Abstract
OBJECTIVES
Dysbiosis leading to abnormal intestinal fermentation has been suggested as a possible etiological mechanism in irritable bowel syndrome (IBS). We aimed to investigate the location and magnitude of altered intestinal bacterial fermentation in IBS and its clinical subtypes.
METHODS
One hundred fourteen IBS patients who satisfied Rome III criteria and 33 healthy controls (HC) were investigated. Intestinal fermentation was assessed using two surrogate measures: intestinal intraluminal pH and fecal short chain fatty acids (SCFAs). Intraluminal pH and intestinal transit time were measured in the small and large bowel using a wireless motility capsule (SmartPill™) in 47 IBS and 10 HC. Fecal SCFAs including acetate, propionate, butyrate and lactate were analyzed by capillary gas chromatography in all enrolled subjects. Correlations between intestinal pH, fecal SCFAs, intestinal transit time and IBS symptom scores were analyzed.
RESULTS
Colonic intraluminal pH levels were significantly lower in IBS patients compared to HC (total colonic pH, 6.8 for IBS vs. 7.3 for HC, P = 0.042). There were no differences in total and segmental pH levels in the small bowel between IBS patients and HC (6.8 vs. 6.8, P = NS). The intraluminal colonic pH differences were consistent in all IBS subtypes. Total SCFAs level was significantly lower in C-IBS patients than in D-IBS and M-IBS patients and HC. The total SCFAs level in all IBS subjects was similar with that of HC. Colonic pH levels correlated positively with colon transit time (CTT) and IBS symptoms severity. Total fecal SCFAs levels correlated negatively with CTT, and positively with stool frequency.
CONCLUSIONS
Colonic intraluminal pH is decreased, suggesting higher colonic fermentation, in IBS patients compared with HC. Fecal SCFAs are not a sensitive marker to estimate intraluminal bacterial fermentation.
Keywords: Irritable Bowel Syndrome, Fermentation, Short chain fatty acids
INTRODUCTION
Irritable bowel syndrome (IBS) is characterized by chronic or recurring abdominal pain or discomfort associated with altered bowel habits and is one of the most common gastrointestinal (GI) disorders.1, 2 IBS is considered a multifactorial disorder associated with visceral hypersensitivity, altered gut motility and dysfunction of the brain-gut axis and immune system. However, the pathophysiology of the disorder is still not completely understood.2–5 Recently, it has been suggested that alterations in the gut microbiota, leading to abnormal intestinal fermentation may be a possible etiological mechanism.6–9
Intestinal fermentation by gut microbiota is a central physiological process by which polymers, including carbohydrates, are biotransformed into end-products, mainly short chain fatty acids (SCFAs) and gases. These intra-luminal fermentation processes have an important role in supplying nutrients and energy to the host.10 However, alterations in intestinal fermentation may lead to certain physiological abnormalities that are often observed in IBS such as intraluminal excessive gas production and altered motility. These alterations in intestinal physiology can be an important factor in provoking or exacerbating IBS symptoms.11, 12 In addition, interventional clinical studies targeting the intestinal microbiota with antibiotics or probiotics demonstrated beneficial effects in some patients with IBS. 19, 20Specifically, in regard to intestinal fermentation, recent studies have shown that diet low in fermentable oligosaccharides, disaccharides, monosaccharides, and polyols (FODMAPs) can offer considerable symptom relief in patients with IBS.13, 14 However, the role of altered intestinal fermentation by intestinal bacteria in the pathogenesis of IBS has not been adequately investigated in this condition.
We hypothesized that bacterial fermentation is altered in certain segments of the GI tract in IBS, and that these alterations are associated with bowel functions and symptoms. The aims of the current study were to investigate the location and magnitude of altered intestinal bacterial fermentation in IBS and its clinical subtypes, and to examine their relation to bowel characteristics and GI symptoms. Intestinal fermentation was assessed by measuring intraluminal pH levels [by a wireless motility capsule (SmartPill™)] and fecal SCFAs concentration as surrogate markers in a cohort of well characterized patients with IBS and healthy controls (HC).
METHODS
Study Population
All subjects were recruited from the University of North Carolina (UNC) healthcare outpatient clinics and from the Chapel Hill general population by advertising. Inclusion criteria included age of 18 years or older, any gender, race, or ethnicity. Healthy controls had no chronic or recurring GI symptoms. IBS patients met the Rome III criteria for IBS and had mild to moderate symptom severity with IBS-symptom severity scale (IBS-SSS) score of 175–300. Additional inclusion criteria included normal physical examination and laboratory tests and a normal colonoscopy within the last 3 years, in patients age 50 or older. Subjects with a history of GI tract surgery (other than appendectomy or cholecystectomy), a history of inflammatory bowel diseases (IBD), celiac disease, lactose malabsorption, or any other diagnosis that could explain their chronic or recurrent bowel symptoms were excluded from the study. In addition, participants were excluded if they had a history of treatment with antibiotics, anti-inflammatory agents including aspirin, non-steroidal anti-inflammatory drugs or steroids, or intentional consumption of probiotics in the past 6 weeks. All subjects were evaluated by a physician to exclude an alternative diagnosis to IBS. The study was approved by the UNC Internal Review Board (IRB) and all subjects provided written consent before participation and were compensated for participating in the study.
