Significance
Gefitinib is a small molecular inhibitor that targets EGFR tyrosine kinases (EGFR-TKI) and has been used as a first-line treatment for advanced lung cancer. However, not all lung cancer patients respond to gefitinib treatment, and resistance to gefitinib has been apparent for lung cancer patients who have undergone treatment for a few months. We observed that FOXO3a expression is inversely correlated with lung cancer patients who responded poorly to EGFR-TKI treatment and identified an underlying mechanism of FOXO3a in EGFR mutation-independent cancer stemness and gefitinib resistance through the epigenetic regulation of NF-κB/miR-155. This finding highlights the potential of targeting the NF-κB/miR-155/FOXO3a pathway as a novel therapeutic strategy for lung cancer with the acquisition of resistance to EGFR-TKIs.
Keywords: EGFR, gefitinib, lung cancer, miR-155, NF-κB
Abstract
Therapy with epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (EGFR-TKIs, such as gefitinib or erlotinib) significantly prolongs survival time for patients with tumors harboring an activated mutation on EGFR; however, up to 40% of lung cancer patients exhibit acquired resistance to EGFR-TKIs with an unknown mechanism. FOXO3a, a transcription factor of the forkhead family, triggers apoptosis, but the mechanistic details involved in EGFR-TKI resistance and cancer stemness remain largely unclear. Here, we observed that a high level of FOXO3a was correlated with EGFR mutation-independent EGFR-TKI sensitivity, the suppression of cancer stemness, and better progression-free survival in lung cancer patients. The suppression of FOXO3a obviously increased gefitinib resistance and enhanced the stem-like properties of lung cancer cells; consistent overexpression of FOXO3a in gefitinib-resistant lung cancer cells reduced these effects. Moreover, we identified that miR-155 targeted the 3′UTR of FOXO3a and was transcriptionally regulated by NF-κB, leading to repressed FOXO3a expression and increased gefitinib resistance, as well as enhanced cancer stemness of lung cancer in vitro and in vivo. Our findings indicate that FOXO3a is a significant factor in EGFR mutation-independent gefitinib resistance and the stemness of lung cancer, and suggest that targeting the NF-κB/miR-155/FOXO3a pathway has potential therapeutic value in lung cancer with the acquisition of resistance to EGFR-TKIs.
Small molecule inhibitors of receptor tyrosine kinase are currently an important treatment for nonsmall cell lung cancer (NSCLC), especially for tumors harboring an activated mutation of epithelial growth factor receptor (EGFR) (1–3). Genetic mutations of L858R or exon 19 deletions in ∼90% of EGFR mutations of lung cancer patients are associated with sensitivity to EGFR tyrosine kinase inhibitors (EGFR-TKIs), such as gefitinib and erlotinib; however, patients who received EGFR-TKI treatment showed a response for ∼10–16 mo (4). Increasing studies have reported several acquired resistance mechanisms, such as T790M site mutation of EGFR (5), hyperactivation of HER2 and receptor tyrosine kinase MET (6, 7), and somatic mutations in Kirsten rat sarcoma viral oncogene homolog (KRAS), BRAF, and phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha (PIK3CA) (8), and indicated that minor subpopulations of cancer stem cells (CSC) are intrinsically more resistant to anticancer drugs or radiation and are responsible for metastasis and recurrence to cancer therapies (9), including the EGFR-TKI resistance of lung cancers (10, 11). Unfortunately, the intrinsic mechanisms of acquired resistance remain unclear for up to 40% of lung cancer patients (12). Therefore, it is necessary to further clarify the underlying mechanism of EGFR-TKI resistance in lung cancer to improve the efficiency of the clinical treatment and develop new therapeutic strategies.
The human FOXO family includes FOXO1, FOXO3a, FOXO4, and FOXO6; FOXO3a is abundant in various tissues and is different from the other three in its tissue specificity (13). Additionally, FOXO3a is a transcription factor that acts as a tumor suppressor by inducing cell cycle arrest, and the down-regulation of FOXO3a is involved in the tumorigenesis of various cancer types (14). Studies indicate that FOXO3a activity is negatively regulated by oncogenic kinases, such as AKT, IKK, and ERK (15–17), and the activation of these oncogenic kinases is associated with FOXO3a suppression, which triggers cancer progression. Because knockdown of FOXO3a in breast cancer results in a reduction of gefitinib-induced cell cycle arrest and cell death (18), and FOXO3a nuclear localization induced by metformin or SN-38 would down-regulate the properties of stem-like cells in breast and ovarian cancer cells (19), we speculate that FOXO3a might be involved in resistance to EGFR-TKIs and the CSC properties of lung cancer and could be another pathway for cancer cells to survive by resisting gefitinib agents.
Here, we found that FOXO3a was negatively correlated with EGFR mutation-independent gefitinib resistance and reduced CSC properties in lung cancer. Moreover, we revealed the down-regulation of FOXO3a through NF-κB activation via miR-155, which confer gefitinib resistance and stemness in lung cancer. Our findings suggest that FOXO3a suppression contributes to acquired gefitinib resistance in NSCLC patients carrying an EGFR-activating mutation.
Results
FOXO3a Expression Is Associated with EGFR-TKI Resistance and Cancer Stemness.
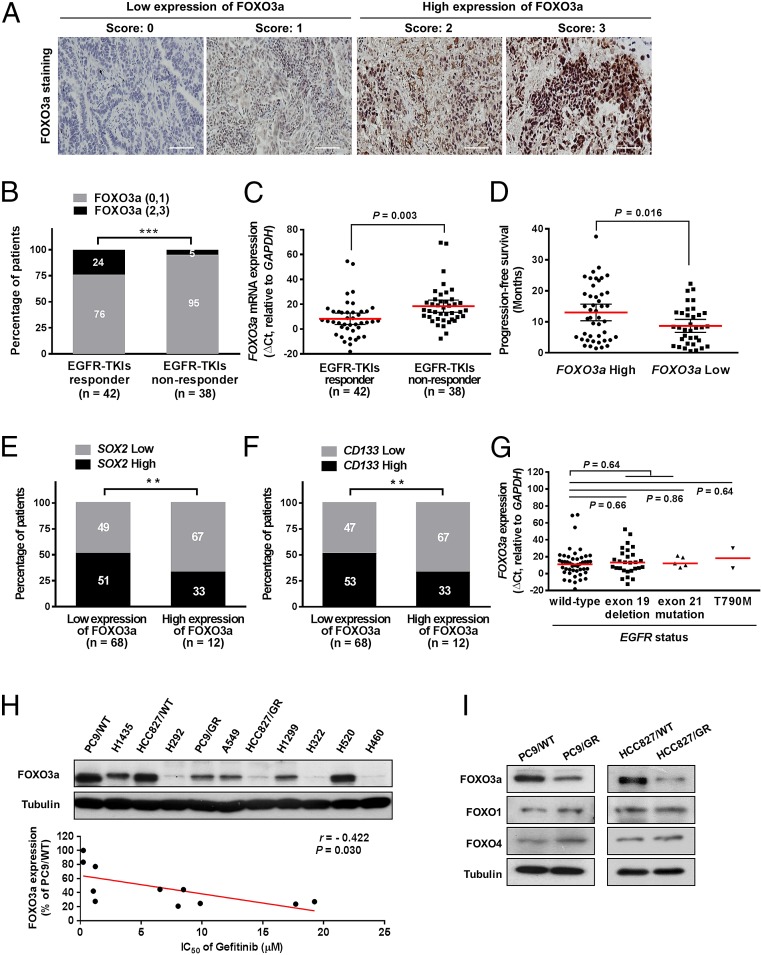
To elicit the clinical significance of FOXO3a in EGFR-TKI resistance, we retrospectively collected and analyzed specimens from a cohort of 80 lung cancer patients who had received EGFR-TKIs (erlotinib or gefitinib), either as front line or salvage treatment. The expression of FOXO3a in lung cancer tissues was detected by immunohistochemical (IHC) staining, and we observed that the nuclear staining intensity of FOXO3a was stronger than cytoplasmic staining (Fig. 1A), and a higher proportion of patients who responded to EGFR-TKI treatment expressed high FOXO3a protein (Fig. 1B) and high FOXO3a mRNA (Fig. 1C) than the nonresponder group. In addition, high levels of FOXO3a were significantly correlated with better survival outcomes (SI Appendix, Fig. S1A), according to an analysis of the online PrognoScan database, and our collected cohort also observed that patients with a high level of FOXO3a taking EGFR-TKI treatment lived for over a year without tumor growth (progression-free survival; PFS of 13.0 mo) versus those with low levels of FOXO3a (PFS of 8.68 mo) (Fig. 1D), suggesting that FOXO3a plays a critical role in response to EGFR-TKIs and survival outcomes of lung cancer patients. We further found that high expression of CSC markers, such as SRY (sex determining region Y)-box 2 (SOX2) and cell surface marker CD133, were significantly inversely correlated with FOXO3a expression (Fig. 1E and SI Appendix, Table S1) and positively with EGFR-TKI nonresponders (SI Appendix, Fig. S1B). However, we analyzed the relationship between FOXO3a expression and EGFR mutation status of lung cancer and observed that FOXO3a is not significantly correlated with wild-type and mutated (exon 19 deletion, T790M or L858R) EGFR in our cohorts (Fig. 1G), and was consistent with observations in the The Cancer Genome Atlas (TCGA), Gene-Expression Omnibus, and Oncomine databases (SI Appendix, Fig. S1 C–E). These findings imply that FOXO3a might be involved in EGFR mutation-independent EGFR-TKI resistance in lung cancer. To further investigate whether FOXO3a expression is associated with EGFR mutation-independent EGFR-TKI resistance, we analyzed the relationship of FOXO3a and gefitinib sensitivity in lung cancer cell lines and found that the FOXO3a protein level was negatively correlated with the IC50 of gefitinib in lung cancer cells (Fig. 1H and SI Appendix, Table S2). Compared with parental PC9/WT cells, gefitinib-resistant PC9 (PC9/GR) cells contained the same exon 19 deletion of EGFR appeared to have a low level of gefitinib sensitivity and FOXO3a expression but not FOXO1 or FOXO4 (Fig. 1I). Consistent effects were observed in parental HCC827/WT cells and HCC827/GR cells with exon 19 deletion of EGFR. These results suggest that the expression of FOXO3a was positively correlated with gefitinib sensitivity and CSC suppression of lung cancer in an EGFR mutation-independent manner.
