Significance
Mature B cells are long-lived cells responsible for the Ab production in the immune system. Canonical NF-κB signaling, one of the two discrete pathways activating transcription factors of the NF-κB family, participates in the generation of a normal mature B-cell compartment. However, the role of these signals specifically in mature B cells is still imperfectly defined. Notably, their role in the persistence of follicular B cells, the main mature B-cell subset, is controversial. Here, we show that canonical NF-κB signaling does not contribute to immediate (short-term) survival of follicular B cells, contrasting with the crucial tonic B-cell antigen receptor survival signals, but is required for their long-term persistence as well as functional fitness.
Keywords: NF-κB, canonical signaling, follicular B cells, persistence
Abstract
Although canonical NF-κB signaling is crucial to generate a normal mature B-cell compartment, its role in the persistence of resting mature B cells is controversial. To resolve this conflict, we ablated NF-κB essential modulator (NEMO) and IκB kinase 2 (IKK2), two essential mediators of the canonical pathway, either early on in B-cell development or specifically in mature B cells. Early ablation severely inhibited the generation of all mature B-cell subsets, but follicular B-cell numbers could be largely rescued by ectopic expression of B-cell lymphoma 2 (Bcl2), despite a persisting block at the transitional stage. Marginal zone (MZ) B and B1 cells were not rescued, indicating a possible role of canonical NF-κB signals beyond the control of cell survival in these subsets. When canonical NF-κB signaling was ablated specifically in mature B cells, the differentiation and/or persistence of MZ B cells was still abrogated, but follicular B-cell numbers were only mildly affected. However, the mutant cells exhibited increased turnover as well as functional deficiencies upon activation, suggesting that canonical NF-κB signals contribute to their long-term persistence and functional fitness.
Mature B cells comprise three major subsets: follicular and marginal zone (MZ) B and B1 cells (1). Two receptors, the B-cell antigen receptor (BCR) and B-cell activating factor of the tumor necrosis factor family receptor (BAFFR), have been shown to control the generation and/or persistence of mature B cells critically (2–5).
Numerous stimuli activate the NF-κB signaling pathways in mature B cells. In mammals, the NF-κB family of transcription factors comprises five members (RelA, RelB, c-Rel, NF-κB1, and NF-κB2) whose activation is initiated by two major signaling pathways (5). The canonical pathway depends on the IκB kinase (IKK) complex, consisting of the structural protein NF-κB essential modulator (NEMO) and the IκB kinases IKK1 and IKK2. This complex triggers the degradation of a specific set of inhibitors of NF-κB and the induction of dimers containing RelA and/or c-Rel. The canonical pathway regulates NF-κB activation downstream of the BCR (5). The stimulation of the alternative pathway, mediated by NF-κB–inducing kinase and IKK1, leads to the partial proteolysis of the inhibitory precursor NF-κB2 and the activation of dimers containing RelB and/or the processed form of NF-κB2 through another set of receptors, including BAFFR (4).
There is abundant evidence for a critical role of the canonical pathway in the generation and/or maintenance of mature B cells. The ablation of NEMO or IKK2 in the B-cell lineage, as well as conditional replacement of the latter by a kinase-dead IKK2, impaired the generation of the three mature B subsets and transitional 2 (T2) cells, a developmental stage preceding the mature stage (6–9). In the case of the kinase-dead IKK2, some mutant cells made it into the mature compartment but were completely outcompeted by WT cells over a period of 4 wk, upon blockade of B-cell generation in the bone marrow (6). Mechanistically, tonic BCR signaling could increase the production of NF-κB2 via canonical NF-κB activation in T2 and follicular B cells, sensitizing B cells to the prosurvival effect of BAFFR (10).
However, other work suggested that canonical signals downstream of the BCR may not be critical for the maintenance of mature follicular B cells. Ectopic expression of a constitutively active form of IKK2 only slightly rescued the acute loss of B cells upon ablation of the BCR in these cells, whereas a full rescue was obtained through signals along the phosphatidylinositol 3-kinase (PI3K)/forkhead box O1 (FOXO1) axis (11). In addition, a study of the role of B cells in a prostate cancer model suggested that the absence of canonical signaling may not result in the rapid disappearance of mature B cells (12).
In the present work, using conditional ablation of NEMO and IKK2 either early during B-cell development or specifically in mature B cells, as well as ectopic expression of B-cell lymphoma 2 (Bcl2), we show that canonical NF-κB signaling contributes to the functional integrity of follicular B cells and supports their long-term persistence through its prosurvival activity. However, absence of canonical NF-κB signaling in these cells only mildly affects their numbers in steady state, in contrast to its severe impact on transitional B-cell numbers.
Results
Ectopic Expression of Bcl2 Allows Accumulation of Follicular B Cells upon NEMO Deletion Early in Development.
