Abstract
How, why, and when consciousness evolved remain hotly debated topics. Addressing these issues requires considering the distribution of consciousness across the animal phylogenetic tree. Here we propose that at least one invertebrate clade, the insects, has a capacity for the most basic aspect of consciousness: subjective experience. In vertebrates the capacity for subjective experience is supported by integrated structures in the midbrain that create a neural simulation of the state of the mobile animal in space. This integrated and egocentric representation of the world from the animal’s perspective is sufficient for subjective experience. Structures in the insect brain perform analogous functions. Therefore, we argue the insect brain also supports a capacity for subjective experience. In both vertebrates and insects this form of behavioral control system evolved as an efficient solution to basic problems of sensory reafference and true navigation. The brain structures that support subjective experience in vertebrates and insects are very different from each other, but in both cases they are basal to each clade. Hence we propose the origins of subjective experience can be traced to the Cambrian.
Keywords: subjective experience, primary consciousness, vertebrate midbrain, central complex
Consciousness is marked by the presence of subjective experience: In the philosopher’s term of art, there is “something it is like” for us to be aware of the world (1). Neurotypical adult humans are obviously conscious. So are young human children, although we recognize that the nature of consciousness in children is different from that of adults and changes rapidly as children develop. Plausibly, some animals also have the capacity for some forms of consciousness—at least, it certainly seems odd to insist that the inner life of a chimp goes on entirely in the dark. However, consciousness also gives out somewhere. Plants do not have it. It would be surprising if jellyfish did. Where to draw the line between what is conscious and what is not, and how to justify drawing that line, remain hotly debated questions.
These debates are especially difficult when it comes to assessing potential consciousness in invertebrates. Methodological challenges are partly to blame. The three most common methods of studying consciousness in humans—verbal report, behavioral demonstrations, and neural correlation with conscious activity—generalize poorly to invertebrate models. Nonhuman animals cannot give verbal reports about what they are experiencing. There have been attempts to deploy to animals behavioral paradigms that are considered evidence of conscious processing when successfully performed by humans (2–4). This introduces a strong bias toward anthropomorphic performance by animals. As skeptics are quick to note, we have no guarantee that animals that behave like humans do so because they have the same subjective experiences that humans do. (Indeed, establishing this is precisely the issue.) Further, the bias toward clever animals is itself distorting. Many invertebrates live comparatively simple lives, without complex forms of communication and social behavior. If one cares about the basic capacity for consciousness and where it came from, one should be prepared to accept that the origins of consciousness may lie in animals that do only very boring, unclever things.
The search for the neural correlates of consciousness (NCCs) (5) is often framed as avoiding the pitfalls of purely behavioral research. NCC research focuses on the contents of particular conscious experiences, rather than the overall capacity for subjective experience (6). NCCs may thus be a poor guide to the more basic shared capacity to have any conscious experiences at all. In practice, NCC work also tends toward the anthropocentric, and the best paradigms are still difficult to extend to invertebrates. NCC results thus tend to be biased toward complex cortical structures and cannot generalize to animals lacking a cortex.
In this review, we will suggest an alternative approach to studying the capacity for subjective experience in invertebrates. Neuroethological approaches have made great advances in determining how neurobiological mechanisms within the insect brain generate adaptive behavior. We argue that knowledge of these mechanisms can ground an evidence-based inquiry into the capacity for consciousness in insects. We begin our argument by discussing the basic features of the simplest forms of consciousness, the capacity to have subjective experience (7). We then consider structures that support the capacity for subjective experience in humans. Following Bjorn Merker (8, 9), we argue that the human midbrain subserves the basic capacity for subjective experience. It does so in virtue of producing an integrated simulation of the state of the animal’s own mobile body within the environment. We then argue that the insect brain performs similar functions, even though the anatomy of the insect brain is very different from that of vertebrates. Insects have specialized brain structures that solve the same basic problems by producing the same kind of unified model. We therefore conclude that if subjective experience is indeed supported by midbrain structures in humans, then insects also have the capacity for a form of subjective experience.
Finally, we propose that a capacity for subjective experience probably evolved early in the history of animal life for specific animal clades. We thus suggest that the study of insects gives a unique comparative perspective on the mechanisms and evolution of consciousness.
Subjective Experience and the Vertebrate Midbrain
Behavioral researchers have realized the need to distinguish different levels of consciousness. There are many classifications (7), but each posits a basic form of consciousness that consists of a direct awareness of the world without further reflection upon that awareness. This basic level has been termed primary consciousness (10), core consciousness (11, 12), phenomenal consciousness (13), or “I” consciousness (14). The defining feature of this most basic level of consciousness is the appearance of the capacity for subjective experience.
Humans are capable of more complex forms of consciousness. We can reflect upon our own mental states, for example, which is why verbal reports are so valuable. The levels corresponding to these higher capacities are variously termed self-reflexive consciousness (7), access consciousness (13), higher-order awareness (10), or “me” consciousness (14). These higher forms of consciousness require more than the mere capacity for subjective experience: the ability to represent one’s own subjective experience to oneself, awareness of oneself as a self, the possession of the concept of experience, or full linguistic capability.
We mention these more complex levels of consciousness to bracket them off. It is contentious whether any other higher vertebrates possess them; we think it exceedingly unlikely that any invertebrates do. Our argument thus concerns only whether insects have subjective experience. Because subjective experience is a simpler phenomenon than self-reflexive consciousness, one might reasonably expect it to be more widespread in the animal kingdom, and evolutionarily older.
There is now considerable evidence that, in humans, subjective experience can exist in the absence of self-reflexive consciousness, and that the two are supported by different neural structures. Midbrain structures, rather than cortex, seem to be especially important. Merker (8, 9), Parvisi and Damasio (11), Damasio and Carvalho (15), and Mashour and Alkire (16) have all argued that the integrated structures of the vertebrate midbrain are sufficient to support the capacity for subjective experience.
Merker (8) notes that subjective experience is remarkably sensitive to damage to midbrain structures. Conversely, there is evidence of preserved consciousness even in patients who lack a cortex (9). Further, although cortical damage can have profound effects on the contents of consciousness, damage to any portion of the cortex alone can spare the basic capacity for subjective experience (17–22). Cortical damage alone can have profound effects on the contents of consciousness, but even massive cortical damage seems to spare subjective experience itself (8, 17, 18). Indeed, there is evidence of residual conscious awareness in patients with severe cortical damage who are otherwise unresponsive to the world, suggesting that preserved subcortical structures may continue to support subjective experience (23, 24). Although the mechanism of anesthetic action is still debated (25), there is increasing evidence that the effect of anesthetics depends on the disconnection of cortical circuits from subcortical structures rather than on their direct cortical activity (26, 27). Anesthetics (28) or electrical stimulation (19), which affect cortical midline structures without affecting subcortical structures, do not abolish consciousness; they instead produce unresponsive but conscious dreamlike states. Conversely, emergence from anesthesia (16, 29) and coma/vegetative state (30) are predicted by the reengagement of subcortical structures and reintegration of those structures with cortical circuits. Other authors have noted the powerful subcortical effect of drugs, endogenous peptides, and direct stimulation on primitive motivational states (12, 16, 31).
In sum, there is good evidence that subcortical structures underlie the basic capacity for subjective experience in humans. This is not to say that the cortex is unimportant for conscious experience, of course. Rather, the proposal is that subcortical structures support the basic capacity for experience, the detailed contents of which might be elaborated by or otherwise depend upon cortical structures (32).
How the Vertebrate Midbrain Supports the Capacity for Subjective Experience
We will adopt and expand the account given by Merker (8), who argues that subjective experience arises from interacting midbrain and basal ganglia structures creating an integrated simulation of the state of the animal’s own mobile body within the environment.