Fig. 1.
FOXO3a expression is associated with the response to gefitinib treatment and stemness in lung cancer. (A) IHC staining yielded scores ranging from 0 to 3, which are representative of the amount of FOXO3a in lung cancer specimens. A score of 0–1 represented low expression of FOXO3a, and 2–3 represented high expression. (Scale bars, 100 μm.) (B) The percentages of patients with high expression (black bar) and low expression of FOXO3a (gray bar) were assigned according to different responses to EGFR-TKIs (responder, n = 42; nonresponder, n = 38). Numbers near bars represent the percentage of patients for each condition. ***P < 0.001 (Pearson’s χ2 test). (C) Expression of FOXO3a mRNA was assigned according to different responses to EGFR-TKIs. FOXO3a expression in tumors was classified according to the median of the individual ΔCt values of patient samples. The median of the individual ΔCt values of patient samples was used as the cut-off value between high and low expression. Lower ΔCt values indicate higher expression of the gene. P = 0.003. (D) Mean PFS of patients with high levels of FOXO3a [n = 45, PFS of 13 mo, 95% confidence interval (CI) = 10.33–15.68] and low levels of FOXO3a (n = 35, PFS of 8.68 mo, 95% CI = 6.59–10.77) taking EGFR-TKI treatment. P = 0.016. (E and F) Patients with high SOX2 (E) and CD133 (F) expression were accompanied by decreased expression of FOXO3a protein. The expression levels of FOXO3a were classified into two groups according to the IHC scoring: low-expression group (score 0 and 1) and high-expression group (score 2 and 3). P values were determined by Pearson’s χ2 test. **P < 0.01. (G) The expression of FOXO3a between wild-type and mutant EGFR in lung cancer. The EGFR mutational status of patients contains wild-type (n = 45), exon 19 deletion (n = 28), exon 21 mutation (L858R and L861Q, n = 5), and T790M (n = 2). (H) Inverse correlation between FOXO3a expression (Upper) and IC50 of gefitinib in human lung cancer cell lines (Lower). The IC50 values were obtained by MTT assay as shown in SI Appendix, Table S2. r = −0.422; P = 0.030 (Pearson's correlation coefficient). (I) Expression levels of FOXO3a, FOXO1, and FOXO4 in PC9/WT and PC9/GR (Left), as well as in HCC827/WT and HCC827/GR cells (Right), were analyzed by Western blot. Tubulin was used as a loading control.
Effects of FOXO3a Involved in Gefitinib Resistance and CSC Properties.
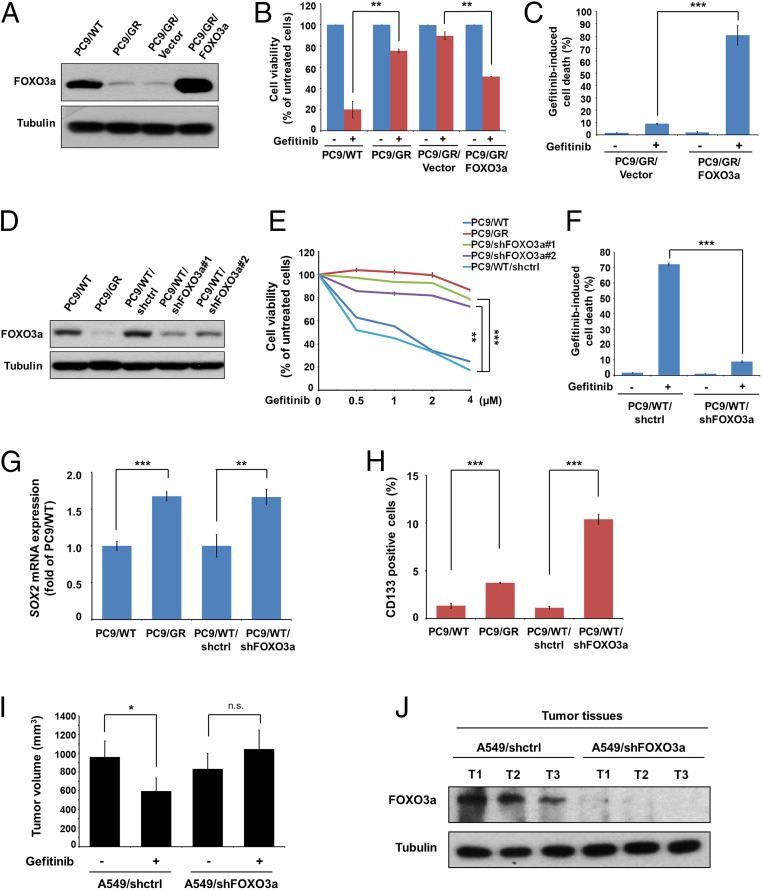
To elucidate whether FOXO3a affects sensitivity to gefitinib, we generated PC9/GR with the overexpression of FOXO3a (Fig. 2A) and found that PC9/GR/FOXO3a cells significantly increased sensitivity to gefitinib (Fig. 2 B and C) and the effects of FOXO3a were consistent in H460 and H292 cells (SI Appendix, Fig. S2 A–F). Additionally, FOXO3a-knockdown PC9/WT cells (PC9/WT/shFOXO3a) (Fig. 2D) exhibited increased resistance to gefitinib and gefitinib-induced apoptotic population (Fig. 2 E and F), which was consistent in HCC827 and A549 cells (SI Appendix, Fig. S2 G and H). We also found that the expression of SOX2, Kruppel-like factor 4 (KLF4), Nanog homeobox (Nanog), the CD133+ and aldehyde dehydrogenases (ALDH)+ cells, sphere formation, and side population were significantly increased in PC9/GR cells compared with PC9/WT cells, and that the CSC properties were increased in the FOXO3a-knockdown cells (Fig. 2 G and H and SI Appendix, Fig. S3 A–D). Moreover, the A549/shFOXO3a-bearing mice showed increased tumor volume in response to gefitinib resistance compared with the A549/shctrl-bearing mice (Fig. 2I), and FOXO3a expression was found in formed tumors (Fig. 2J). The above data suggest that FOXO3a was involved in the gefitinib response and the CSC characteristics of lung cancer cells.
Fig. 2.
FOXO3a is required for gefitinib-induced cell death and CSC markers of lung cancer cells. (A) FOXO3a expression was analyzed by Western blot in PC9/WT, PC/GR, vector control, and FOXO3a-overexpressing PC9/GR (PC9/GR/FOXO3a) cells. (B) PC9/GR/FOXO3a and corresponding vector control cells were treated with 4 μM gefitinib, and cell viability was measured by MTT assay and (C) cell apoptosis was detected by flow cytometric analysis, respectively. (D) Western blot analysis of FOXO3a expression in PC9/WT, PC9/GR, shctrl control, and FOXO3a-knockdown of PC9/WT (PC9/WT/shFOXO3a#1 and #2) cells. (E) The indicated concentrations of gefitinib were added to these cells; cell viability was measured by MTT assay, and (F) cell apoptosis was detected by flow cytometric analysis. (G) The effects of FOXO3a expression mediated mRNA expression of SOX2. Total RNA was harvested for the analysis of SOX2 mRNA by real-time RT-PCR. (H) The effects of FOXO3a expression mediated CD133+ cells. Cells were harvested for flow cytometric analysis to detect CD133+ cells. The results are shown as the means ± SD of three independent experiments, each performed in triplicate. **P < 0.01 and ***P < 0.001 (two-tailed Student's t test). (I) Effects of FOXO3a depletion on the tumor growth of A549 cells were evaluated in the NOD/SCID mice with xenografts after treatment with gefitinib for 2 wk. Tumor volume was calculated as indicated in Materials and Methods. Five mice for each group; *P < 0.05 (two-tailed Student’s t test). (J) Western blot assay to confirm the expression of FOXO3a in the indicated group of tumor samples. Tubulin was used as a loading control.
miR-155 Targeting of FOXO3a Is Correlated with Gefitinib Sensitivity.
