p = 0.0078) and medial (ANOVA and Fisher’s test, F3,14 = 3.740, p = 0.0389) putamen. The putamen showed an increase of A2A receptor mRNA levels with the MPTP lesion in its lateral and medial parts (+61% and +43% in the lateral and medial parts respectively compared to intact-saline-treated monkeys) (Fig. 2). A2A receptor mRNA levels remained elevated in MPTP monkeys treated with L-DOPA, those animals displaying dyskinesias. In MPTP monkeys treated with L-DOPA and MPEP that developed less dyskinesias than those treated with L-DOPA [17], A2A receptor mRNA levels were lower and this was significant compared to MPTP saline treated animals in the lateral putamen a sub-region associated with motor control whereas in the medial part the difference was intermediate, neither significant compared to controls nor MPTP + L-DOPA treated MPTP monkeys. These results are consistent with our previous findings where A2A receptor mRNA levels were elevated in MPTP and dyskinetic MPTP + L-DOPA treated MPTP monkeys and lower in MPTP monkeys treated with L-DOPA and CI-1041, an NMDA glutamate receptor antagonist, that prevented the development of dyskinesias [112]. However, we cannot rule out the possibility that MPEP or CI-1041 treatments could have a direct effect on the expression of A2A receptors thereby associating the change in levels of these receptors to the antagonist treatment themselves rather than to the dyskinetic condition. Indeed in mice, striatal binding of the A2A receptor antagonist ligand [125I]ZM241385 was reduced in brain slices pre-incubated with MPEP compared to untreated animals and this was attributed to a possible change of conformation of the mGlu5-A2A heteromers and consequently the binding affinity of [125I]ZM241385 for A2A receptors [113].
Fig. (2).
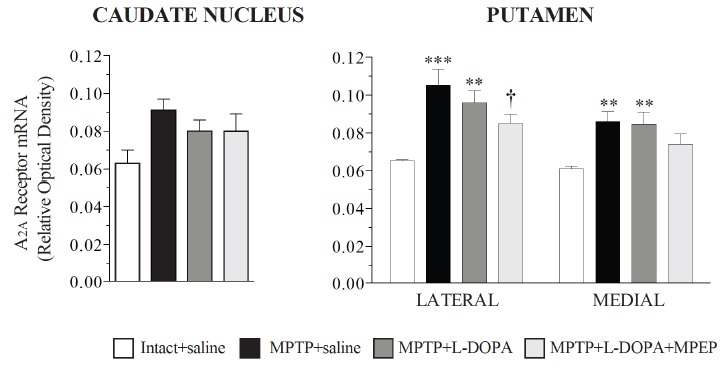
A2A receptor mRNA levels measured by in situ hybridization in the caudate nucleus and putamen of intact monkeys (intact + saline, n=3), saline-treated MPTP monkeys (MPTP + saline, n=4), monkeys chronically treated with L-DOPA (MPTP + L-DOPA, n=5) that developed dyskinesias, and monkeys chronically treated with L-DOPA and MPEP (MPTP + L-DOPA + MPEP, n=5) that developed significantly less dyskinesias. Data are presented as the mean ± S.E.M. **p<0.01, and ***p<0.001 vs respective intact +saline group; †p<0.05 vs MPTP + saline group.
Nevertheless, these findings in MPTP monkeys model well the human condition where we showed that A2A receptor mRNA levels were elevated in PD patients with dyskinesias compared to PD patients without dyskinesias and controls in the putamen while no significant effect was measured in the caudate nucleus [80]. In this regard, post-mortem studies have shown an increase of basal ganglia A2A adenosine receptors and its mRNA levels in PD patients with LID as compared to PD patients without LID and controls [80]. As for post-mortem human brains, A2A receptor levels were only increased in dyskinetic monkeys as compared to untreated monkeys [112]. These results in PD patients were in accordance with a report by Varani et al. (2010) showing elevated A2A receptor mRNA levels (5.2 fold increase) and an overexpression of A2A protein levels (280%), measured by Western blot in the putamen of PD patients, when compared to healthy subjects [114]. In the present study and in our previous ones in MPTP monkeys [112] and PD patients [80] similar changes were observed for A2A receptor mRNA levels and [3H]-SCH 58261 specific binding to A2A receptors supporting that changes of expression of this receptor in PD is the result of altered transcriptional activity and are functionally associated with changes of this receptor protein levels. A2A receptors availability in PD patients with and without LID was evaluated by PET studies and higher levels of A2A receptor binding in PD patients with LID compared to those without LID were reported [115, 116]. This suggests that LID might be the result of altered adenosine transmission and that A2A receptor antagonists could produce potential beneficial antidyskinetic activity. The molecular mechanisms involving A2A receptors in the induction of dyskinesia is not yet fully elucidated. However, it is well known that activation of A2A receptors disrupts the inhibitory actions of D2 receptors within the indirect pathway resulting in a facilitation of DA transmission [117]. In the light of various biochemical and behavioural results showing the functional interaction between A2A and D2 receptors, it was postulated that altered striatal D2-A2A functional interaction in PD creates an imbalance in the activity of the two efferent pathways of the basal ganglia that could be associated with motor alterations inherent to dyskinesia [102, 117]. These changes suggest that both striatal A2A receptors and their mRNA levels are modulated by chronic L-DOPA treatment and might be related to the close interactions with DA and glutamate systems in the basal ganglia. These findings also suggest that the striatal mGlu5–mGlu5 dimeric receptor complexes on the striato-pallidal efferents neurons [104] as well as the putative mGlu5-D2-A2A complexes on the same neuronal population, might be involved in striatal plasticity and hence could be relevant for the management of PD and LID [67, 71, 105].
Discussion
The mechanisms involved in the occurrence of LID are complex and have been investigated in numerous studies using animal models and PD patients. Multiple changes in the basal ganglia dopaminergic systems in D1 and D2 receptors and their signaling pathways have been observed such as modulation in the expression and the activity of subtypes of DA receptors, G proteins, effectors, protein kinases, transcription factors, etc [118]. Abnormal adaptation in the striatum leading to LID may also involve faulty interaction between glutamate and DA inputs and DA-glutamate signaling in the nigrostriatal pathway [119].
Glutamate is the brain most abundant excitatory neuro- transmitter mediating as much as 70% of synaptic transmission in the central nervous system and its overactivity is well documented in PD and LID. An attractive strategy to treat LID is to use anti-glutamatergic adjunct drugs that can modulate basal ganglia dopaminergic neurotransmission [120-122]. The present review showed that a chronic treatment with mGlu5 negative allosteric modulators in 6-OHDA rats and MPTP monkeys treated with L-DOPA normalized changes produced by L-DOPA on D2 receptor and its mRNA, PPE mRNA, PPD mRNA, ERK1/2 and Akt/GSK3β signaling proteins, but not D1 and its mRNA.
In the basal ganglia of post-mortem PD patients with motor complications, changes of glutamate neurotransmission and its receptors such as NMDA, AMPA, mGlu2 and mGlu5 receptors were reported [8, 20, 123]. In these same patients, A2A receptor levels were measured and they were increased in the basal ganglia and associated with LID [80]. These observations in human brains do not provide a causal link between glutamate and adenosine receptors in motor complications. Nevertheless, they show altered adenosine and glutamate receptors in human PD and that normalization of glutamatergic transmission in MPTP monkeys with a mGlu5 receptor negative allosteric modulator normalizes also A2A receptors suggesting possible interactions between these receptors. Moreover, in MPTP monkeys, a chronic treatment with L-DOPA and a NMDA receptor antagonist CI-1041, that prevented LID, was shown to normalize basal ganglia A2A receptors as well as NMDA receptors suggesting the close link between these neurotransmitters in dyskinesias [112].
The interactions of A2A receptors, with dopaminergic and glutamatergic receptors represent an interesting area of research. In the light of theses interactions, new therapeutic approaches could include the combination of an A2A receptor antagonist with dopaminomimetic drugs or with anti- glutamatergic drug. This combination could lead to the use of lower doses of each drug, especially L-DOPA, and could have an impact on PD symptoms and the development of motor complication. For example, A2A receptor antagonists could be combined to mGlu5 receptor negative allosteric modulators and to lower doses of L-DOPA or DA agonists to increase the antiparkinsonian activity of the dopaminergic drugs without increasing or even decreasing dyskinesias [124]. The mGlu5 receptor subtype is highly expressed in striatal medium spiny neurons [16] and plays a key role in modulating the responses mediated by NMDA receptors and L-type calcium channels [125]. In addition, an antagonistic interaction between the D2 receptor and mGlu5 receptors is reported [55]. The response of the basal ganglia to a chronic treatment with a mGlu5 receptor negative allosteric modulators in rodent models of PD show that striatal molecular changes relevant to LID are reversed by MPEP or MTEP, including delta FosB protein [126], prodynorphin mRNA [26], glutamic acid decarboxylase (GAD65 and GAD67) mRNA [127] and phospho-ERK1/2 protein levels [128]. We showed that MPEP prevented dyskinesias and the effects of chronic L-DOPA on various ionotropic and metabotropic glutamate receptors in the basal ganglia of MPTP monkeys thus showing the widespread activity of mGlu5 receptor negative allosteric modulators in the basal ganglia [18]. MPEP did not affect D1 receptor levels and its mRNA but was associated with an increased in D2 receptors levels, its mRNA and its associated signaling proteins.