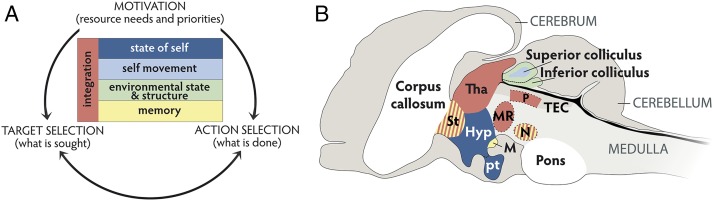
Merker suggests that an important function of the midbrain is to combine interoceptive (stimuli arising from within the body) and exteroceptive (stimuli external to the body) sensory information. Information on the environment, and the location and movement of the animal within it, is processed within the roof of the midbrain (the tectum, or colliculus in mammals). Information about homeostatic needs is processed within the floor of the midbrain (the hypothalamus and associated structures). Nuclei located between these poles integrate this information to produce a unified multimodal neural model of the spatial location of resources relative to the animal, which is coupled to, and weighted by, the extent to which different resources are needed by the animal (8) (Fig. 1A). Vertebrates organize their behavior by reference to this integrated model of the environment rather than by reacting to independent sensory inputs (8, 33).
Fig. 1.
Structures of the vertebrate midbrain (not to scale) supporting the behavioral “core control system.” The vertebrate midbrain supports an integrated multisensory model of the state of the animal in space, which supports effective decision making. (A) Decision making involves an assessment of what is needed and where and how the needed resources can be obtained. Decision making can therefore be considered to involve three domains: internal motivations, target selection, and action selection [adapted from the “selection triangle” proposed by Merker (8)]). These domains interact and can be resolved by referencing an integrated neural model that contains information on the state of self, self movement, environmental state and structure, and memory of prior experience. These elements of the neural model are supported by midbrain structures (B). As a simplification, regions are colored according to their primary function(s) in the neural model described in A. The superior colliculus [part of the tectum (TEC) forming the roof of the midbrain] receives topographically organized multisensory input and creates an integrated neural model of the organism moving in space (8). The floor of the midbrain is formed by the hypothalamic structures (Hyp) and associated nuclei [pituitary (pt) and mammillary bodies (M)] that collate information on the physiological status of the organism referenced with prior experience, to identify needs to maintain a homeostatic optimum (15, 56). Integrative structures within the midbrain and basal ganglia, including the periaqueductal gray (P), substantia nigra (N), thalamus (Tha), striatum (St), and midbrain reticular formation (MR), integrate these sources of information (8, 33) (A). In advanced vertebrates the cortex and hippocampal structures clearly have a very strong input in to this system, but this midbrain system is not dependent on cortical inputs to function (140) and is highly conserved across all vertebrate lineages (33).
In mammals the superficial layers of the superior colliculus (SC) receive topographically organized visual inputs (34), whereas the deeper layers receive topographically organized visual, auditory, and somatosensory input (8, 15, 33, 35). In some species, specialized spatial senses such as infrared and magneto- and electrosenses and echolocation send topographically organized inputs to the SC (8, 36–38). Within a species, the sensory topographic maps in different layers of the SC are superposed, meaning specific regions of space are represented by similar points in each overlaid sensory map (39). This arrangement allows a point of convergence for processed spatially structured information, in turn allowing an integrated sense of space that includes the position, state, and movement of the body (9, 40–42).
Organizing behavior by reference to an integrated simulation of the state of the mobile body in space also provides an efficient neural solution for resolving the confusing sensory input caused by self-motion [the so-called reafference problem (43)]. Bodily motion causes widespread changes in sensory input, yet we seamlessly disambiguate our own movements from the movements of objects around us. In mammals, the multisensory inputs to the SC include inputs from the vestibular system (44), information on eye position (45–47), and somatosensation (8). This allows the influence of self-motion on the sensory fields to be factored out of the constructed sensory model of the environment (42). Resolving the reafference problem is a key function for the mammalian SC, which is why this region is vital for organizing motion in space for directed attention, reaching, and grasping for targets (41, 48–50).
For active animals with well-developed spatial senses, it is computationally more effective to resolve the reafference problem once for a unified sensory model than to resolve it in a dispersed and peripheral way for each sense independently. Further, different senses are affected by reafference in different ways and contribute different information on how the body is moving. Reafference can thus be resolved with greater accuracy and precision by integrating information from multiple senses (9).
For mammals, the processing performed within the SC constructs something akin to a simulation of the moving body in the environment (9). For humans at least, this spatial “model” is further enhanced by processing within the subcortical dorsal pulvinar (one of the thalamic nuclei, part of the basal ganglia) (32), which adds color, three-dimensionality, and an egocentric first-person perspective to the human conscious experience of space. A major benefit of this integrated spatial simulation is that it allows animals to interact with objects in a qualitatively different set of ways than simple stimulus-bound organisms could manage. Animals that navigate by reference to a simulated spatial model can actively seek out and navigate toward hidden objects. It is worth emphasizing what is implied by such an apparently simple navigational ability. A food source concealed behind a barrier, for example, is neither part of an organism’s current sensory input nor accessible by following a simple vector with its origin at the animal’s current position. Navigation requires a unified metric space that allows for effective computation of relative position after both actual and hypothetical translations within that space (51–54). This is beyond the primary sensory input but can be supported by a simulation of the environment constructed from that input (55).
The midbrain also provides the capacity to resolve competing behavioral priorities or motivations and rank needed resources by both urgency and availability. The floor of the midbrain is formed by the hypothalamic structures and associated nuclei whose function is to integrate information from the nervous and endocrine systems, to harvest and respond to information on the physiological status of the organism, and to motivate behavior to maintain a homeostatic optimum (56) (Fig. 1B). Maintaining homeostasis requires actively seeking needed resources; hence, midbrain structures are critical for the initiation and direction of a wide range of goal-directed behavior (15, 56). Integrative structures within the midbrain and basal ganglia, including the periaqueductal gray, substantia nigra, ventral thalamus, striatum, and midbrain reticular formation, combine this information on introspective assessment of state with exteroceptive assessment of location in space to collate and resolve competing motivations, prioritize resources, and select targets and actions (8, 33).
Midbrain and basal ganglia structures thus provide an effective and efficient neural solution for decision making (Fig. 1A). Competing and potentially conflicting behavioral responses that might be stimulated by different inputs can be resolved within a single behavioral control system (33, 57). Target selection and action selection must interact (Fig. 1A) because each domain informs the others. Motivational factors will influence the prioritization of targets and therefore action selection, but the location of targets will also influence which is selected, and which actions should be taken (8). Merker (8, 9) describes this as a “behavioral core control system” within which these decision domains can be resolved. Analyses and modeling of basal ganglia function in vertebrates have emphasized how drawing together all evidence for different possible actions within a unified neural system enables an effective and efficient solution for action selection, without having to posit a higher-level decision maker reflecting on the decision (57–59).
Placing the basic capacity for subjective experience in subcortical structures does not rule out a role for the cortex and other subcortical systems (including hippocampal systems) in conscious experience. The contents of subjective experience will vary enormously between species, and that variation will depend in part on the degree of elaboration of cortical structures. However, the capacity for subjective experience is not dependent on containing any particular contents (9, 32). The basic capacity for subjective experience that the midbrain supports is rather a capacity to have any subjective contents at all.
The midbrain thus provides a forum for specific contents to be integrated together with more basic survival-oriented machinery. However, the modeling function of the midbrain does not require some overseer that reflects on the model [the fallacy of the “Cartesian theater” (60)]. Decisions arise directly from this model, not from some further decision-making process informed by the model.
It is worth distinguishing the current proposal for the substrate of subjective experience from other, related, accounts. Like global workspace theories (61), we emphasize the role of consciousness in bringing together disparate brain processes into a common arena. Global workspace theories have a strongly cortical bias, however, focusing on the contribution of human fronto-parietal regions to reflective self-awareness of our mental states. It is unclear how widely this generalizes. We propose that even for invertebrates—which lack anything remotely like an elaborate cortex—holistic integration is essential for the more basic, evolutionarily ancient behavioral demands of action selection, reafference adjustment, and navigation. Further, insofar as cortical processes can matter to the organism, we argue that they must ultimately be integrated via midbrain mechanisms (58, 62). As Merker (8) puts it, the midbrain control system is “anatomically subcortical [but] functionally supra-cortical.”
Like information integration theories of consciousness (63–65), we claim that consciousness performs an important synthetic role. Unlike information integration theories, however, it matters which information is unified. Subjective experience requires the construction of an integrated neural simulation of the agent in space, weighted by the state of the agent. This simulation is constructed from appropriate integration of afferent, efferent, and homeostatic information. Just integrating information is not sufficient for subjective experience, no matter how complex or well-integrated the information. Information integration theorists have also tended to take a more liberal and graded view of consciousness, suggesting that even simple circuits such as a photodiode attached to memory might have a “modicum of experience” (65). By contrast, our theory implies that there is a definite cutoff point below which there is definitely not subjective experience (a point to which we will return).