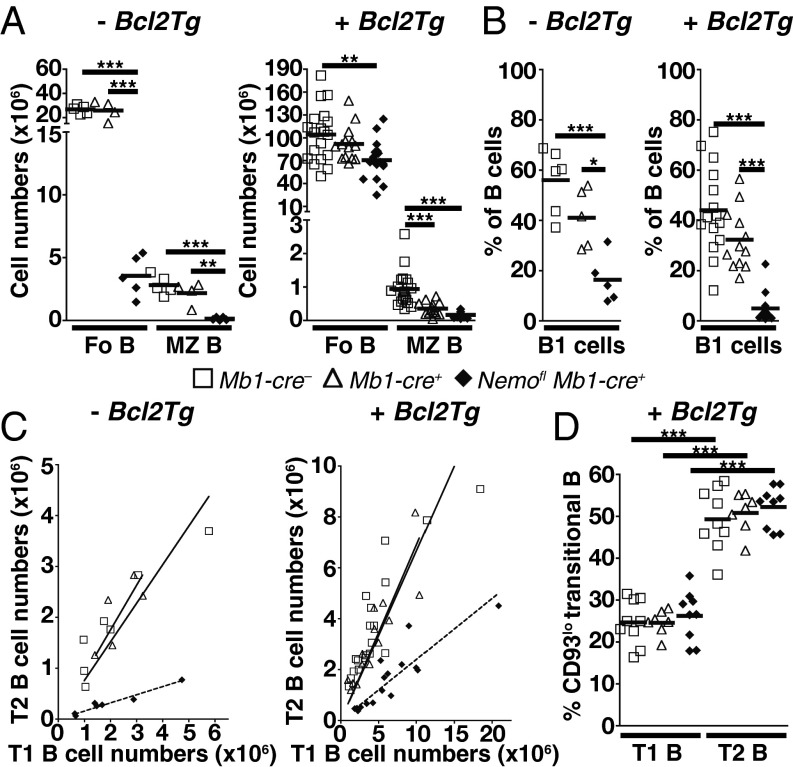
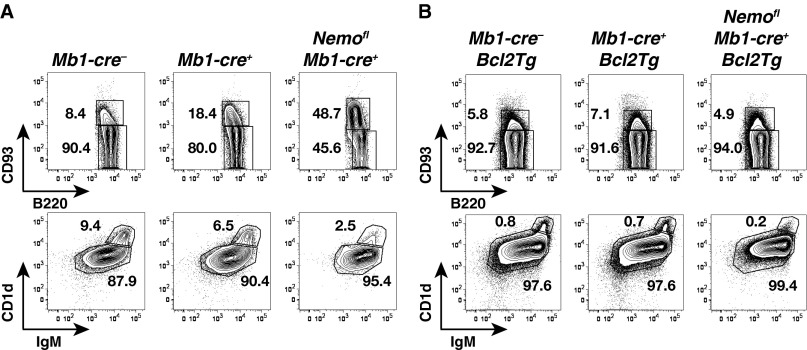
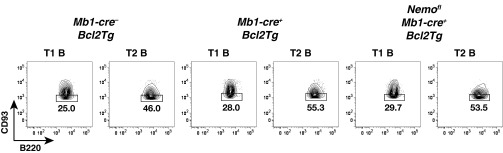
Consistent with previous work (6, 8, 9), the generation of mature B cells was impaired upon ablation of NEMO in the B-cell lineage using Mb1-cre (13) (Fig. 1 A and B and Figs. S1A and S2A). Within the mature compartment, MZ B and B1 cells were more affected than follicular B cells. Because overexpression of Bcl2 in RelA and c-Rel double-deficient hematopoietic cells has been shown to rescue the generation of mature B cells and promote their survival in vitro (14), we assessed whether ectopic expression of Bcl2 leads to the accumulation of mature B cells in the absence of NEMO. Indeed, the generation of follicular B cells was largely rescued in Nemofl Mb1-cre+ Bcl2Tg mice (Fig. 1A and Fig. S1B). In contrast, mutant MZ B cellularity was lower (2- to 5.5-fold) compared with controls and was similar to the MZ B-cell numbers observed in Nemofl Mb1-cre+ mice (Fig. 1A and Fig. S1B). However, the difference between Mb1-cre+ Bcl2Tg and Nemofl Mb1-cre+ Bcl2Tg mice did not reach statistical significance. Surprisingly, MZ B cellularity was also reduced in Mb1-cre+ Bcl2Tg compared with Mb1-cre− Bcl2Tg control mice. In addition, the ectopic expression of Bcl2 in B cells did not rescue NEMO-deficient B1 cells in the peritoneal cavity (Fig. 1B and Fig. S2B).
Fig. 1.
Ectopic expression of Bcl2 rescues follicular (Fo) B-cell generation in the absence of NEMO. (A) Absolute cell numbers of Fo B and MZ B cells in the spleens of Mb1-cre−, Mb1-cre+, and Nemofl Mb1-cre+ mice in the absence of (Left, −Bcl2Tg) or upon (Right, +Bcl2Tg) ectopic expression of Bcl2. Data are pooled from four to 15 experiments (n = 4–19 per genotype). (B) Percentage of B1 cells within B cells in the peritoneal cavity. Data are cumulative from five to 14 experiments (n = 5–15 per group). (C) T2 cellularity as a function of T1 cell numbers. Data are pooled from six to 15 experiments (n = 5–19 per genotype). Solid (controls) and dotted (Nemofl Mb1-cre+) lines represent the best-fitting linear function, forced to go through the origin. (D) Percentage of CD93lo T1 and T2 cells in the spleens of Mb1-cre−, Mb1-cre+, and Nemofl Mb1-cre+ mice upon ectopic expression of Bcl2 (+Bcl2Tg). Data are pooled from nine experiments (n = 7–9 per group). One Mb1-cre+ mouse and one Nemofl Mb1-cre+ mouse were excluded from the analysis in A (Left) and B (Left), respectively, due to aberrantly high cell numbers compared with the other mice of their group. Each symbol indicates one mouse, and horizontal lines signify the mean in A, B, and D. *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001 by one-way ANOVA in A, B, and D.
Fig. S1.
Identification of mature follicular and MZ B cells. Flow cytometry of B220+CD93+ transitional and B220+CD93− mature B cells within splenic B220+CD19+ B cells (Top), as well as IgM+CD1d+ follicular and IgMhiCD1dhi MZ B cells within splenic mature B cells (Bottom) of Mb1-cre−, Mb1-cre+, and Nemofl Mb1-cre+ mice in the absence of Bcl2 (A; n = 4–5 per genotype in four experiments) or upon ectopic expression of Bcl2 (Bcl2Tg) (B; n = 14–19 per genotype in 15 experiments). Numbers adjacent to outlined areas specify the percentage of cells in each gate.
Fig. S2.
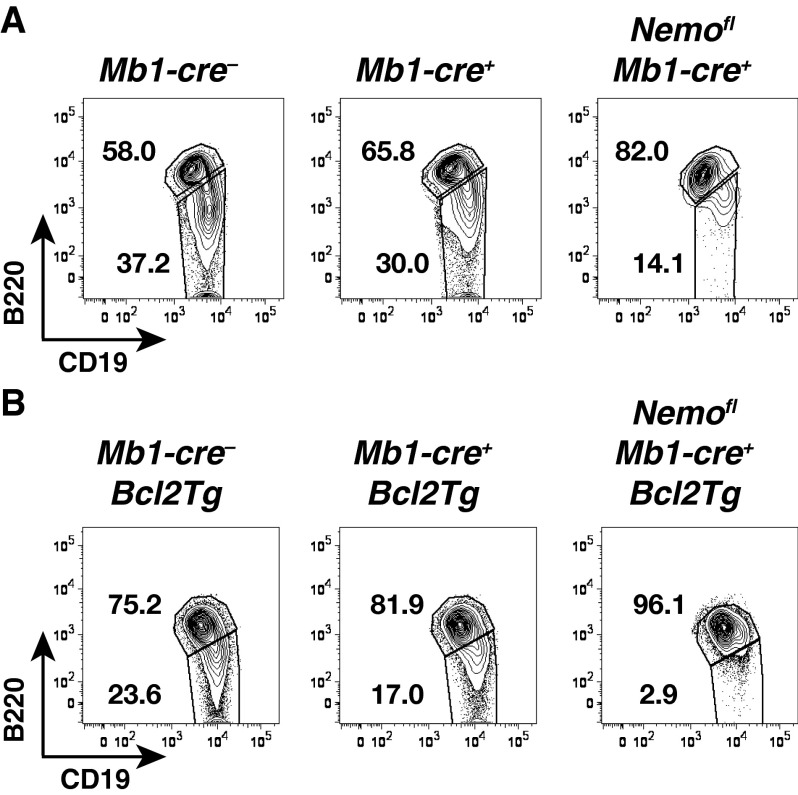
Evaluation of B1 cell proportions. Flow cytometry of B220lo/−CD19+ B1 and B220+CD19+ B2 cells gated on CD19+ B cells in the peritoneal cavity of Mb1-cre−, Mb1-cre+, and Nemofl Mb1-cre+ mice in the absence of Bcl2 (A; n = 5–6 per genotype in five experiments) or upon ectopic expression of Bcl2 (Bcl2Tg) (B; n = 12–15 per genotype in 14 experiments). Numbers adjacent to outlined areas indicate the percentage of cells in the gate.