Conclusion
In rodent and primate models of PD and LID, mGlu5 receptor negative allosteric modulators are well documented to inhibit dyskinesias and have an extensive range of beneficial biochemical effects. In parkinsonian primate and rodent treated with L-DOPA and mGlu5 negative allosteric modulators, crosstalk between mGlu5-D2-A2A receptors seems to be present whereas recent studies reported that L-DOPA disrupts D2-A2A-CB1 receptor heteromers crosstalk in the striatum of hemiparkinsonian rats [103] and primates in the caudate nucleus but not the putamen (two levels were investigated, pre and post commissural) [102]. The mGlu5 negative allosteric modulator treatment in parkinsonian primates and rats affects not only glutamate receptors but also DA and A2A receptors. This may be indirect by restoring glutamate neurotransmission but could also involve the direct interactions of the trio mGlu5-D2-A2A receptor crosstalk [96, 129]. Moreover, supporting a close mGlu5-A2A receptor interaction, MTEP treatment was reported to decrease mice brain A2A receptor specific binding and regulate the conditioned effects of cocaine [113].
Thus, mGlu5 receptor negative allosteric modulators preventing the development of LID and inhibiting the expression of already developed LID not only affects several glutamate receptors but also D2 and A2A receptors, neuropeptides and Akt/GSK3β and ERK1/2 signaling. An abundant literature mainly from rodent models of PD supports the striatal overactivation of ERK1/2 via DA D1 receptors to be associated with dyskinetic behaviors [130]; reviewed in [131] that is critically modulated by striatal mGlu5 receptors. Thus mGlu5 receptor negative allosteric modulators affect various markers of both the striatal direct and indirect output pathways. The implication of these receptor interactions in mental and neurodegenerative diseases and more specifically in the development and expression of PD symptoms and LID needs to be considered and further investigated to find novel targets and ultimately, novel pharmacological treatments.
Acknowledgements
This work was supported by a grant from the Canadian Institutes of Health Research to TDP. N.M. held a professional health care studentship from the Fonds de la recherche en santé du Québec (FRSQ).
CONFLICT OF INTEREST
NM, MM, LG have no conflict of interest. TDP held research contracts from Novartis, Basel, Switzerland.
REFERENCES
- 1.Siderowf A., Stern M. Update on Parkinson disease. Ann. Intern. Med. 2003;138(8):651–658. doi: 10.7326/0003-4819-138-8-200304150-00013. [http://dx.doi.org/10.7326/0003-4819-138-8-200304150-00013]. [PMID: 12693888]. [DOI] [PubMed] [Google Scholar]
- 2.Toulouse A., Sullivan A.M. Progress in Parkinson’s disease-where do we stand? Prog. Neurobiol. 2008;85(4):376–392. doi: 10.1016/j.pneurobio.2008.05.003. [http:// dx.doi.org/10.1016/j.pneurobio.2008.05.003]. [PMID: 18582530]. [DOI] [PubMed] [Google Scholar]
- 3.Mercuri N.B., Bernardi G. The ‘magic’ of L-dopa: why is it the gold standard Parkinson’s disease therapy? Trends Pharmacol. Sci. 2005;26(7):341–344. doi: 10.1016/j.tips.2005.05.002. [http://dx.doi.org/10.1016/j.tips.2005.05.002]. [PMID: 15936832]. [DOI] [PubMed] [Google Scholar]
- 4.Fabbrini G., Brotchie J.M., Grandas F., Nomoto M., Goetz C.G. Levodopa-induced dyskinesias. Mov. Disord. 2007;22(10):1379–1389. doi: 10.1002/mds.21475. [http://dx.doi.org/10.1002/mds.21475]. [PMID: 17427940]. [DOI] [PubMed] [Google Scholar]
- 5.Ahlskog J.E., Muenter M.D. Frequency of levodopa-related dyskinesias and motor fluctuations as estimated from the cumulative literature. Mov. Disord. 2001;16(3):448–458. doi: 10.1002/mds.1090. [http://dx.doi.org/ 10.1002/mds.1090]. [PMID: 11391738]. [DOI] [PubMed] [Google Scholar]
- 6.Hely M.A., Morris J.G., Reid W.G., Trafficante R. Sydney Multicenter Study of Parkinson’s disease: non-L-dopa-responsive problems dominate at 15 years. Mov. Disord. 2005;20(2):190–199. doi: 10.1002/mds.20324. [http://dx.doi.org/10.1002/mds.20324]. [PMID: 15551331]. [DOI] [PubMed] [Google Scholar]
- 7.Jenner P. Molecular mechanisms of L-DOPA-induced dyskinesia. Nat. Rev. Neurosci. 2008;9(9):665–677. doi: 10.1038/nrn2471. [http://dx.doi.org/10. 1038/nrn2471]. [PMID: 18714325]. [DOI] [PubMed] [Google Scholar]
- 8.Calon F., Rajput A.H., Hornykiewicz O., Bédard P.J., Di Paolo T. Levodopa-induced motor complications are associated with alterations of glutamate receptors in Parkinson’s disease. Neurobiol. Dis. 2003;14(3):404–416. doi: 10.1016/j.nbd.2003.07.003. [http://dx.doi.org/10.1016/ j.nbd.2003.07.003]. [PMID: 14678757]. [DOI] [PubMed] [Google Scholar]
- 9.Chase T.N., Oh J.D. Striatal mechanisms and pathogenesis of parkinsonian signs and motor complications. 2000. [PubMed]
- 10.Fox S.H., Katzenschlager R., Lim S.Y., Ravina B., Seppi K., Coelho M., Poewe W., Rascol O., Goetz C.G., Sampaio C. The Movement Disorder Society Evidence-Based Medicine Review Update: Treatments for the motor symptoms of Parkinson’s disease. Mov. Disord. 2011;26(Suppl. 3):S2–S41. doi: 10.1002/mds.23829. [http://dx.doi. org/10.1002/mds.23829]. [PMID: 22021173]. [DOI] [PubMed] [Google Scholar]
- 11.Meissner W.G., Frasier M., Gasser T., Goetz C.G., Lozano A., Piccini P., Obeso J.A., Rascol O., Schapira A., Voon V., Weiner D.M., Tison F., Bezard E. Priorities in Parkinson’s disease research. Nat. Rev. Drug Discov. 2011;10(5):377–393. doi: 10.1038/nrd3430. [http://dx.doi.org/10.1038/nrd3430]. [PMID: 21532567]. [DOI] [PubMed] [Google Scholar]
- 12.Sawada H., Oeda T., Kuno S., Nomoto M., Yamamoto K., Yamamoto M., Hisanaga K., Kawamura T. Amantadine for dyskinesias in Parkinson’s disease: a randomized controlled trial. PLoS One. 2010;5(12):e15298. doi: 10.1371/journal.pone.0015298. [http://dx.doi.org/10.1371/ journal.pone.0015298]. [PMID: 21217832]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schaeffer E., Pilotto A., Berg D. Pharmacological strategies for the management of levodopa-induced dyskinesia in patients with Parkinson’s disease. CNS Drugs. 2014;28(12):1155–1184. doi: 10.1007/s40263-014-0205-z. [http://dx.doi.org/10.1007/s40263-014-0205-z]. [PMID: 25342080]. [DOI] [PubMed] [Google Scholar]
- 14.Verhagen Metman L., Del Dotto P., van den Munckhof P., Fang J., Mouradian M.M., Chase T.N. Amantadine as treatment for dyskinesias and motor fluctuations in Parkinson’s disease. Neurology. 1998;50(5):1323–1326. doi: 10.1212/wnl.50.5.1323. [http://dx.doi.org/10.1212/ WNL.50.5.1323]. [PMID: 9595981]. [DOI] [PubMed] [Google Scholar]