Unified modeling provides effective solutions to the reafference problem and action selection for motile animals with developed spatially coherent senses (such as vision) (9, 66, 67). As such, it is not surprising that the vertebrate midbrain behavioral core control system evolved early on in the group and has been largely conserved since. Lampreys (primitive jawless fish) have functional homologs of all of the key nuclei involved in the vertebrate behavioral core control system (sensu ref. 8) including a well-developed tectum to process spatially structured visual information (68, 69) and equivalents of the basal ganglia system to resolve action and target selection (67, 70). It is thus a commitment of our model that subjective experience is likely to be universal among the vertebrates. What about invertebrate groups?
Consciousness in Insects
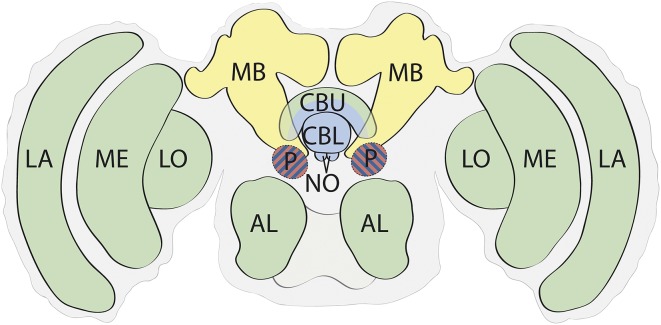
The insects are an extremely diverse group, but all insect brains have a common anatomical plan (Fig. 2). The nervous system contains an enlarged cephalic ganglion (a brain) specialized for sensory processing and integration. This is linked by paired ventral nerve cords to a series of smaller ganglia for the thoracic and abdominal body segments. The insect nervous system has frequently been stereotyped as decentralized (71), with the cephalic ganglion acting simply as a region of sensory input that triggers motor responses organized by the segmental ganglia (8, 71). This interpretation is incorrect and outdated. The insect brain resolves action and target selection, processes sensory information, and clearly executes a command function over the behavioral system (72).
Fig. 2.
Basic functional anatomy of the insect brain (not to scale). The structures of the insect brain create an integrated neural model of the state of the insect in space that is functionally analogous to that described for the vertebrate brain in Fig. 1. Regions are colored to reflect the major functions described in Fig. 1A. Vision and smell are primarily processed by dedicated sensory lobes, which function to refine and enhance sensory representations and enhance distinctions between similar stimuli (95, 141). Primary odor processing is performed in the antennal lobes (AL). Visual processing is performed by the lamina (LA), medulla (ME), and lobula (LO). The MB supports learning and memory (142–146). The CX is anatomically variable between insect orders but typically is composed of the central body upper (CBU), central body lower (CBL), and noduli (NO). It has several specializations for processing spatial information corrected for self movement (75, 76, 83, 87). The protocerebrum (P) is an anatomically complicated region. Modulatory and inhibitory connections to and within the protocerebrum convey information on physiological state (94, 95), and structures within the protocerebrum, particularly the lateral accessory lobe, are involved in integration of information, hence the hatched shading.
A compelling demonstration of the command function of the insect brain for the total behavioral system of the insect is the effect of focused injection of neurotransmitter agonists and antagonists to the region of the central complex (CX) of the insect brain. The parasitoid jewel wasp Ampulex compressa uses its ovipositor to inject venom containing GABA and octopamine antagonists into the CX of its cockroach prey (72, 73). The venom is not paralytic: the cockroach is still able to perform many basic actions. Rather, the pharmacological lesion to the central protocerebrum containing the CX disrupts the cockroach’s behavioral program, rendering it entirely passive so that it will not struggle as the wasp leads the cockroach by the antennae into its burrow. The effect of Ampulex venom on the cockroach brain is thus to eliminate the capacity of the roach to organize and initiate behavior (73). This example shows that the central brain structures are key for the initiation and direction of movement in cockroaches and crickets (74, 75).
Further, the CX of the insect brain seems specialized for the processing of spatial information and organization of movement (76). As such (we argue), it performs functions analogous to the vertebrate tectum/colliculus (Fig. 2). Like the vertebrate tectum/colliculus, the CX plays a crucial role in processing multiple sources of spatial information drawn from different senses. Many insects are sensitive to plane-polarized light, a vital celestial navigational cue. In locusts (Schistocerca gregaria) information on the distribution of polarized light in the sky is processed by the CX, which creates a central polarotopic representation of the sky polarization pattern (77). Outputs from this system could provide compass-like information to stabilize the direction of moving insects relative to the external celestial cue (78). This function for the CX has been described in locusts and butterflies (Danaus plexippus) but may be common across insect orders (79). Further, in S. gregaria (80), the cockroach Blaberus discoidalis (75), and Drosophila melanogaster (81, 82), the CX encodes topographically organized visual information on moving objects and is capable of factoring out the confounding effects of visual motion generated by self-movement from moving objects in the environment (82). In Drosophila, neural activity within the CX encodes the fly’s heading relative to visual landmarks in the environment and presumably enables the fly to maintain a course relative to those landmarks (83). The CX is also vital for flies to learn spatial tasks (84, 85).
Collectively these studies emphasize the capacity of the insect CX to represent visual space such that the insect can orient and navigate. However, the CX processes more than just visual information. In cockroaches (86), Drosophila (83), and crickets (74), the CX also encodes topographically organized mechanosensory and proprioceptive information, corrected for reafference, to resolve the movements and orientation of the body and limbs in space. The CX is necessary for producing actions that involve coordination of limbs on both sides of the body (such as turning), and also for judging distance in targeting and reaching (87). The various topographically organized sensory maps are layered in Drosophila (79), and it would seem the sensory maps are integrated because the encoding of azimuth in the Drosophila CX can switch seamlessly between a reliance on visual landmarks in the light to a reliance on proprioceptive cues in the dark (83).
New electrophysiological studies of the visual interneurons of the lobula of the Drosophila brain have shown how a flying fly can resolve the reafference problem (88). Visual interneurons that register optic flow in the visual field receive a motor-related input when the fly turns, which precisely counters the visual stimulation of the neurons caused by the turn (88). The electrophysiological data support von Holst and Mittelstaedt’s (43) classic theory, and inferences from insect behavioral studies (89–91), that an “efference copy” of a motor action is sent to the visual system to silence the specific image motion caused by a voluntary turn. The motor-related input to the lobula is specific and anticipatory of the motor action, cautiously supporting a forward model of action selection (where modeling of the movement and its consequences precedes the movement itself) (88, 92). Although we still do not know how the efference copy to the lobula is generated, it seems increasingly likely that CX circuits are involved.
One of the crucial functions of the CX is thus to generate a neural simulation of the state of the moving insect in space. This simulation forms part of the insect behavioral core control system (Fig. 2). The CX outputs to, and receives input from, the protocerebrum (P) of the insect brain, especially the lateral accessory lobe which is a point of convergence for sensory information. These include both direct inputs from the sensory lobes and indirect inputs via the CX and mushroom body (MB) pathways (Fig. 2), which are a locus for memory of past experience. The P is premotor, and emerging evidence suggests that competitive processing within structures of the P contributes to effective action selection based on all available sensory information (93–96).
In sum, new functional analyses of the insect brain emphasize how it supports a behavioral core control system that is functionally analogous to that of the vertebrate midbrain (Fig. 2). This no doubt supports much of the dynamic and flexible behavior for which some insects are famous. Some of the central-place foraging ants and bees have remarkable navigational skills and spatial memory and are clearly able to organize their behavior with respect to more than simply their immediate sensory environment. They will perform targeted searches in appropriate locations and at appropriate times (97) for resources they have experienced previously. Several insect species have been shown to be able to plot novel routes based on learned landmarks and goals, evidencing a spatial relation of landmark information (98, 99). The honey bee dance communication system requires a dance follower to determine a flight vector relative to celestial cues from symbolic and stereotyped dance movements (100).
