Abstract
Considering that ligands of metabotropic glutamate and GABA receptors may exert beneficial effects on schizophrenia, we assessed the actions of the first mGlu4-selective orthosteric agonist, LSP4-2022, in several tests reflecting positive, negative, and cognitive symptoms of schizophrenia. Moreover, we investigated the possible involvement of GABAB receptors in LSP4-2022-induced actions. Hyperactivity induced by MK-801 or amphetamine and DOI-induced head twitches in mice were used as the models of positive symptoms. The social interaction test, modified forced swim test (FST), and novel object recognition (NOR) test were used as the models of negative and cognitive symptoms of schizophrenia. LSP4-2022 inhibited hyperactivity (in a dose-dependent manner, 0.5-2 mg/kg) induced by MK-801 or amphetamine and DOI-induced head twitches. In mGlu4 receptor knockout mice, LSP4-2022 was not effective. However, it reversed MK-801-induced impairment in the social interaction test and the MK-801-induced increase of immobility in the modified FST. In the NOR test, LSP4-2022 was active at a dose of 2 mg/kg. GABAB receptor antagonist, CGP55845 (10 mg/kg), reversed LSP4-2022-induced effects in hyperactivity and head twitch tests. At the same time, the simultaneous administration of subeffective doses of LSP4-2022 (0.1 mg/kg) and a positive allosteric modulator of GABAB receptor PAM, GS39783 (0.1 mg/kg), induced clear antipsychotic-like effects in those two tests. Such an interaction between mGlu4 and GABAB receptors was not observed in the social interaction and NOR tests. Therefore, we suggest that the activation of the mGlu4 receptor is a promising approach facilitating the discovery of novel antipsychotic drugs, and that the interplay between mGlu4 and GABAB receptors may become the basis for a novel therapy for schizophrenic patients with predomination of positive symptoms.
Keywords: GABAB, LSP4-2022, mGlu4, schizophrenia
Introduction
A number of our previous studies focused on the role of the glutamatergic system in the pathophysiology and treatment of severe mental disorders. In particular, we concentrated on the role of metabotropic receptors that are linked with G-proteins and mediate the slow synaptic current [1]. In recent years, we have become particularly interested in metabotropic receptors for the two main amino acids in the brain, namely, glutamate and GABA. There are eight types of metabotropic glutamate receptors (mGlu) divided into three groups according to sequence homology, pharmacology, and the second messenger system they activate (mGlu1/5, mGlu2/3, and mGlu4/7/8, respectively) [2], and one type of the metabotropic receptor for GABA, GABAB, that is divided into two subtypes, GABAB1 and GABAB2 [3-6]. The GABAB receptor and mGlu receptors belong to the same receptor family, which is the third family of G-proteins coupled receptors (GPRCs) [1]. Metabotropic receptor ligands for glutamate and GABA have recently been considered as putative candidates for novel psychotropic drugs. The mechanism of their action consists in the ability to regulate the release of glutamate and/or GABA [7]. This ability seems to be important because numerous disorders of the central nervous system result from imbalance between inhibitory and excitatory neurotransmission in the brain. Therefore, the use of metabotropic receptor ligands may restore brain homeostasis. Among all mGlu receptors, the third group seems to be particularly interesting because it is the largest and the least investigated group. It consists of mGlu4, mGlu6, mGlu7, and mGlu8 receptors, with the distribution of the mGlu6 receptor being limited only to the retina. Those receptors have been proposed as one of the most important factors involved in the regulation of glutamate release [2, 8-10] because of their expression in the near fusion of synaptic vesicles. They are negatively linked to adenylyl cyclase activity, and their activation inhibits glutamate release [2, 8, 11]. The first report concerning the antipsychotic-like activity of the nonselective agonist of mGlu4/7/8 receptors, ACPT-1 [12], was published in 2008 [13]. The report concerned the activity of this ligand in animal models of positive symptoms of schizophrenia. In vitro, ACPT-I was characterized as a nonselective agonist of mGlu4 and mGlu8 receptors, activating both subtypes to almost the same extent [EC50=6.5 and EC50=10.1, respectively]. Subsequently, more selective compounds were synthesized, such as LSP1-2111, which better differentiated between mGlu4 and mGlu7/8 receptors because of its 30-fold higher selectivity towards the mGlu4 receptor subtype compared with mGlu7 and mGlu8 receptor [14]. The compound was active in various animal models of schizophrenia, including the models of positive, negative, and cognitive symptoms [15, 16]. The use of selective positive allosteric modulators (PAMs) of the mGlu4 receptor, Lu AF21934 and Lu AF32615, confirmed the theory of targeting the mGlu4 receptor as the novel strategy to search for antipsychotic drugs [17]. We showed that both of these PAMs reversed deficits (in a dose-dependent manner) in preclinical models mimicking positive, negative, and cognitive symptoms of schizophrenia [17]. Simultaneously, the involvement of the mGlu7 receptor subtype was excluded as a potential antipsychotic target [15].
The GABAB receptor is thought to regulate the release of glutamate in the same way as mGlu4 and mGlu7 receptor. Previously, we showed that positive allosteric modulators of the GABAB receptor, such as GS39783 and CGP7930, were active in the animal models of schizophrenia [18, 19]. Therefore, it is possible that the mGlu4 and GABAB receptors may act concomitantly regulating the release of glutamate and thus exerting antipsychotic action.
In the present study, we used the most recent compound selectively activating mGlu4 receptor, LSP4-2022. We investigated the activity of the compound in various animal models including hyperactivity, 2.5-dimethoxy-4-iodoamphetamine (DOI)-induced head twitches, modified forced swim test (FST), social interaction test, and novel object recognition (NOR) test. In the second part of the study, we used a selective PAM of the GABAB receptor, GS39783, and the antagonist CGP55845 to investigate the involvement of the GABAB receptor in LSP4-2022-induced antipsychotic-like action. The chemical structures of glutamatergic or GABAergic tool compounds used in this study are shown in Fig. 1.
Fig. (1).

The chemical structure of glutamatergic and GABAergic tool compounds used in the present study (mGlu4 receptor agonist, LSP4-2022, GABAB receptor PAM GS39783, and GABAB antagonist CGP55845).
Materials and methods
Animals and Housing
Male Albino Swiss mice (20-25 g) were used to assess MK-801- and amphetamine-induced hyperlocomotion and DOI-induced head twitches. mGlu4 knockout mice were also used in behavioral assays. mGlu4 knockout and wild-type C57Bl/6J mice were bred at our Institute. The genotypes of newborn mice were analyzed by polymerase chain reaction, according to [20]. Male Wistar rats (250-300 g) were used in the social interaction and NOR tests. The animals were kept under a 12:12 light-dark cycle at a room temperature of 19 to 21°C, with free access to food and water. Each experimental group consisted of 8 to 10 animals, and the animals were used only once in each test. All the compounds were used at a volume of 10 ml/kg when given to mice and 1 ml/kg when injected to rats. All behavioral measurements were done by an observer blinded to the treatment. All procedures were conducted according to the guidelines of the National Institutes of Health Animal Care and Use Committee and were approved by the Ethics Committee of the Institute of Pharmacology, Polish Academy of Sciences in Kraków, Poland.