- 15.Stocchi F., Tagliati M., Olanow C.W. Treatment of levodopa-induced motor complications. Mov. Disord. 2008;23(Suppl. 3):S599–S612. doi: 10.1002/mds.22052. [http://dx.doi.org/10.1002/mds.22052]. [PMID: 18781681]. [DOI] [PubMed] [Google Scholar]
- 16.Conn P.J., Battaglia G., Marino M.J., Nicoletti F. Metabotropic glutamate receptors in the basal ganglia motor circuit. Nat. Rev. Neurosci. 2005;6(10):787–798. doi: 10.1038/nrn1763. [http://dx.doi.org/10.1038/ nrn1763]. [PMID: 16276355]. [DOI] [PubMed] [Google Scholar]
- 17.Morin N., Grégoire L., Morissette M., Desrayaud S., Gomez-Mancilla B., Gasparini F., Di Paolo T. MPEP, an mGlu5 receptor antagonist, reduces the development of L-DOPA-induced motor complications in de novo parkinsonian monkeys: biochemical correlates. Neuropharmacology. 2013;66:355–364. doi: 10.1016/j.neuropharm.2012.07.036. [http://dx.doi. org/10.1016/j.neuropharm.2012.07.036]. [PMID: 22884464]. [DOI] [PubMed] [Google Scholar]
- 18.Morin N., Morissette M., Grégoire L., Gomez-Mancilla B., Gasparini F., Di Paolo T. Chronic treatment with MPEP, an mGlu5 receptor antagonist, normalizes basal ganglia glutamate neurotransmission in L-DOPA-treated parkinsonian monkeys. Neuropharmacology. 2013;73:216–231. doi: 10.1016/j.neuropharm.2013.05.028. [http://dx.doi.org/10. 1016/j.neuropharm.2013.05.028]. [PMID: 23756168]. [DOI] [PubMed] [Google Scholar]
- 19.Ouattara B., Gasparini F., Morissette M., Grégoire L., Samadi P., Gomez-Mancilla B., Di Paolo T. Effect of L-Dopa on metabotropic glutamate receptor 5 in the brain of parkinsonian monkeys. J. Neurochem. 2010;113(3):715–724. doi: 10.1111/j.1471-4159.2010.06635.x. [http://dx.doi. org/10.1111/j.1471-4159.2010.06635.x]. [PMID: 20132464]. [DOI] [PubMed] [Google Scholar]
- 20.Ouattara B., Grégoire L., Morissette M., Gasparini F., Vranesic I., Bilbe G., Johns D.R., Rajput A., Hornykiewicz O., Rajput A.H., Gomez-Mancilla B., Di Paolo T. Metabotropic glutamate receptor type 5 in levodopa-induced motor complications. Neurobiol. Aging. 2011;32(7):1286–1295. doi: 10.1016/j.neurobiolaging.2009.07.014. [http://dx.doi.org/10.1016/j. neurobiolaging.2009.07.014]. [PMID: 20036444]. [DOI] [PubMed] [Google Scholar]
- 21.Samadi P., Grégoire L., Morissette M., Calon F., Hadj Tahar A., Dridi M., Belanger N., Meltzer L.T., Bédard P.J., Di Paolo T. mGluR5 metabotropic glutamate receptors and dyskinesias in MPTP monkeys. Neurobiol. Aging. 2008;29(7):1040–1051. doi: 10.1016/j.neurobiolaging.2007.02.005. [http:// dx.doi.org/10.1016/j.neurobiolaging.2007.02.005]. [PMID: 17353071]. [DOI] [PubMed] [Google Scholar]
- 22.Dekundy A., Gravius A., Hechenberger M., Pietraszek M., Nagel J., Tober C., van der Elst M., Mela F., Parsons C.G., Danysz W. Pharmacological characterization of MRZ-8676, a novel negative allosteric modulator of subtype 5 metabotropic glutamate receptors (mGluR5): focus on L: -DOPA-induced dyskinesia. J Neural Transm (Vienna) 2011;118(12):1703–1716. doi: 10.1007/s00702-010-0526-0. [http://dx.doi.org/10.1007/s00702-010-0526-0]. [PMID: 21161716]. [DOI] [PubMed] [Google Scholar]
- 23.Dekundy A., Pietraszek M., Schaefer D., Cenci M.A., Danysz W. Effects of group I metabotropic glutamate receptors blockade in experimental models of Parkinson’s disease. Brain Res. Bull. 2006;69(3):318–326. doi: 10.1016/j.brainresbull.2005.12.009. [http://dx.doi.org/10.1016/j.brainresbull. 2005.12.009]. [PMID: 16564428]. [DOI] [PubMed] [Google Scholar]
- 24.Gravius A., Dekundy A., Nagel J., Morè L., Pietraszek M., Danysz W. Investigation on tolerance development to subchronic blockade of mGluR5 in models of learning, anxiety, and levodopa-induced dyskinesia in rats. J Neural Transm (Vienna) 2008;115(12):1609–1619. doi: 10.1007/s00702-008-0098-4. [http://dx.doi.org/10.1007/s00702-008-0098-4]. [PMID: 18690408]. [DOI] [PubMed] [Google Scholar]
- 25.Levandis G., Bazzini E., Armentero M.T., Nappi G., Blandini F. Systemic administration of an mGluR5 antagonist, but not unilateral subthalamic lesion, counteracts l-DOPA-induced dyskinesias in a rodent model of Parkinson’s disease. Neurobiol. Dis. 2008;29(1):161–168. doi: 10.1016/j.nbd.2007.08.011. [http://dx.doi.org/10.1016/j.nbd. 2007.08.011]. [PMID: 17933546]. [DOI] [PubMed] [Google Scholar]
- 26.Mela F., Marti M., Dekundy A., Danysz W., Morari M., Cenci M.A. Antagonism of metabotropic glutamate receptor type 5 attenuates l-DOPA-induced dyskinesia and its molecular and neurochemical correlates in a rat model of Parkinson’s disease. J. Neurochem. 2007;101(2):483–497. doi: 10.1111/j.1471-4159.2007.04456.x. [http://dx.doi.org/10.1111/ j.1471-4159.2007.04456.x]. [PMID: 17359492]. [DOI] [PubMed] [Google Scholar]
- 27.Grégoire L., Morin N., Ouattara B., Gasparini F., Bilbe G., Johns D., Vranesic I., Sahasranaman S., Gomez-Mancilla B., Di Paolo T. The acute antiparkinsonian and antidyskinetic effect of AFQ056, a novel metabotropic glutamate receptor type 5 antagonist, in L-Dopa-treated parkinsonian monkeys. Parkinsonism Relat. Disord. 2011;17(4):270–276. doi: 10.1016/j.parkreldis.2011.01.008. [http://dx.doi.org/10.1016/ j.parkreldis.2011.01.008]. [PMID: 21315648]. [DOI] [PubMed] [Google Scholar]
- 28.Johnston T.H., Fox S.H., McIldowie M.J., Piggott M.J., Brotchie J.M. Reduction of L-DOPA-induced dyskinesia by the selective metabotropic glutamate receptor 5 antagonist 3-[(2-methyl-1,3-thiazol-4-yl)ethynyl]pyridine in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-lesioned macaque model of Parkinson’s disease. J. Pharmacol. Exp. Ther. 2010;333(3):865–873. doi: 10.1124/jpet.110.166629. [http://dx.doi.org/10.1124/jpet.110.166629]. [PMID: 20231306]. [DOI] [PubMed] [Google Scholar]
- 29.Morin N., Grégoire L., Gomez-Mancilla B., Gasparini F., Di Paolo T. Effect of the metabotropic glutamate receptor type 5 antagonists MPEP and MTEP in parkinsonian monkeys. Neuropharmacology. 2010;58(7):981–986. doi: 10.1016/j.neuropharm.2009.12.024. [http://dx.doi.org/10.1016/ j.neuropharm.2009.12.024]. [PMID: 20074579]. [DOI] [PubMed] [Google Scholar]
- 30.Marin C., Bonastre M., Aguilar E., Jiménez A. The metabotropic glutamate receptor antagonist 2-methyl-6-(phenylethynyl) pyridine decreases striatal VGlut2 expression in association with an attenuation of L-DOPA-induced dyskinesias. Synapse. 2011;65(10):1080–1086. doi: 10.1002/syn.20941. [http://dx.doi.org/10.1002/syn.20941]. [PMID: 21484883]. [DOI] [PubMed] [Google Scholar]
- 31.Therapeutics A. http://www.addextherapeutics.com/ investors/press-releases/news-details/article/addex-reportspositive- top-line-phase-iia-data-for-dipraglurant-in-parkinsonsdisease- levodopa-indu/ 2013.