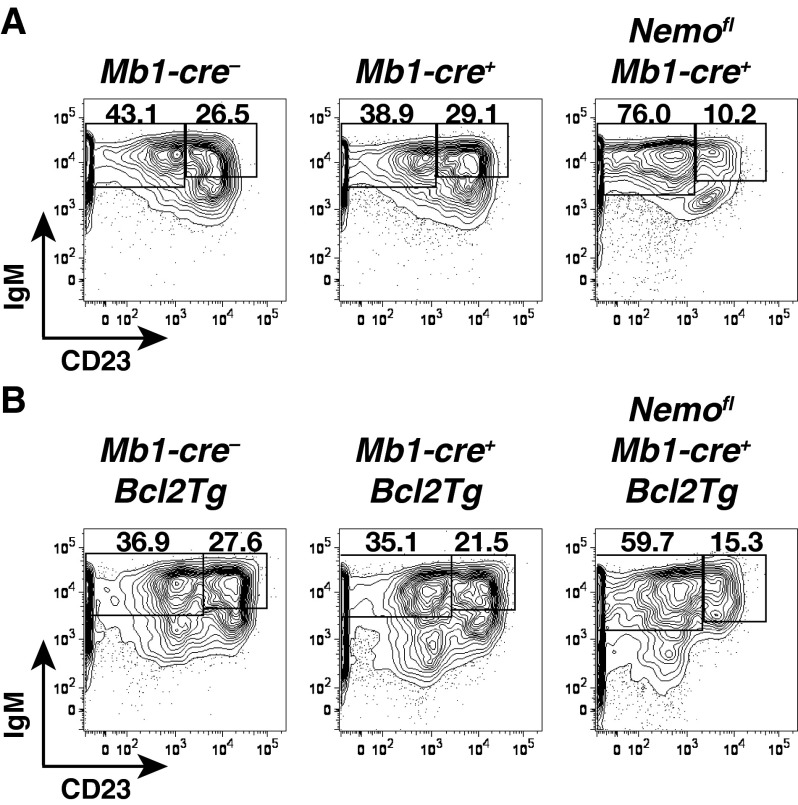
The absence of canonical NF-κB signaling in B cells has previously been shown to affect splenic B-cell development also at the T1 to T2 transition (8, 9). We thus investigated whether the accumulation of mutant follicular B cells could be due to the rescue of T2 cell generation in Nemofl Mb1-cre+ Bcl2Tg mice. T2 cell numbers demonstrated a positive correlation with T1 cellularity (Fig. 1C and Fig. S3), in agreement with T2 cells arising from the T1 subset (15). Notably, the production of NEMO-deficient T2 cells was clearly reduced compared with controls, independent of the overexpression of Bcl2 (Fig. 1C). CD23, used to discriminate T1 and T2 cells (15), has been reported to be an NF-κB target gene (16). We therefore confirmed the identity of the mutant transitional populations using an independent marker, CD93, which is expressed at lower levels on T2 cells (3, 9) (Fig. 1D and Fig. S4). Comparable distributions of CD93lo cells were seen in the transitional subsets of Nemofl Mb1-cre+ Bcl2Tg and control mice, supporting that genuine T1 and T2 cells were detected in the mutant mice.
Fig. S3.
Detection of T1 and T2 B cells. Flow cytometry of IgMhiCD23− T1 and IgMhiCD23+ T2 subsets within B220+CD19+CD93+ transitional B cells in the spleens of Mb1-cre−, Mb1-cre+, and Nemofl Mb1-cre+ mice in the absence of Bcl2 (A; n = 5–7 per genotype in six experiments) or upon ectopic expression of Bcl2 (Bcl2Tg) (B; n = 14–19 per genotype in 15 experiments). Numbers adjacent to outlined areas specify the percentage of cells in the gate.
Fig. S4.
Determination of the percentage of CD93lo cells within T1 and T2 populations. Proportions of CD93loB220+ cells within splenic B220+CD19+CD93+IgMhiCD23− T1 and B220+CD19+CD93+IgMhiCD23+ T2 B cells measured by flow cytometry in Mb1-cre− Bcl2Tg, Mb1-cre+ Bcl2Tg, and Nemofl Mb1-cre+ Bcl2Tg mice (n = 7–9 per genotype in nine experiments). Numbers adjacent to outlined areas specify the percentage of cells in the gate. T2 cells were used as the reference to set the CD93lo gate.
Thus, ectopic expression of Bcl2 permitted the accumulation of NEMO-deficient follicular B cells close to normal cellularity despite a persisting developmental block at the transitional stage. In contrast, the generation of MZ B and B1 cells was not rescued, possibly due to a role for canonical NF-κB signaling beyond cell survival (17), consistent with the inability of a Bcl2 transgene regulated by vav gene regulatory elements to promote the development of MZ B cells in NF-κB1–deficient mice (18).