All these behaviors require a form of neural modeling of space. There is now both behavioral and electrophysiological evidence that the neural modeling of the environment performed by insects involves multiple layers of filtering of sensory information to support selective attention to stimuli that are salient and suppression of representation of irrelevant stimuli (101–104). The amount and amplitude of firing activity within certain defined frequency bands in the central brain of tethered flies in response to visual stimuli is dependent on whether the fly is visually fixed on the stimulus or whether the context of the stimulus has changed (101). This change in neural activity has been described as a neural signature of “salience” in insects (101, 102). The brain region in which this occurs includes the point of interaction between the MB, CX, and P circuits, and the electrophysiological response is dependent on a functioning MB (101, 102).
These findings show how the brain responses to environmental stimuli in insects are not driven simply by the primary sensory input, but rather by egocentric characteristics: whether the context of the stimulus needs to be updated or whether the stimulus is a point of immediate navigational reference (101, 102). In essence, responses depend upon what the insect is attending to at that moment (104). In bees, even activity in the optic lobes is influenced by centrifugal input from the central brain to enhance neural responses to stimuli that are points of visual fixation (103). These exciting new electrophysiological studies demonstrate more compellingly than behavioral studies alone the subjective and egocentric nature of the neural representation of the environment in insects, and their capacity for selective attention supports our assertion that insects have a capacity for subjective experience. We note that the relationship between subjective experience and selective attention in humans remains a topic of considerable debate (105, 106). Whether selective attention is a prerequisite for consciousness (105) or whether consciousness is broader than what we attend to (106) is quite contentious. Because insects clearly have a capacity for selective attention we may safely sidestep this debate for now. However, insect brains could provide powerful experimental systems for future exploration of the relationship between attention and experience in simple animals.
The honey bee particularly is held up as an insect with cognitive capacities that rival those of many mammals (107–109). Without consideration of the underlying mechanisms, this may seem like no more than a curiosity. The systems that underlie these abilities were shaped by evolutionary pressures similar to those that shaped the mammalian midbrain. The insect brain does a similar sort of modeling, for the same reasons, in a similar way. That is strong evidence that the insect brain has the capacity to support subjective experience.
Beyond Insects
Our thesis raises two related questions. First, how widespread is subjective experience among the other invertebrates? Second, when did a capacity for subjective experience arise?
To the question of how widespread subjective experience is among the invertebrates, we answer that it is likely to be more extensive than previously thought, but far from universal. One of the virtues of the account we have endorsed is that it also gives an evidence-based argument for where to draw the line between the haves and have-nots. There are other, simpler ways to organize animal behavior than by creating the kind of integrated neural simulation seen in the insect and vertebrate core control systems. These simpler systems cannot support a capacity for subjective experience.
An informative, if simple, example is the cubozoan box jellyfish Tripedalia cystophora. This species shows dynamic behavior that includes actively hunting its prey. It has a well-developed geosense and visual sense thanks to 24 lensed eyes distributed around the body (110, 111), yet the nervous system is entirely decentralized. Sense organs independently modulate activity in regions of the sensory net and muscle walls to steer the animal (112). The outcome of local sensory input acting on local muscle activity is an adaptive change in the swimming direction and speed of the animal (112), but the simple behavioral control system is entirely decentralized. Although such systems manifest the appearance of adaptive and dynamic targeting, they are nothing more than simple decentralized stimulus–response systems. They could not reasonably be expected to support any form of subjective experience.
Centralization is necessary for subjective experience, but it is not sufficient. The nematode Caenorhabditis elegans possesses a famously simple and well-characterized centralized nervous system that can integrate input from an array of thermo-, mechano-, chemo-, and nociceptors, along with an interoceptive sense of time to organize directed movements (113). Sensory inputs are integrated at the level of an array of interneurons that compete for activation of the motor neurons (113). The simple nervous system of C. elegans can also support various forms of learning such as habituation (114–116) and classical associative conditioning (116–118), enabling adaptive change in behavior with experience.
Nematodes are thus able to integrate multiple forms of sensory input using a centralized nervous system, but it seems that their behavior is organized as responses either to the immediate sensory environment or to immediate interoceptive signals of physiological state. Action selection in nematodes is driven by shifts in global brain dynamics (119), and there is sufficient plasticity in the nematode nervous system for their responses to vary with system state (120). However, there is no evidence that nematodes can actively hunt for things beyond their immediate sensory environment. Hungry nematodes respond to starvation with increased locomotion and dispersal in a random, rather than directed, search (121, 122). By contrast, hungry rodents, ants, and bees will navigate to places where they have previously encountered food. Their internal state of hunger triggers a highly directional and oriented food search focused on locations where food was previously experienced, even if no food stimuli are currently present (123–125). Hence, in mammals and insects homeostatic drives direct behavior to where resources are expected to be, even if they are not currently there. We argue the difference between this behavior and nematode search behavior arises because nematode behavior is organized by reference to their primary sensory input, whereas rodent and insect behavior is organized in response to an integrated and spatial simulation of their environment. Nematodes do possess forms of memory (116) that can change how they react to stimuli (120), but there is no evidence this memory has a spatial component or contributes to a structured model of their environment.
Consequently, even though nematodes have a centralized nervous system and memory, they lack the egocentric modeling of the environment that is required for subjective experience. Further, it is because they lack the capacity to make such models that they are unable to do a variety of tasks that vertebrates and insects handle with ease. As a group, nematodes may have evolved a simplified body plan and nervous system as a consequence of evolving to exploit very stable environments or a parasitic existence (126, 127). In such environments, very simple behavioral control systems are sufficient.
If the insect brain supports subjective experience, then this not only increases the diversity of animals considered to have these abilities—it also requires a reconsideration of when this ability might have appeared on Earth. As noted above, the key structural elements of the vertebrate behavioral core control system are all present and functional in lampreys (128). If these structures are present in the basal vertebrates then they may also have been present in the basal vertebrate species Haikouichthys identified in Cambrian fossil fauna (128). This species had well-developed eyes and a hydrodynamic eel-like body (129). It is reconstructed as an active swimmer (129) and presumably had a version of an operational behavioral core control system for the organization of dynamic and active movement, action selection, and target selection (128).
The insect behavioral core control system is likely to be similarly ancient. Cambrian fossil faunas are famous for their arthropod diversity, many of which had complex jointed limbs and bodies, sensory appendages, and well-developed image-forming compound eyes (130–133). These have been reconstructed as having active lifestyles, and arthropods were the top predators of the period (130–133). At least some of these arthropods had well-developed cephalic ganglia with structural similarities to extant crustacean and insect brains (134). We argue it is likely that a version of the extant insect behavioral core control system must have been present in at least some Cambrian arthropods to support their active foraging and hunting lifestyles. The CX is basal to the insect clade. Although there is some variation in the structure of the CX between insect orders, this structure features in all insects and crustaceans and is likely homologous to the arcuate body of arachnids (87, 135, 136), suggesting a form of the CX evolved before the radiation of insects, crustaceans, and spiders. Trestman (130) has argued that the spatial awareness and agentive behavior enabled by arthropod neural and sensory systems may have contributed to the arthropod radiation in the Cambrian as a consequence of the emergence of new forms of behavior such as hunting. It is plausible that some of the Cambrian fauna within both the basal vertebrate and arthropod groups had a capacity for subjective experience.
It is presently unclear whether the insect and vertebrate behavioral core control systems evolved independently. Strausfeld and Hirth (137) marshal commanding developmental, anatomical, and genetic evidence to argue a possible deep homology of the insect CX and associated structures with vertebrate basal ganglia structures. If this interpretation is correct, it would imply that a brain with a form of higher sensory integration center may even predate the divergence of these groups.
Moving Forward on Invertebrate Consciousness
We have argued that insects possess a capacity for subjective experience. Many find this a counterintuitive result. A natural place to take issue with our argument is with our reliance on Merker’s proposal that the midbrain is sufficient to support subjective experience. Fair enough. Merker’s theory is far from universally accepted, and even otherwise similar theories may not (for all we have said) generalize to invertebrates. With Merker, we have emphasized the importance of a unified perspective on the world as a key feature of subjective experience (14). However, perhaps other neural features are also necessary for subjective experience, such as a representation of a temporal dimension (138). Perhaps insects lack these (though see ref. 139).
However, this kind of disagreement can be fruitful. If insects do not have subjective experience, why not?