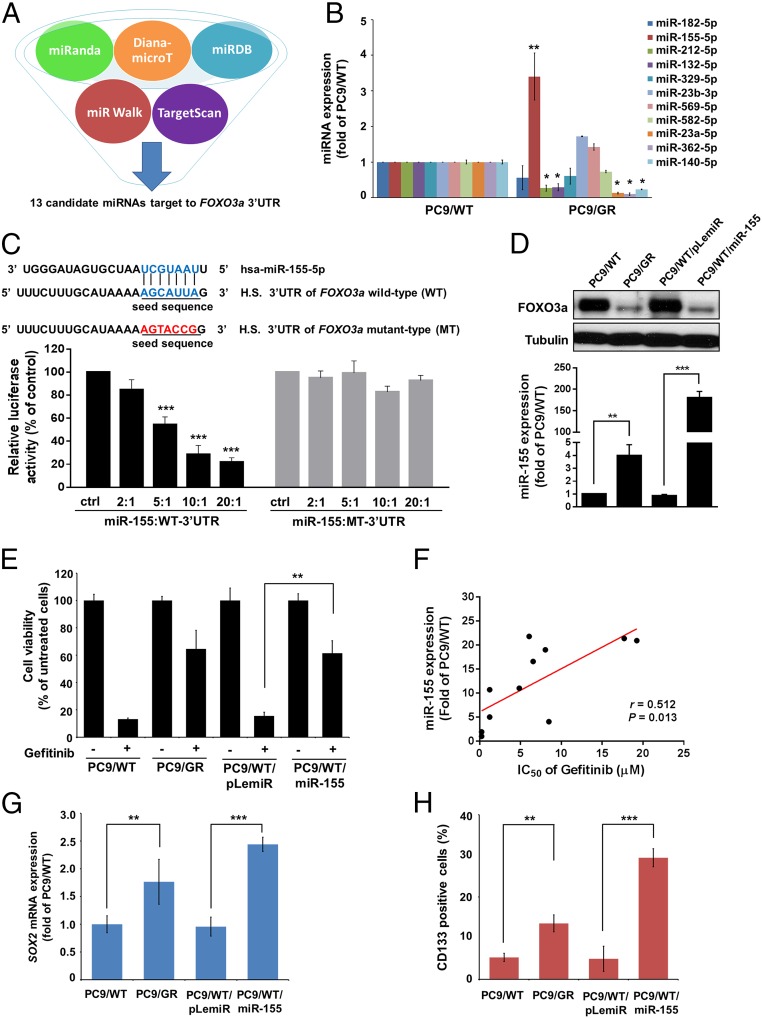
To investigate whether FOXO3a was regulated by microRNAs, we first used five different algorithms (miRanda, DianamicroT, miRDB, miRWalk, and TargetScan) to filter out 13 candidate microRNAs commonly predicted to target to FOXO3a 3′UTR (Fig. 3A). Next, these microRNA candidates were validated by real-time RT-PCR to confirm differential expression, and one of the identified candidates, miR-155, was significantly increased in PC9/GR cells compared with PC9/WT cells (Fig. 3B). Nevertheless, miR-380–3p and miR-495 were not detectable in the PC9/WT and PC9/GR cells. To further identify whether FOXO3a was a direct target of miR-155, the binding sequence of miR-155 on FOXO3a 3′UTR was predicted by miRanda software, and wild-type FOXO3a 3′UTR (WT-3′UTR) and miR-155 binding site mutant FOXO3a 3′UTR (MT-3′UTR) reporters were transfected into PC9/WT cells at different miR-155–to–reporter ratios. Cotransfection with miR-155 and the WT-3′UTR reporter resulted in a significant inhibition of luciferase activity in a dose-dependent manner, but not in the MT-3′UTR reporter (Fig. 3C). Western blot analysis showed that the increased expression of miR-155 clearly reduced the expression of FOXO3a protein (Fig. 3D), and the expression level of miR-155 was also inversely correlated with FOXO3a expression in lung cancer patients by an approach to the TCGA database (SI Appendix, Table S3). Moreover, we found that FOXO3a-mediated gefitinib sensitivity was significantly repressed by miR-155 in PC9/WT cells (Fig. 3E) and observed a positive correlation between miR-155 expression and gefitinib resistance in lung cancer cells (Fig. 3F). PC9/WT cells with overexpression of miR-155 significantly increased the CSC properties, including the expression level of SOX2, KLF4, Nanog, the CD133+, and ALDH+ cells, sphere formation, and side population (Fig. 3 G and H and SI Appendix, Fig. S3 E–H). These results suggest that miR-155 directly hinders FOXO3a expression and is involved in FOXO3a-mediated gefitinib sensitivity and cancer stemness.
Fig. 3.
miR-155 is involved in FOXO3a-mediated gefitinib sensitivity and CSC characteristics. (A) Schematic of the bioinformatic analyses of candidate microRNAs that targeted FOXO3a. The putative microRNAs targeting FOXO3a in miRWalk, TargetScan, miRanda, miRDB, and Diana-microT were searched and 13 candidate microRNAs were filtered out for real-time RT-PCR analysis. (B) Real-time RT-PCR analysis of the differential expression of the 11 microRNAs in PC9/WT compared with PC9/GR cells. (C) A schematic diagram representing the predicted miR-155 binding sequences or mutated versions (Upper). Luciferase activity (Lower) of wild-type FOXO3a-3′UTR (WT-3′UTR) and mutant-type FOXO3a-3′UTR (MT-3′UTR) reporter genes were measured using the Dual-Luciferase Reporter Assay System in PC9/WT cells transfected with miR-155 at different ratios. (D) The effects of miR-155 overexpression on endogenous FOXO3a protein expression in PC9/WT cells. FOXO3a and miR-155 expression were measured by Western blot analysis and real-time RT-PCR, respectively. Tubulin was used as a loading control. (E) The functions of miR-155 in regulating gefitinib sensitivity were assayed by comparing PC9/WT/pLemiR and PC9/WT/miR-155 cells that were treated with or without 4 μM gefitinib. Cell viability was determined by MTT assay. (F) The correlation between miR-155 expression and the IC50 of gefitinib with doses for the lung cancer cell lines. (r = 0.512; P = 0.013; Pearson’s correlation coefficient). Expression of miR-155 affected (G) SOX2 mRNA levels by real-time RT-PCR and (H) CD133+ cells by flow cytometric analysis in PC/WT cells with the expression of pLemiR-155 and control vector. The results are shown as the means ± SD of three independent experiments, each performed in triplicate. *P < 0.05, **P < 0.01, and ***P < 0.001 (two-tailed Student's t test).
miR-155 Represses FOXO3a-Mediated Gefitinib Sensitivity.
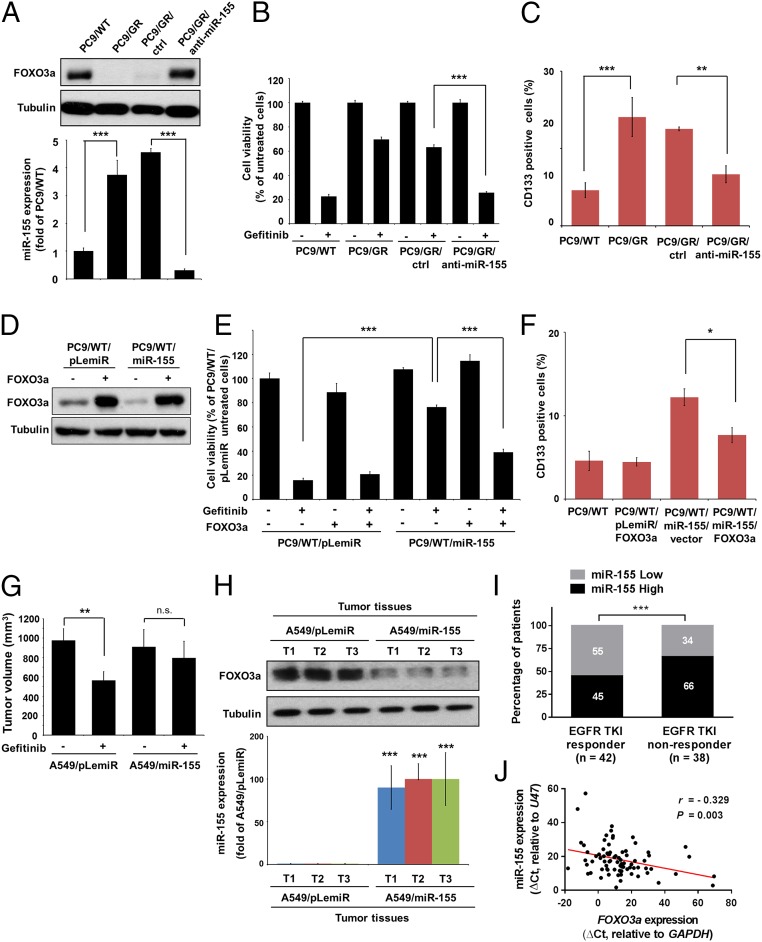
To obtain more detailed information on miR-155–repressed FOXO3a-mediated gefitinib sensitivity, we transfected PC9/GR cells with scramble control oligos (ctrl) or anti–miR-155. FOXO3a protein levels increased along with significantly reduced miR-155 expression upon anti–miR-155 transfection in PC9/GR cells (Fig. 4A). Moreover, the blockage of miR-155 expression increased gefitinib sensitivity (Fig. 4B) and decreased the CSC properties (Fig. 4C and SI Appendix, Fig. S4 A–D). We established a FOXO3a-expressing construct lacking the FOXO3a 3′UTR and transfected it into control- and miR-155–overexpressing PC9/WT cells. The transfection of the FOXO3a-expressing vector successfully restored the expression of FOXO3a protein suppressed by miR-155 in cells (Fig. 4D) and abolished miR-155–mediated gefitinib sensitivity (Fig. 4E) and CSC phenotypes (Fig. 4F and SI Appendix, Fig. S4 E–H). Consistent with the observations made in vitro, mice-bearing tumors derived from A549 cells with the overexpression of miR-155 showed resistance to gefitinib treatment (Fig. 4G) because of FOXO3a depletion in formed tumors (Fig. 4H). By analyzing lung cancer tissue specimens, it was found that a higher proportion (66%) of patients who did not respond to EGFR-TKI treatment expressed a high level of miR-155 than the responder group (Fig. 4I). Additionally, we observed an inverse correlation between miR-155 and FOXO3a expression in lung cancer (Fig. 4J) and in the TCGA database (SI Appendix, Table S3). Taken together, these findings suggest that the expression of miR-155 is increased in patients who are considered to have poor response to EGFR-TKIs, and its expression is inversely correlated with FOXO3a expression.