Drugs
The following drugs were used: LSP4-2022 (mGlu4 receptor agonist) synthesized at the laboratory of Université Paris Descartes, Paris, France, and characterized using H-1, C-13, and P-31 nuclear magnetic resonance spectroscopy and high-performance liquid chromatography–mass spectrometry. The compound was dissolved in saline and administered as an intraperitoneal (IP) injection 45 minutes before the tests. The administration schedule for LSP4-2022 was planned according to our previous studies on its precursor drug, LSP1-2111 [15, 16]. MK-801 (0.35 mg/kg; Sigma-Aldrich, St. Louis, USA) or amphetamine (3 mg/kg; Sigma-Aldrich) was dissolved in NaCl (0.9%), and the doses were selected consistently with our previous studies [13, 15, 16] and those of other authors [21, 22]. GS39783 (Tocris Bioscience, Bristol, United Kingdom) was dissolved in a small amount of ethanol and then titrated with Tween 2% to an appropriate volume. CGP55845 (Tocris Bioscience, Bristol, United Kingdom) was dissolved in 0.9% saline. Both drugs were administered according to our previous studies and those by other authors [18, 23, 24].
Locomotor Activity
The locomotor activity was recorded individually for each animal in OPTO-M3 locomotor activity cages (Columbus Instrument) linked online to a compatible PC. Each cage (13cm×23cm×15cm) was surrounded with an array of photocell beams. Interruptions of these photobeams resulted in horizontal activity defined as ambulation scores. Mice were placed separately into activity cages for an acclimatization period of 30 minutes, following which they were injected with LSP4-2022, CGP55845, GS39783, and their combinations (time of administration and doses were similar as described below for MK-801-induced hyperactivity). Following this, the ambulation scores were measured for 60 minutes.
MK-801-induced Hyperactivity
The locomotor activity was recorded for each animal in locomotor activity cages [25, 26], with small modifications used in our previous studies [13, 15, 16]. Mice were placed separately into actometers for an acclimatization period of 30 minutes, following which they were administered four doses of LSP4-2022, namely, 0.1, 0.5, 1, and 2 mg/kg, 45 minutes before MK-801 or amphetamine administration. Afterwards, the most active dose of the compound (2 mg/kg) was administered together with CGP55845 (10 mg/kg IP; 30 minutes before MK-801). The subeffective dose of the compound (0.1 mg/kg) was administered together with GS39783 (0.1 mg/kg IP; 30 minutes before MK-801), or vehicle and placed again in the same cages. After a specified time period, all mice received an IP injection of MK-801 at a dose of 0.35 mg/kg and were once again placed in the same cage. Following this, the ambulation scores were counted for 60 minutes. All groups were compared with the MK-801 control group. The experiment also included a control group not treated with MK-801.
Head Twitch Test
The experiment was performed according to Pałucha-Poniewiera et al. [13] and Wieronska et al. [15, 16]. In order to habituate mice to the experimental environment, each animal was transferred to a glass cage of 12 cm in diameter and 20 cm in height, lined with sawdust, 30 minutes before the treatment. The head twitches of mice were induced by DOI (2.5 mg/kg IP). Immediately after the treatment, the number of head twitches was counted during a 20-minute session. LSP4-2022 was injected in four doses (0.1, 0.5, 1, and 2 mg/kg), and then the most active dose (2 mg/kg) was administered together with CGP55845, and the subeffective dose with GS39783.
mGluR4 knockout mice and their wild-type littermates were used to confirm the specificity of the ligand. The most active dose of LSP4-2022 (2 mg/kg) was administered both to knockout and wild-type mice.
Modified Forced Swim Test
The experiment was performed according to the method described by Noda et al. [27, 28] and Langen et al. [29]. On day 1, each mouse was placed in a transparent glass cylinder (20 cm high, 8 cm in diameter), which contained water at a temperature of 22°C to 23°C and a depth of 15 cm, and was forced to swim for 180 seconds. The immobility time was calculated as follows: 180 (s) − swimming time (s) = immobility time (s) (T1). Mice were matched according to the results of immobility time in the first measurement of immobility and were divided into various treatment groups.
On day 2, drug treatment was started. Saline or MK-801 (0.4 mg/kg IP) was administered once a day for 13 days. After a one-day break (no MK-801 administration), each mouse (under the condition of MK-801 free) was placed in water again for 180 seconds, and the immobility time was calculated (the second measurement of immobility, T2). LSP4-2022 was administered 45 minutes before the second measurement of immobility. Control animals received the vehicle only, and the same procedure was performed.
The effects of drugs on the spontaneous activity were also examined: mice chronically treated with MK-801 received the tested drug and the spontaneous activity of each mouse was measured for 5 minutes using behavioral analysis systems.
MK-801-induced Deficits in Social Interaction Test in Rats
The social interaction test was performed according to the method described by Satow et al. [22], using a circle made of wood, 90 cm in diameter, and divided into 10×10 cm squares by faint yellow lines. Each social interaction test involving two rats was conducted during the light phase of the light/dark cycle. The body weights of the paired rats were matched within 20 g of variance. All rats were placed in an experimental room and the study was conducted 3.5 hours after the subcutaneous administration of MK-801 at a dose of 0.1 mg/kg subcutaneously (SC). LSP4-2022 was administered in three doses: 0.5, 1, and 2 mg/kg. Then, the subeffective dose of the compound (0.1 mg/kg) was administered together with GS39783 (0.1 mg/kg).
The test box was wiped clean between each trial. Social interaction between two rats was determined as the total time spent participating in social behavior such as sniffing, genital investigation, and chasing and fighting each other. The total number of social episodes was also measured. In addition, control experiments in animals not receiving MK-801 were also conducted to establish if the drugs had any influence on social behavior when given alone.
Novel Object Recognition
The method was adapted from Horiguchi et al. [30, 31] and Dere et al. [32]. The animals were trained and tested in a black wooden circular open field (100 cm in diameter, 35 cm high) with the floor divided into 20-cm square sections. The open field was in a dark room illuminated only by a 25-W bulb. On the first day (adaptation), the animals were allowed to explore the open field for 10 minutes. On the next day (training, T1), the animals were administered the tested drugs, placed in the apparatus, and allowed to explore two identical objects (cylinder-shaped objects with walls painted white, 7 cm in diameter, 11 cm high) for the time required to complete 15 seconds of exploration of either object. For the retention trial (T2) conducted 1 hour later, one of the objects presented in T1 was replaced with a novel object (a prism-shaped object with walls painted black, 5 cm wide, 14 cm high). The rats were returned to the open field for 5 minutes, and the duration of exploration of each object (eg, sitting in close proximity to the objects, sniffing or touching them) was measured separately by a trained observer. All drugs were administered before the training (T1) session. MK-801 (0.1 mg/kg, SC) was given 30 minutes before the session. LSP4-2022 was injected at the doses of 0.5, 1, and 2 mg/kg. Subsequently, a subeffective dose (0.5 mg/kg) was administered together with GS39783. All injections were given at a volume of 1 ml/kg of the body weight. The treatment groups included eight animals.
