Abstract
Cancer is the second leading cause of death worldwide. There is greater need for more effective and less toxic therapeutic and preventive strategies. Natural products are becoming an important research area for novel and bioactive molecules for drug discovery. Phytochemicals and dietary compounds have been used for the treatment of cancer throughout history due to their safety, low toxicity, and general availability. Many active phytochemicals are in human clinical trials. Studies have indicated that daily consumption of dietary phytochemicals have cancer protective effects against carcinogens. They can inhibit, delay, or reverse carcinogenesis by inducing detoxifying and antioxidant enzymes systems, regulating inflammatory and proliferative signaling pathways, and inducing cell cycle arrest and apoptosis. Epidemiological studies have also revealed that high dietary intakes of fruits and vegetables reduce the risk of cancer. This review discusses potential natural cancer preventive compounds, their molecular targets, and their mechanisms of actions.
Keywords: Chemoprevention, Phytochemicals, Activator protein-1, Nuclear factor (NF)-κB, Cyclooxygenase (COX)-2, Apoptosis, Cell cycle arrest, Angiogenesis, Oxidative stress, Curcumin, (−)-epigallocatechin-3-gallate, Resveratrol, Isothyocynates
INTRODUCTION
Cancer is one of the leading causes of death worldwide and the incidence is on the rise in both developing and developed countries. Cancer prevention may be more effective and less costly because cancer is closely associated with lifestyle factors [1]. Natural products have been used for the treatment of various diseases and are becoming an important research area for drug discovery. These products, especially phytochemicals have been extensively studied and have exhibited anti-carcinogenic activities by interfering with the initiation, development and progression of cancer through the modulation of various mechanisms including cellular proliferation, differentiation, apoptosis, angiogenesis, and metastasis [2]. Many successful anti-cancer drugs now available are phytochemicals or their analogues [3], and some are in human clinical trials [4].
Phytochemicals obtained from vegetables, fruits, spices, teas, herbs, and medicinal plants, such as flavanoids, carotenoids, phenolic compounds, and terpenoids, have been extensively investigated for their anti-cancer activities due to their safety, low toxicity, and general availability [1, 5]. It has been estimated that more than two-third of human cancers could be prevented through appropriate lifestyle modification including dietary habits [6]. Recent studies have indicated that a high intake of fruit and vegetables is associated with a decreased risk of human malignancies, including colon, breast, lung, laryngeal, pancreatic, bladder, stomach, esophageal, and oral cancers [5, 7]. Population studies also suggest that a reduced risk of cancer is associated with a high consumption of vegetables and fruits.
Chemoprevention, a means of cancer control by which the occurrence of the disease can be entirely prevented, slowed down, or reversed by use of nontoxic natural or synthetic products, has emerged as a promising and pragmatic medical approach to reduce the risk of cancer. This concept is gaining increasing attention because it is a cost-effective alternative to cancer treatment [1, 8]. Cancer treatment with commercially available synthetic chemotherapeutic agents is limited because of their severe side effects. Various plant products have been reported to possess substantial chemopreventive properties [8]. Approximately, 20% of cancer incidents are preventable by consuming more vegetables and fruits and may potentially prevent approximately 200,000 cancer-related deaths annually [1].
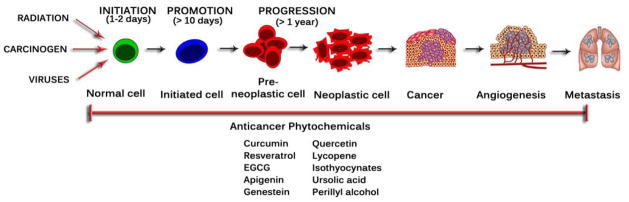
Carcinogenesis is a multi-step process (Fig. 1) which begins with initiation followed by promotion and progression that involve a series of epigenetic and genetic alterations affecting oncogenes and tumor suppressor genes [9]. Tumor initiation is a rapid and irreversible process. This process involves an initial uptake of or exposure to a carcinogenic agent including a distribution of this agent to organs and tissues where metabolic activation and detoxification can occur. Finally this agent and its possible metabolic derivatives covalently interact with target-cell DNA, leading to genotoxic damage [10]. Phytochemicals can prevent these multi-step processes by exerting anti-inflammatory and anti-oxidative stress effects which are mediated by integrated Nrf2, NF-κB, and AP-1 signaling pathways [9]. Many of the antioxidant compounds present in foods of plant origin protect against genotoxicity and other cancer-initiating or -promoting processes [11].
Fig. (1).
Multi-step process in carcinogenesis.
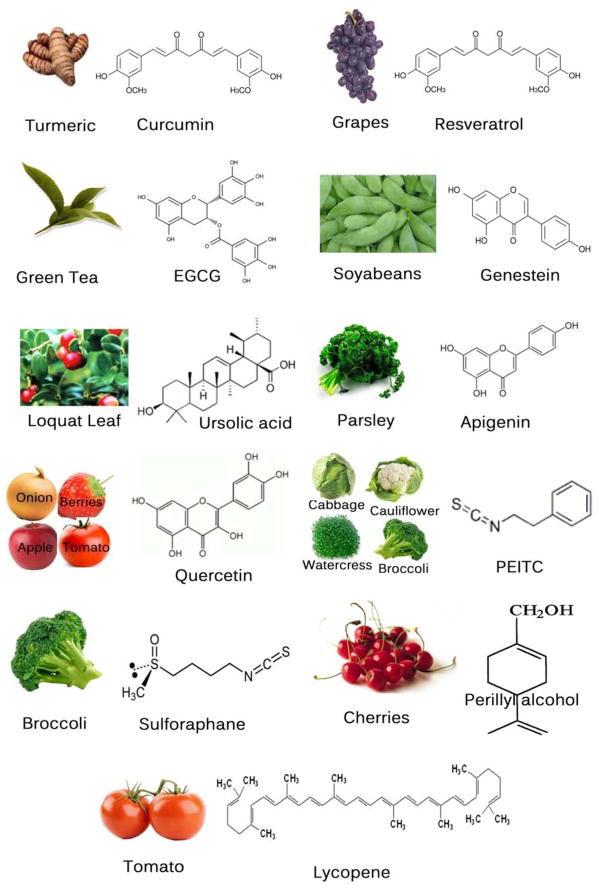
In this review we focus on (a) the usefulness of some promising phytochemicals (Fig. 2), including curcumin, resveratrol, epigallocatechin gallate (EGCG), apigenin, quercetin, genistein, lycopene, ursolic acid, isothyocynates, and perillyl alcohol, and (b) their mechanism of actions as well as molecular targets (Fig. 3 & Table 1), such as antioxidant properties, inhibition of cell cycle, induction of apoptosis, regulation of angiogenesis, inhibition of cyclooxygenase (COX)-2, signal transducers and activator of transcription 3 (STAT3) inhibition, and down-regulation of nuclear factor-kappaB (NF-κB), activator protein-1, and mitogen-activated protein kinases (MAPKs).
Fig. (2).
Chemical structures and sources of natural chemopreventive agents.
Fig. (3).
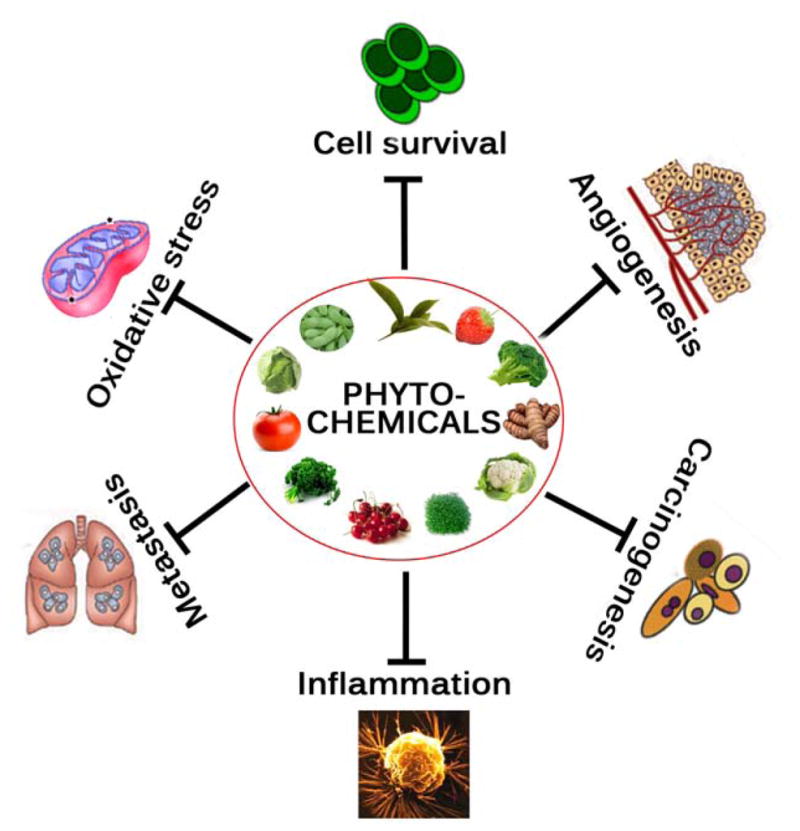
Targets of natural chemopreventive agents.
Table 1.
Molecular Targets for Natural Chemopreventive Agents
| Agent | Natural Source | Mechanism of Action | Molecular Targets |
|---|---|---|---|
| Curcumin | Curcuma longa (turmeric powder) | Anti-oxidant, antiproliferation, anticarcinogenesis, cell cycle arrest, apoptosis, antiinflammation, antiangiogenesis. | EGFR, IGF-1R, AKT, NF-κB, COX-2, LOX, ERK, AP-1, PI3K, VEGF, VEGFR1, MMP-2/9, p53, p21, Bax, iNOS STAT3/5, TRAIL-R1/DR4, G2/M phase arrest, cyclin D1, c-myc, Bcl-2, Bcl-xL, cFLIP, XIAP, c-IAP1, JNK. |
| Resveratrol | Red wine, grapes (mainly in the skin), mulberries, peanuts, vines, pines | Antioxidant, antiproliferation, anticarcinogenesis, cell cycle arrest, apoptosis, antiangiogenesis, antiinflammation | SOD, catalase, glutathione, AKT, AP-1, NF-κB, iNOS, COX-2, STAT3, survivin, p53, p21, BAX, BAK, MMP-2, MMP-9, Bcl-2, Bcl-xL, and Caspase-3. |
| EGCG | Camellia sinensis (green tea) | Antioxidant, anti-mutagenesis, anti-proliferation, anticarcinogenesis, cell cycle arrest, apoptosis, antiinflammation, antiangiogenesis. | p53, p73, p21, Bax, EGFR, AKT, NF-κB, Bcl-2, cyclin D1, COX-2, VEGF, MMP-2/9, STAT3, ERK1/2, AP-1, IL-12, |
| Isothyocynates | cruciferous vegetables, watercress, brussels sprouts, broccoli, cabbage, horseradish, radish | Anticarcinogenesis, Anti-oxidant, antiproliferation, anticarcinogenesis, cell cycle arrest, apoptosis, antiinflammation, antiangiogenesis. | MEK, ERK1/2, PKC, COX-2, iNOS, JNK, p38, c-Jun and c-Fos |
| Quercetin | phytoalexin, skin of red grapes and red wine | Anticarcinogenesis, Anti-oxidant, antiproliferation, anticarcinogenesis, cell cycle arrest, apoptosis, antiinflammation, antiangiogenesis. | MAPK, AKT, Bcl-2, Bax and caspase-3, mTO R |
| Genistein | Soybeans and soy products, red clover (Trifolium pretense), sicilian pistachio (Pistacia vera) | Antioxidant, antiproliferation, antiproliferation, anticarcinogenesis, cell cycle arrest, apoptosis, antiangiogenesis, antiinflammation | AKT, NF-κB, Bcl-2, survivin, cyclin D1, COX-2, MMP-2/9, p53, p21, GADD153, Bax, STAT3/5, ERK1/2, CDK1, AP- 1, IGF-1R |
| Apigenin | Parsley, Petroselinum crispum | Anti-oxidant, antiproliferation, anticarcinogenesis, cell cycle arrest, apoptosis | MAPK, PI3K-AKT, NF-κB, Bcl-2, cyclin D1, COX-2, MMP-2/9, ICAM-1, HIF-1, VEGF |
| Ursolic acid | Loquat leaf, apples, bilberries, cranberries | Anti-oxidant, antiproliferation, anticarcinogenesis, cell cycle arrest, apoptosis. | COX-2, iNOS, TNF-α, IL-1β, IL-2 and IL-6, JNK1/2, MAPK, CyclinD1/CDK4. |
| Perillyl alcohol | cherries, lavender | Antioxidant, antiproliferation (growth inhibition, cell cycle arrest, apoptosis), antiangiogenesis, antiinflammation, | MAPK, AKT bax, p21 and caspase-3 |
| Lycopene | Tomatoes, guava, rosehip, watermelon, papaya, apricot and pink grapefruit; most abundant in red tomatoes | Antioxidant, antiproliferation (growth inhibition, cell cycle arrest, apoptosis), antiangiogenesis, antiinflammation, immunomodulator | Cyclin D1, Bcl-2, Bcl-xL, AKT, BAD, NF-κB, MMP- 9, Sp-1, IGF-BP3 |
Abbreviations: EGFR, epidermal growth factor receptor; EGCG, epigallocatechin-3-gallate; TRAIL, tumor necrosis factor–related apoptosis-inducing ligand; NF-κB, nuclear factor-κB; MMP-2/9, matrix metalloproteinases; IL-12, interleukin 12; Jun-N-terminal kinase; iNOS, inducible nitric oxide synthase; PPAR-γ, peroxisome proliferator-activated receptor-γ; COX-2, cyclo-oxygenase-2; VEGF, vascular endothelial growth factor; VEGFR1, vascular endothelial growth factor receptor 1; TNF-α, tumor necrosis factor- α; JNK, Jun-N-terminal kinase; CDK, cyclin-dependent kinase; ERK, extracelluar signal–regulated kinase; SOD, superoxide dismutase; mTOR, mammalian target of rapanycin; DR, death receptor; PAFR, platelet activating factor receptor; NO, nitric oxide; eNOS, endothelial nitric oxide synthase. ICAM-1 intercellular adhesion molecule 1; Mcl-1 myeloid cell leukemia 1; XIAP, X-chromosome-linked IAP; Bax Bcl-2-associated X protein, Bcl-2 B cell lymphoma 2; AP-1 activator protein 1; IGF-BP3, insulin-like growth factor binding protein 3.
CURCUMIN
Curcumin (diferuloylmethane), a yellow pigment belongs to the class of polyphenols present in the rhizomes of turmeric (Curcuma longa L.), is used in cooking in India and other regions of Asia. It is also used as a cosmetic and in some medical preparations. It has a long history as herbal remedy for a variety of diseases and has been used in Indian and Chinese traditional medicine since 700 AD [12]. Curcumin has been demonstrated to have a wide spectrum of pharmacological properties. Multiple therapeutic activities of curcumin have also been considered to be associated with its antioxidant and anti-inflammatory properties. The anti-inflammatory effect of curcumin is most likely mediated through its ability to inhibit cyclooxygenase-2 (COX-2), lipoxygenase (LOX), and inducible nitric oxide synthase (iNOS) [13]. Curcumin likely exerts its inhibitory effect on cancer development by several mechanisms such as inhibition of carcinogen activation and stimulation of carcinogen detoxification, prevention of oxidative DNA damage, and its capacity to reduce inflammation [8]. Studies revealed that curcumin mediates its anti-inflammatory effects through the downregulation of inflammatory transcription factors (such as NF-κB), enzymes (such as COX-2 and 5 LOX) and cytokines (such as tumor necrosis factor, interleukin 1 and interleukin 6) [14]. Curcumin has also been shown to be beneficial in all 3 stages of carcinogenesis. Much of its beneficial effect is found to be due to its inhibition of the transcription factor NF-κB and subsequent inhibition of proinflammatory pathways [15]. Curcumin has the potential to inhibit the NF-κB and AP-1, the major transcription factors that regulate inflammation, cell proliferation, differentiation and even apoptosis. It has promising chemopreventive and therapeutic potential for various cancers including leukemia, lymphoma and cancers of the gastrointestinal tract, genitourinary system, breast, ovary, head and neck, lung and skin [16]. It has also shown to affect a variety of other key players involved in carcinogenesis, such as cyclooxygenase-2, matrix metallopeptidases 2 and 9, and tumor necrosis factor α-induced vascular cell adhesion molecules [17].
Curcumin inhibits pancreatic tumor growth through mitotic catastrophe by increasing the expression of RNA binding protein CUGBP2, thereby inhibiting the translation of COX-2 and VEGF mRNA [18]. It enhances the effect of cisplatin in suppression of head and neck squamous cell carcinoma via inhibition of IKKβ protein of the NF-κB pathway [19]. Curcumin induces cell cycle arrest at the G2/M phase by decreasing the Cdc2 expression [20]. It inhibits cell proliferation, tumor growth, invasion and in vivo metastasis, and stabilizes the expression of the tumor suppressor Pdcd4 in colorectal cancer [21]. It has been shown to protect against skin, oral, intestinal, and colon carcinogenesis and also to suppress angiogenesis and metastasis in a variety animal tumor models. It also inhibits the proliferation of cancer cells by arresting them in the various phases of the cell cycle and by inducing apoptosis. Curcumin has a capability to inhibit carcinogen bioactivation via suppression of specific cytochrome P450 isozymes, as well as to induce the activity or expression of phase II carcinogen detoxifying enzymes [8]. Combination of phenethyl isothiocyanate and curcumin caused suppression of epidermal growth factor (EGF) receptor phosphorylation and inhibition of EGF-induced phosphorylation of Akt and induction of phosphatidylinositol 3-kinase (PI3K) in PC-3 cells [22]. Menon et al first reported that curcumin inhibits the invasion of B16F10 cells by suppressing MMP-2, thereby inhibiting lung metastasis in an animal model [23]. It has also been shown to suppress the production of cytokines such as interferon-γ (IFN-γ), interleukins, and tumor necrosis factor-α (TNF- α); to inhibit the inducible nitric oxide synthase (iNOS); and to suppress the activation of NF-κB [24, 25]. Curcumin-induced inactivation of NF-κB DNA-binding activity was potentially mediated by Notch-1 signaling pathway [26]. Curcumin regulates tumor cell growth through multiple cell signaling pathways including cell proliferation pathway (cyclin D1, c-myc), cell survival pathway (Bcl-2, Bcl-xL, cFLIP, XIAP, c-IAP1), caspase activation pathway (caspase-8, 3, 9), tumor suppressor pathway (p53, p21) death receptor pathway (DR4, DR5), mitochondrial pathways, and protein kinase pathway (JNK, Akt, and AMPK) [27]. Curcumin also inhibited migration of Colo205 cells through the inhibition of NF-κB and the down-regulation of COX-2 and MMP-2 expression [28]. Curcumin upregulated the expression of TRAIL-R1/DR4, TRAIL-R2/DR5, Bax, Bak, p21/WAF1, and p27/KIP1, and inhibited the activation of NF-κB and its gene products in xenografted tumors [29]. Curcumin suppressed the expression of human epidermal growth factor receptor (HER) 2 and the activity of p21-activated kinase (PAK) 1, a downstream protein of EGFR, to inhibit the proliferation and invasion of gastric cancer cells [30]. Curcumin induces caspase-3-mediated cleavage of β-catenin, leading to inactivation of Wnt/β-catenin signaling in HCT116 intestinal cancer cells [31].
RESVERATROL
Resveratrol (trans-3,5,4′-trihydroxystilbene), a naturally occurring phytoalexin, is found at a high concentration in the skin of red grapes and red wine. Resveratrol is known to have antioxidant, anti-inflammatory and antiproliferative effects on a variety of cancer cells in vitro and in various animal models [32]. It effectively scavenges superoxide, hydroxyl, and peroxynitrite radicals generated from enzymatic and nonenzymatic systems and affords protection against DNA damage caused by these ROS [33, 34]. Resveratrol has been identified as an effective candidate for cancer chemoprevention based on its striking inhibitory effects on cellular events associated with cancer initiation, promotion, and progression [35]. It potentiates antitumor activity by regulating multiple cell-signaling molecules, including drug transporters, cell survival proteins, cell proliferative proteins, and members of the NF-κB and STAT3 signaling pathways [36]. The resveratrol-induced apoptosis was mediated by activation of caspases-9 and -3 and a change in the Bax/Bcl-2 ratio. It regulated the expressions of cyclin D1, E, and Cdk4 as well as cyclin D1/Cdk4 kinase activity in LNCaP cells [37]. It enhanced TRAIL-induced apoptosis through G1 cell cycle arrest and survivin depletion [38]. It can inhibit the activation of p38 MAPK, p53 and p21WAF1 pathway in tumor cells [39]. It has been shown to inhibit COX-2 activity [40], and tumor angiogenesis by regulating MMP-2 and MMP-9 [41, 42]. The inhibition of NF-κB and MMP-9 activities by resveratrol in breast cancer cells blocks their migratory potentials [43]. It is well documented that resveratrol can suppress proliferation and invasion as well as induce apoptosis in a wide variety of tumor cell types [42]. Resveratrol suppressed NF-κB–regulated gene products (COX-2, MMP-3, MMP-9, and VEGF), inhibited anti-apoptosis (Bcl-2, and Bcl-xL) [44]. It can block IL-1-induced activation of NF-κB leading to inhibition of proliferation, S-phase arrest, and induces apoptosis [45]. Resveratrol has been shown to inhibit TNFα-mediated MMP-9 expression in HepG2 cells by downregulation of the NF-κB signaling pathway [46]. Extensive in vitro studies revealed multiple intracellular targets of resveratrol, which affect cell growth, inflammation, apoptosis, angiogenesis, and invasion and metastasis [47]. Resveratrol has been shown to inhibit the phosphatidylinositol- 3 kinase (PI-3K)/AKT and the MAPK pathways, two key survival cascades that are frequently aberrantly activated in human cancers [48, 49]. It has been found to inhibit Mammalian target of rapamycin (mTOR) which is a central controller of cell growth, proliferation, metabolism and angiogenesis [50]. Resveratrol suppressed AP-1 activity by the inhibition of mitogen-activated protein kinase/extracellular signal-regulated kinase kinase (MEK)1 > ERK1/2 signaling [51]. It also regulated the expression of IAPs and Bax, and decreased Akt phosphorylation in PC-3 cells, leading to increased caspase activation and apoptosis [52]. Resveratrol activates MAPK at low concentrations, but at higher concentrations it can inhibit this signal transducing kinase in cancer cells [53]. It inhibited nuclear expression of the AP-1 component proteins, in TPA stimulated mouse skin [54]. It has been shown to inhibit the migration and decrease the focal adhesion kinase (FAK) activity in MDAMB231 cells [55].
APIGENIN
Apigenin, a naturally occurring plant flavone, abundantly present in common fruits and vegetables, possesses anti-oxidant, anti-mutagenic, anti-carcinogenic, anti-inflammatory, anti-growth, and anti-progression properties [56]. Apigenin is effective in carcinogenesis. Topical application of apigenin inhibited dimethyl benzanthracene-induced skin tumors [57], and also diminished UV-induced cancer incidence and increased tumor free survival experiment [58]. Previous studies demonstrated that apigenin promotes metal chelation, scavenges free radicals, and stimulates phase II detoxification enzymes in cell culture and in in vivo tumor models [59]. It has been shown to increase the intracellular concentration of glutathione, enhancing the endogenous defense against oxidative stress [60]. In PC-3 tumor model, treatment with apigenin resulted in 32% and 51% inhibition in tumor growth [57]. The proposed mechanism of anti-tumor activity by apigenin was upregulation of WAF1/p21, KIP1/p27, INK4a/p16, and down-modulation of the protein expression of cyclins D1, D2, E and cyclin-dependent kinases (cdk) [61]. Apigenin has been shown to inhibit the proliferation of human prostate cancer cells by regulating MAPK, PI3K-Akt pathways [62]. It inhibited the LPS-induced cyclooxygenase-2 and nitric oxide synthase-2 activity and expression in mouse macrophages [63]. Apigenin induced G2/M phase cell cycle arrest and reduced the levels of cyclin A, cyclin B, phosphorylated forms of cdc2 and cdc25 in pancreatic cancer cell lines [64]. It also induced growth-inhibition and G2/M phase arrest in two p53-mutant cancer cell lines, HT-29 and MG63. These effects were associated with a marked increase in the protein expression of p21/WAF1 [65]. Previous studies have shown that topical application of apigenin prior to UV irradiation prevents UV-induced tumorigenesis in mice by inhibiting the cell cycle machinery and cyclin-dependent kinases (cdk) [58, 66]. Apigenin has shown promise in inhibiting tumor cell invasion and metastases by regulating protease production [67]. It inhibited lung tumor metastasis in B16-BL6 murine melanoma metastasis model [68]. Apigenin has been shown to be markedly effective against human leukemia cells [69]. It inhibits HIF-1α, GLUT-1, VEGF mRNA, and protein expression in pancreatic cancer cells in both normoxic and hypoxic conditions [70]. Apigenin is a strong inhibitor of ornithine decarboxylase, an enzyme that plays a major role in tumor promotion [57]. Apigenin inhibits the TPA-dependent increase in c-jun and c-fos gene expression and tumor promotion in mouse skin. It also suppresses the TPA-mediated COX-2 expression by blocking Akt signal transduction and arachidonic acid release in HaCaT cells [71]. It has been shown to inhibit proteasome activity and induce apoptosis in human breast cancer MDA-MB-231 cells [72]. Apigenin is a well-known inhibitor of protein-tyrosine kinases and has been shown to block peroxisome proliferation regulated kinase (ERK), a MAPK in isolated hepatocytes [73].
QUERCETIN
Quercetin is a dietary flavonoid abundant in variety of foods including apples, berries, brassica vegetables, grapes, onions, shallots, tea, and tomatoes, as well as many seeds, nuts, barks, and leaves [74]. It usually occurs as O-glycosides, with D-glucose as the most frequent sugar residue. More than 170 different quercetin glycosides have been identified [75]. Among polyphenols, quercetin is one of the most potent antioxidants, as demonstrated in different studies [76, 77]. It has been shown to inhibit oxidative species generating enzymes such as xanthine oxidase (XOD), lipoxygenase (LOX) and NADPH oxidase [78]. It is a potent anticancer agent, exhibiting different activities such as cell cycle regulation, interaction with type II estrogen binding sites, and tyrosine kinase inhibition [79]. Intravenous administration of quercetin inhibited the lymphocyte tyrosine kinase in cancer patients and it is the first tyrosine kinase inhibiting compound tested in a human phase I trial [80]. Studies have revealed that this flavonoid is capable of inhibiting LPS induced inflammation in macrophages [81]. Quercetin could inhibit the gene expression of TNF-α via modulation of NF-κB in human peripheral blood mononuclear cells [82]. It inhibited the growth of multiple cancer cell lines by mitochondrial mediated apoptotic pathway [83]. It has been shown that quercetin reduced the expression of ErbB2 and ErbB3 proteins [84], and potentiated AMPK activation and p53-dependent apoptotic cell death in HT-29 colon cancer cells [85]. Chemopreventive properties of quercetin have been demonstrated in various animal models [86–89], and has been subjected to a Phase I clinical trial in cancer patients [80].
Previous study has shown that quercetin can induce cell cycle arrest at late G1 phase in human leukemic T-cells [90]. Quercetin increased the therapeutic efficacy of cisplatin both in vivo and in vitro [91] and also enhanced the cytotoxic effect of radiation on rat hepatoma cells [92]. It also caused pronounced apoptosis in both transformed and primary leukemia cells but not in normal blood peripheral mononuclear cells. Quercetin-induced apoptosis was accompanied by Mcl-1 downregulation and Bax conformational change and mitochondrial translocation that triggered cytochrome c release [93]. Quercetin modulates UVB-induced c-Fos expression through activation of p38 and cAMP-responsive element binding protein (CREB) [94]. It enhances the expression of p53, caspase-3,-9, cytochrome c in MDA-MB-231 cells [95]. It has been shown to regulate the expression of oncogenes (H-ras, c-myc and K-ras) and anti-oncogenes (p53) [96], cell cycle control protein (p21WAF1 and p27KIP1) [97], and inhibit different tyrosine and serine–threonine kinases associated with cell survival pathways (MAPK, AKT/PKB) [98]. Robaszkiewicz et al. studied the effect of quercetin on A-549 cells proliferation. They found that quercetin acts as both antioxidant and pro-oxidant depending upon the concentration used. Quercetin at lower concentration enhanced the cancer cell prolifearation whereas at higher concentrations showed the concentration dependent cytotoxicity [99]. Previous study has shown that quercetin can prevent metal induced tumorogenesis by inhibiting oxidative DNA damage [100]. It has also been shown to regulate several signal transduction pathways involving MEK/ERK and Nrf2/keap1, which are involved in the processes of inflammation and carcinogenesis [101]. Treatment of quercetin down-regulated the antiapoptotic proteins Bcl-2 and Bcl-xL and also up-regulated the proapoptotic proteins Bax and caspase-3 in prostatic PC-3 carcinoma cells [102]. Quercitrin blocked TPA-induced neoplastic transformation in JB6 P+ cells. Pretreatment of JB6 cells with quercitrin down-regulated transactivation of AP-1 and NF-κB induced by UVB or TPA. [103], Wang et al., reported that quercetin could activate autophagy in gastric cancer cells by regulating of Akt-mTOR and hypoxia-induced factor 1α (HIF-1α) signaling. This was further confirmed in animal model [104]. Previous studies revealed that quercetin can inhibit production of heat shock proteins in several cancers, including breast cancer [105], leukemia [106], and colon cancer [107]. Quercetin has been shown to induce estrogen-induced carcinogenesis in animal models [108, 109].