Fig. 4.
miR-155 mediates gefitinib resistance and CSC of lung cancer cells. (A) FOXO3a and miR-155 were measured by Western blot analysis (Upper) and real-time RT-PCR (Lower) in PC9/WT, PC/GR, and PC9/GR cells with expressing anti–miR-155 (PC9/GR/anti-miR-155) and scramble control oligos (PC9/GR/ctrl), respectively. (B and C) Indicated cells were untreated or treated with 4 μM gefitinib and analyzed for cell viability by MTT assay and CD133+ cells by flow cytometric analysis. **P < 0.01 and ***P < 0.001 (two-tailed Student’s t test). (D) Western blot analysis of FOXO3a expression in PC9/WT cells transiently transfected with miR-155 and FOXO3a. (E and F) Indicated cells were untreated or treated with 4 μM gefitinib and analyzed for cell viability by MTT assay and CD133+ cells by flow cytometric analysis. *P < 0.05 and ***P < 0.001 (two-tailed Student’s t test). (G) Effects of miR-155 overexpression on the tumor growth of A549 cells were evaluated in the NOD/SCID mice with xenografts after treatment with gefitinib for 2 wk. Five mice in each group; **P < 0.01 and n.s., not significant (two-tailed Student's t test). (H) FOXO3a and miR-155 expression were measured by Western blot analysis and real-time RT-PCR in the indicated group of tumor samples, respectively. The results are shown as the means ± SD of three independent experiments, each performed in triplicate. ***P < 0.001 (two-tailed Student's t test). (I) miR-155 expression in patients was assigned according to different responses to EGFR-TKIs. miR-155 expression levels in tumors that were classified into high and low groups according to median of the individual ΔCt values of patient samples. ***P < 0.001 (Pearson’s χ2 test). (J) The inverse correlation between miR-155 and FOXO3a in lung cancer. n = 80; r = −0.329; P = 0.003 (Pearson's correlation coefficient).
NF-κB Is Involved in the Regulation of miR-155 Expression.
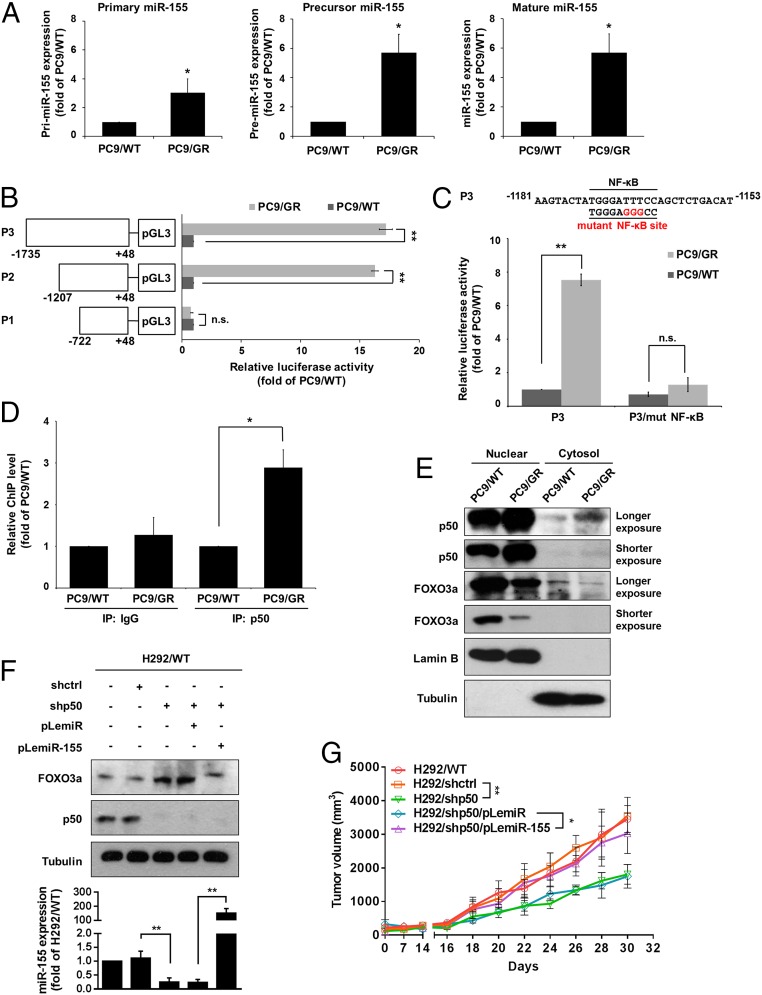
The expression of microRNA is controlled by factors that regulate primary microRNA transcription, maturation, and degradation. We found that primary, precursor, and mature forms of miR-155 were all up-regulated in PC9/GR cells compared with PC9/WT cells (Fig. 5A), thereby supporting the idea that miR-155 expression may occur through transcriptional regulation. We further developed a series of reporter constructs containing various regions of the miR-155 promoter (Fig. 5B, Left). A luciferase reporter assay showed that the region of the miR-155 promoter from −1,207 to −722 bp is required to maintain a high level of miR-155 promoter activity in PC9/GR cells compared with PC9/WT cells (Fig. 5B, Right). In addition, a previous study indicated that NF-κB could bind to a region of the miR-155 promoter (20). To further investigate whether NF-κB is vital for the regulation of miR-155 promoter activity in gefitinib-resistant cells, wild-type or mutant putative NF-κB binding sites of miR-155 promoter were transfected into PC9/WT and PC9/GR cells. As shown in Fig. 5C, mutagenesis of the NF-κB binding site abolished the ability of NF-κB to regulate the luciferase activity of the miR-155 promoter in PC9/GR cells. To verify the direct binding of NF-κB on the miR-155 promoter in PC9/GR cells, we performed a chromatin immunoprecipitation (ChIP) assay and showed that p50, a subunit of NF-κB, increased the binding ability to the miR-155 promoter (Fig. 5D), and a high level of nuclear p50 (Fig. 5E) was found in PC9/GR cells compared with PC9/WT cells, resulting in decreased FOXO3a expression. Moreover, H292 cells with stable knockdown of p50 were shown to decrease miR155 and increase FOXO3a expression but reduced FOXO3a in H292/shp50 cells with the overexpression of miR-155 (Fig. 5F). Mice-bearing tumors derived from H292/shp50 cells exhibited a significant reduction of tumor volume compared with H292/shctrl tumors, but the effects were reversed in mice bearing H292/shp50/miR-155 tumors (Fig. 5G). These findings suggest that NF-κB binds to the miR-155 promoter region and induces miR-155 expression and subsequently decreases FOXO3a expression, resulting in the increased tumorigenicity of lung cancer.
Fig. 5.
miR-155 is transcriptionally regulated by NF-κB of gefitinib-resistant lung cancer cells. (A) Real-time RT-PCR analysis of pri–miR-155, pre–miR-155, and miR-155 expression in PC9/WT and PC9/GR cells. (B) Schematic description of serial deletion reporter constructs of the miR-155 promoter cloned into the pGL3-Basic vector (Left). PC9/WT and PC9/GR cells were transfected with various miR-155 promoter reporters, and the luciferase activity was measured by the dual-luciferase reporter assay (Right). (C) Schematic diagram showing that the NF-κB binding sequences or mutated versions in the miR-155 promoter (Upper), and luciferase activity was measured using the dual-luciferase reporter assay (Lower). (D) ChIP analysis of chromatin extracted from PC9/WT and PC9/GR using polyclonal antibodies directed against the p50, a subunit of NF-κB, followed by real-time RT-PCR analysis to confirm p50 occupancy at the miR-155 promoter. (E) Cytoplasmic and nuclear fractions from PC9/WT and PC9/GR cells were assessed for the presence of FOXO3a, p50, Lamin B (nuclear marker), and Tubulin (cytosolic marker) by Western blot analysis. (F) The FOXO3a and p50 protein expression of H292 cells with stable expression of the indicated transfectants were measured by Western blot (Upper), and miR-155 expression by real-time RT-PCR (Lower), respectively. **P < 0.01 (two-tailed Student's t test). (G) In vivo tumor growth of H292 cells with stable expression of the indicated transfectants. Each point represents the mean ± SEM of tumor volumes of six mice in each group. Tumor volume was calculated as indicated in Materials and Methods. *P < 0.05 and **P < 0.01 (two-tailed Student's t test).
Inhibition of NF-κB Activity Enhances Gefitinib Sensitivity.