What is important, from our perspective, is that either proposing or denying that insects have subjective experience should require telling an evidence-based structural, functional, and comparative story about the insect brain. We have downplayed behavioral data. Critically, we have not relied on evidence of unusual or clever achievements by insects. Rather, we suggest, behavior is important only insofar as it is a guide to understanding the underlying mechanisms by which behavior is generated.
We propose that arguing about what subjective experience is, and what is capable of it, is most productive when appealing to empirical neuroscience. We believe that it is on these structural, functional, and comparative grounds that questions about subjective experience—of insects or of any other animal—ought to be settled.
Acknowledgments
We thank Tim Bayne, Lars Chittka, Jean-Marc Devaud, Stanley Heinze, Michael Gillings, and Peter Godfrey-Smith for constructive discussion and feedback. Figs. 1 and 2 were created by Marcus J. A. Plath. This work was supported by Australian Research Council Future Fellowship FT140100452 (to A.B.B.) and Australian Research Council Future Fellowship FT140100422 (to C.K.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Nagel T. What is it like to be a bat? Philos Rev. 1974;83(4):435–450. [Google Scholar]
- 2.Boly M, et al. Consciousness in humans and non-human animals: Recent advances and future directions. Front Psychol. 2013;4:625. doi: 10.3389/fpsyg.2013.00625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Edelman DB, Seth AK. Animal consciousness: A synthetic approach. Trends Neurosci. 2009;32(9):476–484. doi: 10.1016/j.tins.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 4.Pepperberg IM. The Alex Studies: Cognitive and Communicative Abilities of Grey Parrots. Harvard Univ Press; Cambridge, MA: 2002. [Google Scholar]
- 5.Crick F, Koch C. Towards a neurobiological theory of consciousness. Semin Neurosci. 1990;2:263–275. [Google Scholar]
- 6.Hohwy J. The search for neural correlates of consciousness. Philos Compass. 2007;2(3):461–474. [Google Scholar]
- 7.Morin A. Levels of consciousness and self-awareness: A comparison and integration of various neurocognitive views. Conscious Cogn. 2006;15(2):358–371. doi: 10.1016/j.concog.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 8.Merker B. Consciousness without a cerebral cortex: A challenge for neuroscience and medicine. Behav Brain Sci. 2007;30(1):63–81, discussion 81–134. doi: 10.1017/S0140525X07000891. [DOI] [PubMed] [Google Scholar]
- 9.Merker B. The liabilities of mobility: A selection pressure for the transition to consciousness in animal evolution. Conscious Cogn. 2005;14(1):89–114. doi: 10.1016/S1053-8100(03)00002-3. [DOI] [PubMed] [Google Scholar]
- 10.Edelman GM. Naturalizing consciousness: A theoretical framework. Proc Natl Acad Sci USA. 2003;100(9):5520–5524. doi: 10.1073/pnas.0931349100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parvizi J, Damasio A. Consciousness and the brainstem. Cognition. 2001;79(1-2):135–160. doi: 10.1016/s0010-0277(00)00127-x. [DOI] [PubMed] [Google Scholar]
- 12.Panksepp J. The affective brain and core consciousness: How does neural activity generate emotional feelings? In: Lewis M, Haviland-Jones JM, Barrett LF, editors. Handbook of Emotions. 3rd Ed. Guilford; New York: 2008. pp. 47–67. [Google Scholar]
- 13.Block N. On a confusion about a function of consciousness. Behav Brain Sci. 1995;18(02):227–287. [Google Scholar]
- 14.Christoff K, Cosmelli D, Legrand D, Thompson E. Specifying the self for cognitive neuroscience. Trends Cogn Sci. 2011;15(3):104–112. doi: 10.1016/j.tics.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 15.Damasio A, Carvalho GB. The nature of feelings: Evolutionary and neurobiological origins. Nat Rev Neurosci. 2013;14(2):143–152. doi: 10.1038/nrn3403. [DOI] [PubMed] [Google Scholar]
- 16.Mashour GA, Alkire MT. Evolution of consciousness: Phylogeny, ontogeny, and emergence from general anesthesia. Proc Natl Acad Sci USA. 2013;110(Suppl 2):10357–10364. doi: 10.1073/pnas.1301188110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Damasio A, Damasio H, Tranel D. Persistence of feelings and sentience after bilateral damage of the insula. Cereb Cortex. 2013;23(4):833–846. doi: 10.1093/cercor/bhs077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Philippi CL, et al. Preserved self-awareness following extensive bilateral brain damage to the insula, anterior cingulate, and medial prefrontal cortices. PLoS One. 2012;7(8):e38413. doi: 10.1371/journal.pone.0038413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Herbet G, et al. Disrupting posterior cingulate connectivity disconnects consciousness from the external environment. Neuropsychologia. 2014;56:239–244. doi: 10.1016/j.neuropsychologia.2014.01.020. [DOI] [PubMed] [Google Scholar]
- 20.Kapur N, et al. Bilateral temporal lobe pathology with sparing of medial temporal lobe structures: Lesion profile and pattern of memory disorder. Neuropsychologia. 1994;32(1):23–38. doi: 10.1016/0028-3932(94)90066-3. [DOI] [PubMed] [Google Scholar]
- 21.Friedman-Hill SR, Robertson LC, Treisman A. Parietal contributions to visual feature binding: Evidence from a patient with bilateral lesions. Science. 1995;269(5225):853–855. doi: 10.1126/science.7638604. [DOI] [PubMed] [Google Scholar]
- 22.Damasio A, Van Hoesen G. Emotional disturbances associated with focal lesions of the limbic frontal lobe. In: Satz P, Heilman KM, editors. Neuropsychology of Human Emotion. Guilford; New York: 1983. pp. 85–110. [Google Scholar]
- 23.Owen AM, et al. Detecting residual cognitive function in persistent vegetative state. Neurocase. 2002;8(5):394–403. doi: 10.1076/neur.8.4.394.16184. [DOI] [PubMed] [Google Scholar]
- 24.Klein C, Hohwy J. Variability, convergence and dimensions of consciousness. Behavioral Methods. In: Overgaard M, editor. Consciousness Research. Oxford Univ Press; Oxford: 2015. pp. 249–264. [Google Scholar]
- 25.Hudetz AG. General anesthesia and human brain connectivity. Brain Connect. 2012;2(6):291–302. doi: 10.1089/brain.2012.0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alkire MT, Hudetz AG, Tononi G. Consciousness and anesthesia. Science. 2008;322(5903):876–880. doi: 10.1126/science.1149213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gili T, et al. The thalamus and brainstem act as key hubs in alterations of human brain network connectivity induced by mild propofol sedation. J Neurosci. 2013;33(9):4024–4031. doi: 10.1523/JNEUROSCI.3480-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gyulai FE, Firestone LL, Mintun MA, Winter PM. In vivo imaging of human limbic responses to nitrous oxide inhalation. Anesth Analg. 1996;83(2):291–298. doi: 10.1097/00000539-199608000-00016. [DOI] [PubMed] [Google Scholar]
- 29.Långsjö JW, et al. Returning from oblivion: Imaging the neural core of consciousness. J Neurosci. 2012;32(14):4935–4943. doi: 10.1523/JNEUROSCI.4962-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schiff ND. Recovery of consciousness after brain injury: A mesocircuit hypothesis. Trends Neurosci. 2010;33(1):1–9. doi: 10.1016/j.tins.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Denton D. The Primordial Emotions. Oxford Univ Press; New York: 2006. [Google Scholar]