(−)-EPIGALLOCATECHIN-3-GALLATE
(−)-Epigallocatechin-3-gallate (EGCG) is the most abundant catechin, representing ~16.5 wt % of the water extractable fraction of green tea leaves [110]. EGCG is a strong anti-oxidant and it has been demonstrated to inhibit carcinogen-induced oxidative stress [111]. EGCG has been shown to inhibit NF-κB activity, MAPK pathway, AP-1 activity, and EGFR-mediated downstream signaling pathways [112]. Previous studies have revealed that EGCG suppressed Akt activation in both colon cancer cell lines and in vivo mouse models [112, 113]. Topical application of EGCG to the skin has been shown to decrease the incidence of UVB induced skin cancer in SKH-1 mice [114]. EGCG inhibited the expression and activation of MMPs [115–117] and also increased the expression of the tissue inhibitor of MMPs (TIMP1 and TIMP2) [116], indicating its anti-invasive potential. EGCG combined with taxane showed an increase in the expression of apoptotic genes such as p53, p73, p21, and caspase-3 in in vitro and also in tumor model [118]. It can inhibit the VEGF expression and angiogenesis induced by interleukin-6 (IL-6) in gastric cancer [119]. EGCG treatment increased the levels of E-cadherin on the cell plasma membrane and suppressed the expression of nuclear β-catenin, c-Myc, phospho-Akt, and phospho-Erk in the tumors [120]. It inhibited cell proliferation and induced apoptosis in various types of cancer cells by inhibiting the activation of the epidermal growth factor receptor (EGFR) family of RTKs [121]. EGCG induced apoptosis in gastric cancer cell lines by down-regulating survivin expression [122].
Regulation of angiogenesis is one of the possible mechanisms by which EGCG can inhibit cancer progression [123]. EGCG inhibits IL-6-induced VEGF expression and angiogenesis via suppressing Stat3 activity in gastric cancer [119]. ECCG abolishes the activation of FAK and suppresses more than 50% of cell proliferation without evidence of apoptosis analyzed by PARP cleavage [124]. EGCG inhibits cell proliferation and AKT phosphorylation at Ser473 in MDA-MB-231 and A549 cells [125]. It has been shown to inhibit the activities of a variety of enzymes. Xu et al. reported the chemopreventive effect of EGCG on N-methyl-N′-nitro-N-nitrosoguanidine (MNNG)-induced gastrointestinal cancer in rats [126]. It inhibited the phosphorylation of JNK, JUN, MEK1, MEK2, ERK1, ERK2, and ELK1 (Ets-like protein 1) in JB6 epidermal cell lines [127]. EGCG inhibits HGF-induced tumor growth and invasion in oral cancer [128]. It inhibited AZ521 cell proliferation by preventing β-catenin oncogenic signaling through proteasomal degradation of p68 [129]. EGCG treatment caused dose-dependent cell growth inhibition, cell cycle arrest at the G(0)/G(1) phase, and DNA fragmentation in HT-1080 cells [130]. EGCG showed significant reduction in tumor volume, proliferation, angiogenesis and metastasis and induction in apoptosis, and growth arrest in pancreatic cancer cells. EGCG also inhibited circulating endothelial growth factor receptor 2 (VEGF-R2) positive endothelial cells derived from xenografted mice. Tumor samples from EGCG treated mice showed significantly reduced ERK activity; and enhanced p38 and JNK activities [131].
ISOTHIOCYANATES
Isothiocyanates (ITCs) are electrophilic compounds that play a major role in potential chemopreventive effects associated with high intake of cruciferous vegetables such as watercress, brussel sprouts, broccoli, cabbage, horseradish, radish, and turnip [132]. Cruciferous vegetables have been widely accepted as potential diet components that may decrease the risk of cancer [133]. Epidemiological studies show that dietary intake of ITCs is associated with reduced risk of certain human cancers [134]. ITCs display anticarcinogenic activity by reducing the activation of carcinogens and increase their detoxification. They have been shown to inhibit the carcinogen-activating cytochrome P450 mono-oxygenases and also induce carcinogen-detoxifying phase 2 enzymes [135]. They exhibit anti-tumor activity by regulating multiple pathways including apoptosis, MAPK signaling, oxidative stress, and cell cycle progression [136]. They also inhibited the growth and proliferation of several types of cancer cells, such as prostate cancer [137], breast cancer [138], lung cancer [139], cervical cancer [140], leukaemia [141], and colorectal cancer [142].
ITCs have been divided into six dietary isothiocyanates (ITCs), allyl isothiocyanate (AITC), benzyl isothiocyanate (BITC), phenethyl isothiocyanate (PEITC), sulforaphane (SFN), erucin (ERN), and iberin (IBN) based on biological activities [143]. PEITC and SFN are two of the most widely investigated isothiocyanates from the crucifers, which are highly effective in reducing the risk of cancer, induced by carcinogens in animals as well as cell culture models [144] PEITC suppressed prostate cancer progression by induction of autophagic cell death [145]. They induced cervical cancer cell apoptosis by stimulating DR4 and DR5 through the inactivation of ERK and MEK [146]. They also have been shown to induce G2/M phase arrest and apoptosis in human prostate cancer DU 145 Cell [147].
PEITC induces apoptosis in human osteogenic sarcoma U-2 OS cells through ROS, caspase-3, and NO signaling pathways [148]. Recent studies have revealed that ITCs is able to inhibit the metastasis potential of human hepatoma cells [149], colon cancer cells [150], and breast cancer cells [151]. Previous studies reported that BITC and PEITC inhibited the metastasis potential of highly metastatic lung cancer cells by inducing apoptosis and cell cycle arrest, via targeting the MAPK/AP-1 pathway [152]. PEITC also inhibited the migration and invasionof human gastric cells by regulating the extracellular signal-regulated kinases 1/2 (ERK1/2), protein kinase C (PKC), and nuclear factor-kappaB (NF-κB) signaling pathways [153]. AITC has been shown to decrease the proliferation and viability of human brain malignant glioma GBM 8401 cells in a dose-dependent manner [154].
Shan et al. evaluated the protective effect of SFN on human vascular endothelial cells against lipopolysaccharide-induced inflammatory damage. Treatment of SFN inhibited the expression of COX-2, and iNOS and also suppressed the phosphorylation of ERK1/2, JNK, and p38 activated by lipopolysaccharide [155]. SFN has been shown to inhibit the interleukin-6 (IL-6)-inducible activation of STAT3, which is an oncogenic transcription factor activated in many human malignancies, including prostate cancer [156]. It activates NF-E2 related factor-2 (Nrf2), a well-known chemopreventive target and activates the Nrf2-regulated cytoprotective signaling pathway [157]. Treatment with SFN induced apoptosis in B16F-10, a highly metastatic melanoma cells by activating caspases 3,-9, Bax, p53 and downregulating Bcl-2, caspase-8, Bid, and NF-κB [158]. It reduced the G1 phase cell distribution and also down regulalated the phosphorylation of extracellular-regulated kinase 1/2 (ERK1/2), and induced apoptosis in HCT116 cells [159]. Sulforaphane induces down-regulation of β-catenin in human cervical carcinoma HeLa and hepatocarcinoma HepG2 cells [160].
GENISTEIN
Genistein is an isoflavone compound, found in soy bean and related products such as tofu, soy milk and soy sauce [161], and is a promising cancer chemotherapeutic agent [162]. Treatment of genestein has shown to inhibit the growth of many different cancer cell lines by increasing apoptosis, inducing cell cycle delays, and modulating intracellular signaling pathways [162]. It inhibited tumor growth in mouse models of breast, prostate and skin cancers [163, 164]. Recent studies have demonstrated that genistein inhibited EGF-induced proliferation in colon cancer cells via regulating the PI3K/Akt pathway [165], and also induced apoptosis and cell cycle arrest at G(2)/M phase [166]. Li et al. showed that treatment of genestein enhanced the apoptotic effect of chemotherapy drugs in H460, a lung cancer cell line, through the inactivation of NF-κB [167]. Genistein induced cell cycle arrest and apoptosis in breast cancer cells [168], and also modulated BRCA1 expression in vitro [169] and in vivo [170]. Laboratory investigations demonstrated that the growth inhibitory effects of genistein were linked to the inhibition of PI3K, leading to the inhibition of Akt, and eventual inhibition of NF-κB [10, 167, 171]. Treatment of genistein can inhibit β-catenin-mediated WNT signaling through increasing sFRP2 gene expression by demethylating its silenced promoter in colon cancer cell line DLD-1 [172]. It has the potential to stimulate breast cancer cell death through mobilizing endogenous copper ions and generation of reactive oxygen species (ROS) [6].
Genistein has been shown to decrease the synthesis of prostaglandins in several normal and malignant cells [173–175]. In the cell culture experiments, genistein decreased cyclooxygenase-2 (COX-2) mRNA and protein expression in both human PCa cell lines (LNCaP and PC-3) and primary prostate epithelial cells and increased 15-hydroxyprostaglandin dehydrogenase (15-PGDH) mRNA levels in primary prostate cells [176]. Treatment with genistein combined with prostate tumor irradiation significantly enhanced inhibition of prostate tumor growth and increased mouse survival in metastatic orthotopic PC-3 xenograft tumor model [177, 178]. It also inhibited the activation of NF-κB DNA binding activity and upregulation of APE1/Ref-1 induced by radiation in vitro and in PC-3 prostate tumors in vivo [178, 179]. Genistein is a potent antiangiogenic agent which can inhibit VEGF-induced endothelial cell activation by decreasing protein tyrosine kinase (PTK) activity and MAPK activation [180]. It also decreased the activation of JNK and p38, induced by VEGF [180]. Genistein promotes the tissue-plasminogen activator (tPA) activity in human cervical cancer cells (HeLa S3) and in human umbilical vein endothelial cells (HUVEC) [181]. It has been demonstrated that genistein could inhibit osteolytic bone metastases, suppress bone resorption, increase bone mass and improve bone microstructure in bone metastases of breast cancer [172]. It has the potential to inhibit NF-κB activation and modulate inflammatory pathways [179, 182, 183]. It also induces apoptosis via NF-κB dependent and independent pathways [184]. Pretreatment with this isoflavone inhibited Src/STAT3/HIF-1alpha activation by radiation and nuclear translocation of HIF-1alpha [185].
URSOLIC ACID
Ursolic acid (UA) is a pentacyclic triterpene compound widely found in food, medicinal herbs, apple peel and is able to exhibit a wide range of pharmacological functions, including antioxidant, anti-tumor, and anti-inflammatory activities [186], and is also one of the most promising chemopreventive agents. Ursolic acid can inhibit the activities of DNA polymerase and DNA topoisomerase and decrease the rate of cell proliferation [187]. Ursolic acid attenuated oxidative stress-mediated hepatocellular carcinoma induction by diethylnitrosamine in male Wistar rats [188]. It also decresed the production of pro-inflammatory markers including COX-2, iNOS, TNF-α, IL-1β, IL-2, and IL-6 in LPS-treated mouse brain [186]. Treatment with UA suppressed TPA-mediated induction of COX-2 protein and synthesis of PGE2 in human mammary epithelial cells. It also inhibited TPA-mediated activation of protein kinase C, extracellular signal regulated kinase 1/2 (ERK1/2), c-Jun NH2-terminal kinase 1/2 (JNK1/2), and p38 mitogen-activated protein kinase (MAPK) [189]. Ursolic acid potentiates its anti-tumor effects through suppression of NF-κB and STAT3 pathways in prostate cancer [190]. Harmand et al. reported the apoptotic potential of ursolic acid in HaCat derived keratinocyte cell line. Treatment with UA decreased the viability of HaCat cells in a concentration- and time-dependent manner. In addition, cell cycle analysis revealed that UA treated HaCat cells were blocked predominantly in G1 phase [191]. It also has been found to inhibit the proliferation of MCF-7 cells followed by a significant decrease in CyclinD1/CDK4 expression, which can be regulated by FoxM1 [192].
Ursolic acid potentiates TRAIL-induced apoptosis through activation of ROS and JNK-mediated up-regulation of death receptors and down-regulation of decoy receptor 2 (DcR2) and cell survival proteins [193]. It has been shown to induce apoptosis in highly metastatic melanoma cell line, B16F-10 by activating cells p53 and caspase-3 gene expressions and inhibiting the NF-κB mediated activation of bcl-2 [194]. Ursolic acid inhibits the invasion and metastasis of various cancers such as ovarian carcinoma [195], prostate [196, 197], and breast [198], probably through inhibiting the activity of gelatinase and the expressions of MMP-2 and MMP-9 [180, 197], suppressing CXCR4 expression [196], and modulating c-Jun N-terminal kinase, Akt and mammalian target of rapamycin signalling [198]. It potentiates antiangiogenic activity by regulating VEGF, TIMP-1, MMP-2 and MMP-9 [199]. Shanmugam et al. investigated the effect of ursolic acid (UA) on NF-κB and STAT3 signaling pathways in both androgen-independent (DU145) and androgen-dependent (LNCaP) prostate cancer cell lines. They found that UA inhibited constitutive and TNF-α-induced activation of NF-κB in DU145 and LNCaP cells in a dose-dependent manner. This suppression was mediated through the inhibition of constitutive and TNF-α-induced IκB kinase (IKK) activation, phosphorylation of IκBα and p65, and NF-κB-dependent reporter activity. Moreover, UA suppressed both constitutive and inducible STAT3 activation concomitant with upstream kinases (Src and JAK2) [190]. Ursolic acid can regulate key steps of angiogenesis in vitro, including endothelial cell proliferation, migration, and differentiation [200]. Raphael and Kuttan, investigated the effect of UA on the cell-mediated immune response in metastatic tumor-bearing C57BL/6 mice. They found that intraperitoneal administration of UA (50 mumoles/Kg body) increased natural killer cell activity (NK-activity) and enhanced the antibody dependent cell mediated cytotoxicity (ADCC) in metastatic tumor-bearing animals [201].
LYCOPENE
Lycopene is a most potent antioxidant carotenoid pigment, found in tomatoes, fruits, and vegetables such as red carrots, guava, watermelons, and papayas. High consumption of tomatoes and tomato products containing lycopene has been shown to decrease the risk of cancer [202]. The chemopreventive potential of lycopene can be contributed to its strong antioxidant activity [203]. Epidemiological studies have shown that dietary intake of lycopene is inversely correlated with the risk of many cancers including prostate [204], lung [205], colon [206], leukemia [207] and liver [208]. Lycopene prevents carcinogenesis by interfering with various cell signalling pathways. It regulates the cell cycle progression mainly in the G0/G1 phase via down-regulation of cell cycle regulatory proteins, including cyclin D1, cyclin E, and cyclin-dependent kinases (CDK) 2 and 4 in breast and prostate cancer cell lines [209–211]. A phase II randomized clinical trial of lycopene supplementation before radical prostatectomy showed that lycopene supplementation could decrease the growth of prostate cancer [212]. In another phase II trial combination of lycopene with soy isoflavones more strongly stabilized serum prostate-specific antigen (PSA) levels than lycopene alone in men with prostate cancer [213].
It has been reported that lycopene is able to modulate MAPK signalling in breast cancer cells [214] and is also able to regulate NF-κB activation by inhibiting ROS production [215]. Lycopene effectively inhibited the HMG-CoA reductase expression and cell growth and inactivated the Ras activation in prostate PC-3, colon HCT-116, HT-29 and lung BEN cancer cells [215]. Scolastici et al. studied the antimutagenic potential of lycopene and showed its inhibitory effect on DNA damage induced either directly by hydrogen peroxide (H2O2) or indirectly by n-nitrosodiethylamine (DEN) in HepG2 cells [216]. The most important target of lycopene has been found to be IGF-1 receptor signaling. This signaling regulates the downstream Ras/MAPK and PI3K/Akt signalling pathway, leading to the blockade of cell cycle progression and finally apoptosis [217].
PERILLYL ALCOHOL (POH)
The monoterpene perillyl alcohol is a natural product from cherries, lavender, and other plants [218]. Perillyl alcohol (POH) has chemopreventive activity against rat liver cancer [219] and it inhibits the proliferation of cultured human colon carcinoma cells [220]. It exhibits chemotherapeutic activity against chemically induced rat mammary tumors with little toxicity to the host [221–223]. Perillyl alcohol has been shown to induce regression of 81% of small mammary carcinomas and up to 75% of advanced mammary carcinomas initiated by 7,12-dimethylbenz(a) anthracene (DMBA) in the Wistar rat. Dietary POH was more potent than monoterpene limonene at inducing tumor regression [221]. The chemopreventive activity of POH during the promotion phase of liver carcinogenesis is associated with a marked increase in tumor cell death by apoptosis, or programmed cell death [219]. Chaudhary et al., investigated the chemopreventive effect of perillyl alcohol on DMBA-initiated and 12-O-tetradecanoylphorbol-13-acetate (TPA)-promoted skin tumorigenesis and its possible mechanisms of action in Swiss albino mice [224]. Pretreatment of POH modulated the activities of catalase, glutathione reductase, glutathione peroxidase, glutathione-S-transferase and reduced glutathione contents on TPA-induced skin edema. Perillyl alcohol also suppressed the Ras/Raf/ERK pathway and induced apoptosis [224].
Perillyl alcohol has been shown to inhibit the growth and proliferation of various cancers [225–227]. It induced cell cycle arrest and apoptosis by upregulating the expression of bax, bid, and p21waf1, and also by downregulating cyclinD and cdk2 expression [226]. In a combination treatment, POH and methyl jasmonate blocked cells at the G0/G1 phase of the cell cycle and induced forced apoptosis by the addition of cisplatin in breast cancer cells. Furthermore, POH and methyl jasmonate treatment activated tumor necrosis factor receptor 1 and this was further increased by the addition of cisplatin [228]. Yeruva et al. investigated the effects of POH and its metabolite perillic acid (PA) on the proliferation of non small cell lung cancer (NSCLC, A549, and H520) cells and found that both POH and PA elicited dose-dependent cytotoxicity, and induced cell cycle arrest and apoptosis with increasing expression of bax, p21 and caspase-3 activity in both the cell lines. Perillyl alcohol and PA in a combination treatment were also sensitized the cells to cisplatin and radiation in a dose-dependent manner [229].
MOLECULAR TARGETS FOR NATURAL CHEMOPREVENTIVE AGENTS
Regulation of Reactive Oxygen Species (ROS)
Oxidative stress is defined as an imbalance between production of free radicals and reactive metabolites, so-called oxidants, and their elimination by protective mechanisms, referred to as anti-oxidative systems [230]. Generation of ROS and the corresponding response to oxidative stress are the key factors in the pathogenesis of several human diseases including cancer [231]. There are several studies indicating the relationship between ROS and tumor progressin [232–234]. Epidemiological and laboratory studies together indicate that a high consumption of antioxidant-rich fruits and vegetables can reduce the risk of cancer [235–237]. The mitochondria, the cellular membrane oxidases, such as NADPH oxidase, nitric oxide synthase and myeloperoxidase are major sources of ROS. The byproducts associated with the metabolism of arachidonic acid by cyclooxygenase, lipoxygenase, and cytochrome P450 monooxygenase also result in the production of ROS [238]. The ROS produced in the body include superoxide (O2−), hydroxyl (OH−), hydroperoxyl (HOO−), peroxyl (ROO−), and alkoxyl (RO−) radicals, and the reactive nitrogen species (RNS) include nitric oxide (NO−) and the peroxynitrite anion (ONOO−) [231]. Many tumor promoters generate ROS, and the involvement of ROS –particularly hydrogen peroxide (H2O2) – in tumor promotion is supported by both in vivo and in vitro studies [232, 239]. ROS are also known to play a significant role in the promotion stage of carcinogenesis. Vitamins and phenolic phytochemicals are among the most prevalent antioxidants in fruits and vegetables and are onsidered to prevent ROS-mediated carcinogenicity [231].
Curcumin has antioxidant activity against free radicals and also increases the activity of antioxidant enzymes [240]. Incubation with curcumin resulted in enhanced cellular resistance to oxidative damage [241]. Previous investigation has shown that curcumin prevents lipid peroxidation and DNA strand breakage [242]. Curcumin has been shown to induce phase II detoxifying enzymes (glutathione peroxidase, glutathione reductase, glucose-6-phosphate dehydrogenase and catalase) and enhance the body’s natural antioxidant system, leading to detoxification of mutagens and carcinogens [243]. Antioxidant effects of curcumin are 10-fold more potent than ascorbic acid or resveratrol [244]. Curcumin has been found to increase hepatic GSH, SOD, GPx, GR, GST, and CAT activities in paracetamol-treated rats [245]. It also down regulates nitric oxide formation, a key element in inflammation and possibly in the process of carcinogenesis [246, 247]. Curcumin increased heme oxygenase activity in vascular endothelial cells both in normoxic and hypoxic conditions. This function is important in curcumin-mediated cytoprotection against oxidative stress [241].
Resveratrol is a nutraceutical with well-known antioxidant activity [248]. Pintea et al. studied the protective effect of resveratrol against hydrogen peroxide induced oxidative stress in cultured human RPE cells. They found that it has no cytotoxic effect at concentrations of 25–100 μM in culture media, but showed a protective effect against hydrogen peroxide-induced cytoxicity. Pretreatment with resveratrol induced a significant, dose-dependent increase of superoxide dismutase, glutathione peroxidase, and catalase activities. Moreover, it also enhanced the level of reduced glutathione under both basal and oxidative stress conditions [249]. Spanier et al. have reported that protective effects of resveratrol against oxidative injury is attributed to the upregulation of endogenous cellular antioxidant systems rather than the direct scavenging activity of ROS [250].
EGCG, isolated from green tea, displays several biological and pharmacological properties, antioxidant actions, iron-chelating capabilities, attenuation of lipid peroxidation due to various forms of radicals, and is thought to act as an antioxidant in biological systems [251]. It has also been found to protect and rescue PC12 cells against amyloid β-induced neurotoxicity in a dose dependent manner [252, 253]. In another study, oral supplementation of EGCG significantly decreased the levels of lipid peroxidation and protein carbonyl content in aged rats, possibly by enhancing the GSH redox status and both enzymic and non-enzymic antioxidants status [254]. Laboratory study revealed that EGCG has biphasic action and also acts as prooxidant depending on the concentration and cellular environment [255]. However, at lower concentrations, EGCG has been found to protect cells against the detrimental effects of oxidative stress [253].
Apigenin plays a role in cancer chemoprevention and cancer chemotherapy. It has been found to inhibit lipid peroxidation and protects antioxidant system in N-nitrosodiethylamine administered animals [256]. Apigenin has been found to scavenge free radicals and stimulates phase II detoxification enzymes in culture and tumor model [59]. It also has been shown to increase the level of intracellular glutathione and increase the endogenous defense against oxidative stress [60]. Ursolic acid remarkably inhibited the ethanol-induced oxidative stress in the liver and heart by decreasing lipid peroxidation products (thiobarbituric acid reactive substances (TBARS), lipid hydroperoxides, and conjugated dienes) by enhancing the activities of antioxidant enzymes (superoxide dismutase (SOD), catalase, glutathione peroxidase, and glutathione S-transferase) [257, 258].
Several studies have indicated that lycopene is an effective antioxidant and free radical scavenger [259]. Lycopene possess high number of conjugated double bonds, exhibits higher singlet oxygen quenching ability compared to β-carotene or α-tocopherol [260]. It has been shown to inactivate hydrogen peroxide and nitrogen dioxide [261, 262]. Dietary intake of lycopene significantly reduced lipid and protein oxidation and demonstrated a protective effect against azoxymethane (AOM)-induced colonic preneoplastic lesions [263]. Laboratory studies have shown that lycopene is able to protect lymphocytes against NO2 -induced membrane damage and cell death twice as efficiently as β-carotene [261, 264].
REGULATION OF NUCLEAR FACTOR (NF)-κB ACTIVATION
Nuclear factor (NF)-κB, one of the most investigated transcription factors, has been found to control multiple cellular processes in cancer including inflammation, transformation, proliferation, angiogenesis, invasion, metastasis, chemoresistance, and radioresistance [265]. Vertebrate Rel/NF-κB transcription factors include RelA, RelB, c-Rel, p50/p105 and p52/p100 [266]. They exist as homo or heterodimers, which bind to DNA target sites (κB sites) to influence gene expression. The most common dimer is a p50-RelA heterodimer, specifically called NF-κB. In most normal cells, they are retained in the cytoplasm as an inactive complex through the direct binding of IκB inhibitor. Various signals, including many cytokines, can cause degradation of the IκB protein and the resulting in translocation of the active Rel/NF-κB complex into the nucleus [267–269]. Continuous activation of NF-κB factors is also emerging as a hallmark of various types of solid tumors, including breast [270], ovarian [271], colon [272], pancreatic [273], thyroid [274], bladder [275], and prostate, carcinomas [276], as well as in melanomas [277]. Helbig et al. have demonstrated that NF-κB regulates the motility of breast cancer cells by directly upregulating the expression of the chemokine receptor CXCR4 [278]. The regulation of apoptotic response by NF-κB supports a role in oncogenesis and also in the resistance of tumor cells to chemotherapy [269]. This transcription factor controls the expression of gene products linked with invasion, angiogenesis, and metastasis of cancer [279].
NF-κB stimulates VEGF, proteolytic enzymes such as MMPs, urokinase plasminogen activator (uPA), and cell adhesion molecules such as ICAM-1 [267]. NF-κB regulates the expression of growth factors, cytokines and other factors involved in stress responses, cell proliferation and cell cycle progression [267, 280, 281]. The ability of tumor cells to evade immune surveillance is another contributing factor to tumor progression. The patients with renal cell carcinoma (RCC) showed impaired T-cell signaling as a result of defective NF-κB activation [282]. Therefore, drugs that could inhibit NF-κB activity are found to be useful in cancer chemotherapy and treatment [283].
Curcumin exhibits several pharmacological properties in humans and experimental colitis through inhibition of NF-κB transcription factor in inflammation 9 [284–286]. Curcumin blocked IκB alpha phosphorylation and degradation, leading to abrogation of NF-κB activation [287]. It has been shown to down regulate NF-κB and inhibit IKB kinase, suppressing proliferation and inducing apoptosis [288]. Shishodia et al. studied the effect of curcumin on Human mantle cell lymphoma. Their study has shown that treatment of curcumin suppressed NF-κB activation and also downregulated the expression of this transcription factor regulated gene products (Bcl-2, Bcl-XL, cyclin D1, COX-2, TNF, IL-6, RANK, and RANKL) leading to cell cycle arrest, suppression of proliferation and induction of apoptosis in Human mantle cell lymphoma (MCL) [289]. Curcumin is known to exert anti-inflammatory effects by interrupting NF-κB signaling at multiple levels. Collet and Campbell, have shown that curcumin induces sustained activation of JNK, which promotes apoptosis in human HCT116 colon cancer cells [290]. Curcumin inhibits inducible NF-κB activation and suppresses cancer cell proliferation in oral cancer [291], leukemia and multiple myeloma [292], breast cancer [293], ovarian cancer [294], pancreatic cancer [295], bladder cancer [296], and prostate cancer [297]. Curcumin sensitizes human cancer cells to cell-killing agents through NF-κB pathway. In human pancreatic cancer, the curcumin combination therapy with TNF-related apoptosis inducing ligand (TRAIL) suggests that inhibition of NF-κB stimulates TRAIL-induced apoptosis [298].
Manna et al. investigated the effect of resveratrol on NF-κB activation induced by various inflammatory agents. They showed that resveratrol blocked TNF-α induced activation of NF-κB in a dose- and time-dependent manner. Resveratrol also suppressed TNF-induced phosphorylation and nuclear translocation of the p65 subunit of NF-κB. Suppression of TNF-α induced NF-kappaB activation by resveratrol was not restricted to myeloid cells (U-937); it was also observed in lymphoid (Jurkat) and epithelial (HeLa and H4) cells. Resveratrol also blocked the NF-kappaB activation induced by PMA, LPS, H2O2, okadaic acid, and ceramide [299]. Resveratrol suppressed phosphorylation and subsequent degradation of IκB a, thereby inhibiting activation of NF-κB in TPA-stimulated mouse skin [300].
Apigenin inhibits the transcriptional activity of NF-κB in LPS-stimulated mouse macrophages. The classical proteasome-dependent degradation of the NF-κB inhibitor IκB alpha was observed in apigenin LPS-stimulated human monocytes [301]. Xu et al. demonstrated that treatment of apigenin potentiated the activation-induced cell death (AICD) by inhibiting NF-κB activation and suppressing NF-κB -regulated anti-apoptotic molecules, cFLIP, Bcl-x(L), Mcl-1, XIAP, and IAP, but not Bcl-2. Furthermore, it suppressed NF-κB translocation to nucleus and inhibited IκB-α phosphorylation and degradation in response to TCR stimulation in reactivated peripheral blood CD4 T cells, as well as in leukemic Jurkat T cell lines [302]. Apigenin has been found to inhibit DNA binding and reduce nuclear levels of the p65 and p50 subunits of NF-κB in human prostate carcinoma PC-3 cells. Apigenin blocked the IκB degradation, IκB-α phosphorylation along with significant decrease in IKK-alpha kinase activity.