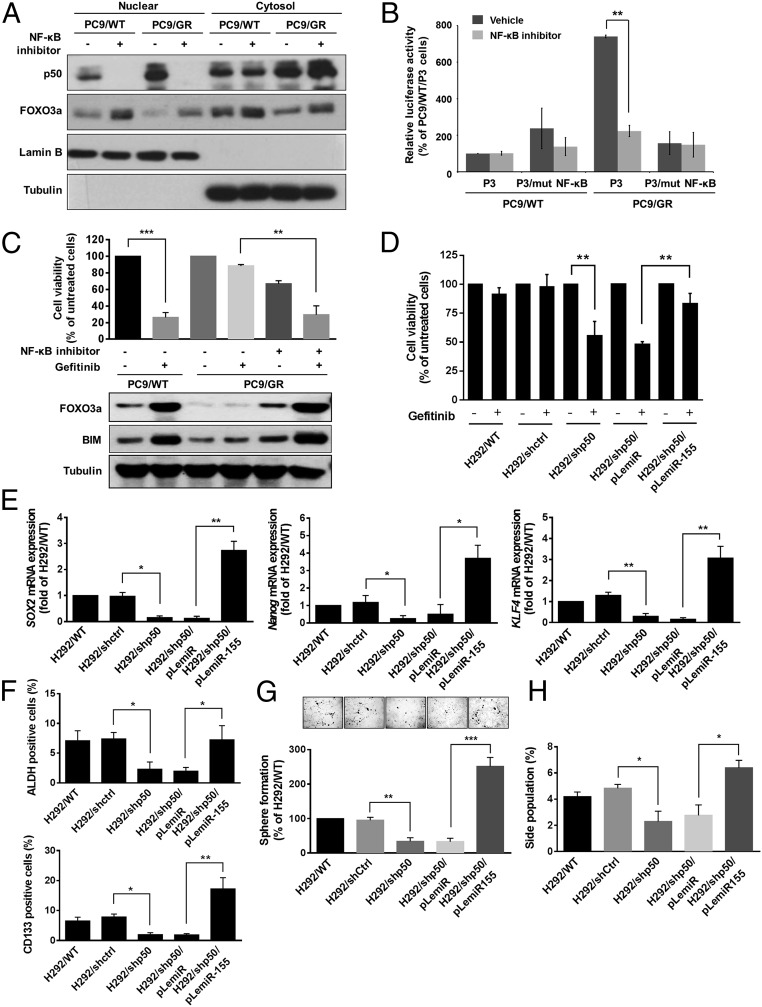
We next determined whether inhibited NF-κB activity causes PC9/GR cells to be more sensitive to gefitinib treatment. As shown in Fig. 6A, treatment with NF-κB inhibitor led to the prevention of nuclear translocation of p50 followed by increased FOXO3a expression in the nucleus. Additionally, activation of NF-κB by TGF-β stimulus in PC9/WT cells increased miR-155 expression, decreased protein levels of FOXO3a and BCL-2–like protein 11 (BIM), which is a FOXO3a-targeted proapoptotic protein, and resulted in enhancement of gefitinib resistance (SI Appendix, Fig. S5 A–C). To further investigate whether NF-κB inactivation was associated with a decrease in the transcriptional activity of miR-155, the reporter assay was used by the transfection of the miR-155 promoter construct containing a wild-type or mutant NF-κB binding site in PC9/WT and PC9/GR cells. The results showed the significantly decreased promoter activity of wild-type miR-155 reporter but not the mutant miR-155 reporter in PC9/GR cells after treatment with specific NF-κB inhibitor (Fig. 6B), resulting in the increased expression of FOXO3a and BIM and sensitivity of PC9/GR cells to gefitinib treatment upon NF-κB inactivation (Fig. 6C). In addition to using a NF-κB inhibitor, a knockdown approach of p50 was also performed and its knockdown efficiency was verified by Western blot analysis, showing significantly decreased miR-155 promoter activity and increased gefitinib sensitivity in PC9/GR cells (SI Appendix, Fig. S5 D–F). Additionally, the suppression of p50 in H292 cells obviously increased gefitinib sensitivity (Fig. 6D) and repressed the CSC properties (Fig. 6 E–H), and those effects of p50 knockdown were reversed by the overexpression of miR-155 in H292/shp50 cells. We further observed that the expression of p50 was positively correlated with miR-155 expression level in lung cancer patients (SI Appendix, Table S3) and was inversely correlated with FOXO3a expression in the Oncomine database (SI Appendix, Table S4); it was also consistent with RelA/p65, another subunit of NF-κB (SI Appendix, Table S4). In combination, these results suggest that NF-κB activity is responsible for generating gefitinib resistance and CSC properties through the suppression of FOXO3a via miR-155 in lung cancer.
Fig. 6.
Inhibition of NF-κB activity results in reduced miR-155 expression and suppressed gefitinib resistance and CSC properties. (A) Western blot analysis of p50, FOXO3a, Lamin B (nuclear marker), and Tubulin (cytosolic marker) in cytoplasmic and nuclear extracts of PC9/WT and PC9/GR cells treated with or without 2 μM NF-κB inhibitor. (B) Reporter assays of miR-155 promoter containing wild-type or mutant NF-κB binding site in PC9/WT and PC9/GR cells treated with NF-κB inhibitor. (C) Cell viability (Upper) was determined by MTT assay and expression of FOXO3a and BIM (Lower) were analyzed by Western blot in PC9/WT and PC9/GR cells treated with NF-κB inhibitor and 4 μM gefitinib. (D) Cell viability of gefitinib treatment was determined by MTT assay in H292 cells with stable expression of the indicated transfectants. The results are shown as the means ± SD of three independent experiments, each performed in triplicate. **P < 0.01 (two-tailed Student's t test). (E) Expression of miR-155 affected mRNA levels of SOX2, Nanog, and KLF4 by real-time RT-PCR, (F) ALDH+ and CD133+ cells by flow cytometric analysis, (G) sphere formation and (H) side population in H292/WT cells with p50 knockdown and miR-155 overexpression. The graph indicated the differences in the sphere numbers per microscopic field at 100× magnification (G, Upper) and that was calculated the percentage of sphere forming cells compared with PC9/WT cells (Lower). The results are shown as the means ± SD of three independent experiments each performed in triplicate. *P < 0.05, **P < 0.01, and ***P < 0.001 (two-tailed Student’s t test).
Physiological Effects of NF-κB/miR-155/FOXO3a Is Related to the Gefitinib Response.
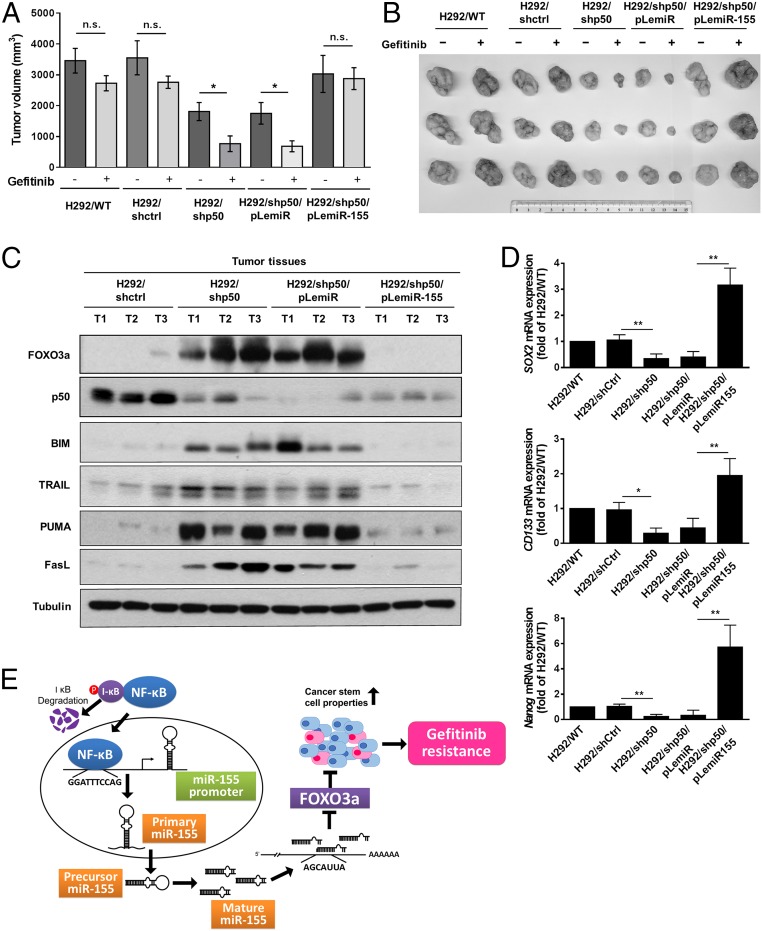
To further assess the ability of NF-κB/miR-155/FOXO3a to affect gefitinib response in lung cancer, H292 cells with stable knockdown of p50 (H292/shp50) were subcutaneously injected into nonobese diabetic (NOD)/SCID mice with gefitinib treatment after inoculation. Compared with the control (H292/shctrl) group, tumors in the H292/shp50 group had statistically significantly less tumor volume after gefitinib treatment for 14 d (Fig. 7 A and B). In addition, the H292/shp50/pLemiR-155–bearing mice showed increased tumor volume and resistance to gefitinib compared with the H292/shp50/pLemiR-bearing mice (Fig. 7 A and B). We confirmed the expression of p50, FOXO3a, and FOXO3a-targeted proapoptotic proteins, including BIM, tumor necrosis factor-related apoptosis inducing ligand (TRAIL), p53 upregulated modulator of apoptosis (PUMA), and cell death surface receptor Fas ligand (FasL), in formed tumors by Western blot (Fig. 7C). We further examined whether these effects are involved in cancer stemness in tumor cells. Consistent with the observations made in vitro, tumors derived from H292 cells with p50 knockdown significantly reduced the expression of SOX2, CD133, and Nanog, and preserved the effects by overexpressing miR-155 in H292/shp50 cells (Fig. 7D). These data demonstrate that the NF-κB/miR-155/FOXO3a axis is critical for gefitinib sensitivity and the suppression of the cancer stemness of lung cancer (Fig. 7E).