Clinical symptom assessments in IBS
Abdominal pain/discomfort, altered bowel habit (loose/watery or hard/lumpy stools), and bloating were assessed using a 10-cm visual analogue scale (VAS; maximum score, 10).15 IBS symptom severity scale (IBS-SSS)16 and IBS specific quality of life (IBS-QOL)17 were also assessed. Stool frequency was measured by number of bowel movements per day and stool consistency determined using the stool Bristol Score.18
Assessment for intraluminal fermentation and intestinal transit
Intestinal fermentation was assessed using two surrogate markers: Intestinal intraluminal pH and fecal SCFAs. Intraluminal pH levels were measured using a wireless motility capsule (SmartPill™; Given Imaging, Yoqneam, Israel). The wireless motility capsule is an FDA approved device that is clinically used to assess intestinal transit. After being swallowed, the capsule constantly measures intraluminal pH, pressure, and temperature as it travels through the GI tract and transmits this information wirelessly to a portable receiver worn by the examinee.19 Using these parameters and a specific software the (SmartPill™) system generate data on gastric emptying time (GET), small bowel transit time (SBTT), colon transit time (CTT) and whole gut transit time (WGTT).19, 20 For the purpose of this study we used the intraluminal pH data as surrogate markers for intestinal fermentation. To enable further accuracy in assessment of localization of intestinal fermentation, the small and large bowel pH measurements were both divided into four quartiles (Q1–4) based on the total time in each (small and large bowel) segment. SBTT, CTT, and WGTT were also assessed as previously described.19, 20
Analysis of SCFA
Fresh stool samples were collected from all subjects on site or at home in the morning of the study visit. Each fecal sample was immediately transferred to the laboratory where it was homogenized and stored at −80°C until analyzed. Fecal acetate, propionate, butyrate, lactate and total SCFAs were quantified in fecal samples in duplicate using capillary gas chromatography with 2-ethyl butyric acid as an internal standard, as described previously.10, 21–23
Statistical Analysis
We used Student t-test when there were two groups being compared and analysis of variance (ANOVA) when there were more than two groups being compared for continuous variables. The chi-square test was used for discrete variables. For post hoc analysis, Tukey’s honest significance test was used. Baseline characteristics were evaluated by Student t-test or Pearson’s chi-square test. Intestinal pH, fecal SCFAs levels and GI transit times were compared between groups by Student t-test or ANOVA. Correlations between colonic pH, fecal SCFAs, CTT, stool frequency and consistency, IBS-SSS, and IBS-QOL were done using Spearman correlation analysis for all study subjects including IBS and HC. All P values were two-tailed, and P values < 0.05 were considered statistically significant. Statistical analyses were performed using SPSS version 18.0 for Windows (SPSS, Inc., Chicago, IL, USA). Unless otherwise stated, data are presented as mean ± standard deviation.
RESULTS
Study population
We investigated a total of 147 subjects (114 IBS and 33 HC). The study IBS population included patients with constipation-predominant IBS (C-IBS, n=26), diarrhea-predominant IBS (D-IBS, n=42), mixed type IBS (M-IBS, n=32) and unspecified type IBS (U-IBS, n=14). The racial distribution was as follows: Caucasian 70.7%, Black 21.1%, and other 8.2%. There were no significant differences in age, sex, body mass index, and race between the study groups (Table 1).
Table 1.
Baseline characteristics of the study groups
| IBS | HC | P - value | |
|---|---|---|---|
| Number of subjects | 114 | 33 | |
| Age (years): mean | 35.4±11.3 | 33.9±13.0 | 0.534 |
| Sex (male:female) | 16:98 | 4:29 | 0.778 |
| Body mass index (kg m−2): mean | 27.1±5.5 | 27.2±9.2 | 0.929 |
| Race | 0.054 | ||
| Caucasian | 84 (73.7%) | 20 (60.6%) | |
| Black | 24 (21.1%) | 7 (21.2%) | |
| Others | 6 (5.3%) | 6 (18.2%) | |
| IBS subtype: number | |||
| Constipation-predominant | 26 (22.8%) | ||
| Diarrhea-predominant | 42 (36.8%) | ||
| Mixed type | 32 (28.1%) | ||
| Unspecified type | 14 (12.3%) |
IBS; irritable bowel syndrome, HC; healthy control
The data are presented as mean ± standard deviation.
Intestinal intraluminal pH
Intestinal intraluminal pH data were collected from 47 patients with IBS and 10 HC. Mean total colonic pH levels were significantly lower in the IBS group compared to HC (6.8 vs. 7.3, P = 0.042). The significant differences were also observed in Q1 (6.4 vs. 6.8, P = 0.011) and Q4 (7.2 vs. 7.8, P = 0.046). The colonic pH levels at the first quartile (Q1) were the lowest among the four quartiles in both groups (Table 2 and Fig. 1). The lower intraluminal colonic pH levels compared to HC were consistent across all IBS subtypes (mean total colonic pH: 7.0 for C-IBS, 7.0 for D-IBS, 6.8 for M-IBS, 6.4 for U-IBS, and 7.3 for HC, respectively) (Table 3).
Table 2.
Small and large bowel intraluminal pH levels
| IBS (n=47) |
HC (n=10) |
P - value | |
|---|---|---|---|
| Small bowel pH | |||
| Q1 small bowel pH | 6.1±0.7 | 6.2±1.0 | 0.885 |
| Q2 small bowel pH | 6.7±0.5 | 6.7±0.4 | 0.840 |
| Q3 small bowel pH | 7.1±0.4 | 7.1±0.4 | 0.994 |
| Q4 small bowel pH | 7.3±0.4 | 7.3±0.5 | 0.878 |
| Total small bowel pH | 6.8±0.4 | 6.8±0.4 | 0.937 |
| Colonic pH | |||
| Q1 Colonic pH | 6.4±0.6 | 6.8±0.4 | 0.011 |
| Q2 Colonic pH | 6.8±0.7 | 7.1±0.5 | 0.173 |
| Q3 Colonic pH | 7.0±0.8 | 7.5±0.6 | 0.081 |
| Q4 Colonic pH | 7.2±0.8 | 7.8±0.8 | 0.046 |
| Total Colonic pH | 6.8±0.7 | 7.3±0.5 | 0.042 |
IBS; irritable bowel syndrome, HC; healthy control, Q; quartile
The data are presented as mean ± standard deviation.
Figure 1.