Apigenin also inhibited TNF-α-induced activation of NF-κB via the IκB-α pathway, thereby sensitizing the cells to TNF-α-induced apoptosis. The inhibition of NF-κB activation correlated with a decreased expression of NF-κB -dependent reporter gene and suppressed expression of NF-κB-regulated genes specifically, Bcl2, cyclin D1, COX-2, MMP-9, iNOS-2, and VEGF, which contribute to prostate cancer progression [303]. Afaq et al. evaluated the effect of EGCG on UVB-mediated modulation of the NF-κB pathway. Treatment of normal human epidermal keratinocytes (NHEK) with EGCG (10–40 μM) for 24 h resulted in a significant inhibition of UVB (40 mJ/cm2)-mediated degradation and phosphorylation of IκB-α and activation of IKK-α, in a dose-dependent manner. Data suggest that EGCG protects against the adverse effects of UV radiation via modulations in NF-κB pathway, and provide a molecular basis for the photochemopreventive effect of EGCG [304]. Pretreatment of normal human bronchial epithelial cells (NHBE) cells with EGCG suppressed CSC-induced phosphorylation of IκB-α, and activation and nuclear translocation of NF-κB /p65, which leads to the significant downregulation of NF-κB -regulated proteins cyclin D1, MMP-9, IL-8, and iNOS [305]. Quercetin has been found to inhibit the NF-κB and their downstream cascade including TNF-α and NO [306]. It binds to the NF-κB in HepG2 cells [307]. Davis et al., demonstrated that the soy isoflavone genistein inhibits the cell growth and induced apoptosis in human prostate cancer, LNCaP and PC3 cells by regulating NF-κB [308]. Treatment with low doses of UA has been found to sensitize cancer cells to chemotherapeutic agents, taxol or cisplatin through suppressing NF-κB [309].
INHIBITION OF ANGIOGENESIS
Angiogenesis is the development of new blood vessels from the pre-existing vascular beds. It is tightly regulated by a large number of proangiogenic and antiangiogenic factors, occurs in response to the increasing demand for nutrients and oxygen experienced by proliferating tumor cells. Angiogenesis plays a pivotal role in tumor growth, invasion, and metastasis [310]. Tumors cannot grow beyond a size of approximately 1–2mm without neovascularization [311]. The angiogenic process involves the activation, proliferation, and migration of endothelial cells toward angiogenic stimuli produced by the tumor [312]. The tumor cells induced secretion of angiogenesis factors which is commonly observed in most aggressive tumors. The process of angiogenesis is mediated by several pro-angiogenic molecules released by both tumor cells and host cells including endothelial cells, epithelial cells, mesothelial cells, and leucocytes [310]. These pro-angiogenic molecules bind to receptors on nearby blood vessels and induce the activation, proliferation, and migration of endothelial cells towards the tumor. Stimuli for the production of pro-angiogenic mediators include hypoxia, cytokines, and growth factors, as well as mutations in oncogenes and tumor suppressor genes [313].
Among various angiogenic factors, VEGF, fibroblast growth factor (FGF) family, interleukin-8 (IL-8), angiogenin, angiotropin, epidermal growth factor (EGF), plateletderived endothelial cell growth factor (PD-ECGF), platelet derived growth factor (PDGF), fibrin, nicotinamide, transforming growth factor- α (TGF-α), TGF-β, and tumor necrosis factor-α (TNF-α) are important regulators [314]. Degradation of basement membrane is a crucial event in angiogenesis. Many proteases are capable of degrading extracellular matrix components. They are of three major groups: Serine proteases, Cathepsins, and Matrix metalloproteases (MMPs). Urokinase plasminogen actvator is the most important member of the serine protease family involved in ECM degradation. It is secreted as an inactive, single chain precursor that requires binding to its cell membrane receptor for activation [313]. Members of this system, including uPA and its receptor (uPAR), are over expressed in several malignant tumors [315]. MMPs are a broad family of zinc-binding endopeptidases, which secreted in inactive proenzymatic forms. MMPs can be subdivided on the basis of preferential extracellular matrix substrate [316]. Among matrix metalloproteinases (MMPs), gelatinases such as MMP-2 and MMP-9 play a key role in degrading most ECM components surrounding tumor tissue [317].
There are several natural products which have been tested for their antiangiogenic potential [318]. Curcumin is found to be a potent angiogenesis inhibitor which downregulates various proangiogenic proteins such as vascular endothelial growth factor and basic fibroblast growth factor [319].
Previous studies have shown that curcumin inhibited vascular endothelial cell proliferation in vitro and capillary tube formation in vivo [288, 320, 321]. Curcumin has been shown to down-regulate the hypoxia-induced mRNA and the protein expression of VEGF, leading to suppression of hypoxia-stimulated angiogenesis [322]. Thaloor et al. studied the effect of curcumin on endothelial cell migration, attachment, and tube formation on matrigel. Curcumin treatment resulted in a dose-dependent inhibition of tube formation and metalloproteinase activities. It also inhibited angiogenesis in a s.c. matrigel plug model in mice [323].
Singh et al. investgated the antiangiogenic potential of EGCG, and found that treatment of EGCG inhibited the tube formation, migration and regulated MMPs [324]. Apigenin has been found to inhibit tumor angiogenesis by downregulating HIF-1 and VEGF expression in human ovarian cancer cells under normoxic condition [325]. Apigenin also suppressed the expression of erythropoietin mRNA, which is a typical hypoxia-inducible gene, via the degradation of HIF-1 α [326].
Genistein has been found to be the most potent angiogenesis inhibitor among the flavanoids compounds. Treatment with genistein caused a dose-dependent inhibition in the expression of VEGF, PDGF, uPA, and MMP-2 and 9, respectively. Furthermore, angiogenesis inhibitors-plasminogen activator inhibitor-1, endostatin, angiostatin, and thrombospondin-1 were also upregulated [327]. Igura et al., studied the effect of resveratrol and quercetin on the inhibition of angiogenesis in vitro. They found that treatment of resveratrol and quercetin inhibited angiogenesis by inhibiting the migration and tube formation in bovine aorta endothelial (BAE) cells [328].
Antiangiogenic potential of UA in both in vitro and in vivo model was investigated. Treatment of ursolic acid inhibited the prolifearation, migration, invasion, and tube formation in human umbilical vein endothelial cells (HUVEC). The levels of serum VEGF, NO, and proinflammatory cytokines were significantly reduced, whereas serum TIMP-1 and IL-2 levels were significantly elevated in UA treated animals compared with those in control animals. Gelatin zymographic analysis also showed the inhibitory effect of UA on the protein expression of matrix metalloproteinases MMP-2 and MMP-9 [199].
The PEITC treatment caused a decrease in survival of HUVECs in a concentration- and time-dependent manner. The tube formation and migration by HUVEC was also inhibited in the presence of PEITC at pharmacologically relevant concentrations. This effect has been associated with suppression of VEGF secretion, downregulation of VEGF receptor 2 protein levels, and inactivation of prosurvival serine-threonine kinase Akt [329]. Sulforaphane showed inhibitory effects on hypoxia-induced VEGF and VEGF receptor (flk-1) expression, and also two angiogenesis-associated transcription factors, HIF-1α and c-Myc in HMEC-1 cells [330]. This compound also caused the inhibition of cell migration and capillary tube formation in HUVECs by regulating the MEK/ERK and PI3K/AKT pathways which synergistically induced FOXO transcriptional activity [331].
REGULATIN OF APOPTOSIS
Cancer is a disorder characterized by uncontrolled proliferation and reduced apoptosis. Apoptosis, a genetically programmed cell-suicide process, is vital for the proper development and functioning of multicellular organisms. It is characterized by typical morphological and biochemical hallmarks, including cell shrinkage, membrane blebbing, nuclear condensation, and fragmentation [332, 333]. Inducing apoptosis is an effective method of treating cancers. There are two major pathways, extrinsic and intrinsic, have been identified for the induction of apoptosis [334]. The most evident morphological signs of apoptosis are cellular shrinkage, membrane blebbing, nuclear condensation, and fragmentation, which are the final steps of consequential signaling cascades [335]. Apoptotic signaling pathways can also be divided into caspase-dependent and -independent or mitochondria-dependent and -independent pathways. Caspases are cysteine proteases and play a central role in the initiation and execution phases of apoptosis.
Activation of caspases is recognized as a key element in the apoptotic process [336]. They are of two types, initiator caspases (caspase 2, 8, 9 and 10), and effector or executioner caspases (caspase 3, 6 and 7). Initiator caspases transmit the death signal generated by the apoptotic stimulus to the effector caspases, which act to cleave the target proteins and thus produce the morphological features of apoptosis [337]. During apoptosis, the released cytochrome c from mitochondria triggers caspase-9 activation whereas ligation of death receptors on the plasma membrane activates caspase-8 [338]. p53 also called ‘guardian of the genome’, the first tumor suppressor gene involved in cell cycle regulation and induction of apoptosis. p53 can be activated by a variety of stimuli such as radiation and anticancer agents, leading to cell cycle arrest and/or apoptosis [339]. It has been found that; p53 is either mutated or overexpressed in most of the human tumors along with antiapoptotic gene bcl-2 that enhances tumor cell proliferation and metastasis [340].
Curcumin has been shown to induce apoptosis in many types of cancer cell lines [341–343]. Previous studies have demonstrated that curcumin-induces apoptosis through a mechanism of downregulating ornithine decarboxylase and along a ROS dependent mitochondria-mediated pathway in human acute promyelocytic leukemia HL-60 cells [344]. Curcumin treatment resulted in cleavage of caspase-3 and poly adenosine diphosphate-ribose polymerase. It also increased the cellular levels of apoptotic Bcl-XL protein and a decreased the cellular content of antiapoptotic protein Bcl-2 [345]. Wu et al., studied the effect of curcumin on human non-small cell lung cancer NCI-H460 cells. They have shown that curcumin induces apoptosis in NCI-H460 cells in a dose-dependent manner. Curcumin treatment upregulated BAX and BAD and down regulated BCL-2, BCL-XL and XIAP. It also has been shown that increase the reactive oxygen species (ROS), intracellular Ca (2+) and endoplasmic reticulum (ER) stress in NCI-H460 cells after exposure to curcumin [346]. It has been suggested that production of reactive oxygen intermediates the release of cytochrome c causing tumor cell apoptosis as a result of curcumin treatment [347]. Curcumin is also able to induce mitochondrial abnormalities and promote p53-dependent apoptosis and the activation of caspase-8 and caspase-3 [348–350].
Resveratrol-treatment inhibited the constitutive expression of phosphatidylinositol 3′-kinase (PI3K) and phosphorylated (active) Akt in human prostate carcinoma LNCaP cells. It also down regulated the level of ant apoptotic protein Bcl-2 and up regulated the proapoptotic members of the Bcl-2 family such as Bax, Bak, Bid, and Bad [351]. Resveratrol activate intracellular Notch-1 and restore wild-type p53 expression in glioblastoma cells. Significant de-phosphorylation of Akt, increased Bax expression and decreased Bcl-2 expression and cleavage of caspase-3 were also observed in resveratrol-induced apoptosis in glioblastoma cells [352].
EGCG inhibited the growth of anaplastic thyroid carcinoma cells in a dose-dependent manner. Treatment of EGCG has been shown to suppress the phosphorylation of EGFR, ERK1/2, JNK, and p38. These changes were correlated with increased p21 and reduced cyclin B1/CDK1 expression. Furthermore, it increased the accumulation of sub-G1 cell, activated caspase-3 and cleaved PARP [353]. Lim and Cha studied the in vitro cytotoxic effect of EGCG against human laryngeal epidermoid carcinoma Hep2 cells. EGCG-treatment increased the p53 level in the cells, with a corresponding decrease in Bcl-2 and Bid protein levels as well as an increase in the Bax level. EGCG also induced the cytoplasmic release of cytochrome c from the mitochondria accompanied by a decreased mitochondrial membrane potential, and subsequently upregulated translocation of apoptosis-inducing factor (AIF) and endonuclease G (EndoG) into the nucleus during the apoptotic process [353].
Treatment with apigenin inhibited the proliferation of MDA-MB-453 human breast cancer cells in a dose- and time-dependent manner. Apigenin activated caspase-9 and -3, accompanied by the cleavage of capases-6, -7, and -8 [354]. Wang et al. reported that the apoptotic rate was significantly increased in tumor cells treated with ursolic acid both in vitro and in vivo. Ursolic acid induced DNA fragmentation, upregulated caspase-3, -8, and -9 and down regulated expression of Bcl-2 in BGC-803 cells [355]. Ursolic acid at non-cytotoxic concentrations, upregulated the tumor suppressor gene p53 and caspase-3 and down-regulated the anti-apoptotic gene Bcl-2 in B16F-10 melanoma cells [194]. Incubation of HepG2 cells with quercetin induced apoptosis by the activating caspase-3 and -9, but not caspase-8. Furthermore, this flavonoid decreased the Bcl-xL:Bcl-xS ratio and increased translocation of Bax to the mitochondrial membrane. It also inhibited the major survival signals, Akt and extracellular regulated kinase (ERK) in the same experiment [356].
REGULATION OF CYCLOOXYGENASE (COX)-2
Cyclooxygenase (COX) is a member of the prostaglandin synthase family of enzymes that plays an important role in the growth and progression of cancer. They are made up of two main types of enzymes, namely COX-1 and COX-2. COX-1 enzyme is constitutively expressed in normal circumstances, whereas COX-2, which is an inducible enzyme, has a major role in inflammatory response [357, 358]. COX-2 is overexpressed in inflammatory states which are induced by a variety of stimulators including cytokines, growth factors, as well as tumor promoters [359]. The specific function of COX-2 is the conversion of arachidonic acid to prostaglandin (PGE(2)), a major metabolite in inflammation. COX-2 has been found to be constitutively expressed in a number of cancers, colon [360, 361], prostate [362, 363] breast [364], where the expression of COX-2 is paralleled by a higher incidence of chemotherapy failure. A study showed that COX-2 was expressed in 24% of adenomas and in 56% of adenocarcinomas [365]. COX-2 protein inhibitors (i.e., NSAIDs and COXIBs) are not recommended for prolonged administration since they may cause severe side effects [366].
Curcumin is a potent inhibitor of COX-2. It inhibits COX-2 via NF-κB downregulation, which is one of the major inducers of COX-2 promoter activation [367]. Celecoxib is a promising preventive drug but its long-term use causes adverse cardiovascular effects [368]. Curcumin in combination with celecoxib have been shown to inhibit colon cancer cell growth by suppressing COX-2 [369]. In another study, curcumin has been found to inhibit PMA-induced COX-2 mRNA and protein levels in H460 cells [370]. Wang et al. studied the effect of curcumin on deoxycholic acid (DCA) induced cell proliferation in human HT-29 colon cancer cell line and its underlying molecular mechanisms. They found that treatment of curcumin inhibited the cell proliferation by regulating COX-2 mRNA transcription, COX-2 protein expression, and PGE(2) synthesis induced by DCA in HT-29 cell line [371].
Resveratrol dose-dependently prevented both COX-2 induction and PGE(2) production in bFGF-stimulated fibroblasts [372]. Studies revealed that apigenin can inhibit the phorbol ester-induced COX-2 expression in the breast cancer cell lines (MCF-10A and MCF-7) [373] and human brain microvascular endothelial cells (HBMEC) [374]. Furthermore, apigenin suppressed the expression of COX-2 by decreasing the intracellular Ca(2+) level and inhibiting NF-κB activation in human mast cell line (HMC-1) [375]. In another study EGCG has been found to inhibit COX-2, PGE(2), and IL-8 expression, induced by IL-1beta in human synovial fibroblasts [376], and the inhibition of COX-2 was associated with inhibition of NF-κB translocation [377]. Genistein in combination with capsaicin exerts anti-inflammatory and anticarcinogenic properties through the modulation of COX-2 in TPA (12-O-tetradecanoylphorbol-13-acetate) -treated rat mammary glands or mammary cancer cell line [378].
INHIBITION OF SIGNAL TRANSDUCERS AND ACTIVATOR OF TRANSCRIPTION 3 (STAT3)
STAT proteins were originally identified as being latent cytoplasmic transcription factors, which were only translocated to the nucleus upon Jak-mediated phosphorylation and dimerization following cytokine-induced activation of Jaks [379]. Constitutive activation of Stat proteins, notably of Stat3 and also Jak- Stat3 signalling pathway has been frequently observed in many primary human tumors [380, 381]. Dysregulated Stat3 has been associated with increased breast cancer cell proliferation, survival, and metastasis [382]. Abberent Stat3 promotes uncontrolled growth and survival through dysregulation of gene expression, including cyclin D1, c-Myc, Bcl-xL, Mcl-1, and survivin genes, thereby contributing to oncogenesis [380, 383, 384]. Constitutive activation of Stat3 signaling also confers resistance to apoptosis in human U266 myeloma cells [385], in breast cancer cells [382] and in other cells [386]. Inhibition of the Stat3 pathway has been shown to induce apoptosis in breast cancer cell lines [387]. Antiapoptotic proteins, such as Bcl-xL and Mcl-1, were shown to be up-regulated in multiple myeloma cells in which constitutive Stat3 activity was induced by IL-6 [385, 388].
Curcumin was shown to inhibit constitutive and IL-6–induced Stat3 activation in human multiple myeloma cells [389]. Stat3 was considered to be a master regulator in human glioma and essential for tumor cell survival and its ability to invade the normal brain [390]. Weissenberger et al. studied the effect of curcumin on Jak/Stat3 activity in glioma cell. Low dose of curcumin treatment inhibited Jak1,2/Stat3 tyrosine-phosphorylation in a dose-dependent fashion. Curcumin downregulated transcription of the Stat3 target genes c-Myc, MMP-9, Snail, and Twist, and of the proliferation marker Ki67. It has also been shown to inhibit cell proliferation by inducing a G2/M phase arrest [391]. Treatment of cancer cells with curcumin induced a dose- and time-dependent decrease of constitutive IL-6 expression and IL-6-induced Stat3 phosphorylation in ovarian and endometrial cancer cells. Stat3 activation was found to be reversible and was returned to control levels 24 h after curcumin removal [392].
Epigallocatechin-3-gallate inhibits growth and angiogenesis of gastric cancer by inhibiting VEGF and Stat3 activation [393]. It blocked Stat3 phosphorylation (Tyr705 and Ser727) and thereby inhibited the collagen production and proliferation in keloid fibroblasts cells [394]. EGCG also markedly inhibited the activation of Stat3 in YCU-H891 (carcinoma of the hypopharynx) cells [395] and BT-474 (human breast cancer cell) cells [396]. Apigenin inhibited Stat3 phosphorylation following exposure to hypoxia. Interestingly, Stat3 inhibition is correlated with VEGF expression [397]. Previous study has shown that luteolin inhibited phosphorylation of STAT3 and inhibited the expression of cyclin D1, survivin, Bcl-x(L), and VEGF [398].
Resveratrol has been found to regulate interleukin (IL)-6–induced ICAM-1 gene expression by attenuating Stat3 phosphorylation [399]. Resveratrol promotes differentiation and apoptosis of medulloblastoma cells by suppressing Stat3 signaling and a range of cancer-associated gene expression [400]. Yang et al., reported that resveratrol can reduce in vivo tumorigenicity and enhance the sensitivity of glioblastoma tumor initiating cells (GBM-TIC) to radiotherapies through the Stat3 pathway [401]. Quercetin inhibited Cu(2+)-oxidized LDL-induced endothelial apoptosis through modulating Jak2-Stat3 pathways [402]. In vitro treatment of activated T cells with quercetin blocked IL-12-induced tyrosine phosphorylation of Jak2, Tyk2, Stat3, and Stat4, resulting in a decrease in IL-12-induced T cell proliferation and Th1 differentiation [403]. Pathak et al. showed that treatment of UA, inhibited both constitutive and IL-6–inducible Stat3 activation in a dose- and time-dependent manner in multiple myeloma cells [404].
REGULATING MITOGEN-ACTIVATED PROTEIN KINASES (MAPKs) PATHWAY
Mitogen-activated protein kinases (MAPKs), comprising a family of serine and threonine kinases of ERK, JNK, and p38, play an important role in the transmission of cell signals through transduction systems to cell nucleus, where they influence the expression of genes that regulate important cellular processes such as cell growth, differentiation, proliferation, survival, migration, and apoptosis [405, 406]. Components of these pathways are mutated or aberrantly expressed in human cancer (e.g., Ras, B-Raf, PI3K, PTEN, Akt) [407]. The Raf/MEK/ERK pathway has a major role in the regulation of apoptosis by the post-translational phosphorylation of apoptotic regulatory molecules including Bad, caspase 9, and Bcl-2 [408]. This pathway also contributes to chemotherapeutic drug resistance as ectopic activation of Raf induces resistance to doxorubicin and paclitaxel in breast cancer cells, and also B-Raf has been found to be mutated in various malignancies including melanoma, thyroid and breast cancer [409]. Similar to other MAPKs, p38 is activated by MAP kinase kinases (MAPKKs/MKKs) with MKK3 and MKK6 being the two main upstream MAPKKs [410]. p38 stimulates a number of downstream substrates, including MAP kinase-activated protein kinase-2 (MAPKAPK-2/MK2), transcription factor-2 (ATF-2), mitogen- and stress-activated kinase (MSK), and p53 [411–413].
Curcumin caused a down-regulation of enhancer of zeste homolog 2 (EZH2) genes through stimulation of three major members of the mitogen-activated protein kinase (MAPK) pathway: c-Jun NH2-terminal kinase (JNK), extracellular signal-regulated kinase (ERK) and p38 kinase in MDA-MB-435 human breast cancer cells [414]. Weir et al. reported that curcumin induced apoptosis in cisplatin-resistant human ovarian cancer cells by modulating p38 and Akt [415]. Curcumin also has been shown to suppress the PMA-induced phosphorylation of ERK, JNK, and p38 MAP kinase in human astroglioma cells [416].
Resveratrol has been found to activate MAP kinases pathways and induce apoptosis in human breast cancer cells MCF-7 [417]. Dietary supplementation of resveratrol attenuated chronic colonic inflammation in mice. Treatment of resveratrol reduced prostaglandin E synthase-1 (PGES-1), COX-2, and iNOS proteins expression, via downregulation of p38, a mitogen-activated protein kinase (MAPK) signal pathway in chronic dextran sulphate sodium (DSS)-induced colitis mice model [418].
Noh et al. found that apigenin could inhibit PMA-induced phosphorylation of p38, which was involved in the down-regulation of the expression of MMP-9 at mRNA levels. Furthermore, the treatment of p38 MAPK inhibitors (SB203580) to Caski cells caused the reduction of MMP-9 expression [419]. Apigenin treatment caused increased phosphorylation of ERK1/2 and JNK1/2 and this sustained activation resulted in decreased ELK-1 phosphorylation and c-FOS expression, leading to inhibition cell survival in human prostate cancer cells [420].
Treatment of EGCG significantly inhibited the phosphorylation of p38 and c-Jun N-terminal kinase (JNK), NF-κB, and AP-1 transcriptional activities in human corneal epithelial cells [421]. In another study, EGCG inhibited Ang II-induced endothelial stress fiber formation and hyperpermeability via inactivation of p38 MAPK/HSP27 pathway [422]. Quercitrin blocked TPA-induced neoplastic transformation in JB6 P+ cells. Pretreatment of JB6 cells with quercitrin down-regulated transactivation of AP-1 and NF-κB induced by UVB or TPA through the inhibition of MAPKs phosphorylation, including ERKs and p38 kinase and the inhibition of JNKs [103]. Ursolic acid inhibited the proliferation and induced apoptosis in HT-29 colon cancer cells by inhibiting the EGFR/MAPK pathway. Treatment with UA suppressed the phosphorylation of EGFR, ERK1/2, p38 MAPK, and JNK, which is well correlated with its growth inhibitory effect [423].
REGULATION OF CELL CYCLE
Cell growth and proliferation are highly regulated events in normal cells, and dysregulation of cell cycle can lead to uncontrolled proliferation and contribute to the malignant phenotype of tumor cells [421]. Cancer cells are characterized by a failure of cell cycle control which results in their over proliferation. Cell cycle checkpoints play pivotal roles in the regulation of critical events, including DNA replication, cellular proliferation, cellular differentiation, and apoptosis [424]. Cell cycle control is a highly regulated process that involves the modulation of cell cycle regulatory proteins, including cyclins (cyclin A, B, Ds, or E); cyclin-dependent kinases (CDKs) (CDK 1, 2, 4, or 6); and CDK inhibitors, such as p21WAF1, p27KIP1, p53, and phosphorylated retinoblastoma (pRb) [425]. Many phytochemicals have been shown to regulate the cell cycle proteins, leading to the inhibition of cancer cell proliferation. In addition, cell cycle checkpoints, such as G1/S and G2/M, are also important targets in cell cycle control [426]. Studies showed that apoptosis was accompanied by a reduction in the number of cells in G0/G1 and an increase in the number of G2/M-phase cells, indicating a cell cycle arrest in G2/M phase [427–429].
Lu et al. investigated the curcumin induced DNA damage, S and G2/M phase arrest in the colorectal carcinoma HCT116 cells [430]. In another study curcumin induced successive G(1)/S and G(2)/M phase arrest accompanied by down-regulation of cyclin D1, cdc2 and cyclin B1 in HOS cells [431]. Treatment of quercetin caused G2/M arrest in human esophageal squamous cell carcinoma cell line through up-regulation of p73 and p21waf1 and subsequent down-regulation of cyclin B1, both at the mRNA and protein levels [432]. Resveratrol (100 μmol/L) induced cell cycle block and accumulation of cells at the G0–G1 phase by 48 h in PANC-1 cells. In AsPC-1 cells, resveratrol caused cell cycle arrest and induced depletion of cells in the S and G2-M phases of the cell cycle [433].
In vitro study showed that EGCG down-regulated cell cyclerelated proteins such as cyclin B1 and CDK1, whereas p21 was up-regulated in ARO cells [353]. Apigenin remarkably inhibited Huh7 cell proliferation and induced cell cycle arrest at G2/M phase and an increased rate of apoptosis [434]. In another study, apigenin blocked the proliferation of two types of leukemia through cell-cycle arrest in G(2)/M phase (myeloid HL60) and G(0)/G(1) phase for (erythroid TF1 cells) [435].
Isothiocyanates induced G(2)/M cell cycle arrest accompanied by mitotic phosphorylation of histone H3 in multiple myeloma [436]. PEITC induced a dose-dependent decrease in cell viability through induction of cell apoptosis and cell cycle arrest in the G(2)/M phase of DU 145 cells. The induction of G(2)/M phase arrest was mediated by the increase of p53 and WEE1 [147].
REGULATION OF ACTIVATOR PROTEIN-1
Activator protein-1 (AP-1) is a transcription factor that regulates genes involved in carcinogenesis, cell proliferation, and progression of benign tumors to malignancy and in metastasis. It is formed by dimerization of activated c-Jun, and c-FOS [437, 438]. When AP-1 is activated, it binds to TPA response elements (TRE) or cAMP response element (CRE) in the promoter region of the target genes [439]. There is a close relationship between c-fos over-expression and tumor development. Furthermore, inactivation or suppression of c-jun leads to the development of papillomas and liver tumors [440]. JNK and p38 MAPK phosphorylation enhances the activity of c-Jun and ATF2 proteins, which in turn activate AP-1 target genes [441]. AP-1 has been found to induce COX-2, uPA, c-Fos, MMP-9, cyclin D1, VEGF [442], and this transcription factor is recognized as a molecular target of many antioxidant and chemopreventive compounds [443]. Inhibition of AP-1 has been shown to block tumor promotion, transformation, progression, and invasion [444].
Curcumin effectively blocks AP-1 and NF-κB signaling pathways and enhances apoptosis in many cancer cell lines [445]. Curcumin also has been reported to inhibit the activation of AP-1 induced by tumor promoters by direct interaction with the AP-1 DNA-binding motif and to inhibit C-Jun N-terminal kinase (JNK) activation by carcinogens [389, 442]. Resveratrol inhibited the c-Fos [446] and suppressed AP-1 DNA binding affinity [54], via MEK1 > ERK1/2 signaling [51]. Treatment of EGCG inhibited phosphorylation of the MAPKs p38, JNK and AP-1 transcriptional activity in human corneal epithelial cells [421]. Electrophoretic mobility shift assay revealed that EGCG inhibits binding of AP-1 proteins to its response elements in synovial fibroblast [447]. Nair et al., showed that treatment of SULF in combination with EGCG attenuated AP-1 activity in PC-3 cells [448].
Previous study showed that treatment of apigenin inhibited c-Fos / AP-1 activity by regulating and Janus kinase 2 (JAK2) / signal transducer and activator of transcription 1/2 (STAT1/2) pathways in interleukin-1β-treated articular chondrocytes [449]. Hwang et al. investigated the protective effects of apigenin and luteolin on immortalized human keratinocytes (HaCaT) against UVA damage, and found that treatment of both flavanoids inhibited UVA-induced collagenolytic MMP-1 production by interfering with Ca(2+)-dependent MAPKs and AP-1 signaling [450]. Li et al. showed that Ras/MAPK/AP-1 signalling pathway is involved in genistein induced G2/M cell cycle arrest in MDA-MB-231 breast cancer cells [451].
CONCLUSION
Natural products are a rich source of cancer chemotherapy drugs, and primarily target rapidly proliferating tumor cells. Chemoprevention by edible phytochemicals is of great interest and is considered to be an inexpensive, readily applicable, acceptable, and accessible approach to cancer control and management. Several phytochemicals are in preclinical or clinical trials for cancer chemoprevention. Epidemiological studies have shown that high dietary consumption of vegetables and fruits reduced the risk of cancer. Severe toxicity is the major drawback in conventional radiotherapy and chemotherapy. Both methods exert toxic side effects, such as nausea, vomiting, mucosal ulceration, alopecia, pulmonary fibrosis, cardiac, and hepatic toxicity. Use of natural compounds as an adjunct to chemotherapy and radiation may reduce treatment toxicities as well as increase the therapeutic index. Studies showed that phytochemicals, including curcumin, reseveratrol, epigallocatechin gallate, apigenin, quercetin, genistein, lycopene, ursolic acid, isothyocynates, and perillyl alcohol exerted anticancer effects by multiple signal transduction pathways. They inhibited the reactive oxygen species production, NF-κB activation, angiogenesis, AP-1 and COX-2; regulated Stat3 and MAPKs pathway; and induced cell cycle arrest and apoptosis; in various experimental models. Based on these available evidences, it can be concluded that the daily consumption of fruits and vegetables rich in above discussed components could be beneficial for the cancer prevention. The better strategy to identify the compounds for cancer prevention is through mechanism based investigation. The understanding of molecular mechanism of a specific plant derived compound against a particular type of cancer will leads to the invention of novel drug and drug targets for therapeutic intervention.