Fig. 7.
NF-κB/miR-155–mediated FOXO3a suppression is required for gefitinib resistance and stemness of lung cancer in vivo. (A) Mice were implanted subcutaneously with the indicated cell lines for ∼100 mm3 and were treated with vehicle and gefitinib for 14 d. Each point represents the mean ± SEM of tumor volumes of six mice in each group. Tumor volume was calculated as indicated in Materials and Methods. *P < 0.05 and n.s., not significant (two-tailed Student's t test). (B) The indicated tumors with vehicle and gefitinib treatment for 14 d were dissected from the surrounding tissue. (C) Western blot assay to confirm the expression of FOXO3a, p50, BIM, TRAIL, PUMA, and FasL in the indicated group of tumor samples. Tubulin was used as a loading control. (D) The relative expression of SOX2, CD133, and Nanog in the indicated group of tumor samples were measured by real-time RT-PCR analysis. The results are shown as the means ± SD of three independent experiments each performed in triplicate. *P < 0.05 and **P < 0.01 (two-tailed Student's t test). (E) Model of the regulatory signaling networks of NF-κB/miR-155/FOXO3a in gefitinib resistance and CSC properties of lung cancer. NF-κB activates the transcription of miR-155, promoting the targeting of the 3′UTR of FOXO3a and decreasing the expression of FOXO3a.
Discussion
The molecular mechanisms of acquired and intrinsic resistance to EGFR-TKIs in the presence of EGFR-sensitizing mutations are relatively unknown for up to 40% of patients. The presence of T790M or other exon 20-resistant mutations has been reported in only 5% of lung cancer patients with EGFR-TKI treatment (5). The activation of EGFR downstream signals, such as PIK3CA and KRAS mutations, seem to be indicators of resistance and poor survival in NSCLC patients with exposure to EGFR-TKI therapy, but only in 4.1% and 6.7% of patients, respectively (21). Resistance to EGFR-TKIs have also been found in lung cancer patients with 7.3% MET amplification (22) or with 5% EML4–ALK fusion in NSCLC patients (23). Recent studies showed that most NSCLC patients with the EGFR mutation carry a proapoptotic protein, BIM, that restores apoptotic responses in oncogene-addicted cancers and intrinsic resistance to EGFR-TKI therapy (24, 25), and its mutation was detected in 12.5% of lung cancer patients in East Asia (26). More importantly, FOXO3a has been found to increase several target genes, such as TRAIL, PUMA, FasL, and BIM, which is essential for the gefitinib-induced killing of NSCLC cells (14, 27), and the suppression of FOXO3a in breast cancer cells result in a reduction of gefitinib-induced cell cycle arrest and cell death (18). These results are consistent with our observations, which showed that 24% of lung cancer patients with high FOXO3a have a better response to EGFR-TKIs and progression-free survival outcomes, suggesting that FOXO3a expression is involved in the regulation of the EGFR-TKI–induced apoptotic response and is a good marker for predicting the therapeutic effects of EGFR-TKIs in lung cancer.
Current studies reveal that NF-κB activation promotes resistance to EGFR inhibitors and might be a possible mechanism and therapeutic strategy for EGFR-TKI resistance in cancer treatments (28). Additionally, the suppression of the Fas/NF-κB pathway specifically enhances erlotinib-induced cell death in EGFR mutant cells and tumor models (29). Consistently, we found that NF-κB–driven miR-155 expression suppressed the FOXO3a protein level in gefitinib-resistant lung cancer, and the suppression of NF-κB activity and expression contributed to the resensitivity to gefitinib treatment in vitro and in vivo, implying that the combination of both the NF-κB and EGFR inhibitors might be a potential therapeutic strategy to improve tumors with resistance to EGFR-TKIs. In addition to the fact that NF-κB–activating signal cascades promote resistance to chemotherapy through the transcriptional induction of multidrug resistance gene-1 (30), which promotes the CSC properties of lung cancer (31), increasing the expression of CSC markers has previously been considered to associate with acquired EGFR-TKI resistance and metastasis in lung cancer (32, 33). CD133 expression is linked to a resistant phenotype in NSCLC and suggests that the detection of CD133+ cells may be useful to predict the efficacy of cytotoxic therapy for lung cancer (34). In addition, SOX2 expression maintained the self-renewal of lung cancer cells and was positively correlated with the metastatic progression of NSCLC (35, 36), suggesting that CD133 and SOX2 expression plays an important role in studying the properties of cancer stem cells and the therapeutic response in lung cancer. Our study observed that SOX2 and CD133 expression were positively correlated with EGFR-TKI nonresponders and low expression levels of FOXO3a in lung cancer patients, and we found that NF-κB/miR-155 decreased FOXO3a expression to enhance CSC characteristics in vitro and in vivo, indicating that the NF-κB–driven suppression of FOXO3a plays a crucial role in CSC properties and EGFR-TKI resistance of lung cancer.
miR-155 is transcribed from the host gene, BIC (B-cell integration cluster), also termed MIR155HG gene, which is located on chromosome 21 (37). Previous studies reported that TGF-β–mediated Smad4 expression induces miR-155 promoter activity and enriches miR-155 expression to contribute to cell migration and invasion (38), and found that NF-κB, STAT5, or CCAAT/enhancer binding protein beta (C/EBPβ) could bind to miR-155 promoter region and induce miR-155 expression in colon cancer, cutaneous T-cell lymphoma, or fat cells (20, 39, 40). miR-155 has been considered an “oncomicroRNA” by targeting several tumor suppressors, including SOCS1, FOXO3a, RhoA, C/EBPβ, PP2A/C, and von Hippel–Lindau tumor suppressor (VHL), and it has been found to promote the EMT, invasion, metastasis, growth, and angiogenesis of cancer cells (20, 41–43). In addition, the up-regulation of miR-155 has been reported to promote tumor angiogenesis by targeting VHL and is associated with poor prognosis for breast cancer (43); additionally, the loss of VHL has been reported to induce NF-κB activity (44). These findings support our results that miR-155 decreased VHL expression, which in turn activated NF-κB; this may contribute to the positive feedback activation of miR-155 and promote gefitinib resistance and cancer stemness. Collectively, the significance of this negative correlation between miR-155 and FOXO3a of lung cancer indicates that miR-155 may be a critical therapeutic target in lung cancer, especially in EGFR-TKI–resistant patients.
A previous study indicated an important role for NF-κB in regulating the miR-155 expression of immune cell development and function (45); however, the role of NF-κB–mediated miR-155 in cancer progression and gefitinib resistance and cancer stemness were not investigated. Consistent with previous studies, our findings used a computational design to search the microRNA database, and we identified and verified that miR-155 is able to induce gefitinib resistance by directly inhibiting FOXO3a at the posttranscriptional level and that NF-κB increases gefitinib resistance by inducing miR-155 expression. Moreover, miR-140 was found to be a critical suppressor, inhibiting NF-κB activity (46, 47). In addition to miR-155, we also found that miR-140 was significantly reduced in resistant cells (PC9/GR), which may confer synergistic effects with miR-155 in mediating gefitinib resistance.
In this study, we found that NF-κB/miR-155 decreased FOXO3a expression to enhance CSC characteristics and resistance to gefitinib in lung cancer, suggesting that FOXO3a could be an independent prognostic marker of lung cancer and that the NF-κB/miR-155/FOXO3a axis plays an important role in lung cancer patients with acquired resistance to EGFR-TKIs.
Materials and Methods
Specimens.
Lung cancer specimens were obtained from the China Medical University Hospital with Institutional Review Board approval (CMUH102-REC2-035), and written informed consent was obtained from all patients. Between December 2005 and September 2011, a total of 80 cases of stage IV lung adenocarcinoma patients, including 37 males and 43 females with an age ranging from 31 to 85 y (median, 69 y), were enrolled in this retrospective study. Patients who had ever received EGFR-TKI (erlotinib or gefitinib) therapy between 5.7 and 37.5 mo were evaluated for their clinical response according to RECIST 1.1 criteria (48), and patients who had progressive disease and stable disease for 4 mo or less were regarded as EGFR-TKI nonresponders (n = 38). The status of complete response, partial response, and stable disease for more than 4 mo were classified as EGFR-TKIs responders (n = 42). Details of EGFR mutational analysis, immunohistochemical staining, and real-time PCR quantification of lung cancer are provided in SI Appendix, Materials and Methods.
Construction of Expression Vectors, Plasmids, and Anti-miR.