Statistical Analysis
Data were presented as means ± standard error of the mean. The statistical analysis of the data was performed using the Statistically 10 package (StatSoft Inc., OK, USA). One-way ANOVA followed by the Newman–Keuls post-hoc comparison was performed. A P value of less than 0.05 was considered statistically significant.
Results
The Effects of LSP4-2022 on MK-801- and Amphetamine-induced Hyperactivity in Mice
LSP4-2022 was administered at the doses of 0.1, 0.5, 1, and 2 mg/kg. One-way ANOVA followed by the Newman–Keuls multiple comparison test revealed a significant effect at the doses of 0.5, 1, and 2 mg/kg [F(4.51)=9, P<0.001] for MK-801-induced hyperactivity and at a dose of 0.5 mg/kg [F(3.43)=3.57, P<0.05] for amphetamine-induced hyperactivity (2A, B).
Fig. (2).

Effects on MK-801- and amphetamine-induced hyperactivity (A and B, respectively). LSP4-2022 was administered 45 minutes before the administration of MK-801 or amphetamine, after 30 minutes of habituation to activity cages. Data are presented as means ± SEM. Doses in mg/kg are indicated in parentheses. #P<0.001 versus control, *P<0.05, **P<0.01, and ***P<0.001 versus MK-801- or amphetamine-treated group.
The Effects of the Combined Administration of CGP55845 and LSP4-2022 on MK-801-induced Hyper- activity in Mice
LSP4-2022 administered at a dose of 2 mg/kg reversed MK-801-induced hyperactivity (P<0.001). CGP55845, a GABAB receptor antagonist, administered at a dose of 10 mg/kg IP did not have any effect on its own. The administration of CGP55845 with LSP4-2022 resulted in the inhibition of LSP4-2022-induced effect in the hyperactivity test. The one-way ANOVA main effects revealed a significant effect of the LSP4-2022–CGP55845 interaction [F(4.41)=11.93, P<0.001] (Fig. 3A).
The Effects of the Combined Administration of Subeffective Doses of GS39783 and LSP4-2022 on MK-801-induced Hyperactivity in Mice
LSP4-2022 was administered at a dose of 0.1 mg/kg, and GS39783 (GABAB receptor PAM) —at a dose of 0.1 mg/kg. None of the compounds showed any effect when administered separately. The simultaneous administration of the subeffective doses of the GABAB receptor PAM and mGlu4 receptor agonist induced reversal of hyperactivity. The one-way ANOVA main effects revealed a significant effect of the GS39783–LSP4-2022 interaction [F(4.42)=6.7, P<0.002]. The post-hoc Newman–Keuls analysis revealed a significant effect of the GS39783–LSP4-2022 interaction when compared with MK-801-treated animals, P<0.05 (Fig. 3B).
Control Experiments: The Effects of LSP4-2022, CGP55845, and GS39783 on Locomotor Activity in Mice Habituated to Activity Meters
The two-way ANOVA revealed that LSP4-2022, CGP55845 (10 mg/kg, 30 minutes before the test), and GS39783 (0.1 mg/kg, 30 minutes before the test) did not change the locomotor activity of mice adapted to activity meters for 30 minutes. No significant effect of the simultaneous administration of LSP4-2022 with CGP55845 or of LSP4-2022 (1 mg/kg) with GS39783 was observed (Fig. 3c, D).
Fig. (3).

Effects on MK-801-induced hyperactivity in mice habituated to activity cages. The simultaneous administration of LSP4-2022 with GABAB receptor antagonist, CGP55845 (A), and of the subeffective doses of LSP4-2022 and GABAB positive modulator, GS39783 (B). Control experiments with the combined administration of LSP4-2022 with CGP55845 (C) and GS39783 (D) on the locomotor activity in mice habituated to activity cages and not given MK-801. Data are presented as means ± SEM. Doses in mg/kg are indicated in parentheses. #P<0.01 versus control group, *P<0.05 and **P<0.01 versus MK-801-treated group and @P<0.01 versus LSP4-2022-treated group.
The Effects of LSP4-2022 on DOI-induced Head Twitches in Mice
LSP4-2022 was administered in four doses: 0.1, 0.5, 1, and 2 mg/kg. One-way ANOVA followed by the Newman–Keuls comparison revealed a statistically significant effect of the 0.5-2 mg/kg dose [F(4.44)=7.29, P<0.01] (Fig.4A). At the same time, the experiments performed on knockout mice revealed that the action of the compound was specific because it had no effect in mGlu4 receptor knockout animals P<0.3 (Fig. 4B).
Fig. (4).

Effects on DOI-induced head twitches. The dose-dependent studies of LSP4-2022 (A) and the use of mGluR4 knockout mice to evaluate the specificity of the compound (B) are presented. Data are presented as means ± SEM. Doses in mg/kg are indicated in parentheses. *P<0.05 versus DOI-treated group.
The Effects of the Combined Administration of CGP55845 and LSP4-2022 on DOI-induced Head Twitches in Mice
LSP4-2022 administered at a dose of 2 mg/kg significantly inhibited the number of DOI-induced head twitches (P<0.01). CGP55845 administered at a dose of 10 mg/kg did not have any effect on its own. The simultaneous administration of LSP4-2022 and CGP55845 resulted in the inhibition of LSP4-2022-induced effect [F(3.22)=15.21, P<0.001]. The post-hoc Newman–Keuls analysis revealed a significant effect of the LSP4-2022–CGP55845 interaction when compared with the LSP4-2022-treated group, P<0.001 (Fig. 5A).
Fig. (5).

Effects on DOI-induced head twitches. The simultaneous administration of LSP4-2022 with GABAB receptor antagonist, CGP55845 (A), and of the subeffective doses of LSP4-2022 and GABAB positive modulator, GS39783 (B). Data are presented as means ± SEM. Doses in mg/kg are indicated in parentheses. **P<0.01 and ***P<0.001 versus DOI-treated group and #P<0.01 vs LSP4-2022 group.
The Effect of the Combined Administration of GS 39783 and a Subeffective Dose of LSP4-2022 on DOI-induced Head Twitches in Mice
LSP4-2022 was administered at a dose of 0.1 mg/kg, and GS39783 at a dose of 0.1 mg/kg. None of the drugs showed any effects when administered on its own. The simultaneous administration of subeffective doses of GABAB and mGlu4 receptor PAM induced a clear reduction in the number of DOI-induced head twitches. The one-way ANOVA main effects revealed a significant effect of the LSP4-2022–GS39783 interaction [F(3.29)=8.83, P<0.001]. The post-hoc Newman–Keuls analysis revealed a significant effect of the LSP4-2022–GS39783 interaction when compared with DOI-treated animals, P<0.001 (Fig. 5B).