Acknowledgments
Declared none.
Footnotes
CONFLICT OF INTEREST
The author(s) confirm that this article content has no conflict of interest.
References
- 1.Amin A, Kucuk O, Khuri FR, Shin DM. Perspectives for cancer prevention with natural compounds. J Clin Oncol. 2009;27(16):2712. doi: 10.1200/JCO.2008.20.6235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen D, Dou QP. Tea polyphenols and their roles in cancer prevention and chemotherapy. Int J Mol Sci. 2008;9(7):1196–1206. doi: 10.3390/ijms9071196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mondal S, Bandyopadhyay S, Ghosh M, Mukhopadhyay S, Roy S, Mandal C. Natural Products: Promising Resources For Cancer Drug Discovery. Anticancer Agents Med Chem. 2011 doi: 10.2174/187152012798764697. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 4.Sultana N. Clinically useful anticancer, antitumor, and antiwrinkle agent, ursolic acid and related derivatives as medicinally important natural product. J Enzyme Inhib Med Chem. 2011:1–27. doi: 10.3109/14756366.2010.546793. [DOI] [PubMed] [Google Scholar]
- 5.Nishino H, Tokuda H, Satomi Y, Masuda M, Onozuka M, Yamaguchi S, Takayasu J, Tsuruta J, Takemura M, Ii T. Cancer chemoprevention by phytochemicals and their related compounds. Asian Pac J Cancer Prev. 2000;1(1):49–55. [PubMed] [Google Scholar]
- 6.Ullah MF, Ahmad A, Zubair H, Khan HY, Wang Z, Sarkar FH, Hadi SM. Soy isoflavone genistein induces cell death in breast cancer cells through mobilization of endogenous copper ions and generation of reactive oxygen species. Mol Nutr Food Res. 2011;55(4):553–9. doi: 10.1002/mnfr.201000329. [DOI] [PubMed] [Google Scholar]
- 7.Nigam N, George J, Srivastava S, Roy P, Bhui K, Singh M, Shukla Y. Induction of apoptosis by [6]-gingerol associated with the modulation of p53 and involvement of mitochondrial signaling pathway in B [a] P-induced mouse skin tumorigenesis. Cancer Chemother Pharmacol. 2010;65(4):687–696. doi: 10.1007/s00280-009-1074-x. [DOI] [PubMed] [Google Scholar]
- 8.Surh YJ, Chun KS. Cancer chemopreventive effects of curcumin. In: Aggarwal BB, Surh YJ, Shishodia S, editors. The Molecular Targets and Therapeutic Uses of urcumin in Health and Disease. Springer; 2007. pp. 149–172. [DOI] [PubMed] [Google Scholar]
- 9.Shu L, Cheung KL, Khor TO, Chen C, Kong AN. Phytochemicals: cancer chemoprevention and suppression of tumor onset and metastasis. Cancer Metastasis Rev. 2010;29(3):483–502. doi: 10.1007/s10555-010-9239-y. [DOI] [PubMed] [Google Scholar]
- 10.Surh YJ. Cancer chemoprevention with dietary phytochemicals. Nat Rev Cancer. 2003;3(10):768–780. doi: 10.1038/nrc1189. [DOI] [PubMed] [Google Scholar]
- 11.de Kok TMCM, de Waard P, Wilms LC, van Breda SGJ. Antioxidative and antigenotoxic properties of vegetables and dietary phytochemicals: The value of genomics biomarkers in molecular epidemiology. Mol Nutr Food Res. 2010;54(2):208–217. doi: 10.1002/mnfr.200900288. [DOI] [PubMed] [Google Scholar]
- 12.Rajasekaran SA. Therapeutic potential of curcumin in gastrointestinal diseases. World J Gastrointest Pathophysiol. 2011;2(1):1–14. doi: 10.4291/wjgp.v2.i1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Menon VP, Sudheer AR. Antioxidant and anti-inflammatory properties of curcumin. In: Aggarwal BB, Surh YJ, Shishodia S, editors. The Molecular Targets and Therapeutic Uses of urcumin in Health and Disease. Springer; 2007. pp. 105–125. [DOI] [PubMed] [Google Scholar]
- 14.Aggarwal BB, Sung B. Pharmacological basis for the role of curcumin in chronic diseases: an age-old spice with modern targets. Trends Pharmacol Sci. 2009;30(2):85–94. doi: 10.1016/j.tips.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 15.Thangapazham RL, Sharma A, Maheshwari RK. Multiple molecular targets in cancer chemoprevention by curcumin. AAPS J. 2006;8(3):443–449. doi: 10.1208/aapsj080352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anand P, Sundaram C, Jhurani S, Kunnumakkara AB, Aggarwal BB. Curcumin and cancer: An. Cancer lett. 2008;267(1):133–164. doi: 10.1016/j.canlet.2008.03.025. [DOI] [PubMed] [Google Scholar]
- 17.Park J, Conteas CN. Anti-carcinogenic properties of curcumin on colorectal cancer. World J Gastrointest Oncol. 2010;2(4):169. doi: 10.4251/wjgo.v2.i4.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Subramaniam D, Ramalingam S, Linehan DC, Dieckgraefe BK, Postier RG, Houchen CW, Jensen RA, Anant S. RNA Binding Protein CUGBP2/CELF2 Mediates Curcumin-Induced Mitotic Catastrophe of Pancreatic Cancer Cells. PloS one. 2011;6(2):e16958. doi: 10.1371/journal.pone.0016958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Duarte VM, Han E, Veena MS, Salvado A, Suh JD, Liang LJ, Faull KF, Srivatsan ES, Wang MB. Curcumin enhances the effect of cisplatin in suppression of head and neck squamous cell carcinoma via inhibition of IKK protein of the NF B pathway. Mol Cancer Ther. 2010;9(10):2665–2675. doi: 10.1158/1535-7163.MCT-10-0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cheng CY, Lin YH, Su CC. Curcumin inhibits the proliferation of human hepatocellular carcinoma J5 cells by inducing endoplasmic reticulum stress and mitochondrial dysfunction. Int J Mol Med. 2010;26(5):673–678. doi: 10.3892/ijmm_00000513. [DOI] [PubMed] [Google Scholar]
- 21.Mudduluru G, George-William JN, Muppala S, Asangani IA, Regalla K, Nelson LD, Allgayer H. Curcumin regulates miR-21 expression and inhibits invasion and metastasis in colorectal cancer. Biosci Rep. 2011;31(3):185–197. doi: 10.1042/BSR20100065. [DOI] [PubMed] [Google Scholar]
- 22.Kim JH, Xu C, Keum YS, Reddy B, Conney A, Kong ANT. Inhibition of EGFR signaling in human prostate cancer PC-3 cells by combination treatment with -phenylethyl isothiocyanate and curcumin. Carcinogenesis. 2006;27(3):475–482. doi: 10.1093/carcin/bgi272. [DOI] [PubMed] [Google Scholar]
- 23.Menon LG, Kuttan R, Kuttan G. Anti-metastatic activity of curcumin and catechin. Cancer lett. 1999;141(1–2):159–165. doi: 10.1016/s0304-3835(99)00098-1. [DOI] [PubMed] [Google Scholar]
- 24.Rao CV. The Molecular Targets and Therapeutic Uses of Curcumin in Health and Disease. 2007. Regulation of COX and LOX by curcumin; pp. 213–226. [DOI] [PubMed] [Google Scholar]
- 25.Kim YS, Young MR, Bobe G, Colburn NH, Milner JA. Bioactive food components, inflammatory targets, and cancer prevention. Cancer Prev Res (Phila) 2009;2(3):200–208. doi: 10.1158/1940-6207.CAPR-08-0141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang Z, Zhang Y, Banerjee S, Li Y, Sarkar FH. Notch 1 down regulation by curcumin is associated with the inhibition of cell growth and the induction of apoptosis in pancreatic cancer cells. Cancer. 2006;106(11):2503–2513. doi: 10.1002/cncr.21904. [DOI] [PubMed] [Google Scholar]
- 27.Ravindran J, Prasad S, Aggarwal BB. Curcumin and cancer cells: how many ways can curry kill tumor cells selectively? AAPS J. 2009;11(3):495–510. doi: 10.1208/s12248-009-9128-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Su CC, Chen GWEI, Lin JG, Wu LIITZU, Chung JG. Curcumin inhibits cell migration of human colon cancer colo 205 cells through the inhibition of nuclear factor kappa B/p65 and down-regulates cyclooxygenase-2 and matrix metalloproteinase-2 expressions. Anticancer Res. 2006;26(2A):1281–1288. [PubMed] [Google Scholar]
- 29.Shankar S, Ganapathy S, Chen Q, Srivastava RK. Curcumin sensitizes TRAIL-resistant xenografts: molecular mechanisms of apoptosis, metastasis and angiogenesis. Mol Cancer. 2008;7(1):16. doi: 10.1186/1476-4598-7-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cai XZ, Wang J, Li X, Wang GL, Liu FN, Cheng MS, Li F. Curcumin suppresses proliferation and invasion in human gastric cancer cells by downregulation of PAK1 activity and cyclin D1 expression. Cancer Biol Ther. 2009;8(14):1360–1368. doi: 10.4161/cbt.8.14.8720. [DOI] [PubMed] [Google Scholar]
- 31.Jaiswal AS, Marlow BP, Gupta N, Narayan S. Catenin-mediated transactivation and cell-cell adhesion pathways are important in curcumin (diferuylmethane)-induced growth arrest and apoptosis in colon cancer cells. Oncogene. 2002;21(55):8414–8427. doi: 10.1038/sj.onc.1205947. [DOI] [PubMed] [Google Scholar]
- 32.Gagliano N, Aldini G, Colombo G, Rossi R, Colombo R, Gioia M, Milzani A, Dalle-Donne I. The potential of resveratrol against human gliomas. Anticancer Drugs. 2010;21(2):140–150. doi: 10.1097/CAD.0b013e32833498f1. [DOI] [PubMed] [Google Scholar]
- 33.Lee KW, Lee HJ. The roles of polyphenols in cancer chemoprevention. Biofactors. 2006;26(2):105–121. doi: 10.1002/biof.5520260202. [DOI] [PubMed] [Google Scholar]
- 34.Leonard SS, Xia C, Jiang BH, Stinefelt B, Klandorf H, Harris GK, Shi X. Resveratrol scavenges reactive oxygen species and effects radical-induced cellular responses. Biochem Biophys Res Commun. 2003;309(4):1017–1026. doi: 10.1016/j.bbrc.2003.08.105. [DOI] [PubMed] [Google Scholar]
- 35.Zhu HL. Resveratrol and Its Analogues: Promising Antitumor Agents. Anticancer Agents Med Chem. 2011;11(5):479–490. doi: 10.2174/187152011795677427. [DOI] [PubMed] [Google Scholar]
- 36.Gupta SC, Kannappan R, Reuter S, Kim JH, Aggarwal BB. Chemosensitization of tumors by resveratrol. Ann N Y Acad Sci. 2011;1215(1):150–160. doi: 10.1111/j.1749-6632.2010.05852.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Benitez DA, Pozo-Guisado E, Alvarez-Barrientos A, Fernandez-Salguero PM, Castellon EA. Mechanisms involved in resveratrol-induced apoptosis and cell cycle arrest in prostate cancer-derived cell lines. J Androl. 2007;28(2):282–293. doi: 10.2164/jandrol.106.000968. [DOI] [PubMed] [Google Scholar]
- 38.Fulda S, Debatin KM. Resveratrol-mediated sensitisation to TRAIL-induced apoptosis depends on death receptor and mitochondrial signalling. Eur J Cancer. 2005;41(5):786–798. doi: 10.1016/j.ejca.2004.12.020. [DOI] [PubMed] [Google Scholar]
- 39.Heiss EH, Schilder YDC, Dirsch VM. Chronic treatment with resveratrol induces redox stress-and ataxia telangiectasia-mutated (ATM)-dependent senescence in p53-positive cancer cells. J Biol Chem. 2007;282(37):26759–26766. doi: 10.1074/jbc.M703229200. [DOI] [PubMed] [Google Scholar]
- 40.Subbaramaiah K, Chung WJ, Michaluart P, Telang N, Tanabe T, Inoue H, Jang M, Pezzuto JM, Dannenberg AJ. Resveratrol inhibits cyclooxygenase-2 transcription and activity in phorbol ester-treated human mammary epithelial cells. J Biol Chem. 1998;273(34):21875–21882. doi: 10.1074/jbc.273.34.21875. [DOI] [PubMed] [Google Scholar]
- 41.Cao Y, Fu ZD, Wang F, Liu HY, Han R. Anti-angiogenic activity of resveratrol, a natural compound from medicinal plants. J Asian Nat Prod Res. 2005;7(3):205–213. doi: 10.1080/10286020410001690190. [DOI] [PubMed] [Google Scholar]
- 42.Sun C, Hu Y, Liu X, Wu T, Wang Y, He W, Wei W. Resveratrol downregulates the constitutional activation of nuclear factor-[kappa] B in multiple myeloma cells, leading to suppression of proliferation and invasion, arrest of cell cycle, and induction of apoptosis. Cancer Genet Cytogenet. 2006;165(1):9–19. doi: 10.1016/j.cancergencyto.2005.06.016. [DOI] [PubMed] [Google Scholar]
- 43.Pozo Guisado E, Merino JM, Mulero Navarro S, Lorenzo Benayas MJ, Centeno F, Alvarez Barrientos A, Salguero PMF. Resveratrol induced apoptosis in MCF 7 human breast cancer cells involves a caspase independent mechanism with downregulation of Bcl 2 and NF B. Int J Cancer. 2005;115(1):74–84. doi: 10.1002/ijc.20856. [DOI] [PubMed] [Google Scholar]
- 44.Csaki C, Mobasheri A, Shakibaei M. Synergistic chondro-protective effects of curcumin and resveratrol in human articular chondrocytes: inhibition of IL-1beta-induced NF-kappaB-mediated inflammation and apoptosis. Arthritis Res Ther. 2009;11(6):R165. doi: 10.1186/ar2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Estrov Z, Shishodia S, Faderl S, Harris D, Van Q, Kantarjian HM, Talpaz M, Aggarwal BB. Resveratrol blocks interleukin-1β–induced activation of the nuclear transcription factor NF-κB, inhibits proliferation, causes S-phase arrest, and induces apoptosis of acute myeloid leukemia cells. Blood. 2003;102(3):987–995. doi: 10.1182/blood-2002-11-3550. [DOI] [PubMed] [Google Scholar]
- 46.Yu H, Pan C, Wu W, Zhao S, Zhang H. Effects of resveratrol on matrix metalloproteinase-9 expression in hepatoma cells. Zhong Xi Yi Jie He Xue Bao. 2008;6(3):270–273. doi: 10.3736/jcim20080310. [DOI] [PubMed] [Google Scholar]
- 47.Athar M, Back JH, Kopelovich L, Bickers DR, Kim AL. Multiple molecular targets of resveratrol: Anti-carcinogenic mechanisms. Arch Biochem Biophys. 2009;486(2):95–102. doi: 10.1016/j.abb.2009.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fulda S. Modulation of apoptosis by natural products for cancer therapy. Planta Med. 2010;76(11):1075–1079. doi: 10.1055/s-0030-1249961. [DOI] [PubMed] [Google Scholar]
- 49.Shaw RJ, Cantley LC. Ras, PI (3) K and mTOR signalling controls tumour cell growth. Nature. 2006;441(7092):424–430. doi: 10.1038/nature04869. [DOI] [PubMed] [Google Scholar]
- 50.Zhou H, Luo Y, Huang S. Updates of mTOR inhibitors. Anticancer Agents Med Chem. 2010;10(7):571–581. doi: 10.2174/187152010793498663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim AL, Zhu Y, Zhu H, Han L, Kopelovich L, Bickers DR, Athar M. Resveratrol inhibits proliferation of human epidermoid carcinoma A431 cells by modulating MEK1 and AP 1 signalling pathways. Exp Dermatol. 2006;15(7):538–546. doi: 10.1111/j.1600-0625.2006.00445.x. [DOI] [PubMed] [Google Scholar]
- 52.Gill C, Walsh SE, Morrissey C, Fitzpatrick JM, Watson RWG. Resveratrol sensitizes androgen independent prostate cancer cells to death receptor mediated apoptosis through multiple mechanisms. Prostate. 2007;67(15):1641–1653. doi: 10.1002/pros.20653. [DOI] [PubMed] [Google Scholar]
- 53.Miloso M, Bertelli AAE, Nicolini G, Tredici G. Resveratrol-induced activation of the mitogen-activated protein kinases, ERK1 and ERK2, in human neuroblastoma SH-SY5Y cells. Neurosci Lett. 1999;264(1–3):141–144. doi: 10.1016/s0304-3940(99)00194-9. [DOI] [PubMed] [Google Scholar]
- 54.Kundu JK, Shin YK, Surh YJ. Resveratrol modulates phorbol ester-induced pro-inflammatory signal transduction pathways in mouse skinin vivo: NF-[kappa] B and AP-1 as prime targets. Biochem Pharmacol. 2006;72(11):1506–1515. doi: 10.1016/j.bcp.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 55.Azios NG, Dharmawardhane SF. Resveratrol and estradiol exert disparate effects on cell migration, cell surface actin structures, and focal adhesion assembly in MDA-MB-231 human breast cancer cells. Neoplasia. 2005;7(2):128–140. doi: 10.1593/neo.04346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Patel D, Shukla S, Gupta S. Apigenin and cancer chemoprevention: Progress, potential and promise (Review) Int J Oncol. 2006;30(1):233–245. [PubMed] [Google Scholar]
- 57.Wei H, Tye L, Bresnick E, Birt DF. Inhibitory effect of apigenin, a plant flavonoid, on epidermal ornithine decarboxylase and skin tumor promotion in mice. Cancer Res. 1990;50(3):499–502. [PubMed] [Google Scholar]
- 58.Birt D, Mitchell D, Gold B, Pour P, Pinch H. Inhibition of ultraviolet light induced skin carcinogenesis in SKH-1 mice by apigenin, a plant flavonoid. Anticancer Res. 1997;17(1A):85–91. [PubMed] [Google Scholar]
- 59.Middleton E, Kandaswami C, Theoharides TC. The effects of plant flavonoids on mammalian cells: implications for inflammation, heart disease, and cancer. Pharmacol Rev. 2000;52(4):673–751. [PubMed] [Google Scholar]
- 60.Myhrstad MCW, Carlsen H, Nordström O, Blomhoff R, Moskaug J. Flavonoids increase the intracellular glutathione level by transactivation of the [gamma]-glutamylcysteine synthetase catalytical subunit promoter. Free Radic Biol Med. 2002;32(5):386–393. doi: 10.1016/s0891-5849(01)00812-7. [DOI] [PubMed] [Google Scholar]
- 61.Shukla S, Gupta S. Apigenin: a promising molecule for cancer prevention. Pharm Res. 2010;27(6):962–978. doi: 10.1007/s11095-010-0089-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shukla S, Gupta S. Apigenin-induced cell cycle arrest is mediated by modulation of MAPK, PI3KAkt, and loss of cyclin D1 associated retinoblastoma dephosphorylation in human prostate cancer cells. Cell Cycle. 2007;6:1102–1114. doi: 10.4161/cc.6.9.4146. [DOI] [PubMed] [Google Scholar]
- 63.Liang YC, Huang YT, Tsai SH, Lin-Shiau SY, Chen CF, Lin JK. Suppression of inducible cyclooxygenase and inducible nitric oxide synthase by apigenin and related flavonoids in mouse macrophages. Carcinogenesis. 1999;20(10):1945. doi: 10.1093/carcin/20.10.1945. [DOI] [PubMed] [Google Scholar]
- 64.Ujiki MB, Ding XZ, Salabat MR, Bentrem DJ, Golkar L, Milam B, Talamonti MS, Bell RH, Iwamura T, Adrian TE. Apigenin inhibits pancreatic cancer cell proliferation through G2/M cell cycle arrest. Mol Cancer. 2006;5(1):76. doi: 10.1186/1476-4598-5-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Takagaki N, Sowa Y, Oki T, Nakanishi R, Yogosawa S, Sakai T. Apigenin induces cell cycle arrest and p21/WAF1 expression in a p53-independent pathway. Int J Oncol. 2005;26(1):185–189. [PubMed] [Google Scholar]
- 66.McVean M, Weinberg WC, Pelling JC. A p21waf1 independent pathway for inhibitory phosphorylation of cyclin dependent kinase p34cdc2 and concomitant G2/M arrest by the chemopreventive flavonoid apigenin. Mol Carcinog. 2002;33(1):36–43. doi: 10.1002/mc.10016. [DOI] [PubMed] [Google Scholar]
- 67.Lindenmeyer F, Li H, Menashi S, Soria C, Lu H. Apigenin acts on the tumor cell invasion process and regulates protease production. Nutr Cancer. 2001;39(1):139–147. doi: 10.1207/S15327914nc391_19. [DOI] [PubMed] [Google Scholar]
- 68.Caltagirone S, Rossi C, Poggi A, Ranelletti FO, Natali PG, Brunetti M, Aiello FB, Piantelli M. Flavonoids apigenin and quercetin inhibit melanoma growth and metastatic potential. Int J Cancer. 2000;87(4):595–600. doi: 10.1002/1097-0215(20000815)87:4<595::aid-ijc21>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 69.Wang IK, Lin-Shiau SY, Lin JK. Induction of apoptosis by apigenin and related flavonoids through cytochrome c release and activation of caspase-9 and caspase-3 in leukaemia HL-60 cells. Eur J Cancer. 1999;35(10):1517–1525. [PubMed] [Google Scholar]
- 70.Melstrom LG, Salabat MR, Ding XZ, Strouch MJ, Grippo PJ, Mirzoeva S, Pelling JC, Bentrem DJ. Apigenin Down-Regulates the Hypoxia Response Genes: HIF-1 [alpha], GLUT-1, and VEGF in Human Pancreatic Cancer Cells. J Surg Res. 2010;167(2):173–81. doi: 10.1016/j.jss.2010.10.041. [DOI] [PubMed] [Google Scholar]
- 71.Balasubramanian S, Eckert RL. Keratinocyte proliferation, differentiation, and apoptosis--Differential mechanisms of regulation by curcumin, EGCG and apigenin. Toxicol Appl Pharmacol. 2007;224(3):214–219. doi: 10.1016/j.taap.2007.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chen D, Landis-Piwowar KR, Chen MS, Dou QP. Inhibition of proteasome activity by the dietary flavonoid apigenin is associated with growth inhibition in cultured breast cancer cells and xenografts. Breast Cancer Res. 2007;9(6):R80. doi: 10.1186/bcr1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mounho BJ, Thrall BD. The extracellular signal-regulated kinase pathway contributes to mitogenic and antiapoptotic effects of peroxisome proliferators in vitro. Toxicol Appl Pharmacol. 1999;159(2):125–133. doi: 10.1006/taap.1999.8740. [DOI] [PubMed] [Google Scholar]
- 74.Kelly G. Quercetin monograph. Alternative medicine review: a journal of clinical therapeutic. 2011;16(2):172. [PubMed] [Google Scholar]
- 75.Yang CS, Landau JM, Huang MT, Newmark HL. Inhibition of carcinogenesis by dietary polyphenolic compounds. Annu Rev Nutr. 2001;21(1):381–406. doi: 10.1146/annurev.nutr.21.1.381. [DOI] [PubMed] [Google Scholar]
- 76.Bors W, Heller W, Michel C, Saran M. [36] Flavonoids as antioxidants: Determination of radical-scavenging efficiencies. Methods Enzymol. 1990;186:343–355. doi: 10.1016/0076-6879(90)86128-i. [DOI] [PubMed] [Google Scholar]
- 77.Prior RL. Fruits and vegetables in the prevention of cellular oxidative damage. Am J Clin Nutr. 2003;78(3):570S. doi: 10.1093/ajcn/78.3.570S. [DOI] [PubMed] [Google Scholar]
- 78.Day AJ, Bao Y, Morgan MRA, Williamson G. Conjugation position of quercetin glucuronides and effect on biological activity. Free Radic Biol Med. 2000;29(12):1234–1243. doi: 10.1016/s0891-5849(00)00416-0. [DOI] [PubMed] [Google Scholar]
- 79.Lamson D, Brignall M. Antioxidants and cancer, part 3: quercetin. Alternative medicine review: a journal of clinical therapeutic. 2000;5(3):196. [PubMed] [Google Scholar]
- 80.Ferry DR, Smith A, Malkhandi J, Fyfe DW, deTakats PG, Anderson D, Baker J, Kerr DJ. Phase I clinical trial of the flavonoid quercetin: pharmacokinetics and evidence for in vivo tyrosine kinase inhibition. Clin Cancer Res. 1996;2(4):659. [PubMed] [Google Scholar]
- 81.Manjeet KR, Ghosh B. Quercetin inhibits LPS-induced nitric oxide and tumor necrosis factor-alpha production in murine macrophages. Int J Immunopharmacol. 1999;21(7):435. doi: 10.1016/s0192-0561(99)00024-7. [DOI] [PubMed] [Google Scholar]
- 82.Nair MP, Mahajan S, Reynolds JL, Aalinkeel R, Nair H, Schwartz SA, Kandaswami C. The Flavonoid Quercetin Inhibits Proinflammatory Cytokine (Tumor Necrosis Factor Alpha) Gene Expression in Normal Peripheral Blood Mononuclear Cells via Modulation of the NF-{kappa}{beta} System. Clin Vaccine Immunol. 2006;13(3):319. doi: 10.1128/CVI.13.3.319-328.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhang Q, Zhao XH, Wang ZJ. Flavones and flavonols exert cytotoxic effects on a human oesophageal adenocarcinoma cell line (OE33) by causing G2/M arrest and inducing apoptosis. Food Chem Toxicol. 2008;46(6):2042–2053. doi: 10.1016/j.fct.2008.01.049. [DOI] [PubMed] [Google Scholar]
- 84.Kim WK, Bang MH, Kim ES, Kang NE, Jung KC, Cho HJ, Park JHY. Quercetin decreases the expression of ErbB2 and ErbB3 proteins in HT-29 human colon cancer cells. J Nutr Biochem. 2005;16(3):155–162. doi: 10.1016/j.jnutbio.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 85.Kim HJ, Kim SK, Kim BS, Lee SH, Park YS, Park BK, Kim SJ, Kim J, Choi C, Kim JS. Apoptotic effect of quercetin on HT-29 colon cancer cells via the AMPK signaling pathway. J Agric Food Chem. 2010;58(15):8643–50. doi: 10.1021/jf101510z. [DOI] [PubMed] [Google Scholar]
- 86.Makita H, Tanaka T, Fujitsuka H, Tatematsu N, Satoh K, Hara A, Mori H. Chemoprevention of 4-nitroquinoline 1-oxide-induced rat oral carcinogenesis by the dietary flavonoids chalcone, 2-hydroxychalcone, and quercetin. Cancer Res. 1996;56(21):4904–4909. [PubMed] [Google Scholar]
- 87.De S, Chakraborty J, Chakraborty R, Das S. Chemopreventive activity of quercetin during carcinogenesis in cervix uteri in mice. Phytother Res. 2000;14(5):347–351. doi: 10.1002/1099-1573(200008)14:5<347::aid-ptr613>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 88.De S, Chakraborty R, Ghosh S, Sengupta A, Das S. Comparative evaluation of cancer chemopreventive efficacy of alpha-tocopherol and quercetin in a murine model. J Exp Clin Cancer Res. 2004;23(2):251–258. [PubMed] [Google Scholar]
- 89.Khanduja K, Gandhi R, Pathania V, Syal N. Prevention of N-nitrosodiethylamine-induced lung tumorigenesis by ellagic acid and quercetin in mice. Food Chem Toxicol. 1999;37(4):313–318. doi: 10.1016/s0278-6915(99)00021-6. [DOI] [PubMed] [Google Scholar]
- 90.Yoshida M, Sakai T, Hosokawa N, Marui N, Matsumoto K, Fujioka A, Nishino H, Aoike A. The effect of quercetin on cell cycle progression and growth of human gastric cancer cells. FEBS Lett. 1990;260(1):10–13. doi: 10.1016/0014-5793(90)80053-l. [DOI] [PubMed] [Google Scholar]
- 91.Hofmann J, Fiebig HH, Winterhalter BR, Berger DP, Grunicke H. Enhancement of the antiproliferative activity of cis diamminedichloroplatinum (II) by quercetin. Int J Cancer. 1990;45(3):536–539. doi: 10.1002/ijc.2910450327. [DOI] [PubMed] [Google Scholar]
- 92.van Rijn J, van den Berg J. Flavonoids as enhancers of x-ray-induced cell damage in hepatoma cells. Clin Cancer Res. 1997;3(10):1775–1779. [PubMed] [Google Scholar]