- 32.Berg D., Godau J., Trenkwalder C., Eggert K., Csoti I., Storch A., Huber H., Morelli-Canelo M., Stamelou M., Ries V., Wolz M., Schneider C., Di Paolo T., Gasparini F., Hariry S., Vandemeulebroecke M., Abi-Saab W., Cooke K., Johns D., Gomez-Mancilla B. AFQ056 treatment of levodopa-induced dyskinesias: results of 2 randomized controlled trials. Mov. Disord. 2011;26(7):1243–1250. doi: 10.1002/mds.23616. [http://dx.doi.org/10.1002/mds.23616]. [PMID: 21484867]. [DOI] [PubMed] [Google Scholar]
- 33.Stocchi F., Rascol O., Destee A., Hattori N., Hauser R.A., Lang A.E., Poewe W., Stacy M., Tolosa E., Gao H., Nagel J., Merschhemke M., Graf A., Kenney C., Trenkwalder C. AFQ056 in Parkinson patients with levodopa-induced dyskinesia: 13-week, randomized, dose-finding study. Mov. Disord. 2013;28(13):1838–1846. doi: 10.1002/mds.25561. [http://dx.doi.org/10.1002/mds.25561]. [PMID: 23853029]. [DOI] [PubMed] [Google Scholar]
- 34.Stocchi F., Balaguer E., Trekwalder C., Shah A., Dronamraju N., Tran M., Kenney C., Graf A. 12-Week, Double-Blind, Placebo-Controlled, Fixed-Dose Study Of Immediate Release AFQ056, An mGluR5 Receptor Antagonist, In Parkinson's Disease Patients With Moderate-To-Severe L-Dopa Induced Dyskinesias. Mov. Disord. 2014;29(Suppl. 1):723. [Google Scholar]
- 35.Trenkwalder C., Kulisevsky J., Poewe W., Shah A., Han G., Tran M., Kenney C., Graf A. 13-Week, Double-Blind, Placebo-Controlled, Fixed-Dose Study Of Modified Release AFQ056, An mGluR5 Receptor Antagonist, In Parkinson's Disease Patients With Moderate-To-Severe L-Dopa Induced Dyskinesias. Mov. Disord. 2014;29(Suppl. 1):733. [Google Scholar]
- 36.Cenci M.A., Lundblad M. Post- versus presynaptic plasticity in L-DOPA-induced dyskinesia. J. Neurochem. 2006;99(2):381–392. doi: 10.1111/j.1471-4159.2006.04124.x. [http://dx.doi.org/10.1111/j.1471-4159.2006.04124.x]. [PMID: 16942598]. [DOI] [PubMed] [Google Scholar]
- 37.Blandini F., Armentero M.T. New pharmacological avenues for the treatment of L-DOPA-induced dyskinesias in Parkinson’s disease: targeting glutamate and adenosine receptors. Expert Opin. Investig. Drugs. 2012;21(2):153–168. doi: 10.1517/13543784.2012.651457. [http://dx.doi.org/10.1517/ 13543784.2012.651457]. [PMID: 22233485]. [DOI] [PubMed] [Google Scholar]
- 38.Beaulieu J.M., Gainetdinov R.R. The physiology, signaling, and pharmacology of dopamine receptors. Pharmacol. Rev. 2011;63(1):182–217. doi: 10.1124/pr.110.002642. [http://dx.doi.org/10.1124/pr.110.002642]. [PMID: 21303898]. [DOI] [PubMed] [Google Scholar]
- 39.DeLong M.R., Wichmann T. Circuits and circuit disorders of the basal ganglia. Arch. Neurol. 2007;64(1):20–24. doi: 10.1001/archneur.64.1.20. [http://dx.doi.org/ 10.1001/archneur.64.1.20]. [PMID: 17210805]. [DOI] [PubMed] [Google Scholar]
- 40.Hurd Y.L., Suzuki M., Sedvall G.C. D1 and D2 dopamine receptor mRNA expression in whole hemisphere sections of the human brain. J. Chem. Neuroanat. 2001;22(1-2):127–137. doi: 10.1016/s0891-0618(01)00122-3. [http:// dx.doi.org/10.1016/S0891-0618(01)00122-3]. [PMID: 11470560]. [DOI] [PubMed] [Google Scholar]
- 41.Creese I., Burt D.R., Snyder S.H. Dopamine receptor binding enhancement accompanies lesion-induced behavioral super- sensitivity. Science. 1977;197(4303):596–598. doi: 10.1126/science.877576. [http://dx.doi.org/ 10.1126/science.877576]. [PMID: 877576]. [DOI] [PubMed] [Google Scholar]
- 42.Lee T., Seeman P., Rajput A., Farley I.J., Hornykiewicz O. Receptor basis for dopaminergic supersensitivity in Parkinson’s disease. Nature. 1978;273(5657):59–61. doi: 10.1038/273059a0. [http://dx.doi.org/10.1038/ 273059a0]. [PMID: 692671]. [DOI] [PubMed] [Google Scholar]
- 43.Guttman M., Seeman P., Reynolds G.P., Riederer P., Jellinger K., Tourtellotte W.W. Dopamine D2 receptor density remains constant in treated Parkinson’s disease. Ann. Neurol. 1986;19(5):487–492. doi: 10.1002/ana.410190510. [http://dx.doi.org/10.1002/ana.410190510]. [PMID: 2940960]. [DOI] [PubMed] [Google Scholar]
- 44.Quik M., Spokes E.G., Mackay A.V., Bannister R. Alterations in [3H]spiperone binding in human caudate nucleus, substantia nigra and frontal cortex in the Shy-Drager syndrome and Parkinson’s disease. J. Neurol. Sci. 1979;43(3):429–437. doi: 10.1016/0022-510x(79)90021-2. [http://dx.doi.org/ 10.1016/0022-510X(79)90021-2]. [PMID: 521836]. [DOI] [PubMed] [Google Scholar]
- 45.Rinne U.K., Lönnberg P., Koskinen V. Dopamine receptors in the Parkinsonian brain. J. Neural Transm. 1981;51(1-2):97–106. doi: 10.1007/BF01664007. [http://dx.doi.org/10.1007/BF01664007]. [PMID: 6114985]. [DOI] [PubMed] [Google Scholar]
- 46.Bédard P.J., Di Paolo T., Falardeau P., Boucher R. Chronic treatment with L-DOPA, but not bromocriptine induces dyskinesia in MPTP-parkinsonian monkeys. Correlation with [3H]spiperone binding. Brain Res. 1986;379(2):294–299. doi: 10.1016/0006-8993(86)90783-3. [http://dx.doi.org/ 10.1016/0006-8993(86)90783-3]. [PMID: 3488796]. [DOI] [PubMed] [Google Scholar]
- 47.Falardeau P., Bouchard S., Bédard P.J., Boucher R., Di Paolo T. Behavioral and biochemical effect of chronic treatment with D-1 and/or D-2 dopamine agonists in MPTP monkeys. Eur. J. Pharmacol. 1988;150(1-2):59–66. doi: 10.1016/0014-2999(88)90750-9. [http://dx.doi.org/10.1016/ 0014-2999(88)90750-9]. [PMID: 3261249]. [DOI] [PubMed] [Google Scholar]
- 48.Gagnon C., Bédard P.J., Di Paolo T. Effect of chronic treatment of MPTP monkeys with dopamine D-1 and/or D-2 receptor agonists. Eur. J. Pharmacol. 1990;178(1):115–120. doi: 10.1016/0014-2999(90)94802-5. [http://dx.doi. org/10.1016/0014-2999(90)94802-5]. [PMID: 1970537]. [DOI] [PubMed] [Google Scholar]
- 49.Berretta S., Parthasarathy H.B., Graybiel A.M. Local release of GABAergic inhibition in the motor cortex induces immediate-early gene expression in indirect pathway neurons of the striatum. J. Neurosci. 1997;17(12):4752–4763. doi: 10.1523/JNEUROSCI.17-12-04752.1997. [PMID: 9169535]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Goulet M., Grondin R., Morissette M., Maltais S., Falardeau P., Bédard P.J., Di Paolo T. Regulation by chronic treatment with cabergoline of dopamine D1 and D2 receptor levels and their expression in the striatum of Parkinsonian-monkeys. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2000;24(4):607–617. doi: 10.1016/s0278-5846(00)00096-8. [http:// dx.doi.org/10.1016/S0278-5846(00)00096-8]. [PMID: 10958154]. [DOI] [PubMed] [Google Scholar]
- 51.Goulet M., Morissette M., Calon F., Blanchet P.J., Falardeau P., Bédard P.J., Di Paolo T. Continuous or pulsatile chronic D2 dopamine receptor agonist (U91356A) treatment of drug-naive 4-phenyl-1,2,3,6-tetrahydropyridine monkeys differentially regulates brain D1 and D2 receptor expression: in situ hybridization histo- chemical analysis. Neuroscience. 1997;79(2):497–507. doi: 10.1016/s0306-4522(96)00689-6. [http:// dx.doi.org/10.1016/S0306-4522(96)00689-6]. [PMID: 9200732]. [DOI] [PubMed] [Google Scholar]
- 52.Morissette M., Goulet M., Calon F., Falardeau P., Blanchet P.J., Bédard P.J., Di Paolo T. Changes of D1 and D2 dopamine receptor mRNA in the brains of monkeys lesioned with 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine: correction with chronic administration of L-3,4-dihydroxyphenylalanine. Mol. Pharmacol. 1996;50(5):1073–1079. [PMID: 8913337]. [PubMed] [Google Scholar]