The Effect of LSP4-2022 in the Modified Forced Swim Test
MK-801 administered chronically (14 days) at a dose of 0.4 mg/kg induced an increase in the immobility time of about 300% of the control level. LSP4-2022 reversed this MK-801-induced prolongation in the immobility time at all tested doses, that is, 0.5, 1, and 2 mg/kg. The one-way ANOVA revealed a statistical effect of LSP4-2022 with F(4.45)=3.68, P<0.05 (Fig. 6A).
Fig. (6).

Effect on modified forced swim test (A) and spontaneous locomotor activity (B) in mice chronically treated with MK-801 (13 days; 0.4 mg/kg IP). Data are presented as means ± SEM. Doses in mg/kg are indicated in parentheses. #P<0.05 versus controls, *P<0.05 versus MK-801-treated group.
The chronic administration of MK-801 did not induce any changes in spontaneous locomotor activity when measured for 5 minutes. However, the simultaneous administration of LSP4-2022 at a dose of 0.5 mg/kg 45 minutes before the measurement of the locomotor activity significantly increased the number of ambulation scores [F(4.44)=5.104, P<0.01] (Fig. 6B).
The Effect of LSP4-2022 in the Social Interaction Test in Rats
MK-801 induced a typical decrease both in the number (Fig. 7A) and duration (Fig. 7B) of social episodes measured during the 8-minute test. LSP4-2022 was administered at three doses: 0.5, 1, and 2 mg/kg. The one-way ANOVA followed by the Newman–Keuls comparison revealed that all three doses attenuated the MK-801-induced deficits in both parameters, that is, the time of interaction [F(4.20)=15.81] and the number of episodes [F(4.20)=12.65]. The compound did not have any own effects at the highest investigated dose (Fig. 7).
Fig. (7).

Effects on MK-801-induced deficits in social interaction. The time of social interaction (A) and the number of episodes of social contacts (B) were measured. The dose-dependent studies of LSP4-2022 were performed. The graphs also include the control experiments with the most active dose administered to rats not treated with MK-801. Data are presented as means ± SEM. Doses in mg/kg are indicated in parentheses. #P<0.01 versus controls, *P<0.05 and **P<0.01 versus MK-801-treated group.
The Effect of the Combined Administration of GS39783 and LSP4-2022 in the Social Interaction Test in Rats
LSP4-2022 was given at a subeffective dose of 0.1 mg/kg, 45 minutes before the test. GS39783 was administered at a subeffective dose of 0.1 mg/kg. The simultaneous administration of LSP4-2022 and GS39783 did not alter the effects induced by MK-801, which produced significant changes in behavior when administered on its own (Fig. 8A, B, left part of the graph). Neither LSP4-2022 nor GS39783 alone or in combination induced changes in behavior (Fig. 8A, B, right part of the graph).
Fig. (8).
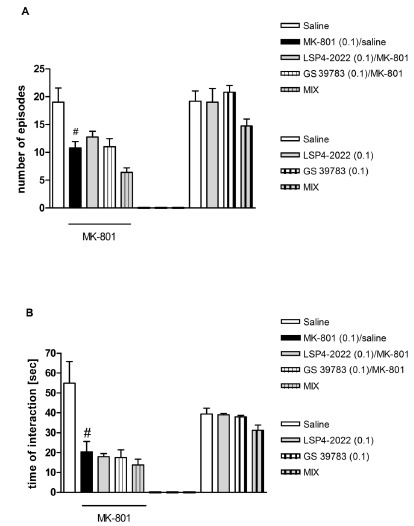
Effects on MK-801-induced deficits in social interaction. The number of episodes of social contacts (A) and the time of social interaction (B) were measured. The effect of the combined administration of LSP4-2022 and GABAB receptor positive modulator, GS39783, was measured. The graphs also include the control experiments with the same treatment group (excluding MK-801 administration). Data are presented as means ± SEM. Doses in mg/kg are indicated in parentheses. #P<0.01 versus controls.
The one-way ANOVA revealed no changes between the particular groups not injected with MK-801.
The Effect of LSP4-2022 in the Novel Object Recognition Test in Rats
LSP4-2022 was administered at three doses: 0.5, 1, and 2 mg/kg. The one-way ANOVA followed by Newman–Keuls comparison revealed that only the effect of the higher dose (2 mg/kg) was statistically significant [F(3.36)=7.09, P<0.001] (Fig. 9A).
Fig. (9).

Effects on MK-801-induced deficits in NOR. The dose-dependent study of LSP4-2022 (A) and the combined administration of LSP4-2022 with CGP55845 (B) or GS39783 (C). The graphs also include control experiments with the same treatment group. Data are presented as means ± SEM. Doses in mg/kg are indicated in parentheses. #P<0.01 versus controls, ***P<0.001 versus MK-801-treated group.
The Effect of the Combined Administration of GS39783 and LSP4-2022 in the Novel Object Recognition Test in Rats
LSP4-2022 was administered at a subeffective dose of 0.5 mg/kg, 45 minutes before the test. GS39783 was given at a dose of 0.1 mg/kg, 30 minutes before the test, and did not show any effect. GS39783, when administered with LSP4-2022, showed no effect on the behavior of MK-801-treated animals in the NOR test (Fig. 8B). Neither LSP4-2022 nor GS39783 alone or in combination induced changes in behavior (Fig. 9B, right part of the graph).
Discussion
The present study expands on our recent reports showing that the novel compounds stimulating mGlu4 receptor, both orthosteric agonists and PAMs, exhibit antipsychotic-like activity in animal models, widely used to study antipsychotic-like activity of drugs [15-17, 33]. Herein, we describe the activity of the agonist of group III mGlu receptors, LSP4-2022 [34]. LSP4-2022 is the successor of the former compound, LSP1-2111, preferential mGlu4 receptor agonist, whose activity was described in our previous studies [15, 16, 35]. The in vitro potency of LSP4-2022 to mGlu4 receptor is higher than that of LSP1-2111 or any other known mGlu4 agonist or PAM (EC50=0.11 ±0.02 µM). Simultaneously, the affinity of LSP4-2022 to mGlu7 receptor remains significant, although it is weaker than that to mGlu4 receptor (EC50=11.6 ±1.9 µM) [34]. Our study conducted in 2012 revealed that the activation of mGlu7 receptor did not induce any antipsychotic-like effect in rodents [15]. Therefore, on the basis of our previous results, we assumed that the stimulation of mGlu4 receptor, and not mGlu7 receptor, is responsible for the observed antipsychotic-like effect. Additionally, LSP4-2022 has been tested against 34 GPCRs including GABAB receptor in the laboratory of J.P Pin at the Institute for Functional Genomics in Montpellier, France, and showed to have no effects on these targets. The paper is currently being prepared with Abderazack Belhocine as the first author (personal communication). A study by Cajina et al. concerning LSP1-2111, a close analog of LSP4-2022, provides an indirect evidence for LSP4-2022 selectivity [36]. The drug was tested in a broad binding screen against 70 GPCRs, ion channels, and enzymes as well as a functional screen toward 56 GPCRs and demonstrated a highly selective cross-reactivity profile for LSP1-2111.