- 93.Cheng S, Gao N, Zhang Z, Chen G, Budhraja A, Ke Z, Son Y, Wang X, Luo J, Shi X. Quercetin Induces Tumor-Selective Apoptosis through Downregulation of Mcl-1 and Activation of Bax. Clin Cancer Res. 2010;16(23):5679–5691. doi: 10.1158/1078-0432.CCR-10-1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Olson ER, Melton T, Dickinson SE, Dong Z, Alberts DS, Bowden GT. Quercetin potentiates UVB-induced c-Fos expression: Implications for its use as a chemopreventive agent. Cancer Prev Res (Phila) 2010;3(7):876–884. doi: 10.1158/1940-6207.CAPR-09-0220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chien SY, Wu YC, Chung JG, Yang JS, Lu HF, Tsou MF, Wood W, Kuo SJ, Chen DR. Quercetin-induced apoptosis acts through mitochondrial-and caspase-3-dependent pathways in human breast cancer MDA-MB-231 cells. Hum Exp Toxicol. 2009;28(8):493–503. doi: 10.1177/0960327109107002. [DOI] [PubMed] [Google Scholar]
- 96.Ranelletti FO, Maggiano N, Serra FG, Ricci R, Larocca LM, Lanza P, Scambia G, Fattorossi A, Capelli A, Piantelli M. Quercetin inhibits p21 RAS expression in human colon cancer cell lines and in primary colorectal tumors. Int J Cancer. 2000;85(3):438–445. [PubMed] [Google Scholar]
- 97.Casagrande F, Darbon JM. Effects of structurally related flavonoids on cell cycle progression of human melanoma cells: regulation of cyclin-dependent kinases CDK2 and CDK11. Biochem Pharmacol. 2001;61(10):1205–1215. doi: 10.1016/s0006-2952(01)00583-4. [DOI] [PubMed] [Google Scholar]
- 98.Spencer JPE, Rice-Evans C, Williams RJ. Modulation of pro-survival Akt/PKB and ERK1/2 signalling cascades by quercetin and its in vivo metabolites underlie their action on neuronal viability. J Biol Chem. 2003;278(37):34783–34793. doi: 10.1074/jbc.M305063200. [DOI] [PubMed] [Google Scholar]
- 99.Robaszkiewicz A, Balcerczyk A, Bartosz G. Antioxidative and prooxidative effects of quercetin on A549 cells. Cell Biol Int. 2007;31(10):1245–1250. doi: 10.1016/j.cellbi.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 100.Murota K, Terao J. Antioxidative flavonoid quercetin: implication of its intestinal absorption and metabolism* 1. Arch Biochem Biophys. 2003;417(1):12–17. doi: 10.1016/s0003-9861(03)00284-4. [DOI] [PubMed] [Google Scholar]
- 101.Murakami A, Ashida H, Terao J. Multitargeted cancer prevention by quercetin. Cancer Lett. 2008;269(2):315–325. doi: 10.1016/j.canlet.2008.03.046. [DOI] [PubMed] [Google Scholar]
- 102.Vijayababu M, Kanagaraj P, Arunkumar A, Ilangovan R, Aruldhas M, Arunakaran J. Quercetin-induced growth inhibition and cell death in prostatic carcinoma cells (PC-3) are associated with increase in p21 and hypophosphorylated retinoblastoma proteins expression. J Cancer Res Clin Oncol. 2005;131(11):765–771. doi: 10.1007/s00432-005-0005-4. [DOI] [PubMed] [Google Scholar]
- 103.Ding M, Zhao J, Bowman L, Lu Y, Shi X. Inhibition of AP-1 and MAPK signaling and activation of Nrf2/ARE pathway by quercitrin. Int J Oncol. 2010;36(1):59–67. [PubMed] [Google Scholar]
- 104.Wang K, Liu R, Li J, Mao J, Lei Y, Wu J, Zeng J, Zhang T, Wu H, Chen L. Quercetin induces protective autophagy in gastric cancer cells: Involvement of Akt-mTOR-and hypoxia-induced factor 1 -mediated signaling. Autophagy. 2011;7(9):966–978. doi: 10.4161/auto.7.9.15863. [DOI] [PubMed] [Google Scholar]
- 105.Hansen R, Oesterreich S, Lemieux P, Sarge K, Fuqua S. Quercetin inhibits heat shock protein induction but not heat shock factor DNA-binding in human breast carcinoma cells. Biochem Biophys Res Commun. 1997;239(3):851–856. doi: 10.1006/bbrc.1997.7572. [DOI] [PubMed] [Google Scholar]
- 106.Elia G, Amici C, Rossi A, Santoro MG. Modulation of prostaglandin A1-induced thermotolerance by quercetin in human leukemic cells: role of heat shock protein 70. Cancer Res. 1996;56(1):210–217. [PubMed] [Google Scholar]
- 107.Koishi M, Hosokawa N, Sato M, Nakai A, Hirayoshi K, Hiraoka M, Abe M, Nagata K. Quercetin, an inhibitor of heat shock protein synthesis, inhibits the acquisition of thermotolerance in a human colon carcinoma cell line. Jpn J Cancer Res. 1992;83(11):1216–1222. doi: 10.1111/j.1349-7006.1992.tb02748.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Singh B, Mense SM, Bhat NK, Putty S, Guthiel WA, Remotti F, Bhat HK. Dietary quercetin exacerbates the development of estrogen-induced breast tumors in female ACI rats. Toxicol Appl Pharmacol. 2010;247(2):83–90. doi: 10.1016/j.taap.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zhu B, Liehr J. Quercetin increases the severity of estradiol-induced tumorigenesis in hamster kidney. Toxicol Appl Pharmacol. 1994;125(1):149–158. doi: 10.1006/taap.1994.1059. [DOI] [PubMed] [Google Scholar]
- 110.Tachibana H. Green tea polyphenol sensing. Proc Jpn Acad Ser B Phys Biol Sci. 2011;87(3):66–80. doi: 10.2183/pjab.87.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Higdon JV, Frei B. Tea catechins and polyphenols: health effects, metabolism, and antioxidant functions. Crit Rev Food Sci Nutr. 2003;43(1):89–143. doi: 10.1080/10408690390826464. [DOI] [PubMed] [Google Scholar]
- 112.Shimizu M, Deguchi A, Lim JTE, Moriwaki H, Kopelovich L, Weinstein IB. (−)-Epigallocatechin Gallate and Polyphenon E Inhibit Growth and Activation of the Epidermal Growth Factor Receptor and Human Epidermal Growth Factor Receptor-2 Signaling Pathways in Human Colon Cancer Cells. Clin Cancer Res. 2005;11(7):2735–2746. doi: 10.1158/1078-0432.CCR-04-2014. [DOI] [PubMed] [Google Scholar]
- 113.Bose M, Hao X, Ju J, Husain A, Park S, Lambert JD, Yang CS. Inhibition Of Tumorigenesis in Apc Min/+ Mice by a Combination of (−)-Epigallocatechin-3-gallate and Fish Oil. J Agri Food Chem. 2007;55(19):7695–7700. doi: 10.1021/jf071004r. [DOI] [PubMed] [Google Scholar]
- 114.Lu YP, Lou YR, Liao J, Xie JG, Peng QY, Yang CS, Conney AH. Administration of green tea or caffeine enhances the disappearance of UVB-induced patches of mutant p53 positive epidermal cells in SKH-1 mice. Carcinogenesis. 2005;26(8):1465–1472. doi: 10.1093/carcin/bgi086. [DOI] [PubMed] [Google Scholar]
- 115.Garbisa S, Biggin S, Cavallarin N, Sartor L, Benelli R, Albini A. Tumor invasion: molecular shears blunted by green tea. Nat Med. 1999;5(11):1216–1216. doi: 10.1038/15145. [DOI] [PubMed] [Google Scholar]
- 116.Garbisa S, Sartor L, Biggin S, Salvato B, Benelli R. Tumor gelatinases and invasion inhibited by the green tea flavanol epigallocatechin 3 gallate. Cancer. 2001;91(4):822–832. doi: 10.1002/1097-0142(20010215)91:4<822::aid-cncr1070>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 117.Annabi B, Lachambre MP, Bousquet-Gagnon N, Pagé M, Gingras D, Béliveau R. Green tea polyphenol (−)-epigallocatechin 3-gallate inhibits MMP-2 secretion and MT1-MMP-driven migration in glioblastoma cells. Biochim Biophys Acta. 2002;1542(1–3):209–220. doi: 10.1016/s0167-4889(01)00187-2. [DOI] [PubMed] [Google Scholar]
- 118.Stearns ME, Wang M. Synergistic Effects of the Green Tea Extract Epigallocatechin-3-gallate and Taxane in Eradication of Malignant Human Prostate Tumors. Transl Oncol. 2011;4(3):147–156. doi: 10.1593/tlo.10286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zhu BH, Chen HY, Zhan WH, Wang CY, Cai SR, Wang Z, Zhang CH, He YL. (−)-Epigallocatechin-3-gallate inhibits VEGF expression induced by IL-6 via Stat3 in gastric cancer. World J Gastroenterol. 2011;17(18):2315–2325. doi: 10.3748/wjg.v17.i18.2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ju J, Hong J, Zhou J, Pan Z, Bose M, Liao J, Yang G, Liu YY, Hou Z, Lin Y. Inhibition of intestinal tumorigenesis in Apcmin/+ mice by (−)-epigallocatechin-3-gallate, the major catechin in green tea. Cancer Res. 2005;65(22):10623–10631. doi: 10.1158/0008-5472.CAN-05-1949. [DOI] [PubMed] [Google Scholar]
- 121.Shimizu M, Adachi S, Masuda M, Kozawa O, Moriwaki H. Cancer chemoprevention with green tea catechins by targeting receptor tyrosine kinases. Mol Nutr Food Res. 2011;55(6):832–43. doi: 10.1002/mnfr.201000622. [DOI] [PubMed] [Google Scholar]
- 122.Onoda C, Kuribayashi K, Nirasawa S, Tsuji N, Tanaka M, Kobayashi D, Watanabe N. Epigallocatechin-3-gallate induces apoptosis in gastric cancer cell lines by down-regulating survivin expression. Int J Oncol. 2011;38(5):1403–8. doi: 10.3892/ijo.2011.951. [DOI] [PubMed] [Google Scholar]
- 123.Tudoran O, Soritau O, Balacescu O, Balacescu L, Braicu C, Rus M, Gherman C, Virag P, Irimie F, Neagoe IB. Early transcriptional pattern of angiogenesis induced by EGCG treatment in cervical tumor cells. J Cell Mol Med. 2011;10:1582–4934. doi: 10.1111/j.1582-4934.2011.01346.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Vu H, Beppu Y, Chi H, Sasaki K, Yamamoto H, Xinh P, Tanii T, Hara Y, Watanabe T, Sato Y, Ohdomari I. Green tea epigalocatechin gallate exhibits anticancer effect in human pancreatic carcinoma cells via inhibition of both FAK and IGF-1R. J Biomed Biotechnol. 2010;2010:290516. doi: 10.1155/2010/290516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Van Aller GS, Carson JD, Tang W, Peng H, Zhao L, Copeland RA, Tummino PJ, Luo L. Epigallocatechin gallate (EGCG): a major component of green tea, is a dual phosphoinositide-3-kinase/mTOR inhibitor. Biochem Biophys Res Commun. 2011;406(2):194–199. doi: 10.1016/j.bbrc.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 126.Xu Q, Yang CH, Liu Q, Jin XF, Xu XT, Tong JL, Xiao SD, Ran ZH. Chemopreventive effect of epigallocatechin-3-gallate (EGCG) and folic acid on the N-methyl-N′-nitro-N-nitrosoguanidine (MNNG)-induced gastrointestinal cancer in rat model. J Dig Dis. 2011;12(3):181–187. doi: 10.1111/j.1751-2980.2011.00494.x. [DOI] [PubMed] [Google Scholar]
- 127.Dong Z, Ma W, Huang C, Yang CS. Inhibition of tumor promoter-induced activator protein 1 activation and cell transformation by tea polyphenols,(−)-epigallocatechin gallate, and theaflavins. Cancer Res. 1997;57(19):4414–4419. [PubMed] [Google Scholar]
- 128.Koh YW, Choi EC, Kang SU, Hwang HS, Lee MH, Pyun JH, Park RH, Lee YD, Kim CH. Green tea (−)-epigallocatechin-3-gallate inhibits HGF-induced progression in oral cavity cancer through suppression of HGF/c-Met. J Nutr Biochem. 2011;22(11):1074–83. doi: 10.1016/j.jnutbio.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 129.Tanaka T, Ishii T, Mizuno D, Mori T, Yamaji R, Nakamura Y, Kumazawa S, Nakayama T, Akagawa M. (−)-Epigallocatechin-3-gallate suppresses growth of AZ521 human gastric cancer cells by targeting the DEAD box RNA helicase p68. Free Radic Biol Med. 2011;50(10):1324–35. doi: 10.1016/j.freeradbiomed.2011.01.024. [DOI] [PubMed] [Google Scholar]
- 130.Lee MH, Han DW, Hyon SH, Park JC. Apoptosis of human fibrosarcoma HT-1080 cells by epigallocatechin-3-O-gallate via induction of p53 and caspases as well as suppression of Bcl-2 and phosphorylated nuclear factor- B. Apoptosis. 2011;16(1):75–85. doi: 10.1007/s10495-010-0548-y. [DOI] [PubMed] [Google Scholar]
- 131.Shankar S, Ganapathy S, Hingorani S, Srivastava R. EGCG inhibits growth, invasion, angiogenesis and metastasis of pancreatic cancer. Front Biosci. 2008;13:440–442. doi: 10.2741/2691. [DOI] [PubMed] [Google Scholar]
- 132.Cavell BE, Alwi SSS, Donlevy A, Packham G. Anti-angiogenic effects of dietary isothiocyanates; mechanisms of action and implications for human health. Biochem Pharmacol. 2010;81(3):327–36. doi: 10.1016/j.bcp.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 133.Lam TK, Gallicchio L, Lindsley K, Shiels M, Hammond E, Tao XG, Chen L, Robinson KA, Caulfield LE, Herman JG. Cruciferous vegetable consumption and lung cancer risk: a systematic review. Cancer Epidemiol Biomarkers Prev. 2009;18(1):184–195. doi: 10.1158/1055-9965.EPI-08-0710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Mi L, Di Pasqua AJ, Chung FL. Proteins as Binding Targets of Isothiocyanates in Cancer Prevention. Carcinogenesis. 2011;32(10):1405–13. doi: 10.1093/carcin/bgr111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Nakamura Y. Chemoprevention by isothiocyanates: molecular basis of apoptosis induction. Forum Nutr. 2009;61:170–81. doi: 10.1159/000212749. [DOI] [PubMed] [Google Scholar]
- 136.Wu X, Zhou Q, Xu K. Are isothiocyanates potential anti-cancer drugs? Acta Pharmacol Sin. 2009;30(5):501–512. doi: 10.1038/aps.2009.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Gong A, He M, Krishna Vanaja D, Yin P, Karnes RJ, Young CYF. Phenethyl isothiocyanate inhibits STAT3 activation in prostate cancer cells. Mol Nutr Food Res. 2009;53(7):878–886. doi: 10.1002/mnfr.200800253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kang L, Ding L, Wang ZY. Isothiocyanates repress estrogen receptor expression in breast cancer cells. Oncol Rep. 2009;21(1):185–192. [PMC free article] [PubMed] [Google Scholar]
- 139.Mi L, Gan N, Cheema A, Dakshanamurthy S, Wang X, Yang DCH, Chung FL. Cancer preventive isothiocyanates induce selective degradation of cellular -and -tubulins by proteasomes. J Biol Chem. 2009;284(25):17039–17051. doi: 10.1074/jbc.M901789200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Mukherjee S, Dey S, Bhattacharya R, Roy M. Isothiocyanates sensitize the effect of chemotherapeutic drugs via modulation of protein kinase C and telomerase in cervical cancer cells. Mol Cell Biochem. 2009;330(1):9–22. doi: 10.1007/s11010-009-0095-4. [DOI] [PubMed] [Google Scholar]
- 141.Xu K, Thornalley P. Signal transduction activated by the cancer chemopreventive isothiocyanates: cleavage of BID protein, tyrosine phosphorylation and activation of JNK. Br J Cancer. 2001;84(5):670–673. doi: 10.1054/bjoc.2000.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Prawan A, Saw CLL, Khor TO, Keum YS, Yu S, Hu L, Kong AN. Anti-NF-[kappa] B and anti-inflammatory activities of synthetic isothiocyanates: Effect of chemical structures and cellular signaling. Chem Biol Interact. 2009;179(2–3):202–211. doi: 10.1016/j.cbi.2008.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Jakubikova J, Bao Y, Sedlak J. Isothiocyanates induce cell cycle arrest, apoptosis and mitochondrial potential depolarization in HL-60 and multidrug-resistant cell lines. Anticancer Res. 2005;25(5):3375. [PubMed] [Google Scholar]
- 144.Cheung KL, Kong AN. Molecular targets of dietary phenethyl isothiocyanate and sulforaphane for cancer chemoprevention. AAPS J. 2010;12(1):87–97. doi: 10.1208/s12248-009-9162-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Powolny AA, Bommareddy A, Hahm ER, Normolle DP, Beumer JH, Nelson JB, Singh SV. Chemopreventative potential of the cruciferous vegetable constituent phenethyl isothiocyanate in a mouse model of prostate cancer. J Natl Cancer Inst. 2011;103(7):571–584. doi: 10.1093/jnci/djr029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Huong L, Shim J, Choi K, Shin J, Choi E, Kim H, Lee S, Kim S, Cho N, Cho S. Effect of -phenylethyl isothiocyanate from Cruciferous Vegetables on Growth Inhibition and Apoptosis of Cervical cancer cells through the Induction of Death Receptor 4 and 5. J Agric Food Chem. 2011;59(15):8124–31. doi: 10.1021/jf2006358. [DOI] [PubMed] [Google Scholar]
- 147.Tang NOUY, Huang YAT, Yu CSHU, Ko YC, Wu SH, Ji BINC, Yang JAIS, Yang JL, Hsia TEC, Chen YAYIN. Phenethyl Isothiocyanate (PEITC) Promotes G2/M Phase Arrest via p53 Expression and Induces Apoptosis through Caspase-and Mitochondria-dependent Signaling Pathways in Human Prostate Cancer DU 145 Cells. Anticancer Res. 2011;31(5):1691–1702. [PubMed] [Google Scholar]
- 148.Wu CL, Huang AC, Yang JS, Liao CL, Lu HF, Chou ST, Ma CY, Hsia TC, Ko YC, Chung JG. Benzyl isothiocyanate (BITC) and phenethyl isothiocyanate (PEITC)-mediated generation of reactive oxygen species causes cell cycle arrest and induces apoptosis via activation of caspase-3, mitochondria dysfunction and nitric oxide (NO) in human osteogenic sarcoma U-2 OS cells. J Orthop Res. 2011;29(8):1199–1209. doi: 10.1002/jor.21350. [DOI] [PubMed] [Google Scholar]
- 149.Hwang ES, Lee HJ. Benzyl isothiocyanate inhibits metalloproteinase-2/-9 expression by suppressing the mitogen-activated protein kinase in SK-Hep1 human hepatoma cells. Food Chem Toxicol. 2008;46(7):2358–2364. doi: 10.1016/j.fct.2008.03.016. [DOI] [PubMed] [Google Scholar]
- 150.Lai KC, Huang AC, Hsu SC, Kuo CL, Yang JS, Wu SH, Chung JG. Benzyl isothiocyanate (BITC) inhibits migration and invasion of human colon cancer HT29 cells by inhibiting matrix metalloproteinase-2/-9 and urokinase plasminogen (uPA) through PKC and MAPK signaling pathway. J Agric Food Chem. 2010;58(5):2935–2942. doi: 10.1021/jf9036694. [DOI] [PubMed] [Google Scholar]
- 151.Hunakova L, Sedlakova O, Cholujova D, Gronesova P, Duraj J, Sedlak J, Altanerova V, Horvathova E, Matuskova M, Kucerova L. Modulation of markers associated with aggressive phenotype in MDA-MB-231 breast carcinoma cells by sulforaphane. Neoplasma. 2009;56(5):435–440. doi: 10.4149/neo_2009_06_548. [DOI] [PubMed] [Google Scholar]
- 152.Yan H, Zhu Y, Liu B, Wu H, Li Y, Wu X, Zhou Q, Xu K. Mitogen-activated protein kinase mediates the apoptosis of highly metastatic human non-small cell lung cancer cells induced by isothiocyanates. Br J Nutr. 2011;1(1):1–13. doi: 10.1017/S0007114511002315. [DOI] [PubMed] [Google Scholar]
- 153.Yang MEIDUE, Lai KCHI, Lai TY, Hsu SHUC, Kuo CLIN, Yu CSHU, Lin ML, Yang JAIS, Kuo H, Wu SH. Phenethyl Isothiocyanate Inhibits Migration and Invasion of Human Gastric Cancer AGS Cells through Suppressing MAPK and NF-κB Signal Pathways. Anticancer Res. 2010;30(6):2135–2143. [PubMed] [Google Scholar]
- 154.Chen NG, Chen KT, Lu CC, Lan YH, Lai CH, Chung YT, Yang JS, Lin YC. Allyl isothiocyanate triggers G2/M phase arrest and apoptosis in human brain malignant glioma GBM 8401 cells through a mitochondria-dependent pathway. Oncol Rep. 2010;24(2):449–455. doi: 10.3892/or_00000878. [DOI] [PubMed] [Google Scholar]
- 155.Shan Y, Zhao R, Geng W, Lin N, Wang X, Du X, Wang S. Protective effect of sulforaphane on human vascular endothelial cells against lipopolysaccharide-induced inflammatory damage. Cardiovasc Toxicol. 2010;10(2):139–145. doi: 10.1007/s12012-010-9072-0. [DOI] [PubMed] [Google Scholar]
- 156.Hahm ER, Singh SV. Sulforaphane Inhibits Constitutive and Interleukin-6–Induced Activation of Signal Transducer and Activator of Transcription 3 in Prostate Cancer Cells. Cancer Prev Res (Phila) 2010;3(4):484–494. doi: 10.1158/1940-6207.CAPR-09-0250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Ding Y, Paonessa JD, Randall KL, Argoti D, Chen L, Vouros P, Zhang Y. Sulforaphane inhibits 4-aminobiphenyl-induced DNA damage in bladder cells and tissues. Carcinogenesis. 2010;31(11):1999–2003. doi: 10.1093/carcin/bgq183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Hamsa TP, Thejass P, Kuttan G. Induction of apoptosis by sulforaphane in highly metastatic B16F-10 melanoma cells. Drug Chem Toxicol. 2011;34(3):332–340. doi: 10.3109/01480545.2010.538694. [DOI] [PubMed] [Google Scholar]
- 159.Zeng H, Trujillo O, Moyer M, Botnen J. Prolonged Sulforaphane Treatment Activates Survival Signaling in Nontumorigenic NCM460 Colon Cells but Apoptotic Signaling in Tumorigenic HCT116 Colon Cells. Nutr Cancer. 2011;63(2):248–255. doi: 10.1080/01635581.2011.523500. [DOI] [PubMed] [Google Scholar]
- 160.Park SY, Kim GIY, Bae SJ, Yoo YH, Choi YH. Induction of apoptosis by isothiocyanate sulforaphane in human cervical carcinoma HeLa and hepatocarcinoma HepG2 cells through activation of caspase-3. Oncol Rep. 2007;18(1):181–187. [PubMed] [Google Scholar]
- 161.Fotsis T, Pepper M, Adlercreutz H, Hase T, Montesano R, Schweigerer L. Genistein, a dietary ingested isoflavonoid, inhibits cell proliferation and in vitro angiogenesis. J Nutr. 1995;125(3 Suppl):790S–797S. doi: 10.1093/jn/125.suppl_3.790S. [DOI] [PubMed] [Google Scholar]
- 162.Li W, Frame LT, Hirsch S, Cobos E. Genistein and hematological malignancies. Cancer lett. 2010;296(1):1–8. doi: 10.1016/j.canlet.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 163.Barnes S. Effect of genistein on in vitro and in vivo models of cancer. J Nutr. 1995;125(3 Suppl):777S. doi: 10.1093/jn/125.3_Suppl.777S. [DOI] [PubMed] [Google Scholar]
- 164.Lamartiniere CA, Cotroneo MS, Fritz WA, Wang J, Mentor-Marcel R, Elgavish A. Genistein chemoprevention: timing and mechanisms of action in murine mammary and prostate. J Nutr. 2002;132(3):552S. doi: 10.1093/jn/132.3.552S. [DOI] [PubMed] [Google Scholar]
- 165.Qi W, Weber CR, Wasland K, Savkovic SD. Genistein inhibits proliferation of colon cancer cells by attenuating a negative effect of epidermal growth factor on tumor suppressor FOXO3 activity. BMC Cancer. 2011;11:219. doi: 10.1186/1471-2407-11-219. Epub 2011/06/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Fan Y, Li G, Wang Y, Ren Q, Shi H. Effects of genistein on colon cancer cells in vitro and in vivo and its mechanism of action. Zhonghua zhong liu za zhi [Chinese Journal of Oncology] 2010;32(1):4. [PubMed] [Google Scholar]
- 167.Li M, Zhang Z, Hill DL, Chen X, Wang H, Zhang R. Genistein, a dietary isoflavone, down-regulates the MDM2 oncogene at both transcriptional and posttranslational levels. Cancer Res. 2005;65(18):8200. doi: 10.1158/0008-5472.CAN-05-1302. [DOI] [PubMed] [Google Scholar]
- 168.Magee PJ, Rowland IR. Phyto-oestrogens, their mechanism of action: current evidence for a role in breast and prostate cancer. Br J Nutr. 2004;91(4):513–532. doi: 10.1079/BJN20031075. [DOI] [PubMed] [Google Scholar]
- 169.Fan S, Meng Q, Auborn K, Carter T, Rosen E. BRCA1 and BRCA2 as molecular targets for phytochemicals indole-3-carbinol and genistein in breast and prostate cancer cells. Br J Cancer. 2006;94(3):407–426. doi: 10.1038/sj.bjc.6602935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Vissac-Sabatier C, Coxam V, Déchelotte P, Picherit C, Horcajada MN, Davicco MJ, Lebecque P, Bignon YJ, Bernard-Gallon D. Phytoestrogen-rich diets modulate expression of Brca1 and Brca2 tumor suppressor genes in mammary glands of female Wistar rats. Cancer Res. 2003;63(20):6607. [PubMed] [Google Scholar]
- 171.El-Rayes BF, Ali S, Ali IF, Philip PA, Abbruzzese J, Sarkar FH. Potentiation of the effect of erlotinib by genistein in pancreatic cancer: the role of Akt and nuclear factor- B. Cancer Res. 2006;66(21):10553. doi: 10.1158/0008-5472.CAN-06-2333. [DOI] [PubMed] [Google Scholar]
- 172.Zhang Y, Chen H. Genistein attenuates WNT signaling by up-regulating sFRP2 in a human colon cancer cell line. Exp Biol Med. 2011;236(6):714. doi: 10.1258/ebm.2011.010347. [DOI] [PubMed] [Google Scholar]
- 173.Blanco A, Habib A, Levy-Toledano S, Maclouf J. Involvement of tyrosine kinases in the induction of cyclo-oxygenase-2 in human endothelial cells. Biochem J. 1995;312(Pt 2):419. doi: 10.1042/bj3120419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Lin CH, Wu CH, Thum WY, Ho YS, Lee HM. Involvement of p38 mitogen-activated protein kinase in PLL-AGE-induced cyclooxgenase-2 expression. Eur J Pharmacol. 2002;438(3):143–152. doi: 10.1016/s0014-2999(02)01285-2. [DOI] [PubMed] [Google Scholar]
- 175.Luo SF, Wang CC, Chien CS, Hsiao LD, Yang CM. Induction of cyclooxygenase-2 by lipopolysaccharide in canine tracheal smooth muscle cells: involvement of p42/p44 and p38 mitogen-activated protein kinases and nuclear factor-[kappa] B pathways. Cellular signalling. 2003;15(5):497–509. doi: 10.1016/s0898-6568(02)00135-3. [DOI] [PubMed] [Google Scholar]
- 176.Swami S, Krishnan AV, Moreno J, Bhattacharyya RS, Gardner C, Brooks JD, Peehl DM, Feldman D. Inhibition of prostaglandin synthesis and actions by genistein in human prostate cancer cells and by soy isoflavones in prostate cancer patients. Int J Cancer. 2009;124(9):2050–2059. doi: 10.1002/ijc.24161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Hillman GG, Wang Y, Kucuk O, Che M, Doerge DR, Yudelev M, Joiner MC, Marples B, Forman JD, Sarkar FH. Genistein potentiates inhibition of tumor growth by radiation in a prostate cancer orthotopic model. Mol Cancer Ther. 2004;3(10):1271. [PubMed] [Google Scholar]
- 178.Raffoul JJ, Banerjee S, Singh-Gupta V, Knoll ZE, Fite A, Zhang H, Abrams J, Sarkar FH, Hillman GG. Down-regulation of apurinic/apyrimidinic endonuclease 1/redox factor-1 expression by soy isoflavones enhances prostate cancer radiotherapy in vitro and in vivo. Cancer Res. 2007;67(5):2141. doi: 10.1158/0008-5472.CAN-06-2147. [DOI] [PubMed] [Google Scholar]