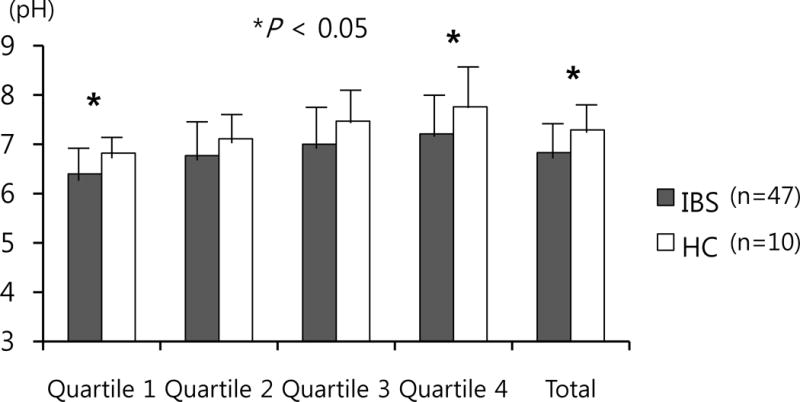
Colonic pH levels. Quartile 1 and 4, and mean total colonic pH levels were significantly lower in irritable bowel syndrome (n=47) compared to healthy control (n=10). The pH in the proximal colon (quartile 1) was the lowest in both groups. * P < 0.05
Table 3.
Large bowel intraluminal pH levels according to IBS subtype
| C-IBS (n=13) |
D-IBS (n=14) |
M-IBS (n=12) |
U-IBS (n=8) |
HC (n=10) |
|
|---|---|---|---|---|---|
| Q1 Colonic pH | 6.4±0.5 | 6.7±0.7 | 6.4±0.5 | 5.9±0.3a | 6.8±0.4 |
| Q2 Colonic pH | 7.0±0.9 | 6.9±0.8 | 6.5±0.6 | 6.5±0.4 | 7.1±0.5 |
| Q3 Colonic pH | 7.1±0.8 | 7.0±0.6 | 7.1±0.9 | 6.7±0.8 | 7.5±0.6 |
| Q4 Colonic pH | 7.3±0.8 | 7.3±0.7 | 7.2±1.0 | 7.0±0.7 | 7.8±0.8 |
| Total Colonic pH | 7.0±0.7 | 7.0±0.6 | 6.8±0.7 | 6.4±0.6b | 7.3±0.5 |
IBS; irritable bowel syndrome, C-IBS; constipation-predominant IBS, D-IBS; diarrhea- predominant IBS, M-IBS; mixed type IBS, U-IBS; unspecified type IBS, HC; healthy control, Q; quartile
P < 0.05, compared to Q1 colonic pHs of D-IBS and HC;
P < 0.05, compared to total colonic pH of HC
The data are presented as mean ± standard deviation.
There were no intraluminal pH differences in the 4 quartiles and total small bowel between IBS and HC (mean total small bowel pH: 6.8 for IBS vs. 6.8 for HC, P = 0.937) (Table 2). Also, there were no significant intraluminal small bowel pH differences between the groups based on IBS subtypes.
In subgroup analyses, small and large bowel pH levels were not different according to gender, race and age (≤ 45 vs. > 45).
Fecal short chain fatty acids
Fecal SCFAs were measured in all enrolled subjects (114 IBS and 33 HC). Mean acetate, propionate, butyrate, lactate and total fecal SCFAs levels were not significantly different between all IBS and HC (total SCFAs level: 92.1 mM vs. 92.0 mM, P = 0.996, respectively). Acetate had the highest levels followed by propionate and butyrate in both IBS and HC. Lactate was not detected except for two cases (6.4 mM in a M-IBS subject and 8.1 mM in a D-IBS subject). The SCFAs profiles were similar across the different IBS subtypes (Table 4 and Fig. 2). However, acetate, propionate, butyrate, and total SCFAs levels were lower in C-IBS than in all the other subtypes, and were significantly lower compared to D-IBS. The propionate level in C-IBS was significantly lower than in any other group.
Table 4.
Fecal short chain fatty acids levels
| C-IBS (n=26) |
D-IBS (n=42) |
M-IBS (n=32) |
U-IBS (n=14) |
IBS, total (n=114) |
HC (n=33) |
P - valuea | |
|---|---|---|---|---|---|---|---|
| Acetate (mM) | 47.8±23.4b | 61.3±23.2 | 59.2±21.2 | 63.9±40.9 | 58.0±25.8 | 56.6±18.5 | 0.777 |
| Propionate (mM) | 13.6±5.8c | 20.1±8.9 | 18.4±10.2 | 20.4±14.5 | 18.2±9.8 | 20.0±8.0 | 0.331 |
| Butyrate (mM) | 11.6±9.5d | 16.4±8.9 | 15.8±10.5 | 21.5±22.8 | 15.8±12.1 | 15.4±8.9 | 0.875 |
| Total SCFA (mM) | 73.0±35.7e | 98.1±36.7 | 93.7±37.0 | 105.9±77.0 | 92.1±44.1 | 92.0±33.3 | 0.996 |
IBS, irritable bowel syndrome; C-IBS, constipation-predominant IBS; D-IBS, diarrhea- predominant IBS; M-IBS, mixed type IBS; U-IBS, unspecified type IBS; HC, healthy control; SCFA, short chain fatty acid
P-value for comparison between total IBS and HC;
P < 0.05, compared to acetate level of D-IBS;
P < 0.05, compared to propionate levels of all the other groups;
P < 0.05, compared to butyrate level of D-IBS;
P < 0.05, compared to total SCFAs level of D-IBS, M-IBS and HC
The data are presented as mean ± standard deviation.
Figure 2.