- 53.Aubert I., Guigoni C., Håkansson K., Li Q., Dovero S., Barthe N., Bioulac B.H., Gross C.E., Fisone G., Bloch B., Bezard E. Increased D1 dopamine receptor signaling in levodopa-induced dyskinesia. Ann. Neurol. 2005;57(1):17–26. doi: 10.1002/ana.20296. [http://dx.doi.org/ 10.1002/ana.20296]. [PMID: 15514976]. [DOI] [PubMed] [Google Scholar]
- 54.Herrero M.T., Augood S.J., Asensi H., Hirsch E.C., Agid Y., Obeso J.A., Emson P.C. Effects of L-DOPA-therapy on dopamine D2 receptor mRNA expression in the striatum of MPTP-intoxicated parkinsonian monkeys. Brain Res. Mol. Brain Res. 1996;42(1):149–155. doi: 10.1016/s0169-328x(96)00157-x. [http://dx.doi.org/10.1016/S0169-328X(96)00157-X]. [PMID: 8915594]. [DOI] [PubMed] [Google Scholar]
- 55.Fuxe K., Marcellino D., Rivera A., Diaz-Cabiale Z., Filip M., Gago B., Roberts D.C., Langel U., Genedani S., Ferraro L., de la Calle A., Narvaez J., Tanganelli S., Woods A., Agnati L.F. Receptor-receptor interactions within receptor mosaics. Impact on neuropsychopharmacology. Brain Res. Brain Res. Rev. 2008;58(2):415–452. doi: 10.1016/j.brainresrev.2007.11.007. [http://dx.doi.org/10.1016/j.brainresrev.2007.11.007]. [PMID: 18222544]. [DOI] [PubMed] [Google Scholar]
- 56.Samadi P., Rouillard C., Bédard P.J., Di Paolo T. Functional neurochemistry of the basal ganglia. Handb. Clin. Neurol. 2007;83:19–66. doi: 10.1016/S0072-9752(07)83002-8. [http://dx.doi.org/10.1016/S0072-9752(07)83002-8]. [PMID: 18808909]. [DOI] [PubMed] [Google Scholar]
- 57.Cosford N.D., Tehrani L., Roppe J., Schweiger E., Smith N.D., Anderson J., Bristow L., Brodkin J., Jiang X., McDonald I., Rao S., Washburn M., Varney M.A. 3-[(2-Methyl-1,3-thiazol-4-yl)ethynyl]-pyridine: a potent and highly selective metabotropic glutamate subtype 5 receptor antagonist with anxiolytic activity. J. Med. Chem. 2003;46(2):204–206. doi: 10.1021/jm025570j. [http://dx.doi.org/10.1021/ jm025570j]. [PMID: 12519057]. [DOI] [PubMed] [Google Scholar]
- 58.Gasparini F., Lingenhöhl K., Stoehr N., Flor P.J., Heinrich M., Vranesic I., Biollaz M., Allgeier H., Heckendorn R., Urwyler S., Varney M.A., Johnson E.C., Hess S.D., Rao S.P., Sacaan A.I., Santori E.M., Veliçelebi G., Kuhn R. 2-Methyl-6-(phenylethynyl)-pyridine (MPEP), a potent, selective and systemically active mGlu5 receptor antagonist. Neuropharmacology. 1999;38(10):1493–1503. doi: 10.1016/s0028-3908(99)00082-9. [http://dx.doi.org/10.1016/S0028-3908(99) 00082-9]. [PMID: 10530811]. [DOI] [PubMed] [Google Scholar]
- 59.Lea P.M., IV, Faden A.I. Metabotropic glutamate receptor subtype 5 antagonists MPEP and MTEP. CNS Drug Rev. 2006;12(2):149–166. doi: 10.1111/j.1527-3458.2006.00149.x. [http://dx.doi.org/10.1111/j.1527-3458.2006.00149.x]. [PMID: 16958988]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mathiesen J.M., Svendsen N., Bräuner-Osborne H., Thomsen C., Ramirez M.T. Positive allosteric modulation of the human metabotropic glutamate receptor 4 (hmGluR4) by SIB-1893 and MPEP. Br. J. Pharmacol. 2003;138(6):1026–1030. doi: 10.1038/sj.bjp.0705159. [http://dx.doi. org/10.1038/sj.bjp.0705159]. [PMID: 12684257]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nagel J., Greco S., Parsons C.G., Flik G., Tober C., Klein K.U., Danysz W. Brain concentrations of mGluR5 negative allosteric modulator MTEP in relation to receptor occupancy--Comparison to MPEP. Pharmacol. Rep. 2015;67(3):624–630. doi: 10.1016/j.pharep.2015.01.004. [http://dx.doi.org/10.1016/j.pharep.2015.01.004]. [PMID: 25933979]. [DOI] [PubMed] [Google Scholar]
- 62.Dunwiddie T.V., Masino S.A. The role and regulation of adenosine in the central nervous system. Annu. Rev. Neurosci. 2001;24:31–55. doi: 10.1146/annurev.neuro.24.1.31. [http://dx.doi.org/10.1146/annurev.neuro.24.1.31]. [PMID: 11283304]. [DOI] [PubMed] [Google Scholar]
- 63.Ribeiro J.A., Sebastião A.M., de Mendonça A. Adenosine receptors in the nervous system: pathophysiological implications. Prog. Neurobiol. 2002;68(6):377–392. doi: 10.1016/s0301-0082(02)00155-7. [http://dx.doi.org/10.1016/ S0301-0082(02)00155-7]. [PMID: 12576292]. [DOI] [PubMed] [Google Scholar]
- 64.Ferré S., Ciruela F., Canals M., Marcellino D., Burgueno J., Casadó V., Hillion J., Torvinen M., Fanelli F., Benedetti Pd Pd., Goldberg S.R., Bouvier M., Fuxe K., Agnati L.F., Lluis C., Franco R., Woods A. Adenosine A2A-dopamine D2 receptor-receptor heteromers. Targets for neuro-psychiatric disorders. Parkinsonism Relat. Disord. 2004;10(5):265–271. doi: 10.1016/j.parkreldis.2004.02.014. [http://dx.doi. org/10.1016/j.parkreldis.2004.02.014]. [PMID: 15196504]. [DOI] [PubMed] [Google Scholar]
- 65.Kase H. New aspects of physiological and pathophysiological functions of adenosine A2A receptor in basal ganglia. Biosci. Biotechnol. Biochem. 2001;65(7):1447–1457. doi: 10.1271/bbb.65.1447. [http://dx.doi.org/ 10.1271/bbb.65.1447]. [PMID: 11515525]. [DOI] [PubMed] [Google Scholar]
- 66.Martinez-Mir M.I., Probst A., Palacios J.M. Adenosine A2 receptors: selective localization in the human basal ganglia and alterations with disease. Neuroscience. 1991;42(3):697–706. doi: 10.1016/0306-4522(91)90038-p. [http:// dx.doi.org/10.1016/0306-4522(91)90038-P]. [PMID: 1835521]. [DOI] [PubMed] [Google Scholar]
- 67.Morelli M., Di Paolo T., Wardas J., Calon F., Xiao D., Schwarzschild M.A. Role of adenosine A2A receptors in parkinsonian motor impairment and l-DOPA-induced motor complications. Prog. Neurobiol. 2007;83(5):293–309. doi: 10.1016/j.pneurobio.2007.07.001. [http://dx. doi.org/10.1016/j.pneurobio.2007.07.001]. [PMID: 17826884]. [DOI] [PubMed] [Google Scholar]
- 68.Schiffmann S.N., Jacobs O., Vanderhaeghen J.J. Striatal restricted adenosine A2 receptor (RDC8) is expressed by enkephalin but not by substance P neurons: an in situ hybridization histochemistry study. J. Neurochem. 1991;57(3):1062–1067. doi: 10.1111/j.1471-4159.1991.tb08257.x. [http://dx.doi.org/10.1111/j.1471-4159.1991.tb08257.x]. [PMID: 1713612]. [DOI] [PubMed] [Google Scholar]
- 69.Svenningsson P., Le Moine C., Aubert I., Burbaud P., Fredholm B.B., Bloch B. Cellular distribution of adenosine A2A receptor mRNA in the primate striatum. J. Comp. Neurol. 1998;399(2):229–240. doi: 10.1002/(sici)1096-9861(19980921)399:2<229::aid-cne6>3.0.co;2-2. [http://dx.doi.org/10.1002/(SICI)1096-9861(19980921) 399:2<229:AID-CNE6>3.0.CO;2-2]. [PMID: 9721905]. [DOI] [PubMed] [Google Scholar]
- 70.Domenici M.R., Pepponi R., Martire A., Tebano M.T., Potenza R.L., Popoli P. Permissive role of adenosine A2A receptors on metabotropic glutamate receptor 5 (mGluR5)-mediated effects in the striatum. J. Neurochem. 2004;90(5):1276–1279. doi: 10.1111/j.1471-4159.2004.02607.x. [http://dx.doi. org/10.1111/j.1471-4159.2004.02607.x]. [PMID: 15312183]. [DOI] [PubMed] [Google Scholar]
- 71.Ferré S., Karcz-Kubicha M., Hope B.T., Popoli P., Burgueño J., Gutiérrez M.A., Casadó V., Fuxe K., Goldberg S.R., Lluis C., Franco R., Ciruela F. Synergistic interaction between adenosine A2A and glutamate mGlu5 receptors: implications for striatal neuronal function. Proc. Natl. Acad. Sci. USA. 2002;99(18):11940–11945. doi: 10.1073/pnas.172393799. [http://dx.doi.org/10.1073/pnas.172393799]. [PMID: 12189203]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fredholm B.B., IJzerman A.P., Jacobson K.A., Klotz K.N., Linden J. International Union of Pharmacology. XXV. Nomenclature and classification of adenosine receptors. Pharmacol. Rev. 2001;53(4):527–552. [PMID: 11734617]. [PMC free article] [PubMed] [Google Scholar]