- 32.Merker B. The efference cascade, consciousness, and its self: Naturalizing the first person pivot of action control. Front Psychol. 2013;4:501. doi: 10.3389/fpsyg.2013.00501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McHaffie JG, Stanford TR, Stein BE, Coizet V, Redgrave P. Subcortical loops through the basal ganglia. Trends Neurosci. 2005;28(8):401–407. doi: 10.1016/j.tins.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 34.Klier EM, Wang H, Crawford JD. The superior colliculus encodes gaze commands in retinal coordinates. Nat Neurosci. 2001;4(6):627–632. doi: 10.1038/88450. [DOI] [PubMed] [Google Scholar]
- 35.Harting JK, Updyke BV, Van Lieshout DP. Corticotectal projections in the cat: Anterograde transport studies of twenty-five cortical areas. J Comp Neurol. 1992;324(3):379–414. doi: 10.1002/cne.903240308. [DOI] [PubMed] [Google Scholar]
- 36.Ulanovsky N, Moss CF. What the bat’s voice tells the bat’s brain. Proc Natl Acad Sci USA. 2008;105(25):8491–8498. doi: 10.1073/pnas.0703550105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nemec P, Altmann J, Marhold S, Burda H, Oelschläger HHA. Neuroanatomy of magnetoreception: The superior colliculus involved in magnetic orientation in a mammal. Science. 2001;294(5541):366–368. doi: 10.1126/science.1063351. [DOI] [PubMed] [Google Scholar]
- 38.Bastian J. Vision and electroreception: Integration of sensory information in the optic tectum of weakly electric fish Apteronotus albifrons. J Comp Physiol. 1982;147:287–298. [Google Scholar]
- 39.May PJ. The mammalian superior colliculus: Laminar structure and connections. Prog Brain Res. 2006;151:321–378. doi: 10.1016/S0079-6123(05)51011-2. [DOI] [PubMed] [Google Scholar]
- 40.Masino T. Brainstem control of orienting movements: Intrinsic coordinate systems and underlying circuitry. Brain Behav Evol. 1992;40(2–3):98–111. doi: 10.1159/000113906. [DOI] [PubMed] [Google Scholar]
- 41.Zénon A, Krauzlis RJ. Attention deficits without cortical neuronal deficits. Nature. 2012;489(7416):434–437. doi: 10.1038/nature11497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sparks DL. Neural cartography: Sensory and motor maps in the superior colliculus. Brain Behav Evol. 1988;31(1):49–56. doi: 10.1159/000116575. [DOI] [PubMed] [Google Scholar]
- 43.von Holst E, Mittelstaedt H. Das reafferenzprincip (wechselwirkungen zwischen zentralnervensystem und peripherie) Naturwissen. 1950;37:464–476. [Google Scholar]
- 44.Frens MA, Suzuki Y, Scherberger H, Hepp K, Henn V. The collicular code of saccade direction depends on the roll orientation of the head relative to gravity. Exp Brain Res. 1998;120(3):283–290. doi: 10.1007/s002210050402. [DOI] [PubMed] [Google Scholar]
- 45.Van Opstal AJ, Hepp K, Suzuki Y, Henn V. Influence of eye position on activity in monkey superior colliculus. J Neurophysiol. 1995;74(4):1593–1610. doi: 10.1152/jn.1995.74.4.1593. [DOI] [PubMed] [Google Scholar]
- 46.Groh JM, Sparks DL. Saccades to somatosensory targets. III. Eye-position-dependent somatosensory activity in primate superior colliculus. J Neurophysiol. 1996;75(1):439–453. doi: 10.1152/jn.1996.75.1.439. [DOI] [PubMed] [Google Scholar]
- 47.Knox PC, Donaldson IML. The effect of afferent signals from extraocular muscles on visual responses of cells in the optic tectum of the pigeon. Proc R Soc Lond B Biol Sci. 1995;259:285–291. [Google Scholar]
- 48.Krauzlis RJ, Liston D, Carello CD. Target selection and the superior colliculus: Goals, choices and hypotheses. Vision Res. 2004;44(12):1445–1451. doi: 10.1016/j.visres.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 49.McPeek RM, Keller EL. Deficits in saccade target selection after inactivation of superior colliculus. Nat Neurosci. 2004;7(7):757–763. doi: 10.1038/nn1269. [DOI] [PubMed] [Google Scholar]
- 50.Horwitz GD, Newsome WT. Separate signals for target selection and movement specification in the superior colliculus. Science. 1999;284(5417):1158–1161. doi: 10.1126/science.284.5417.1158. [DOI] [PubMed] [Google Scholar]
- 51.Collett M. How navigational guidance systems are combined in a desert ant. Curr Biol. 2012;22(10):927–932. doi: 10.1016/j.cub.2012.03.049. [DOI] [PubMed] [Google Scholar]
- 52.Collett M, Collett TS. The learning and maintenance of local vectors in desert ant navigation. J Exp Biol. 2009;212(Pt 7):895–900. doi: 10.1242/jeb.024521. [DOI] [PubMed] [Google Scholar]
- 53.Collett TS, Baron J. Learnt sensori-motor mappings in honeybees: Interpolation and its possible relevance to navigation. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 1995;117:287–298. [Google Scholar]
- 54.Collett TS, Rees JA. View-based navigation in Hymenoptera: Multiple strategies of landmark guidance in the approach to a feeder. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 1997;181:47–58. [Google Scholar]
- 55.Revonsuo A. Consciousness, dreams, and virtual realities. Philos Psychol. 1995;8:35–58. [Google Scholar]
- 56.Swanson LW. Cerebral hemisphere regulation of motivated behavior. Brain Res. 2000;886(1–2):113–164. doi: 10.1016/s0006-8993(00)02905-x. [DOI] [PubMed] [Google Scholar]
- 57.Redgrave P, Prescott TJ, Gurney K. The basal ganglia: A vertebrate solution to the selection problem? Neuroscience. 1999;89(4):1009–1023. doi: 10.1016/s0306-4522(98)00319-4. [DOI] [PubMed] [Google Scholar]
- 58.Bogacz R, Gurney K. The basal ganglia and cortex implement optimal decision making between alternative actions. Neural Comput. 2007;19(2):442–477. doi: 10.1162/neco.2007.19.2.442. [DOI] [PubMed] [Google Scholar]
- 59.Gurney K, Prescott TJ, Redgrave P. A computational model of action selection in the basal ganglia. I. A new functional anatomy. Biol Cybern. 2001;84(6):401–410. doi: 10.1007/PL00007984. [DOI] [PubMed] [Google Scholar]
- 60.Dennett DC. Consciousness Explained. Little, Brown; Boston: 1991. [Google Scholar]
- 61.Baars BJ. Global workspace theory of consciousness: Toward a cognitive neuroscience of human experience. Prog Brain Res. 2005;150:45–53. doi: 10.1016/S0079-6123(05)50004-9. [DOI] [PubMed] [Google Scholar]
- 62.Gurney KN, Humphries MD, Redgrave P. A new framework for cortico-striatal plasticity: Behavioural theory meets in vitro data at the reinforcement-action interface. PLoS Biol. 2015;13(1):e1002034. doi: 10.1371/journal.pbio.1002034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tononi G. An information integration theory of consciousness. BMC Neurosci. 2004;5(42):42. doi: 10.1186/1471-2202-5-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tononi G. Consciousness as integrated information: A provisional manifesto. Biol Bull. 2008;215(3):216–242. doi: 10.2307/25470707. [DOI] [PubMed] [Google Scholar]
- 65.Tononi G, Koch C. Consciousness: Here, there and everywhere? Phil Trans R Soc B. 2015;370:20140167. doi: 10.1098/rstb.2014.0167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stephenson-Jones M, Samuelsson E, Ericsson J, Robertson B, Grillner S. Evolutionary conservation of the basal ganglia as a common vertebrate mechanism for action selection. Curr Biol. 2011;21(13):1081–1091. doi: 10.1016/j.cub.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 67.Grillner S, Robertson B, Stephenson-Jones M. The evolutionary origin of the vertebrate basal ganglia and its role in action selection. J Physiol. 2013;591(22):5425–5431. doi: 10.1113/jphysiol.2012.246660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zompa IC, Dubuc R. A mesencephalic relay for visual inputs to reticulospinal neurones in lampreys. Brain Res. 1996;718(1-2):221–227. doi: 10.1016/0006-8993(96)00131-x. [DOI] [PubMed] [Google Scholar]
- 69.Zompa IC, Dubuc R. Electrophysiological and neuropharmacological study of tectoreticular pathways in lampreys. Brain Res. 1998;804(2):238–252. doi: 10.1016/s0006-8993(98)00650-7. [DOI] [PubMed] [Google Scholar]