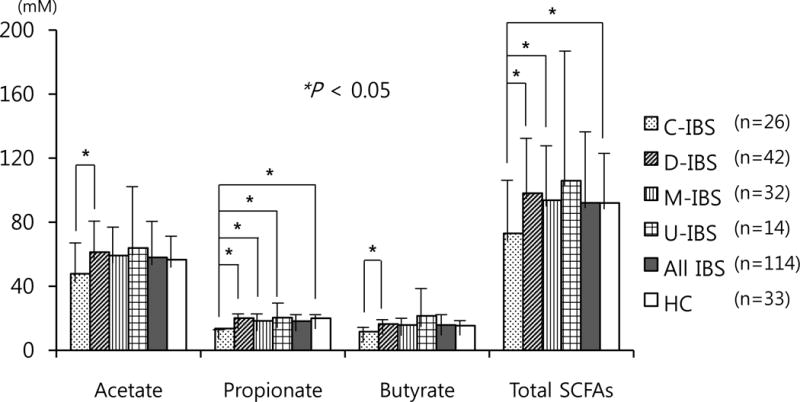
Fecal short chain fatty acids (SCFAs) levels by irritable bowel syndrome subtypes. Total SCFAs level was significantly lower in constipation-predominant irritable bowel syndrome (C-IBS) than in diarrhea-predominant IBS, mixed type IBS and healthy control (HC). Total SCFAs level averaged across all IBS was similar with that in HC. Propionate level in C-IBS was significantly lower than those of the other groups. Acetate and butyrate levels were significantly lower in C-IBS than in D-IBS. * P < 0.05.
Intestinal transit time
Intestinal transit time data were collected from 47 patients with IBS and 10 HC. Overall the average intestinal transit times including SBTT, CTT, and WGTT were not significantly different between IBS patients and HC. However, as expected, CTT and WGTT were significantly longer in C-IBS than those in D-IBS and M-IBS. There were no significant differences in SBTT between the groups. The whole gut and segmental transit times are presented in Table 5.
Table 5.
Gastrointestinal transit times according to IBS subtype
| C-IBS (n=13) |
D-IBS (n=14) |
M-IBS (n=12) |
U-IBS (n=8) |
IBS, total (n=47) |
HC (n=10) |
P - valuea | |
|---|---|---|---|---|---|---|---|
| WGTT (hours) | 57.0±27.9b | 30.1±18.4 | 30.1±14.4 | 45.3±25.2 | 39.6±24.0 | 48.2±31.9 | 0.344 |
| SBTT (hours) | 4.4±2.1 | 4.1±1.1 | 3.7±1.2 | 3.9±1.1 | 4.0±1.4 | 3.6±1.3 | 0.348 |
| CTT (hours) | 44.6±24.7c | 22.4±16.0 | 23.1±14.7 | 39.1±24.6 | 31.1±21.6 | 34.7±21.1 | 0.632 |
IBS, irritable bowel syndrome; C-IBS, constipation-predominant IBS; D-IBS, diarrhea- predominant IBS; M-IBS, mixed type IBS; U-IBS, unspecified type IBS; HC, healthy control; WGTT, whole gut transit time; SBTT, small bowel transit time; CTT, colon transit time
P - value for comparison between total IBS and HC;
P < 0.05, compared to WGTTs of D-IBS and M-IBS;
P < 0.05, compared to CTTs of D-IBS and M-IBS;
Correlation between colonic pH, fecal SCFAs, CTT and symptoms
Colonic pH levels positively correlated with CTT (Spearman correlation coefficient, SCC = 0.33, P = 0.013), and had a negative correlation trend with fecal SCFAs levels (SCC = −0.23, P = 0.079). Acetate, propionate, butyrate and total SCFAs levels correlated negatively with CTT, and positively with stool frequency (total SCFAs vs. CTT, SCC = −0.44, P = 0.001; total SCFAs vs. stool frequency, SCC = 0.45, P < 0.001).
Colonic pH levels positively correlated with IBS-SSS (SCC = 0.49, P = 0.014), and similar positive trends were noted for abdominal pain and bloating with negative trends for IBS-QOL. CTT had correlation trend positively with IBS symptom scores and negatively with IBS-QOL. Fecal SCFAs levels had no correlation with IBS symptom scores and QOL. The results of Spearman correlation analyses are presented in Table 6.
Table 6.
Spearman correlation coefficient in correlation analyses between variables
| Total Colon pH | Total SCFAs | CTT | Stool consistency | Stool frequency | Abdominal pain | Bloating | IBS-SSS | IBS-QOL | |
|---|---|---|---|---|---|---|---|---|---|
| Total Colon pH | 1.00 | −0.23 | 0.33b | −0.30 | −0.10 | 0.29 | 0.29 | 0.49b | −0.35 |
| Total SCFAs | −0.23 | 1.00 | −0.44a | −0.01 | 0.45a | −0.14 | 0.01 | −0.33 | 0.12 |
| CTT | 0.33b | −0.44a | 1.00 | −0.64 | −0.40 | 0.41 | 0.30 | 0.39 | −0.28 |
SCFA, short chain fatty acid; CTT, colon transit time; IBS, irritable bowel syndrome; IBS-SSS, IBS-symptom severity scale score; IBS-QOL, IBS-quality of life
P < 0.01;
P < 0.05
DISCUSSION
IBS is a common condition that affects nearly 10% of the general population,1, 2 and contributes to a significant decrease in quality of life along with an increase in healthcare utilization. In spite of its high prevalence and impact on quality of life, the pathophysiological mechanisms underlying the disease remains unknown. Recently, the possible role of the intestinal microbiota in the pathogenesis of IBS has gained much interest.4, 8, 24 In this study, we investigated the role of intestinal bacterial fermentation in IBS.
Intraluminal intestinal fermentation by colonic bacteria produces gases such as hydrogen and carbon dioxide, and SCFAs as secondary by-products.10, 25, 26 The predominant SCFAs produced by bacterial fermentation are acetate, butyrate, and propionate. Lactate, which is a precursor for propionate and butyrate, is itself a by-product of fermentation. SCFAs are naturally acidic and cause a measurable drop in the pH of the intestinal lumen.26–28 In this study we have measured intraluminal pH levels and fecal SCFA concentrations as surrogate markers for intraluminal intestinal fermentation and used these factors to assess the location and magnitude of intestinal bacterial fermentation in a well characterized cohort of patients with IBS and HC.
We found that the mean total colonic pH levels were significantly lower in IBS compared to HC potentially indicating higher intraluminal bacterial fermentation in the IBS group. Of great importance is our finding of consistently higher bacterial fermentation in the large bowel of patients with IBS compared to HC while there were no differences in the pH levels in the small bowel between those two groups. Notably these findings were consistent in all small bowel and large bowel segments and across all IBS subtypes.