- 179.Raffoul J, Wang Y, Kucuk O, Forman J, Sarkar F, Hillman G. Genistein inhibits radiation-induced activation of NF-κB in prostate cancer cells promoting apoptosis and G2/M cell cycle arrest. BMC Cancer. 2006;6(1):107. doi: 10.1186/1471-2407-6-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Yu X, Zhu J, Mi M, Chen W, Pan Q, Wei M. Anti-angiogenic genistein inhibits VEGF-induced endothelial cell activation by decreasing PTK activity and MAPK activation. Med Oncol. 2010:1–9. doi: 10.1007/s12032-010-9770-2. [DOI] [PubMed] [Google Scholar]
- 181.Yatagai C, Singu T, Maruyama M, Sumi H. Genistein and Its Analogue Enhanced Tissue Plasminogen Activator Activity in HeLa S3. Pathophysiol Haemost Thromb. 2007;36(6):298–304. doi: 10.1159/000296280. [DOI] [PubMed] [Google Scholar]
- 182.Gadgeel SM, Ali S, Philip PA, Wozniak A, Sarkar FH. Genistein enhances the effect of epidermal growth factor receptor tyrosine kinase inhibitors and inhibits nuclear factor kappa B in nonsmall cell lung cancer cell lines. Cancer. 2009;115(10):2165–2176. doi: 10.1002/cncr.24250. [DOI] [PubMed] [Google Scholar]
- 183.Valsecchi AE, Franchi S, Panerai AE, Rossi A, Sacerdote P, Colleoni M. The soy isoflavone genistein reverses oxidative and inflammatory state, neuropathic pain, neurotrophic and vasculature deficits in diabetes mouse model. Eur J Pharmacol. 2010 doi: 10.1016/j.ejphar.2010.10.060. [DOI] [PubMed] [Google Scholar]
- 184.Lee YK, Park OJ. Soybean isoflavone genistein regulates apoptosis through NF-κB dependent and independent pathways. Exp Toxicol Pathol. 2011 doi: 10.1016/j.etp.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 185.Singh Gupta V, Zhang H, Banerjee S, Kong D, Raffoul JJ, Sarkar FH, Hillman GG. Radiation induced HIF 1 cell survival pathway is inhibited by soy isoflavones in prostate cancer cells. Int J Cancer. 2009;124(7):1675–1684. doi: 10.1002/ijc.24015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Wang YJ, Lu J, Wu D, Zheng Z, Zheng YL, Wang X, Ruan J, Sun X, Shan Q, Zhang Z. Ursolic acid attenuates lipopolysaccharide-induced cognitive deficits in mouse brain through suppressing p38/NF-[kappa] B mediated inflammatory pathways. Neurobiol Learn Mem. 2011;96(2):156–65. doi: 10.1016/j.nlm.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 187.Mizushina Y, Iida A, Ohta K, Sugawara F, Sakaguchi K. Novel triterpenoids inhibit both DNA polymerase and DNA topoisomerase. Biochem J. 2000;3:757–63. [PMC free article] [PubMed] [Google Scholar]
- 188.Gayathri R, Priya D, Gunassekaran G, Sakthisekaran D. Ursolic acid attenuates oxidative stress-mediated hepatocellular carcinoma induction by diethylnitrosamine in male Wistar rats. Asian Pac J Cancer Prev. 2009;10(5):933–938. [PubMed] [Google Scholar]
- 189.Subbaramaiah K, Michaluart P, Sporn MB, Dannenberg AJ. Ursolic acid inhibits cyclooxygenase-2 transcription in human mammary epithelial cells. Cancer Res. 2000;60(9):2399–2404. [PubMed] [Google Scholar]
- 190.Shanmugam MK, Rajendran P, Li F, Nema T, Vali S, Abbasi T, Kapoor S, Sharma A, Kumar AP, Ho PC, Hui KM, Sethi G. Ursolic acid inhibits multiple cell survival pathways leading to suppression of growth of prostate cancer xenograft in nude mice. J Mol Med (Berl) 2011;89(7):713–727. doi: 10.1007/s00109-011-0746-2. [DOI] [PubMed] [Google Scholar]
- 191.Harmand PO, Duval R, Liagre B, Jayat-Vignoles C, Beneytout JL, Delage C, Simon A. Ursolic acid induces apoptosis through caspase-3 activation and cell cycle arrest in HaCat cells. Int J Oncol. 2003;23(1):105–112. [PubMed] [Google Scholar]
- 192.Wang J, Ren T, Xi T. Ursolic acid induces apoptosis by suppressing the expression of FoxM1 in MCF-7 human breast cancer cells. Med Oncol. 2010:1–6. doi: 10.1007/s12032-010-9777-8. [DOI] [PubMed] [Google Scholar]
- 193.Prasad S, Yadav VR, Kannappan R, Aggarwal BB. Ursolic Acid, a Pentacyclin Triterpene, Potentiates TRAIL-induced Apoptosis through p53-independent Up-regulation of Death Receptors. J Biol Chem. 2011;286(7):5546–5557. doi: 10.1074/jbc.M110.183699. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 194.Manu K, Kuttan G. Ursolic acid induces apoptosis by activating p53 and caspase-3 gene expressions and suppressing NF-[kappa] B mediated activation of bcl-2 in B16F-10 melanoma cells. Int Immunopharmacol. 2008;8(7):974–981. doi: 10.1016/j.intimp.2008.02.013. [DOI] [PubMed] [Google Scholar]
- 195.Yu LB, Wang J, Ma BZ, Sun WZ. Inhibitive effect of ursolic acid on the invasion and metastasis of ovarian carcinoma cells HO-8910PM. Sichuan Da Xue Xue Bao Yi Xue Ban. 2010;41(6):986–988. 1038. [PubMed] [Google Scholar]
- 196.Shanmugam MK, Manu KA, Ong TH, Ramachandran L, Surana R, Bist P, Lim LHK, Kumar AP, Hui KM, Sethi G. Inhibition of CXCR4/CXCL12 signaling axis by ursolic acid leads to suppression of metastasis in transgenic adenocarcinoma of mouse prostate model. Int J Cancer. 2011;129(7):1552–63. doi: 10.1002/ijc.26120. [DOI] [PubMed] [Google Scholar]
- 197.Zhang Y, Kong C, Zeng Y, Wang L, Li Z, Wang H, Xu C, Sun Y. Ursolic acid induces PC 3 cell apoptosis via activation of JNK and inhibition of Akt pathways in vitro. Mol Carcinog. 2010;49(4):374–385. doi: 10.1002/mc.20610. [DOI] [PubMed] [Google Scholar]
- 198.Yeh CT, Wu CH, Yen GC. Ursolic acid, a naturally occurring triterpenoid, suppresses migration and invasion of human breast cancer cells by modulating c Jun N terminal kinase, Akt and mammalian target of rapamycin signaling. Mol Nutr Food Res. 2010;54(9):1285–1295. doi: 10.1002/mnfr.200900414. [DOI] [PubMed] [Google Scholar]
- 199.Kanjoormana M, Kuttan G. Antiangiogenic activity of ursolic acid. Integr Cancer Ther. 2010;9(2):224–235. doi: 10.1177/1534735410367647. [DOI] [PubMed] [Google Scholar]
- 200.Cárdenas C, Quesada AR, Medina MÁ. Effects of ursolic acid on different steps of the angiogenic process. Biochem Biophys Res Commun. 2004;320(2):402–408. doi: 10.1016/j.bbrc.2004.05.183. [DOI] [PubMed] [Google Scholar]
- 201.Raphael T, Kuttan G. Effect of naturally occurring triterpenoids ursolic acid and glycyrrhizic acid on the cell-mediated immune responses of metastatic tumor-bearing animals. Immunopharmacol Immunotoxicol. 2008;30(2):243–255. doi: 10.1080/08923970701675044. [DOI] [PubMed] [Google Scholar]
- 202.Rahman MA, Amin AR, Shin DM. Chemopreventive potential of natural compounds in head and neck cancer. Nutr Cancer. 2010;62(7):973–987. doi: 10.1080/01635581.2010.509538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Krinsky NI. The Antioxidant and Biological Properties of the Carotenoidsa. Ann N Y Acad Sci. 1998;854(1):443–447. doi: 10.1111/j.1749-6632.1998.tb09923.x. [DOI] [PubMed] [Google Scholar]
- 204.Obermüller-Jevic UC, Olano-Martin E, Corbacho AM, Eiserich JP, van der Vliet A, Valacchi G, Cross CE, Packer L. Lycopene inhibits the growth of normal human prostate epithelial cells in vitro. J Nutr. 2003;133(11):3356–3360. doi: 10.1093/jn/133.11.3356. [DOI] [PubMed] [Google Scholar]
- 205.Levy J, Bosin E, Feldman B, Giat Y, Miinster A, Danilenko M, Sharoni Y. Lycopene is a more potent inhibitor of human cancer cell proliferation than either alpha-carotene or beta-carotene. Nutr Cancer. 1995;24(3):257–266. doi: 10.1080/01635589509514415. [DOI] [PubMed] [Google Scholar]
- 206.Salman H, Bergman M, Djaldetti M, Bessler H. Lycopene affects proliferation and apoptosis of four malignant cell lines. Biomed Pharmacother. 2007;61(6):366–369. doi: 10.1016/j.biopha.2007.02.015. [DOI] [PubMed] [Google Scholar]
- 207.Amir H, Karas M, Giat J, Danilenko M, Levy R, Yermiahu T, Levy J, Sharoni Y. Lycopene and 1, 25-dihydroxyvitamin D3 cooperate in the inhibition of cell cycle progression and induction of differentiation in HL-60 leukemic cells. Nutr Cancer. 1999;33(1):105–112. doi: 10.1080/01635589909514756. [DOI] [PubMed] [Google Scholar]
- 208.Hwang ES, Lee HJ. Inhibitory effects of lycopene on the adhesion, invasion, and migration of SK-Hep1 human hepatoma cells. Exp Biol Med. 2006;231(3):322–327. doi: 10.1177/153537020623100313. [DOI] [PubMed] [Google Scholar]
- 209.Nahum A, Hirsch K, Danilenko M, Watts C, Prall O, Levy J, Sharoni Y. Lycopene inhibition of cell cycle progression in breast and endometrial cancer cells is associated with reduction in cyclin D levels and retention of p27 (Kip1) in the cyclin E-cdk2 complexes. Oncogene. 2001;20(26):3428–3436. doi: 10.1038/sj.onc.1204452. [DOI] [PubMed] [Google Scholar]
- 210.Nahum A, Zeller L, Danilenko M, Prall OWJ, Watts CKW, Sutherland RL, Levy J, Sharoni Y. Lycopene inhibition of IGF-induced cancer cell growth depends on the level of cyclin D1. Eur J Nutr. 2006;45(5):275–282. doi: 10.1007/s00394-006-0595-x. [DOI] [PubMed] [Google Scholar]
- 211.Siler U, Barella L, Spitzer V, Schnorr J, Lein M, Goralczyk R, Wertz K. Lycopene and vitamin E interfere with autocrine/paracrine loops in the Dunning prostate cancer model. FASEB J. 2004;18(9):1019–1021. doi: 10.1096/fj.03-1116fje. [DOI] [PubMed] [Google Scholar]
- 212.Kucuk O, Sarkar FH, Sakr W, Djuric Z, Pollak MN, Khachik F, Li YW, Banerjee M, Grignon D, Bertram JS. Phase II randomized clinical trial of lycopene supplementation before radical prostatectomy. Cancer Epidemiol Biomarkers Prev. 2001;10(8):861–868. [PubMed] [Google Scholar]
- 213.Vaishampayan U, Hussain M, Banerjee M, Seren S, Sarkar FH, Fontana J, Forman JD, Cher ML, Powell I, Pontes JE. Lycopene and soy isoflavones in the treatment of prostate cancer. Nutr Cancer. 2007;59(1):1–7. doi: 10.1080/01635580701413934. [DOI] [PubMed] [Google Scholar]
- 214.Chalabi N, Satih S, Delort L, Bignon YJ, Bernard-Gallon DJ. Expression profiling by whole-genome microarray hybridization reveals differential gene expression in breast cancer cell lines after lycopene exposure. Biochim Biophys Acta. 2007;1769(2):124–130. doi: 10.1016/j.bbaexp.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 215.Palozza P, Colangelo M, Simone R, Catalano A, Boninsegna A, Lanza P, Monego G, Ranelletti FO. Lycopene induces cell growth inhibition by altering mevalonate pathway and Ras signaling in cancer cell lines. Carcinogenesis. 2010;31(10):1813–1821. doi: 10.1093/carcin/bgq157. [DOI] [PubMed] [Google Scholar]
- 216.Scolastici C, Alves de Lima R, Barbisan L, Ferreira A, Ribeiro D, Salvadori D. Antigenotoxicity and antimutagenicity of lycopene in HepG2 cell line evaluated by the comet assay and micronucleus test. Toxicol In Vitro. 2008;22(2):510–514. doi: 10.1016/j.tiv.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 217.Kelkel M, Schumacher M, Dicato M, Diederich M. Antioxidant and anti-proliferative properties of lycopene. Free Radic Res. 2011:1–16. doi: 10.3109/10715762.2011.564168. [DOI] [PubMed] [Google Scholar]
- 218.Stark MJ, Burke YD, McKinzie JH, Ayoubi AS, Crowell PL. Chemotherapy of pancreatic cancer with the monoterpene perillyl alcohol. Cancer lett. 1995;96(1):15–21. doi: 10.1016/0304-3835(95)03912-g. [DOI] [PubMed] [Google Scholar]
- 219.Mills JJ, Chari RS, Boyer IJ, Gould MN, Jirtle RL. Induction of apoptosis in liver tumors by the monoterpene perillyl alcohol. Cancer Res. 1995;55(5):979–983. [PubMed] [Google Scholar]
- 220.Crowell PL, Ren Z, Lin S, Vedejs E, Gould MN. Structure-activity relationships among monoterpene inhibitors of protein isoprenylation and cell proliferation. Biochem Pharmacol. 1994;47(8):1405–1415. doi: 10.1016/0006-2952(94)90341-7. [DOI] [PubMed] [Google Scholar]
- 221.Haag JD, Gould MN. Mammary carcinoma regression induced by perillyl alcohol, a hydroxylated analog of limonene. Cancer Chemother Pharmacol. 1994;34(6):477–483. doi: 10.1007/BF00685658. [DOI] [PubMed] [Google Scholar]
- 222.Elegbede J, Elson C, Tanner M, Qureshi A, Gould M. Regression of rat primary mammary tumors following dietary d-limonene. J Natl Cancer Inst. 1986;76(2):323–325. [PubMed] [Google Scholar]
- 223.Haag JD, Lindstrom MJ, Gould MN. Limonene-induced regression of mammary carcinomas. Cancer Res. 1992;52(14):4021–4026. [PubMed] [Google Scholar]
- 224.Chaudhary SC, Alam MS, Siddiqui M, Athar M. Perillyl alcohol attenuates Ras-ERK signaling to inhibit murine skin inflammation and tumorigenesis. Chem Biol Interact. 2009;179(2–3):145–153. doi: 10.1016/j.cbi.2008.12.016. [DOI] [PubMed] [Google Scholar]
- 225.Elegbede JA, Flores R, Wang RC. Perillyl alcohol and perillaldehyde induced cell cycle arrest and cell death in BroTo and A549 cells cultured in vitro. Life Sci. 2003;73(22):2831–2840. doi: 10.1016/s0024-3205(03)00701-x. [DOI] [PubMed] [Google Scholar]
- 226.Xu M, Floyd HS, Greth SM, Chang WCL, Lohman K, Stoyanova R, Kucera GL, Kute TE, Willingham MC, Miller MS. Perillyl alcohol-mediated inhibition of lung cancer cell line proliferation: potential mechanisms for its chemotherapeutic effects. Toxicol Appl Pharmacol. 2004;195(2):232–246. doi: 10.1016/j.taap.2003.11.013. [DOI] [PubMed] [Google Scholar]
- 227.Bardon S, Foussard V, Fournel S, Loubat A. Monoterpenes inhibit proliferation of human colon cancer cells by modulating cell cycle-related protein expression. Cancer Lett. 2002;181(2):187–194. doi: 10.1016/s0304-3835(02)00047-2. [DOI] [PubMed] [Google Scholar]
- 228.Yeruva L, Hall C, Elegbede JA, Carper SW. Perillyl alcohol and methyl jasmonate sensitize cancer cells to cisplatin. Anticancer Drugs. 2010;21(1):1–9. doi: 10.1097/CAD.0b013e32832a68ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Yeruva L, Pierre KJ, Elegbede A, Wang RC, Carper SW. Perillyl alcohol and perillic acid induced cell cycle arrest and apoptosis in non small cell lung cancer cells. Cancer Lett. 2007;257(2):216–226. doi: 10.1016/j.canlet.2007.07.020. [DOI] [PubMed] [Google Scholar]
- 230.ura ková Z. Some current insights into oxidative stress. Physiol Res. 2010;59:459–469. doi: 10.33549/physiolres.931844. [DOI] [PubMed] [Google Scholar]
- 231.Lee KW, Lee HJ. Biphasic effects of dietary antioxidants on oxidative stress-mediated carcinogenesis. Mech Ageing Dev. 2006;127(5):424–431. doi: 10.1016/j.mad.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 232.Cerutti PA. Prooxidant states and tumor promotion. Science. 1985;227(4685):375–381. doi: 10.1126/science.2981433. [DOI] [PubMed] [Google Scholar]
- 233.Konturek PC, Kania J, Konturek JW, Nikiforuk A, Konturek SJ, Hahn EGH. pylori infection, atrophic gastritis, cytokines, gastrin, COX-2, PPAR gamma and impaired apoptosis in gastric carcinogenesis. Med Sci Monit. 2003;9(7):SR53–SR66. [PubMed] [Google Scholar]
- 234.Nishigori C, Hattori Y, Toyokuni S. Role of reactive oxygen species in skin carcinogenesis. Antioxid Redox Signal. 2004;6(3):561–570. doi: 10.1089/152308604773934314. [DOI] [PubMed] [Google Scholar]
- 235.Doll R. An overview of the epidemiological evidence linking diet and cancer. Proc Nutr Soc. 1990;49(02):119–131. doi: 10.1079/pns19900018. [DOI] [PubMed] [Google Scholar]
- 236.Doll R, Peto R. The causes of cancer: quantitative estimates of avoidable risks of cancer in the United States today. J Natl Cancer Inst. 1981;66(6):1191–1308. [PubMed] [Google Scholar]
- 237.La Vecchia C, Altieri A, Tavani A. Vegetables, fruit, antioxidants and cancer: a review of Italian studies. Eur J Nutr. 2001;40(6):261–267. doi: 10.1007/s394-001-8354-9. [DOI] [PubMed] [Google Scholar]
- 238.Tardif JC. Antioxidants: the good, the bad and the ugly. Can J Cardiol. 2006;22(Suppl B):61B–65B. doi: 10.1016/s0828-282x(06)70988-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Trosko JE, Chang CC. Modulation of cell cell communication in the cause and chemoprevention/chemotherapy of cancer. Biofactors. 2000;12(1 4):259–263. doi: 10.1002/biof.5520120139. [DOI] [PubMed] [Google Scholar]
- 240.Joe B, Vijaykumar M, Lokesh B. Biological properties of curcumin-cellular and molecular mechanisms of action. Crit Rev Food Sci Nutr. 2004;44(2):97–111. doi: 10.1080/10408690490424702. [DOI] [PubMed] [Google Scholar]
- 241.Motterlini R, Foresti R, Bassi R, Green CJ. Curcumin, an antioxidant and anti-inflammatory agent, induces heme oxygenase-1 and protects endothelial cells against oxidative stress. Free Radic Biol Med. 2000;28(8):1303–1312. doi: 10.1016/s0891-5849(00)00294-x. [DOI] [PubMed] [Google Scholar]
- 242.Wright JS. Predicting the antioxidant activity of curcumin and curcuminoids. J Mol Struct. 2002;591(1–3):207–217. [Google Scholar]
- 243.Iqbal M, Sharma SD, Okazaki Y, Fujisawa M, Okada S. Dietary supplementation of curcumin enhances antioxidant and phase II metabolizing enzymes in ddY male mice: possible role in protection against chemical carcinogenesis and toxicity. Pharmacol Toxicol. 2003;92(1):33–38. doi: 10.1034/j.1600-0773.2003.920106.x. [DOI] [PubMed] [Google Scholar]
- 244.Song EK, Cho H, Kim JS, Kim NY, An NH, Kim JA, Lee SH, Kim YC. Diarylheptanoids with free radical scavenging and hepatoprotective activity in vitro from Curcuma longa. Planta Med. 2001;67(9):876–877. doi: 10.1055/s-2001-18860. [DOI] [PubMed] [Google Scholar]
- 245.Farghaly HS, Hussein MA. Protective Effect of Curcumin Against Paracetamol-induced Liver Damage. Aust J Bas Appl Sci. 2010;4(9):4266–4274. [Google Scholar]
- 246.Pan MH, Lin-Shiau SY, Lin JK. Comparative studies on the suppression of nitric oxide synthase by curcumin and its hydrogenated metabolites through down-regulation of I [kappa] B kinase and NF [kappa] B activation in macrophages. Biochem Pharmacol. 2000;60(11):1665–1676. doi: 10.1016/s0006-2952(00)00489-5. [DOI] [PubMed] [Google Scholar]
- 247.Brouet I, Ohshima H. Curcumin, an anti-tumor promoter and anti-inflammatory agent, inhibits induction of nitric oxide synthase in activated macrophages. Biochem Biophys Res Commun. 1995;206(2):533–540. doi: 10.1006/bbrc.1995.1076. [DOI] [PubMed] [Google Scholar]
- 248.Palsamy P, Sivakumar S, Subramanian S. Resveratrol attenuates hyperglycemia-mediated oxidative stress, proinflammatory cytokines and protects hepatocytes ultrastructure in streptozotocin-nicotinamide-induced experimental diabetic rats. Chem Biol Interact. 2010;186(2):200–210. doi: 10.1016/j.cbi.2010.03.028. [DOI] [PubMed] [Google Scholar]
- 249.Pintea A, Rugin D, Pop R, Bunea A, Socaciu C, Diehl HA. Antioxidant Effect of Trans-Resveratrol in Cultured Human Retinal Pigment Epithelial Cells. J Ocul Pharmacol Ther. 2011;27(4):315–21. doi: 10.1089/jop.2010.0144. [DOI] [PubMed] [Google Scholar]
- 250.Spanier G, Xu H, Xia N, Tobias S, Deng S, Wojnowski L, Forstermann U, Li H. Resveratrol reduces endothelial oxidative stress by modulating the gene expression of superoxide dismutase 1 (SOD1), glutathione peroxidase 1 (GPx1) and NADPH oxidase subunit (Nox4) J Physiol Pharmacol. 2009;60(Suppl 4):111–116. [PubMed] [Google Scholar]
- 251.Valcic S, Muders A, Jacobsen NE, Liebler DC, Timmermann BN. Antioxidant chemistry of green tea catechins. Identification of products of the reaction of (−)-epigallocatechin gallate with peroxyl radicals. Chem Res Toxicol. 1999;12(4):382–386. doi: 10.1021/tx990003t. [DOI] [PubMed] [Google Scholar]
- 252.Levites Y, Amit T, Mandel S, Youdim MB. Neuroprotection and neurorescue against Abeta toxicity and PKC-dependent release of nonamyloidogenic soluble precursor protein by green tea polyphenol (−)-epigallocatechin-3-gallate. FASEB J. 2003;17(8):952–954. doi: 10.1096/fj.02-0881fje. [DOI] [PubMed] [Google Scholar]
- 253.Bastianetto S, Yao ZX, Papadopoulos V, Quirion R. Neuroprotective effects of green and black teas and their catechin gallate esters against amyloid induced toxicity. Eur J Neurosci. 2006;23(1):55–64. doi: 10.1111/j.1460-9568.2005.04532.x. [DOI] [PubMed] [Google Scholar]
- 254.Senthil Kumaran V, Arulmathi K, Srividhya R, Kalaiselvi P. Repletion of antioxidant status by EGCG and retardation of oxidative damage induced macromolecular anomalies in aged rats. Exp Gerontol. 2008;43(3):176–183. doi: 10.1016/j.exger.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 255.Raza H, John A. Green tea polyphenol epigallocatechin-3-gallate differentially modulates oxidative stress in PC12 cell compartments. Toxicol Appl Pharmacol. 2005;207(3):212–220. doi: 10.1016/j.taap.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 256.Prince Vijeya Singh J, Selvendiran K, Mumtaz Banu S, Padmavathi R, Sakthisekaran D. Protective role of Apigenin on the status of lipid peroxidation and antioxidant defense against hepatocarcinogenesis in Wistar albino rats. Phytomedicine. 2004;11(4):309–314. doi: 10.1078/0944711041495254. [DOI] [PubMed] [Google Scholar]
- 257.Saravanan R, Pugalendi V. Impact of ursolic acid on chronic ethanol-induced oxidative stress in the rat heart. Pharmacol Rep. 2006;58(1):41. [PubMed] [Google Scholar]
- 258.Saravanan R, Viswanathan P, Pugalendi KV. Protective effect of ursolic acid on ethanol-mediated experimental liver damage in rats. Life Sci. 2006;78(7):713–718. doi: 10.1016/j.lfs.2005.05.060. [DOI] [PubMed] [Google Scholar]
- 259.Rao AV, Agarwal S. Role of antioxidant lycopene in cancer and heart disease. J Am Coll Nutr. 2000;19(5):563–569. doi: 10.1080/07315724.2000.10718953. [DOI] [PubMed] [Google Scholar]
- 260.Di Mascio P, Kaiser S, Sies H. Lycopene as the most efficient biological carotenoid singlet oxygen quencher* 1. Arch Biochem Biophys. 1989;274(2):532–538. doi: 10.1016/0003-9861(89)90467-0. [DOI] [PubMed] [Google Scholar]
- 261.Böhm F, Tinkler J, Truscott T. Carotenoids protect against cell membrane damage by the nitrogen dioxide radical. Nat Med. 1995;1(2):98–99. doi: 10.1038/nm0295-98. [DOI] [PubMed] [Google Scholar]
- 262.Lu Y, Etoh H, Watanabe N, Ina K, Ukai N, Oshima S, Ojima F, Sakamoto H, Ishiguro Y. A new carotenoid, hydrogen peroxide oxidation products from lycopene. Bioscience, biotech biochem. 1995;59(11):2153–2155. [Google Scholar]
- 263.Jain CK, Agarwal S, Rao AV. The effect of dietary lycopene on bioavailability, tissue distribution, in vivo antioxidant properties and colonic preneoplasia in rats. Nutr Res. 1999;19(9):1383–1391. [Google Scholar]
- 264.Tinkler JH, Böhm F, Schalch W, Truscott TG. Dietary carotenoids protect human cells from damage. J Photochem Photobiol B. 1994;26(3):283–285. doi: 10.1016/1011-1344(94)07049-0. [DOI] [PubMed] [Google Scholar]
- 265.Chaturvedi M, Sung B, Yadav V, Kannappan R, Aggarwal B. NF-κB addiction and its role in cancer:‘one size does not fit all’. Oncogene. 2010;30(14):1615–1630. doi: 10.1038/onc.2010.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Chen FE, Ghosh G. structural views. Oncogene. 1999;18(49):6845–6852. doi: 10.1038/sj.onc.1203224. [DOI] [PubMed] [Google Scholar]
- 267.Pahl HL. Activators and target genes of Rel/NF-kappaB transcription factors. Oncogene. 1999;18(49):6853–6866. doi: 10.1038/sj.onc.1203239. [DOI] [PubMed] [Google Scholar]
- 268.Karin M. How NF-kappaB is activated: the role of the IkappaB kinase (IKK) complex. Oncogene. 1999;18(49):6867–6874. doi: 10.1038/sj.onc.1203219. [DOI] [PubMed] [Google Scholar]
- 269.Rayet B, Gelinas C. Aberrant rel/nfkb genes and activity in human cancer. Oncogene. 1999;18(49):6938–6947. doi: 10.1038/sj.onc.1203221. [DOI] [PubMed] [Google Scholar]
- 270.Nakshatri H, Bhat-Nakshatri P, Martin DA, Goulet RJ, Jr, Sledge GW., Jr Constitutive activation of NF-kappaB during progression of breast cancer to hormone-independent growth. Mol Cell Biol. 1997;17(7):3629–3639. doi: 10.1128/mcb.17.7.3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Bours V, Dejardin E, Goujon-Letawe F, Merville MP, Castronovo V. The NF-[kappa] B transcription factor and cancer: high expression of NF-[kappa] B-and I [kappa] B-related proteins in tumor cell lines. Biochem Pharmacol. 1994;47(1):145–149. doi: 10.1016/0006-2952(94)90448-0. [DOI] [PubMed] [Google Scholar]
- 272.Dejardin E, Deregowski V, Chapelier M, Jacobs N, Gielen J, Merville MP, Bours V. Regulation of NF-kappaB activity by I kappaB-related proteins in adenocarcinoma cells. Oncogene. 1999;18(16):2567–2577. doi: 10.1038/sj.onc.1202599. [DOI] [PubMed] [Google Scholar]
- 273.Wang W, Abbruzzese JL, Evans DB, Larry L, Cleary KR, Chiao PJ. The nuclear factor- B RelA transcription factor is constitutively activated in human pancreatic adenocarcinoma cells. Clin Cancer Res. 1999;5(1):119–127. [PubMed] [Google Scholar]