Full-length human FOXO3a was amplified by PCR using cDNA of HeLa cells and cloned into the NheI and NotI sites of pCDH-CMV-MCS-EF1-copGFP+Puro (System Biosciences). The hsa-miR-155 stem-loop sequence plus its up- and downstream-flanking regions was amplified by PCR using genomic DNA of HeLa cells and cloned into the XhoI and NotI sites of pLemiR-empty vector (Open Biosystem). All primer sequences of cDNA and microRNA constructs are shown in SI Appendix, Table S5 (lowercase is representative of the additional sequence, and the restriction enzyme site is underlined). All constructs were confirmed by DNA sequencing. The lentiviral FOXO3a shRNA clones TRCN0000010334 and TRCN0000235488, p50 shRNA clones TRCN0000006518 and TRCN0000006520, the pLKO.1-shLuc vector TRCN0000072244 that was shRNA against luciferase acted as controls; the pMD2.G plasmid and pCMVdeltaR8.91 plasmid were purchased from the National RNAi Core Facility at Academia Sinica, Taipei, Taiwan. The pRL-TK, pGL3-basic, and pmirGLO Dual-Luciferase vectors were obtained from Promega. Anti–miR-155, the microRNA inhibitor (Ambion), was a single-stranded chemically modified oligonucleotide designed to inhibit endogenous miR-155. The anti-miR negative control is a random sequence that has been extensively tested in human cell lines and tissues and was validated to produce no identifiable effects on known microRNA function.
ChIP Assay.
The ChIP assay was performed with the EZ ChIP kit (Millipore) as previously described (49). Briefly, cells were fixed with 1% formaldehyde, washed, and lysed. The cell lysates were sonicated to shear DNA to sizes of 300–1,000 bp. Protein–DNA complexes were precipitated with either nonimmune IgG or target protein p50 (Abcam) overnight at 4 °C with rotation. After reverse cross-link of protein–DNA complexes to free DNA, real-time RT-PCR were performed with the LightCycler 480 (Roche) by using LightCycler FastStart DNA Master SYBR Green I kit (Roche) and specific primers (SI Appendix, Table S5). Cycling conditions were 95 °C for 10 min followed by 50 cycles of 95 °C for 15 s, 60 °C for 1 min. A dissociation procedure was performed to generate a melting curve for confirmation of amplification specificity. The relative occupancy of the immunoprecipitated factor at a locus was estimated by using the comparative threshold method (50) and expressed as fold-enrichment versus PC9/WT cells.
Cellular Fractionation.
The cytosolic and nuclear fractions were extracted as previously described (51). Briefly, cells were washed twice with ice-cold PBS, harvested by scraping with a rubber policeman, and lysed in buffer A (20 mM Hepes, pH 7.0, 10 mM KCl, 2 mM MgCl2, 0.5% Nonidet P-40, 1 mM Na3VO4, 10 mM NaF) containing protease inhibitor mixture (Roche). After 10-min incubation on ice, cells were homogenized by 15–20 strokes in a tightly fitting Dounce homogenizer and centrifuged 5 min at 1,500 × g to sediment the nuclei. The supernatant is the cytosolic fraction. To remove contamination from cytoplasmic membranes, the nuclear pellet was washed three times with buffer A. To extract nuclear proteins, the isolated nuclei were resuspended in NETN lysis buffer (20 mM Tris⋅HCl, pH 8.0, 150 mM NaCl, 0.5% Nonidet P-40, and 1 mM EDTA) containing protease inhibitor mixture (Roche) and the mixture was sonicated briefly to aid nuclear lysis. After centrifugation at 16,100 × g for 20 min at 4 °C, the nuclear lysates were collected. Cytosolic fraction and nuclear fractions were analyzed by Western blot (SI Appendix).
Animal Studies.
All animal work was done in accordance with a protocol approved by the Institutional Animal Care and Use Committee of National Health Research Institutes. Four- to 6-wk-old NOD.CB17-Prkdcscid/J severe combined immunodeficient (NOD/SCID) male mice (supplied by LASCO) were used for tumor growth in a xenograft studies. Next, 5 × 106 cells/100 μL in PBS mixed with equal volume of Matrigel (BD Biosciences) were subcutaneously injected into the back of mice. When tumor volumes reached ∼100 mm3, as determined by measuring tumor length and width using calipers and calculating volume through the equation [1/2 (length × width2)], mice were randomly allocated into groups of six mice to receive gefitinib or vehicle by oral gavage at 100 mg/kg every 2 d and tumor volumes were measured every 2 d.
Statistical Analysis.
All statistical analyses were performed with Prism 6 software. Data of in vitro experiments were approximately normally distributed and presented as the means ± SD from at least three independent experiments, each performed in triplicate. Statistical evaluation of variance among experimental groups was similar and performed with a two-tailed Student’s t test for comparison between two groups. Pearson’s χ2 test was used to compare the relationship between FOXO3a, miR-155 expression, and clinicopathological factors.
Supplementary Material
Acknowledgments
We thank the National RNAi Core Facility (Academia Sinica, Taiwan) for providing specific shRNAs; Ms. Fang-Yu Tsai, Dr. I-Shou Chang, and Dr. Shih-Sheng Jiang of the Taiwan Bioinformatics Institute Core Facility for assistance in using Oncomine (National Core Facility Program for Biotechnology, NSC 100-2319-B-400-001). The results shown here are in part based upon data generated by the TCGA Research Network (cancergenome.nih.gov/). We thank the Cell Sorting Core Facility of the First Core Laboratory and the Center of Genomic Medicine, National Taiwan University, for the services they provided. This work was supported by Ministry of Science and Technology (MOST) (National Science Council; NSC) of Taiwan Grants NSC 101-2320-B-400-016-MY3, NSC 102-2314-B-038-028-MY3, MOST 103-2314-B-038-059, MOST 104-2314-B-038-002, MOST 104-2321-B-400-018-MY3, and MOST 104-2320-B-400-015-MY3 and by National Health Research Institutes of Taiwan Grants CA-102-PP-41, CA-103-PP-35, CA-104-PP-12, MOHW104-TDU-B-212-124-008, and CA-105-PP-12.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1522612113/-/DCSupplemental.
References
- 1.Paez JG, et al. EGFR mutations in lung cancer: Correlation with clinical response to gefitinib therapy. Science. 2004;304(5676):1497–1500. doi: 10.1126/science.1099314. [DOI] [PubMed] [Google Scholar]
- 2.Keedy VL, et al. American Society of Clinical Oncology provisional clinical opinion: Epidermal growth factor receptor (EGFR) mutation testing for patients with advanced non-small-cell lung cancer considering first-line EGFR tyrosine kinase inhibitor therapy. J Clin Oncol. 2011;29(15):2121–2127. doi: 10.1200/JCO.2010.31.8923. [DOI] [PubMed] [Google Scholar]
- 3.Su KY, et al. Pretreatment epidermal growth factor receptor (EGFR) T790M mutation predicts shorter EGFR tyrosine kinase inhibitor response duration in patients with non-small-cell lung cancer. J Clin Oncol. 2012;30(4):433–440. doi: 10.1200/JCO.2011.38.3224. [DOI] [PubMed] [Google Scholar]
- 4.Maemondo M, et al. North-East Japan Study Group Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362(25):2380–2388. doi: 10.1056/NEJMoa0909530. [DOI] [PubMed] [Google Scholar]
- 5.Sharma SV, Bell DW, Settleman J, Haber DA. Epidermal growth factor receptor mutations in lung cancer. Nat Rev Cancer. 2007;7(3):169–181. doi: 10.1038/nrc2088. [DOI] [PubMed] [Google Scholar]
- 6.Scheffler M, et al. Spatial tumor heterogeneity in lung cancer with acquired epidermal growth factor receptor-tyrosine kinase inhibitor resistance: Targeting high-level MET-amplification and EGFR T790M mutation occurring at different sites in the same patient. J Thorac Oncol. 2015;10(6):e40–e43. doi: 10.1097/JTO.0000000000000503. [DOI] [PubMed] [Google Scholar]
- 7.Takezawa K, et al. HER2 amplification: A potential mechanism of acquired resistance to EGFR inhibition in EGFR-mutant lung cancers that lack the second-site EGFRT790M mutation. Cancer Discov. 2012;2(10):922–933. doi: 10.1158/2159-8290.CD-12-0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bertotti A, Sassi F. Molecular pathways: Sensitivity and resistance to anti-EGFR antibodies. Clin Cancer Res. 2015;21(15):3377–3383. doi: 10.1158/1078-0432.CCR-14-0848. [DOI] [PubMed] [Google Scholar]
- 9.Trumpp A, Wiestler OD. Mechanisms of disease: Cancer stem cells—Targeting the evil twin. Nat Clin Pract Oncol. 2008;5(6):337–347. doi: 10.1038/ncponc1110. [DOI] [PubMed] [Google Scholar]
- 10.Murakami A, et al. Hypoxia increases gefitinib-resistant lung cancer stem cells through the activation of insulin-like growth factor 1 receptor. PLoS One. 2014;9(1):e86459. doi: 10.1371/journal.pone.0086459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shien K, et al. Acquired resistance to EGFR inhibitors is associated with a manifestation of stem cell-like properties in cancer cells. Cancer Res. 2013;73(10):3051–3061. doi: 10.1158/0008-5472.CAN-12-4136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Takeda M, et al. De novo resistance to epidermal growth factor receptor-tyrosine kinase inhibitors in EGFR mutation-positive patients with non-small cell lung cancer. J Thorac Oncol. 2010;5(3):399–400. doi: 10.1097/JTO.0b013e3181cee47e. [DOI] [PubMed] [Google Scholar]