The intraluminal pH levels were lower at the proximal colon than the distal colon in both IBS and HC groups when we divided the quartiles based on the transit time. However, the quartiles based on transit time might not be the same with those based on geography, because if some patients might have mixtures of very rapid transit through one part of the colon, and then very slow transit through the next, although these two segments might differ pathophysiologically. Although those cases were not considered common findings, it may be a limitation of our study.
Small intestinal bacterial overgrowth (SIBO) is a condition in which an excess of colonic bacteria colonize the small intestine. The symptoms of SIBO and IBS overlap29 and it has been suggested that the prevalence of SIBO is increased in IBS or that SIBO may have an etiological role in the pathogenesis of IBS, particularly in view of the improvement of symptoms with antibiotic treatment.30–33 However, the link between SIBO and IBS is still unclear and there are some controversies regarding the real prevalence and the importance of SIBO in the pathogenesis of IBS.34 Our study findings indicate an increased bacterial fermentation in the large but not in the small bowel of patients with IBS, thus supporting the hypothesis of altered microbial composition and/or function in the colon rather than in the small bowel (e.g., in SIBO) in the pathogenesis of IBS. In addition, our finding of significant differences in total and segmental CTT but not in SBTT between IBS and its subtypes and HC, further support the importance of altered colonic- rather than small bowel physiology in the pathogenesis of the disorder.
The importance of SCFAs as possible etiological factors in the pathogenesis of IBS is suggested by observations from animal and human studies. Animal studies demonstrate that SCFA can initiate high-amplitude propagated colonic contractions35 and accelerated intestinal transit and motility via intestinal release of 5-hydroxytryptamine.36 Intracolonic infusion of 0.5% acetic acid enhanced sensitivity to colorectal distension.37 In a human study, the total concentration of SCFAs in jejunal secretion was approximately four times higher in patients with SIBO than in healthy subjects.38 In addition, a recent study reported higher counts of acetic- and propionic-acid producing bacteria (Veillonella and Lactobacillus) in IBS patients and an association between the higher levels of these SCFAs and GI symptoms and QOL.39 However, the studies in this area have generated conflicting findings on fecal organic acids. For example, a study conducted in patients with D-IBS showed that fecal SCFAs were decreased compared to HC.40 In our study, there were no significant differences in the mean levels of fecal SCFAs between IBS (all subtypes) and HC and the levels of fecal SCFAs did not correlate with IBS symptom severity. However, we were able to demonstrate that fecal SCFAs levels in C-IBS patients were significantly lower than in D-IBS patients, and tended to be lower than those in all the other IBS subtypes and HC. In addition, as expected, SCFAs levels negatively correlated with CTT which is consistent with our finding of a positive correlation between colonic pH and CTT, indicating that higher fermentation with higher production of SCFA is associated with increased motility and shorter transit times. An alternative explanation can be reduced absorption of SCFAs in patients with rapid intestinal transit due to a shorter transit time. The difficulties in interpretation of these findings reflect the multifactorial determinants of fecal SCFAs. Intraluminal SCFAs are very efficiently absorbed in the colon and only 5% to 10% of the SCFAs produced by bacterial fermentation are excreted and can be measured in the stool.41–43 The correlation between fecal SCFAs levels and CTT and the differences between IBS subtypes suggest that fecal SCFAs levels may be controlled by CTT more dominantly than fermentation degree, thus, fecal SCFAs may not be a sensitive marker to estimate intraluminal bacterial fermentation.
In addition to magnitude and location, we examined the associations between intestinal fermentation and clinical presentation of IBS. Unlike what we expected the colonic pH levels positively correlated with IBS symptom scores including IBS-SSS. This finding suggests that other factors such as abnormal intestinal motility and psychological disturbances may be more important than bacterial fermentation in determining symptoms severity.
The wireless motility SmartPill™ capsule measures pH, pressure and temperature in real time throughout the GI tract.19 Overall, pH profiles in the GI tract are characterized by an abrupt rise in pH from acid to near neutral as the capsule exited the stomach, a slow continued rise in pH through the small bowel until reaching the large bowel where pH falls more than 1 unit, and subsequently, there is a slow rise in pH through the colon.19, 44 The pH drop at the ileocecal junction is well documented in studies using ingestible radiotelemetry capsules.45, 46 Time between an abrupt rise of pH in duodenum and a fall of >1 pH unit in ileocolonic junction was taken as SBTT. CTT was assessed by measuring the time required from cecal entry to body exit (loss of signal and/or an abrupt temperature drop).19 A recent scintigraphic study using a radioisotope-labeled WMC has validated this pH change at the ileocolonic junction and has shown that the fall in pH observed with WMC corresponds to the time of arrival of the WMC into the cecum or ascending colon.47 Several studies have confirmed the utility of WMC in quantifying CTT when compared to radiopaque marker method. There was good agreement between the WMC and radiopaque marker transit results.20, 44 The WMC is a validated test, however there are some confounding issues with the test. The pH drop at the ileocecal junction is occasionally (<5%) not clearly identifiable, and device failure is reported in <3% of cases. In our study, the pH landmarks were well identified in all cases and there were no capsule failures.
To our knowledge there has been no previous study to evaluate bacterial fermentation in the whole intestine by assessing intraluminal pH changes in patients with IBS. However, our study may have some limitations. Firstly, although subjects were asked to keep their regular diet during the study, we did not have detailed dietary data and could not control for dietary effects on intestinal physiology and fermentation processes including intraluminal pH and SCFAs production. Second, we did not investigate the intestinal bacterial community, so we do not know which bacterial species or genera were altered and how these alterations relate to the variables of interest. Third, we did not evaluate gas production, such as hydrogen and methane which might be associated with symptom generation. Finally, the analyses of the wireless motility capsule (SmartPill™) data included a relatively small number of subjects and involved some multiple comparisons; particularly in sub-analysis by IBS subtypes. Further large scale studies, including the analyses of dietary factors, gas production and bacterial communities are needed. These studies may enable to associate alterations in bacterial fermentation with specific diet or bacterial communities and provide insights on how these associations relate to the altered physiology and symptoms observed in IBS.