- 70.Stephenson-Jones M, Ericsson J, Robertson B, Grillner S. Evolution of the basal ganglia: Dual-output pathways conserved throughout vertebrate phylogeny. J Comp Neurol. 2012;520(13):2957–2973. doi: 10.1002/cne.23087. [DOI] [PubMed] [Google Scholar]
- 71.Altman JS, Kien J. New models for motor control. Neural Comput. 1989;1:173–183. [Google Scholar]
- 72.Strausfeld NJ. Arthropod Brains: Evolution, Functional Elegance, and Historical Significance. Belknap; Cambridge, MA: 2012. [Google Scholar]
- 73.Libersat F, Gal R. Wasp voodoo rituals, venom-cocktails, and the zombification of cockroach hosts. Integr Comp Biol. 2014;54(2):129–142. doi: 10.1093/icb/icu006. [DOI] [PubMed] [Google Scholar]
- 74.Kai K, Okada J. Characterization of locomotor-related spike activity in protocerebrum of freely walking cricket. Zoolog Sci. 2013;30(7):591–601. doi: 10.2108/zsj.30.591. [DOI] [PubMed] [Google Scholar]
- 75.Kathman ND, Kesavan M, Ritzmann RE. Encoding wide-field motion and direction in the central complex of the cockroach Blaberus discoidalis. J Exp Biol. 2014;217(Pt 22):4079–4090. doi: 10.1242/jeb.112391. [DOI] [PubMed] [Google Scholar]
- 76.Plath JA, Barron AB. Current progress in understanding the functions of the insect central complex. Curr Opin Insect Sci. 2015;12:11–18. [Google Scholar]
- 77.Heinze S, Homberg U. Maplike representation of celestial E-vector orientations in the brain of an insect. Science. 2007;315(5814):995–997. doi: 10.1126/science.1135531. [DOI] [PubMed] [Google Scholar]
- 78.Bockhorst T, Homberg U. Amplitude and dynamics of polarization-plane signaling in the central complex of the locust brain. J Neurophysiol. 2015;113(9):3291–3311. doi: 10.1152/jn.00742.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lin CY, et al. A comprehensive wiring diagram of the protocerebral bridge for visual information processing in the Drosophila brain. Cell Reports. 2013;3(5):1739–1753. doi: 10.1016/j.celrep.2013.04.022. [DOI] [PubMed] [Google Scholar]
- 80.Rosner R, Homberg U. Widespread sensitivity to looming stimuli and small moving objects in the central complex of an insect brain. J Neurosci. 2013;33(19):8122–8133. doi: 10.1523/JNEUROSCI.5390-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Weir PT, Schnell B, Dickinson MH. Central complex neurons exhibit behaviorally gated responses to visual motion in Drosophila. J Neurophysiol. 2014;111(1):62–71. doi: 10.1152/jn.00593.2013. [DOI] [PubMed] [Google Scholar]
- 82.Seelig JD, Jayaraman V. Feature detection and orientation tuning in the Drosophila central complex. Nature. 2013;503(7475):262–266. doi: 10.1038/nature12601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Seelig JD, Jayaraman V. Neural dynamics for landmark orientation and angular path integration. Nature. 2015;521(7551):186–191. doi: 10.1038/nature14446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Neuser K, Triphan T, Mronz M, Poeck B, Strauss R. Analysis of a spatial orientation memory in Drosophila. Nature. 2008;453(7199):1244–1247. doi: 10.1038/nature07003. [DOI] [PubMed] [Google Scholar]
- 85.Ofstad TA, Zuker CS, Reiser MB. Visual place learning in Drosophila melanogaster. Nature. 2011;474(7350):204–207. doi: 10.1038/nature10131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Guo P, Ritzmann RE. Neural activity in the central complex of the cockroach brain is linked to turning behaviors. J Exp Biol. 2013;216(Pt 6):992–1002. doi: 10.1242/jeb.080473. [DOI] [PubMed] [Google Scholar]
- 87.Pfeiffer K, Homberg U. Organization and functional roles of the central complex in the insect brain. Annu Rev Entomol. 2014;59:165–184. doi: 10.1146/annurev-ento-011613-162031. [DOI] [PubMed] [Google Scholar]
- 88.Kim AJ, Fitzgerald JK, Maimon G. Cellular evidence for efference copy in Drosophila visuomotor processing. Nat Neurosci. 2015;18(9):1247–1255. doi: 10.1038/nn.4083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Heisenberg M, Wolf R. On the fine structure of yaw torque in visual flight orientation of Drosophila melanogaster. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 1979;130:113–130. [Google Scholar]
- 90.Collett TS. Angular tracking of the opotmotor response: An analysis of visual reflex interaction in a hoverfly. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 1980;140:145–158. [Google Scholar]
- 91.Collett T, Land M. How hoverflies compute interception courses. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 1978;125:191–204. [Google Scholar]
- 92.Webb B. Neural mechanisms for prediction: Do insects have forward models? Trends Neurosci. 2004;27(5):278–282. doi: 10.1016/j.tins.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 93.Liang L, et al. GABAergic projection neurons route selective olfactory inputs to specific higher-order neurons. Neuron. 2013;79(5):917–931. doi: 10.1016/j.neuron.2013.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Parnas M, Lin AC, Huetteroth W, Miesenböck G. Odor discrimination in Drosophila: From neural population codes to behavior. Neuron. 2013;79(5):932–944. doi: 10.1016/j.neuron.2013.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Galizia CG. Olfactory coding in the insect brain: Data and conjectures. Eur J Neurosci. 2014;39(11):1784–1795. doi: 10.1111/ejn.12558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Barron AB, Gurney KN, Meah LFS, Vasilaki E, Marshall JAR. Decision-making and action selection in insects: Inspiration from vertebrate-based theories. Front Behav Neurosci. 2015;9:216. doi: 10.3389/fnbeh.2015.00216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pahl M, Zhu H, Pix W, Tautz J, Zhang S. Circadian timed episodic-like memory - A bee knows what to do when, and also where. J Exp Biol. 2007;210(Pt 20):3559–3567. doi: 10.1242/jeb.005488. [DOI] [PubMed] [Google Scholar]
- 98.Collett TS, Graham P, Harris RA. Novel landmark-guided routes in ants. J Exp Biol. 2007;210(Pt 12):2025–2032. doi: 10.1242/jeb.000315. [DOI] [PubMed] [Google Scholar]
- 99.Narendra A, Gourmaud S, Zeil J. Mapping the navigational knowledge of individually foraging ants, Myrmecia croslandi. Proc Biol Sci. 2013;280(1765):20130683. doi: 10.1098/rspb.2013.0683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Dyer FC. The biology of the dance language. Annu Rev Entomol. 2002;47:917–949. doi: 10.1146/annurev.ento.47.091201.145306. [DOI] [PubMed] [Google Scholar]
- 101.van Swinderen B, Greenspan RJ. Salience modulates 20-30 Hz brain activity in Drosophila. Nat Neurosci. 2003;6(6):579–586. doi: 10.1038/nn1054. [DOI] [PubMed] [Google Scholar]
- 102.Swinderen Bv. The remote roots of consciousness in fruit-fly selective attention? BioEssays. 2005;27(3):321–330. doi: 10.1002/bies.20195. [DOI] [PubMed] [Google Scholar]
- 103.Paulk AC, et al. Selective attention in the honeybee optic lobes precedes behavioral choices. Proc Natl Acad Sci USA. 2014;111(13):5006–5011. doi: 10.1073/pnas.1323297111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sareen P, Wolf R, Heisenberg M. Attracting the attention of a fly. Proc Natl Acad Sci USA. 2011;108(17):7230–7235. doi: 10.1073/pnas.1102522108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Prinz JJ. The Conscious Brain: How Attention Engenders Experience. Oxford Univ Press; Oxford: 2012. [Google Scholar]
- 106.Block N. Perceptual consciousness overflows cognitive access. Trends Cogn Sci. 2011;15(12):567–575. doi: 10.1016/j.tics.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 107.Avarguès-Weber A, Giurfa M. Conceptual learning by miniature brains. Proc R Soc Lond B. 2013;280:1907. doi: 10.1098/rspb.2013.1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Giurfa M. The amazing mini-brain: Lessons from a honey bee. Bee World. 2003;84:5–18. [Google Scholar]