- 13.Fu Z, Tindall DJ. FOXOs, cancer and regulation of apoptosis. Oncogene. 2008;27(16):2312–2319. doi: 10.1038/onc.2008.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nho RS, Hergert P. FoxO3a and disease progression. World J Biol Chem. 2014;5(3):346–354. doi: 10.4331/wjbc.v5.i3.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chapuis N, et al. IκB kinase overcomes PI3K/Akt and ERK/MAPK to control FOXO3a activity in acute myeloid leukemia. Blood. 2010;116(20):4240–4250. doi: 10.1182/blood-2009-12-260711. [DOI] [PubMed] [Google Scholar]
- 16.Brunet A, et al. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96(6):857–868. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- 17.Hu MC, et al. IkappaB kinase promotes tumorigenesis through inhibition of forkhead FOXO3a. Cell. 2004;117(2):225–237. doi: 10.1016/s0092-8674(04)00302-2. [DOI] [PubMed] [Google Scholar]
- 18.Krol J, et al. The transcription factor FOXO3a is a crucial cellular target of gefitinib (Iressa) in breast cancer cells. Mol Cancer Ther. 2007;6(12 Pt 1):3169–3179. doi: 10.1158/1535-7163.MCT-07-0507. [DOI] [PubMed] [Google Scholar]
- 19.Hu T, et al. Reprogramming ovarian and breast cancer cells into non-cancerous cells by low-dose metformin or SN-38 through FOXO3 activation. Sci Rep. 2014;4:5810–5822. doi: 10.1038/srep05810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bakirtzi K, et al. Neurotensin signaling activates microRNAs-21 and -155 and Akt, promotes tumor growth in mice, and is increased in human colon tumors. Gastroenterology. 2011;141(5):1749–1761.e1. doi: 10.1053/j.gastro.2011.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ludovini V, et al. Phosphoinositide-3-kinase catalytic alpha and KRAS mutations are important predictors of resistance to therapy with epidermal growth factor receptor tyrosine kinase inhibitors in patients with advanced non-small cell lung cancer. J Thorac Oncol. 2011;6(4):707–715. doi: 10.1097/JTO.0b013e31820a3a6b. [DOI] [PubMed] [Google Scholar]
- 22.Siegelin MD, Borczuk AC. Epidermal growth factor receptor mutations in lung adenocarcinoma. Lab Invest. 2014;94(2):129–137. doi: 10.1038/labinvest.2013.147. [DOI] [PubMed] [Google Scholar]
- 23.Inamura K, et al. EML4-ALK lung cancers are characterized by rare other mutations, a TTF-1 cell lineage, an acinar histology, and young onset. Mod Pathol. 2009;22(4):508–515. doi: 10.1038/modpathol.2009.2. [DOI] [PubMed] [Google Scholar]
- 24.Ng KP, et al. A common BIM deletion polymorphism mediates intrinsic resistance and inferior responses to tyrosine kinase inhibitors in cancer. Nat Med. 2012;18(4):521–528. doi: 10.1038/nm.2713. [DOI] [PubMed] [Google Scholar]
- 25.Faber AC, et al. BIM expression in treatment-naive cancers predicts responsiveness to kinase inhibitors. Cancer Discov. 2011;1(4):352–365. doi: 10.1158/2159-8290.CD-11-0106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cheng EH, Sawyers CL. In cancer drug resistance, germline matters too. Nat Med. 2012;18(4):494–496. doi: 10.1038/nm.2725. [DOI] [PubMed] [Google Scholar]
- 27.Cragg MS, Kuroda J, Puthalakath H, Huang DC, Strasser A. Gefitinib-induced killing of NSCLC cell lines expressing mutant EGFR requires BIM and can be enhanced by BH3 mimetics. PLoS Med. 2007;4(10):1681–1689, discussion 1690. doi: 10.1371/journal.pmed.0040316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shostak K, Chariot A. EGFR and NF-κB: Partners in cancer. Trends Mol Med. 2015;21(6):385–393. doi: 10.1016/j.molmed.2015.04.001. [DOI] [PubMed] [Google Scholar]
- 29.Bivona TG, et al. FAS and NF-κB signalling modulate dependence of lung cancers on mutant EGFR. Nature. 2011;471(7339):523–526. doi: 10.1038/nature09870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bentires-Alj M, et al. NF-kappaB transcription factor induces drug resistance through MDR1 expression in cancer cells. Oncogene. 2003;22(1):90–97. doi: 10.1038/sj.onc.1206056. [DOI] [PubMed] [Google Scholar]
- 31.Ho MM, Ng AV, Lam S, Hung JY. Side population in human lung cancer cell lines and tumors is enriched with stem-like cancer cells. Cancer Res. 2007;67(10):4827–4833. doi: 10.1158/0008-5472.CAN-06-3557. [DOI] [PubMed] [Google Scholar]
- 32.Corominas-Faja B, et al. Stem cell-like ALDH(bright) cellular states in EGFR-mutant non-small cell lung cancer: A novel mechanism of acquired resistance to erlotinib targetable with the natural polyphenol silibinin. Cell Cycle. 2013;12(21):3390–3404. doi: 10.4161/cc.26417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Perona R, López-Ayllón BD, de Castro Carpeño J, Belda-Iniesta C. A role for cancer stem cells in drug resistance and metastasis in non-small-cell lung cancer. Clin Transl Oncol. 2011;13(5):289–293. doi: 10.1007/s12094-011-0656-3. [DOI] [PubMed] [Google Scholar]
- 34.Salnikov AV, et al. CD133 is indicative for a resistance phenotype but does not represent a prognostic marker for survival of non-small cell lung cancer patients. Int J Cancer. 2010;126(4):950–958. doi: 10.1002/ijc.24822. [DOI] [PubMed] [Google Scholar]
- 35.Singh S, et al. EGFR/Src/Akt signaling modulates Sox2 expression and self-renewal of stem-like side-population cells in non-small cell lung cancer. Mol Cancer. 2012;11:73. doi: 10.1186/1476-4598-11-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xu C, et al. β-Catenin/POU5F1/SOX2 transcription factor complex mediates IGF-I receptor signaling and predicts poor prognosis in lung adenocarcinoma. Cancer Res. 2013;73(10):3181–3189. doi: 10.1158/0008-5472.CAN-12-4403. [DOI] [PubMed] [Google Scholar]
- 37.Tam W, Dahlberg JE. miR-155/BIC as an oncogenic microRNA. Genes Chromosomes Cancer. 2006;45(2):211–212. doi: 10.1002/gcc.20282. [DOI] [PubMed] [Google Scholar]
- 38.Kong W, et al. MicroRNA-155 is regulated by the transforming growth factor beta/Smad pathway and contributes to epithelial cell plasticity by targeting RhoA. Mol Cell Biol. 2008;28(22):6773–6784. doi: 10.1128/MCB.00941-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen Y, et al. miR-155 regulates differentiation of brown and beige adipocytes via a bistable circuit. Nat Commun. 2013;4:1769. doi: 10.1038/ncomms2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kopp KL, et al. STAT5-mediated expression of oncogenic miR-155 in cutaneous T-cell lymphoma. Cell Cycle. 2013;12(12):1939–1947. doi: 10.4161/cc.24987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Johansson J, et al. MiR-155-mediated loss of C/EBPβ shifts the TGF-β response from growth inhibition to epithelial-mesenchymal transition, invasion and metastasis in breast cancer. Oncogene. 2013;32(50):5614–5624. doi: 10.1038/onc.2013.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Czyzyk-Krzeska MF, Zhang X. MiR-155 at the heart of oncogenic pathways. Oncogene. 2014;33(6):677–678. doi: 10.1038/onc.2013.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kong W, et al. Upregulation of miRNA-155 promotes tumour angiogenesis by targeting VHL and is associated with poor prognosis and triple-negative breast cancer. Oncogene. 2014;33(6):679–689. doi: 10.1038/onc.2012.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.An J, Rettig MB. Mechanism of von Hippel-Lindau protein-mediated suppression of nuclear factor kappa B activity. Mol Cell Biol. 2005;25(17):7546–7556. doi: 10.1128/MCB.25.17.7546-7556.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Baltimore D, Boldin MP, O’Connell RM, Rao DS, Taganov KD. MicroRNAs: New regulators of immune cell development and function. Nat Immunol. 2008;9(8):839–845. doi: 10.1038/ni.f.209. [DOI] [PubMed] [Google Scholar]
- 46.Takata A, et al. MicroRNA-22 and microRNA-140 suppress NF-κB activity by regulating the expression of NF-κB coactivators. Biochem Biophys Res Commun. 2011;411(4):826–831. doi: 10.1016/j.bbrc.2011.07.048. [DOI] [PubMed] [Google Scholar]
- 47.Takata A, et al. A miRNA machinery component DDX20 controls NF-κB via microRNA-140 function. Biochem Biophys Res Commun. 2012;420(3):564–569. doi: 10.1016/j.bbrc.2012.03.034. [DOI] [PubMed] [Google Scholar]
- 48.Eisenhauer EA, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45(2):228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 49.Yu YH, et al. MiR-520h-mediated FOXC2 regulation is critical for inhibition of lung cancer progression by resveratrol. Oncogene. 2013;32(4):431–443. doi: 10.1038/onc.2012.74. [DOI] [PubMed] [Google Scholar]
- 50.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3(6):1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 51.Yu YL, et al. Nuclear EGFR suppresses ribonuclease activity of polynucleotide phosphorylase through DNAPK-mediated phosphorylation at serine 776. J Biol Chem. 2012;287(37):31015–31026. doi: 10.1074/jbc.M112.358077. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.