In conclusion, we found that colonic intraluminal pH is decreased, suggesting increased bacterial fermentation, in IBS patients across all subtypes compared with HC, and that this process is more prominent in the proximal colon. The altered intestinal fermentation and transit in IBS appear to be in the colon and not in the small bowel. Higher levels of fecal SCFAs and lower levels of intraluminal pH are associated with decreased CTT. The hypothesized model of our study results was illustrated in the figure 3. Our study further support the notion that altered colonic bacterial fermentation has an important etiological role for in the pathogenesis of IBS. Future studies should investigate the degree to which altered intestinal fermentation is a cause of IBS symptoms or an effect of IBS-related altered physiology by, if possible, direct measurement of bacterial fermentation, and clarify the possible clinical benefit of targeting intestinal fermentation in the treatment of functional GI disorders.
Figure 3.
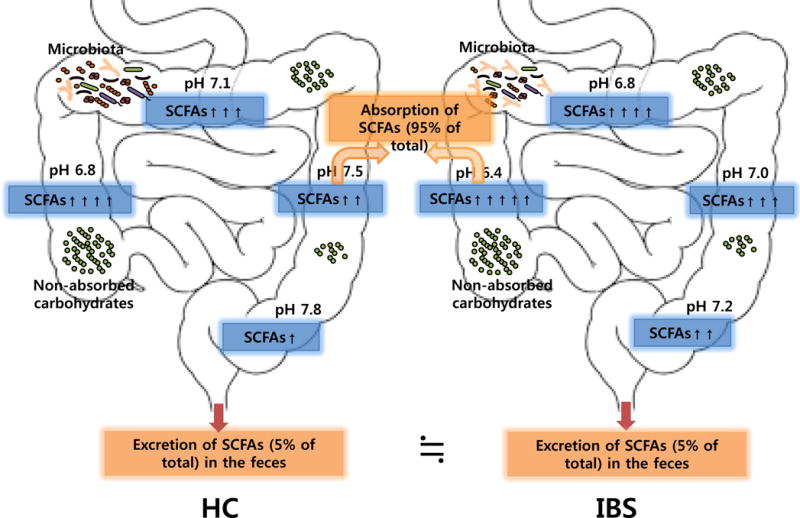
Hypothesized model. Colonic intraluminal pH is decreased and SCFAs production is increased suggesting higher fermentation in patients with IBS compared with HC. SCFAs are effectively absorbed (> 95% of SCFAs) in the GI tract and only a small portion (~5% of total SCFAs) is excretion in feces. Both processes can affect and are affected by intestinal transit. Abbreviations: SCFAs, short chain fatty acids; IBS, irritable bowel syndrome; HC, healthy control.
Study Highlights.
WHAT IS CURRENT KNOWLEDGE
-
✓
Alterations in the gut microbiota play a role in irritable bowel syndrome (IBS).
-
✓
Alterations in intestinal fermentation lead to certain physiological abnormalities such as intraluminal excessive gas production and altered motility.
-
✓
Diet low in fermentation substrates such as poorly absorbable, highly fermentable carbohydrates offer considerable symptom relief in IBS.
-
✓
The understanding of the role of altered intestinal fermentation, including its magnitude and location, in the pathogenesis of IBS is not completely understood.
WHAT IS NEW HERE
-
✓
Colonic intraluminal pH decreased, indicating higher intestinal fermentation, in IBS patients across all subtypes compared with healthy controls.
-
✓
The intestinal fermentation in IBS is altered in the colon but not in the small bowel.
-
✓
Higher levels of fecal SCFAs and lower levels of intraluminal pH are associated with decreased colon transit time.
Acknowledgments
The authors would like to thank Ms. Jenny C. Martin for her contribution to the SCFA analyses.
Financial support: This work was supported by grants K23 DK075621 (YR) and P30 DK34987 (JG). The project described was supported by the National Center for Advancing Translational Sciences (NCATS), National Institutes of Health, through Grant Award Number 1UL1TR001111. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Conflict of interest: The authors declare no conflict of interest.
Guarantor of the article: Yehuda Ringel, MD
Specific author contributions: T.R. and Y.R. planned and performed the study, interpreted the results and reviewed and edited the manuscript; D.T. collected and arranged the data; C.C. and A.R. analyzed and interpreted data, and drafted this article; J.G. provided statistical support; K.S. contributed to the analysis of fecal SCFAs; D.M. contributed to the wireless motility capsule (SmartPill™) studies; All authors have read and approved the final draft of this paper.
Potential competing interests: None.