- 109.Giurfa M. Cognitive neuroethology: Dissecting non-elemental learning in a honeybee brain. Curr Opin Neurobiol. 2003;13(6):726–735. doi: 10.1016/j.conb.2003.10.015. [DOI] [PubMed] [Google Scholar]
- 110.Kingsford MJ, Mooney CJ. The ecology of box jellyfishes (Cubozoa) In: Pitt KA, Lucas CH, editors. Jellyfish Blooms. Springer; New York: 2014. pp. 267–303. [Google Scholar]
- 111.Garm A, Oskarsson M, Nilsson D-E. Box jellyfish use terrestrial visual cues for navigation. Curr Biol. 2011;21(9):798–803. doi: 10.1016/j.cub.2011.03.054. [DOI] [PubMed] [Google Scholar]
- 112.Petie R, Garm A, Nilsson D-E. Visual control of steering in the box jellyfish Tripedalia cystophora. J Exp Biol. 2011;214(Pt 17):2809–2815. doi: 10.1242/jeb.057190. [DOI] [PubMed] [Google Scholar]
- 113.de Bono M, Maricq AV. Neuronal substrates of complex behaviors in C. elegans. Annu Rev Neurosci. 2005;28:451–501. doi: 10.1146/annurev.neuro.27.070203.144259. [DOI] [PubMed] [Google Scholar]
- 114.Rankin CH, Beck CD, Chiba CM. Caenorhabditis elegans: A new model system for the study of learning and memory. Behav Brain Res. 1990;37(1):89–92. doi: 10.1016/0166-4328(90)90074-o. [DOI] [PubMed] [Google Scholar]
- 115.Rose JK, Kaun KR, Rankin CH. A new group-training procedure for habituation demonstrates that presynaptic glutamate release contributes to long-term memory in Caenorhabditis elegans. Learn Mem. 2002;9(3):130–137. doi: 10.1101/lm.46802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ardiel EL, Rankin CH. An elegant mind: Learning and memory in Caenorhabditis elegans. Learn Mem. 2010;17(4):191–201. doi: 10.1101/lm.960510. [DOI] [PubMed] [Google Scholar]
- 117.Nuttley WM, Atkinson-Leadbeater KP, Van Der Kooy D. Serotonin mediates food-odor associative learning in the nematode Caenorhabditiselegans. Proc Natl Acad Sci USA. 2002;99(19):12449–12454. doi: 10.1073/pnas.192101699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wen JY, et al. Mutations that prevent associative learning in C. elegans. Behav Neurosci. 1997;111(2):354–368. doi: 10.1037//0735-7044.111.2.354. [DOI] [PubMed] [Google Scholar]
- 119.Kato S, et al. Global brain dynamics embed the motor command sequence of Caenorhabditis elegans. Cell. 2015;163(3):656–669. doi: 10.1016/j.cell.2015.09.034. [DOI] [PubMed] [Google Scholar]
- 120.Gordus A, Pokala N, Levy S, Flavell SW, Bargmann CI. Feedback from network states generates variability in a probabilistic olfactory circuit. Cell. 2015;161(2):215–227. doi: 10.1016/j.cell.2015.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lüersen K, Faust U, Gottschling D-C, Döring F. Gait-specific adaptation of locomotor activity in response to dietary restriction in Caenorhabditis elegans. J Exp Biol. 2014;217(Pt 14):2480–2488. doi: 10.1242/jeb.099382. [DOI] [PubMed] [Google Scholar]
- 122.Artyukhin AB, Yim JJ, Cheong Cheong M, Avery L. Starvation-induced collective behavior in C. elegans. Sci Rep. 2015;5:10647. doi: 10.1038/srep10647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Oades RD, Isaacson RL. The development of food search behavior by rats: The effects of hippocampal damage and haloperidol. Behav Biol. 1978;24(3):327–337. doi: 10.1016/s0091-6773(79)90184-6. [DOI] [PubMed] [Google Scholar]
- 124.Seeley TD. The Wisdom of the Hive. Harvard Univ Press; Cambridge, MA: 1995. p. 295. [Google Scholar]
- 125.Wehner R. Life as a cataglyphologist--And beyond. Annu Rev Entomol. 2013;58:1–18. doi: 10.1146/annurev-ento-120811-153641. [DOI] [PubMed] [Google Scholar]
- 126.Wallace RL, Ricci C, Melone G. A cladistic analysis of pseudocoelomate (Aschelminth) morphology. Invertebr Biol. 1996;115(2):104–112. [Google Scholar]
- 127.Telford MJ, Bourlat SJ, Economou A, Papillon D, Rota-Stabelli O. The evolution of the Ecdysozoa. Philos Trans R Soc Lond B Biol Sci. 2008;363(1496):1529–1537. doi: 10.1098/rstb.2007.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Feinberg TE, Mallatt J. The evolutionary and genetic origins of consciousness in the Cambrian Period over 500 million years ago. Front Psychol. 2013;4(667):667. doi: 10.3389/fpsyg.2013.00667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Shu D-G, et al. Head and back bone of the Early Cambrian vertebrate Haikouichthys. Nature. 2003;421(6922):526–529. doi: 10.1038/nature01264. [DOI] [PubMed] [Google Scholar]
- 130.Trestman M. The Cambrian explosion and the origins of embodied cognition. Biol Theory. 2013;8:80–92. [Google Scholar]
- 131.Parker A. In the Blink of an Eye: How Vision Sparked the Big Bang of Evolution. Basic; New York: 2004. [Google Scholar]
- 132.Conway Morris S. The Crucible of Creation: The Burgess Shale and the Rise of Animals. Oxford Univ Press; Oxford: 1998. p. 242. [Google Scholar]
- 133.Gould SJ. Wonderful Life: The Burgess Shale and the Nature of History. Norton; London: 1990. p. 352. [Google Scholar]
- 134.Ma X, Hou X, Edgecombe GD, Strausfeld NJ. Complex brain and optic lobes in an early Cambrian arthropod. Nature. 2012;490(7419):258–261. doi: 10.1038/nature11495. [DOI] [PubMed] [Google Scholar]
- 135.Loesel R, Nässel DR, Strausfeld NJ. Common design in a unique midline neuropil in the brains of arthropods. Arthropod Struct Dev. 2002;31(1):77–91. doi: 10.1016/S1467-8039(02)00017-8. [DOI] [PubMed] [Google Scholar]
- 136.Homberg U. Evolution of the central complex in the arthropod brain with respect to the visual system. Arthropod Struct Dev. 2008;37(5):347–362. doi: 10.1016/j.asd.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 137.Strausfeld NJ, Hirth F. Deep homology of arthropod central complex and vertebrate basal ganglia. Science. 2013;340(6129):157–161. doi: 10.1126/science.1231828. [DOI] [PubMed] [Google Scholar]
- 138.Kant I. Critique of Pure Reason: The Cambridge Edition of the Works of Immanuel Kant. Cambridge Univ Press; Cambridge, UK: 1999. [Google Scholar]
- 139.Skorupski P, Chittka L. Animal cognition: An insect’s sense of time? Curr Biol. 2006;16(19):R851–R853. doi: 10.1016/j.cub.2006.08.069. [DOI] [PubMed] [Google Scholar]
- 140.Bjursten LM, Norrsell K, Norrsell U. Behavioural repertory of cats without cerebral cortex from infancy. Exp Brain Res. 1976;25(2):115–130. doi: 10.1007/BF00234897. [DOI] [PubMed] [Google Scholar]
- 141.Horridge A. What the honeybee sees: A review of the recognition system of Apis mellifera. Physiol Entomol. 2005;30:2–13. [Google Scholar]
- 142.Menzel R, Giurfa M. Cognitive architecture of a mini-brain: The honeybee. Trends Cogn Sci. 2001;5(2):62–71. doi: 10.1016/s1364-6613(00)01601-6. [DOI] [PubMed] [Google Scholar]
- 143.Heisenberg M. Mushroom body memoir: From maps to models. Nat Rev Neurosci. 2003;4(4):266–275. doi: 10.1038/nrn1074. [DOI] [PubMed] [Google Scholar]
- 144.Schwaerzel M, et al. Dopamine and octopamine differentiate between aversive and appetitive olfactory memories in Drosophila. J Neurosci. 2003;23(33):10495–10502. doi: 10.1523/JNEUROSCI.23-33-10495.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Huerta R, Nowotny T, García-Sanchez M, Abarbanel HDL, Rabinovich MI. Learning classification in the olfactory system of insects. Neural Comput. 2004;16(8):1601–1640. doi: 10.1162/089976604774201613. [DOI] [PubMed] [Google Scholar]
- 146.Bazhenov M, Huerta R, Smith BH. A computational framework for understanding decision making through integration of basic learning rules. J Neurosci. 2013;33(13):5686–5697. doi: 10.1523/JNEUROSCI.4145-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]