In the present study, we performed standard behavioral procedures to evaluate the antipsychotic-like activity of LSP4-2022, namely, hyperactivity, DOI-induced head twitches, modified FST, social interaction test, and NOR test. We used mGluR4 knockout animals (mGluR4-/-) to confirm the specific mGluR4-dependent action of the compound. Hyperactivity and DOI-induced head twitches are widely used animal models reflecting the positive symptoms of schizophrenia, while the modified FST and social interaction test are popular animal models of negative symptoms of schizophrenia. The modified FST was introduced by Noda [27-29]. Repeated MK-801 treatment (13 days, 0.4 mg/kg) significantly increased immobility in the modified FST, without affecting locomotor activity. Mice were forced to swim twice for 3 minutes, during which the immobility time was measured. After the first trial (T1), the chronic administration of MK-801 was started and continued for 13 days, and then the second trial (T2) was performed. The increase in immobility was counted as the difference between T2 and T1. The test is considered as a model of depression-like negative symptoms of schizophrenia. The social interaction test was used in our previous studies and was adapted from Satow et al. [16, 17, 22]. Social withdrawal is one of the most common negative symptoms observed in schizophrenia patients; therefore, the social interaction test has a significant face and construct validity. Atypical antipsychotic drugs reverse MK-801-induce behavioral changes both in the modified FST and social interaction test.
For cognitive symptoms, we used the NOR test, which is a well-validated model of recognition memory. It is widely used in studies that explore cognitive impairment in rodent models of schizophrenia. It is relevant to short-term visual memory, and MK-801-induced deficits are selectively reversed by the administration of novel generations of antipsychotics [30, 37-40].
In all our experiments in the present study, the orthosteric agonist of mGlu4 receptor, LSP4-2022, induced a clear antipsychotic-like effect in a dose-dependent manner, reversing MK-801-induced disturbances. The action of the compound was observed at relatively small doses, from 0.5 mg/kg to 2 mg/kg. The lower and higher doses were relatively less effective in all tests, with the exception of the NOR test, in which only the highest dose (2 mg/kg) reversed the MK-80-induced effect. The drug did not induce any behavioral activity when given to animals not treated with MK-801. Only in the locomotor activity test performed as a control experiment for the modified FST, the administration of the lowest dose of LSP4-2022 (0.5 mg/kg) increased the spontaneous locomotor activity of mice chronically treated with MK-801 (Fig. 5B). Such an increase in the immobility time was not observed in mice treated with the doses of 1 and 2 mg/kg. Additionally, using mGluR4 knockout mice, we confirmed the specificity of the ligand because it was inactive at a dose of 2 mg/kg in the DOI-induced head twitches test.
In the second part of the study, we investigated the possible involvement of GABAB receptor in the action of LSP4-2022. The GABAergic system plays an important role in the development of schizophrenia because according to the GABAergic theory of psychosis, the deficit in GABA synthesis was observed in postmortem studies of schizophrenic patients [41]. Our previous publications showed that GABAB receptor agonists/PAMs were effective in the animal models of positive symptoms [18] as well as in those of negative and cognitive symptoms [19]. We also showed that the concomitant stimulation of GABAB and mGlu5 receptors with subeffective doses of selective PAMs of those receptors (GS39783 and CDPPB, respectively) induced clear antipsychotic-like effects in the animal models of schizophrenia [19]. This interplay between mGlu5 and GABAB receptors was evident in the models of negative and cognitive symptoms, but not in the model of positive symptoms of schizophrenia, such as the DOI-induced head twitches [19].
In the present set of experiments, we used selective GABAB receptor antagonist, CGP55845, to block the action of the effective doses of LSP4-2022. On the other hand, the subeffective dose of LSP4-2022 was administered with the subeffective dose of GS39783. The effect of LSP4-2022 was abolished by the administration of GABAB receptor antagonist only in hyperactivity and DOI-induced head twitches tests, and, simultaneously, the administration of subeffective doses of LSP4-2022 and GABAB PAM GS39783 induced clear antipsychotic-like effects in these tests. The interplay between mGlu4 and GABAB receptors was not observed in the models of negative and cognitive symptoms of schizophrenia, contrary to our previous results observed for the interaction between mGlu5 and GABAB receptors [19]. At the moment, we cannot fully explain the mechanisms that underlie the reported interactions because further studies are needed to elucidate these issues. One of the possibility is that mGlu4 and GABAB receptors are not expressed by the same neurons in the brain regions responsible for the performance of the social interaction and NOR tests (such as the piriform cortex). In contrast, they can be coexpressed in the V layer of the prefrontal cortex, where both GABAB and mGlu4 receptor stimulators attenuated a DOI-induced increase in the frequency of spontaneous EPSCs [17, 18], and their effect was considered as primarily presynaptic. As the effect of DOI occurs via the induction of glutamate release caused by activation of 5-HT2A receptors placed postsynaptically on pyramidal neurons [43], the concomitant stimulation of presynaptic GABAB and mGlu4 receptors expressed on the presynaptic site of the same neuron may counteract the effect of DOI.
We are aware that in studies with simultaneous administration of up to 3 compounds, the lack of drug–drug interactions should be confirmed. Unfortunately, we did not have any drug exposure studies for either LSP4-2022 or GS39783. However, except LSP4-2022, which is a new compound (the close analog of LSP1-2111, described in the work of Cajina et al. [36]), all the other compounds have been widely used in animal studies. A variety of controls that were performed in our studies minimized the risk of unwanted effects and possible drug–drug interactions. In each paradigm, there were 4 groups of MK-801-treated animals: vehicle-receiving (solvent given in the same time as tested compound), tested drug 1, tested drug 2, and tested drugs 1+2. Additionally, the same investigation scheme was performed for animals not receiving MK-801. According to our knowledge, experience, and practice, as well as a vast number of studies by other authors, such an experimental design is sufficient to consider the results as reliable.
Among the currently used antipsychotics, there are no drugs with significant affinity to glutamatergic or GABAergic receptors. Various preclinical studies have indicated that activation of amino acidergic receptors can induce strong antipsychotic-like effects in the models of positive, negative, and cognitive symptoms. So far, the clinical trials were performed with mGlu2/3 receptors modulators. Unfortunately, the studies with LY2140023 were not conclusive because the positive control, olanzapine, did not show any effects [44]. However, there are some studies demonstrating positive results [45, 46]. The major benefit of using glutamatergic agents is their unique tolerability profile characterized by a low incidence of some adverse events such as extrapyramidal symptoms or weight gain that have been reported for the currently used dopaminergic antipsychotic drugs [44-47].