References
- 1.Drossman DA, Camilleri M, Mayer EA, et al. AGA technical review on irritable bowel syndrome. Gastroenterology. 2002;123:2108–31. doi: 10.1053/gast.2002.37095. [DOI] [PubMed] [Google Scholar]
- 2.Spiller R, Aziz Q, Creed F, et al. Guidelines on the irritable bowel syndrome: mechanisms and practical management. Gut. 2007;56:1770–98. doi: 10.1136/gut.2007.119446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barbara G, Cremon C, Carini G, et al. The immune system in irritable bowel syndrome. J Neurogastroenterol Motil. 2011;17:349–59. doi: 10.5056/jnm.2011.17.4.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Camilleri M. Peripheral mechanisms in irritable bowel syndrome. N Engl J Med. 2012;367:1626–35. doi: 10.1056/NEJMra1207068. [DOI] [PubMed] [Google Scholar]
- 5.Camilleri M, Lasch K, Zhou W. Irritable bowel syndrome: methods, mechanisms, and pathophysiology. The confluence of increased permeability, inflammation, and pain in irritable bowel syndrome. Am J Physiol Gastrointest Liver Physiol. 2012;303:G775–85. doi: 10.1152/ajpgi.00155.2012. [DOI] [PubMed] [Google Scholar]
- 6.Carroll IM, Ringel-Kulka T, Keku TO, et al. Molecular analysis of the luminal- and mucosal-associated intestinal microbiota in diarrhea-predominant irritable bowel syndrome. Am J Physiol Gastrointest Liver Physiol. 2011;301:G799–807. doi: 10.1152/ajpgi.00154.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carroll IM, Ringel-Kulka T, Siddle JP, et al. Alterations in composition and diversity of the intestinal microbiota in patients with diarrhea-predominant irritable bowel syndrome. Neurogastroenterol Motil. 2012;24:521–30. e248. doi: 10.1111/j.1365-2982.2012.01891.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee BJ, Bak YT. Irritable bowel syndrome, gut microbiota and probiotics. J Neurogastroenterol Motil. 2011;17:252–66. doi: 10.5056/jnm.2011.17.3.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ringel Y, Maharshak N. Intestinal microbiota and immune function in the pathogenesis of irritable bowel syndrome. Am J Physiol Gastrointest Liver Physiol. 2013;305:G529–41. doi: 10.1152/ajpgi.00207.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chassard C, Dapoigny M, Scott KP, et al. Functional dysbiosis within the gut microbiota of patients with constipated-irritable bowel syndrome. Aliment Pharmacol Ther. 2012;35:828–38. doi: 10.1111/j.1365-2036.2012.05007.x. [DOI] [PubMed] [Google Scholar]
- 11.Dapoigny M, Stockbrugger RW, Azpiroz F, et al. Role of alimentation in irritable bowel syndrome. Digestion. 2003;67:225–33. doi: 10.1159/000072061. [DOI] [PubMed] [Google Scholar]
- 12.King TS, Elia M, Hunter JO. Abnormal colonic fermentation in irritable bowel syndrome. Lancet. 1998;352:1187–9. doi: 10.1016/s0140-6736(98)02146-1. [DOI] [PubMed] [Google Scholar]
- 13.Halmos EP, Power VA, Shepherd SJ, et al. A diet low in FODMAPs reduces symptoms of irritable bowel syndrome. Gastroenterology. 2014;146:67–75.e5. doi: 10.1053/j.gastro.2013.09.046. [DOI] [PubMed] [Google Scholar]
- 14.Staudacher HM, Lomer MC, Anderson JL, et al. Fermentable carbohydrate restriction reduces luminal bifidobacteria and gastrointestinal symptoms in patients with irritable bowel syndrome. J Nutr. 2012;142:1510–8. doi: 10.3945/jn.112.159285. [DOI] [PubMed] [Google Scholar]
- 15.Veldhuyzen van Zanten SJ, Talley NJ, Bytzer P, et al. Design of treatment trials for functional gastrointestinal disorders. Gut. 1999;45(Suppl 2):II69–77. doi: 10.1136/gut.45.2008.ii69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Francis CY, Morris J, Whorwell PJ. The irritable bowel severity scoring system: a simple method of monitoring irritable bowel syndrome and its progress. Aliment Pharmacol Ther. 1997;11:395–402. doi: 10.1046/j.1365-2036.1997.142318000.x. [DOI] [PubMed] [Google Scholar]
- 17.Drossman DA, Patrick DL, Whitehead WE, et al. Further validation of the IBS-QOL: a disease-specific quality-of-life questionnaire. Am J Gastroenterol. 2000;95:999–1007. doi: 10.1111/j.1572-0241.2000.01941.x. [DOI] [PubMed] [Google Scholar]
- 18.Lewis SJ, Heaton KW. Stool form scale as a useful guide to intestinal transit time. Scand J Gastroenterol. 1997;32:920–4. doi: 10.3109/00365529709011203. [DOI] [PubMed] [Google Scholar]
- 19.Rao SS, Camilleri M, Hasler WL, et al. Evaluation of gastrointestinal transit in clinical practice: position paper of the American and European Neurogastroenterology and Motility Societies. Neurogastroenterol Motil. 2011;23:8–23. doi: 10.1111/j.1365-2982.2010.01612.x. [DOI] [PubMed] [Google Scholar]
- 20.Rao SS, Kuo B, McCallum RW, et al. Investigation of colonic and whole-gut transit with wireless motility capsule and radiopaque markers in constipation. Clin Gastroenterol Hepatol. 2009;7:537–44. doi: 10.1016/j.cgh.2009.01.017. [DOI] [PubMed] [Google Scholar]
- 21.Chassard C, Scott KP, Marquet P, et al. Assessment of metabolic diversity within the intestinal microbiota from healthy humans using combined molecular and cultural approaches. FEMS Microbiol Ecol. 2008;66:496–504. doi: 10.1111/j.1574-6941.2008.00595.x. [DOI] [PubMed] [Google Scholar]
- 22.Matulova M, Nouaille R, Capek P, et al. Degradation of wheat straw by Fibrobacter succinogenes S85: a liquid- and solid-state nuclear magnetic resonance study. Appl Environ Microbiol. 2005;71:1247–53. doi: 10.1128/AEM.71.3.1247-1253.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Richardson A, Calder A, Srewart C, et al. Simultaneous determination of volatile and non-volatile acidic fermentation products of anaerobes by capillary gas chromatography. Letters in applied Microbiology. 1989;9:5–8. [Google Scholar]
