Lieberman and Eisenberger (1) claim that the “dorsal anterior cingulate cortex (dACC) is selective for pain.” This surprising conclusion contradicts a large body of evidence showing robust dACC responses to nonpainful conditions. Electrophysiological and optogenetic studies have identified neuronal populations activated during foraging behavior, attention, emotion, reward expectancy, skeletomotor and visceromotor activity, and other functions (e.g., refs. 2–5). Only a small minority of dACC neurons are pain-related.
Lieberman and Eisenberger (1) later propose that the dACC responds to “enduring survival-relevant goals,” including hunger and social rejection. This hypothesis appears inconsistent with selectivity for pain, with attention- (3) and motor-coding (4) dACC neurons, and with demonstrations of dissociable representations of pain and rejection in dACC (6). We agree that dACC subserves survival-relevant functions; however, acceptance of dACC as “pain-selective” will lead the field down the wrong track.
Lieberman and Eisenberger’s (1) conclusions are based on Neurosynth.org (7), a database of activation coordinates and words used in >11,000 neuroimaging studies. The claim of pain selectivity is based on a statistical preference in dACC activation studies for the use of pain-related words, compared with a modest number of alternatives (e.g., “salience”). Neurosynth analyses are based on word frequencies in published papers. They may not reflect the actual processes studied, and are not linked specifically to particular brain locations. They are subject to biases in how different literatures use words and label brain areas (e.g., “salience” has multiple meanings, and dACC is also called anterior mid-cingulate cortex). Neurosynth is useful for exploring structure-to-function mappings across a large literature, but it cannot provide definitive inferences about specific brain regions.
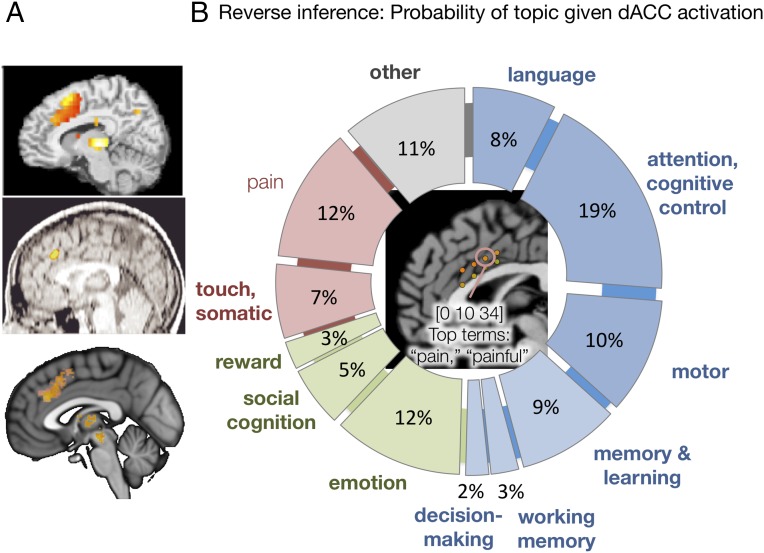
Nonetheless, Lieberman and Eisenberger (1) wrote that the “best interpretation of dACC activity is in terms of pain…,” implying that dACC activity can be used as an indicator for pain and/or related survival-relevant functions. This sets a dangerous precedent, and would yield erroneous conclusions in many instances (Fig. 1A). In addition, Lieberman and Eisenberger’s claims of selectivity are based on a flawed method of making “reverse inferences” (RI), inferences based on the posterior probability of a mental state (S) given regional fMRI activation. Valid RI requires estimating the probability of S given activation using Bayes’ rule. Unlike prior metaanalyses testing RI (e.g., ref. 7), Lieberman and Eisenberger’s analyses do not do this. Rather, they use a nonstandard comparison of Z-scores for pain with other individual keywords. This has been extensively critiqued elsewhere (www.talyarkoni.org/blog/2015/12/14/still-not-selective-comment-on-comment-on-comment-on-lieberman-eisenberger-2015/) and is not equivalent to estimating posterior probabilities. In fact, using the same database, we estimate the probability of a study inducing physical pain given activity in pain-selective dACC at ∼12%, on par with language, emotion, attention, and memory (Fig. 1B).
Fig. 1.