The GABAergic agents are widely used in clinical practice. The best known agents are benzodiazepines, common anxiolytic drugs [48]. The GABAB receptor agonist, baclofen, have been used in clinical practice for several decades now [49]. No trials have been performed with GABAB receptor positive modulators in humans. Only one compound, ADX71441, a novel orally active GABAB PAM, was on track for Phase 1 clinical testing in the first half of 2013 (Addex information). However, the available data concerning the efficacy of GABAergic drugs in schizophrenic patients are limited, and more research is needed in this field.
As the most effective and safe neuroleptics are characterized by multidirectional action, activating different classes of receptors, mainly dopaminergic, serotonergic, histaminergic, and muscarinic, we assume that the activation of one type of receptors may not be sufficient to induce antipsychotic-like effect. On the other hand, the use of lower doses of the compounds may reduce the risk of adverse effects that are often associated with higher doses. In a series of our previous experiments, we suggested that the interaction between mGlu4 and 5-HT1A receptors may be a good antipsychotic target [16, 33]. In the present study, we showed that the therapy based on the interaction between mGlu4 and GABAB receptors may prove beneficial for patients with a predominance of positive symptoms.
ACKNOWLEDGEMENTS
The study was supported by the Statutory Funds of the Polish Academy of Science and by the National Science Centre, Grant no. 2012/6/ 06/A/NZ7/00014 (MAESTRO) granted to A. Pilc. We would also like to thank Delphine Rigault for the synthesis of LSP4-2022.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
REFERENCES
- 1.Bockaert J., Perroy J., Bécamel C., Marin P., Fagni L. GPCR interacting proteins (GIPs) in the nervous system: Roles in physiology and pathologies. Annu. Rev. Pharmacol. Toxicol. 2010;50:89–109. doi: 10.1146/annurev.pharmtox. [DOI] [PubMed] [Google Scholar]
- 2.Conn P.J., Pin J.P. Pharmacology and functions of metabotropic glutamate receptors. Annu. Rev. Pharmacol. Toxicol. 1997;37:205–237. doi: 10.1146/annurev.pharmtox.37.1.205. [DOI] [PubMed] [Google Scholar]
- 3.Craig MT., McBain C.J. The emerging role of GABAB receptors as regulators of network dynamics: fast actions from a 'slow' receptor? Curr. Opin. Neurobiol. 2014;26:15–21. doi: 10.1016/j.conb.2013.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Filip M., Frankowska M., Sadakierska-Chudy A, Suder A., Szumiec L., Mierzejewski P., Bienkowski P, Przegaliński E., Cryan J.F. GABAB receptors as a therapeutic strategy in substance use disorders: Focus on positive allosteric modulators. Neuropharmacol. 2015;88C:36–47. doi: 10.1016/j.neuropharm.2014.06.016. [DOI] [PubMed] [Google Scholar]
- 5.Kaupmann K., Huggel K., Heid J., Flor P.J., Bischoff S., Mickel S.J., McMaster G., Angst C., Bittiger H., Froestl W. Bettler. B. Expression cloning of GABA(B) receptors uncovers similarity to metabotropic glutamate receptors. Nature. 1997;386(6622):239–246. doi: 10.1038/386239a0. [DOI] [PubMed] [Google Scholar]
- 6.Hill D.R., Bowery N.G. 3H-baclofen and 3H-GABA bind to bicuculline-insensitive GABA B sites in rat brain. Nature. 1981;290(5802):149–152. doi: 10.1038/290149a0. [DOI] [PubMed] [Google Scholar]
- 7.Mercier M.S., Lodge D. Group III metabotropic glutamate receptors: pharmacology, physiology and therapeutic potential. Neurochem. Res. 2014;39(10):1876–1894. doi: 10.1007/s11064-014-1415-y. [DOI] [PubMed] [Google Scholar]
- 8.Pin J.P., Duvoisin R. The metabotropic glutamate receptors: structure and functions. Neuropharmacol. 1995;34(1):1–26. doi: 10.1016/0028-3908(94)00129-g. [DOI] [PubMed] [Google Scholar]
- 9.Schoepp D.D., Marek G.J. Preclinical pharmacology of mGlu2/3 receptor agonists: novel agents for schizophrenia? Curr. Drug Targets CNS Neurol. Disord. 2002;1(2):215–225. doi: 10.2174/1568007024606177. [DOI] [PubMed] [Google Scholar]
- 10.Wierońska J.M., Pilc A. Metabotropic glutamate receptors in the tripartite synapse as a target for new psychotropic drugs. Neurochem. Int. 2009;5(1-3):85–97. doi: 10.1016/j.neuint.2009.02.019. [DOI] [PubMed] [Google Scholar]
- 11.Conn P.J., Lindsley C.W., Jones C.K. Activation of metabotropic glutamate receptors as a novel approach for the treatment of schizophrenia. Trends Pharmacol. Sci. 2009;30(1):25–31. doi: 10.1016/j.tips.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Acher F.C., Tellier F.J., Azerad R., Brabet I.N., Fagni L. ; Pin J.P. Synthesis and pharmacological characterization of aminocyclopentanetricarboxylic acids: new tools to discriminate between metabotropic glutamate receptor subtypes. J. Med. Chem. 1997;40(19):3119–3129. doi: 10.1021/jm970207b. [DOI] [PubMed] [Google Scholar]
- 13.Pałucha-Poniewiera A., Kłodzińska A., Stachowicz K., et al. Peripheral administration of group III mGlu receptor agonist ACPT-I exerts potential antipsychotic effects in rodents. Neuropharmacol. 2008;55(4):517–524. doi: 10.1016/j.neuropharm.2008.06.033. [DOI] [PubMed] [Google Scholar]
- 14.Beurrier C., Lopez S., Révy D., et al. Electrophysiological and behavioral evidence that modulation of metabotropic glutamate receptor 4 with a new agonist reverses experimental parkinsonism. FASEB J. 2009;23(10):3619–3628. doi: 10.1096/fj.09-131789. [DOI] [PubMed] [Google Scholar]