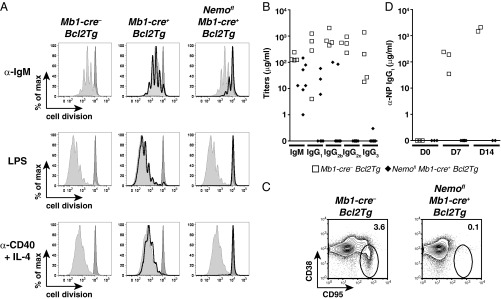
Peripheral B cells from Ikk2fl CD19-cre+ mice showed altered in vitro and in vivo responses to stimulation (7), but these results could have been partly due to the reduced numbers of mature B cells in these mice. The large numbers of follicular B cells in Nemofl Mb1-cre+ Bcl2Tg mice allowed us to examine their responses to various kinds of stimulation. The NEMO-deficient B cells overexpressing Bcl2 exhibited an impaired proliferative response to various mitogenic stimuli in vitro compared with control B cells overexpressing Bcl2 (Fig. 2A). Consistent with defective B-cell functions, IgM and IgG serum Ab levels were strongly reduced or undetectable (Fig. 2B). In the T-cell–dependent response to immunization with (4-hydroxy-3-nitrophenyl)acetyl (NP)–chicken γ-globulin (CGG), the frequency of germinal center cells was strongly reduced and the production of anti-NP IgG1 Abs was abolished (Fig. 2 C and D). These data show that canonical NF-κB signals are essential for the functional activity of follicular B cells, and complement a large body of evidence for the multifaceted role of this pathway in the control of humoral immunity (5, 17, 19).
Fig. 2.
Mature B cells from Nemofl Mb1-cre+ Bcl2Tg mice are functionally defective. (A) Proliferation of live splenic mature B cells from Mb1-cre− Bcl2Tg (light gray-filled histogram), Mb1-cre+ Bcl2Tg (black histogram), and Nemofl Mb1-cre+ Bcl2Tg (black histogram) mice that were MACS-purified; labeled with cell proliferation dye eFluor 450; and stimulated with 10 μg/mL anti-IgM (α-IgM), 20 μg/mL LPS, or 1 μg/mL anti-CD40 + 25 ng/mL IL-4 (α-CD40 + IL-4) for 4 d. The dark gray-filled histogram shows resting Mb1-cre− Bcl2Tg B cells. At least three mice per genotype were analyzed in independent experiments. max, maximum. (B) Serum Ab isotype titers in sera of naive mice (n = 4–7 per genotype). (C) Flow cytometry of CD38loCD95+ germinal center B cells within splenic B220+ cells of mutant and control mice 14 d after immunization with NP-CGG. Numbers adjacent to outlined areas specify the percentage of cells in each gate. Five mice were analyzed per genotype in two experiments. (D) Serum titers of NP-specific IgG1 (n = 2–6 per group). D0, unimmunized mice; D7 and D14, day 7 and day 14 postimmunization. One control mouse at D7 did not respond to the immunization and was excluded from the analysis. Data are representative of two experiments. In B and D, symbols indicate individual mice.
Long-Term Persistence of Follicular B Cells Requires Canonical NF-κB Signaling.
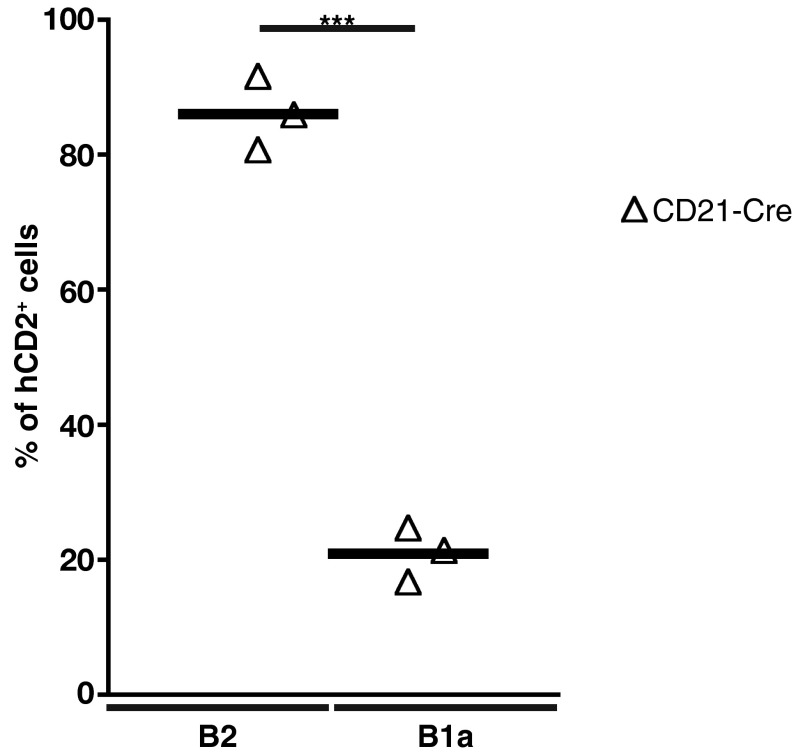
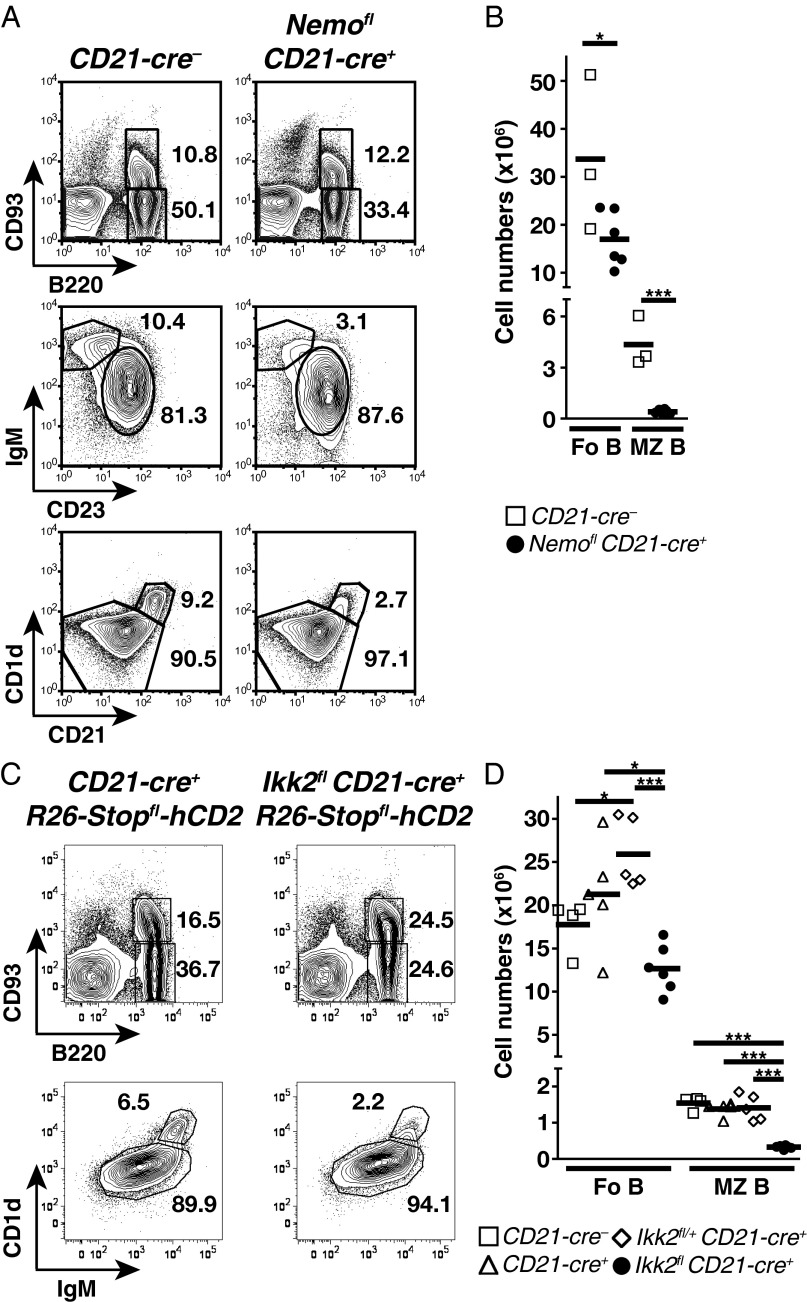
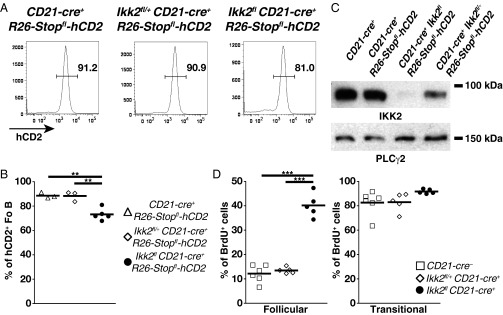
To evaluate directly the contribution of canonical signaling to the maintenance of mature B cells, we ablated NEMO using CD21-cre (3). We excluded B1 cells from the analysis because, in our hands, CD21-cre proved to be poorly expressed in the prototypical CD5+ B1a subset (Fig. S5). A large follicular B-cell population was detected in the spleens of Nemofl CD21-cre+ mice, whereas MZ B cells were essentially lost (Fig. 3 A and B). Similar results were obtained upon IKK2 ablation through CD21-cre (Fig. 3 C and D). These data contrast with the small mature B-cell compartment detected in the absence of NEMO or IKK2 using the CD19-cre