- 73.Rosin D.L., Hettinger B.D., Lee A., Linden J. Anatomy of adenosine A2A receptors in brain: morphological substrates for integration of striatal function. Neurology. 2003;61(11) Suppl. 6:S12–S18. doi: 10.1212/01.wnl.0000095205.33940.99. [http://dx.doi.org/10.1212/01.WNL.0000095205.33940. 99]. [PMID: 14663003]. [DOI] [PubMed] [Google Scholar]
- 74.Rosin D.L., Robeva A., Woodard R.L., Guyenet P.G., Linden J. Immunohistochemical localization of adenosine A2A receptors in the rat central nervous system. J. Comp. Neurol. 1998;401(2):163–186. [http://dx.doi.org/10.1002/(SICI)1096-9861(19981116) 401:2<163:AID-CNE2>3.0.CO;2-D]. [PMID: 9822147]. [PubMed] [Google Scholar]
- 75.Ferré S., Fredholm B.B., Morelli M., Popoli P., Fuxe K. Adenosine-dopamine receptor-receptor interactions as an integrative mechanism in the basal ganglia. Trends Neurosci. 1997;20(10):482–487. doi: 10.1016/s0166-2236(97)01096-5. [http://dx.doi.org/10.1016/S0166-2236(97)01096-5]. [PMID: 9347617]. [DOI] [PubMed] [Google Scholar]
- 76.Svenningsson P., Le Moine C., Fisone G., Fredholm B.B. Distribution, biochemistry and function of striatal adenosine A2A receptors. Prog. Neurobiol. 1999;59(4):355–396. doi: 10.1016/s0301-0082(99)00011-8. [http://dx.doi. org/10.1016/S0301-0082(99)00011-8]. [PMID: 10501634]. [DOI] [PubMed] [Google Scholar]
- 77.Fink J.S., Weaver D.R., Rivkees S.A., Peterfreund R.A., Pollack A.E., Adler E.M., Reppert S.M. Molecular cloning of the rat A2 adenosine receptor: selective co-expression with D2 dopamine receptors in rat striatum. Brain Res. Mol. Brain Res. 1992;14(3):186–195. doi: 10.1016/0169-328x(92)90173-9. [http://dx.doi.org/10.1016/0169-328X(92) 90173-9]. [PMID: 1279342]. [DOI] [PubMed] [Google Scholar]
- 78.Pollack A.E., Harrison M.B., Wooten G.F., Fink J.S. Differential localization of A2a adenosine receptor mRNA with D1 and D2 dopamine receptor mRNA in striatal output pathways following a selective lesion of striatonigral neurons. Brain Res. 1993;631(1):161–166. doi: 10.1016/0006-8993(93)91204-6. [http://dx.doi.org/10.1016/0006-8993(93)91204-6]. [PMID: 8298989]. [DOI] [PubMed] [Google Scholar]
- 79.Chase T.N., Bibbiani F., Bara-Jimenez W., Dimitrova T., Oh-Lee J.D. Translating A2A antagonist KW6002 from animal models to parkinsonian patients. Neurology. 2003;61(11) Suppl. 6:S107–S111. doi: 10.1212/01.wnl.0000095223.08711.48. [http://dx.doi.org/10.1212/01.WNL.0000095223.08711.48]. [PMID: 14663022]. [DOI] [PubMed] [Google Scholar]
- 80.Calon F., Dridi M., Hornykiewicz O., Bédard P.J., Rajput A.H., Di Paolo T. Increased adenosine A2A receptors in the brain of Parkinson’s disease patients with dyskinesias. Brain. 2004;127(Pt 5):1075–1084. doi: 10.1093/brain/awh128. [http://dx.doi.org/10.1093/brain/awh128]. [PMID: 15033896]. [DOI] [PubMed] [Google Scholar]
- 81.Grondin R., Bédard P.J., Hadj Tahar A., Grégoire L., Mori A., Kase H. Antiparkinsonian effect of a new selective adenosine A2A receptor antagonist in MPTP-treated monkeys. Neurology. 1999;52(8):1673–1677. doi: 10.1212/wnl.52.8.1673. [http://dx.doi.org/10.1212/WNL.52.8.1673]. [PMID: 10331698]. [DOI] [PubMed] [Google Scholar]
- 82.Kanda T., Jackson M.J., Smith L.A., Pearce R.K., Nakamura J., Kase H., Kuwana Y., Jenner P. Combined use of the adenosine A(2A) antagonist KW-6002 with L-DOPA or with selective D1 or D2 dopamine agonists increases antiparkinsonian activity but not dyskinesia in MPTP-treated monkeys. Exp. Neurol. 2000;162(2):321–327. doi: 10.1006/exnr.2000.7350. [http://dx.doi.org/10.1006/exnr.2000.7350]. [PMID: 10739638]. [DOI] [PubMed] [Google Scholar]
- 83.Morelli M., Pinna A. Interaction between dopamine and adenosine A2A receptors as a basis for the treatment of Parkinson’s disease. Neurol. Sci. 2001;22(1):71–72. doi: 10.1007/s100720170052. [http://dx.doi.org/10. 1007/s100720170052]. [PMID: 11487207]. [DOI] [PubMed] [Google Scholar]
- 84.Bara-Jimenez W., Sherzai A., Dimitrova T., Favit A., Bibbiani F., Gillespie M., Morris M.J., Mouradian M.M., Chase T.N. Adenosine A(2A) receptor antagonist treatment of Parkinson’s disease. Neurology. 2003;61(3):293–296. doi: 10.1212/01.wnl.0000073136.00548.d4. [http://dx.doi.org/10.1212/01. WNL.0000073136.00548.D4]. [PMID: 12913186]. [DOI] [PubMed] [Google Scholar]
- 85.Hodgson R.A., Bedard P.J., Varty G.B., Kazdoba T.M., Di Paolo T., Grzelak M.E., Pond A.J., Hadjtahar A., Belanger N., Gregoire L., Dare A., Neustadt B.R., Stamford A.W., Hunter J.C. Preladenant, a selective A(2A) receptor antagonist, is active in primate models of movement disorders. Exp. Neurol. 2010;225(2):384–390. doi: 10.1016/j.expneurol.2010.07.011. [http://dx.doi.org/10.1016/j.expneurol.2010.07.011]. [PMID: 20655910]. [DOI] [PubMed] [Google Scholar]
- 86.Salamone J.D. Preladenant, a novel adenosine A(2A) receptor antagonist for the potential treatment of parkinsonism and other disorders. IDrugs. 2010;13(10):723–731. [PMID: 20878595]. [PubMed] [Google Scholar]
- 87.Varty G.B., Hodgson R.A., Pond A.J., Grzelak M.E., Parker E.M., Hunter J.C. The effects of adenosine A2A receptor antagonists on haloperidol-induced movement disorders in primates. Psychopharmacology (Berl.) 2008;200(3):393–401. doi: 10.1007/s00213-008-1214-8. [http://dx.doi.org/10.1007/s00213-008-1214-8]. [PMID: 18594798]. [DOI] [PubMed] [Google Scholar]
- 88.Jenner P. An overview of adenosine A2A receptor antagonists in Parkinson’s disease. Int. Rev. Neurobiol. 2014;119:71–86. doi: 10.1016/B978-0-12-801022-8.00003-9. [http:// dx.doi.org/10.1016/B978-0-12-801022-8.00003-9]. [PMID: 25175961]. [DOI] [PubMed] [Google Scholar]
- 89.Kanda T., Uchida S. Clinical/pharmacological aspect of adenosine A2A receptor antagonist for dyskinesia. Int. Rev. Neurobiol. 2014;119:127–150. doi: 10.1016/B978-0-12-801022-8.00006-4. [http://dx.doi.org/10.1016/B978-0-12-801022-8.00006-4]. [PMID: 25175964]. [DOI] [PubMed] [Google Scholar]
- 90.Preti D., Baraldi P.G., Moorman A.R., Borea P.A., Varani K. History and perspectives of A2A adenosine receptor antagonists as potential therapeutic agents. Med. Res. Rev. 2015;35(4):790–848. doi: 10.1002/med.21344. [http://dx.doi.org/10.1002/med.21344]. [PMID: 25821194]. [DOI] [PubMed] [Google Scholar]
- 91.Tomiyama M. Adenosine receptors and dyskinesia in patho- physiology. Int. Rev. Neurobiol. 2014;119:117–126. doi: 10.1016/B978-0-12-801022-8.00005-2. [http://dx. doi.org/10.1016/B978-0-12-801022-8.00005-2]. [PMID: 25175963]. [DOI] [PubMed] [Google Scholar]
- 92.Uchida S., Tashiro T., Kawai-Uchida M., Mori A., Jenner P., Kanda T. Adenosine A2A-receptor antagonist istradefylline enhances the motor response of L-DOPA without worsening dyskinesia in MPTP-treated common marmosets. J. Pharmacol. Sci. 2014;124(4):480–485. doi: 10.1254/jphs.13250fp. [http://dx.doi.org/10.1254/jphs. 13250FP]. [PMID: 24681641]. [DOI] [PubMed] [Google Scholar]