- 15.Wierońska J.M., Stachowicz K., Acher F., Lech T., Pilc A. Opposing efficacy of group III mGlu receptor activators, LSP1-2111 and AMN082, in animal models of positive symptoms of schizophrenia. Psychopharmacology (Berl.) 2012;220(3):481–494. doi: 10.1007/s00213-011-2502-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wierońska J.M., Acher F.C., Sławińska A., Gruca P., Lasoń-Tyburkiewicz M., Papp M., Pilc A. The antipsychotic-like effects of the mGlu group III orthosteric agonist, LSP1-2111, involves 5-HT1A signalling. Psychopharmacology (Berl.) 2013;227(4):711–725. doi: 10.1007/s00213-013-3005-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sławińska A., Wierońska J.M., Stachowicz K., Marciniak M., Lasoń-Tyburkiewicz M., Gruca P., Papp M., Kusek M., Tokarski K., Doller D., Pilc A. The antipsychotic-like effects of positive allosteric modulators of metabotropic glutamate mGlu4 receptors in rodents. Br. J. Pharmacol. 2013;169(8):1824–1839. doi: 10.1111/bph.12254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wierońska J.M., Kusek M., Tokarski K., Wabno J., Pilc A. The GABA B receptor agonist CGP44532 and the positive modulator GS39783 reverse some behavioral changes related to positive syndromes of psychosis in mice. Br. J. Pharmacol. 2011;163(5):1034–1047. doi: 10.1111/j.1476-5381.2011.01301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wierońska J.M., Kłeczek N., Woźniak M., Gruca P., Łasoń-Tyburkiewicz M., Papp M., Brański P., Burnat G., Pilc A. A. mGlu5-GABAB interplay in antipsychotic-like action in animal models of positive, negative and cognitive symptoms of schizophrenia. Neurochem. Int. 2015 doi: 10.1016/j.neuint.2015.03.010. [DOI] [PubMed] [Google Scholar]
- 20.Sansig G., Bushell T.J., Clarke V.R., Rozov A., Burnashev N., Portet C., Gasparini F., Schmutz M., Klebs K., Shigemoto R., Flor P.J., Kuhn R., Knoepfel T., Schroeder M., Hampson D.R., Collett V.J., Zhang C., Duvoisin R.M., Collingridge G.L., van Der Putten H. Increased seizure susceptibility in mice lacking metabotropic glutamate receptor 7. J. Neurosci. 2001;21(22):8734–8745. doi: 10.1523/JNEUROSCI.21-22-08734.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Geyer M.A., Ellenbroek B. Animal behavior models of the mechanisms underlying antipsychotic atypicality. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2003;27(7):1071–1079. doi: 10.1016/j.pnpbp.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 22.Satow A., Suzuki G., Maehara S., Hikichi H., Murai T., Murai T., Kawagoe-Takaki H., Hata M., Ito S., Ozaki S., Kawamoto H., Ohta H. Unique antipsychotic activities of the selective metabotropic glutamate receptor 1 allosteric antagonist 2-cyclopropyl-5-[1-(2-fluoro-3-pyridinyl)-5-methyl-1H-1,2,3-triazol-4-yl]-2,3-dihydro-1H-isoindol-1-one. J. Pharmacol. Exp. Ther. 2009;330(1):179–190. doi: 10.1124/jpet.109.151118. [DOI] [PubMed] [Google Scholar]
- 23.Slattery D.A., Desrayaud S., Cryan J.F. GABAB receptor antagonist-mediated antidepressant-like behavior is serotonin-dependent. J. Pharmacol. Exp. Ther. 2005;312(1):290–296. doi: 10.1124/jpet.104.073536. [DOI] [PubMed] [Google Scholar]
- 24.Cryan J.F., Kelly P.H., Chaperon F., Gentsch C., Mombereau C., Lingenhoehl K., Froestl W., Bettler B., Kaupmann K., Spooren W.P. Behavioral characterization of the novel GABAB receptor-positive modulator GS39783 (N,N'-dicyclopentyl-2-methylsulfanyl-5-nitro-pyrimidine-4,6-diamine): anxiolytic-like activity without side effects associated with baclofen or benzodiazepines. J. Pharmacol. Exp. Ther. 2004;310(3):952–963. doi: 10.1124/jpet.104.066753. [DOI] [PubMed] [Google Scholar]
- 25.Rorick-Kehn L.M., Johnson B.G., Knitowski K.M., et al. In vivo pharmacological characterization of the structurally novel, potent, selective mGlu2/3 receptor agonist LY404039 in animal models of psychiatric disorders. Psychopharmacology (Berl.) 2007;193(1):121–136. doi: 10.1007/s00213-007-0758-3. [DOI] [PubMed] [Google Scholar]
- 26.Rorick-Kehn L.M., Johnson B.G., Burkey J.L., Wright R.A., Calligaro D.O., Marek G.J., Nisenbaum E.S., Catlow J.T., Kingston A.E., Giera D.D., Herin M.F., Monn J.A., McKinzie D.L., Schoepp D.D. Pharmacological and pharmacokinetic properties of a structurally novel, potent, and selective meta- botropic glutamate 2/3 receptor agonist: in vitro characterization of agonist (-)-(1R,4S,5S,6S)-4-amino-2-sulfonylbicyclo[3.1.0]-hexane-4,6-dicarboxylic acid (LY404039). J. Pharmacol. Exp. Ther. 2007;321(1):308–317. doi: 10.1124/jpet.106.110809. [DOI] [PubMed] [Google Scholar]
- 27.Noda Y., Kamei H., Mamiya T., Furukawa H., Nabeshima T. T. Repeated phencyclidine treatment induces negative symptom-like behavior in forced swimming test in mice: imbalance of prefrontal serotonergic and dopaminergic functions. Neuropsychopharmacol. 2000;23(4):375–387. doi: 10.1016/S0893-133X(00)00138-X. [DOI] [PubMed] [Google Scholar]
- 28.Noda Y., Yamada K., Furukawa H., Nabeshima T. Enhancement of immobility in a forced swimming test by subacute or repeated treatment with phencyclidine: a new model of schizophrenia. Br. J. Pharmacol. 1995;116(5):2531–2537. doi: 10.1111/j.1476-5381.1995.tb15106.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Langen B., Dost R., Egerland U., Stange H., Hoefgen N. Effect of PDE10A inhibitors on MK-801-induced immobility in the forced swim test. Psychopharmacology (Berl.) 2012;221(2):249–259. doi: 10.1007/s00213-011-2567-y. [DOI] [PubMed] [Google Scholar]
- 30.Horiguchi M., Huang M., Meltzer H.Y. The role of 5-hydroxytryptamine 7 receptors in the phencyclidine-induced novel object recognition deficit in rats. J. Pharmacol. Exp. Ther. 2011;338(2):605–614. doi: 10.1124/jpet.111.180638. [DOI] [PubMed] [Google Scholar]
- 31.Horiguchi M., Huang M., Meltzer H.Y. Interaction of mGlu2/3 agonism with clozapine and lurasidone to restore novel object recognition in subchronic phencyclidine-treated rats. Psychopharmacology (Berl.) 2011;217(1):13–24. doi: 10.1007/s00213-011-2251-2. [DOI] [PubMed] [Google Scholar]