The dACC is not selective for pain. (A) dACC activation in studies of (Top) cognitive control [go/no-go (8)], (Middle) response conflict (9), and (Bottom) reward (10). If pain were considered the “best interpretation” of dACC activity, these studies would be erroneously classified as pain-related. (B) Posterior probabilities of study topic given activation in the pain-selective dACC, using empirical priors. Center shows Lieberman and Eisenberger’s (1) pain-selective dACC coordinates. They find that seven out of eight of these locations are highly selective for pain, including the one circled [Montreal Neurological Institute (MNI) coordinates 0, 10, 34]. The top terms in Neurosynth’s “reverse inference” list are indeed pain-related. However, this does not reflect the actual topics studied or the relevant posterior probabilities. We (T.D.W. and laboratory members) manually identified the topics of the first 240 (of 647 total) studies reporting brain activation within 8 mm of MNI coordinates 0, 10, 34 based on the paper titles and abstracts, and used them to calculate the posterior probabilities shown in the figure. The probability that the study topic was pain was 12%. More broadly, 50% of dACC activation studies were focused on cognition (blue), 20% were focused on socioemotional processes (green), and 19% were focused on pain, somatosensation, or other somatic referents (red). The remaining studies were difficult to categorize or irrelevant (mainly resting state and methodological studies). This analysis does not imply that there is no pain-related information in the dACC, but it does imply that dACC is not especially selective for either pain or a special class of survival-relevant functions. Images courtesy of (A, Top) ref. 8, with permission from Elsevier, and (A, Middle) ref. 9. A, Bottom is a schematic based on findings in ref. 10.
Finally, the seductive notion of a single, one- or two-word “best interpretation” of the dACC’s ∼550 million neurons is based on a flawed premise. dACC neurons code for a broad spectrum of mental processes. Instead of trying to find the single word or phrase that best characterizes dACC function, we should use rigorously designed experiments to identify signals within dACC that are common and/or specific to particular information processing functions (e.g., ref. 6), and use information distributed across multiple brain regions to perform meaningful structure-to-function mapping.
Footnotes
The authors declare no conflict of interest.
References
- 1.Lieberman MD, Eisenberger NI. The dorsal anterior cingulate cortex is selective for pain: Results from large-scale reverse inference. Proc Natl Acad Sci USA. 2015;112(49):15250–15255. doi: 10.1073/pnas.1515083112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kvitsiani D, et al. Distinct behavioural and network correlates of two interneuron types in prefrontal cortex. Nature. 2013;498(7454):363–366. doi: 10.1038/nature12176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davis KD, et al. Human anterior cingulate cortex neurons encode cognitive and emotional demands. J Neurosci. 2005;25(37):8402–8406. doi: 10.1523/JNEUROSCI.2315-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Picard N, Strick PL. Motor areas of the medial wall: A review of their location and functional activation. Cereb Cortex. 1996;6(3):342–353. doi: 10.1093/cercor/6.3.342. [DOI] [PubMed] [Google Scholar]
- 5.Shidara M, Richmond BJ. Anterior cingulate: Single neuronal signals related to degree of reward expectancy. Science. 2002;296(5573):1709–1711. doi: 10.1126/science.1069504. [DOI] [PubMed] [Google Scholar]
- 6.Woo CW, et al. Separate neural representations for physical pain and social rejection. Nat Commun. 2014;5:5380. doi: 10.1038/ncomms6380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yarkoni T, Poldrack RA, Nichols TE, Van Essen DC, Wager TD. Large-scale automated synthesis of human functional neuroimaging data. Nat Methods. 2011;8(8):665–670. doi: 10.1038/nmeth.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wager TD, et al. Common and unique components of response inhibition revealed by fMRI. Neuroimage. 2005;27(2):323–340. doi: 10.1016/j.neuroimage.2005.01.054. [DOI] [PubMed] [Google Scholar]
- 9.Botvinick M, Nystrom LE, Fissell K, Carter CS, Cohen JD. Conflict monitoring versus selection-for-action in anterior cingulate cortex. Nature. 1999;402(6758):179–181. doi: 10.1038/46035. [DOI] [PubMed] [Google Scholar]
- 10.Bartra O, McGuire JT, Kable JW. The valuation system: A coordinate-based meta-analysis of BOLD fMRI experiments examining neural correlates of subjective value. Neuroimage. 2013;76:412–427. doi: 10.1016/j.neuroimage.2013.02.063. [DOI] [PMC free article] [PubMed] [Google Scholar]