or Mb1-cre allele (6–9). Nemofl and Ikk2fl loxP-flanked exons are efficiently eliminated upon Cre-mediated recombination in B cells (6, 8), which we verified in the case of follicular B cells from Ikk2fl CD21-cre+ mice (Fig. 4). The mild reduction of follicular B-cell numbers in Ikk2fl CD21-cre+ mice was not due to the accumulation of cells that had escaped IKK2 deletion. Indeed, the majority of these cells expressed a Cre-inducible truncated human CD2 reporter (Fig. 4 A and B), and the reporter-positive cells demonstrated dramatically reduced IKK2 protein levels compared with controls (Fig. 4C).
Fig. S5.
Proportions of hCD2+ cells within the B1a and B2 cell subsets. The percentage of hCD2+ B220+CD19+ B2 and B220lo/−CD19+CD5+CD43+ B1a cells in the peritoneal cavity of CD21-cre+ R26-Stopfl-hCD2 mice was determined by flow cytometry. Data are pooled from three experiments (n = 3). Each symbol indicates one mouse, and horizontal lines signify the mean. ***P ≤ 0.001 by Student’s t test.
Fig. 3.
Mild reduction of Fo B-cell numbers upon deletion of NEMO or IKK2 in mature B cells. (A) Flow cytometry of B220+CD93+ transitional and B220+CD93− mature B cells within lymphocytes (Top), IgM+CD23+ Fo B cells within mature B cells (Middle), and CD1dhiCD21hi MZ B cells within CD19+ B cells (Bottom) in the spleens of CD21-cre− and Nemofl CD21-cre+ mice. (B) Absolute cell numbers of Fo and MZ B cells in CD21-cre− and Nemofl CD21-cre+ mice. Data are cumulative from two experiments (n = 3–6 per group). (C) Flow cytometry of transitional and mature B cells among lymphocytes (Top) and CD1d+IgM+ Fo and CD1dhiIgMhi MZ B cells within mature B cells (Bottom) in the spleens of CD21-cre+ R26-Stopfl-hCD2 and Ikk2fl CD21-cre+ R26-Stopfl-hCD2 mice. (D) Absolute cell numbers of Fo and MZ B cells in CD21-cre−, CD21-cre+, Ikk2fl/+ CD21-cre+, and Ikk2fl CD21-cre+ mice. Data are pooled from six experiments (n = 4–6 per genotype). In A and C, numbers adjacent to outlined areas specify the percentage of cells in the gate. Symbols represent individual mice, and horizontal bars signify the mean in B and D. *P ≤ 0.05; ***P ≤ 0.001 by Student’s t test in B and one-way ANOVA in D.
Fig. 4.
IKK2-deficient Fo B cells persist but exhibit increased turnover. (A) Flow cytometry of human CD2 (hCD2) expression on Fo B cells in the spleens of CD21-cre+ R26-Stopfl-hCD2, Ikk2fl/+ CD21-cre+ R26-Stopfl-hCD2 and Ikk2fl CD21-cre+ R26-Stopfl-hCD2 mice. Numbers adjacent to outlined areas specify the percentage of cells in each gate. (B) Proportions of hCD2+ Fo B cells. Data are cumulative from five experiments (n = 3–5 per group). (C) Western blot analysis of IKK2 and PLCγ2 protein levels in flow cytometry-purified splenic B220+CD93−CD1d+IgM+hCD2+ Fo B cells from control and mutant mice; Fo B cells from CD21-cre+ mice were hCD2−. Western blotting was performed twice. (D) Proportions of splenic BrdU+ B220+CD19+CD93−IgM+CD23+ Fo (Left) and B220+CD19+CD93+ transitional (Right) B cells in mice given BrdU in drinking water for 14 d, as determined by flow cytometry. Data are cumulative from two experiments (n = 5–6 per group). One Ikk2fl CD21-cre+ mouse in D was excluded from the analysis due to sickness. In B and D, symbols represent individual mice and horizontal bars signify the mean. **P ≤ 0.01; ***P ≤ 0.001 by one-way ANOVA in B and D.