- 93.Ciruela F., Casadó V., Rodrigues R.J., Luján R., Burgueño J., Canals M., Borycz J., Rebola N., Goldberg S.R., Mallol J., Cortés A., Canela E.I., López-Giménez J.F., Milligan G., Lluis C., Cunha R.A., Ferré S., Franco R. Presynaptic control of striatal glutamatergic neurotransmission by adenosine A1-A2A receptor heteromers. J. Neurosci. 2006;26(7):2080–2087. doi: 10.1523/JNEUROSCI.3574-05.2006. [http://dx.doi. org/10.1523/JNEUROSCI.3574-05.2006]. [PMID: 16481441]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ciruela F., Ferré S., Casadó V., Cortés A., Cunha R.A., Lluis C., Franco R. Heterodimeric adenosine receptors: a device to regulate neurotransmitter release. Cell. Mol. Life Sci. 2006;63(21):2427–2431. doi: 10.1007/s00018-006-6216-2. [http://dx.doi.org/10.1007/s00018-006-6216-2]. [PMID: 17058035]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Schiffmann S.N., Fisone G., Moresco R., Cunha R.A., Ferré S. Adenosine A2A receptors and basal ganglia physiology. Prog. Neurobiol. 2007;83(5):277–292. doi: 10.1016/j.pneurobio.2007.05.001. [http://dx.doi.org/10.1016/ j.pneurobio.2007.05.001]. [PMID: 17646043]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Fuxe K., Ferré S., Genedani S., Franco R., Agnati L.F. Adenosine receptor-dopamine receptor interactions in the basal ganglia and their relevance for brain function. Physiol. Behav. 2007;92(1-2):210–217. doi: 10.1016/j.physbeh.2007.05.034. [http://dx.doi.org/10.1016/j.physbeh. 2007.05.034]. [PMID: 17572452]. [DOI] [PubMed] [Google Scholar]
- 97.Fuxe K., Ferré S., Zoli M., Agnati L.F. Integrated events in central dopamine transmission as analyzed at multiple levels. Evidence for intramembrane adenosine A2A/dopamine D2 and adenosine A1/dopamine D1 receptor interactions in the basal ganglia. Brain Res. Brain Res. Rev. 1998;26(2-3):258–273. doi: 10.1016/s0165-0173(97)00049-0. [http:// dx.doi.org/10.1016/S0165-0173(97)00049-0]. [PMID: 9651540]. [DOI] [PubMed] [Google Scholar]
- 98.Canals M., Marcellino D., Fanelli F., Ciruela F., de Benedetti P., Goldberg S.R., Neve K., Fuxe K., Agnati L.F., Woods A.S., Ferré S., Lluis C., Bouvier M., Franco R. Adenosine A2A-dopamine D2 receptor-receptor heteromerization: qualitative and quantitative assessment by fluorescence and bioluminescence energy transfer. J. Biol. Chem. 2003;278(47):46741–46749. doi: 10.1074/jbc.M306451200. [http://dx.doi.org/10.1074/jbc.M306451200]. [PMID: 12933819]. [DOI] [PubMed] [Google Scholar]
- 99.Hillion J., Canals M., Torvinen M., Casado V., Scott R., Terasmaa A., Hansson A., Watson S., Olah M.E., Mallol J., Canela E.I., Zoli M., Agnati L.F., Ibanez C.F., Lluis C., Franco R., Ferre S., Fuxe K. Coaggregation, cointernalization, and codesensitization of adenosine A2A receptors and dopamine D2 receptors. J. Biol. Chem. 2002;277(20):18091–18097. doi: 10.1074/jbc.M107731200. [http:// dx.doi.org/10.1074/jbc.M107731200]. [PMID: 11872740]. [DOI] [PubMed] [Google Scholar]
- 100.Torvinen M., Torri C., Tombesi A., Marcellino D., Watson S., Lluis C., Franco R., Fuxe K., Agnati L.F. Trafficking of adenosine A2A and dopamine D2 receptors. J. Mol. Neurosci. 2005;25(2):191–200. doi: 10.1385/JMN:25:2:191. [http://dx.doi.org/10.1385/JMN:25:2:191]. [PMID: 15784967]. [DOI] [PubMed] [Google Scholar]
- 101.Salim H., Ferré S., Dalal A., Peterfreund R.A., Fuxe K., Vincent J.D., Lledo P.M. Activation of adenosine A1 and A2A receptors modulates dopamine D2 receptor-induced responses in stably transfected human neuroblastoma cells. J. Neurochem. 2000;74(1):432–439. doi: 10.1046/j.1471-4159.2000.0740432.x. [http://dx.doi.org/10.1046/j.1471-4159.2000. 0740432.x]. [PMID: 10617149]. [DOI] [PubMed] [Google Scholar]
- 102.Bonaventura J., Rico A.J., Moreno E., Sierra S., Sánchez M., Luquin N., Farré D., Müller C.E., Martínez-Pinilla E., Cortés A., Mallol J., Armentero M.T., Pinna A., Canela E.I., Lluís C., McCormick P.J., Lanciego J.L., Casadó V., Franco R. L-DOPA-treatment in primates disrupts the expression of A(2A) adenosine-CB(1) cannabinoid-D(2) dopamine receptor heteromers in the caudate nucleus. Neuropharmacology. 2014;79:90–100. doi: 10.1016/j.neuropharm.2013.10.036. [http:// dx.doi.org/10.1016/j.neuropharm.2013.10.036]. [PMID: 24230991]. [DOI] [PubMed] [Google Scholar]
- 103.Pinna A., Bonaventura J., Farré D., Sánchez M., Simola N., Mallol J., Lluís C., Costa G., Baqi Y., Müller C.E., Cortés A., McCormick P., Canela E.I., Martínez-Pinilla E., Lanciego J.L., Casadó V., Armentero M.T., Franco R. L-DOPA disrupts adenosine A(2A)-cannabinoid CB(1)-dopamine D(2) receptor heteromer cross-talk in the striatum of hemiparkinsonian rats: biochemical and behavioral studies. Exp. Neurol. 2014;253:180–191. doi: 10.1016/j.expneurol.2013.12.021. [http://dx.doi.org/10.1016/j.expneurol.2013.12.021]. [PMID: 24412491]. [DOI] [PubMed] [Google Scholar]
- 104.Bogenpohl J.W., Ritter S.L., Hall R.A., Smith Y. Adenosine A2A receptor in the monkey basal ganglia: ultrastructural localization and colocalization with the metabotropic glutamate receptor 5 in the striatum. J. Comp. Neurol. 2012;520(3):570–589. doi: 10.1002/cne.22751. [http://dx.doi.org/10.1002/cne.22751]. [PMID: 21858817]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Fuxe K., Agnati L.F., Jacobsen K., Hillion J., Canals M., Torvinen M., Tinner-Staines B., Staines W., Rosin D., Terasmaa A., Popoli P., Leo G., Vergoni V., Lluis C., Ciruela F., Franco R., Ferré S. Receptor heteromerization in adenosine A2A receptor signaling: relevance for striatal function and Parkinson’s disease. Neurology. 2003;61(11) Suppl. 6:S19–S23. doi: 10.1212/01.wnl.0000095206.44418.5c. [http://dx.doi.org/10.1212/01.WNL.0000095206.44418.5C]. [PMID: 14663004]. [DOI] [PubMed] [Google Scholar]
- 106.Nishi A., Liu F., Matsuyama S., Hamada M., Higashi H., Nairn A.C., Greengard P. Metabotropic mGlu5 receptors regulate adenosine A2A receptor signaling. Proc. Natl. Acad. Sci. USA. 2003;100(3):1322–1327. doi: 10.1073/pnas.0237126100. [http://dx.doi.org/10.1073/pnas.0237126100]. [PMID: 12538871]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Nash J.E., Brotchie J.M. A common signaling pathway for striatal NMDA and adenosine A2a receptors: implications for the treatment of Parkinson’s disease. J. Neurosci. 2000;20(20):7782–7789. doi: 10.1523/JNEUROSCI.20-20-07782.2000. [PMID: 11027242]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kachroo A., Orlando L.R., Grandy D.K., Chen J.F., Young A.B., Schwarzschild M.A. Interactions between metabotropic glutamate 5 and adenosine A2A receptors in normal and parkinsonian mice. J. Neurosci. 2005;25(45):10414–10419. doi: 10.1523/JNEUROSCI.3660-05.2005. [http://dx.doi.org/ 10.1523/JNEUROSCI.3660-05.2005]. [PMID: 16280580]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Morin N., Jourdain V.A., Morissette M., Grégoire L., Di Paolo T. Long-term treatment with l-DOPA and an mGlu5 receptor antagonist prevents changes in brain basal ganglia dopamine receptors, their associated signaling proteins and neuropeptides in parkinsonian monkeys. Neuropharmacology. 2014;79:688–706. doi: 10.1016/j.neuropharm.2014.01.014. [http://dx.doi.org/10.1016/j.neuropharm.2014.01.014]. [PMID: 24456747]. [DOI] [PubMed] [Google Scholar]
- 110.Furlong T.J., Pierce K.D., Selbie L.A., Shine J. Molecular characterization of a human brain adenosine A2 receptor. Brain Res. Mol. Brain Res. 1992;15(1-2):62–66. doi: 10.1016/0169-328x(92)90152-2. [http://dx.doi.org/10. 1016/0169-328X(92)90152-2]. [PMID: 1331670]. [DOI] [PubMed] [Google Scholar]
- 111.Schiffmann S.N., Libert F., Vassart G., Vanderhaeghen J.J. Distribution of adenosine A2 receptor mRNA in the human brain. Neurosci. Lett. 1991;130(2):177–181. doi: 10.1016/0304-3940(91)90391-6. [http://dx.doi.org/10.1016/ 0304-3940(91)90391-6]. [PMID: 1795877]. [DOI] [PubMed] [Google Scholar]
- 112.Morissette M., Dridi M., Calon F., Hadj Tahar A., Meltzer L.T., Bédard P.J., Di Paolo T. Prevention of dyskinesia by an NMDA receptor antagonist in MPTP monkeys: effect on adenosine A2A receptors. Synapse. 2006;60(3):239–250. doi: 10.1002/syn.20295. [http://dx.doi.org/10. 1002/syn.20295]. [PMID: 16739115]. [DOI] [PubMed] [Google Scholar]