- 274.Visconti R, Cerutti J, Battista S, Fedele M, Trapasso F, Zeki K, Miano MP, de Nigris F, Casalino L, Curcio F. Expression of the neoplastic phenotype by human thyroid carcinoma cell lines requires NFkappaB p65 protein expression. Oncogene. 1997;15(16):1987–1994. doi: 10.1038/sj.onc.1201373. [DOI] [PubMed] [Google Scholar]
- 275.Sumitomo M, Tachibana M, Murai M, Hayakawa M, Nakamura H, Shimizu N, Ozu C, Asakura H, Takayanagi A. Induction of apoptosis of cytokine-producing bladder cancer cells by adenovirus-mediated I kappa B alpha overexpression. Hum Gene Ther. 1999;10(1):37–48. doi: 10.1089/10430349950019174. [DOI] [PubMed] [Google Scholar]
- 276.Herrmann JL, Beham AW, Sarkiss M, Chiao PJ, Rands MT, Bruckheimer EM, Brisbay S, McDonnell TJ. Bcl-2 Suppresses Apoptosis Resulting from Disruption of the NF-[kappa] B Survival Pathway. Exp Cell Res. 1997;237(1):101–109. doi: 10.1006/excr.1997.3737. [DOI] [PubMed] [Google Scholar]
- 277.Devalaraja MN, Wang DZ, Ballard DW, Richmond A. Tumor Biology-Elevated Constitutive IkB Kinase Activity and IkB-a Phosphorylation in Hs294T Melanoma Cells Lead to Increased Basal MGSA/GRO-a Transcription. Cancer Research-Paper Edition. 1999;59(6):1372–1377. [PubMed] [Google Scholar]
- 278.Helbig G, Christopherson KW, Bhat-Nakshatri P, Kumar S, Kishimoto H, Miller KD, Broxmeyer HE, Nakshatri H. NF-κB promotes breast cancer cell migration and metastasis by inducing the expression of the chemokine receptor CXCR4. J Biol Chem. 2003;278(24):21631. doi: 10.1074/jbc.M300609200. [DOI] [PubMed] [Google Scholar]
- 279.Coope H, Atkinson P, Huhse B, Belich M, Janzen J, Holman M, Klaus G, Johnston L, Ley S. CD40 regulates the processing of NF-κB2 p100 to p52. EMBO J. 2002;21(20):5375–5385. doi: 10.1093/emboj/cdf542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Ghosh S, May MJ, Kopp EB. NF-κB and Rel proteins: evolutionarily conserved mediators of immune responses. Annu Rev Immunol. 1998;16(1):225–260. doi: 10.1146/annurev.immunol.16.1.225. [DOI] [PubMed] [Google Scholar]
- 281.Siebenlist U, Franzoso G, Brown K. Structure, regulation and function of NF-kappa B. Annu Rev Cell Biol. 1994;10:405–455. doi: 10.1146/annurev.cb.10.110194.002201. [DOI] [PubMed] [Google Scholar]
- 282.Uzzo RG, Clark PE, Rayman P, Bloom T, Rybicki L, Novick AC, Bukowski RM, Finke JH. Alterations in NF B activation in T lymphocytes of patients with renal cell carcinoma. J Natl Cancer Inst. 1999;91(8):718. doi: 10.1093/jnci/91.8.718. [DOI] [PubMed] [Google Scholar]
- 283.Hoffmann A, Xia Y, Verma IM. Inflammatory tales of liver cancer. Cancer Cell. 2007;11(2):99–101. doi: 10.1016/j.ccr.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 284.Pari L, Tewas D, Eckel J. Role of curcumin in health and disease. Arch Physiol Biochem. 2008;114(2):127–149. doi: 10.1080/13813450802033958. [DOI] [PubMed] [Google Scholar]
- 285.Kozuch PL, Hanauer SB. Treatment of inflammatory bowel disease: a review of medical therapy. World J Gastroenterol. 2008;14(3):354–377. doi: 10.3748/wjg.14.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Billerey Larmonier C, Uno JK, Larmonier N, Midura AJ, Timmermann B, Ghishan FK, Kiela PR. Protective effects of dietary curcumin in mouse model of chemically induced colitis are strain dependent. Inflamm Bowel Dis. 2008;14(6):780–793. doi: 10.1002/ibd.20348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Divya CS, Pillai MR. Antitumor action of curcumin in human papillomavirus associated cells involves downregulation of viral oncogenes, prevention of NFkB and AP 1 translocation, and modulation of apoptosis. Mol Carcinog. 2006;45(5):320–332. doi: 10.1002/mc.20170. [DOI] [PubMed] [Google Scholar]
- 288.Singh S, Khar A. Biological effects of curcumin and its role in cancer chemoprevention and therapy. Anticancer Agents Med Chem. 2006;6(3):259–270. doi: 10.2174/187152006776930918. [DOI] [PubMed] [Google Scholar]
- 289.Shishodia S, Amin HM, Lai R, Aggarwal BB. Curcumin (diferuloylmethane) inhibits constitutive NF-[kappa] B activation, induces G1/S arrest, suppresses proliferation, and induces apoptosis in mantle cell lymphoma. Biochem Pharmacol. 2005;70(5):700–713. doi: 10.1016/j.bcp.2005.04.043. [DOI] [PubMed] [Google Scholar]
- 290.Collett GP, Campbell FC. Curcumin induces c-jun N-terminal kinase-dependent apoptosis in HCT116 human colon cancer cells. Carcinogenesis. 2004;25(11):2183–2189. doi: 10.1093/carcin/bgh233. [DOI] [PubMed] [Google Scholar]
- 291.Sharma C, Kaur J, Shishodia S, Aggarwal BB, Ralhan R. Curcumin down regulates smokeless tobacco-induced NF-[kappa] B activation and COX-2 expression in human oral premalignant and cancer cells. Toxicology. 2006;228(1):1–15. doi: 10.1016/j.tox.2006.07.027. [DOI] [PubMed] [Google Scholar]
- 292.Alaikov T, Konstantinov SM, Tzanova T, Dinev K, Topashika Ancheva M, Berger MR. Antineoplastic and anticlastogenic properties of curcumin. Ann N Y Acad Sci. 2007;1095(1):355–370. doi: 10.1196/annals.1397.039. [DOI] [PubMed] [Google Scholar]
- 293.Bachmeier BE, Nerlich AG, Iancu CM, Cilli M, Schleicher E, Vené R, Dell’Eva R, Jochum M, Albini A, Pfeffer U. The chemopreventive polyphenol Curcumin prevents hematogenous breast cancer metastases in immunodeficient mice. Cell Physiol Biochem. 2010;19(1–4):137–152. doi: 10.1159/000099202. [DOI] [PubMed] [Google Scholar]
- 294.Lin YG, Kunnumakkara AB, Nair A, Merritt WM, Han LY, Armaiz-Pena GN, Kamat AA, Spannuth WA, Gershenson DM, Lutgendorf SK. Curcumin inhibits tumor growth and angiogenesis in ovarian carcinoma by targeting the nuclear factor- B pathway. Clin Cancer Res. 2007;13(11):3423–3430. doi: 10.1158/1078-0432.CCR-06-3072. [DOI] [PubMed] [Google Scholar]
- 295.Kunnumakkara AB, Guha S, Krishnan S, Diagaradjane P, Gelovani J, Aggarwal BB. Curcumin Potentiates Antitumor Activity of Gemcitabine in an Orthotopic Model of Pancreatic Cancer through Suppression of Proliferation, Angiogenesis, and Inhibition of Nuclear Factor-κB–Regulated Gene Products. Cancer Res. 2007;67(8):3853–3861. doi: 10.1158/0008-5472.CAN-06-4257. [DOI] [PubMed] [Google Scholar]
- 296.Kamat AM, Sethi G, Aggarwal BB. Curcumin potentiates the apoptotic effects of chemotherapeutic agents and cytokines through down-regulation of nuclear factor-κB and nuclear factor-κB–regulated gene products in IFN-α–sensitive and IFN-α–resistant human bladder cancer cells. Mol Cancer Ther. 2007;6(3):1022–1030. doi: 10.1158/1535-7163.MCT-06-0545. [DOI] [PubMed] [Google Scholar]
- 297.Deeb D, Jiang H, Gao X, Hafner MS, Wong H, Divine G, Chapman RA, Dulchavsky SA, Gautam SC. Curcumin sensitizes prostate cancer cells to tumor necrosis factor–related apoptosis-inducing ligand/Apo2L by inhibiting nuclear factor- B through suppression of I B phosphorylation. Mol Cancer Ther. 2004;3(7):803–812. [PubMed] [Google Scholar]
- 298.Khanbolooki S, Nawrocki ST, Arumugam T, Andtbacka R, Pino MS, Kurzrock R, Logsdon CD, Abbruzzese JL, McConkey DJ. Nuclear factor- B maintains TRAIL resistance in human pancreatic cancer cells. Mol Cancer Ther. 2006;5(9):2251–2260. doi: 10.1158/1535-7163.MCT-06-0075. [DOI] [PubMed] [Google Scholar]
- 299.Manna SK, Mukhopadhyay A, Aggarwal BB. Resveratrol suppresses TNF-induced activation of nuclear transcription factors NF-κB, activator protein-1, and apoptosis: potential role of reactive oxygen intermediates and lipid peroxidation. J Immunol. 2000;164(12):6509–6519. doi: 10.4049/jimmunol.164.12.6509. [DOI] [PubMed] [Google Scholar]
- 300.Kundu JK, Shin YK, Kim SH, Surh YJ. Resveratrol inhibits phorbol ester-induced expression of COX-2 and activation of NF-κB in mouse skin by blocking I B kinase activity. Carcinogenesis. 2006;27(7):1465–1474. doi: 10.1093/carcin/bgi349. [DOI] [PubMed] [Google Scholar]
- 301.Nicholas C, Batra S, Vargo MA, Voss OH, Gavrilin MA, Wewers MD, Guttridge DC, Grotewold E, Doseff AI. Apigenin blocks lipopolysaccharide-induced lethality in vivo and proinflammatory cytokines expression by inactivating NF-κB through the suppression of p65 phosphorylation. J Immunol. 2007;179(10):7121–7127. doi: 10.4049/jimmunol.179.10.7121. [DOI] [PubMed] [Google Scholar]
- 302.Xu L, Zhang L, Bertucci AM, Pope RM, Datta SK. Apigenin, a dietary flavonoid, sensitizes human T cells for activation-induced cell death by inhibiting PKB/Akt and NF-[kappa] B activation pathway. Immunol Lett. 2008;121(1):74–83. doi: 10.1016/j.imlet.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Shukla S, Gupta S. Apigenin inhibits constitutive and TNFalpha-mediated NF-kappaB activation and induces apoptosis in human prostate carcinoma PC-3 cells: Correlation with NF-kappaB responsive genes. P Am Assoc Cancer Res. 2004;2004(1):516. doi: 10.1158/1078-0432.ccr-03-0586. [DOI] [PubMed] [Google Scholar]
- 304.Afaq F, Adhami VM, Ahmad N, Mukhtar H. Inhibition of ultraviolet B-mediated activation of nuclear factor B in normal human epidermal keratinocytes by green tea Constituent (−)-epigallocatechin-3-gallate. Oncogene. 2003;22(7):1035–1044. doi: 10.1038/sj.onc.1206206. [DOI] [PubMed] [Google Scholar]
- 305.Syed D, Afaq F, Kweon M, Hadi N, Bhatia N, Spiegelman V, Mukhtar H. Green tea polyphenol EGCG suppresses cigarette smoke condensate-induced NF-κB activation in normal human bronchial epithelial cells. Oncogene. 2006;26(5):673–682. doi: 10.1038/sj.onc.1209829. [DOI] [PubMed] [Google Scholar]
- 306.Bharrhan S, Chopra K, Arora SK, Toor JS, Rishi P. Down-regulation of NF-{kappa}B signalling by polyphenolic compounds prevents endotoxin-induced liver injury in a rat model. Innate Immun. 2011 doi: 10.1177/1753425910393369. Epub 2011/01/18. [DOI] [PubMed] [Google Scholar]
- 307.Musonda CA, Chipman JK. Quercetin inhibits hydrogen peroxide (H2O2)-induced NF-kappaB DNA binding activity and DNA damage in HepG2 cells. Carcinogenesis. 1998;19(9):1583–1589. doi: 10.1093/carcin/19.9.1583. [DOI] [PubMed] [Google Scholar]
- 308.Davis JN, Kucuk O, Sarkar FH. Genistein inhibits NF-kappa B activation in prostate cancer cells. Nutr Cancer. 1999;35(2):167–174. doi: 10.1207/S15327914NC352_11. [DOI] [PubMed] [Google Scholar]
- 309.Li Y, Xing D, Chen Q, Chen WR. Enhancement of chemotherapeutic agent-induced apoptosis by inhibition of NF-kappaB using ursolic acid. Int J Cancer. 2010;127(2):462–473. doi: 10.1002/ijc.25044. [DOI] [PubMed] [Google Scholar]
- 310.Kuttan G, Pratheeshkumar P. Invasion and Metastasis:Major Obstacles to Curative Cancer Therapy. In: Klein SA, Beckler JP, editors. Metastatic Melanoma: Symptoms, Diagnoses and Treatments. Nova Science Publishers, Inc; 2011. pp. 1–27. [Google Scholar]
- 311.Folkman J. What is the evidence that tumors are angiogenesis dependent? J Natl Cancer Inst. 1990;82(1):4–6. doi: 10.1093/jnci/82.1.4. [DOI] [PubMed] [Google Scholar]
- 312.McMahon G. VEGF receptor signaling in tumor angiogenesis. Oncologist. 2000;5(Supplement 1):3–10. doi: 10.1634/theoncologist.5-suppl_1-3. [DOI] [PubMed] [Google Scholar]
- 313.Neal CP, Berry DP. Basic principles of the molecular biology of cancer II: angiogenesis, invasion and metastasis. Surgery (Oxford) 2006;24(4):120–125. [Google Scholar]
- 314.Ellis L, Fidler I. Angiogenesis and metastasis. Eur J Cancer. 1996;32(14):2451–2460. doi: 10.1016/s0959-8049(96)00389-9. [DOI] [PubMed] [Google Scholar]
- 315.Mekkawy AH, Morris DL, Pourgholami MH. Urokinase plasminogen activator system as a potential target for cancer therapy. Future Oncol. 2009;5(9):1487–1499. doi: 10.2217/fon.09.108. [DOI] [PubMed] [Google Scholar]
- 316.Kessenbrock K, Plaks V, Werb Z. Matrix metalloproteinases: regulators of the tumor microenvironment. Cell. 2010;141(1):52–67. doi: 10.1016/j.cell.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Davidson B, Goldberg I, Gotlieb WH, Kopolovic J, Risberg B, Ben-Baruch G, Reich R. Coordinated expression of integrin subunits, matrix metalloproteinases (MMP), angiogenic genes and Ets transcription factors in advanced-stage ovarian carcinoma: A possible activation pathway? Cancer Metastasis Rev. 2003;22(1):103–115. doi: 10.1023/a:1022272204045. [DOI] [PubMed] [Google Scholar]
- 318.Varinska L, Mirossay L, Mojzisova G, Mojzis J. Antiangogenic effect of selected phytochemicals. Pharmazie. 2010;65(1):57–63. [PubMed] [Google Scholar]
- 319.Bhandarkar SS, Arbiser JL. Curcumin as an inhibitor of angiogenesis. The Molecular Targets and Therapeutic Uses of Curcumin in Health and Disease. 2007:185–195. doi: 10.1007/978-0-387-46401-5_7. [DOI] [PubMed] [Google Scholar]
- 320.Gururaj AE, Belakavadi M, Venkatesh DA, Marmé D, Salimath BP. Molecular mechanisms of anti-angiogenic effect of curcumin. Biochem Biophys Res Commun. 2002;297(4):934–942. doi: 10.1016/s0006-291x(02)02306-9. [DOI] [PubMed] [Google Scholar]
- 321.Mohan R, Sivak J, Ashton P, Russo LA, Pham BQ, Kasahara N, Raizman MB, Fini ME. Curcuminoids inhibit the angiogenic response stimulated by fibroblast growth factor-2, including expression of matrix metalloproteinase gelatinase B. J Biol Chem. 2000;275(14):10405–10412. doi: 10.1074/jbc.275.14.10405. [DOI] [PubMed] [Google Scholar]
- 322.Bae MK, Kim SH, Jeong JW. Curcumin inhibits hypoxia-induced angiogenesis via down-regulation of HIF-1. Oncol Rep. 2006;15(6):1557–1562. [PubMed] [Google Scholar]
- 323.Thaloor D, Singh AK, Sidhu GS, Prasad PV, Kleinman HK, Maheshwari RK. Inhibition of angiogenic differentiation of human umbilical vein endothelial cells by curcumin. Cell Growth Differ. 1998;9(4):305–312. [PubMed] [Google Scholar]
- 324.Singh AK, Seth P, Anthony P, Husain MM, Madhavan S, Mukhtar H, Maheshwari RK. Green tea constituent epigallocatechin-3-gallate inhibits angiogenic differentiation of human endothelial cells. Arch Biochem Biophys. 2002;401(1):29–37. doi: 10.1016/S0003-9861(02)00013-9. [DOI] [PubMed] [Google Scholar]
- 325.Fang J, Zhou Q, Liu LZ, Xia C, Hu X, Shi X, Jiang BH. Apigenin inhibits tumor angiogenesis through decreasing HIF-1 and VEGF expression. Carcinogenesis. 2006;28(4):858–864. doi: 10.1093/carcin/bgl205. [DOI] [PubMed] [Google Scholar]
- 326.Osada M, Imaoka S, Funae Y. Apigenin suppresses the expression of VEGF, an important factor for angiogenesis, in endothelial cells via degradation of HIF-1 [alpha] protein. FEBS Lett. 2004;575(1–3):59–63. doi: 10.1016/j.febslet.2004.08.036. [DOI] [PubMed] [Google Scholar]
- 327.Su SJ, Yeh TM, Chuang WJ, Ho CL, Chang KL, Cheng HL, Liu HS, Hsu PY, Chow NH. The novel targets for anti-angiogenesis of genistein on human cancer cells. Biochem Pharmacol. 2005;69(2):307–318. doi: 10.1016/j.bcp.2004.09.025. [DOI] [PubMed] [Google Scholar]
- 328.Igura K, Ohta T, Kuroda Y, Kaji K. Resveratrol and quercetin inhibit angiogenesis in vitro. Cancer lett. 2001;171(1):11–16. doi: 10.1016/s0304-3835(01)00443-8. [DOI] [PubMed] [Google Scholar]
- 329.Xiao D, Singh SV. Phenethyl isothiocyanate inhibits angiogenesis in vitro and ex vivo. Cancer Res. 2007;67(5):2239–2246. doi: 10.1158/0008-5472.CAN-06-3645. [DOI] [PubMed] [Google Scholar]
- 330.Bertl E, Bartsch H, Gerhäuser C. Inhibition of angiogenesis and endothelial cell functions are novel sulforaphane-mediated mechanisms in chemoprevention. Mol Cancer Ther. 2006;5(3):575–585. doi: 10.1158/1535-7163.MCT-05-0324. [DOI] [PubMed] [Google Scholar]
- 331.Davis R, Sing KP, Kurzrock R, Shankar S. Sulforaphane inhibits angiogenesis through activation of FOXO transcription factors. Oncol Rep. 2009;22(6):1473–1478. doi: 10.3892/or_00000589. [DOI] [PubMed] [Google Scholar]
- 332.Heemels MT, Dhand R, Allen L. The biochemistry of apoptosis. Nature. 2000;407:770–776. doi: 10.1038/35037710. [DOI] [PubMed] [Google Scholar]
- 333.Hengartner MO. The biochemistry of apoptosis. Chemistry. 2002;101(102):145–153. [Google Scholar]
- 334.Sprick MR, Walczak H. The interplay between the Bcl-2 family and death receptor-mediated apoptosis. Biochim Biophys Acta. 2004;1644(2–3):125–132. doi: 10.1016/j.bbamcr.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 335.Huppertz B, Frank HG, Kaufmann P. The apoptosis cascade—morphological and immunohistochemical methods for its visualization. Anat Embryol (Berl) 1999;200(1):1–18. doi: 10.1007/s004290050254. [DOI] [PubMed] [Google Scholar]
- 336.Lorenzo HK, Susin SA. Mitochondrial effectors in caspase-independent cell death. FEBS Lett. 2004;557(1–3):14–20. doi: 10.1016/s0014-5793(03)01464-9. [DOI] [PubMed] [Google Scholar]
- 337.Ulukaya E, Acilan C, Yilmaz Y. Apoptosis: why and how does it occur in biology? Cell Biochem Funct. 2011;29(6):468–80. doi: 10.1002/cbf.1774. [DOI] [PubMed] [Google Scholar]
- 338.Gogada R, Prabhu V, Amadori M, Scott R, Hashmi S, Chandra D. Resveratrol Induces p53-Independent, XIAP-Mediated Bax Oligomerization on Mitochondria to Initiate Cytochrome c Release and Caspase Activation. J Biol Chem. 2011;286(33):28749–60. doi: 10.1074/jbc.M110.202440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Vogelstein B, Lane D, Levine AJ. Surfing the p53 network. Nature. 2000;408(6810):307–310. doi: 10.1038/35042675. [DOI] [PubMed] [Google Scholar]
- 340.Hainaut P, Hollstein M. p53 and human cancer: the first ten thousand mutations. Adv Cancer Res. 1999;77:81–86. 86a, 87–137. doi: 10.1016/s0065-230x(08)60785-x. [DOI] [PubMed] [Google Scholar]
- 341.Zanotto-Filho A, Braganhol E, Edelweiss MI, Behr GA, Zanin R, Schröder R, Simões-Pires A, Battastini AMO, Moreira JCF. The curry spice curcumin selectively inhibits cancer cells growth in vitro and in preclinical model of glioblastoma. J Nutr Biochem. 2011 Jul 18; doi: 10.1016/j.jnutbio.2011.02.015. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 342.Ibrahim A, El-meligy A, Lungu G, Fetaih H, Dessouki A, Stoica G, Barhoumi R. Curcumin induces apoptosis in a murine mammary gland adenocarcinoma cell line through the mitochondrial pathway. Eur J Pharmacol. 2011;668(1–2):127–32. doi: 10.1016/j.ejphar.2011.06.048. [DOI] [PubMed] [Google Scholar]
- 343.Liu HS, Ke CS, Cheng HC, Huang CYF, Su CL. Curcumin-induced Mitotic Spindle Defect and Cell Cycle Arrest in Human Bladder Cancer Cells is partly through Inhibition of Aurora-A. Mol Pharmacol. 2011;80(4):638–46. doi: 10.1124/mol.111.072512. [DOI] [PubMed] [Google Scholar]
- 344.Liao YF, Hung HC, Hour TC, Hsu PC, Kao MC, Tsay GJ, Liu GY. Curcumin induces apoptosis through an ornithine decarboxylase-dependent pathway in human promyelocytic leukemia HL-60 cells. Life Sci. 2008;82(7–8):367–375. doi: 10.1016/j.lfs.2007.11.022. [DOI] [PubMed] [Google Scholar]
- 345.Walters DK, Muff R, Langsam B, Born W, Fuchs B. Cytotoxic effects of curcumin on osteosarcoma cell lines. Invest New Drugs. 2008;26(4):289–297. doi: 10.1007/s10637-007-9099-7. [DOI] [PubMed] [Google Scholar]
- 346.Wu SH, Hang LWEN, Yang JAIS, Chen HYI, Lin HUIYI, Chiang JOHUA, Lu CHIC, Yang JL, Lai TY, Ko YC. Curcumin induces apoptosis in human non-small cell lung cancer NCI-H460 cells through ER stress and caspase cascade-and mitochondria-dependent pathways. Anticancer Res. 2010;30(6):2125–2133. [PubMed] [Google Scholar]
- 347.Bhaumik S, Anjum R, Rangaraj N, Pardhasaradhi B, Khar A. Curcumin mediated apoptosis in AK-5 tumor cells involves the production of reactive oxygen intermediates. FEBS Lett. 1999;456(2):311–314. doi: 10.1016/s0014-5793(99)00969-2. [DOI] [PubMed] [Google Scholar]
- 348.Mukhopadhyay A, Bueso-Ramos C, Chatterjee D, Pantazis P, Aggarwal BB. Curcumin downregulates cell survival mechanisms in human prostate cancer cell lines. Oncogene. 2001;20(52):7597–7609. doi: 10.1038/sj.onc.1204997. [DOI] [PubMed] [Google Scholar]
- 349.Anto RJ, Mukhopadhyay A, Denning K, Aggarwal BB. Curcumin (diferuloylmethane) induces apoptosis through activation of caspase-8, BID cleavage and cytochrome c release: its suppression by ectopic expression of Bcl-2 and Bcl-xl. Carcinogenesis. 2002;23(1):143–150. doi: 10.1093/carcin/23.1.143. [DOI] [PubMed] [Google Scholar]
- 350.Morin D, Barthélémy S, Zini R, Labidalle S, Tillement JP. Curcumin induces the mitochondrial permeability transition pore mediated by membrane protein thiol oxidation. FEBS Lett. 2001;495(1–2):131–136. doi: 10.1016/s0014-5793(01)02376-6. [DOI] [PubMed] [Google Scholar]
- 351.Aziz MH, Nihal M, Fu VX, Jarrard DF, Ahmad N. Resveratrol-caused apoptosis of human prostate carcinoma LNCaP cells is mediated via modulation of phosphatidylinositol 3 - kinase/Akt pathway and Bcl-2 family proteins. Mol Cancer Ther. 2006;5(5):1335–1341. doi: 10.1158/1535-7163.MCT-05-0526. [DOI] [PubMed] [Google Scholar]
- 352.Lin H, Xiong W, Zhang X, Liu B, Zhang W, Zhang Y, Cheng J, Huang H. Notch-1 activation-dependent p53 restoration contributes to resveratrol-induced apoptosis in glioblastoma cells. Oncol Rep. 2011;26(4):925–30. doi: 10.3892/or.2011.1380. [DOI] [PubMed] [Google Scholar]
- 353.Lim YC, Cha YY. Epigallocatechin 3 gallate induces growth inhibition and apoptosis of human anaplastic thyroid carcinoma cells through suppression of EGFR/ERK pathway and cyclin B1/CDK1 complex. J Surg Oncol. 2011;104(7):776–80. doi: 10.1002/jso.21999. [DOI] [PubMed] [Google Scholar]
- 354.Choi EJ, Kim GH. Apigenin induces apoptosis through a mitochondria/caspase-pathway in human breast cancer MDA-MB-453 cells. J Clin Biochem Nutr. 2009;44(3):260–265. doi: 10.3164/jcbn.08-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Xuemei W, Fan Z, Ling Y, Ying M, Hai L, Xiaowen Z, Jialing Z. Ursolic Acid Inhibits Proliferation and Induces Apoptosis of Cancer Cells In Vitro and In Vivo. J Biomed Biotechnol. 2011;2011:419343. doi: 10.1155/2011/419343. Epub 2011 May 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Granado-Serrano AB, Martín MA, Bravo L, Goya L, Ramos S. Quercetin induces apoptosis via caspase activation, regulation of Bcl-2, and inhibition of PI-3-kinase/Akt and ERK pathways in a human hepatoma cell line (HepG2) J Nutr. 2006;136(11):2715–2721. doi: 10.1093/jn/136.11.2715. [DOI] [PubMed] [Google Scholar]
- 357.Nesaretnam K, Meganathan P. Tocotrienols: inflammation and cancer. Ann N Y Acad Sci. 2011;1229(1):18–22. doi: 10.1111/j.1749-6632.2011.06088.x. [DOI] [PubMed] [Google Scholar]
- 358.Mukherjee D, Nissen SE, Topol EJ. Risk of cardiovascular events associated with selective COX-2 inhibitors. JAMA. 2001;286(8):954–959. doi: 10.1001/jama.286.8.954. [DOI] [PubMed] [Google Scholar]
- 359.Xing LL, Wang ZN, Jiang L, Zhang Y, Xu YY, Li J, Luo Y, Zhang X. Cyclooxygenase 2 polymorphism and colorectal cancer:-765G> C variant modifies risk associated with smoking and body mass index. World J Gastroenterol. 2008;14(11):1785. doi: 10.3748/wjg.14.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Brown JR, DuBois RN. COX-2: a molecular target for colorectal cancer prevention. J Clin Oncol. 2005;23(12):2840–2855. doi: 10.1200/JCO.2005.09.051. [DOI] [PubMed] [Google Scholar]
- 361.Eberhart CE, Coffey R, Radhika A, Giardiello F, Ferrenbach S, DuBois R. Up-regulation of cyclooxygenase 2 gene expression in human colorectal adenomas and adenocarcinomas. Gastroenterology. 1994;107(4):1183–1188. doi: 10.1016/0016-5085(94)90246-1. [DOI] [PubMed] [Google Scholar]
- 362.Cai Y, Lee YF, Li G, Liu S, Bao BY, Huang J, Hsu CL, Chang C. A new prostate cancer therapeutic approach: Combination of androgen ablation with COX 2 inhibitor. Int J Cancer. 2008;123(1):195–201. doi: 10.1002/ijc.23481. [DOI] [PubMed] [Google Scholar]
- 363.Aparicio Gallego G, Diaz Prado S, Jimenez Fonseca P, Garcia Campelo R, Cassinello Espinosa J, Anton Aparicio L. Cyclooxygenase-2 (COX-2): a molecular target in prostate cancer. Clin Transl Oncol. 2007;9(11):694–702. doi: 10.1007/s12094-007-0126-0. [DOI] [PubMed] [Google Scholar]
- 364.Zhao X, Goswami M, Pokhriyal N, Ma H, Du H, Yao J, Victor TA, Polyak K, Sturgis CD, Band H. Cyclooxygenase-2 expression during immortalization and breast cancer progression. Cancer Res. 2008;68(2):467–475. doi: 10.1158/0008-5472.CAN-07-0782. [DOI] [PubMed] [Google Scholar]
- 365.Dore M, Lanthier I, Sirois J. Cyclooxygenase-2 expression in canine mammary tumors. Vet Pathol. 2003;40(2):207–212. doi: 10.1354/vp.40-2-207. [DOI] [PubMed] [Google Scholar]