Residual splenic mature B cells expressing a kinase-dead IKK2 have been shown to display an increased turnover compared with controls (6). Thus, we assessed whether follicular B-cell persistence was altered in Ikk2fl CD21-cre+ mice by measuring BrdU incorporation into their DNA after 2 wk of labeling. Labeling efficiency was similar among the various mice, as indicated by comparable proportions of BrdU+ transitional B cells (Fig. 4D). In agreement with previous work (3, 20), follicular B cells from CD21-cre− and Ikk2fl/+ CD21-cre+ controls showed low proportions (12–13%) of BrdU+ cells. In contrast, 40% of IKK2-deficient follicular B cells had incorporated BrdU (Fig. 4D), suggesting increased turnover.
Overall, the present results suggest that the main function of canonical NF-κB signaling in follicular B-cell homeostasis is indeed the control of cell survival, as demonstrated by the ability of a Bcl2 transgene to rescue a substantial compartment of these cells even under conditions where the ablation of canonical signaling in the B-cell lineage leads to a severe developmental block at the transitional B-cell stage (8, 9). These data are in line with earlier work showing the accumulation of mature B cells in mice reconstituted with RelA and c-Rel double-deficient fetal liver cells overexpressing Bcl2 (14).
Discussion
Whereas ablation of components of the BCR in mature B cells led to a steady state in which BCR-deficient cells were a minority of the mature B-cell population because of their rapid elimination (2, 3, 11), NEMO or IKK2 ablation by CD21-cre resulted in only a moderate reduction of follicular B-cell numbers. These data indicate that follicular B cells do not require continuous canonical NF-κB signaling for their persistence, and contrasts with the rapid loss of B cells upon BCR deletion (2, 3). Quite fittingly, the latter process can be rescued by constitutive PI3K activation, but not by canonical NF-κB activity (11). However, the homeostasis of follicular B cells unable to signal through the canonical NF-κB pathway is clearly different from the homeostasis of their WT counterparts. Whereas the latter are long-lived cells with average life spans of months (21), the mutant follicular B cells appear to have a limited life span, probably on the order of a few weeks as indicated by the BrdU labeling data. These results complement our previous demonstration of the failure of such cells to compete with WT cells in vivo (6). Together with the accumulation of NEMO-deficient follicular B cells upon ectopic expression of Bcl2, these data raise the possibility that canonical NF-κB signals may determine the fitness of mature follicular B cells in their competition for survival niches in the peripheral immune system (22).
The ablation of NEMO or IKK2 early during B-cell development results in strongly reduced numbers of follicular B cells (6–9), in stark contrast to the effects of the deletion of these two molecules specifically in mature B cells. This difference likely reflects the critical role of canonical NF-κB signaling in transitional B cells (6, 8, 9). In combination with the limited life span of follicular B cells, the shortage of newly generated mature B cells is expected to lead to the suboptimal filling of the follicular B compartment, in the absence of canonical NF-κB signaling.
Compared with follicular B cells, MZ B and B1 cells demonstrate a stronger dependency on canonical NF-κB signals for their development and/or persistence. Thus, MZ B-cell numbers were strongly reduced in Nemofl and Ikk2fl CD21-cre+ mice, and the ectopic expression of Bcl2 failed to rescue the generation and/or maintenance of NEMO-deficient MZ B and B1 cells. These results are suggestive of a role beyond the control of survival for canonical NF-κB signaling in MZ B and B1 cells. It is noteworthy in this context that cyclin D2, a regulator of the cell cycle and a target of NF-κB, contributes to the development of B1 cells (23).
Although an important signaling pathway activated in B cells upon engagement of BAFFR is the alternative NF-κB signaling pathway (4), there is also evidence for cross-talk between the alternative and canonical pathways, both in the sense of BAFFR signaling resulting in canonical NF-κB activity (8, 24) and of canonical signals up-regulating the expression of components of the alternative pathway (8, 10, 17, 19). Thus, BAFFR might be involved in the activation of survival signals in mature B cells through both the canonical and alternative NF-κB signaling pathways, and/or canonical signals could contribute to an enhanced sensitivity of the corresponding cells to survival signals downstream of BAFFR. In this scenario, the occasional engagement of the BCR on resting mature B cells by antigens in the environment, with a resulting activation of the canonical NF-κB pathway, might enhance the competitive fitness of the cells in their ability to access survival niches (22). The activation of canonical NF-κB signaling downstream of the BCR and/or BAFFR could be mediated by Bruton’s tyrosine kinase (Btk) (5, 25). Indeed, similar to the behavior of follicular B cells in the absence of canonical NF-κB signals, ectopic expression of Bcl2 rescues the generation of follicular B cells in x-linked immunodeficiency (xid) mice, which bear a mutation in Btk, and CD23+ transitional and follicular xid B cells are outcompeted by WT B cells (26, 27).
Materials and Methods
Mice.
Nemofl, Ikk2fl, Mb1-cre, CD21-cre, and R26-Stopfl-hCD2 mice have been described (3, 6, 13, 28, 29). Bcl2 transgenic Eμ-Bcl-2-22 (Bcl2Tg) mice were obtained from The Jackson Laboratory (30). Mice were generated on or backcrossed to the C57BL/6 genetic background. Animal care and mouse work were conducted according to the guidelines of the Institutional Animal Care and Use Committee of Harvard University, the Immune Disease Institute, the Max Delbrück Center for Molecular Medicine, the Landesamt für Gesundheit und Soziales, and the Bundesministerium für Wissenschaft und Forschung.
For simplicity and clarity, the given denominations include genotypes as follows (+, f, Δ, and y indicate WT, loxP-flanked, deleted alleles, and the y sex chromosome, respectively): Mb1-cre−: Nemof/f, Nemof/y, and Nemo+/y; Nemofl Mb1-cre+: Nemof/f Mb1-cre+ and Nemof/y Mb1-cre+; CD21-cre−: Nemof/y or Ikk2f/Δ and Ikk2+/Δ; Nemofl CD21-cre+: Nemof/y CD21-cre+; Ikk2fl/+ CD21-cre+: Ikk2f/+ CD21-cre+ and Ikk2+/Δ CD21-cre+; Ikk2fl CD21-cre+: Ikk2f/Δ CD21-cre+. Previous work (6) suggested that B-cell development is unaffected in mice bearing either a heterozygous deletion of Ikk2 or loxP-flanked Ikk2 or Nemo alleles. The presence of single Ikk2-deleted alleles in some of our mice resulted from the occasional deletion of loxP-flanked alleles by CD21-cre in the germ line (11).