- 113.Brown R.M., Duncan J.R., Stagnitti M.R., Ledent C., Lawrence A.J. mGlu5 and adenosine A2A receptor interactions regulate the conditioned effects of cocaine. Int. J. Neuropsychopharmacol. 2012;15(7):995–1001. doi: 10.1017/S146114571100126X. [http://dx.doi.org/10.1017/S146114571100126X]. [PMID: 21816123]. [DOI] [PubMed] [Google Scholar]
- 114.Varani K., Vincenzi F., Tosi A., Gessi S., Casetta I., Granieri G., Fazio P., Leung E., MacLennan S., Granieri E., Borea P.A. A2A adenosine receptor overexpression and functionality, as well as TNF-alpha levels, correlate with motor symptoms in Parkinson’s disease. FASEB J. 2010;24(2):587–598. doi: 10.1096/fj.09-141044. [http://dx.doi.org/10. 1096/fj.09-141044]. [PMID: 19776336]. [DOI] [PubMed] [Google Scholar]
- 115.Mishina M., Ishiwata K., Naganawa M., Kimura Y., Kitamura S., Suzuki M., Hashimoto M., Ishibashi K., Oda K., Sakata M., Hamamoto M., Kobayashi S., Katayama Y., Ishii K. Adenosine A(2A) receptors measured with [C]TMSX PET in the striata of Parkinson’s disease patients. PLoS One. 2011;6(2):e17338. doi: 10.1371/journal.pone.0017338. [http://dx.doi.org/10.1371/journal.pone.0017338]. [PMID: 21386999]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ramlackhansingh A.F., Bose S.K., Ahmed I., Turkheimer F.E., Pavese N., Brooks D.J. Adenosine 2A receptor availability in dyskinetic and nondyskinetic patients with Parkinson disease. Neurology. 2011;76(21):1811–1816. doi: 10.1212/WNL.0b013e31821ccce4. [http://dx.doi.org/10.1212/ WNL.0b013e31821ccce4]. [PMID: 21606452]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Schwarzschild M.A., Agnati L., Fuxe K., Chen J.F., Morelli M. Targeting adenosine A2A receptors in Parkinson’s disease. Trends Neurosci. 2006;29(11):647–654. doi: 10.1016/j.tins.2006.09.004. [http://dx.doi.org/10.1016/ j.tins.2006.09.004]. [PMID: 17030429]. [DOI] [PubMed] [Google Scholar]
- 118.Huot P., Johnston T.H., Koprich J.B., Fox S.H., Brotchie J.M. The pharmacology of L-DOPA-induced dyskinesia in Parkinson’s disease. Pharmacol. Rev. 2013;65(1):171–222. doi: 10.1124/pr.111.005678. [http://dx.doi.org/ 10.1124/pr.111.005678]. [PMID: 23319549]. [DOI] [PubMed] [Google Scholar]
- 119.Ghiglieri V., Bagetta V., Pendolino V., Picconi B., Calabresi P. Corticostriatal Plastic Changes in Experimental L-DOPA-Induced Dyskinesia. Dyskinesia. Parkinson's disease. 2012;2012:358176. doi: 10.1155/2012/358176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Bonsi P., Cuomo D., Picconi B., Sciamanna G., Tscherter A., Tolu M., Bernardi G., Calabresi P., Pisani A. Striatal meta- botropic glutamate receptors as a target for pharmacotherapy in Parkinson’s disease. Amino Acids. 2007;32(2):189–195. doi: 10.1007/s00726-006-0320-3. [http:// dx.doi.org/10.1007/s00726-006-0320-3]. [PMID: 16715415]. [DOI] [PubMed] [Google Scholar]
- 121.Duty S. Targeting glutamate receptors to tackle the pathogenesis, clinical symptoms and levodopa-induced dyskinesia associated with Parkinson’s disease. CNS Drugs. 2012;26(12):1017–1032. doi: 10.1007/s40263-012-0016-z. [http://dx.doi.org/10.1007/s40263-012-0016-z]. [PMID: 23114872]. [DOI] [PubMed] [Google Scholar]
- 122.Johnson K.A., Conn P.J., Niswender C.M. Glutamate receptors as therapeutic targets for Parkinson’s disease. CNS Neurol. Disord. Drug Targets. 2009;8(6):475–491. doi: 10.2174/187152709789824606. [http://dx.doi.org/10.2174/ 187152709789824606]. [PMID: 19702565]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Samadi P., Rajput A., Calon F., Grégoire L., Hornykiewicz O., Rajput A.H., Di Paolo T. Metabotropic glutamate receptor II in the brains of Parkinsonian patients. J. Neuropathol. Exp. Neurol. 2009;68(4):374–382. doi: 10.1097/NEN.0b013e31819cabe4. [http://dx.doi.org/10.1097/NEN.0b013 e31819cabe4]. [PMID: 19287314]. [DOI] [PubMed] [Google Scholar]
- 124.Jones C.K., Bubser M., Thompson A.D., Dickerson J.W., Turle-Lorenzo N., Amalric M., Blobaum A.L., Bridges T.M., Morrison R.D., Jadhav S., Engers D.W., Italiano K., Bode J., Daniels J.S., Lindsley C.W., Hopkins C.R., Conn P.J., Niswender C.M. The metabotropic glutamate receptor 4-positive allosteric modulator VU0364770 produces efficacy alone and in combination with L-DOPA or an adenosine 2A antagonist in preclinical rodent models of Parkinson’s disease. J. Pharmacol. Exp. Ther. 2012;340(2):404–421. doi: 10.1124/jpet.111.187443. [http://dx.doi.org/10.1124/jpet. 111.187443]. [PMID: 22088953]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Gubellini P., Pisani A., Centonze D., Bernardi G., Calabresi P. Metabotropic glutamate receptors and striatal synaptic plasticity: implications for neurological diseases. Prog. Neurobiol. 2004;74(5):271–300. doi: 10.1016/j.pneurobio.2004.09.005. [http://dx.doi.org/10.1016/j.pneurobio.2004.09. 005]. [PMID: 15582223]. [DOI] [PubMed] [Google Scholar]
- 126.Jiménez A., Bonastre M., Aguilar E., Marin C. Effect of the metabotropic glutamate antagonist MPEP on striatal expression of the Homer family proteins in levodopa-treated hemiparkinsonian rats. Psychopharmacology (Berl.) 2009;206(2):233–242. doi: 10.1007/s00213-009-1600-x. [http://dx.doi.org/10.1007/s00213-009-1600-x]. [PMID: 19636538]. [DOI] [PubMed] [Google Scholar]
- 127.Yamamoto N., Soghomonian J.J. Metabotropic glutamate mGluR5 receptor blockade opposes abnormal involuntary movements and the increases in glutamic acid decarboxylase mRNA levels induced by l-DOPA in striatal neurons of 6-hydroxydopamine-lesioned rats. Neuroscience. 2009;163(4):1171–1180. doi: 10.1016/j.neuroscience.2009.07.060. [http://dx.doi.org/10. 1016/j.neuroscience.2009.07.060]. [PMID: 19660528]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Rylander D., Recchia A., Mela F., Dekundy A., Danysz W., Cenci M.A. Pharmacological modulation of glutamate transmission in a rat model of L-DOPA-induced dyskinesia: effects on motor behavior and striatal nuclear signaling. J. Pharmacol. Exp. Ther. 2009;330(1):227–235. doi: 10.1124/jpet.108.150425. [http://dx.doi.org/10.1124/jpet. 108.150425]. [PMID: 19357321]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Fiorentini C., Savoia P., Savoldi D., Missale C. Receptor heteromers in Parkinson’s disease and L-DOPA-induced dyskinesia. CNS Neurol. Disord. Drug Targets. 2013;12(8):1101–1113. [PMID: 24040823]. [PubMed] [Google Scholar]
- 130.Fieblinger T., Sebastianutto I., Alcacer C., Bimpisidis Z., Maslava N., Sandberg S., Engblom D., Cenci M.A. Mechanisms of dopamine D1 receptor-mediated ERK1/2 activation in the parkinsonian striatum and their modulation by metabotropic glutamate receptor type 5. J. Neurosci. 2014;34(13):4728–4740. doi: 10.1523/JNEUROSCI.2702-13.2014. [http://dx.doi.org/10.1523/JNEUROSCI.2702-13.2014]. [PMID: 24672017]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Cenci M.A., Konradi C. Maladaptive striatal plasticity in L-DOPA-induced dyskinesia. Prog. Brain Res. 2010;183:209–233. doi: 10.1016/S0079-6123(10)83011-0. [http://dx.doi.org/10.1016/S0079-6123(10)83011-0]. [PMID: 20696322]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Rylander D., Iderberg H., Li Q., Dekundy A., Zhang J., Li H., Baishen R., Danysz W., Bezard E., Cenci M.A. A mGluR5 antagonist under clinical development improves L-DOPA-induced dyskinesia in parkinsonian rats and monkeys. Neurobiol. Dis. 2010;39(3):352–361. doi: 10.1016/j.nbd.2010.05.001. [http://dx.doi.org/10.1016/j.nbd.2010.05. 001]. [PMID: 20452425]. [DOI] [PubMed] [Google Scholar]