- 32.Dere E., Huston J.P., De Souza Silva M.A. The pharmacology, neuroanatomy and neurogenetics of one-trial object recognition in rodents. Neurosci. Biobehav. Rev. 2007;31(5):673–704. doi: 10.1016/j.neubiorev.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 33.Wierońska J.M., Sławińska A., Lasoń-Tyburkiewicz M., Gruca P., Papp M., Zorn S.H., Doller D., Kłeczek N., Noworyta-Sokołowska K., Gołembiowska K., Pilc A. The antipsychoticlike effects in rodents of the positive allosteric modulator Lu AF21934 involve 5-HT1A receptor signaling: mechanistic studies. Psychopharmacol (Berl). 2014 doi: 10.1007/s00213-014-3657-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goudet C., Vilar B., Courtiol T., Deltheil T., Bessiron T., Brabet I., Oueslati N., Rigault D., Bertrand H.O., McLean H., Daniel H., Amalric M., Acher F., Pin J.P. A novel selective metabotropic glutamate receptor 4 agonist reveals new possibilities for developing subtype selective ligands with therapeutic potential. FASEB J. 2012;26(4):1682–1693. doi: 10.1096/fj.11-195941. [DOI] [PubMed] [Google Scholar]
- 35.Wierońska J.M., Stachowicz K., Pałucha-Poniewiera A., Acher F., Brański P., Pilc A. Metabotropic glutamate receptor 4 novel agonist LSP1-2111 with anxiolytic, but not antidepressant-like activity, mediated by serotonergic and GABAergic systems. Neuropharmacol. 2010;59((7-8)):627–634. doi: 10.1016/j.neuropharm.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 36.Cajina M., Nattini M., Song D., Smagin G., Jørgensen E.B., Chandrasena G., Bundgaard C., Toft D.B., Huang X., Acher F., Doller D. Qualification of LSP1-2111 as a Brain Penetrant Group III Metabotropic Glutamate Receptor Orthosteric Agonist. ACS Med. Chem. Lett. 2013;5(2):119–123. doi: 10.1021/ml400338f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Snigdha S., Horiguchi M., Huang M., Li Z., Shahid M., Neill J.C., Meltzer H.Y. Attenuation of phencyclidine-induced object recognition deficits by the combination of atypical antipsychotic drugs and pimavanserin (ACP 103), a 5-hydroxytryptamine(2A) receptor inverse agonist. J. Pharmacol. Exp. Ther. 2010;332(2):622–631. doi: 10.1124/jpet.109.156349. [DOI] [PubMed] [Google Scholar]
- 38.Meltzer H.Y., Horiguchi M., Massey B.W. The role of serotonin in the NMDA receptor antagonist models of psychosis and cognitive impairment. Psychopharmacology (Berl.) 2011;213(2-3):289–305. doi: 10.1007/s00213-010-2137-8. [DOI] [PubMed] [Google Scholar]
- 39.Horiguchi M., Hannaway K.E., Adelekun A.E., Jayathilake K., Meltzer H.Y. Prevention of the phencyclidine-induced impairment in novel object recognition in female rats by co-administration of lurasidone or tandospirone, a 5-HT(1A) partial agonist. Neuropsychopharmacol. 2012;37(10):2175–2183. doi: 10.1038/npp.2012.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Meltzer H.Y., Rajagopal L., Huang M., Oyamada Y., Kwon S., Horiguchi M. Translating the N-methyl-D-aspartate receptor antagonist model of schizophrenia to treatments for cognitive impairment in schizophrenia. Int. J. Neuropsychopharmacol. 2013;16(10):2181–2194. doi: 10.1017/S1461145713000928. [DOI] [PubMed] [Google Scholar]
- 41.Guidotti A., Auta J., Davis J.M., Dong E., Grayson D.R., Veldic M., Zhang X., Costa E. GABAergic dysfunction in schizophrenia: new treatment strategies on the horizon. sychopharmacol(Berl). 2005;180((2)):191–205. doi: 10.1007/s00213-005-2212-8. [DOI] [PubMed] [Google Scholar]
- 42.Dong E., Agis-Balboa R.C., Simonini M.V., Grayson D.R., Costa E., Guidotti A. Reelin and glutamic acid decarboxylase67 promoter remodeling in an epigenetic methionine-induced mouse model of schizophrenia. Proc. Natl. Acad. Sci. USA. 2005;102(35):12578–12583. doi: 10.1073/pnas.0505394102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Aghajanian G.K., Marek G.J. Serotonin model of schizophrenia: emerging role of glutamate mechanisms. Brain Res. Brain Res. 2000;31:302–312. doi: 10.1016/s0165-0173(99)00046-6. [DOI] [PubMed] [Google Scholar]
- 44.Kinon B.J., Zhang L., Millen B.A., Osuntokun O.O., Williams J.E., Kollack-Walker S., Jackson K., Kryzhanovskaya L., Jarkova N., HBBI Study Group A multicenter, inpatient, phase 2, double-blind, placebo-controlled dose-ranging study of LY2140023 monohydrate in patients with DSM-IV schizophrenia. J. Clin. Psychopharmacol. 2011;31(3):349–355. doi: 10.1097/JCP.0b013e318218dcd5. [DOI] [PubMed] [Google Scholar]
- 45.Stauffer V.L., Baygani S.K., Kinon B.J., Krikke-Workel J.O. A short-term, multicenter, placebo-controlled, randomized withdrawal study of a metabotropic glutamate 2/3 receptor agonist using an electronic patient-reported outcome device in patients with schizophrenia. J. Clin. Psychopharmacol. 2014;34(5):552–558. doi: 10.1097/JCP.0000000000000187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Adams D.H., Kinon B.J., Baygani S., et al. A long-term, phase 2, multicenter, randomized, open-label, comparative safety study of pomaglumetad methionil (LY2140023 monohydrate) versus atypical antipsychotic standard of care in patients with schizophrenia. BMC Psychiatry. 2013;13(1):143. doi: 10.1186/1471-244X-13-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Patil S.T., Zhang L., Martenyi F., Lowe S.L., Jackson K.A., Andreev B.V., Avedisova A.S., Bardenstein L.M., Gurovich I.Y., Morozova M.A., Mosolov S.N., Neznanov N.G., Reznik A.M., Smulevich A.B., Tochilov V.A., Johnson B.G., Monn J.A., Schoepp D.D. Activation of mGlu2/3 receptors as a new approach to treat schizophrenia: a randomized Phase 2 clinical trial. Nat. Med. 2007;13(9):1102–1107. doi: 10.1038/nm1632. [DOI] [PubMed] [Google Scholar]
- 48.Sternbach L.H. The benzodiazepine story. J. Med. Chem. 1979;22(1):1–7. doi: 10.1021/jm00187a001. [DOI] [PubMed] [Google Scholar]
- 49.Bowery N.G. GABAB receptor: a site of therapeutic benefit. Curr. Opin. Pharmacol. 2006;6(1):37–43. doi: 10.1016/j.coph.2005.10.002. [DOI] [PubMed] [Google Scholar]