- 24.Simren M, Barbara G, Flint HJ, et al. Intestinal microbiota in functional bowel disorders: a Rome foundation report. Gut. 2013;62:159–76. doi: 10.1136/gutjnl-2012-302167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hamer HM, De Preter V, Windey K, et al. Functional analysis of colonic bacterial metabolism: relevant to health? Am J Physiol Gastrointest Liver Physiol. 2012;302:G1–9. doi: 10.1152/ajpgi.00048.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wong JM, de Souza R, Kendall CW, et al. Colonic health: fermentation and short chain fatty acids. J Clin Gastroenterol. 2006;40:235–43. doi: 10.1097/00004836-200603000-00015. [DOI] [PubMed] [Google Scholar]
- 27.Eswaran S, Muir J, Chey WD. Fiber and functional gastrointestinal disorders. Am J Gastroenterol. 2013;108:718–27. doi: 10.1038/ajg.2013.63. [DOI] [PubMed] [Google Scholar]
- 28.Layden BT, Angueira AR, Brodsky M, et al. Short chain fatty acids and their receptors: new metabolic targets. Transl Res. 2013;161:131–40. doi: 10.1016/j.trsl.2012.10.007. [DOI] [PubMed] [Google Scholar]
- 29.Pimentel M, Soffer EE, Chow EJ, et al. Lower frequency of MMC is found in IBS subjects with abnormal lactulose breath test, suggesting bacterial overgrowth. Dig Dis Sci. 2002;47:2639–43. doi: 10.1023/a:1021039032413. [DOI] [PubMed] [Google Scholar]
- 30.Majewski M, McCallum RW. Results of small intestinal bacterial overgrowth testing in irritable bowel syndrome patients: clinical profiles and effects of antibiotic trial. Adv Med Sci. 2007;52:139–42. [PubMed] [Google Scholar]
- 31.Majewski M, Reddymasu SC, Sostarich S, et al. Efficacy of rifaximin, a nonabsorbed oral antibiotic, in the treatment of small intestinal bacterial overgrowth. Am J Med Sci. 2007;333:266–70. doi: 10.1097/MAJ.0b013e3180536784. [DOI] [PubMed] [Google Scholar]
- 32.Pimentel M, Chow EJ, Lin HC. Eradication of small intestinal bacterial overgrowth reduces symptoms of irritable bowel syndrome. Am J Gastroenterol. 2000;95:3503–6. doi: 10.1111/j.1572-0241.2000.03368.x. [DOI] [PubMed] [Google Scholar]
- 33.Pimentel M, Chow EJ, Lin HC. Normalization of lactulose breath testing correlates with symptom improvement in irritable bowel syndrome. a double-blind, randomized, placebo-controlled study. Am J Gastroenterol. 2003;98:412–9. doi: 10.1111/j.1572-0241.2003.07234.x. [DOI] [PubMed] [Google Scholar]
- 34.Yu D, Cheeseman F, Vanner S. Combined oro-caecal scintigraphy and lactulose hydrogen breath testing demonstrate that breath testing detects oro-caecal transit, not small intestinal bacterial overgrowth in patients with IBS. Gut. 2011;60:334–40. doi: 10.1136/gut.2009.205476. [DOI] [PubMed] [Google Scholar]
- 35.Kamath PS, Hoepfner MT, Phillips SF. Short-chain fatty acids stimulate motility of the canine ileum. Am J Physiol. 1987;253:G427–33. doi: 10.1152/ajpgi.1987.253.4.G427. [DOI] [PubMed] [Google Scholar]
- 36.Fukumoto S, Tatewaki M, Yamada T, et al. Short-chain fatty acids stimulate colonic transit via intraluminal 5-HT release in rats. Am J Physiol Regul Integr Comp Physiol. 2003;284:R1269–76. doi: 10.1152/ajpregu.00442.2002. [DOI] [PubMed] [Google Scholar]
- 37.Winston J, Shenoy M, Medley D, et al. The vanilloid receptor initiates and maintains colonic hypersensitivity induced by neonatal colon irritation in rats. Gastroenterology. 2007;132:615–27. doi: 10.1053/j.gastro.2006.11.014. [DOI] [PubMed] [Google Scholar]
- 38.Hoverstad T, Bjorneklett A, Fausa O, et al. Short-chain fatty acids in the small-bowel bacterial overgrowth syndrome. Scand J Gastroenterol. 1985;20:492–9. doi: 10.3109/00365528509089686. [DOI] [PubMed] [Google Scholar]
- 39.Tana C, Umesaki Y, Imaoka A, et al. Altered profiles of intestinal microbiota and organic acids may be the origin of symptoms in irritable bowel syndrome. Neurogastroenterol Motil. 2010;22:512–9. e114–5. doi: 10.1111/j.1365-2982.2009.01427.x. [DOI] [PubMed] [Google Scholar]
- 40.Treem WR, Ahsan N, Kastoff G, et al. Fecal short-chain fatty acids in patients with diarrhea-predominant irritable bowel syndrome: in vitro studies of carbohydrate fermentation. J Pediatr Gastroenterol Nutr. 1996;23:280–6. doi: 10.1097/00005176-199610000-00013. [DOI] [PubMed] [Google Scholar]
- 41.Roediger WE, Moore A. Effect of short-chaim fatty acid on sodium absorption in isolated human colon perfused through the vascular bed. Dig Dis Sci. 1981;26:100–6. doi: 10.1007/BF01312224. [DOI] [PubMed] [Google Scholar]
- 42.Ruppin H, Bar-Meir S, Soergel KH, et al. Absorption of short-chain fatty acids by the colon. Gastroenterology. 1980;78:1500–7. [PubMed] [Google Scholar]
- 43.McNeil NI, Cummings JH, James WP. Short chain fatty acid absorption by the human large intestine. Gut. 1978;19:819–22. doi: 10.1136/gut.19.9.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Camilleri M, Thorne NK, Ringel Y, et al. Wireless pH-motility capsule for colonic transit: prospective comparison with radiopaque markers in chronic constipation. Neurogastroenterol Motil. 2010;22:874–82, e233. doi: 10.1111/j.1365-2982.2010.01517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Evans DF, Pye G, Bramley R, et al. Measurement of gastrointestinal pH profiles in normal ambulant human subjects. Gut. 1988;29:1035–41. doi: 10.1136/gut.29.8.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Watson BW, Meldrum SJ, Riddle HC, et al. pH profile of gut as measured by radiotelemetry capsule. Br Med J. 1972;2:104–6. doi: 10.1136/bmj.2.5805.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zarate N, Mohammed SD, O’Shaughnessy E, et al. Accurate localization of a fall in pH within the ileocecal region: validation using a dual-scintigraphic technique. Am J Physiol Gastrointest Liver Physiol. 2010;299:G1276–86. doi: 10.1152/ajpgi.00127.2010. [DOI] [PubMed] [Google Scholar]