Flow Cytometry.
Cell suspensions from the spleen and peritoneal cavity were stained with the following Abs coupled to FITC, phycoerythrin (PE), peridinin chlorophyll (PerCP), PerCP-Cy5.5, allophycocyanin (APC), PE-Cy7, Pacific Blue, Brilliant violet (BV) 421, BV605, or BV785: anti-CD1d (1B1), anti-human CD2 (TS1/8), anti-CD19 (6D5 and 1D3), anti-CD21 (7G6 and 7E9), anti-CD23 (B3B4), anti-CD38 (90), anti-CD93 (AA4.1), anti-CD95 (Jo2), anti-B220 (RA3-6B2) and anti-IgM (II/41 and goat anti-mouse Fab) purchased from Affymetrix eBioscience, BD Biosciences, Biolegend, and Jackson ImmunoResearch Laboratories. Data were recorded on a FACSCalibur, FACSCanto II, or LSRFortessa (all from BD Biosciences) and analyzed with FlowJo software (TreeStar).
In Vitro B-Cell Proliferation and Isotype Class Switching.
Splenic B cells were purified by magnetic depletion using anti-CD43 beads (Miltenyi Biotec), labeled with 10 μM Cell Proliferation Dye eFluor 450 (Affymetrix eBioscience), 10 μM Cell Proliferation Dye eFluor 670 (Affymetrix eBioscience), or 2.5 nM carboxyfluorescein diacetate succinimidyl ester (Molecular Probes), and cultured in six-well plates at 106 cells per 4 mL of DMEM (Gibco) supplemented with 10% (vol/vol) FCS, 2 mM l-glutamine, 10 mM Hepes, 1 mM sodium pyruvate, 1× nonessential amino acids, 1× penicillin/streptomycin, and 50 μM β-mercaptoethanol. Cells were either left untreated or stimulated with 10 μg/mL F(ab′)2 fragment anti-IgM (Jackson ImmunoResearch Laboratories), 20 μg/mL LPS (Sigma), and 1 or 2 μg/mL anti-CD40 (HM40-3, Biolegend) plus 25 or 100 ng/mL IL-4 (R&D Systems) for 4 d. Dead cells were stained using 1 μg/mL propidium iodide (Sigma), 10 nM TO-PRO-3 (Molecular Probes), or AnnexinV-FITC (Biolegend). Cell division of live B cells was subsequently determined by flow cytometry.
Immunization and Serum Ab Titers.
Mice were immunized and Ab titers were determined as previously described (31). Briefly, animals were injected i.p. with 100 μg of NP-CGG (Biosearch Technologies) in alum (Sigma). The presence of germinal center B cells in the spleen was determined by flow cytometry at day 14 postimmunization. Blood was collected from unimmunized mice, as well as 7 and 14 d after the injection of NP-CGG, to measure serum Ig titers or NP-specific IgG1 by ELISA.
Western Blotting.
Flow cytometry-purified (FACSAriaII, BD Biosciences) B220+CD93−IgM+CD1d+hCD2+ or B220+CD93−IgM+CD1d+hCD2− follicular B cells were lysed using a whole-cell extract buffer [25 mM Hepes (pH 7.9), 0.3 M NaCl, 1.5 mM MgCl2, 0.2 mM EDTA, 0.5% Triton X-100, 10 mM NaF, 10 mM Na-pyrophosphate, 100 μM Na-o-vanadate, 2 mM DTT] completed with protease inhibitors (aprotinin and PMSF). Proteins were separated by SDS/PAGE, and transferred on PVDF membranes (Millipore). The expression of IKK2 and PLCγ2 was visualized using 10AG2 (Millipore) and Q20 (Santa Cruz Biotechnology) primary Abs, anti-mouse and anti-rabbit IgG secondary Abs coupled to HRP (Jackson ImmunoResearch Laboratories), and ECL detection reagent (Amersham).
BrdU Labeling.
Mice were given 1 mg/mL BrdU (Sigma) in the drinking water for 14 d. BrdU incorporation into the DNA of transitional and follicular B cells was determined by flow cytometry using the FITC BrdU Flow Kit (BD Biosciences).
Statistics and Graphs.
Prism software (GraphPad Software) was used to perform statistical analysis of the data, compute best-fitting linear function, and generate graphs. Statistical significance of data was determined using a one-way analysis of variance (ANOVA) followed by a post hoc Tukey’s multiple comparisons test or a two-tailed unpaired Student’s t test.
Acknowledgments
We thank T. Chakraborty, P. S. Danielian, and M. Bamberg for help with in vitro experiments and mouse analysis, as well as N. Barteneva, K. Shortman, H. P. Rahn, and K. Rautenberg for support with flow cytometry; Y. Choi and U. Ziebold for discussion and sharing material; and J. Cernoch, C. Grosse, D. Ghitza, A. Pellerin, J. Grundy, J. Xia, J. Kunert, A. Ullmann, and X. Richter for technical assistance. This work was supported by grants from the US NIH and the European Research Council Advanced Grant 268921 (to K.R.). M.J. was supported by the Deutsche Forschungsgemeinschaft and Charité/Max Delbrück Center for Molecular Medicine.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1604529113/-/DCSupplemental.