- 366.Cerella C, Sobolewski C, Dicato M, Diederich M. Targeting COX-2 expression by natural compounds: A promising alternative strategy to synthetic COX-2 inhibitors for cancer chemoprevention and therapy. Biochem Pharmacol. 2010;80(12):1801–1815. doi: 10.1016/j.bcp.2010.06.050. [DOI] [PubMed] [Google Scholar]
- 367.Plummer SM, Holloway KA, Manson MM, Munks RJL, Kaptein A, Farrow S, Howells L. Inhibition of cyclo-oxygenase 2 expression in colon cells by the chemopreventive agent curcumin involves inhibition of NF-kB activation via the NIK/IKK signalling complex. Oncogene. 1999;18(44):6013–6020. doi: 10.1038/sj.onc.1202980. [DOI] [PubMed] [Google Scholar]
- 368.Arber N, Eagle CJ, Spicak J, Rácz I, Dite P, Hajer J, Zavoral M, Lechuga MJ, Gerletti P, Tang J. Celecoxib for the prevention of colorectal adenomatous polyps. N Engl J Med. 2006;355(9):885–895. doi: 10.1056/NEJMoa061652. [DOI] [PubMed] [Google Scholar]
- 369.Lev-Ari S, Strier L, Kazanov D, Madar-Shapiro L, Dvory-Sobol H, Pinchuk I, Marian B, Lichtenberg D, Arber N. Celecoxib and curcumin synergistically inhibit the growth of colorectal cancer cells. Clin Cancer Res. 2005;11(18):6738–6744. doi: 10.1158/1078-0432.CCR-05-0171. [DOI] [PubMed] [Google Scholar]
- 370.Xiao J, Tan Y, Pan Y, Liang G, Qu C, Zhang X, Zhang Y, Li X, Yang H. A New Cyclooxygenase-2 Inhibitor,(1E, 4E)-1, 5-Bis (2-bromophenyl) penta-1, 4-dien-3-one (GL63) Suppresses Cyclooxygenase-2 Gene Expression in Human Lung Epithelial Cancer Cells: Coupled mRNA Stabilization and Posttranscriptional Inhibition. Biol Pharm Bull. 2010;33(7):1170–1175. doi: 10.1248/bpb.33.1170. [DOI] [PubMed] [Google Scholar]
- 371.Wang B, Zhai C, Fang W, Chen X, Jiang K, Wang Y. The inhibitory effect of curcumin on the proliferation of HT-29 colonic cancer cell induced by deoxycholic acid. Zhonghua Nei Ke Za Zhi. 2009;48(9):760–763. [PubMed] [Google Scholar]
- 372.Uchiyama T, Toda K, Takahashi S. Resveratrol inhibits angiogenic response of cultured endothelial F-2 cells to vascular endothelial growth factor, but not to basic fibroblast growth factor. Biol Pharm Bull. 2010;33(7):1095–1100. doi: 10.1248/bpb.33.1095. [DOI] [PubMed] [Google Scholar]
- 373.Lau GTY, Leung LK. The dietary flavonoid apigenin blocks phorbol 12-myristate 13-acetate-induced COX-2 transcriptional activity in breast cell lines. Food Chem Toxicol. 2010;48(10):3022–7. doi: 10.1016/j.fct.2010.07.046. [DOI] [PubMed] [Google Scholar]
- 374.Tahanian E, Sanchez LA, Shiao TC, Roy R, Annabi B. Flavonoids targeting of I B phosphorylation abrogates carcinogen-induced MMP-9 and COX-2 expression in human brain endothelial cells. Drug Des Devel Ther. 2011;5:299–309. doi: 10.2147/DDDT.S19931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Kang OH, Lee JH, Kwon DY. Apigenin inhibits release of inflammatory mediators by blocking the NF-κB activation pathways in the HMC-1 cells. Immunopharmacol Immunotoxicol. 2010:1–7. doi: 10.3109/08923973.2010.538851. [DOI] [PubMed] [Google Scholar]
- 376.Huang GS, Tseng CY, Lee CH, Su SL, Lee HS. Effects of (−)-epigallocatechin-3-gallate on cyclooxygenase 2, PGE 2, and IL-8 expression induced by IL-1 in human synovial fibroblasts. Rheumatol Int. 2010;30(9):1197–1203. doi: 10.1007/s00296-009-1128-8. [DOI] [PubMed] [Google Scholar]
- 377.Heinecke L, Grzanna M, Au A, Mochal C, Rashmir-Raven A, Frondoza C. Inhibition of cyclooxygenase-2 expression and prostaglandin E2 production in chondrocytes by avocado soybean unsaponifiables and epigallocatechin gallate. Osteoarthritis Cartilage. 2010;18(2):220–227. doi: 10.1016/j.joca.2009.08.015. [DOI] [PubMed] [Google Scholar]
- 378.Hwang JT, Lee YK, Shin JI, Park OJ. Anti inflammatory and Anticarcinogenic Effect of Genistein Alone or in Combination with Capsaicin in TPA Treated Rat Mammary Glands or Mammary Cancer Cell Line. Ann N Y Acad Sci. 2009;1171(1):415–420. doi: 10.1111/j.1749-6632.2009.04696.x. [DOI] [PubMed] [Google Scholar]
- 379.Darnell J, Kerr IM, Stark GR. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science. 1994;264(5164):1415–1421. doi: 10.1126/science.8197455. [DOI] [PubMed] [Google Scholar]
- 380.Turkson J. STAT proteins as novel targets for cancer drug discovery. Expert Opin Ther Targets. 2004;8(5):409–422. doi: 10.1517/14728222.8.5.409. [DOI] [PubMed] [Google Scholar]
- 381.Mitchell TJ, John S. Signal transducer and activator of transcription (STAT) signalling and T cell lymphomas. Immunology. 2005;114(3):301–312. doi: 10.1111/j.1365-2567.2005.02091.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Sato T, Neilson LM, Peck AR, Liu C, Tran TH, Witkiewicz A, Hyslop T, Nevalainen MT, Sauter G, Rui H. Signal transducer and activator of transcription-3 and breast cancer prognosis. Am J Cancer Res. 2011;1(3):347–55. [PMC free article] [PubMed] [Google Scholar]
- 383.Huang M, Page C, Reynolds RK, Lin J. Constitutive activation of stat 3 oncogene product in human ovarian carcinoma cells. Gynecol Oncol. 2000;79(1):67–73. doi: 10.1006/gyno.2000.5931. [DOI] [PubMed] [Google Scholar]
- 384.Bromberg JF, Wrzeszczynska MH, Devgan G, Zhao Y, Pestell RG, Albanese C, Darnell JE. Stat3 as an Oncogene. Cell. 1999;98(3):295–303. doi: 10.1016/s0092-8674(00)81959-5. [DOI] [PubMed] [Google Scholar]
- 385.Catlett-Falcone R, Landowski TH, Oshiro MM, Turkson J, Levitzki A, Savino R, Ciliberto G, Moscinski L, Fernández-Luna JL, Nu ez G. Constitutive activation of Stat3 signaling confers resistance to apoptosis in human U266 myeloma cells. Immunity. 1999;10(1):105–115. doi: 10.1016/s1074-7613(00)80011-4. [DOI] [PubMed] [Google Scholar]
- 386.Shen Y, Devgan G, Darnell JE, Bromberg JF. Constitutively activated Stat3 protects fibroblasts from serum withdrawal and UV-induced apoptosis and antagonizes the proapoptotic effects of activated Stat1. Proc Natl Acad Sci U S A. 2001;98(4):1543–1548. doi: 10.1073/pnas.041588198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387.Garcia R, Bowman TL, NIU G, HUA Y, Minton S, Muro-Cacho CA, Cox CE, Falcone R, Fairclough R, Parsons S. Constitutive activation of Stat3 by the Src and JAK tyrosine kinases participates in growth regulation of human breast carcinoma cells. Oncogene. 2001;20(20):2499–2513. doi: 10.1038/sj.onc.1204349. [DOI] [PubMed] [Google Scholar]
- 388.Catlett-Falcone R, Dalton WS, Jove R. STAT proteins as novel targets for cancer therapy. Curr Opin Oncol. 1999;11(6):490–496. doi: 10.1097/00001622-199911000-00010. [DOI] [PubMed] [Google Scholar]
- 389.Bharti AC, Donato N, Aggarwal BB. Curcumin (diferuloylmethane) inhibits constitutive and IL-6-inducible STAT3 phosphorylation in human multiple myeloma cells. J Immunol. 2003;171(7):3863–3871. doi: 10.4049/jimmunol.171.7.3863. [DOI] [PubMed] [Google Scholar]
- 390.Carro MS, Lim WK, Alvarez MJ, Bollo RJ, Zhao X, Snyder EY, Sulman EP, Anne SL, Doetsch F, Colman H. The transcriptional network for mesenchymal transformation of brain tumours. Nature. 2009;463(7279):318–325. doi: 10.1038/nature08712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391.Weissenberger J, Priester M, Bernreuther C, Rakel S, Glatzel M, Seifert V, Kögel D. Dietary Curcumin Attenuates Glioma Growth in a Syngeneic Mouse Model by Inhibition of the JAK1, 2/STAT3 Signaling Pathway. Clin Cancer Res. 2010;16(23):5781–5795. doi: 10.1158/1078-0432.CCR-10-0446. [DOI] [PubMed] [Google Scholar]
- 392.Saydmohammed M, Joseph D, Syed V. Curcumin suppresses constitutive activation of STAT 3 by up regulating protein inhibitor of activated STAT 3 (PIAS 3) in ovarian and endometrial cancer cells. J Cell Biochem. 2010;110(2):447–456. doi: 10.1002/jcb.22558. [DOI] [PubMed] [Google Scholar]
- 393.Zhu B, Zhan W, He Y, Cai S, Wang Z, Zhang C. Epigallocatechin-3-gallate inhibits growth and angiogenesis of gastric cancer and its molecular mechanism. Zhonghua Wei Chang Wai Ke Za Zhi. 2009;12(1):82–85. [PubMed] [Google Scholar]
- 394.Park G, Yoon BS, Moon JH, Kim B, Jun EK, Oh S, Kim H, Song HJ, Noh JY, Oh CH. Green tea polyphenol epigallocatechin-3-gallate suppresses collagen production and proliferation in keloid fibroblasts via inhibition of the STAT3-signaling pathway. J Invest Dermatol. 2008;128(10):2429–2441. doi: 10.1038/jid.2008.103. [DOI] [PubMed] [Google Scholar]
- 395.Masuda M, Suzui M, Weinstein IB. Effects of epigallocatechin-3-gallate on growth, epidermal growth factor receptor signaling pathways, gene expression, and chemosensitivity in human head and neck squamous cell carcinoma cell lines. Clin Cancer Res. 2001;7(12):4220–4229. [PubMed] [Google Scholar]
- 396.Masuda M, Suzui M, Lim JTE, Weinstein IB. Epigallocatechin-3-gallate inhibits activation of HER-2/neu and downstream signaling pathways in human head and neck and breast carcinoma cells. Clin Cancer Res. 2003;9(9):3486–3491. [PubMed] [Google Scholar]
- 397.Ansó E, Zuazo A, Irigoyen M, Urdaci MC, Rouzaut A, Martínez-Irujo JJ. Flavonoids inhibit hypoxia-induced vascular endothelial growth factor expression by a HIF-1 independent mechanism. Biochem Pharmacol. 2010;79(11):1600–1609. doi: 10.1016/j.bcp.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 398.Selvendiran K, Koga H, Ueno T, Yoshida T, Maeyama M, Torimura T, Yano H, Kojiro M, Sata M. Luteolin promotes degradation in signal transducer and activator of transcription 3 in human hepatoma cells: an implication for the antitumor potential of flavonoids. Cancer Res. 2006;66(9):4826–4834. doi: 10.1158/0008-5472.CAN-05-4062. [DOI] [PubMed] [Google Scholar]
- 399.Wung B, Hsu M, Wu C, Hsieh C. Resveratrol suppresses IL-6-induced ICAM-1 gene expression in endothelial cells: effects on the inhibition of STAT3 phosphorylation. Life Sci. 2005;78(4):389–397. doi: 10.1016/j.lfs.2005.04.052. [DOI] [PubMed] [Google Scholar]
- 400.Wen S, Li H, Wu ML, Fan SH, Wang Q, Shu XH, Kong QY, Chen XY, Liu J. Inhibition of NF-κB signaling commits resveratrol-treated medulloblastoma cells to apoptosis without neuronal differentiation. J Neurooncol. 2011;104(1):169–77. doi: 10.1007/s11060-010-0496-y. [DOI] [PubMed] [Google Scholar]
- 401.Yang YP, Chang YL, Huang PI, Chiou GY, Tseng LM, Chiou SH, Chen MH, Chen MT, Shih YH, Chang CH. Resveratrol suppresses tumorigenicity and enhances radiosensitivity in primary glioblastoma tumor initiating cells by inhibiting the STAT3 axis. J Cell Physiol. 2011 doi: 10.1002/jcp.22806. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 402.Choi JS, Kang SW, Li J, Kim JL, Bae JY, Kim DS, Shin SY, Jun JG, Wang MH, Kang YH. Blockade of oxidized LDL-triggered endothelial apoptosis by quercetin and rutin through differential signaling pathways involving JAK2. J Agric Food Chem. 2009;57(5):2079–2086. doi: 10.1021/jf803390m. [DOI] [PubMed] [Google Scholar]
- 403.Muthian G, Bright JJ. Quercetin, a flavonoid phytoestrogen, ameliorates experimental allergic encephalomyelitis by blocking IL-12 signaling through JAK-STAT pathway in T lymphocyte. J Clin Immunol. 2004;24(5):542–552. doi: 10.1023/B:JOCI.0000040925.55682.a5. [DOI] [PubMed] [Google Scholar]
- 404.Pathak AK, Bhutani M, Nair AS, Ahn KS, Chakraborty A, Kadara H, Guha S, Sethi G, Aggarwal BB. Ursolic acid inhibits STAT3 activation pathway leading to suppression of proliferation and chemosensitization of human multiple myeloma cells. Mol Cancer Res. 2007;5(9):943–955. doi: 10.1158/1541-7786.MCR-06-0348. [DOI] [PubMed] [Google Scholar]
- 405.Min L, He B, Hui L. Mitogen-activated protein kinases in hepatocellular carcinoma development. Semin Cancer Biol. 2011;21(1):10–20. doi: 10.1016/j.semcancer.2010.10.011. Epub 2010/10/26. [DOI] [PubMed] [Google Scholar]
- 406.Brzezianska E, Pastuszak-Lewandoska D. A minireview: the role of MAPK/ERK and PI3K/Akt pathways in thyroid follicular cell-derived neoplasm. Front Biosci. 2011;16:422–439. doi: 10.2741/3696. [DOI] [PubMed] [Google Scholar]
- 407.McCubrey JA, Steelman LS, Abrams SL, Lee JT, Chang F, Bertrand FE, Navolanic PM, Terrian DM, Franklin RA, D’Assoro AB. Roles of the RAF/MEK/ERK and PI3K/PTEN/AKT pathways in malignant transformation and drug resistance. Adv Enzyme Regul. 2006;46(1):249–279. doi: 10.1016/j.advenzreg.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 408.Steelman L, Pohnert S, Shelton J, Franklin R, Bertrand F, McCubrey J. JAK/STAT, Raf/MEK/ERK, PI3K/Akt and BCR-ABL in cell cycle progression and leukemogenesis. Leukemia. 2004;18(2):189–218. doi: 10.1038/sj.leu.2403241. [DOI] [PubMed] [Google Scholar]
- 409.Garnett MJ, Marais R. Guilty as charged:: B-RAF is a human Oncogene. Cancer Cell. 2004;6(4):313–319. doi: 10.1016/j.ccr.2004.09.022. [DOI] [PubMed] [Google Scholar]
- 410.Enslen H, Raingeaud J, Davis RJ. Selective activation of p38 mitogen-activated protein (MAP) kinase isoforms by the MAP kinase kinases MKK3 and MKK6. J Biol Chem. 1998;273(3):1741–1748. doi: 10.1074/jbc.273.3.1741. [DOI] [PubMed] [Google Scholar]
- 411.Keller D, Zeng X, Li X, Kapoor M, Iordanov MS, Taya Y, Lozano G, Magun B, Lu H. The p38MAPK inhibitor SB203580 alleviates ultraviolet-induced phosphorylation at serine 389 but not serine 15 and activation of p53. Biochem Biophys Res Commun. 1999;261(2):464–471. doi: 10.1006/bbrc.1999.1023. [DOI] [PubMed] [Google Scholar]
- 412.Sanchez-Prieto R, Rojas JM, Taya Y, Gutkind JS. A role for the p38 mitogen-activated protein kinase pathway in the transcriptional activation of p53 on genotoxic stress by chemotherapeutic agents. Cancer Res. 2000;60(9):2464–2472. [PubMed] [Google Scholar]
- 413.Thomas SL, Zhao J, Li Z, Lou B, Du Y, Purcell J, Snyder JP, Khuri FR, Liotta D, Fu H. Activation of the p38 pathway by a novel monoketone curcumin analog, EF24, suggests a potential combination strategy. Biochem Pharmacol. 2010;80(9):1309–1316. doi: 10.1016/j.bcp.2010.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 414.Hua WF, Fu YS, Liao YJ, Xia WJ, Chen YC, Zeng YX, Kung HF, Xie D. Curcumin induces down-regulation of EZH2 expression through the MAPK pathway in MDA-MB-435 human breast cancer cells. Eur J Pharmacol. 2010;637(1–3):16–21. doi: 10.1016/j.ejphar.2010.03.051. [DOI] [PubMed] [Google Scholar]
- 415.Weir NM, Selvendiran K, Kutala VK, Tong L, Vishwanath S, Rajaram M, Tridandapani S, Anant S, Kuppusamy P. Curcumin induces G2/M arrest and apoptosis in cisplatin-resistant human ovarian cancer cells by modulating Akt and p38 MAPK. Cancer Biol Ther. 2007;6(2):178–184. doi: 10.4161/cbt.6.2.3577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 416.Woo MS, Jung SH, Kim SY, Hyun JW, Ko KH, Kim WK, Kim HS. Curcumin suppresses phorbol ester-induced matrix metalloproteinase-9 expression by inhibiting the PKC to MAPK signaling pathways in human astroglioma cells. Biochem Biophys Res Commun. 2005;335(4):1017–1025. doi: 10.1016/j.bbrc.2005.07.174. [DOI] [PubMed] [Google Scholar]
- 417.Filomeni G, Graziani I, Rotilio G, Ciriolo M. trans-Resveratrol induces apoptosis in human breast cancer cells MCF-7 by the activation of MAP kinases pathways. Genes Nutr. 2007;2(3):295–305. doi: 10.1007/s12263-007-0059-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 418.Sánchez-Fidalgo S, Cárdeno A, Villegas I, Talero E, de la Lastra CA. Dietary supplementation of resveratrol attenuates chronic colonic inflammation in mice. Eur J Pharmacol. 2010;633(1–3):78–84. doi: 10.1016/j.ejphar.2010.01.025. [DOI] [PubMed] [Google Scholar]
- 419.Noh HJ, SungG EG, Kim JY, Lee TJ, Song IH. Suppression of phorbol-12-myristate-13-acetate-induced tumor cell invasion by apigenin via the inhibition of p38 mitogen-activated protein kinase-dependent matrix metalloproteinase-9 expression. Oncol Rep. 2010;24(1):277–283. doi: 10.3892/or_00000857. [DOI] [PubMed] [Google Scholar]
- 420.Shukla S, Gupta S. Apigenin-Induced Cell Cycle Arrest is Mediated by Modulation of MAPK, PI3K-Akt and Loss of Cyclin D1 Associated Retinoblastoma Dephosphorylation in Human Prostate Cancer Cells. Cell Cycle. 2007;6(9):1102–1114. doi: 10.4161/cc.6.9.4146. [DOI] [PubMed] [Google Scholar]
- 421.Cavet ME, Harrington KL, Vollmer TR, Ward KW, Zhang JZ. Anti-inflammatory and anti-oxidative effects of the green tea polyphenol epigallocatechin gallate in human corneal epithelial cells. Mol Vis. 2011;17:533–542. [PMC free article] [PubMed] [Google Scholar]
- 422.Yang D, Liu J, Tian C, Zeng Y, Zheng Y, Fang Q, Li H. Epigallocatechin gallate inhibits angiotensin II-induced endothelial barrier dysfunction via inhibition of the p38 MAPK/HSP27 pathway. Acta Pharmacol Sin. 2010;31(10):1401–1406. doi: 10.1038/aps.2010.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 423.Shan J, Xuan Y, Zheng S, Dong Q, Zhang S. Ursolic acid inhibits proliferation and induces apoptosis of HT-29 colon cancer cells by inhibiting the EGFR/MAPK pathway. J Zhejiang Univ Sci B. 2009;10(9):668–674. doi: 10.1631/jzus.B0920149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 424.Lee YS, Sohn KC, Kim KH, Cho MJ, Hur GM, Yoon TJ, Kim SK, Lee K, Lee JH, Kim CD. Role of protein kinase C delta in X ray induced apoptosis of keratinocyte. Exp Dermatol. 2009;18(1):50–56. doi: 10.1111/j.1600-0625.2008.00761.x. [DOI] [PubMed] [Google Scholar]
- 425.Araújo JR, Gon alves P, Martel F. Chemopreventive effect of dietary polyphenols in colorectal cancer cell lines. Nutr Res. 2011;31(2):77–87. doi: 10.1016/j.nutres.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 426.Ramos S. Effects of dietary flavonoids on apoptotic pathways related to cancer chemoprevention. J Nutr Biochem. 2007;18(7):427–442. doi: 10.1016/j.jnutbio.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 427.Jun DY, Park HS, Kim JS, Park W, Song BH, Kim HS, Taub D, Kim YH. 17 [alpha]-Estradiol arrests cell cycle progression at G2/M and induces apoptotic cell death in human acute leukemia Jurkat T cells. Toxicol Appl Pharmacol. 2008;231(3):401–412. doi: 10.1016/j.taap.2008.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 428.Kuo PL, Chen CY, Tzeng TF, Lin CC, Hsu YL. Involvement of reactive oxygen species/c-Jun NH2-terminal kinase pathway in kotomolide A induces apoptosis in human breast cancer cells. Toxicol Appl Pharmacol. 2008;229(2):215–226. doi: 10.1016/j.taap.2008.01.034. [DOI] [PubMed] [Google Scholar]
- 429.Sánchez CA, Rodríguez E, Varela E, Zapata E, Páez A, Massó FA, Montaño LF, López-Marure R. Statin-induced inhibition of MCF-7 breast cancer cell proliferation is related to cell cycle arrest and apoptotic and necrotic cell death mediated by an enhanced oxidative stress. Cancer invest. 2008;26(7):698–707. doi: 10.1080/07357900701874658. [DOI] [PubMed] [Google Scholar]
- 430.Lu JJ, Cai YJ, Ding J. Curcumin induces DNA damage and caffeine-insensitive cell cycle arrest in colorectal carcinoma HCT116 cells. Mol Cell Biochem. 2011;354(1–2):247–52. doi: 10.1007/s11010-011-0824-3. [DOI] [PubMed] [Google Scholar]
- 431.LEE DUKSU, LEE MIK, KIM JHEE. Curcumin induces cell cycle arrest and apoptosis in human osteosarcoma (HOS) cells. Anticancer Res. 2009;29(12):5039–5044. [PubMed] [Google Scholar]
- 432.Zhang Q, Zhao XH, Wang ZJ. Cytotoxicity of flavones and flavonols to a human esophageal squamous cell carcinoma cell line (KYSE-510) by induction of G2/M arrest and apoptosis. Toxicol In Vitro. 2009;23(5):797–807. doi: 10.1016/j.tiv.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 433.Cui J, Sun R, Yu Y, Gou S, Zhao G, Wang C. Antiproliferative effect of resveratrol in pancreatic cancer cells. Phytother Res. 2010;24(11):1637–1644. doi: 10.1002/ptr.3157. [DOI] [PubMed] [Google Scholar]
- 434.Cai J, Zhao XL, Liu AW, Nian H, Zhang SH. Apigenin inhibits hepatoma cell growth through alteration of gene expression patterns. Phytomedicine. 2011;18(5):366–373. doi: 10.1016/j.phymed.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 435.Ruela-de-Sousa R, Fuhler G, Blom N, Ferreira C, Aoyama H, Peppelenbosch M. Cytotoxicity of apigenin on leukemia cell lines: implications for prevention and therapy. Cell Death Dis. 2010;1(1):e19. doi: 10.1038/cddis.2009.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 436.Jakubikova J, Cervi D, Ooi M, Kim K, Nahar S, Klippel S, Cholujova D, Leiba M, Daley JF, Delmore J. Anti-tumor activity and signaling events triggered by the isothiocyanates, sulforaphane and PEITC in multiple myeloma. Haematologica. 2011;96(8):1170–9. doi: 10.3324/haematol.2010.029363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 437.Karin M, Liu Z, Zandi E. AP-1 function and regulation. Curr Opin Cell Biol. 1997;9(2):240–246. doi: 10.1016/s0955-0674(97)80068-3. [DOI] [PubMed] [Google Scholar]
- 438.Patel VB, Misra S, Patel BB, Majumdar APN. Colorectal cancer: chemopreventive role of curcumin and resveratrol. Nutr Cancer. 2010;62(7):958–967. doi: 10.1080/01635581.2010.510259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 439.Shehzad A, Wahid F, Lee YS. Curcumin in cancer chemoprevention: molecular targets, pharmacokinetics, bioavailability, and clinical trials. Arch Pharm (Weinheim) 2010;343(9):489–99. doi: 10.1002/ardp.200900319. [DOI] [PubMed] [Google Scholar]
- 440.Young MR, Li JJ, Rincón M, Flavell RA, Sathyanarayana B, Hunziker R, Colburn N. Transgenic mice demonstrate AP-1 (activator protein-1) transactivation is required for tumor promotion. Proc Natl Acad Sci U S A. 1999;96(17):9827–9832. doi: 10.1073/pnas.96.17.9827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 441.Funakoshi Tago M, Tago K, Sonoda Y, Tominaga S, Kasahara T. TRAF6 and C SRC induce synergistic AP 1 activation via PI3 kinase–AKT–JNK pathway. Eur J Biochem. 2003;270(6):1257–1268. doi: 10.1046/j.1432-1033.2003.03487.x. [DOI] [PubMed] [Google Scholar]
- 442.Aggarwal BB, Shishodia S. Molecular targets of dietary agents for prevention and therapy of cancer. Biochem Pharmacol. 2006;71(10):1397–1421. doi: 10.1016/j.bcp.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 443.Matthews CP, Colburn NH, Young MR. AP-1 a target for cancer prevention. Curr Cancer Drug Targets. 2007;7(4):317–324. doi: 10.2174/156800907780809723. [DOI] [PubMed] [Google Scholar]
- 444.Jacobs-Helber SM, Wickrema A, Birrer MJ, Sawyer ST. AP1 regulation of proliferation and initiation of apoptosis in erythropoietin-dependent erythroid cells. Mol Cell Biol. 1998;18(7):3699–3707. doi: 10.1128/mcb.18.7.3699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 445.Dhandapani KM, Mahesh VB, Brann DW. Curcumin suppresses growth and chemoresistance of human glioblastoma cells via AP 1 and NF B transcription factors. J Neurochem. 2007;102(2):522–538. doi: 10.1111/j.1471-4159.2007.04633.x. [DOI] [PubMed] [Google Scholar]
- 446.Jang M, Pezzuto JM. Effects of resveratrol on 12-O-tetradecanoylphorbol-13-acetate-induced oxidative events and gene expression in mouse skin. Cancer lett. 1998;134(1):81–89. doi: 10.1016/s0304-3835(98)00250-x. [DOI] [PubMed] [Google Scholar]
- 447.Yun HJ, Yoo WH, Han MK, Lee YR, Kim JS, Lee SI. Epigallocatechin-3-gallate suppresses TNF-alpha -induced production of MMP-1 and -3 in rheumatoid arthritis synovial fibroblasts. Rheumatol Int. 2008;29(1):23–29. doi: 10.1007/s00296-008-0597-5. [DOI] [PubMed] [Google Scholar]
- 448.Nair S, Barve A, Khor TO, Shen G, Lin W, Chan JY, Cai L, Kong AN. Regulation of Nrf2-and AP-1-mediated gene expression by epigallocatechin-3-gallate and sulforaphane in prostate of Nrf2-knockout or C57BL/6J mice and PC-3 AP-1 human prostate cancer cells. Acta Pharmacol Sin. 2010;31(9):1223–40. doi: 10.1038/aps.2010.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 449.Lim H, Park H, Kim HP. Effects of Flavonoids on Matrix Metalloproteinase-13 Expression of Interleukin-1β–Treated Articular Chondrocytes and Their Cellular Mechanisms: Inhibition of c-Fos/AP-1 and JAK/STAT Signaling Pathways. J Pharmacol Sci. 2011;116(2):221–231. doi: 10.1254/jphs.11014fp. [DOI] [PubMed] [Google Scholar]
- 450.Hwang YP, Oh KN, Yun HJ, Jeong HG. The flavonoids apigenin and luteolin suppress ultraviolet A-induced matrix metalloproteinase-1 expression via MAPKs and AP-1-dependent signaling in HaCaT cells. J Dermatol Sci. 2011;61(1):23–31. doi: 10.1016/j.jdermsci.2010.10.016. [DOI] [PubMed] [Google Scholar]
- 451.Li Z, Li J, Mo B, Hu C, Liu H, Qi H, Wang X, Xu J, editors. Genistein induces G 2/M cell cycle arrest via stable activation of ERK1/2 pathway in MDA-MB-231 breast cancer cells. Springer; 2009. [DOI] [PubMed] [Google Scholar]