References
- 1.Swanson CL, Pelanda R, Torres RM. Division of labor during primary humoral immunity. Immunol Res. 2013;55(1-3):277–286. doi: 10.1007/s12026-012-8372-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lam KP, Kühn R, Rajewsky K. In vivo ablation of surface immunoglobulin on mature B cells by inducible gene targeting results in rapid cell death. Cell. 1997;90(6):1073–1083. doi: 10.1016/s0092-8674(00)80373-6. [DOI] [PubMed] [Google Scholar]
- 3.Kraus M, Alimzhanov MB, Rajewsky N, Rajewsky K. Survival of resting mature B lymphocytes depends on BCR signaling via the Igalpha/β heterodimer. Cell. 2004;117(6):787–800. doi: 10.1016/j.cell.2004.05.014. [DOI] [PubMed] [Google Scholar]
- 4.Gardam S, Brink R. Non-Canonical NF-κB Signaling Initiated by BAFF Influences B Cell Biology at Multiple Junctures. Front Immunol. 2014;4:509. doi: 10.3389/fimmu.2013.00509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sasaki Y, Iwai K. Roles of the NF-κB pathway in B-lymphocyte biology. Curr Top Microbiol Immunol. 2016;393:177–209. doi: 10.1007/82_2015_479. [DOI] [PubMed] [Google Scholar]
- 6.Pasparakis M, Schmidt-Supprian M, Rajewsky K. IkappaB kinase signaling is essential for maintenance of mature B cells. J Exp Med. 2002;196(6):743–752. doi: 10.1084/jem.20020907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li Z-W, Omori SA, Labuda T, Karin M, Rickert RC. IKK beta is required for peripheral B cell survival and proliferation. J Immunol. 2003;170(9):4630–4637. doi: 10.4049/jimmunol.170.9.4630. [DOI] [PubMed] [Google Scholar]
- 8.Sasaki Y, et al. Canonical NF-kappaB activity, dispensable for B cell development, replaces BAFF-receptor signals and promotes B cell proliferation upon activation. Immunity. 2006;24(6):729–739. doi: 10.1016/j.immuni.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 9.Derudder E, et al. Development of immunoglobulin λ-chain-positive B cells, but not editing of immunoglobulin κ-chain, depends on NF-kappaB signals. Nat Immunol. 2009;10(6):647–654. doi: 10.1038/ni.1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stadanlick JE, et al. Tonic B cell antigen receptor signals supply an NF-kappaB substrate for prosurvival BLyS signaling. Nat Immunol. 2008;9(12):1379–1387. doi: 10.1038/ni.1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Srinivasan L, et al. PI3 kinase signals BCR-dependent mature B cell survival. Cell. 2009;139(3):573–586. doi: 10.1016/j.cell.2009.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ammirante M, Luo J-L, Grivennikov S, Nedospasov S, Karin M. B-cell-derived lymphotoxin promotes castration-resistant prostate cancer. Nature. 2010;464(7286):302–305. doi: 10.1038/nature08782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hobeika E, et al. Testing gene function early in the B cell lineage in mb1-cre mice. Proc Natl Acad Sci USA. 2006;103(37):13789–13794. doi: 10.1073/pnas.0605944103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grossmann M, et al. The anti-apoptotic activities of Rel and RelA required during B-cell maturation involve the regulation of Bcl-2 expression. EMBO J. 2000;19(23):6351–6360. doi: 10.1093/emboj/19.23.6351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Allman D, et al. Resolution of three nonproliferative immature splenic B cell subsets reveals multiple selection points during peripheral B cell maturation. J Immunol. 2001;167(12):6834–6840. doi: 10.4049/jimmunol.167.12.6834. [DOI] [PubMed] [Google Scholar]
- 16.Debnath I, Roundy KM, Weis JJ, Weis JH. Defining in vivo transcription factor complexes of the murine CD21 and CD23 genes. J Immunol. 2007;178(11):7139–7150. doi: 10.4049/jimmunol.178.11.7139. [DOI] [PubMed] [Google Scholar]
- 17.Gerondakis S, Siebenlist U. Roles of the NF-kappaB pathway in lymphocyte development and function. Cold Spring Harb Perspect Biol. 2010;2(5):a000182. doi: 10.1101/cshperspect.a000182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Banerjee A, et al. NF-kappaB1 and c-Rel cooperate to promote the survival of TLR4-activated B cells by neutralizing Bim via distinct mechanisms. Blood. 2008;112(13):5063–5073. doi: 10.1182/blood-2007-10-120832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaileh M, Sen R. NF-κB function in B lymphocytes. Immunol Rev. 2012;246(1):254–271. doi: 10.1111/j.1600-065X.2012.01106.x. [DOI] [PubMed] [Google Scholar]
- 20.Förster I, Rajewsky K. The bulk of the peripheral B-cell pool in mice is stable and not rapidly renewed from the bone marrow. Proc Natl Acad Sci USA. 1990;87(12):4781–4784. doi: 10.1073/pnas.87.12.4781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hao Z, Rajewsky K. Homeostasis of peripheral B cells in the absence of B cell influx from the bone marrow. J Exp Med. 2001;194(8):1151–1164. doi: 10.1084/jem.194.8.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cancro MP, Kearney JF. B cell positive selection: Road map to the primary repertoire? J Immunol. 2004;173(1):15–19. doi: 10.4049/jimmunol.173.1.15. [DOI] [PubMed] [Google Scholar]
- 23.Solvason N, et al. Cyclin D2 is essential for BCR-mediated proliferation and CD5 B cell development. Int Immunol. 2000;12(5):631–638. doi: 10.1093/intimm/12.5.631. [DOI] [PubMed] [Google Scholar]
- 24.Enzler T, et al. Alternative and classical NF-κ B signaling retain autoreactive B cells in the splenic marginal zone and result in lupus-like disease. Immunity. 2006;25(3):403–415. doi: 10.1016/j.immuni.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 25.Shinners NP, et al. Bruton’s tyrosine kinase mediates NF-kappa B activation and B cell survival by B cell-activating factor receptor of the TNF-R family. J Immunol. 2007;179(6):3872–3880. doi: 10.4049/jimmunol.179.6.3872. [DOI] [PubMed] [Google Scholar]
- 26.Woodland RT, Schmidt MR, Korsmeyer SJ, Gravel KA. Regulation of B cell survival in xid mice by the proto-oncogene bcl-2. J Immunol. 1996;156(6):2143–2154. [PubMed] [Google Scholar]
- 27.Lindsley RC, Thomas M, Srivastava B, Allman D. Generation of peripheral B cells occurs via two spatially and temporally distinct pathways. Blood. 2007;109(6):2521–2528. doi: 10.1182/blood-2006-04-018085. [DOI] [PubMed] [Google Scholar]
- 28.Schmidt-Supprian M, et al. NEMO/IKK gamma-deficient mice model incontinentia pigmenti. Mol Cell. 2000;5(6):981–992. doi: 10.1016/s1097-2765(00)80263-4. [DOI] [PubMed] [Google Scholar]
- 29.Calado DP, et al. The cell-cycle regulator c-Myc is essential for the formation and maintenance of germinal centers. Nat Immunol. 2012;13(11):1092–1100. doi: 10.1038/ni.2418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Strasser A, et al. Enforced BCL2 expression in B-lymphoid cells prolongs antibody responses and elicits autoimmune disease. Proc Natl Acad Sci USA. 1991;88(19):8661–8665. doi: 10.1073/pnas.88.19.8661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schmidt-Supprian M, et al. I kappa B kinase 2 deficiency in T cells leads to defects in priming, B cell help, germinal center reactions, and homeostatic expansion. J Immunol. 2004;173(3):1612–1619. doi: 10.4049/jimmunol.173.3.1612. [DOI] [PubMed] [Google Scholar]